Abstract
Zanthoxylum armatum (ZA) a commonly used medicinal plant was investigated for phytochemical, anti-nociceptive, anti-inflammatory and antipyretic effects. Extract and total alkaloids from fruit and leaves significantly (p < 0.001) reduced the rectal temperature in mice. The effects of bark and root extracts were less significant. In writhing and tail flick methods both the extract and total alkaloids from fruit showed significant (p < 0.05 and p < 0.001) antinociceptive activity. The fruit extract and crude alkaloids showed significant (p < 0.01) lowering of inflammation of paw edema in mice. Crude alkaloids from fruit and leaves showed significant enzyme inhibition with lower IC50 values for 15 and 69 against COX and 21 and 62 μg/ml against LOX. This study rationalize the usage of this spice in traditional medicine for management of pain and inflammation involving LOX and COX inhibition as possible mechanism. GC-MS analysis revealed the presence of various constituents which might contributed towards the pain and inflammation alleviation.
Keywords: Zanthoxylum armatum, Fruits and leaves, Anti-inflammatory, Antinociceptive, COX, LOX, GC-MS analysis, Chemistry, Organic chemistry, Biological sciences, Pharmaceutical science, Health sciences, Pharmacology, Alternative medicine
Zanthoxylum armatum; Fruits and leaves; anti-inflammatory; antinociceptive; COX; LOX; GC-MS analysis; Chemistry; Organic chemistry; Biological Sciences; Pharmaceutical Science; Health Sciences; Pharmacology; Alternative Medicine
1. Introduction
Presently, a plethora of drugs is available with reputed analgesic and anti-inflammatory activities. It is also known that available drugs are not always suitable for all the patients because of the limitation of potency, adverse effects and tolerability issues. Moreover, there is always a space for the search of best alternatives (Vongtau et al., 2004). Medicinal plants have been employed efficiently from the earlier times for the treatment of said ailments and many have led to the isolation of bioactive compounds now available in the market. Among them, the most important compounds are known as alkaloids. These are the largest class of compounds present in medicinal plants and have a great diversity as well. More than 12,000 different alkaloids have so far been isolated and showed a wide variety of biological and pharmacological activities (Lukhoba et al., 2006; Salminen et al., 2011). Among such medicinal plants, a well-known Asian traditional medicine is Zanthoxylum armatum DC (Rutaceae) which is well known traditional medicine in Asia (Joshi and Edington, 1990; Kala et al., 2005; Kunwar et al., 2013). It is an evergreen, thorny shrub or small tree attaining a height up to 6 m. It is found in India, Pakistan to eastward China, Korea and Japan (Phuyal et al., 2019). It is commonly known as Timur and has been traditionally used for the treatment of various diseases such as abdominal pain, headache, fever and inflammation (Mushtaq et al., 2019; Nooreen et al., 2019). Different parts of the Z. armatum such as fruits, stem, leaves, and bark have been utilized in many native medicinal systems to cure gas trouble, fever, and appetizer. It is effective to relieve stomach pain, toothache and inflammation. There is an enormous demand of Zanthoxylum armatum in the national as well as in international marketplace due to its pharmacological values (Phuyal et al., 2019) and traditional background. In ethnomedicine, we found that fruit, stem, and leaves showed analgesic, anti-inflammatory and antipyretic effects (Mushtaq et al., 2019).
The plant is rich in alkaloids contents and isolated alkaloids are known to have a variety of biological and pharmacological properties such as larvicidal, antinociceptive, antioxidant, antibiotic, hepatoprotective, antiplasmodial, cytotoxic, antiproliferative, anthelminthic, antiviral and antifungal (Negi et al., 2011). This work is based on the hypothesis that the alkaloids from this spice are not tested so for anti-inflammatory, analgesic and antinociceptive activities and to rationalize its traditional use. Based on traditional background this study was designed to conduct the antipyretic, anti-inflammatory, and antinociceptive actions of Z. armatum (fruit, stem, leaves, and root) both in-vitro and in-vivo using methanol extract and total alkaloid fractions.
2. Materials and methods
2.1. Drugs and chemicals
All the chemical and drugs were of standard quality and were procured from Sigma-Aldrich Chemical (St. Louis, MO, USA) unless otherwise stated. The test substances were prepared fresh before application in in-vivo as well as in vitro assays.
2.2. Plant collection and preparation of extract
The plant Zanthoxylum armatum was collected from Salhad, district Abbottabad, KPK Pakistan in September 2018. After confirmation by taxonomist Dr. Muhammad Nazir, the specimen was placed in the herbarium of COMSATS University Islamabad, Abbottabad campus under voucher no CUHA-198. The fruit, leaves, stem bark and root bark were washed cautiously and kept for drying at 25 °C, ground and was made into a fine powder and was preserved in an airtight container. The powder drug was soaked with 95% methanol at room temperature, filtered and the filtrate was concentrated under vacuum and preserved for further use.
2.3. Extraction of total alkaloids
Alkaloid fraction was separated by accurately weighing 5 g extract (each of fruit, stem bark, leaves, and root bark) of Z. armatum. Solution of 200 ml 10% acetic acid in ethanol was added to the extract. . The mixture was kept undisturbed for 4 h. The mixture was filtered and evaporated on a water bath until 1/4 of the original volume remains. Then precipitated alkaloids were collected and washed with dilute NH4OH, filtered and dried. The different chemical tests were performed for the confirmation of alkaloids (Gupta et al., 2013).
2.4. In vivo activities
2.4.1. Ethical clearance
The animals were procured from the National Institute of Health (NIH) Islamabad. Animals were further bred in the Animal House of Department of Pharmacy, CUI (Abbottabad). Animals were given with standard diet and water when needed at the standard circumstance in standard cages. The animal's experiments were permitted by the Research Ethical Committee (REC) Department of Pharmacy, CUI (Abbottabad) PHM-Eth/CF-M04/11–24. The experimental animals were familiarized in house for at least 7 days before the start of experiments. All the animals were placed at room temperature (25 ± 2 °C) and humidity (50 ± 5%), which were kept at the light and dark cycle for 12 h (Muhammad et al., 2017). Healthy Albino mice of either gender were selected for experimental purposes.
2.4.2. Anti-inflammatory activity
2.4.2.1. Carrageenan-induced mice paw edema
The anti-inflammatory property of Z. armatum (fruit ZAF, leaves ZAL, stem bark ZAB and root bark ZAR and corresponding total alkaloids ZAFA (alkaloids from fruit), ZALA (alkaloids from leaves), ZABA (alkaloids from stem bark), and ZARA (alkaloids from root bark), respectively) was investigated using the model of carrageenan-induced mice paw edema. Inflammation was induced as previously employed protocol (Kifayatullah et al., 2019). Methanolic extract, total alkaloid fraction, standard (Diclofenac sodium) and normal saline as vehicle were administered intraperitoneal 30 min before the injection of carrageenan. The water displacement method was used to measure the paw volume using Plythysmometer before and after carrageenan injection for 1, 3, and 5 h.
2.4.3. Antipyretic activity
Antipyretic property of Z. armatum (ZAF, ZAL, ZAB, ZAR/ZAFA, ZALA, ZABA, and ZARA) was tested in mice. Animals were divided into five groups of five animals each and weighing 18–22 g. Hyperthermia was induced in animals according to the method (S. Muhammad et al., 2017). An initial rectal body temperature of animals was noted with a clinical thermometer. The animal was fasted overnight but supplied with drinking water ad libitum. Rectal temperature of mice was recorded after 17 h. Induction of pyrexia was confirmed by 0.5 °C rise in temperature. The animals with a raised temperature of less than 0.5 °C were excluded. The extract, crude alkaloids, standard (Paracetamol), and normal saline (vehicle) were administered intraperitoneal and the rectal temperature of mice was noted at 30 min intervals for 5 h subsequent to the administration of plant extract.
