Abstract
Xenotransplantation has been considered an alternative to the moderate shortage of donor organs for transplantation. To achieve successful xenotransplatation, there is the need to overcome immune rejection. Although, hyperacute rejection has been overcome by α1,3-galactosyltransferase knockout pig, cellular immune rejection remains as a subsequent barrier. Interleukin-10 (IL-10) is known as an anti-inflammatory and immunomodulatory cytokine which has been shown to limit inflammatory responses by inhibiting macrophage activation in several animal experiments. To study the effect of human IL-10 (hIL-10) on pig-to-human xenotransplantation, porcine kidney epithelial cell line (PK(15)) expressing hIL-10 was established. The cytotoxicity of macrophages decreased by hIL-10 from transgenic cells. Furthermore, there is a decreased production of pro-inflammatory cytokines, tumor necrosis factor-α and interleukin-23, and increased anti-inflammatory cytokines like IL-10, but not transforming growth factor beta, in the presence of hIL-10. Also, macrophage polarization toward M2-like phenotype were induced by hIL-10 from transgenic PK(15) cells. Finally, we suggest that the cytotoxicity of human macrophages was reduced by hIL-10 from transgenic cells, inducing M2-like macrophage polarization. Therefore, these results show that hIL-10 transgenic pig can be used as a model to overcome acute immune rejection in pig-to-human xenotransplantation.
Keywords: IL-10, Macrophage, Porcine, Xenotransplantation
Highlights
-
•
The effect of human IL-10 (hIL-10) on pig-to-human xenotransplantation was studied.
-
•
Cytotoxicity of macrophages decreased by hIL-10 from transgenic cells.
-
•
hIL-10 induced macrophage polarization toward M2-like phenotype.
1. Introduction
Currently, txenotransplantation is be an alternative therapeutic technology for overcoming the shortage of organ donation [1]. However, there are three main limitations which are social ethical problem, zoonosis and immunological rejection [2]. To overcome immune rejection following xenotransplantation, genetic manipulation of α1,3-galactosyltransferase (GalT), Non-Gal-antigens and complement regulatory genes relating to hyperacute rejection was researched [3]. However, in the acute rejection initiated by the deposition of antibody and complement [4] followed by sequential T cell responses [5], macrophages, which are antigen-presenting cells (APCs), acted as the bridge between innate and adaptive immunity.
According to recent studies, Interleukin-10 (IL-10) is an immunoregulatory and anti-inflammatory cytokine having a central role related to the inactivation of immune cells [5,6]. Among immune cells, IL-10 can reduce macrophage activation and proliferation through STAT3 signaling [7]. M2 macrophages induced by IL-10 have the characteristics functional inhibitory markers and the ability to reduce inflammation, phagocytosis capacity, producing extracellular matrix components, angiogenic and chemotactic factors [[8], [9], [10]]. Also, IL-10 secreted from macrophages in IL-10 autocrine manner may inhibit the activation of CD4+ and CD8+ cells and promote the induction of IL-10-secreting regulatory T cells [[11], [12], [13], [14]].
In a xenotransplantation of pancreatic islet from rat to mice, macrophage activity was confirmed to be reduced with IL-10/Fc administration, however T cell infiltration was not decreased, and survivability was prolonged [15]. In addition, IL-10 overexpression through lentivirus injection into a wound decreased inflammation, making environment conducive for wound healing [16].
Thus, we wondered if the cytotoxicity of THP-1-differentiated-macrophage could decrease against human porcine cells expressing human IL-10 (hIL-10) than the wild type cells. We also examined whether macrophage polarization could be changed by IL-10 secreted from transgenic cells in vitro. The results of this study showed that there was reduced cytotoxicity by hIL-10 expression in transgenic pig cells and M2 macrophage polarization could be induced by secreted-IL-10-reaction with macrophages.
