Abstract
In patients with myocardial infarction (MI), cardiac rupture is an uncommon but catastrophic complication. In the mouse model of nonreperfused MI, reported rupture rates are highly variable and depend not only on the genetic background and sex of animals but also on the method used for documentation of rupture. In most studies, diagnosis of cardiac rupture is based on visual inspection during autopsy; however, criteria are poorly defined. We performed systematic histopathological analysis of whole hearts from C57BL/6J mice dying after nonreperfused MI and evaluated the reliability of autopsy-based criteria in identification of rupture. Moreover, we compared the cell biological environment of the infarct between rupture-related and rupture-independent deaths. Histopathological analysis documented rupture in 50% of mice dying during the first week post-MI. Identification of a gross rupture site was highly specific but had low sensitivity; in contrast, hemothorax had high sensitivity but low specificity. Mice with rupture had lower myofibroblast infiltration, accentuated macrophage influx, and a trend toward reduced collagen content in the infarct. Male mice had increased mortality and higher incidence of rupture. However, infarct myeloid cells harvested from male and female mice at the peak of the incidence of rupture had comparable inflammatory gene expression. In conclusion, the reliability of autopsy in documentation of rupture in infarcted mice is dependent on the specific criteria used. Macrophage-driven inflammation and reduced activation of collagen-secreting reparative myofibroblasts may be involved in the pathogenesis of post-MI cardiac rupture.
NEW & NOTEWORTHY We show that cardiac rupture accounts for 50% of deaths in C57BL/6J mice undergoing nonreperfused myocardial infarction protocols. Overestimation of rupture events in published studies likely reflects the low specificity of hemothorax as a criterion for documentation of rupture. In contrast, identification of a gross rupture site has high specificity and low sensitivity. We also show that mice dying of rupture have increased macrophage influx and attenuated myofibroblast infiltration in the infarct. These findings are consistent with a role for perturbations in the balance between inflammatory and reparative responses in the pathogenesis of postinfarction cardiac rupture. We also report that the male predilection for rupture in infarcted mice is not associated with increased inflammatory activation of myeloid cells.
Listen to this article’s corresponding podcast at: https://ajpheart.podbean.com/e/smoking-effects-on-ventricular-repolarization/.
Keywords: cardiac rupture, fibroblast, inflammation, macrophage, myocardial infarction
INTRODUCTION
Despite a steady reduction in mortality rates over the past three decades, acute myocardial infarction (MI) remains a deadly disease. In-hospital mortality for ST elevation MI (STEMI) is 4.6% for men and 7.4% for women (90). Patients with non-STEMI have a slightly lower in-hospital mortality than STEMI patients (3.9 and 4.8% for men and women, respectively) (90). In human MI patients, early death is most often due to dysrhythmias or acute pump failure leading to cardiogenic shock (47, 73). In the era of interventional reperfusion, mechanical complications of acute MI (such as left ventricular free wall rupture, ventricular septal rupture, or acute mitral regurgitation due to rupture of the papillary muscle) have become uncommon. The incidence of rupture has decreased from as high as 20% in the 1970s and 1980s to as low as 1–3% in the current era (28). However, the consequences of rupture remain catastrophic, as it may account for 5–20% of all in-hospital mortality after MI (28). It should be noted that the true incidence of free wall rupture may be underestimated, as many sudden pre-hospitalization deaths in MI patients may be due to rupture (41). Patients surviving acute MI remain at high risk for adverse remodeling of the ventricle, which is typically associated with development of chronic heart failure, increased incidence of arrhythmias, and increased late mortality.
Understanding the cellular mechanisms and molecular pathways involved in cardiac injury, repair, and remodeling is critical for the development of new therapeutic strategies for MI patients. Over the past 25 years, mouse models of MI have emerged as key tools for dissection of molecular mechanisms and for designing and testing therapeutic interventions (57, 66). Undoubtedly, the strength of the mouse model lies in the systematic analysis of functional, cell biological, molecular, and proteomic end points that, when coupled with relevant genetic or pharmacological interventions, can contribute to dissection of cellular responses and molecular networks. However, in clinical trials, mortality rate and incidence of adverse cardiac events represent the most important end points when the effects of various therapeutic approaches in acute MI are tested. Thus, acquisition of translationally relevant insights from mouse models requires systematic study of mortality end points and careful identification of the cause of death. Unfortunately, systematic pathological analysis of the cause of death in mice undergoing MI protocols is challenging and often impractical. It is widely believed that in mouse models of nonreperfused MI cardiac rupture is the predominant cause of death, typically occurring during the first week after coronary occlusion (60, 65). In most studies, rupture is assessed through visual inspection by identifying a blood clot around the heart and in the chest cavity (hemothorax) or a perforation site in the infarcted ventricular wall (27, 65, 71, 97).
Considering the obvious challenges in identifying a rupture site that is a few micrometers wide through visual inspection, we performed systematic histopathological analysis of the whole heart to identify structural causes of death in mice dying after nonreperfused MI. In contrast to the very high incidence of rupture reported in the majority of studies using gross pathologic criteria, only 50% of deaths during the first week post-MI could be attributed to histopathologically documented rupture. The reliability of visual inspection for identification of rupture was dependent on the specific criteria used: identification of a rupture site had high specificity but low sensitivity, whereas addition of hemothorax as a criterion markedly increased sensitivity but reduced specificity. Mice with rupture had lower cardiac myofibroblast infiltration and accentuated macrophage influx compared with animals with -non-rupture-related deaths. Male mice had increased mortality and higher incidence of rupture; however, this was not associated with consistent differences in inflammatory gene expression in myeloid cells.
METHODS
Mouse model of nonreperfused MI.
Animal studies were approved by the Institutional Animal Care and Use Committee at Albert Einstein College of Medicine and conformed with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health. A model of nonreperfused myocardial infarction induced through coronary ligation was used, as previously described by our group (80). Female and male C57BL/6J mice (49 mice: 20 males and 29 females), 2–4 mo of age, were anesthetized using inhaled isoflurane (4% for induction, 2% for maintenance). For analgesia, buprenorphine (0.05–0.2 mg/kg sc) was administered at the time of surgery and ~12 h thereafter for 2 days. Additional doses of analgesics were given if the animals appeared to be experiencing pain (based on criteria such as immobility and failure to eat). Intraoperatively, heart rate, respiratory rate and electrocardiogram were continuously monitored, and the depth of anesthesia was assessed using the toe pinch method. The left anterior descending coronary artery was occluded. Mice were followed for 7 days. Dead mice underwent autopsy to determine the cause of death. Mice with rupture were identified through visual inspection. Criteria for rupture included visualization of a perforation site (rupture site, RS) and a thrombus or blood in the chest (hemothorax, Hem). Hearts were then harvested for histological analysis, fixed in zinc-formalin (Anatech, Ltd., Fisher Scientific) and embedded in paraffin.
Histological analysis.
For histopathological analysis, murine hearts were fixed in zinc-formalin (Z-fix; Anatech, Battle Creek, MI), and embedded in paraffin. Infarcted hearts were sectioned from base to apex at 250-μm intervals, thus reconstructing the whole heart, as previously described (10, 11, 40). Ten sections (5 μm thick) were cut at each level. To determine the final infarct size, the first section at each partition was stained with hematoxylin and eosin (H&E). To identify the site of the rupture, sections from all levels were carefully examined.
Quantitative morphometry.
Morphometric parameters were quantitatively assessed using Zen 2.6 Pro software (Zeiss Microscopy, White Plains, NY). The infarcted and noninfarcted areas were measured at each level, and the volume of the infarct and of the noninfarcted remodeling myocardium at each level was calculated as: infarct volume = infarct area × 300 μm (250 μm + 10 sections × 5 μm = 300 μm) and volume of noninfarcted myocardium = noninfarcted area × 300 μm. The total volume of the infarcted and noninfarcted myocardium was calculated as the sum of the volumes of each partition. Scar size was measured by dividing the volume of the infarct to the total volume of the left ventricle (left ventricular volume = infarct volume + volume of noninfarcted myocardium) and was expressed as a percentage.
To measure infarct thickness, maximum and minimum dimensions were measured from the endocardial to the epicardial surface of the transmural infarct wall at each level. The average minimum and average maximum wall thicknesses were then calculated. Similarly, maximum and minimum thickness of the noninfarcted segments was measured from all levels, and the average maximum and minimum thickness was calculated. Mean wall thickness of the infarct and of the noninfarcted area was the average of the maximum and minimum wall thickness.
Infarct length was calculated by measuring the length of the endocardial surface of the transmural infarct at each level, cutting through (excluding) the papillary muscles. The average length from all levels was reported as length of the infarct.
Immunohistochemistry.
For histopathological analysis, murine hearts were fixed in zinc-formalin and embedded in paraffin. For comparisons of histological parameters between mice with and without rupture, only mice that died between 3 and 7 days after coronary occlusion were included; thus, one mouse that died in the absence of rupture 2 days after coronary occlusion was excluded (rupture group: 5.0 ± 0.53 days, n = 7; no rupture group: 5.16 ± 0.4, n = 6). Picrosirius red staining was used to label the collagen fibers, as previously described (1). To identify myofibroblasts in the infarct, sections were stained with an anti-α-smooth muscle actin (α-SMA) antibody (1:100 dilution, Sigma, F3777), as previously described (80). Myofibroblasts were identified as spindle-shaped α-SMA-positive cells located outside the vascular media. Macrophages were stained using anti-Mac2 antibody (CL8942AP; Cedarlane, Burlington, ON, Canada) in 1:200 dilution, as previously described (25). Briefly, sections were deparaffinized, and heat-mediated antigen retrieval was performed for 25 min during incubation of the slides in citrate buffer, pH 6.0 (Sigma, C9999). Sections were then blocked with 10% serum of the species in which the secondary antibody was raised for 1 h and then incubated overnight at 4°C with the primary antibody. Sections were then washed and incubated with a fluorescently labeled secondary antibody for 1 h at room temperature. Autofluorescence quenching was performed using TrueBlack Lipofuscin Autofluorescence Quencher (23007; Biotium, Fremont, CA). Slides were mounted using Fluoro-Gel II with DAPI (17985; EMS, Hatfield, PA). Slides were then scanned using Zen 2.6 Pro software and the Zeiss Imager M2 microscope (Carl Zeiss Microscopy, White Plains, NY).
Machine learning-based quantitative analysis of myofibroblast density.
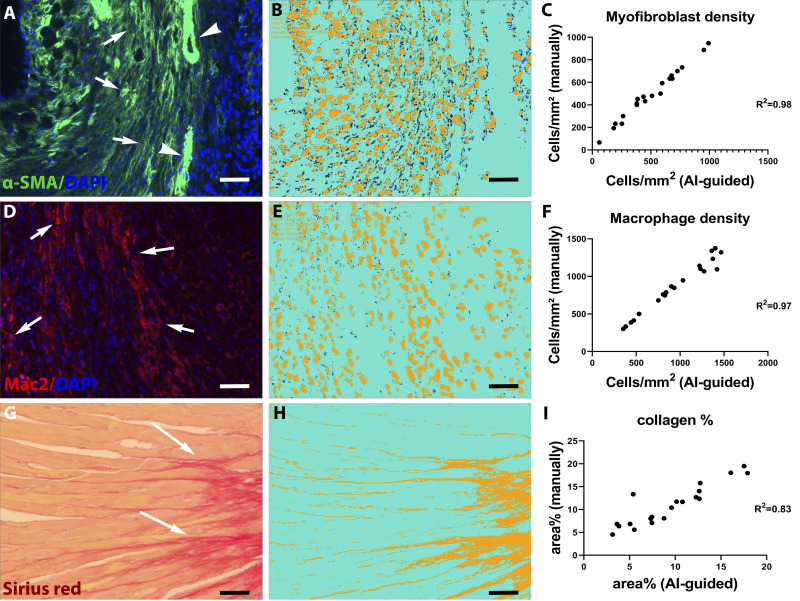
Using default algorithms of the Intellesis Trainable Segmentation module of Zen 2.6 Pro software, an artificial intelligence (AI)-based model was trained on multiple fields of different regions of the myocardium to segment the images and identify myofibroblasts. Objects of interest were defined as the DAPI-α-SMA double-positive profiles, excluding vascular smooth muscle cells (VSMCs). The unstained myocardium (including VSMCs) was considered as background. By use of the Image Analysis module, specific settings were incorporated in the trained model to count the segmented objects. Automatic analysis of 10 fields from two different levels of the murine heart was performed using the trained model. Validation of the AI model was performed by manually counting myofibroblast density in 20 fields from four different mice with ImageJ software. There was an excellent correlation (r = 0.98) between the AI-based quantification and the myofibroblast density derived through manual counting (Fig. 1, A–C).