2.4.4. Analgesic activity
2.4.4.1. Writhing test
The analgesic property Z. armatum (ZAF, ZAL, ZAB, ZAR/ZAFA, ZALA, ZABA, and ZARA) was studied by using the writhing response test in mice. Albino mice of either sex weighing 18–22 g were selected. The writhing was induced by an intraperitoneal injection of 0.7% acetic acid at a dose of 10 ml/kg body weight. Test samples, standard (Diclofenac sodium) and normal saline were injected intraperitoneal into the mice 30 min before acetic acid and the number of writhing were noted after 5 min of acetic acid injection for 30 min (S. Muhammad et al., 2017).
2.4.4.2. Tail immersion test
The animals were distributed into five groups and withdrawn from food before 2 h of the start of the experiment. Methanolic extract, crude alkaloid fractions, standard (Tramadol) and control vehicle were feed orally 60 min before the test. About 2–3cm of the tail of each of the mice were immersed into a water bath which contained hot water sustained at a temperature of 55 ± 5 °C. The time taken for the mice to flick/withdraw its tail from the warm water known as the reaction time was noted. The cut off time or time of no response was taken as 30 s. Degree of analgesia was calculated by the tail-flick response (S. Muhammad et al., 2017).
2.5. In vitro studies
2.5.1. LOX-5 inhibitory assay
The in-vitro study was performed to estimate lipoxygenases (LOX) inhibitory activity of Zanthoxylum armatum crude alkaloids. Lipoxygenases (5-LOX) enzymes are responsible for the conversion of arachidonic acid into the potent bioactive inflammatory mediator such as leukotrienes, thromboxanes, and prostaglandins (Shrivastava et al., 2017).
Inhibition studies of various concentrations of plant crude alkaloid (1000, 500, 250, 125 and 62.5 μg/ml) as well as reference compound viz., quercitrin (10, 50 and 100 μg/ml) was checked at 234 nm through UV-VIS spectrophotometer. A mixture was made composed of 160 ml of 100 mM Na3PO4 buffer with pH 8.0, 10 μl of extract solution and 20 μl of soybean LOX solution (167 U/ml). This mixture was placed for incubation at 25 °C for 10 min. The reaction was then originated by adding of 10 μl of the substrate (sodium linoleic acid solution). The enzymatic conversion of sodium linoleic acid to form (9Z, 11E) (13S)-13-hydroperoxyoctadeca-9, 11-dienoate was determined by observing the change of absorbance at 234 nm over a period of 6 min using UV-VIS spectrophotometer. The negative control was prepared by replacing 10 μl samples with 2.47 ml mixture of Na3PO4 buffer (5 ml) and DMSO (25 μl) into the quartz. All the reactions were carried out in triplicates. The percentage of inhibition was calculated. The IC50 value was determined by graphical method (Shrivastava et al., 2017).
2.5.2. COX inhibitory assay
Human prostaglandin H synthase isozyme (hPGHS-I) expression occurs in cos-1 cells. The COX activity was determined by using microsomal membranes (ca. 5 mg protein/ml in 0.1 M Tris HCL, pH 7.4). The membrane was sham-transfected cos-l cells. During the assay the temperature was kept at 37 °C. The starting rate of O2 was monitored employing O2 electrode (Instech Laboratories, Plymouth). The test mixture was consisted of 3 ml of 0.1 M Tris HCI′3 ml of 0.1 M Tris HCI (pH 8.0), 1 mmol phenol, 85 μg Hb and 100 p mol Arachidonic acid. Reaction was started with the addition of 5–25 μg of microsomal protein at a volume of 10–20 μl. Instant cyclooxygenase inhibitory activity was measured by adding aliquots of microsomal suspensions of hPGHS1 (10 μmol 02/min cyclooxygenase activity/aliquot) to test mixtures comprising 10 μmol arachidonate and 1000 μM concentrations of the extract/total alkaloids. The results were compared with the standard drug Aspirin (Kelm et al., 2000).
2.6. GC-MS investigation
A combined extract of all parts of Z. armatum with chloroform was investigated using GCMS instrument for nitrogen containing compounds. The experiments were carried out according to the method described (Safaei-Ghomi et al., 2009).
A Claurus 600 GC-MS, Electron Impact Ionization detector, installed with turbo mass software version 5.4.0 PerkinElmer, USA was used for analysis. Helium was used with flow rate of 1 ml/min as carrier. Column used as Elite 5MS 250 μm internal diameter, 30 m length. The column was coated with 0.25 μm dimethylpolysiloxane (Rtx-1). Auto injector with split of 1:20 was fixed. The mass spectra scan rate was 2.86 scans/second. Injection temperature was set at 250 Celsius. Ionization source was I.E 70 eV. Initial temperature was held at 50 °C for 2 min. The temperature was ramped from 50 °C to 100 °C with a rate of 15 °C/min and held for 1 min. The second ramp was 100–250 °C with the same rate and held for 1 min. And finally temperature was ramped to 300 °C and held for 0.5 min.
2.7. Statistical analysis
The results obtained were represented as the mean ± SEM (Standard error mean). The data were analyzed by using One way ANOVA followed by Dunnett's test (S. Muhammad et al., 2017).
3. Results
3.1. Antipyretic activity
Effect of methanol extract of Z. armatum fruit (ZAF) and isolated total alkaloid fraction (ZAFA) on pyrexia induced body temperature in mice is shown in Table 1. The subcutaneous injection of 10 ml kg–1 of 15% w/v of yeast suspension prominently raised the rectal temperature after 19 h. It was found that ZAF at doses of 300 mg/kg caused a significant (p < 0.01) decrease of body temperature in mice up to 5 h. Similarly, ZAFA caused a significant (p < 0.001) lowering of rectal temperature in mice after 5 h and these result were comparable to Paracetamol which was used as a standard drug. The sample ZAFA (100 mg/kg) showed a similar pattern of pyrexia control as shown by standard drug and temperature was started reducing from 1st h.
Table 1.
Antipyretic activity of Z. armatum fruit (ZAF), leaves (ZAL) extracts and isolated total alkaloid fraction (ZAFA), (ZALA) on pyrexia induced body temperature in mice.
| Treatment | Dose mg/kg | Rectal temperature after drug administration |
||||||
|---|---|---|---|---|---|---|---|---|
| Initial temp. | Pyrexia temp. | 1 h | 2 h | 3h | 4 h | 5 h | ||
| Control | 10 mL | 36.7 ± 0.09 | 39.2 ± 0.09 | 39.1 ± 0.10 | 39.0 ± 0.11 | 38.9 ± 0.08 | 38.9 ± 0.06 | 39 ± 0.18 |
| Paracetamol | 150 | 36.6 ± 0.1 | 39.2 ± 0.09 | 38.7 ± 0.08 | 37.7 ± 0.1 | 37.5 ± 0.07 | 37.3 ± 0.06∗∗ | 37.1 ± 0.08∗∗∗ |
| ZAF | 100 | 36.6 ± 0.08 | 39.2 ± 0.1 | 39.0 ± 0.1 | 38.8 ± 0.05 | 38.5 ± 0.1 | 38.1 ± 0.1 | 37.9 ± 0.09 |
| ZAF | 200 | 36.7 ± 0.09 | 39.2 ± 0.1 | 39.1 ± 1.1 | 38.7 ± 0.1 | 38.3 ± 0.1 | 37.9 ± 0.14 | 37.7 ± 0.08 |
| ZAF | 300 | 36.6 ± 0.09 | 39.1 ± 0.08 | 39.0 ± 0.06 | 38.3 ± 0.2 | 37.8 ± 0.1 | 37.5 ± 0.08 | 37.3 ± 0.06∗∗ |
| ZAFA | 50 | 36.6 ± 0.08 | 38.4 ± 0.2 | 39.4 ± 0.2 | 38.5 ± 0.2 | 38.2 ± 0.1 | 37.9 ± 0.12 | 37.8 ± 0.08 |
| ZAFA | 100 | 36.4 ± 0.1 | 38.4 ± 0.2 | 38.4 ± 0.2 | 37.6 ± 0.2 | 37.6 ± 0.2 | 37.4 ± 0.14 | 37.2 ± 0.08∗∗∗ |
| ZAL | 100 | 36.67 ± 0.08 | 39.38 ± 0.17 | 39.87 ± 0.1 | 39.77 ± 0.1 | 38.75 ± 0.1 | 38.59 ± 0.1 | 38.23 ± 0.07 |
| ZAL | 200 | 36.87 ± 0.20 | 39.21 ± 0.17 | 39.08 ± 0.0 | 38.85 ± 0.0 | 38.55 ± 0.1 | 38.13 ± 0.12 | 37.95 ± 0.09 |
| ZAL | 300 | 36.84 ± 0.20 | 39.65 ± 0.20 | 38.88 ± 0.1 | 38.56 ± 014 | 37.87 ± 0.0 | 37.71 ± 0.11 | 37.46 ± 0.19∗∗ |
| ZALA | 50 | 36.43 ± 0.09 | 39.44 ± 0.17 | 39.34 ± 0.1 | 39.11 ± 0.1 | 38.67 ± 0.1 | 38.58 ± 0.11 | 38.22 ± 0.07 |
| ZALA | 100 | 36.43 ± 0.09 | 39.11 ± 0.2 | 38.99 ± 0.1 | 38.11 ± 0.14 | 37.9 ± 0.08 | 37.550.11 | 37.12 ± 0.19∗∗∗ |
Data represented as mean ± SEM values of six animals. The data was examined by ANOVA followed by Dunnett's test using Graph pad prism software which indicate that data are statistically significant at <0.05∗, <0.01∗∗ and <0.001∗∗∗ as compare with control.