2. Materials and methods
2.1. Cell culture
THP-1 cell line (American Type Culture Collection, TIB-202) was cultured in RPMI-1640 (Gibco, 22240-089) containing 10% of heat inactivated fetal bovine serum (FBS, Hycolone, SH30919.03), 1% MEM non-essential amino acid solution (Gibco, 11140), 1% Penicillin/Streptomycin (P/S, Gibco, 15140), and 0.1 mM β-mercaptoethanol (β-ME, Sigma, M7522) at 37 °C in an incubator with a 5% CO2 atmosphere. To obtain macrophages, THP-1 were differentiated with 10 ng/ml phorbol-12-myristate-13-acetate (PMA, Sigma, P8139), and 500 ng/mL ionomycin (Sigma, I3909) for 48 hours, with media changed the next day for 24 hours. Porcine Kidney epithelial cell line (PK(15), American Type Culture Collection, CCL-33) line was maintained in Dulbeco's Modified Eagle's Medium (DMEM, Hyclone, SH30243.01) containing 10% FBS, 1% MEM non-essential amino acid solution, 1% P/S, and 0.1 mM β-ME at 37 °C in an incubator with a 5% CO2 atmosphere. PK(15) cell was transfected with pcDNA3.1/hygromycin (−) -hIL-10 using Lipofectamine 2000 (Invitrogen, 11668-027) following the manufacturer's instructions. From 24 hours post-transfection, transgenic cells were added with 300 μg/mL hygromycin (Sigma, H3274) for 2 weeks. After 300 μg/mL hygromycin selection, transfected cells were transferred to the cell-culture-dish and maintained in complete DMEM at 37 °C in an incubator with a 5% CO2 atmosphere.
2.2. Gene manipulation of IL-10
Human interleukin-10 (hIL-10) complementary DNAs (cDNA) were synthesized by reverse transcription-polymerase chain reaction (RT-PCR) from mRNA of THP-1 cells using the PCR cloning primers. The PCR product was inserted to pcDNA3.1/hygromycin (−) (Invitrogen) with restriction enzymes, NotⅠ and BamHⅠ.
2.3. Quantitative PCR
Total RNA was isolated from PK(15), PK(15)-hIL10, THP-1-derieved-macrophages or THP-1-derieved-macrophages treated with culture soup by using Trizol (Life Technologies, 15596018). cDNAs were synthesized by using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems™, 4368814) (Table 1). SYBR® Premix Ex taq™Ⅱ (Takara, RR820) -base quantitative PCR (qPCR) was conducted using human primers to Ccr7, Cd163, Gapdh, Il10, Il23, Tnfα and Tgfβ. The protocol for performing qPCR, was as follows: 95 °C for 10 minutes, 40 cycles of 95 °C for 15 seconds, and 60 °C for 1 minutes on QuantStudio 5 Real-Time PCR System (Applied Biosystems). mRNA levels were calculated to Gapdh and reported as relative mRNA expression (△△Ct) or fold change.
Table 1.
Primers used in quantitative RT-PCR, TM, 60 ℃.
| Species | Gene | Sequence | |
|---|---|---|---|
| Human |
GAPDH | F | 5′_CCACTCCTCCACCTTTGAC_3′ |
| R | 5′_ACCCTGTTGCTGTAGCCA_3′ | ||
| IL-10 | F | 5′_ATGCACAGCTCAGCACTGCTCTGT_3′ | |
| R | 5′_TCAGTTTCGTATCTTCATTGTCATGTAGGC_3′ | ||
| IL-10-qPCR | F | 5′_GCTGTCATCGATTTCTTCCC_3′ | |
| R | 5′_TCAAACTCACTCATGGCTTTGT_3′ | ||
| CCR7 | F | 5′_AGTCTTCCAGCTGCCCTACA_3′ | |
| R | 5′_TCGTAGGCGATGTTGAGTTG_3′ | ||
| CD163 | F | 5′_CCAGTCCCAAACACTGTCCT_3′ | |
| R | 5′_CACTCTCTATGCAGGCCACA_3′ | ||
| IL-23 | F | 5′_AGCCAACTCCTGCAGCCTGA_3′ | |
| R | 5′_TGCGAAGGATTTTGAAGCGG_3′ | ||
| TNF-α | F | 5′_ACTGCACAGCAGTTCCACAG_3′ | |
| R | 5′_ACTCTGGTTGGCTTCCTTCA_3′ | ||
| TGF-β |
F | 5′_CCCTGGACACCAACTATTGC_3′ | |
| R |
5′_GCAGAAGTTGGCATGGTAGC_3′ |
||
| Porcine | GAPDH | F | 5′_GCCATCACTGCCACCCAGAA_3′ |
| R | 5′_GCCAGTGAGCTTCCCGTTGA_3′ |
2.4. Cytotoxicity assay
THP-1 cells were seeded in 96 well culture plate (SPL, 30096) with 10 ng/mL PMA and 500 ng/mL ionomycin for 48 hours, with media changed the next day for 24 hours. After media change on differentiated THP-1 cell, Target cells, PK(15) cells and PK(15)-hIL10 which is transfected human IL -10 in the PK(15) cell line were labelled with CellTrace™ CFSE (Life technologies™, C34554) and seeded in a 96 well plate with THP-1-dereived-macropahges. THP-1-differentiated-macrophages were co-cultured with target cells for 24 hours with E:T ratio; 1:1, 2:1, 5:1, 10:1, and 20:1. Co-cultured cells were stained by 7-AAD (BD, 559925) in 100 μL of PBS. The cytotoxicity of the macrophage was analyzed with the FACS Calibur flow cytometer (Becton Dickinson) as the percentage of dead cells (CFSE+7-AAD+).