Fig. 1.
Validation of artificial intelligence (AI)-based quantification protocols of myofibroblast density, macrophage density, and collagen content. A–C: α-smooth muscle actin (α-SMA) immunofluorescence was used to identify myofibroblasts as spindle-shaped immunoreactive cells located outside the vascular media (A, arrows). Arrowheads show vascular smooth muscle cells (VSMCs). The Intellesis Trainable Segmentation module of Zen 2.6 Pro software (Carl Zeiss Microscopy, New York, NY), an AI-based model, was trained on multiple fields of different regions of the myocardium to segment the images and identify myofibroblasts. Objects of interest were defined as the DAPI-α-SMA double-positive profiles, excluding VSMCs. B: unstained myocardium (including VSMCs) was considered the background. The trained AI-based model exhibited excellent correlation with manual measurement of myofibroblast density in the same fields (r = 0.98, P < 0.0001, n = 20). D–F: Mac2 immunofluorescence was used to identify macrophages (arrows). The AI-based quantification model was validated, showing excellent correlation with manual counts (r = 0.97, P < 0.0001, n = 20). G–I: Picrosirius red staining was used to label collagen fibers. The AI-based quantification model showed excellent correlation with manual quantification of collagen content (r = 0.83, P < 0.0001, n = 20). Scale bar, 20 μm.
Machine learning-based quantitative analysis of macrophage density.
Similarly, an AI-based model was trained to segment the images and identify macrophages. DAPI-Mac2 double-positive objects were identified as the objects of interest, while the unstained myocardium was considered the background. By use of the Image Analysis module, specific settings were incorporated in the trained model to count the segmented objects. Automatic analysis of 10 fields from two different levels of the murine heart was performed using the trained model. Validation of the AI model was performed by manually counting macrophage densities in 20 fields from different mice with ImageJ software. There was an excellent correlation (r = 0.97) between the AI-based quantification and the manual counts (Fig. 1, D–F).
Machine learning-based quantitative analysis of collagen content.
An AI-based model was trained on multiple fields of different regions of the myocardium to segment Picrosirius red-stained collagen fibers. Red fibrillar staining was considered the object of interest, while the unstained myocardium was considered the background. By use of the Image Analysis module, specific settings were incorporated in the trained model to count the segmented objects. Automatic analysis of 10 fields from two different levels of the murine heart was performed using the trained model. Validation of the AI model was performed by quantifying the collagen content in 20 fields from different mice with ImageJ software. There was an excellent correlation (r = 0.83) between the AI-based quantification and the measurements using the standard approach (Fig. 1, G–I).
Dual immunofluorescence for identification and quantification of M1 and M2 macrophages.
Dual immunofluorescent staining was performed to identify M1 and M2 macrophages. Staining for inducible nitric oxide synthase (iNOS) using a rabbit anti-iNOS antibody (dilution 1:100; Abcam, Cambridge, MA) was used as an M1 marker, whereas staining for arginase-1 (Arg1) using a rabbit anti- Arg1 antibody (dilution 1:100; Genetex, Irvine, CA) was used as an M2 marker. Sections were deparaffinized, and heat-mediated antigen retrieval was performed for 25 min during incubation of the slides in citrate buffer, pH 6.0 (Sigma, C9999). Sections were then blocked with 10% serum for 1 h and then incubated overnight at 4°C with Mac2 antibody together with anti-iNOS or anti-Arg1 antibodies. Sections were then washed and incubated with fluorescently labeled secondary antibodies for 1 h at room temperature. Autofluorescence quenching was performed using TrueBlack Lipofuscin Autofluorescence Quencher (23007, Biotium). Slides were mounted using Fluoro-Gel II with DAPI (17985, EMS). Sections were then scanned using Zen 2.6 Pro software and the Zeiss Imager M2 microscope. Mac2-positive cells were identified as macrophages. iNOS+/Mac2+ cells were manually counted using ImageJ software to assess the density of M1 macrophages. Similarly, Arg1+/Mac2+ cells were manually counted to assess the density of M2 macrophages.
Isolation of infarct myeloid cells and mRNA extraction.
Four male and four female C57BL/6J mice underwent 4-day coronary occlusion protocols to harvest myeloid cells. Myocardial tissue from individual mice was finely minced and placed into a digestion buffer cocktail (1 mL) of 0.5 mg/mL Liberase TH research grade, no. 05401151001, Millipore Sigma), 20 U/mL DNase I ((no. D4513, Sigma-Aldrich), 10 mmol/L HEPES (Invitrogen), and 0.1% sodium azide in HBSS with Ca2+ and Mg2+ (Invitrogen). The tissue was then shaken at 37°C for 5 min, and cells were then passed through a 40-μm nylon mesh. The digestion process was repeated four times, cell suspension was collected after it was passed through nylon mesh each time, and finally the total pooled cell suspension was centrifuged (10 min, 500 g, 4°C). Up to 108 cells were reconstituted with 180 μL of MACS buffer (Miltenyi Biotec, 130-091-376). Cells in MACS buffer were incubated with 20 µL of Anti-Ly-6G MicroBeads UltraPure (Cat. No. 130-120-337), incubated for 10 min at 4°C, and then washed once and centrifuged. The cells were resuspended in MACS buffer and passed through a MS column (Cat. No. 130-042-201) in a MACS separator (Miltenyi Biotec, 130-090-312). The magnetically labeled Ly6G+ cells were retained on the column. The unlabeled cells (Ly-6G negative flow through) were collected and washed once with MACS buffer (Ly6G- cells) and centrifuged at 500 g for 10 min. Subsequently, the cell pellet (per 108 Ly6G– cells) was again resuspended in 180 μL of MACS buffer, incubated with 20 µL of CD11b microBeads (Miltenyi Biotec, Cat. No. 130-049-601) at 4°C for 15 min, and then washed once and centrifuged. Resuspended cells went through a MACS column set in a MACS separator. The magnetically labeled CD11b+ cells were retained on the column. Ly6G-CD11b+ cells (macrophages) were flushed out and harvested for RNA isolation.
PCR array.
Gene expression in macrophages harvested from male and female mice was compared after 4 days of coronary occlusion. For PCR array, total RNA was extracted using the TRIzol reagent (Qiagen, 79306). A total of 400 ng of RNA was transcribed into cDNA using the RT2 first-strand kit (Qiagen, 330404). Quantitative PCR was then performed using the RT2 Profiler Mouse PCR Array Chemokines & Receptors (PAMM-022ZE-4) from Qiagen according to the manufacturer’s protocol. The same thermal profile conditions were used for all primer sets: 95°C for 10 min, 40 cycles at 95°C for 15 s, followed at 60°C for 1 min. The data obtained were analyzed using the ΔCq method. Gene expression levels were normalized to the levels of GAPDH.
Statistical analysis.
For all analyses, normal distribution was tested using the Shapiro–Wilk normality test. For comparisons of two groups unpaired two-tailed Student’s t test using (when appropriate) Welch’s correction for unequal variances was performed. The Mann–Whitney test was used for comparisons between two groups that did not show Gaussian distribution. For comparisons of multiple groups, one-way ANOVA was performed followed by Tukey’s multiple comparison test. The Kruskal–Wallis test, followed by Dunn’s multiple comparison posttest was used when one or more groups did not show Gaussian distribution. Survival analysis was performed using the Kaplan–Meier method. Mortality was compared using the log rank test. Correlation analysis between manual and AI-based quantifications was done using the Pearson correlation coefficient (Pearson’s r). Data are expressed as means ± SE. Statistical significance was set at P < 0.05.
RESULTS
Male C57BL/6J mice exhibit higher mortality than female animals following nonreperfused MI.
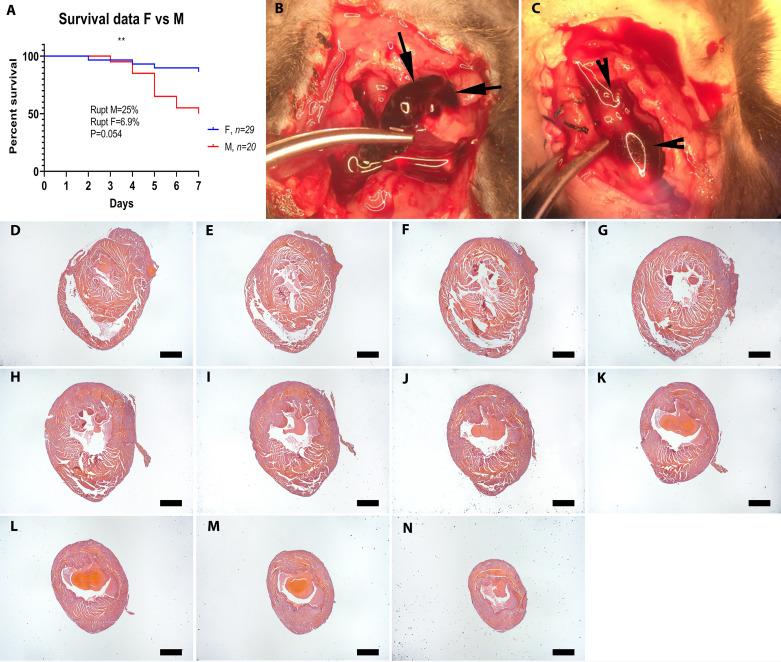
Forty-nine mice on a C57BL/6J background (20 males and 29 females) underwent nonreperfused MI protocols and were followed for 7 days. Mortality during the first week post-MI was 28.6%; deaths occurred between days 2 and 7 (mean: 4.9 ± 0.38, n = 14). Male animals had significantly higher mortality than female mice (Fig. 2A, males: 50% vs, females: 13.79%, P = 0.0067). There was no significant difference in the time of death between male and female animals [male, 5 ± 0.37 days, n = 10; female, 4.5 ± 1.04 days, n = 4, P = not significant (NS)].
Fig. 2.
Sex-specific mortality in infarcted mice and use of visual inspection vs. systematic histological analysis to identify the cause of death. A: when compared with female (F) C57BL/6J mice, male (M) animals had significantly higher post-myocardial infarction (MI) mortality (**P < 0.009), and a trend toward increased cardiac rupture rates (P = 0.054, n = 29 F, 20 M). To identify rupture-related deaths, visual inspection criteria were used. Criteria for rupture included a blood clot or blood in the chest (hemothorax; B and C, arrows) or presence of a cardiac rupture site. D–N: systematic histological analysis was performed by sectioning the entire heart from base to apex at 300-mm partitions and staining the first section of each partition (D–N shows consecutive myocardial sections used to systematically study the cause the death. Scale bar, 1 mm.
Visual inspection has low specificity for identification of cardiac rupture in mice dying following MI.