Effect of Z. armatum leaves extract (ZAL) and a total crude fraction (ZALA) on pyrexia induced body temperature in mice is presented in Table 1. The maximum effect of ZAL was with 300 mg/kg dose and was significant (p < 0.01) at 5 h time. Similarly, ZALA showed significant results (p < 0.001) at 100 mg/kg dose and was equipotent with Paracetamol used as standard drug.
Effect of methanol extract of Z. armatum stem bark (ZAB) and isolated total alkaloid fraction (ZABA) on pyrexia induced body temperature in mice is presented in Table 2. It was noted that ZAB at all doses caused less significant (p < 0.05) decrease of body temperature in mice up to 5 h of extract administration as compared to ZAF and ZAL. Similarly, ZABA showed less significant (p < 0.05) lowering of rectal temperature in mice after 5 h.
Table 2.
Antipyretic activity of Z. armatum stem bark (ZAB) extract and isolated total alkaloid fraction (ZABA) on pyrexia induced body temperature in mice.
| Treatment | Dose mg/kg | Rectal temperature after drug administration |
||||||
|---|---|---|---|---|---|---|---|---|
| Initial temp. | Pyrexia temp. | 1 h | 2 h | 3h | 4 h | 5 h | ||
| Control (NS) | 10 mL | 36.72 ± 0.99 | 39.23 ± 0.09 | 39.11 ± 0.10 | 39.06 ± 0.11 | 38.99 ± 0.08 | 38.94 ± 0.06 | 39.01 ± 0.18 |
| Paracetamol | 150mg | 36.65 ± 0.11 | 39.27 ± 0.09 | 38.78 ± 0.08 | 37.76 ± 0.11 | 37.57 ± 0.07 | 37.36 ± 0.06∗∗ | 37.18 ± 0.08∗∗∗ |
| ZAB | 100 | 36.71 ± 0.09 | 39.31 ± 0.10 | 39.27 ± 0.09 | 39.2 ± 0.08 | 38.96 ± 0.11 | 38.9 ± 0.10 | 38.71 ± 0.09 |
| ZAB | 200 | 36.72 ± 0.09 | 39.21 ± 0.10 | 38.7 ± 0.09 | 37.94 ± 0.18 | 37.72 ± 0.15 | 37.7 ± 0.10 | 37.58 ± 0.08∗ |
| ZAB | 300 | 36.4 ± 0.09 | 39.21 ± 0.10 | 38.7 ± 0.09 | 37.99 ± 0.1 | 37.55 ± 0.15 | 37.43 ± 0.10∗ | 37.43 ± 0.08∗ |
| ZABA | 50 | 36.4 ± 0.09 | 39.2 ± 0.10 | 38.7 ± 0.09 | 38.43 ± 0.1 | 37.88 ± 0.15 | 38.15 ± 0.10 | 37.76 ± 0.08 |
| ZABA | 100 | 36.4 ± 0.09 | 39.01 ± 0.10 | 38.9 ± 0.09 | 37.9 ± 0.1 | 37.77 ± 0.15 | 37.76 ± 0.10 | 37.55 ± 0.08∗ |
Data represented as mean ± SEM values of six animals. The data was examined by ANOVA followed by Dunnett's test using Graph pad prism software which indicate that data are statistically significant at <0.05∗, <0.01∗∗ and <0.001∗∗∗ as compare with control.
Effect of methanol extract of Z. armatum root bark (ZAR) and isolated total alkaloid fraction (ZARA) on pyrexia induced body temperature in mice was not significant during 5 h study period from any of the sample used as compared with the standard drug Paracetamol (Data not shown).
3.2. Analgesic activity
The effect of extract of Z. armatum fruit (ZAF) and isolated alkaloids (ZAFA) was investigated for writhing response in mice and the results are presented in Table 3. It was found that both ZAF and ZAFA caused a dose-dependent inhibition on the writhing response in 10 min time. The pain was more significantly reduced by ZAF 300 mg/kg dose (40.99%, p > 0.05) and 100 mg/kg of ZAFA (45.91%, p > 0.001) and this result was similar to the standard drug Paracetamol (46.33%).
Table 3.
Acetic acid induced Writhing test for Z. armatum fruit (ZAF) bark (ZAB) extracts, and crude alkaloids (ZAFA and (ZABA).
| Treatment | Dose (mg/kg) | No of Writhing in 10 min | % inhibition of writhing |
|---|---|---|---|
| Saline | 10 mL/kg | 40.67 ± 0.55 | 0% |
| Diclofenac | 10 | 21.83 ± 0.40 | 46.33∗∗∗ |
| ZAF | 100 | 31.83 ± 0.60 | 21.74 |
| ZAF | 200 | 25.17 ± 0.30 | 38.12 |
| ZAF | 300 | 24 ± 4.81 | 40.99∗ |
| ZAFA | 50 | 24 ± 0.21 | 40.98∗ |
| ZAFA | 100 | 22 ± 0.25 | 45.91∗∗∗ |
| ZAB | 100 | 30.33 ± 0.61 | 18.95 |
| ZAB | 200 | 27 ± 0.44 | 33.62 |
| ZAB | 300 | 25.83 ± 0.30 | 36.49∗ |
| ZABA | 50 | 28 ± 0.30 | 31.16 |
| ZABA | 100 | 25 ± 0.40 | 38.53∗∗ |
Comparison of different doses with standard drug (Diclofenac) in acetic induced writhing mice. Data represented as mean ± SEM values of six animals. The data was analyzed by ANOVA followed by Dunnett's test using Graph pad prism software which indicate that data are statistically significant at <0.05∗, <0.01∗∗ and <0.001∗∗∗ as compare with control.
The effect of ZAL, ZAR, ZALA, and ZARA from Z. armatum on writhing response in mice showed no significant effect in relieving the pain as compared to standard drug Paracetamol (Data not shown).
The effect of extract of Z. armatum fruit (ZAB) and isolated alkaloids (ZABA) was investigated for writhing response in mice and the results are tabulated in Table 3. It was found that both ZAB and ZABA caused a significant inhibition of writhing in mice at 300 mg/kg (36.49%, p > 0.05) and 100 mg/kg (38.53%, p > 0.001) dose-dependent inhibition on the writhing response in 10 min time.