2.5. Western blotting
For hIL-10 detection, the supernatant of PK(15) and PK(15)-hIL10 was collected and centrifuged at 300 g for 10 minutes. The samples were lyophilized using Freezone Plus (Labconco, 7960040) and the protein concentration was determined with the Bradford assay (Bio-rad, 500-0006). Proteins (5 μg/well) were added 2 X Laemli sample buffer (Bio-rad, 1610737) and reduced. Samples were separated by electrophoresis (Life technologies™, B1000) on 4 %–12% Bis-Tris polyacrylamide gels (Invitrogen, NW04120BOX), and the bands were transferred to nitrocellulose membrane (Bio-rad, 1620115). The membranes were blocked in Dulbecco’s Phosphate Buffered Saline (Welgene, LB001-02) with 0.1% tween 20 (Sigma, P9416)/5% skim milk (BD, 232100) for 1 hour at room temperature. Primary and secondary antibodies were diluted at 1:1000 and 1:5000, respectively for blotting. Quantitation and imaging of western blots were done using LAS 3000 imaging system (Fuji), following the manufacturer's instructions.
2.6. Antibody and reagents
To perform flow cytometry and Western blot, the following materials were used: goat anti-mouse IgG antibody, peroxidase conjugated, H+L (Millipore, AP124P), anti-human IL-10 (Peprotech, 500-M86), recombinant hIL-10 (Peprotech, 200-10), and western blotting luminol reagent (Santa Cruz, sc-2048).
2.7. Statistical analysis
Data were analyzed with GraphPad Prism 7 (GraphPad Software, USA) and represented as mean ± SD. All experiments were performed in triplicates. Statistical analyses were performed using a two-tailed Student's t-test. Differences were considered statistically significant at p < 0.05.
3. Result
3.1. Evaluation of hIL-10 gene manipulation and protein secretion in the PK(15)
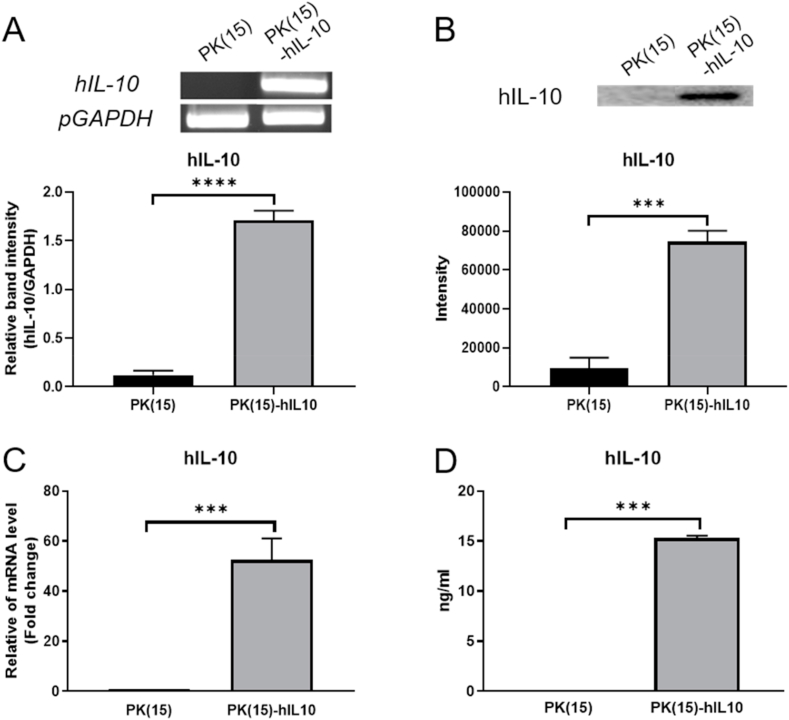