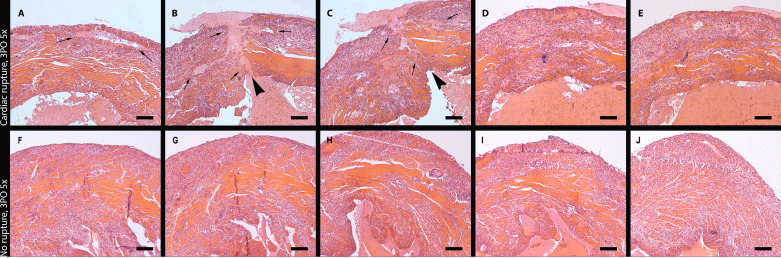
The majority of published studies use visual inspection criteria to determine whether rupture is the cause of death in mice undergoing MI protocols. In our study, visual inspection during autopsy (Fig. 2, B and C) suggested that 10 of 14 deaths (71.4%) were due to cardiac rupture based on the identification of a rupture site or the presence of hemothorax. To validate the findings of visual inspection, we performed systematic histopathological analysis of all dead mouse hearts, examining myocardial sections from base (Fig. 2D) to apex (Fig. 2N) at 250-mm intervals (Fig. 2, D–N). Histological evidence of rupture was found in only 50% of dead mice (Fig. 3, A–E), typically involving the left ventricular free wall at an apical level. In the other 50% of the dead mice, histological analysis documented the absence of rupture (Fig. 3, F–J). Next, we validated specific gross-pathological criteria for documentation of rupture, using systematic histological analysis as the “gold standard.” The presence of hemothorax, or the combined criterion of hemothorax and an identifiable rupture site had a high sensitivity (85.7%) but a relatively low specificity (42.8%) for documentation of rupture in infarcted mice (Table 1). On the other hand, identification of a rupture site or the combined presence of hemothorax and a rupture site were highly specific (85.7%) but had low sensitivity (28.6%). Both rupture-related and rupture-independent deaths were more common in male mice (25% of male mice and only 6.9% of female mice had post-MI cardiac rupture, P = 0.054). There was no significant difference in the time of death due to rupture (mean: 5 ± 0.53 days, n = 7, range: 3–7 days) versus no rupture (4.7 ± 0.56 days, n = 7, range: 2–7 days) for male (rupture: 4.8 ± 0.58, n = 5 versus no rupture: 5.2 ± 0.49, n = 5) or female mice (rupture: 5.5 ± 1.5, n = 2; versus no rupture: 3.5 ± 1.5, n = 2).
Fig. 3.
Histological documentation of left ventricular free wall rupture in mice dying after myocardial infarction (MI). A–E: consecutive sections show the site of the intramural rupture track (arrowheads) in an animal dying of rupture 3 days after permanent coronary occlusion. Arrows show the borders of the ruptured area. F–J: systematic histological analysis of myocardial sections documents the absence of rupture in a representative mouse that died 3 days after coronary occlusion. Scale bar, 200 μm.
Table 1.
Sensitivity and specificity of gross pathological criteria used for documentation of rupture
| RS | Hem | RS and Hem | RS or Hem | |
|---|---|---|---|---|
| Sensitivity | 28.6 | 85.7 | 28.6 | 85.7 |
| Specificity | 85.7 | 42.8 | 85.7 | 42.8 |
RS, rupture site; Hem, hemothorax.
Mice dying of rupture and animals with rupture-independent deaths have comparable infarct size and infarct wall thickness.
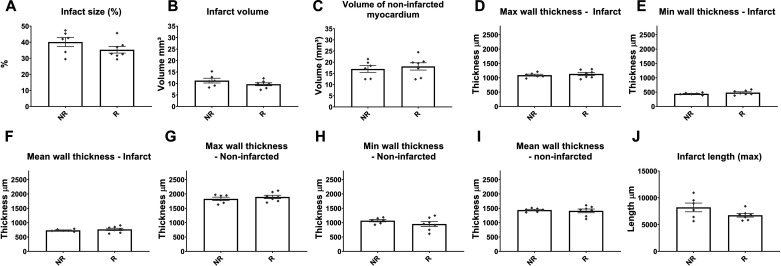
To examine whether rupture was associated with larger infarcts, we compared the morphometric characteristics of the infarcted and noninfarcted myocardial segments between mice dying of rupture and animals that died from non-rupture-related causes. Systematic quantitative analysis of hearts sectioned serially from base to apex showed that mice with rupture and those dying from other infarct-related causes had comparable infarct size (Fig. 4A), infarct volume (Fig. 4B), and volume of noninfarcted myocardium (Fig. 4C), maximal, minimal, and mean thickness of the infarcted and noninfarcted myocardium (Fig. 4, D–I) and infarct length (Fig. 4I). Thus, rupture of the infarcted heart was independent of the size of the infarct or the thickness of the infarcted and noninfarcted segments.
Fig. 4.
Rupture is not associated with larger infarcts or thinner infarct walls. Comparison of morphometric end points between mice dying of rupture (R) and animals dying in the absence of rupture (no rupture, NR) showed no significant differences between groups. Infarct size (A), infarct volume (B), volume of noninfarcted myocardium (C), maximal, minimal, and mean thickness of the infarcted wall (D, E, F, respectively), maximal, minimal, and mean wall thickness of the noninfarcted myocardial segments (G, H, I, respectively), and infarct length (J) were comparable between groups (P = not significant, n = 6–7/group). Time points studied histologically were comparable between groups, as there was no significant difference in time of death (R group: 5.0 ± 0.53 days, n = 7; R group: 5.16 ± 0.4, n = 6).
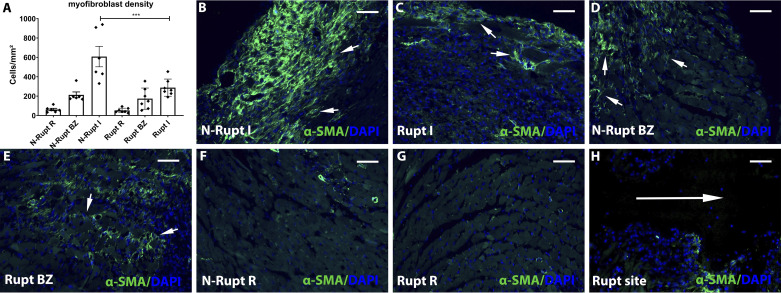
Mice dying of rupture have lower myofibroblast density and a trend toward reduced collagen content in the infarcted area.
We next examined whether cardiac rupture might reflect impaired infiltration of the infarct with activated myofibroblasts. Myofibroblast density in the infarct zone was markedly lower in mice dying of cardiac rupture compared with animals dying due to rupture-independent pathology (P < 0.001; Fig. 5, A–C). Very few myofibroblasts could be identified in the area around the rupture track (Fig. 5H). In contrast, no significant differences were noted in myofibroblast density in border and remote areas of the noninfarcted myocardium (Fig. 5, A and D–G).
Fig. 5.
Rupture is associated with attenuated myofibroblast infiltration in the healing infarct. A: artificial intelligence (AI)-based quantitative analysis showed that mice dying of cardiac rupture had markedly lower myofibroblast density in the infarcted myocardium (I) compared with mice dying the absence of rupture (***P < 0.0001, n = 6–7/group). No significant differences were noted in the border zone (BZ) and in the remote remodeling myocardium (R). B–H: representative images show identification of α-smooth muscle actin (α-SMA) + myofibroblasts in infarcted hearts (short arrows). Infarcted segments in mice dying of rupture (C) have attenuated myofibroblast infiltration. H: please note the absence of myofibroblasts in the rupture site (long arrow). Time points studied histologically were comparable between groups, as there was no significant difference in the time of death (rupture group: 5.0 ± 0.53 days, n = 7; no rupture group: 5.16 ± 0.4, n = 6). Scale bar, 50 μm.
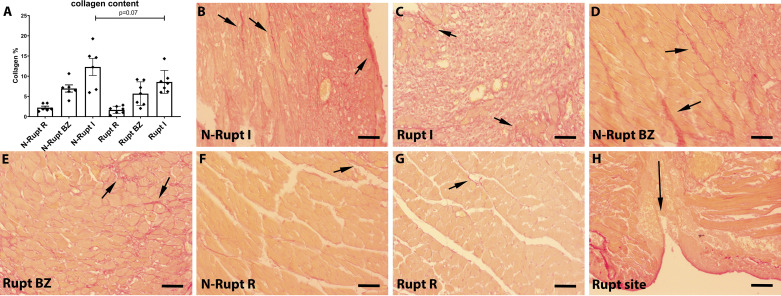
Picrosirius red staining was used to label collagen fibers. Mice dying from cardiac rupture exhibited a trend toward reduced collagen content in the infarct zone (P = 0.07; Fig. 6, A–C). Collagen was virtually absent in the areas neighboring the rupture site (Fig. 6H). No significant differences in collagen content were noted in the noninfarcted border and remote myocardial segments (Fig. 6, A and D–G).
Fig. 6.
Rupture is associated with a trend toward reduced collagen deposition in the healing infarct. A: quantitative analysis using a machine learning approach showed that collagen content in the infarct (I) was lower in mice dying of rupture (Rupt) compared with animals dying without rupture (N-rupt). However, the difference did not reach statistical significance (P = 0.07, n = 6–7/group). No significant differences were noted in the border zone (BZ) and in the remote remodeling myocardium (R). B–H: representative images show labeling of collagen fibers using Picrosirius red staining (short arrows). H: collagen was absent in the rupture site (long arrow). Time points studied histologically were comparable between groups, as there was no significant difference in the time of death (rupture group: 5.0 ± 0.53 days, n = 7; no rupture group: 5.16 ± 0.4, n = 6). Scale bar, 50 μm.
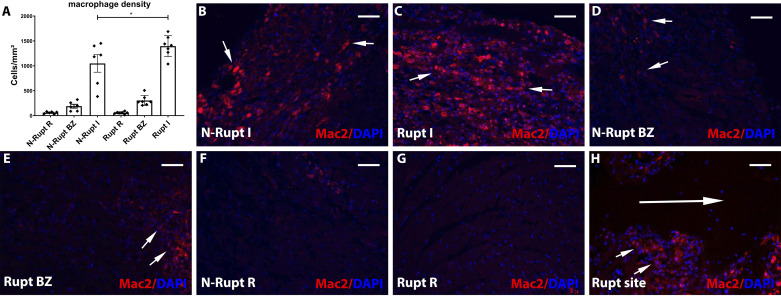
Mice dying of rupture have higher macrophage density in the infarct zone.
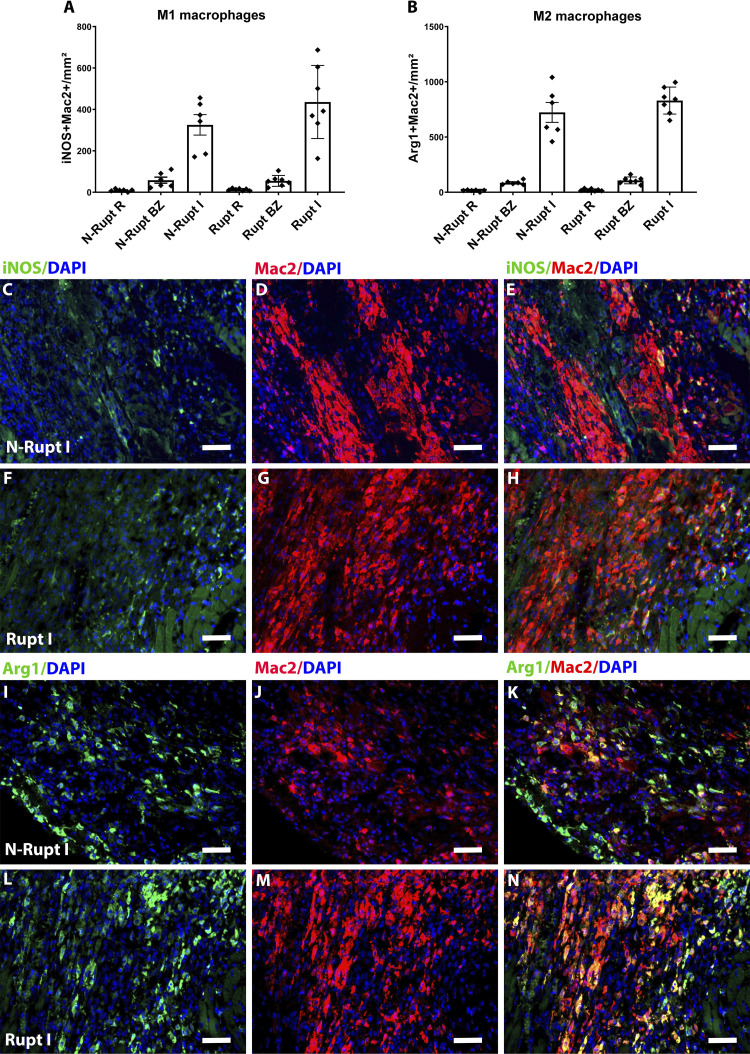
Mac2 immunohistochemistry was used to label macrophages. Mice dying of rupture had significantly higher macrophage density in the infarct zone (P < 0.05; Fig. 7, A–C). Abundant Mac2+ macrophages were found along the edges of the rupture track (Fig. 7H). In contrast, macrophage density in the noninfarcted border and remote myocardial segments was comparable between groups (Fig. 7, A and D–G). To examine potential associations between rupture-related death and macrophage polarization, we identified M1-like and M2-like macrophages by using the M1 marker iNOS and the M2 marker Arg1. No significant differences were noted in the density of M1 or M2 macrophages between animals with rupture and mice that died without rupture (Fig. 8).