Analgesic effect of fruit extract (ZAF) and crude alkaloids (ZAFA) was evaluated by performing tail-flick test. The time in which mice flick the tail from the water bath was noted for all the doses of whole extracts and crude alkaloids and was compared with the effect of the standard drug (tramadol). The effect of ZAF (at 200 and 300 mg/kg) and ZAFA (100 mg/kg) was significant (p < 0.05 and p < 0.001) and was more pronounced in case of crude alkaloids as compared with standard drug Tramadol. It showed that crude alkaloids have the ability to reduce the pain in mice and effect was similar to standard drug. Table 4.
Table 4.
Tail flick test of Z. armatum (ZAF), (ZAL) extracts and crude alkaloids (ZAFA) and (ZALA) in mice.
| Treatment | Dose mg/kg) | Tail flick response |
||||
|---|---|---|---|---|---|---|
| 0 min | 30 min | 60 min | 90 min | 120 min | ||
| Normal Saline | 10 ml | 3.66 ± 0.42 | 3.66 ± 0.42 | 3.50 ± 0.34 | 3.33 ± 0.42 | 3.33 ± 0.21 |
| Tramadol | 30 | 3.33 ± 0.33 | 5.66 ± 0.33∗∗∗ | 5.83 ± 0.40∗∗∗ | 6.16 ± 0.30∗∗∗ | 5.83 ± 0.30∗∗∗ |
| ZAF | 100 | 3.66 ± 0.42 | 3.83 ± 0.42 | 3.83 ± 0.30 | 3.50 ± 0.30 | 3.66 ± 0.21 |
| ZAF | 200 | 3.50 ± 0.42 | 4.50 ± 0.22 | 4.66 ± 0.21 | 4.33 ± 0.33 | 4.66 ± 0.49∗ |
| ZAF | 300 | 3.33 ± 0.33 | 5.50 ± 0.42 | 5.66 ± 0.33∗∗∗ | 5.73 ± 0.30∗∗∗ | 5.66 ± 0.33∗∗∗ |
| ZAFA | 50 | 2.21 ± 0.06 | 2.73 ± 0.05 | 3.54 ± 0.06 | 3.80 ± 0.05 | 4.28 ± 0.06∗ |
| ZAFA | 100 | 2.15 ± 0.04 | 3.70 ± 0.19 | 4.55 ± 0.15∗ | 5.10 ± 0.14∗∗ | 5.99 ± 0.08∗∗∗ |
| ZAL | 100 | 3.16 ± 0.47 | 3.33 ± 0.49 | 3.00 ± 0.36 | 3.16 ± 0.30 | 3.00 ± 0.25 |
| ZAL | 200 | 3.16 ± 0.40 | 3.50 ± 0.42 | 3.66 ± 0.42 | 4.00 ± 0.51 | 3.83 ± 0.47 |
| ZAL | 300 | 3.14 ± 0.30 | 4.16 ± 0.47 | 4.83 ± 0.40 | 4.83 ± 0.30 | 4.33 ± 0.33∗ |
| ZALA | 50 | 2.12 ± 0.06 | 2.48 ± 0.08 | 3.29 ± 0.17 | 3.49 ± 0.06 | 3.78 ± 0.07 |
| ZALA | 100 | 2.28 ± 0.15 | 3.27 ± 0.10 | 3.88 ± 0.13 | 4.47 ± 0.04∗ | 5.22 ± 0.04∗∗ |
Data represented as mean ± SEM values of six animals. The data was analyzed by ANOVA followed by Dunnett's test using Graph pad prism software which indicate that data are statistically significant at <0.05∗, <0.01∗∗ and <0.001∗∗∗ as compare with control.
Analgesic effect of ZAL and ZALA was evaluated by performing the tail-flick test as well and it was found that crude alkaloids showed a pain-reducing effect. Although the effect was less significant as compared with crude alkaloids isolated from fruit extract. The effect of extract ZAL at 300 mg/kg dose showed a significant effect (p < 0.05) and crude alkaloids at 100 mg/kg showed a significant effect (p < 0.01). Table 5.
Table 5.
Tail flick test of Z. armatum (ZAB), (ZAR) extracts and crude alkaloids (ZABA) and (ZARA) in mice.
| Treatment | Dose (mg/kg) | Tail flick response |
||||
|---|---|---|---|---|---|---|
| 0 min | 30 min | 60 min | 90 min | 120 min | ||
| Normal Saline | 10 ml | 3.66 ± 0.42 | 3.66 ± 0.42 | 3.50 ± 0.34 | 3.33 ± 0.42 | 3.33 ± 0.21 |
| Tramadol | 30 | 3.33 ± 0.33 | 5.66 ± 0.33∗∗∗ | 5.83 ± 0.40∗∗∗ | 6.16 ± 0.30∗∗∗ | 5.83 ± 0.30∗∗∗ |
| ZAB | 100 | 3.33 ± 0.33 | 3.50 ± 0.42 | 3.33 ± 0.33 | 3.33 ± 0.21 | 3.16 ± 0.40 |
| ZAB | 200 | 3.16 ± 0.47 | 3.83 ± 0.30 | 4.33 ± 0.21 | 4.16 ± 0.30 | 4.16 ± 0.30 |
| ZAB | 300 | 3.16 ± 0.30 | 4.83 ± 0.30 | 5.16 ± 0.30 | 5.33 ± 0.33 | 5.0 ± 0.25∗∗ |
| ZABA | 50 | 2.12 ± 0.07 | 2.63 ± 0.11 | 3.10 ± 0.10 | 3.69 ± 0.07 | 3.76 ± 0.04 |
| ZABA | 100 | 2.28 ± 0.15 | 3.34 ± 0.11 | 4.22 ± 0.17 | 4.34 ± 0.13 | 5.04 ± 0.19∗∗ |
| ZAR | 100 | 3.00 ± 0.36 | 2.83 ± 0.40 | 3.16 ± 0.40 | 3.16 ± 0.40 | 3.33 ± 0.42 |
| ZAR | 200 | 3.00 ± 0.36 | 3.33 ± 0.33 | 3.50 ± 0.34 | 3.66 ± 0.42 | 3.83 ± 0.60 |
| ZAR | 300 | 3.16 ± 0.16 | 3.66 ± 0.42 | 4.50 ± 0.50 | 4.50 ± 0.22 | 4.00 ± 0.36 |
| ZARA | 50 | 2.12 ± 0.06 | 2.44 ± 0.07 | 3.31 ± 0.06 | 3.40 ± 0.04 | 3.65 ± 0.10 |
| ZARA | 100 | 2.28 ± 0.15 | 3.27 ± 0.10 | 3.88 ± 0.13 | 4.47 ± 0.04∗ | 5.09 ± 0.03∗∗ |
Data represented as mean ± SEM values of six animals. The data was analyzed by ANOVA followed by Dunnett's test using Graph pad prism software which indicate that data are statistically significant at <0.05∗, <0.01∗∗ and <0.001∗∗∗ as compare with control.
Analgesic effect of bark extract (ZAB) and crude alkaloids (ZABA) was also evaluated in a similar manner. The effect was significant in pain reduction in mice. The effect of extract ZAB at 300 mg/kg dose showed a significant effect (p < 0.01) and ZABA at 100 mg/kg showed a significant effect (p < 0.01). The effect of extract (ZAR) and (ZARA) was noted and shortly the pain-reducing ability was less significant. Table 5.
3.3. Anti-inflammatory activity
Results indicated that carrageenan-induced paw edema was decreased significantly (p < 0.01) with a dose of 300 mg/kg of fruit extract (ZAF) and crude alkaloids (ZAFA) at 100 mg/kg.
After 3 h of carrageenan injection the swelling of paw was maximum and mean volume increase was 100% in the control group. Statistical analysis showed that the edema inhibition of extract and crude alkaloids at higher doses were a little less from the effect exerted by standard drug (diclofenac). In this study, the extract and crude alkaloids showed considerable anti-inflammatory activity in comparison with the drug reference. Table 6.
Table 6.