To determine the reduced cytotoxicity effect of hIL-10 on pig-to-human xenotransplantation in vitro, hIL-10 expressing PK(15) cells were established using CMV-promoter, which was whole body expressed. The genetic expression of hIL-10 on PK(15) cells was detected by qPCR and the secreted protein of hIL-10 was quantified using the Western blot. According to the result, the mRNA levels of hIL-10 in transgenic cells were significantly higher than those in normal PK(15) cells (Fig. 1A and C). The secreted protein of hIL-10 was detected in the culture soup of PK(15)-hIL10 cells and the result of the quantification that was 15.31 ± 0.1297 ng/mL (Fig. 1B and D). It means that the hIL-10 secreting porcine cell line was well established and that it can be used as xenogenic antigen model of the human immune system with IL-10 supplement.
Fig. 1.
Establishment of PK(15) expressing hIL-10. The expression levels of hIL-10 determined by (A) RT-PCR, (B) Western blot, (C) real-time quantitative PCR and (D) ELISA. Data are presented as mean ± S. D (n = 3 biological and technical triplicates). (***P < 0.0005 and ****P < 0.0001).
3.2. Inhibition of xenogenic cytotoxicity by hIL-10 secretion in PK(15) cells
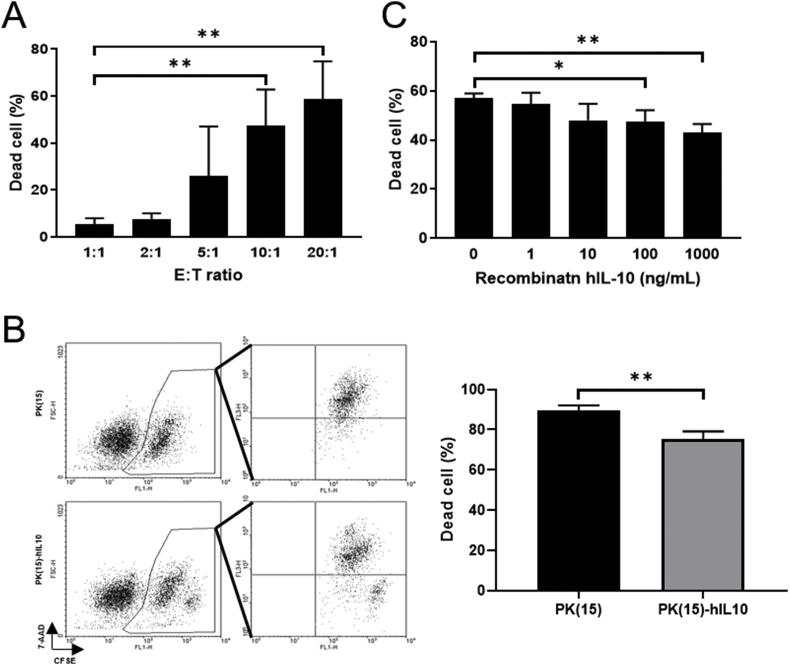
To figure out the immune modulating effect of hIL-10 in pig-to-human co-culture system, firstly, the best effector to target ratios for the cytotoxicity assays were validated. According to the result, the E:T ratio of 10:1 was sufficient to induce the killing of PK(15) cells after 24 hours by THP-1-derived-macrophage co-culture (Fig. 2A). Based on this result, cytotoxicity assay was conducted at an E:T ratio of 10:1. The proportion of dead cells which occurred through THP-1-derived-macrophages was decreased (14.17% ± 2.652%) in the PK(15)-hIL10 group compared to the PK(15) group (Fig. 2B). This suggests that hIL-10 secreted by porcine cells can play the role of immune modulation in xenogenic cytotoxicity.