Fig. 7.
Rupture is associated with increased macrophage infiltration in the infarcted segment. A: quantitative analysis using a machine learning approach showed that macrophage density in the infarct (I) was lower in mice dying of rupture (Rupt) compared with animals dying without rupture (N-rupt) (*P < 0.05, n = 6–7/group). No significant differences in macrophage density were noted in the border zone (BZ) and in the remote remodeling myocardium (R). B–H: representative images show labeling of macrophages using Mac2 immunofluorescence (short arrows). Mice dying of rupture had significantly increased macrophage infiltration (C). H: a significant number of Mac2+ macrophages (short arrows) are noted in the edge of the rupture site (long arrow). Time points studied histologically were comparable between groups, as there was no significant difference in the time of death (rupture group: 5.0 ± 0.53 days, n = 7; no rupture group: 5.16 ± 0.4, n = 6). Scale bar, 50 μm.
Fig. 8.
Rupture is not associated with significant changes in the density of M1 vs. M2 macrophages. Quantitative analysis showed no significant differences in the density of inducible NO synthase (iNOS)+/Mac2+ M1-like macrophages (A) and arginase-1 (Arg1)+/Mac2+ M2-like macrophages (B) between groups (n = 6–7/groups). C–H: dual immunofluorescence for macrophage marker Mac2 (red) and M1 marker iNOS was used to identify M1 macrophages. I–N: dual immunofluorescence for Mac2 (red), and M2 marker Arg1 was used to identify M2 macrophages. Time points studied histologically were comparable between groups, as there was no significant difference in the time of death (rupture group: 5.0 ± 0.53 days, n = 7; no rupture group: 5.16 ± 0.4, n = 6). Scale bar, 50 μm.
Infarct myeloid cells harvested from male and female mice have comparable inflammatory gene expression profiles.
To examine whether the increased incidence of rupture in male mice reflected overactive inflammatory responses in myeloid cells, we compared cytokine and chemokine gene expression in sorted myocardial CD11b+ myeloid cells harvested from male and female mice after 4 days of permanent coronary occlusion (the time point corresponding to the peak incidence of cardiac rupture). Expressions of most inflammatory mediators involved in postinfarction remodeling [including C-C motif ligand (CCL)2, CCL3, CCL4, CCL5, CCL9, C-X3-C motif ligand (CX3CL)1, C-X-C motif ligand (CXCL)10, IL-1β, IL-6, and TNFα] were comparable between cells harvested from male and female animals (Table 2). Expressions of CCL8 and CXCL2 were significantly higher in cells harvested from female mouse infarcts. There were no significant differences in β2-integrin, chemokine receptor or Toll-like receptor (TLR)2 and TLR4 levels between groups (Table 2). Thus, the increased incidence of cardiac rupture in male mice could not be attributed to differences in leukocyte inflammatory gene expression levels.
Table 2.
Inflammatory gene expression in infarct myeloid cells from male and female mice
| Gene Name | Gene: GAPDH |
P Value | |
|---|---|---|---|
| M | F | ||
| Complement component 5a receptor 1 | 0.01384 | 0.01186 | 0.7648 |
| Chemokine (C-C motif) ligand 11 | 0.001534 | 0.005988 | 0.1437 |
| Chemokine (C-C motif) ligand 12 | 0.004248 | 0.002895 | 0.7157 |
| Chemokine (C-C motif) ligand 17 | 0.001263 | 0.002920 | 0.4909 |
| Chemokine (C-C motif) ligand 19 | 0.004525 | 0.004905 | 0.9018 |
| Chemokine (C-C motif) ligand 2 | 0.01989 | 0.01860 | 0.9172 |
| Chemokine (C-C motif) ligand 22 | 0.002595 | 0.0006138 | 0.4856 |
| Chemokine (C-C motif) ligand 24 | 0.001781 | 0.0004637 | 0.4692 |
| Chemokine (C-C motif) ligand 25 | 0.0005090 | ND | 0.3559 |
| Chemokine (C-C motif) ligand 26 | 0.001375 | 0.002190 | 0.4123 |
| Chemokine (C-C motif) ligand 3 | 0.01030 | 0.009001 | 0.8329 |
| Chemokine (C-C motif) ligand 4 | 0.008491 | 0.01264 | 0.4811 |
| Chemokine (C-C motif) ligand 5 | 0.004289 | 0.002758 | 0.5280 |
| Chemokine (C-C motif) ligand 6 | 0.1727 | 0.07732 | 0.3568 |
| Chemokine (C-C motif) ligand 7 | 0.01639 | 0.02507 | 0.3653 |
| Chemokine (C-C motif) ligand 8 | 0.005768 | 0.01586 | 0.0257 |
| Chemokine (C-C motif) ligand 9 | 0.009111 | 0.003166 | 0.1254 |
| Chemokine (C-C motif) receptor 1 | 0.006143 | 0.01291 | 0.2774 |
| Chemokine (C-C motif) receptor 10 | 0.003331 | ND | 0.4226 |
| Chemokine (C-C motif) receptor 1-like 1 | 0.003002 | ND | 0.4226 |
| Chemokine (C-C motif) receptor 2 | 0.007806 | 0.01448 | 0.4072 |
| Chemokine (C-C motif) receptor 3 | 0.006727 | 0.004839 | 0.5371 |
| Chemokine (C-C motif) receptor 4 | 0.0005084 | 0.001546 | 0.2750 |
| Chemokine (C-C motif) receptor 5 | 0.004950 | 0.01017 | 0.2352 |
| Chemokine (C-C motif) receptor 6 | 0.0003573 | 0.0005493 | 0.7640 |
| Chemokine (C-C motif) receptor 7 | 0.005574 | 0.007365 | 0.6991 |
| Chemokine (C-C motif) receptor 8 | ND | ND | |
| Chemokine (C-C motif) receptor 9 | 0.003901 | ND | 0.3912 |
| Chemokine (C-C motif) receptor-like 1 | 0.0006560 | 0.0006533 | 0.9972 |
| Chemokine (C-C motif) receptor-like 2 | 0.004157 | 0.001810 | 0.3649 |
| Chemokine-like receptor 1 | 0.003178 | 0.002272 | 0.7852 |
| Cklf-like Marvel Tm domain containing 2a | ND | 0.0004849 | 0.3559 |
| Cklf-like Marvel Tm domain containing 3 | 0.01266 | 0.01048 | 0.7589 |
| Cklf-like Marvel Tm domain containing 4 | 0.003410 | 0.0006223 | 0.4047 |
| Cklf-like Marvel Tm domain containing 5 | ND | ND | |
| Cklf-like Marvel Tm domain containing 6 | 0.003730 | 0.01734 | 0.0642 |
| Chemokine (C-X3-C motif) ligand 1 | 0.001861 | 0.001978 | 0.9398 |
| Chemokine (C-X3-C) receptor 1 | 0.001722 | 0.002541 | 0.6002 |
| Chemokine (C-X-C motif) ligand 1 | 0.002253 | 0.002495 | 0.8746 |
| Chemokine (C-X-C motif) ligand 10 | 0.01534 | 0.008975 | 0.0814 |
| Chemokine (C-X-C motif) ligand 11 | ND | ND | |
| Chemokine (C-X-C motif) ligand 12 | 0.05222 | 0.1065 | 0.3271 |
| Chemokine (C-X-C motif) ligand 13 | 0.002535 | 0.007473 | 0.1327 |
| Chemokine (C-X-C motif) ligand 14 | 0.0001556 | ND | 0.3559 |
| Chemokine (C-X-C motif) ligand 15 | 0.0009104 | 0.0005166 | 0.6322 |
| Chemokine (C-X-C motif) ligand 16 | 0.008578 | 0.009264 | 0.8886 |
| Chemokine (C-X-C motif) ligand 2 | 0.01357 | 0.02451 | 0.0080 |
| Chemokine (C-X-C motif) ligand 3 | 0.003016 | 0.002059 | 0.4415 |
| Chemokine (C-X-C motif) ligand 5 | 0.003116 | 0.001180 | 0.5320 |
| Chemokine (C-X-C motif) ligand 9 | 0.0001775 | ND | 0.3559 |
| Chemokine (C-X-C motif) receptor 1 | ND | ND | |
| Chemokine (C-X-C motif) receptor 2 | 0.0004524 | ND | 0.4226 |
| Chemokine (C-X-C motif) receptor 3 | ND | ND | |
| Chemokine (C-X-C motif) receptor 4 | 0.02041 | 0.01743 | 0.7842 |
| Chemokine (C-X-C motif) receptor 5 | 0.001828 | 0.1638 | |
| Chemokine (C-X-C motif) receptor 6 | ND | 0.001712 | 0.3559 |
| Chemokine (C-X-C motif) receptor 7 | 0.001689 | 0.002191 | 0.7732 |
| Duffy blood group, chemokine receptor | ND | 0.0005270 | 0.3559 |
| Formyl peptide receptor 1 | 0.001797 | 0.001522 | 0.9107 |
| G protein-coupled receptor 17 | 0.002156 | 0.001442 | 0.6295 |
| Hypoxia-inducible factor 1α | 0.01286 | 0.01349 | 0.9058 |
| Interferon-γ | ND | ND | |
| Interleukin-16 | 0.0009023 | 0.0002709 | 0.3070 |
| Interleukin-1β | 0.04332 | 0.03605 | 0.6744 |
| Interleukin-4 | 0.003591 | 0.00005937 | 0.3314 |
| Interleukin-6 | 0.01221 | 0.008579 | 0.5687 |
| Integrin-αM | 0.02596 | 0.02443 | 0.8738 |
| Integrin-β2 | 0.04285 | 0.04131 | 0.9220 |
| Mitogen-activated protein kinase-1 | 0.02609 | 0.01908 | 0.4511 |
| Mitogen-activated protein kinase-14 | 0.008821 | 0.009484 | 0.8759 |
| Platelet factor 4 | 0.06353 | 0.04216 | 0.2131 |
| Pro-platelet basic protein | 0.006339 | 0.007147 | 0.8091 |
| Slit homolog 2 (Drosophila) | 0.0004225 | ND | 0.1389 |
| Transforming growth factor-β1 | 0.05729 | 0.05662 | 0.9558 |
| Toll-like receptor 2 | 0.01014 | 0.008979 | 0.7833 |
| Toll-like receptor 4 | 0.003651 | 0.0007350 | 0.1473 |
| Tumor necrosis factor | 0.001179 | 0.0009175 | 0.8138 |
| Thymidine phosphorylase | ND | ND | |
| Chemokine (C motif) ligand 1 | 0.006992 | 0.4226 | |
| Chemokine (C motif) receptor 1 | ND | ND | |
M, male; F, female; ND, not determined.
DISCUSSION
The majority of published studies suggest that left ventricular free wall rupture is the predominant cause of death in mice undergoing nonreperfused MI protocols (27, 65, 71, 97). However, there is substantial variation in the reported rupture rates, which is dependent not only on sex and the genetic background of the animals but also on the specific criteria used for documentation of rupture (Table 3). Although most studies use gross pathology to document rupture, the specific criteria vary. Typically, studies using hemothorax as a diagnostic criterion for rupture report very high rupture rates, whereas investigations requiring identification of a perforation site upon autopsy report much lower rupture rates (Table 4). Our study clarifies the basis for the conflicting findings. Systematic histological analysis of the infarcted heart from base to apex (Fig. 2) identified a rupture site in only 50% of mice dying following nonreperfused MI (Figs. 2 and 3), consistent with other histopathological studies in C57BL/6 mice (Table 3). Our findings show that hemothorax is highly sensitive for identification of rupture but has relatively low specificity. In contrast, gross identification of a perforation site is very specific for rupture but has low sensitivity, missing a large number of rupture events (Table 1).
Table 3.