Anti-inflammatory effect of Z. armatum fruit (ZAF) extract and crude alkaloids (ZAFA) in carrageenan induced paw edema in mice.
| Treatment | Dose mg/kg | NPS | 1 h | 2 h | 3 h | 4 h | 5 h |
|---|---|---|---|---|---|---|---|
| Normal saline | 10 mL | 0.64 ± 0.04 | 0.6 ± 0.06 | 0.66 ± 0.05 | 0.66 ± 0.06 | 0.62 ± 0.06 | 0.62 ± 0.06 |
| Diclofenac | 10 mg | 0.28 ± 0.04 | 0.5 ± 0.06 | 0.4 ± 0.06 | 0.3 ± 0.06 | 0.3 ± 0.04 | 0.2 ± 0.046∗∗∗ |
| ZAF | 100 | 0.5 ± 0.03 | 0.6 ± 0.03 | 0.64 ± 0.03 | 0.64 ± 0.27 | 0.58 ± 0.01 | 0.57 ± 0.01 |
| ZAF | 200 | 0.48 ± 0.03 | 0.6 ± 0.03 | 0.61 ± 0.02 | 0.58 ± 0.01 | 0.55 ± 0.01 | 0.52 ± 0.01 |
| ZAF | 300 | 0.2 ± 0.04 | 0.5 ± 0.03 | 0.53 ± 0.03 | 0.43 ± 0.02 | 0.43 ± 0.02 | 0.33 ± 0.02∗∗ |
| ZAFA | 50 | 0.5 ± 0.03 | 0.6 ± 0.03 | 0.61 ± 0.02 | 0.59 ± 0.01 | 0.58 ± 0.01 | 0.56 ± 0.01 |
| ZAFA | 100 | 0.3 ± 0.04 | 0.4 ± 0.03 | 0.52 ± 0.03 | 0.51 ± 0.02 | 0.43 ± 0.02 | 0.33 ± 0.02∗∗ |
Data represented as mean ± SEM values of six animals. The data was analyzed by ANOVA followed by Dunnett's test using Graph pad prism software which indicate that data are statistically significant at <0.05∗, <0.01∗∗ and <0.001∗∗∗ as compare with control.
Anti-inflammatory activity of bark, leaves and root extracts and crude alkaloids from Z. armatum did not show any significant activity (Data not shown).
3.4. LOX and COX inhibition
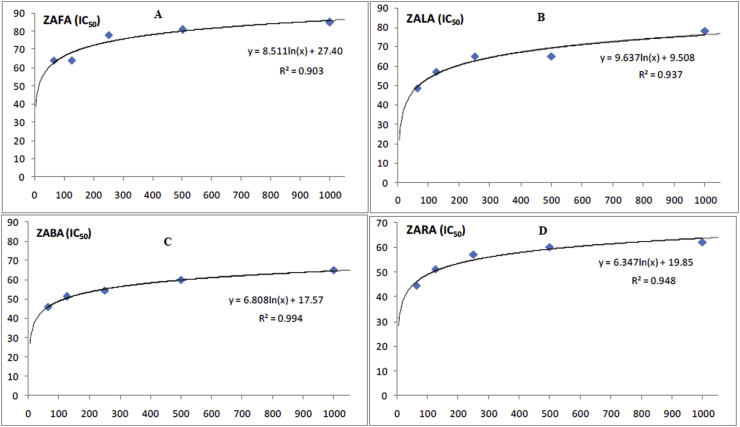
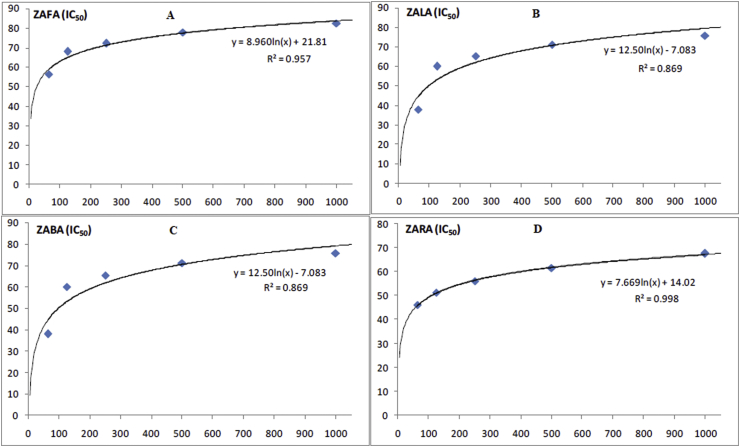
The crude alkaloids from fruit (ZAFA), leaves (ZALA), bark (ZABA), and root bark (ZARA) were investigated for in vitro anti-inflammatory activity in lipoxygenase (5-LOX) inhibition assay. The crude alkaloids inhibited the enzyme 5-LOX with IC50 (μg/ml) 15, 69.48, 110 and 115.38 respectively. This shows that maximum inhibitory activity was exhibited by crude alkaloids from fruit extract of Z. armatum followed by crude alkaloids from leaves extract.
In a similar way the (ZAFA), (ZALA), (ZABA), and (ZARA) were used in cyclooxygenase (COX-2) inhibitory assay and the respective inhibition in IC50 (μg/ml) was calculated. The respective values were 21, 62, 98, 109.03 μg/ml. Again the crude alkaloids from fruit extract showed very good activity followed by crude alkaloids from leaves extract. Figures 1 and 2 showed the curves representing the enzyme inhibition activities.
Figure 1.
Graphical representation of the IC 50 values of ZAFA (2A), ZALA (2B), ZABA (2C) and ZARA (2D) against COX-2 enzyme.
Figure 2.
Graphical representation of the IC50 values of ZAFA (3A), ZALA (3B), ZABA (3C) and ZARA (3D) against 5-LOX enzyme.
3.5. GCMS investigation
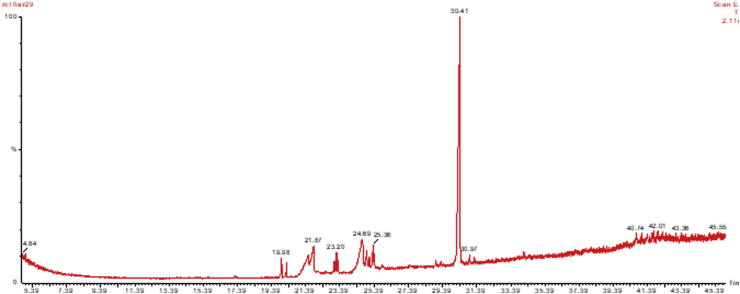
GC-MS analysis showed many compounds but nitrogen containing compounds are important. Table 7 shows the list of compounds identified. Some of the important compounds containing nitrogen gives the indication of presence of various groups of alkaloids like 2-Ethoxy-3-methylpyrazine, Butanoic acid, 4-hydroximino-4-(2-thienyl)-, methyl ester, 3-Aminocrotononitrile, 2-Methoxy-6-methylpyrimidine 1-oxide, 2,5-Diaminobenzoic acid, 1H-Imidazole-2-carboxylic acid, 2-Mercapto-1,3,5-triazole, Piperidin-4-carboxylic acid, Pyrrolidine, 1-(2-chloroethyl, Pent-3-yn-2-ol, 2-cyclopropyl-5-(1-piperidyl), and 2-Propenal, 2-(diethylamino)-3-(dimethylamino). The chromatograph also showed the presence salicylate compound 2-Ethylhexyl salicylate. The salicylate compound may also have contributed towards the bioactivity Table 1, Table 2 and Table 7. Figure 3.
Table 7.