Fig. 2.
Measurement of cytotoxicity on THP-1-derieved-macrophages by secreted hIL-10. (A) E:T ratio optimization for THP-1-derived-macrophages versus PK(15) cells in cell to cell cytotoxicity assay. (B) Result of the cytotoxicity assay in IL-10 expressing PK(15) cells. (C) Cytotoxicity assay after treatment with rhIL-10-dose-dependent manners. Data are presented as mean ± S. D (n = 3 biological replicates). (*P < 0.05, **P < 0.005).
In order to determine whether or not the decrement in the cytotoxicity of PK(15)-hIL10 was due to the effects of hIL-10, recombinant protein hIL-10 (rhIL-10), an assay was performed under co-culture with human macrophage and PK(15) cells. We found the cytotoxicity of THP-1-derieved-macrophage to normal PK(15) cells significantly decreased from 100 ng/mL rhIL-10 treatment (Fig. 2C). These results show that the secreted hIL-10 from transgenic cells had the ability to reduce the xenogenic cytotoxicity of THP-1-derieved-macrophages.
3.3. hIL-10 induces M2 macrophage polarization xenogenic antigen exposure condition
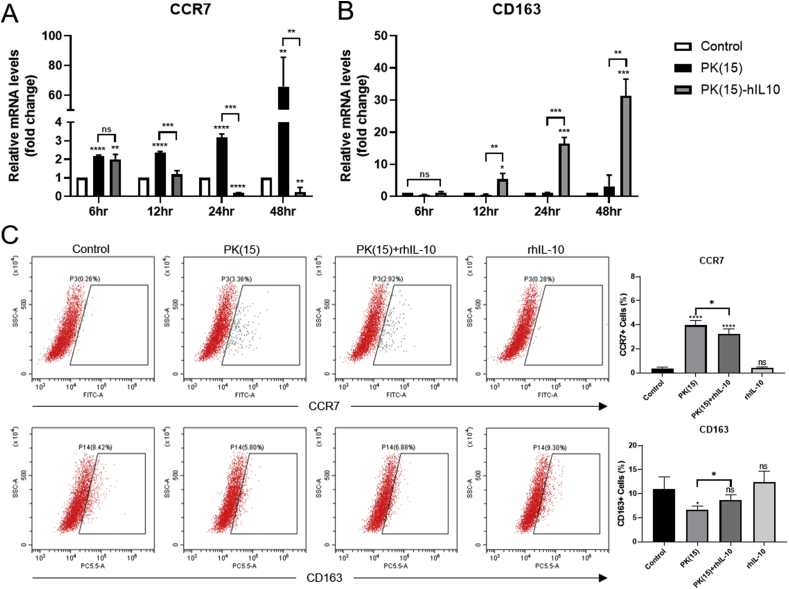
In previous studies, IL-10-stimulated-macropahges could be induced towards M2 macrophage polarization [17,18]. To determine whether M2 polarization was induced by IL-10 with xenogenic antigen, the THP-1-derieved-macrophages were treated with the culture soup of PK(15) and PK(15)-hIL10 for 48 hours. The mRNA levels of CCR7 and CD163 as M1 and M2 markers, respectively, were quantified through qPCR analysis. There were decreasing CCR7 mRNA expressions in PK(15)-hIL10 group, while they increased in PK(15) group in a time-dependent manner and there was significant difference between the PK(15) and PK(15)-hIL10 group from 12 hours (Fig. 3A). Also, the CD163 mRNA expression increased in the group treated with the culture soup of PK(15)-hIL10 compared to the WT conditions (Fig. 3B). To investigate that the markers of macrophage polarization were regulated by IL-10, THP-1-dereived-macrophages were treated with culture soup of PK(15) or containing 100 ng/mL rhIL-10 for 24 hours. Compared with the PK(15) group, rhIL-10 decreased CCR7 and increased CD163 in PK(15)-containing rhIL-10 group (Fig. 3C). These data show that the macrophages stimulated secreted hIL-10 with xenogenic antigens were induced toward M2-like macrophages polarization.