Rupture-related mortality in wild-type mice undergoing nonreperfused myocardial infarction protocols
| Strain | Sex | Age, wk | Mortality, % | Duration of Follow-up | Rupture rates, rupture × 100/total deaths | Rupture Assessment Criteria | Ref. |
|---|---|---|---|---|---|---|---|
| C57BL/6J | M+F | 18 | 45 | 7 | 33 | Hem or RS | (43) |
| C57BL/6 | M | 8–10 | 35.6 | 28 | 78.7 | Autopsy (not otherwise specified) | (46) |
| C57BL/6J | M+F | 10–12 | 35 | 28 | 100 | Hem and RS | (14) |
| C57BL/6 | M | 12 | 46.6 | 56 | 92 | Hem and RS | (87) |
| C57BL/6 | M | 8–12 | 22.7 | 7 | 80 | Hem and RS | (93) |
| C57BL/6J | M+F | 12 | 42 | 28 | 63 (M), 38.5 (F) | Hem and RS | (48) |
| C57BL/6 | M | 8–10 | 52 | 7 | 60.6 | Hem | (45) |
| C57BI/6J | M+F | N/A | 45 | 14 | 100 | Autopsy (not otherwise specified) | (15) |
| C57BL/6 | M | 8–16 | 75 | 28 | 100 | Autopsy (not otherwise specified) | (2) |
| N/A | M+F | N/A | 12 | 7 | 100 | Autopsy (not otherwise specified) | (26) |
| C57Bl/6J | M+F | N/A | 40 | 7 | 100 | Autopsy (not otherwise specified) | (75) |
| C57BL/6 | M | 10 | 55 | 28 | 96 | Hem and/or RS | (96) |
| C57BL/6J | M | 8–12 | 40 | 28 | 100 | Autopsy (not otherwise specified) | (77) |
| C57BL/6 | M | 8–12 | 41 | 56 | 80 | RS | (7) |
| C57BL/6 | M | 8–12 | 20 | 14 | 83 | Autopsy (not otherwise specified) | (12) |
| C57BL/6 | M | 12–20 | 65 | 14 | 26.1 | Autopsy (not otherwise specified) | (84) |
| C57BL/6/129/svj | M | 8–12 | 34.6 | 42 | 11.5 | RS | (50) |
| C57BL/6 | M | 8–12 | 39.3 | 28 | 57.7 | Histology | (64) |
| C57BL/6 | M | 10–12 | 35 | 28 | 85 | Autopsy (not otherwise specified) | (53) |
| C57BL/6J | M+F | 8–12 | 16 | 28 | 73 | Hem or RS | (85) |
| C57BL/6 | M | 12 | 58 | 28 | 92 | Autopsy (not otherwise specified) | (81) |
| C57BL/6 | M | 10–12 | 48 | 28 | 62.5 | Hem and RS | (58) |
| C57BL/6 | M+F | N/A | 44 | 28 | 63.6 | Histology | (46) |
| C57BL/6J | M | 16 | 60 | 28 | 100 | Autopsy (not otherwise specified) | (39) |
| C57BL/6 × FVB | F | 12–14 | 15 | 7 | 100 | Hem and/or RS | (83) |
| C57BL/6 | M | 10–16 | 51 | 28 | 70 | Hem or RS | (97) |
| C57BL/6 | M | 10 | 50 | 7 | 100 | Autopsy (not otherwise specified) | (92) |
| C57BL/6 | M | 9–12 | 50 | 28 | 87.4 | Hem and RS | (82) |
| C57BL/6 | M+F | 7–8 | 60 | 120 | 100 | Autopsy (not otherwise specified) | (70) |
| C57BL/6 | M | 8–10 | 22 | 14 | 100 | Hem and RS | (71) |
| C57Bl/6 | M | 8–10 | 53 | 7 | 55.8 | Histology | (59) |
| C57BL/6 | M | 8 | 31 | 5 | 33.6 | RS | (54) |
| C57BL/6 | M | 8–10 | 41 | 28 | 69.7 | Hem and RS | (44) |
| C57BL/6 | M | 9–12 | 27.1 | 7 | 100 | Hem | (63) |
| C57BL/6 | M+F | N/A | 40 | 28 | 85.5 | Hem | (34) |
| C57BL/6 | M | 10–12 | 19.8 | 28 | 100 | Autopsy (not otherwise specified) | (3) |
| C57BL/6 | M+F | 12–16 | 6 | 14 | 50 | RS and Histology | (88) |
| C57BL/6J | M | 11–12 | 28 | 10 | 75 | Hem and Infusion | (67) |
| FVB | M+F | N/A | 43.2 | 20 | 55 | Hem | (86) |
| C57BL/6 | M | N/A | 66 | 7 | 40 | Autopsy (not otherwise specified) | (61) |
| C57BL/6 | M | 8–28 | 25 | 30 | 33.6 | Infusion | (32) |
| C57BL/6 | M | 10–12 | 40 | 28 | 80 | Hem | (49) |
| FVB/njcl | F | 8–12 | 35 | 28 | 88 | Hem | (37) |
| C57BL/6J | M | 10–18 | 15% | 14 | 60 | Histology | (78) |
| C57BL/6 | M | N/A | 40 | 10 | 100 | Autopsy (not otherwise specified) | (69), |
| C57BL/6J | M | 8 | 46 | 7 | 68 | Autopsy (not otherwise specified) | (79) |
| C57BL/6J | M | 12–13 | 54 | 28 | 38.5 | Histology | (65) |
| C57BL/6 | M | 12–16 | 23.5 | 7 | 100 | Autopsy (not otherwise specified) | (42) |
| C57BL/6 | M | 8–12 | N/A | N/A | 30 | RS | (36) |
| BalbC | M | 10–12 | 6 | 28 | 33.3 | Hem | (89) |
| C57BL/6 | M | 10–12 | 19 | 28 | 81.8 | Hem | (89) |
| FVB | M | 10–12 | 37 | 28 | 45.8 | Hem | (89) |
| 129S6 | M | 10–12 | 42.8 | 28 | 93.3 | Hem | (89) |
| Swiss | M | 10–12 | 14.5 | 28 | 75 | Hem | (89) |
| FVB/N | M+F | 12–16 | 16.5 (M), 15.7 (F) | 7 | 7.8 (M), 0 (F) | Hem and RS | (27) |
| C57BL/6 | M+F | 12–16 | 25.5 (M), 11.2 (F) | 7 | 56 (M), 45.5 (F) | Hem and RS | (27) |
| 129sv | M+F | 12–16 | 45.6 (M), 11.3 (F) | 7 | 93.4 (M), 72.8 (F) | Hem and RS | (27) |
M, male; F, female; N/A, not available; Hem, hemothorax (clot or blood in chest); RS, rupture site in gross pathology.
Table 4.
Postinfarction rupture rates in male C57Bl6 mice based on assessment through various strategies
| Strategy | Rupture Deaths (%) (mean) | Rupture Deaths (%) median | Rupture Deaths (%) (range) | Number of Studies (n) |
|---|---|---|---|---|
| Gross pathology (any criteria) | 77.9 | 81.8 | 26.1–100 | 31 |
| RS | 47.9 | 33.6 | 30–80 | 3 |
| Hem | 80.6 | 80.9 | 60.6–100 | 4 |
| RS + Hem | 77.7 | 75.0 | 56–100 | 10 |
| Gross pathology (criteria not specified) | 83.8 | 96.0 | 26.1–100 | 14 |
| Histology | 53 | 56.8 | 38.5–60 | 4 |
RS, rupture site, Hem, hemothorax.
Moreover, our experiments provide a systematic characterization of the histopathological characteristics of cardiac rupture in mice. When compared with infarcted mice dying in the absence of rupture, ruptured hearts had comparable infarct size and infarct wall thickness but exhibited increased macrophage infiltration accompanied by reduced myofibroblast content. Male mice had a significantly higher incidence of rupture than female animals. However, the increased susceptibility of male mice to rupture could not be explained by increased inflammatory activation of immune cells. Myeloid cells isolated from male and female mouse infarcts had comparable expressions of a wide range of inflammatory genes (Table 2).
Why are mice so susceptible to post-MI cardiac rupture?
Even when the most rigorous and specific approaches for documentation are used, the incidence of rupture in young adult mice with coronary occlusion remains remarkably high compared with the incidence of similar events in human MI patients. Several factors related to the characteristics of the MI model and the quality of cardiac repair in mice may explain the high susceptibility of mice to rupture. First, the extensive transmural MI induced by coronary occlusion in mice and the absence of significant collateral circulation increase the likelihood of cardiac rupture. In human MI patients, rupture is more common in individuals with single-vessel disease that lack collateral circulation (62, 72), a situation more likely to result in transmural infarction. The mouse model of permanent coronary occlusion recapitulates this clinical scenario. Second, the absence of significant hypertrophy and fibrosis in young adult mice increases the likelihood of rupture. In contrast, in human patients MI typically affects older subjects who may exhibit baseline hypertrophic and fibrotic changes due to preexisting coronary disease or other comorbid conditions (such as hypertension, diabetes, obesity, and metabolic dysfunction). The typical patient with rupture has a first infarct without any preexisting fibrotic or hypertrophic remodeling (21). Third, myocardial scars in mice exhibit marked thinning during infarct healing compared with large animals (11, 17) and may be more susceptible to rupture. According to Laplace’s law, tension in the ventricular wall is proportional to the radius and inversely proportional to the thickness of the wall. Although direct comparisons with human infarcts are impossible, the marked reduction in wall thickness in mouse infarcts, coupled with the impressive dilative remodeling in infarcted mouse hearts, may significantly increase wall stress, resulting in rupture. Finally, patients with MI are typically admitted to the coronary care unit, resting and receiving treatment. The continuous activity of infarcted mice may result in exercise-related systemic and intracardiac pressure increases that may precipitate rupture.
The cell biological response in ruptured hearts.
Following MI, massive necrosis of cardiomyocytes triggers an intense myocardial inflammatory reaction, inducing recruitment of neutrophils (13) and proinflammatory monocytes (18). Subsequent activation of fibrogenic macrophages in the infarct results in secretion of growth factors, such as TGFβ, that mediate formation of organized myofibroblast arrays in the infarct (52), triggering secretion and deposition of extracellular matrix (ECM) proteins. Dysregulation in the sequence of inflammatory and reparative cellular responses may play an important role in the pathogenesis of cardiac rupture.
In human patients, left ventricular free wall rupture can occur as early as a few hours or as late as several weeks after MI (94). Early rupture has been suggested to account for a significant percentage of pre-hospitalization deaths in MI patients (41). These rupture events occur before the infarct is infiltrated with significant numbers of leukocytes (6) and well before replacement with scar tissue. Thus, rupture events occurring during the first 24 h after MI cannot be attributed to overactive inflammatory cell infiltration or to perturbed activation of reparative cells. Massive cardiomyocyte death, accompanied by rapid activation of proteases in the interstitium of myocardial segments subjected to high wall stress, may account for early rupture in human patients. In C57BL6J mice, we did not observe rupture-related deaths within the first 24 h of MI. Thus, the cellular mechanisms underlying early rupture cannot be studied using the mouse model.
All rupture events in mice with nonreperfused MI occurred between days 3 and 7 following coronary occlusion at time points corresponding to the inflammatory and early reparative phase of cardiac repair (23). Comparison of the cellular composition of mice dying with and without rupture identified several distinct characteristics of ruptured hearts:
First, macrophage density is significantly increased in infarcted segments of mice dying of rupture, and significant numbers of macrophages are noted in the edge of the rupture track (Fig. 7). Increased influx of proinflammatory macrophages may result in local activation of proteases, thus degrading the cardiac ECM and precipitating rupture. To examine whether rupture is associated with perturbations in the ratio of proinflammatory vs. anti-inflammatory macrophages, we performed immunofluorescent staining for the M1 marker iNOS and the M2 marker Arg1. No significant differences in the density of M1-like and M2-like macrophages were noted in animals dying with or without rupture (Fig. 8). The findings do not support the notion that macrophage polarization is directly involved in cardiac rupture. However, it should be emphasized that systematic study of inflammatory mediator expression (rather than assessment of single M1/M2 markers) is required to gain insights into the phenotypic characteristics of macrophages in tissues. Unfortunately, harvesting macrophages from ruptured hearts to examine relations between inflammatory gene expression and rupture events is impractical.