GC-MS analysis of chloroform extract of various parts (fruit, bark, leaves and roots bark) of Zanthoxylum armatum.
| No | Retention time | Structure | Compound Name and formula |
|---|---|---|---|
| 1 | 15.730 |
Name: 1-Propene, 3,3′-oxybis-Formula: C6H10O MW: 98 |
|
| 2 | 17.301 | 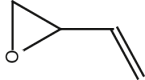 |
Name: Ethenyloxirane Formula: C4H6O MW: 70 |
| 3 | 19.031 |
Name: 1,2-Heptadiene Formula: C7H12 MW: 96 |
|
| 4 | 19.982 | 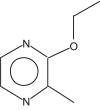 |
Name: 2-Ethoxy-3-methylpyrazine Formula: C7H10N2O MW: 138 |
| 5 | 20.297 | 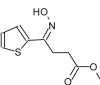 |
Name: Butanoic acid, 4-hydroximino-4-(2-thienyl)-, methyl ester Formula: C9H11NO3S MW: 213 |
| 6 | 21.542 | 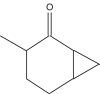 |
Name: 2-Norcaranone, 3-methyl-Formula: C8H12O MW: 124 |
| 7 | 21.858 |
Name: Palmitamide Formula: C16H33NO MW: 255 |
|
| 8 | 23.073 | 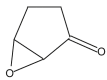 |
9,10- Anthracenedione Formula: C14H8O2 Molecular weight: 208.2121 |
| 9 | 23.203 | 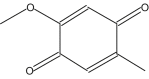 |
Name: 4-Methoxytoluquinone 2,5- Formula: C8H8O3 MW: 152 |
| 10 | 23.293 | 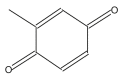 |
Name: Toluquinone Formula: C7H6O2 MW: 122 |
| 11 | 24.69 | 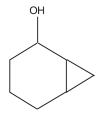 |
Name: Bicyclo [4.1.0]heptan-2-ol Formula: C7H12O MW: 112 |
| 12 | 24.98 | 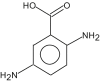 |
Name: 3-Aminocrotononitrile Formula: C4H6N2 MW: 82 |
| 13 | 25.13 | 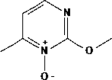 |
Name: 2-Methoxy-6-methylpyrimidine 1-oxide Formula: C6H8N2O2 MW: 140.14 |
| 14 | 25.36 | 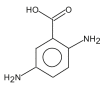 |
Name: 1.2,5-Diaminobenzoic acid Formula: C7H8N2O2 MW: 152 |
| 15 | 25.434 | 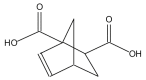 |
Name: Bicyclo [2.2.1]hept-5-ene-1,2-dicarboxylic acid Formula: C9H10O4 MW: 182 |
| 16 | 25.87 | 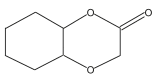 |
Name: 1,4-Dioxa-2-decalone Formula: C8H12O3 MW: 156 |
| 17 | 27.450 | 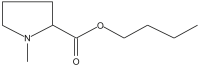 |
Name: Proline, N-methyl-, butyl ester Formula: C10H19NO2 MW: 185 |
| 18 | 29.001 | 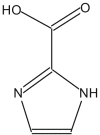 |
Name: 1H-Imidazole-2-carboxylic acid Formula: C4H4N2O2 MW: 112 |
| 19 | 29.291 | 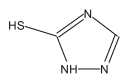 |
Name: 2-Mercapto-1,3,5-triazole Formula: C2H3N3S MW: 101 |
| 20 | 29.301 | 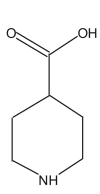 |
Name: Piperidin-4-carboxylic acid Formula: C6H11NO2 MW: 129 |
| 21 | 30.411 | 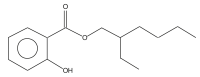 |
Name: 2-Ethylhexyl salicylate Formula: C15H22O3 MW: 250.33 |
| 22 | 34.173 | 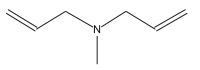 |
Name: Methyldiallylamine Formula: C7H13N MW: 111 |
| 23 | 34.453 | 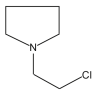 |
Name: Pyrrolidine, 1-(2-chloroethyl)-Formula: C6H12ClN MW: |
| 24 | 44.012 | 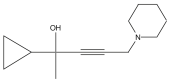 |
Name: Pent-3-yn-2-ol, 2-cyclopropyl-5-(1-piperidyl)-Formula: C13H21NO MW: 207 |
| 25 | 45.057 | 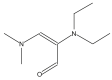 |
Name: 2-Propenal, 2-(diethylamino)-3-(dimethylamino)-Formula: C9H18N2O MW: 170 |
Figure 3.
GC-MS chromatograph of chloroform extract of various parts of Z. armatum.
4. Discussions
Nonsteroidal anti-inflammatory drugs (NSAIDs) are extensively used drugs for the alleviation of pain and inflammation. Side effects of such drugs are sometimes worst and include damage in the gut in the form of ulcers and hemorrhage (Fiorucci et al., 2001). Herbal medicines as alternative have also been used for the same purpose with good efficiency and with few side effects (Dharmasiri et al., 2003). Extracts isolated from plants contains a number of constituents and many works synergistically (Gilani, 2005). The plant extract can be used to obtain more purified fraction and such fractions are of special interest these days. Zanthoxylum armatum is commonly known as prickly ash conventionally used for management of fever, pain, and inflammation. In the present study, the potential effects of an alkaloid fraction from various parts of Zanthoxylum armatum have been evaluated both in vitro and in vivo. For this purpose, firstly we have tested extracts of various parts of Z. armatum for analgesic, antipyretic and anti-inflammatory activities.
Brewer's yeast is used of induction of fever mainly contain protein which is associated with fever development. It may occur due to the production of inflammatory cytokines in the inflammatory reaction. Paracetamol is used for the treatment of fever decrease the levels of prostaglandins hence antipyretic messages in the brain. Effect of methanol extract of Z. armatum fruit (ZAF) and isolated total alkaloid fraction (ZAFA) on pyrexia induced body temperature in mice was tested. The subcutaneous injection of 10 ml kg−1 of 15% w/v of yeast suspension markedly elevated the rectal temperature after 19 h of administration. It was found that ZAF and ZAFA effect was significant (p < 0.01, p < 0.001 respectively) in lowering of rectal temperature in mice after 5 h and these result were comparable to Paracetamol. The sample ZAFA (100 mg/kg) showed a similar pattern of pyrexia control as shown by standard drug and temperature was started reducing from 1st h. The maximum effect of ZAL was with 300 mg/kg dose and was significant (p < 0.01) and ZALA showed significant results (p < 0.001) at 100 mg/kg dose and were equipotent with Paracetamol used as standard drug. It was found that ZAB and ZABA at all doses caused less significant (p < 0.05) reduction of body temperature in mice as compared to ZAF and ZAL. The root extract and crude alkaloids showed that there was no significant change in pyrexia during the study period from any of the sample used as compared with the standard drug Paracetamol.
It is assumed that abdominal contraction is induced by local peritoneal receptor activation which includes prostanoids mediators. In the result peritoneal fluid, PGE2, PGF2, and Lipooxygenase are increased in mice. The nociceptive properties of acetic acid may be due to release of cytokines, TNF-α, interleukin- 1β, interleukin-8, and resident peritoneal macrophages and mast cell. In the present study in order to determine the inhibitory effect of the alkaloids on fever (Küpeli et al., 2002).
The writhing response to acute nociception has been used to test the analgesic activity of Z. armatum in mice. In the writhing test, intraperitoneal injection of 1% acetic acid markedly caused in writhing reflex in untreated control group. It was found that both ZAF and ZAFA caused a dose-dependent inhibition on the writhing response induced by acetic acid in 10 min time. The pain was more significantly reduced by ZAF (p > 0.05) and ZAFA (p > 0.001) and this result was similar to the standard drug Paracetamol. The effect of ZAL, ZAR, ZALA, and ZARA from Z. armatum on writhing response in mice showed no significant effect in relieving the pain. Similarly, from bark extract, both ZAB and ZABA caused a significant inhibition of writhing in mice (p > 0.05) and (p > 0.001) dose-dependent inhibition on the writhing response induced by acetic acid. Z. armatum is a well-known traditional spice used as condiments and medicine and has been employed to manage pain in stomach, toothache, and pain of sickle cell disease (Danton et al., 2019).