Fig. 3.
Analysis of M2 Macrophage polarization induced secreted hIL-10 from PK(15) cells in time-dependent-manners. The expression level of (A) CCR7, M1 macrophage marker, and (B) CD163, M2 macrophage marker, were analyzed by qPCR. (C) CCR7 and CD163 were analyzed by flow cytometry. Data are presented as mean ± S. D (n = 3 biological and technical replicates). (*P < 0.05, **P < 0.005, ***P < 0.0005, ****P < 0.0001, ns, not significant *compared to THP-1-derived-macrophages treated normal media.)
3.4. Cytokine profiling of human macrophage caused by hIL-10 with xenogenic antigen
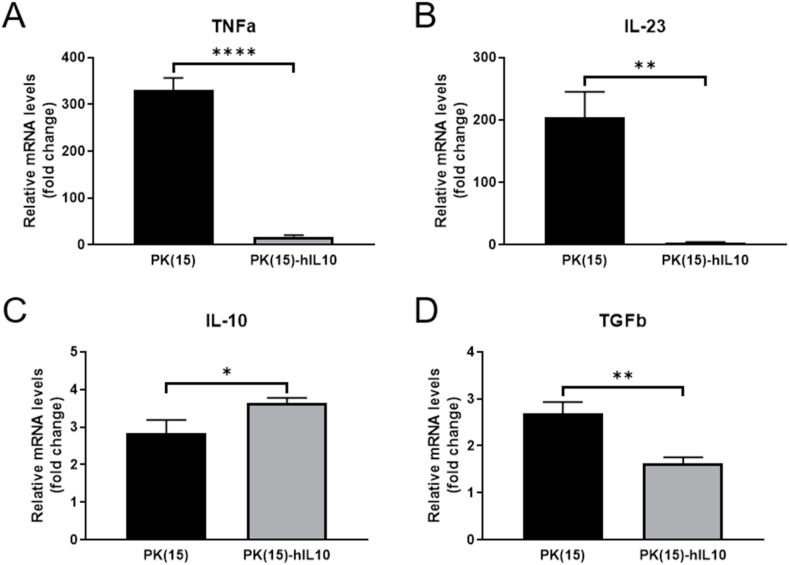
The regulated effect of macrophages treated hIL-10 with xenogenic antigens was investigated by determining, the gene expression profile of THP-1-derived-macrophages in xenograft status, using qPCR analysis. While confirming the gene expression pattern of the culture soup-treated macrophage at 48 hours, a significant decrease in the expression of TNFα and IL-23 was observed in the PK(15)-hIL10 group compared to the PK(15) group. On the other hand, in the PK(15)-hIL10 group, IL-10, an anti-inflammatory cytokine, was confirmed to increase while not TGFβ (Fig. 4A–D). These results indicate that the expression of pro-inflammatory cytokine in macrophage is reduced by the secreted hIL-10, and, conversely, the anti-inflammatory cytokine is increased, thereby reducing the cytotoxicity of the target cells.
Fig. 4.
The quantification of cytokine expression levels in THP-1-derieved-macrophages treated hIL-10 with xenogenic antigen. The mRNA expression levels of pro-inflammatory cytokines (A) TNFα, (B) IL-23 and anti-inflammatory cytokines (C) IL-10, (D) TGFβ determined by qPCR. Data are presented as mean ± S. D (n = 3 technical replicates). (*P < 0.05, **P < 0.005, ****P < 0.0005).
4. Discussion
Previous reports have shown that IL-10 promotes M2-like polarization giving rise to M2-like functional phenotypes that have similar properties to IL-4- or IL-13-activated macrophages [18,19]. Focused on xenotransplantation, we confirmed that the secreted IL-10 from transgenic porcine cells can regulate the polarization of human macrophages. In this study, hIL-10-expressing PK(15) cells exhibited reduced cytotoxicity of macrophages than WT PK(15) cells. These results confirm that macrophage polarization was induced toward M2 stage by secreted IL-10 from PK(15)-hIL10 cells, as reported in previous studies [17,20,21].