Second, ruptured hearts exhibited reduced myofibroblast density in the infarct zone (Fig. 5). Recruitment and activation of fibroblasts play a crucial role in repair of the infarcted heart, generating a scar that protects the ventricle from catastrophic mechanical failure (52, 69). In mice with reduced activation of myofibroblasts, attenuated collagen synthesis may fall below the threshold needed to maintain the structural integrity of the infarct wall, thus leading to cardiac rupture. This notion was also supported by findings showing a trend toward reduced collagen content in ruptured hearts (Fig. 6).
Balance between proinflammatory and matrix-preserving cascades in the pathogenesis of cardiac rupture.
A large body of evidence derived from in vivo experiments in genetically manipulated mice suggests that uncontrolled activation of proinflammatory signaling cascades (reviewed in Table 5), impaired fibroblast activation, and overactive matrix degradation (reviewed in Table 6) are the predominant mechanisms that mediate postinfarction cardiac rupture. It should be noted that in most studies documentation of rupture was based on visual inspection criteria; thus, the findings may more accurately reflect effects on total mortality rather than rupture-specific deaths.
Table 5.
Inflammatory signals involved in the pathogenesis of cardiac rupture
| Gene/Protein | Mechanism | Ref. |
|---|---|---|
| Cyclic GMP-AMP synthase (cGAS) | cGAS was suggested to cause rupture following MI by acting as a pattern recognition receptor that stimulates proinflammatory programs and by inhibiting activation of reparative macrophages. | (8) |
| CD8 | Mice deficient in functional CD8+ T cells had improved survival but increased incidence of cardiac rupture, suggesting that CD8+ T cells may be involved in effective scar formation. | (43) |
| IL-35 | IL-35 protected from post-MI cardiac rupture, promoting reparative macrophage responses and improving repair. | (46) |
| Toll-like receptor 7 (TLR7) | TLR7 was implicated in cardiac rupture through effects on leukocyte cytokine expression. | (14) |
| Muscle atrophy F-box (MAFbx) | The E3 ubiquitin ligase MAFbx was implicated in cardiac rupture post-MI, presumably by inducing inflammatory cell infiltration. | (87) |
| Heat shock protein-B1 (HSPB1) | Cardiomyocyte-specific HSPB1 signaling was found to protect from post-MI cardiac rupture by inhibiting inflammatory activation. | (93) |
| 12/15-Lipoxygenase (LOX) | 12/15 LOX was implicated in the pathogenesis of cardiac rupture, presumably by enhancing synthesis of proinflammatory lipid mediators. | (48) |
| Apoptosis inhibitor of macrophage (AIM) | AIM was implicated in the pathogenesis of post-MI cardiac rupture, presumably through recruitment of proinflammatory macrophages. | (45) |
| CD36 | Activation of a CD36-Mertk axis protects from cardiac rupture by promoting phagocytosis of dead cells by activated macrophages. | (15) |
| Granulocyte/macrophage colony-stimulating factor (GM-CSF) | Fibroblast-derived GM-CSF was implicated in post-MI cardiac rupture, presumably through recruitment of proteolytic/inflammatory neutrophils and monocytes. | (2) |
| Glucocorticoid receptor (GR) | Inactivation of GR altered the functional differentiation of monocyte-derived macrophages in the infarcted myocardium and was suggested to cause higher rupture-related mortality. | (26) |
| β-Adrenergic receptor (β2AR) | Myeloid β2ARKO (through bone marrow transplantation) mice displayed 100% mortality resulting from post-MI cardiac rupture. β2ARKO mice had reduced leukocyte infiltration in infarcted hearts. | (30) |
| Calpastatin | Male mice overexpressing calpastatin had increased rates of post-MI cardiac rupture. KO mice exhibited reduced infiltration of M2 macrophages and CD4+ T cells. | (91) |
| TGFβ receptor 1 (TβRI) | Conditional cardiomyocyte specific TGFβ receptor 1 knockout displayed marked decline in neutrophil recruitment and attenuated MMP9 activity with reduced post-MI cardiac rupture rates. | (75) |
| MIF | MIF-deficient mice had lower rates of post-MI cardiac rupture, associated with reduced myocardial leukocyte infiltration, and reduced activity of MMP-2 and -9, p38, and JNK MAPK. | (96) |
| IL-23 | IL-23KO mice exhibited increased post-MI cardiac rupture rates, presumably through higher expression of proinflammatory cytokines and increased infiltration with immune cells. | (77) |
| Haptoglobin | Haptoglobin-deficient mice displayed increased post-MI cardiac rupture rates, presumably through increased leukocyte infiltration in the infarct, reduced PAI-1 activity and enhanced VEGFα expression. | (4) |
| 5-Lipoxygenase | 5-Lipoxygenase-null mice exhibited higher post-MI cardiac rupture rates, with more abundant proinflammatory macrophages and decreased collagen deposition and fibroblast migration. | (7) |
| D6 | D6-null mice exhibited increased post-MI cardiac rupture rates, presumably through enhanced infiltration of neutrophils and Ly6Chi monocytes and increased MMP-9 and -2 activity in the infarct. | (12) |
| Fibulin-2 | Fibulin-2 mice exhibited lower post-MI cardiac rupture rates with attenuated inflammatory cell infiltration and MMP-2 and -9 expression. | (84) |
| GDF-15 | GDF-15 deficient mice had enhanced recruitment of PMN leukocytes associated with increased rates of post-MI cardiac rupture. | (50) |
| Syndecan-4 (Syn4) | Syn4 KO mice exhibited increased rates of post-MI cardiac rupture, associated with suppressed inflammation and impaired granulation tissue formation in the heart after MI. | (64) |
| Gp130 | Cardiomyocyte-specific Gp130 KO was associated with increased post-MI cardiac rupture rates, presumably through enhanced STAT3 activation and increased expression of IL-6. | (38) |
| Timp4 | Timp4−/− mice had increased post-MI cardiac rupture rates, associated with increased neutrophil infiltration. | (53) |
| Class A macrophage scavenger receptor (SR-A) | SR-A−/− mice exhibited higher rates of post-MI cardiac rupture, presumably due to augmented gelatinolytic activity, increased MMP-9 and TNFα and reduced IL-10 mRNA in the infarcted myocardium. | (85) |
| TNFα | TNFα−/− mice exhibited markedly reduced post-MI cardiac rupture rates, presumably due to reduced inflammatory cell infiltration, cytokines, and MMP-9 and -2 expression in the infarct. | (81) |
| FrzA | FrzA overexpression protected from rupture, attenuating leukocyte infiltration and reducing MMP9 and MMP2 expression in the infarct. | (5) |
MI, myocardial infarction; KO, knockout.
Table 6.
Role of fibroblast activation and extracellular matrix remodeling in cardiac rupture
| Gene/Protein | Mechanism | Ref. |
|---|---|---|
| Hsp47 | Fibroblast-specific activation of Hsp47 was found to protect from cardiac rupture my promoting reparative myofibroblast activation. | (51) |
| TLR9 | TLR9 signaling protects from rupture by activating myofibroblasts possibly through an interaction with HMGB1. | (58) |
| Smad3 | Myofibroblast-specific Smad3, but not Smad2, signaling protects from late rupture post-MI by triggering an integrin-mediated response in myofibroblasts, thus contributing to formation of an organized scar. | (40, 52) |
| IL-35 | IL-35 protects from post-MI cardiac rupture promoting reparative macrophage responses and improving repair. | (46) |
| miR-144 | miR-144 was implicated in the pathogenesis of cardiac rupture, presumably through effects on ECM remodeling. | (35) |
| Mast cell protease 4 | Chymase mast cell protease 4 was implicated in post-MI cardiac rupture, presumably through effects on matrix remodeling. | (39) |
| Heme oxygenase-1 (Hmox1) | Mice deficient in Hmox1 had lower rates of post-MI cardiac rupture, with greater collagen type I production compared with wild-type mice. | (83) |
| Dectin-2 | Dectin-2 KO mice demonstrated lower post-MI cardiac rupture rates with increased expression of α-smooth muscle actin and collagen I/III and reduced MMP-2 and -9 expression. | (97) |
| miR-155 | mir-155-deficient mice exhibited lower rates of post-MI cardiac rupture, associated with more abundant myofibroblasts and a– increased collagen disposition in the infarct. | (92) |
| CD39 | CD39-deficient mice demonstrated lower post-MI cardiac rupture rates associated with elevated fibrin and collagen deposition and increased reparative macrophage influx to the infarcted area. | (82). |
| NEIL3 | Neil3−/− mice showed increased post-MI myocardial rupture, presumably through ECM dysregulation and increased levels of MMP-2. | (70) |
| TLR9 | TLR9 was suggested to reduce post-MI cardiac rupture rates presumably through proliferation and differentiation of cardiac fibroblasts. | (71) |
| Hand1 | Hand1+/− mice had decreased cardiac rupture rates associated with lower MMP-9 activity. | (59) |
| CD28 | CD28KO mice had higher post-MI cardiac rupture rates, lower collagen deposition, and lower myofibroblast numbers. | (54) |
| Twinkle | Twinkle overexpression reduced post-MI cardiac rupture rates presumably through suppression of MMP-2 and -9 in the border zone of the infarct. | (44) |
| Follistatin-like 1 | Conditional ablation of Fstl1 in S100a4-expressing fibroblast lineage cells was reported to reduce numbers of myofibroblast and expression of ECM proteins in the infarct, and demonstrated increased post-MI cardiac rupture. | (63) |
| Girdin | Girdin S1416A knock-in mice (in which the Akt phosphorylation site is replaced with alanin) had reduced cardiac myofibroblast proliferation and collagen deposition that was implicated in higher rates of post-MI cardiac rupture. | (34) |
| Sirtuin 7 | Sirt7−/− mice showed high susceptibility to cardiac rupture, associated with reduced myofibroblast differentiation and perturbed TGFβ responses. | (3) |
| Osteoglycin | Osteoglycin-null mice had significantly increased post-MI cardiac rupture rates. Tissue disruption and impaired collagen fibrilogenesis were implicated. | (88) |
| Phospholipase A2 receptor (PLA2R) | PLA2R-deficient mice exhibited higher rates of post-MI cardiac rupture, associated with decreased numbers of myofibroblasts and attenuated collagen deposition in the infarcted myocardium. PLA2R was implicated in migration and proliferation of myofibroblasts through interactions with integrin-β1. | (67) |
| Melusin | Melusin overexpression reduced post-MI cardiac rupture rates, increasing matricellular protein expression in the infarcted area. | (86) |
| MMP-28 | MMP-28 deletion increased post-MI cardiac rupture rates, presumably through impaired M2 macrophage activation and reduced deposition of ECM proteins. | (61) |
| Syndecan-4 | Syndecan-4 KO mice exhibited increased rates of post-MI cardiac rupture, associated with suppressed inflammation and impaired granulation tissue formation in the heart post-MI. | (64) |
| TIMP3 | TIMP3−/− mice exhibited increased rates of post-MI cardiac rupture presumably through increased MMP activity, activated EGFR signaling, decreased myofibroblast numbers, and collagen deposition. | (32), (49) |
| Bcrp1/Abcg2 | Bcrp1/Abcg2 KO mice exhibited higher post-MI cardiac rupture rates, with reduced capillary, myofibroblast, and macrophage densities in the peri-infarction area. | (37) |
| SPARC | SPARC-null mice exhibited increased rates of post-MI rupture, associated with disorganized granulation tissue formation and defective scar maturation. | (78) |
| Biglycan | Biglycan-deficient mice had higher post-MI rupture rates. Impaired collagen matrix organization was implicated. | (95) |
| Periostin | Periostin−/− mice had increased post-MI rupture rates, associated with lower numbers of myofibroblasts and impaired collagen fibril formation in the infarct. | (69, 79) |
| MMP-2 | MMP-2-null mice exhibited reduced post-MI cardiac rupture, presumably through reduced gelatinolytic activity, and attenuated ECM degradation. | (65) |
| Factor XIII | FXIII−/− mice had markedly higher post-MI cardiac rupture rates, associated with lower collagen I and higher MMP-9 levels. | (68) |
| Angiotensin II receptor (AT2R) | AT2R-null mice had increased post-MI rupture rates, associated with reduced collagen deposition in the infarct. | (42) |
| uPA, TIMP1 | uPA−/− mice exhibited low post-MI cardiac rupture rates. In addition, gene transfer of TIMP1 protected mice from rupture-related death. | (36) |
MI, myocardial infarction; KO, knockout; MMP, matrix metalloproteinase.