The writhing test is the best assay used for the estimation of the analgesic activity of plant materials. It has also been described that when acetic acid injected into the peritoneal cavity it promotes an increase of COX and LOX products in peritoneal fluids along with the release of several other inflammatory mediators which finally stimulate the primary afferent nociceptors entering the dorsal horn of the central nervous system (Subedi et al., 2016). The extract and crude alkaloids from Z. armatum at different doses were capable to decrease writhing reaction pointedly and this was probably facilitated by prostaglandins inhibition mechanism (Kifayatullah et al., 2019; Subedi et al., 2016).
The plant-based biological benefits such as analgesic, antipyretic and anti-inflammatory potentials are mediated by secondary metabolites such as alkaloids (Kifayatullah et al., 2019). In the case of the tail immersion test increase in pain reaction time or latency period indicated the level of analgesia of the plant extract. The tail immersion models have been used to study centrally acting analgesics. Z. armatum activity showed that the noteworthy analgesic activities might be through inhibition of the prostaglandin pathway (Ezeja et al., 2011).
Analgesic effect of fruit extract (ZAF) and crude alkaloids (ZAFA) was evaluated by performing the tail-flick test and was compared with the effect of the standard drug (tramadol). The effect of ZAF and ZAFA was significant (p < 0.05 and p < 0.001) and was more pronounced in case of crude alkaloids as compared with standard drug Tramadol. It showed that crude alkaloids have the ability to reduce the pain in mice.
Analgesic effect of ZAL and ZALA was evaluated and the effect was less significant as compared with crude alkaloids isolated from fruit extract. The effect of extract ZAL at higher dose showed significance effect (p < 0.05) and crude alkaloids also showed significance effect (p < 0.01). Analgesic effect of ZAB and ZABA was significant in pain reduction in mice. The effect of extract ZAB and ZABA showed significance effect (p < 0.01). The effect of extract (ZAR) and (ZARA) was noted and shortly the pain-reducing ability was less significant.
Inflammation is a common phenomenon which may occur due to the reaction of tissue toward injury. The carrageenan test has long been used as the most acceptable method to investigate new anti-inflammatory drugs (Just et al., 1998). Subcutaneous injection of carrageenan induces a biphasic inflammation which is categorized by signs of edema and pain. Pro-inflammatory mediators which include histamine, tachykinins, serotonin, and bradykinin are responsible for the induction. Prostaglandins then initiate the last phase of the edema via the action of COX-2 together with inducible nitric oxide synthase which increases the vascular permeability of vessels causing edema (Posadas et al., 2004). Therapeutic doses of Z. armatum was administered to the carrageenan induce inflammation models which pointedly reduce the paw edema treatment procedure. Paw edema inhibition in mice conceivably preventing the pro-inflammatory mediators of edema synthesis and their release at the target site. However, its exact mechanism is still needed to be established properly. Standard drug (Diclofenac) which is a non-selective COX inhibitor which prevents the synthesis of the arachidonic acid metabolites such as prostaglandins, thromboxanes, and leukotrienes, etc. was compared with Z. armatum extract and crude alkaloids which suppressed edema in the same manner as that of standard drug (N. Muhammad, Saeed and Khan, 2012).
Results showed that carrageenan-induced paw edema was decreased significantly (p < 0.01) with fruit extract (ZAF) and crude alkaloids (ZAFA). The degree of swelling of the carrageenan-injected paws was maximal 3 h after injection and the mean increase in volume at that time was about 100% in the control group. Statistical analysis showed that the edema inhibition of extract and crude alkaloids at higher doses were a little less from the effect exerted by standard drug (diclofenac). Anti-inflammatory activity of bark, leaves and root extracts and crude alkaloids from Z. armatum did not show any significant activity as evident from results. However, the bark extract did show some good activity at ZAB 300 mg/kg and ZABA 100 mg/kg dose and showed a decreasing trend in inflammation in mice paw edema.
The extract and crude alkaloids from Z. armatum were also tested in vitro for cyclooxygenase inhibition assay to explore the mechanism of action. It has been reported that different groups of alkaloids showed promising COX inhibitory activities. Alkaloids also have the ability to reduce the inflammatory responses by suppressing COX 2. The famous COX-2 inhibitors rofecoxib, celecoxib, parecoxib, and etoricoxib. These drugs have also been advised not to use in patients with osteoarthritis or rheumatoid arthritis unless there is no other option. Therefore, search for new COX inhibitors and especially from natural sources is the demand of the time. A lot of studies have shown alkaloids as potent inhibitors of cyclooxygenase-2 and suppressors of PGE2. This role is helpful in reducing inflammation and growth of tumor cells (Hashmi et al., 2018).
The role of lipoxygenase in pathophysiology of many inflammatory diseases is important. Previous experimental work showed that secondary metabolites from plants including terpenes, coumarins, flavonoids, and alkaloids showed inhibitory activity against 5-lipoxygenase enzyme. This study revealed the potential of lipoxygenase inhibition by Z. armatum extracts as well as total alkaloids from different parts of plant. Therefore, it might contribute in the crucial search for alternative therapies for inflammation. The enzymes COX-1, COX-2, and LOX have got utmost importance due to the fact that these are important target for discovery of new anti-inflammatory drugs (Shrivastava et al., 2017).
Alkaloids are major constituent of Zanthoxylum due to which it showed different pharmacological activities (Negi et al., 2011). Alkaloids are the important constituent of this genus which includes quinolines, isoquinolines, and amide alkaloids due to which it showed various pharmacological activities including anti-inflammatory, analgesic, and antioxidant (Souto et al., 2011). Three new alkaloids zanthocadinanine, 7-methoxy-8-demethoxynitidine and zanthonitiside are obtained from genus Zanthoxylum which is used for the treatment of numerous ailments such as fever, gingivitis, and toothache. Several classes of alkaloid are responsible for anti-inflammatory and analgesic properties (Adebayo et al., 2015). The alkaloid obtained from the medicinal plant is responsible for the analgesic activity. The analgesic activity of alkaloids inhibits pain via opioid receptors in the CNS (Asante et al., 2019).
GC-MS analysis of chloroform soluble fraction indicates the presence of various nitrogen containing compounds which might contribute toward the analgesic and anti-inflammatory activity. Moreover, Salicylate compound 2-Ethylhexyl salicylate was also detected in the extract which is also known for such activities. Therefore, our results indicate that crude alkaloids isolated from different parts of ZA might have exerted their effect to alleviate the pain, inflammation, and fever in mice.
5. Conclusion
In conclusion, the present study suggests that the total alkaloid fraction from Z. armatum showed analgesic, anti-inflammatory, and antipyretic activities. This was evident from in vivo activities as well as well supported by in vitro enzyme inhibition assay. The results acquired by performing different activities showed that Z. armatum, the important condiment spice and ethnomedicine, have the potential to treat different ailments and conditions including fever, pain, and inflammation by a possible mechanism involving COX and LOX enzyme inhibition.
Declarations
Author contribution statement
F. Alam: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
K.M. Din: Conceived and designed the experiments; Performed the experiments.