In previous studies, macrophages were induced toward M1 macrophages by the supernatant of PK(15) in vitro culture with xenoantigens [22,23]. Also, we confirmed that the level of CCR7 was decreased while the level of CD163 was increased in THP-1-derieved-macrophages co-cultured with hIL-10. In addition, the cytokine levels of M2 macrophages were up-regulated except those of TGFβ, while the cytokine levels of M1 macrophages were down-regulated in THP-1-derived-macrophages which was co-cultured with hIL-10. The reduced cytotoxic effect of macrophages was thought to be due to the M2 macrophage polarization by the secreted hIL-10.
IL-10 is known as the immunomodulatory cytokine that regulates macrophage proliferation and inhibits pro-inflammatory cytokines such as IL-1β, IL-6, and TNFα expressed by macrophages [24]. In this study, TNFα and IL-23 pro-inflammatory cytokine, were down-regulated by IL-10 with xenogenic antigen in THP-1-derieved-macrophages. Decreased IL-23 that expands Th17 cells and maintains their characters means that the role of IL-10 in xenogenic status can inhibit the Th17 axis [25]. Staples, K. J et al. demonstrated that IL-10 induces IL-10 in macrophages in an autocrine [14]. In our study, the autocrine reaction of IL-10 confirmed that the level of IL-10 mRNA expression increased with a significant difference compared to the WT condition. This indicates that IL-10 with xenogenic antigen could induce M2 macrophage polarization. In the previous study, after co-culture of M2 macrophages and M1 macrophages, the cytokine expression pattern of M1 macrophages was determined. As a result, the expression level of IL-10 increased, and TGFβ did not show significant difference in M1 macrophages. In this case, inflammatory macrophages were deactivated by M2 macrophages [26]. One the other hand, in this study, we suggested that macrophages which have cytotoxicity against PK(15) cells were deactivated by PK(15)-hIL10-secreted IL-10. However, we think it is necessary to investigate why the TGFβ expression decreased in macrophages treated secreted IL-10.
In this study, we found that hIL-10 expressing from porcine cells induces macrophage polarization into M2-like macrophages, reducing the cytotoxic effect of human macrophages. Therefore, these findings suggest that hIL-10 transgenic pig may be considered a useful model to overcome xenograft rejection.
CRediT authorship contribution statement
Young Kyu Kim: Methodology, Software, Validation, Data curation, Writing - original draft, Writing - review & editing, Visualization. Sang Eun Kim: Methodology, Validation, Visualization. Hyo Chang Park: Methodology, Validation. Jeong Ho Hwang: Conceptualization, Supervision, Funding acquisition, Writing - review & editing. Hoon Taek Lee: Conceptualization, Supervision, Funding acquisition, Writing - review & editing.
Declaration of competing interest
Authors declare no conflict of interest including any financial, personal or other relationships with other people or organization that could inappropriately influence, or be perceived to influence the work.
Acknowledgements
One of the authors thank the support from the Korea Institute of Toxicology (KIT, Korea) grant funded by the Ministry of science and ICT (MIST, Korea). [Project number: KK-1911].
Contributor Information
Jeong Ho Hwang, Email: jeongho.hwang@kitox.re.kr.
Hoon Taek Lee, Email: htl3675@konkuk.ac.kr.