Direct activation of inflammatory cascades in leukocytes (14) and indirect stimulation of proinflammatory signals through release of cardiomyocyte- or fibroblast-derived mediators (93) (2) have been implicated in the pathogenesis of rupture. Moreover, loss of critical suppressive signals that inhibit or restrain inflammation has also been found to increase the incidence of cardiac rupture accentuating leukocyte infiltration (50). However, inflammation is not uniformly detrimental: some inflammatory pathways are critical for repair of the infarcted heart and protect from rupture, presumably by activating matrix-secreting mesenchymal cells (29, 64).
On the other hand, activation of reparative fibroblasts and stimulation of matrix-preserving pathways play a critical role in protection of the infarcted heart from rupture. Several members of the matrix metalloproteinase (MMP) family may promote rupture by degrading the cardiac ECM (36, 65) Several matricellular proteins that promote myofibroblast activation [such as periostin (69, 79), osteoglycin (88), and SPARC (secreted protein, acidic, cysteine-rich) (78)] were found to protect from cardiac rupture. Moreover, fibroblast-specific TGFβ/Smad3 signaling prevents late rupture following MI by stimulating integrin-dependent activation of infarct myofibroblasts (52). Considering that excessive ECM deposition in the infarcted heart may promote adverse remodeling and cause diastolic dysfunction, timely activation and suppression of fibroblast-driven matrix-preserving pathways are needed to protect from rupture while preventing exaggerated fibrosis (24). A recent study using single-cell transcriptomics suggested that mouse strains with lower susceptibility to cardiac rupture have early activation of matrix-producing myofibroblasts (22).
The male predilection for cardiac rupture: only in mice?
Our findings confirm the increased susceptibility of male mice to cardiac rupture, reported in many published studies (9, 20, 27). Published studies have suggested sex-specific patterns of inflammatory mediator synthesis and release in male and female patients with myocardial infarction (31, 76). The reduced incidence of rupture in female mice has been attributed to reduced myocardial inflammation and lower levels of matrix-degrading proteases at time points corresponding to the peak of rupture (20). We used a PCR array to examine whether male mice have increased inflammatory gene expression in myeloid cells. Our findings did not show significant differences between male and female mice in levels of a wide range of inflammatory mediators, including the chemokines CCL2, CCL3, CCL4, CCL5, CCL9, CX3CL1, and CXCL10 and the cytokines IL-1β, IL-6, and TNFα, β2-integrin and TLRs (Table 2). Our findings suggest that the increased susceptibility of male C57BL/6 mice to cardiac rupture is not due to differences in the gene expression profile of myeloid cells. Studies in mouse models of cardiac injury have suggested sex-specific differences in inflammatory gene expression patterns in neutrophils (16) and dendritic cells (74). Cardiomyocytes, fibroblasts (19), and vascular cells may also exhibit sex-specific differences in expression of inflammatory mediators or proteins involved in matrix remodeling that may explain the protective effects of female sex in postinfarction rupture. It should be emphasized that the male predilection for cardiac rupture seems to be specific to mice. In human patients, several studies have demonstrated that female sex is associated with an increased risk of rupture after MI (33, 55, 56). The species specificity may reflect, at least in part, the much younger age of mice undergoing experimental MI protocols compared with the human population. The vast majority of women suffering MI are elderly and postmenopausal. The protection noted in female mice may reflect hormonal actions of estrogens in young premenopausal animals (9).
Conclusions.
Cardiac rupture remains a catastrophic complication and a significant cause of death for patients with MI. The mouse model of nonreperfused MI is associated with a high incidence of rupture. Although histopathology is the gold standard for documentation of cardiac rupture, systematic histological analysis of all mice dying following MI may be impractical, considering the costly and highly laborious process required for documentation. Thus, most published studies have used gross pathology for rupture assessment and have generated highly variable results. Our study demonstrates that, if appropriately performed, gross pathology can be a useful alternative to histology but has significant limitations depending on the specific autopsy criteria used for documentation of rupture. Hemothorax is highly sensitive but has relatively low specificity, whereas gross identification of a perforation site is very specific but lacks sensitivity. Moreover, we characterize the cellular composition of the infarct in mice dying with or without rupture. We show that rupture is associated with increased macrophage infiltration and reduced myofibroblast recruitment in the infarcted segments. In the era of single-cell transcriptomics, identification of subsets of immune and reparative cells that may be associated with cardiac rupture may provide key insights into the cellular mechanisms underpinning this catastrophic complication.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01 HL-76246, R01 HL-85440, and R01 HL-149407 and United States Department of Defense Grants PR151029 and PR181464.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.H. and N.G.F. conceived and designed research; A.H. and A.V.S. performed experiments; A.H. and A.V.S. analyzed data; A.H. and N.G.F. interpreted results of experiments; A.H. prepared figures; A.H. and N.G.F. drafted manuscript; A.H., A.V.S., and N.G.F. edited and revised manuscript; A.H., A.V.S., and N.G.F. approved final version of manuscript.
REFERENCES
- 1.Alex L, Russo I, Holoborodko V, Frangogiannis NG. Characterization of a mouse model of obesity-related fibrotic cardiomyopathy that recapitulates features of human heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol 315: H934–H949, 2018. doi: 10.1152/ajpheart.00238.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anzai A, Choi JL, He S, Fenn AM, Nairz M, Rattik S, McAlpine CS, Mindur JE, Chan CT, Iwamoto Y, Tricot B, Wojtkiewicz GR, Weissleder R, Libby P, Nahrendorf M, Stone JR, Becher B, Swirski FK. The infarcted myocardium solicits GM-CSF for the detrimental oversupply of inflammatory leukocytes. J Exp Med 214: 3293–3310, 2017. doi: 10.1084/jem.20170689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araki S, Izumiya Y, Rokutanda T, Ianni A, Hanatani S, Kimura Y, Onoue Y, Senokuchi T, Yoshizawa T, Yasuda O, Koitabashi N, Kurabayashi M, Braun T, Bober E, Yamagata K, Ogawa H. Sirt7 contributes to myocardial tissue repair by maintaining transforming growth factor-β signaling pathway. Circulation 132: 1081–1093, 2015. doi: 10.1161/CIRCULATIONAHA.114.014821. [DOI] [PubMed] [Google Scholar]
- 4.Arslan F, Smeets MB, Buttari B, Profumo E, Riganò R, Akeroyd L, Kara E, Timmers L, Sluijter JP, van Middelaar B, den Ouden K, Pasterkamp G, Lim SK, de Kleijn DP. Lack of haptoglobin results in unbalanced VEGFα/angiopoietin-1 expression, intramural hemorrhage and impaired wound healing after myocardial infarction. J Mol Cell Cardiol 56: 116–128, 2013. doi: 10.1016/j.yjmcc.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Barandon L, Couffinhal T, Ezan J, Dufourcq P, Costet P, Alzieu P, Leroux L, Moreau C, Dare D, Duplàa C. Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation 108: 2282–2289, 2003. doi: 10.1161/01.CIR.0000093186.22847.4C. [DOI] [PubMed] [Google Scholar]
- 6.Becker AE, van Mantgem JP. Cardiac tamponade. A study of 50 hearts. Eur J Cardiol 3: 349–358, 1975. [PubMed] [Google Scholar]
- 7.Blömer N, Pachel C, Hofmann U, Nordbeck P, Bauer W, Mathes D, Frey A, Bayer B, Vogel B, Ertl G, Bauersachs J, Frantz S. 5-Lipoxygenase facilitates healing after myocardial infarction. Basic Res Cardiol 108: 367, 2013. doi: 10.1007/s00395-013-0367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao DJ, Schiattarella GG, Villalobos E, Jiang N, May HI, Li T, Chen ZJ, Gillette TG, Hill JA. Cytosolic DNA sensing promotes macrophage transformation and governs myocardial ischemic injury. Circulation 137: 2613–2634, 2018. doi: 10.1161/CIRCULATIONAHA.117.031046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavasin MA, Tao ZY, Yu AL, Yang XP. Testosterone enhances early cardiac remodeling after myocardial infarction, causing rupture and degrading cardiac function. Am J Physiol Heart Circ Physiol 290: H2043–H2050, 2006. doi: 10.1152/ajpheart.01121.2005. [DOI] [PubMed] [Google Scholar]
- 10.Chen B, Huang S, Su Y, Wu YJ, Hanna A, Brickshawana A, Graff J, Frangogiannis NG. Macrophage Smad3 Protects the infarcted heart, stimulating phagocytosis and regulating inflammation. Circ Res 125: 55–70, 2019. doi: 10.1161/CIRCRESAHA.119.315069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christia P, Bujak M, Gonzalez-Quesada C, Chen W, Dobaczewski M, Reddy A, Frangogiannis NG. Systematic characterization of myocardial inflammation, repair, and remodeling in a mouse model of reperfused myocardial infarction. J Histochem Cytochem 61: 555–570, 2013. doi: 10.1369/0022155413493912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cochain C, Auvynet C, Poupel L, Vilar J, Dumeau E, Richart A, Récalde A, Zouggari Y, Yin KY, Bruneval P, Renault G, Marchiol C, Bonnin P, Lévy B, Bonecchi R, Locati M, Combadière C, Silvestre JS. The chemokine decoy receptor D6 prevents excessive inflammation and adverse ventricular remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol 32: 2206–2213, 2012. doi: 10.1161/ATVBAHA.112.254409. [DOI] [PubMed] [Google Scholar]
- 13.Daseke MJ 2nd, Valerio FM, Kalusche WJ, Ma Y, DeLeon-Pennell KY, Lindsey ML. Neutrophil proteome shifts over the myocardial infarction time continuum. Basic Res Cardiol 114: 37, 2019. doi: 10.1007/s00395-019-0746-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Kleijn DP, Chong SY, Wang X, Yatim SM, Fairhurst AM, Vernooij F, Zharkova O, Chan MY, Foo RS, Timmers L, Lam CS, Wang JW. Toll-like receptor 7 deficiency promotes survival and reduces adverse left ventricular remodelling after myocardial infarction. Cardiovasc Res 115: 1791–1803, 2019. doi: 10.1093/cvr/cvz057. [DOI] [PubMed] [Google Scholar]