A. Sadiq and A. Khan: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
M.S. Jan, R. Rasheed, S. Jan, A. Mehmood: and A.M. Minhas: Performed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Adebayo S.A., Dzoyem J.P., Shai L.J., Eloff J.N. The anti-inflammatory and antioxidant activity of 25 plant species used traditionally to treat pain in southern African. BMC Compl. Alternative Med. 2015;15(1):159. doi: 10.1186/s12906-015-0669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asante D.-B., Henneh I.T., Acheampong D.O., Kyei F., Adokoh C.K., Ofori E.G., Domey N.K., Adakudugu E., Tangella L.P., Ameyaw E.O. Anti-inflammatory, anti-nociceptive and antipyretic activity of young and old leaves of Vernonia amygdalina. Biomed. Pharmacother. 2019;111:1187–1203. doi: 10.1016/j.biopha.2018.12.147. [DOI] [PubMed] [Google Scholar]
- Danton O., Somboro A., Fofana B., Diallo D., Sidibé L., Rubat-Coudert C., Marchand F., Eschalier A., Ducki S., Chalard P. Ethnopharmacological survey of plants used in the traditional treatment of pain conditions in Mali. J. Herb. Med. 2019:100271. [Google Scholar]
- Dharmasiri M., Jayakody J., Galhena G., Liyanage S., Ratnasooriya W. Anti-inflammatory and analgesic activities of mature fresh leaves of Vitex negundo. J. Ethnopharmacol. 2003;87(2-3):199–206. doi: 10.1016/s0378-8741(03)00159-4. [DOI] [PubMed] [Google Scholar]
- Ezeja M., Omeh Y., Ezeigbo I., Ekechukwu A. Evaluation of the analgesic activity of the methanolic stem bark extract of Dialium guineense (Wild) Ann. Med. Health Sci. Res. 2011;1(1):55–62. [PMC free article] [PubMed] [Google Scholar]
- Fiorucci S., Antonelli E., Morelli A. Mechanism of non-steroidal anti-inflammatory drug-gastropathy. Dig. Liver Dis. 2001;33:S35–S43. doi: 10.1016/s1590-8658(01)80157-2. [DOI] [PubMed] [Google Scholar]
- Gilani A.H. Trends in ethnopharmacology. J. Ethnopharmacol. 2005;100(1-2):43–49. doi: 10.1016/j.jep.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Gupta M., Thakur S., Sharma A., Gupta S. Qualitative and quantitative analysis of phytochemicals and pharmacological value of some dye yielding medicinal plants. Oriental J. Chem. 2013;29(2):475–481. [Google Scholar]
- Hashmi M.A., Khan A., Farooq U., Khan S. Alkaloids as cyclooxygenase inhibitors in anticancer drug discovery. Curr. Protein Pept. Sci. 2018;19(3):292–301. doi: 10.2174/1389203718666170106103031. [DOI] [PubMed] [Google Scholar]
- Joshi A., Edington J. The use of medicinal plants by two village communities in the central development region of Nepal. Econ. Bot. 1990;44(1):71–83. [Google Scholar]
- Just M.J., Recio M.C., Giner R.M., Cuéllar M.J., Máñez S., Bilia A.R., Ríos J.-L. Anti-inflammatory activity of unusual lupane saponins from Bupleurum fruticescens. Planta Med. 1998;64(5):404–407. doi: 10.1055/s-2006-957469. [DOI] [PubMed] [Google Scholar]
- Kala C.P., Farooquee N.A., Dhar U. Traditional uses and conservation of timur (Zanthoxylum armatum DC.) through social institutions in Uttaranchal Himalaya, India. Conserv. Soc. 2005;3(1):224. [Google Scholar]
- Kelm M., Nair M., Strasburg G., DeWitt D. Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine. 2000;7(1):7–13. doi: 10.1016/S0944-7113(00)80015-X. [DOI] [PubMed] [Google Scholar]
- Kifayatullah M., Rahim H., Jan N.U., Chishti K.A., Ullah I., Abbas S. In vivo analgesic, antipyretic and anti-inflammatory activities of ethanol extract of pericampylus glaucus in experimental animals. Sains Malays. 2019;48(3):629–635. [Google Scholar]
- Kunwar R.M., Mahat L., Acharya R.P., Bussmann R.W. Medicinal plants, traditional medicine, markets and management in far-west Nepal. J. Ethnobiol. Ethnomed. 2013;9(1):24. doi: 10.1186/1746-4269-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küpeli E., Koşar M., Yeşilada E., Başer K.H.C. A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life Sci. 2002;72(6):645–657. doi: 10.1016/s0024-3205(02)02200-2. [DOI] [PubMed] [Google Scholar]
- Lukhoba C.W., Simmonds M.S., Paton A.J. Plectranthus: a review of ethnobotanical uses. J. Ethnopharmacol. 2006;103(1):1–24. doi: 10.1016/j.jep.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Muhammad N., Saeed M., Khan H. Antipyretic, analgesic and anti-inflammatory activity of Viola betonicifolia whole plant. BMC Compl. Alternative Med. 2012;12(1):59. doi: 10.1186/1472-6882-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad S., Aleem A.A., Muhammad N., Khalid M., Khan A., Farooq U., Ahmad I., Khan F.A., Bukhari S.M. Investigation of antipyretic activity of viola serpens on Breweris yeast induced pyrexia IN mice. Acta Pol. Pharm. 2017;74(6):1797–1802. [Google Scholar]
- Mushtaq M.N., Ghimire S., Akhtar M.S., Adhikari A., Auger C., Schini-Kerth V.B. Tambulin is a major active compound of a methanolic extract of fruits of Zanthoxylum armatum DC causing endothelium-independent relaxations in porcine coronary artery rings via the cyclic AMP and cyclic GMP relaxing pathways. Phytomedicine. 2019;53:163–170. doi: 10.1016/j.phymed.2018.09.020. [DOI] [PubMed] [Google Scholar]
- Negi J., Bisht V., Bh A., Singh P., Sundriyal R. Chemical constituents and biological activities of the genus Zanthoxylum: a review. Afr. J. Pure Appl. Chem. 2011;5(12):412–416. [Google Scholar]
- Nooreen Z., Kumar A., Bawankule D.U., Tandon S., Ali M., Xuan T.D., Ahmad A. New chemical constituents from the fruits of Z anthoxylum armatum and its in vitro anti-inflammatory profile. Nat. Prod. Res. 2019;33(5):665–672. doi: 10.1080/14786419.2017.1405404. [DOI] [PubMed] [Google Scholar]
- Phuyal N., Jha P.K., Raturi P.P., Rajbhandary S. Zanthoxylum armatum DC.: current knowledge, gaps and opportunities in Nepal. J. Ethnopharmacol. 2019;229:326–341. doi: 10.1016/j.jep.2018.08.010. [DOI] [PubMed] [Google Scholar]
- Posadas I., Bucci M., Roviezzo F., Rossi A., Parente L., Sautebin L., Cirino G. Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br. J. Pharmacol. 2004;142(2):331–338. doi: 10.1038/sj.bjp.0705650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaei-Ghomi J., Ebrahimabadi A.H., Djafari-Bidgoli Z., Batooli H. GC/MS analysis and in vitro antioxidant activity of essential oil and methanol extracts of Thymus caramanicus Jalas and its main constituent carvacrol. Food Chem. 2009;115(4):1524–1528. [Google Scholar]
- Salminen K.A., Meyer A., Jerabkova L., Korhonen L.E., Rahnasto M., Juvonen R.O., Imming P., Raunio H. Inhibition of human drug metabolizing cytochrome P450 enzymes by plant isoquinoline alkaloids. Phytomedicine. 2011;18(6):533–538. doi: 10.1016/j.phymed.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Shrivastava S.K., Srivastava P., Bandresh R., Tripathi P.N., Tripathi A. Design, synthesis, and biological evaluation of some novel indolizine derivatives as dual cyclooxygenase and lipoxygenase inhibitor for anti-inflammatory activity. Bioorg. Med. Chem. 2017;25(16):4424–4432. doi: 10.1016/j.bmc.2017.06.027. [DOI] [PubMed] [Google Scholar]
- Souto A.L., Tavares J.F., Da Silva M.S., Diniz M.d.F.F. M., de Athayde-Filho P.F., Barbosa Filho J.M. Anti-inflammatory activity of alkaloids: an update from 2000 to 2010. Molecules. 2011;16(10):8515–8534. doi: 10.3390/molecules16108515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subedi N.K., Rahman S., Akbar M.A. Analgesic and antipyretic activities of methanol extract and its fraction from the root of Schoenoplectus grossus. Evid. base Compl. Alternative Med. 2016;2016:3820704. doi: 10.1155/2016/3820704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vongtau H., Abbah J., Ngazal I., Kunle O., Chindo B., Otsapa P., Gamaniel K. Anti-nociceptive and anti-inflammatory activities of the methanolic extract of Parinari polyandra stem bark in rats and mice. J. Ethnopharmacol. 2004;90(1):115–121. doi: 10.1016/j.jep.2003.09.038. [DOI] [PubMed] [Google Scholar]