References
- 1.Ekser B., Cooper D.K.C., Tector A.J. The need for xenotransplantation as a source of organs and cells for clinical transplantation. Int. J. Surg. 2015;23:199–204. doi: 10.1016/j.ijsu.2015.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekser B., Li P., Cooper D.K.C. Xenotransplantation: past, present, and future. Curr. Opin. Organ Transplant. 2017;22:513–521. doi: 10.1097/MOT.0000000000000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sykes M., Sachs D.H. Transplanting organs from pigs to humans. Sci. Immunol. 2019;4 doi: 10.1126/sciimmunol.aau6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper D.K.C., Ekser B., Tector A.J. Immunobiological barriers to xenotransplantation. Int. J. Surg. 2015;23:211–216. doi: 10.1016/j.ijsu.2015.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scalea J., Hanecamp I., Robson S.C., Yamada K. T-cell-mediated immunological barriers to xenotransplantation. Xenotransplantation. 2012;19:23–30. doi: 10.1111/j.1399-3089.2011.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saraiva M., O'Garra A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 7.Anne-Marie O'Farrell Y.L., Moore Kevin W., Alice L.-F. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathway. EMBO J. 1998;17:13. doi: 10.1093/emboj/17.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediat. Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lurier E.B., Dalton D., Dampier W., Raman P., Nassiri S., Ferraro N.M., Rajagopalan R., Sarmady M., Spiller K.L. Transcriptome analysis of IL-10-stimulated (M2c) macrophages by next-generation sequencing. Immunobiology. 2017;222:847–856. doi: 10.1016/j.imbio.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chazaud B. Macrophages: supportive cells for tissue repair and regeneration. Immunobiology. 2014;219:172–178. doi: 10.1016/j.imbio.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Blazar B.R., Taylor P.A., Panoskaltsis-Mortari A., Narula S.K., Smith S.R., Roncarolo M.G., Vallera D.A. Interleukin-10 dose-dependent regulation of CD4+ and CD8+ T cell-mediated graft-versus-host disease. Transplantation. 1998;66:1220–1229. doi: 10.1097/00007890-199811150-00018. [DOI] [PubMed] [Google Scholar]
- 12.Groux H., Bigler M., de Vries J.E., Roncarolo M.G. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J. Exp. Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawrylowicz C.M., O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat. Rev. Immunol. 2005;5:271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 14.Staples K.J., Smallie T., Williams L.M., Foey A., Burke B., Foxwell B.M., Ziegler-Heitbrock L. IL-10 induces IL-10 in primary human monocyte-derived macrophages via the transcription factor Stat3. J. Immunol. 2007;178:4779–4785. doi: 10.4049/jimmunol.178.8.4779. [DOI] [PubMed] [Google Scholar]
- 15.Feng X., Zheng X.X., Yi S., Lehnert A.M., Strom T.B., O'Connell P.J. IL-10/Fc inhibits macrophage function and prolongs pancreatic islet xenograft survival. Transplantation. 1999;68:1775–1783. doi: 10.1097/00007890-199912150-00023. [DOI] [PubMed] [Google Scholar]
- 16.Peranteau W.H., Zhang L., Muvarak N., Badillo A.T., Radu A., Zoltick P.W., Liechty K.W. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J. Invest. Dermatol. 2008;128:1852–1860. doi: 10.1038/sj.jid.5701232. [DOI] [PubMed] [Google Scholar]
- 17.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biswas S.K., Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat. Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 20.Lang R., Patel D., Morris J.J., Rutschman R.L., Murray P.J. Shaping gene expression in activated and resting primary macrophages by IL-10. J. Immunol. 2002;169:2253–2263. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 21.Fabriek B.O., Dijkstra C.D., van den Berg T.K. The macrophage scavenger receptor CD163. Immunobiology. 2005;210:153–160. doi: 10.1016/j.imbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Jung S.H., Hwang J.H., Kim S.E., Kim Y.K., Park H.C., Lee H.T. Human galectin-9 on the porcine cells affects the cytotoxic activity of M1-differentiated THP-1 cells through inducing a shift in M2-differentiated THP-1 cells. Xenotransplantation. 2017:24. doi: 10.1111/xen.12305. [DOI] [PubMed] [Google Scholar]
- 23.Jung S.H., Hwang J.H., Kim S.E., Young Kyu K., Park H.C., Lee H.T. The potentiating effect of hTFPI in the presence of hCD47 reduces the cytotoxicity of human macrophages. Xenotransplantation. 2017;24 doi: 10.1111/xen.12301. [DOI] [PubMed] [Google Scholar]
- 24.Moore K.W., de Waal Malefyt R., Coffman R.L., O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 25.Langrish C.L., McKenzie B.S., Wilson N.J., de Waal Malefyt R., Kastelein R.A., Cua D.J. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol. Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 26.Cao Q., Wang Y., Zheng D., Sun Y., Wang Y., Lee V.W., Zheng G., Tan T.K., Ince J., Alexander S.I., Harris D.C. IL-10/TGF-beta-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J. Am. Soc. Nephrol. 2010;21:933–942. doi: 10.1681/ASN.2009060592. [DOI] [PMC free article] [PubMed] [Google Scholar]