- 15.Dehn S, Thorp EB. Myeloid receptor CD36 is required for early phagocytosis of myocardial infarcts and induction of Nr4a1-dependent mechanisms of cardiac repair. FASEB J 32: 254–264, 2018. doi: 10.1096/fj.201700450r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLeon-Pennell KY, Mouton AJ, Ero OK, Ma Y, Padmanabhan Iyer R, Flynn ER, Espinoza I, Musani SK, Vasan RS, Hall ME, Fox ER, Lindsey ML. LXR/RXR signaling and neutrophil phenotype following myocardial infarction classify sex differences in remodeling. Basic Res Cardiol 113: 40, 2018. doi: 10.1007/s00395-018-0699-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol 164: 665–677, 2004. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 96: 881–889, 2005. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 19.Dworatzek E, Mahmoodzadeh S, Schriever C, Kusumoto K, Kramer L, Santos G, Fliegner D, Leung YK, Ho SM, Zimmermann WH, Lutz S, Regitz-Zagrosek V. Sex-specific regulation of collagen I and III expression by 17β-estradiol in cardiac fibroblasts: role of estrogen receptors. Cardiovasc Res 115: 315–327, 2019. doi: 10.1093/cvr/cvy185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang L, Gao XM, Moore XL, Kiriazis H, Su Y, Ming Z, Lim YL, Dart AM, Du XJ. Differences in inflammation, MMP activation and collagen damage account for gender difference in murine cardiac rupture following myocardial infarction. J Mol Cell Cardiol 43: 535–544, 2007. doi: 10.1016/j.yjmcc.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Figueras J, Alcalde O, Barrabés JA, Serra V, Alguersuari J, Cortadellas J, Lidón RM. Changes in hospital mortality rates in 425 patients with acute ST-elevation myocardial infarction and cardiac rupture over a 30-year period. Circulation 118: 2783–2789, 2008. doi: 10.1161/CIRCULATIONAHA.108.776690. [DOI] [PubMed] [Google Scholar]
- 22.Forte E, Skelly DA, Chen M, Daigle S, Morelli KA, Hon O, Philip VM, Costa MW, Rosenthal NA, Furtado MB. Dynamic interstitial cell response during myocardial infarction predicts resilience to rupture in genetically diverse mice. Cell Rep 30: 3149–3163, 2020. doi: 10.1016/j.celrep.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol 11: 255–265, 2014. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frangogiannis NG. The extracellular matrix in myocardial injury, repair, and remodeling. J Clin Invest 127: 1600–1612, 2017. doi: 10.1172/JCI87491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frunza O, Russo I, Saxena A, Shinde AV, Humeres C, Hanif W, Rai V, Su Y, Frangogiannis NG. Myocardial galectin-3 expression is associated with remodeling of the pressure-overloaded heart and may delay the hypertrophic response without affecting survival, dysfunction, and cardiac fibrosis. Am J Pathol 186: 1114–1127, 2016. doi: 10.1016/j.ajpath.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galuppo P, Vettorazzi S, Hövelmann J, Scholz CJ, Tuckermann JP, Bauersachs J, Fraccarollo D. The glucocorticoid receptor in monocyte-derived macrophages is critical for cardiac infarct repair and remodeling. FASEB J 31: 5122–5132, 2017. doi: 10.1096/fj.201700317R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao XM, Xu Q, Kiriazis H, Dart AM, Du XJ. Mouse model of post-infarct ventricular rupture: time course, strain- and gender-dependency, tensile strength, and histopathology. Cardiovasc Res 65: 469–477, 2005. doi: 10.1016/j.cardiores.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Gao XM, White DA, Dart AM, Du XJ. Post-infarct cardiac rupture: recent insights on pathogenesis and therapeutic interventions. Pharmacol Ther 134: 156–179, 2012. doi: 10.1016/j.pharmthera.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Grisanti LA, Gumpert AM, Traynham CJ, Gorsky JE, Repas AA, Gao E, Carter RL, Yu D, Calvert JW, García AP, Ibáñez B, Rabinowitz JE, Koch WJ, Tilley DG. Leukocyte-expressed β2-adrenergic receptors are essential for survival after acute myocardial injury. Circulation 134: 153–167, 2016. doi: 10.1161/CIRCULATIONAHA.116.022304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grisanti LA, Traynham CJ, Repas AA, Gao E, Koch WJ, Tilley DG. β2-Adrenergic receptor-dependent chemokine receptor 2 expression regulates leukocyte recruitment to the heart following acute injury. Proc Natl Acad Sci USA 113: 15126–15131, 2016. doi: 10.1073/pnas.1611023114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halade GV, Kain V, Dillion C, Beasley M, Dudenbostel T, Oparil S, Limdi NA. Race-based and sex-based differences in bioactive lipid mediators after myocardial infarction. ESC Heart Fail 7: 1700–1710, 2020. doi: 10.1002/ehf2.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammoud L, Lu X, Lei M, Feng Q. Deficiency in TIMP-3 increases cardiac rupture and mortality post-myocardial infarction via EGFR signaling: beneficial effects of cetuximab. Basic Res Cardiol 106: 459–471, 2011. doi: 10.1007/s00395-010-0147-7. [DOI] [PubMed] [Google Scholar]
- 33.Hao Z, Ma J, Dai J, Shao Q, Shen L, He B, Jiang L. A real-world analysis of cardiac rupture on incidence, risk factors and in-hospital outcomes in 4190 ST-elevation myocardial infarction patients from 2004 to 2015. Coron Artery Dis 31: 424–429, 2020. doi: 10.1097/MCA.0000000000000877. [DOI] [PubMed] [Google Scholar]
- 34.Hayano S, Takefuji M, Maeda K, Noda T, Ichimiya H, Kobayashi K, Enomoto A, Asai N, Takahashi M, Murohara T. Akt-dependent Girdin phosphorylation regulates repair processes after acute myocardial infarction. J Mol Cell Cardiol 88: 55–63, 2015. doi: 10.1016/j.yjmcc.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 35.He Q, Wang F, Honda T, James J, Li J, Redington A. Loss of miR-144 signaling interrupts extracellular matrix remodeling after myocardial infarction leading to worsened cardiac function. Sci Rep 8: 16886, 2018. doi: 10.1038/s41598-018-35314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, Dyspersin GD, Cleutjens JP, Shipley M, Angellilo A, Levi M, Nübe O, Baker A, Keshet E, Lupu F, Herbert JM, Smits JF, Shapiro SD, Baes M, Borgers M, Collen D, Daemen MJ, Carmeliet P. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med 5: 1135–1142, 1999. doi: 10.1038/13459. [DOI] [PubMed] [Google Scholar]
- 37.Higashikuni Y, Sainz J, Nakamura K, Takaoka M, Enomoto S, Iwata H, Sahara M, Tanaka K, Koibuchi N, Ito S, Kusuhara H, Sugiyama Y, Hirata Y, Nagai R, Sata M. The ATP-binding cassette transporter BCRP1/ABCG2 plays a pivotal role in cardiac repair after myocardial infarction via modulation of microvascular endothelial cell survival and function. Arterioscler Thromb Vasc Biol 30: 2128–2135, 2010. doi: 10.1161/ATVBAHA.110.211755. [DOI] [PubMed] [Google Scholar]
- 38.Hilfiker-Kleiner D, Shukla P, Klein G, Schaefer A, Stapel B, Hoch M, Müller W, Scherr M, Theilmeier G, Ernst M, Hilfiker A, Drexler H. Continuous glycoprotein-130-mediated signal transducer and activator of transcription-3 activation promotes inflammation, left ventricular rupture, and adverse outcome in subacute myocardial infarction. Circulation 122: 145–155, 2010. doi: 10.1161/CIRCULATIONAHA.109.933127. [DOI] [PubMed] [Google Scholar]
- 39.Houde M, Schwertani A, Touil H, Desbiens L, Sarrhini O, Lecomte R, Lepage M, Gagnon H, Takai S, Pejler G, Jacques D, Gobeil F Jr, Day R, D’Orléans-Juste P. Mouse mast cell protease 4 deletion protects heart function and survival after permanent myocardial infarction. Front Pharmacol 9: 868, 2018. doi: 10.3389/fphar.2018.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang S, Chen B, Su Y, Alex L, Humeres C, Shinde AV, Conway SJ, Frangogiannis NG. Distinct roles of myofibroblast-specific Smad2 and Smad3 signaling in repair and remodeling of the infarcted heart. J Mol Cell Cardiol 132: 84–97, 2019. doi: 10.1016/j.yjmcc.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hutchins KD, Skurnick J, Lavenhar M, Natarajan GA. Cardiac rupture in acute myocardial infarction: a reassessment. Am J Forensic Med Pathol 23: 78–82, 2002. doi: 10.1097/00000433-200203000-00017. [DOI] [PubMed] [Google Scholar]
- 42.Ichihara S, Senbonmatsu T, Price E Jr, Ichiki T, Gaffney FA, Inagami T. Targeted deletion of angiotensin II type 2 receptor caused cardiac rupture after acute myocardial infarction. Circulation 106: 2244–2249, 2002. doi: 10.1161/01.CIR.0000033826.52681.37. [DOI] [PubMed] [Google Scholar]
- 43.Ilatovskaya DV, Pitts C, Clayton J, Domondon M, Troncoso M, Pippin S, DeLeon-Pennell KY. CD8+ T-cells negatively regulate inflammation post-myocardial infarction. Am J Physiol Heart Circ Physiol 317: H581–H596, 2019. doi: 10.1152/ajpheart.00112.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inoue T, Ikeda M, Ide T, Fujino T, Matsuo Y, Arai S, Saku K, Sunagawa K. Twinkle overexpression prevents cardiac rupture after myocardial infarction by alleviating impaired mitochondrial biogenesis. Am J Physiol Heart Circ Physiol 311: H509–H519, 2016. doi: 10.1152/ajpheart.00044.2016. [DOI] [PubMed] [Google Scholar]
- 45.Ishikawa S, Noma T, Fu HY, Matsuzaki T, Ishizawa M, Ishikawa K, Murakami K, Nishimoto N, Nishiyama A, Minamino T. Apoptosis inhibitor of macrophage depletion decreased M1 macrophage accumulation and the incidence of cardiac rupture after myocardial infarction in mice. PLoS One 12: e0187894, 2017. doi: 10.1371/journal.pone.0187894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jia D, Jiang H, Weng X, Wu J, Bai P, Yang W, Wang Z, Hu K, Sun A, Ge J. Interleukin-35 promotes macrophage survival and improves wound healing after myocardial infarction in mice. Circ Res 124: 1323–1336, 2019. doi: 10.1161/CIRCRESAHA.118.314569. [DOI] [PubMed] [Google Scholar]
- 47.Julian DG. Why do patients die after myocardial infarction? J Cardiovasc Pharmacol 18, Suppl 2: S80–S82, 1991. doi: 10.1097/00005344-199106182-00017. [DOI] [PubMed] [Google Scholar]
- 48.Kain V, Ingle KA, Kabarowski J, Barnes S, Limdi NA, Prabhu SD, Halade GV. Genetic deletion of 12/15 lipoxygenase promotes effective resolution of inflammation following myocardial infarction. J Mol Cell Cardiol 118: 70–80, 2018. doi: 10.1016/j.yjmcc.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kandalam V, Basu R, Abraham T, Wang X, Awad A, Wang W, Lopaschuk GD, Maeda N, Oudit GY, Kassiri Z. Early activation of matrix metalloproteinases underlies the exacerbated systolic and diastolic dysfunction in mice lacking TIMP3 following myocardial infarction. Am J Physiol Heart Circ Physiol 299: H1012–H1023, 2010. doi: 10.1152/ajpheart.00246.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kempf T, Zarbock A, Widera C, Butz S, Stadtmann A, Rossaint J, Bolomini-Vittori M, Korf-Klingebiel M, Napp LC, Hansen B, Kanwischer A, Bavendiek U, Beutel G, Hapke M, Sauer MG, Laudanna C, Hogg N, Vestweber D, Wollert KC. GDF-15 is an inhibitor of leukocyte integrin activation required for survival after myocardial infarction in mice. Nat Med 17: 581–588, 2011. doi: 10.1038/nm.2354. [DOI] [PubMed] [Google Scholar]
- 51.Khalil H, Kanisicak O, Vagnozzi RJ, Johansen AK, Maliken BD, Prasad V, Boyer JG, Brody MJ, Schips T, Kilian KK, Correll RN, Kawasaki K, Nagata K, Molkentin JD. Cell-specific ablation of Hsp47 defines the collagen-producing cells in the injured heart. JCI Insight 4: e128722, 2019. doi: 10.1172/jci.insight.128722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kong P, Shinde AV, Su Y, Russo I, Chen B, Saxena A, Conway SJ, Graff JM, Frangogiannis NG. Opposing actions of fibroblast and cardiomyocyte Smad3 signaling in the infarcted myocardium. Circulation 137: 707–724, 2018. doi: 10.1161/CIRCULATIONAHA.117.029622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koskivirta I, Kassiri Z, Rahkonen O, Kiviranta R, Oudit GY, McKee TD, Kytö V, Saraste A, Jokinen E, Liu PP, Vuorio E, Khokha R. Mice with tissue inhibitor of metalloproteinases 4 (Timp4) deletion succumb to induced myocardial infarction but not to cardiac pressure overload. J Biol Chem 285: 24487–24493, 2010. doi: 10.1074/jbc.M110.136820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kubota A, Hasegawa H, Tadokoro H, Hirose M, Kobara Y, Yamada-Inagawa T, Takemura G, Kobayashi Y, Takano H. Deletion of CD28 co-stimulatory signals exacerbates left ventricular remodeling and increases cardiac rupture after myocardial infarction. Circ J 80: 1971–1979, 2016. doi: 10.1253/circj.CJ-16-0327. [DOI] [PubMed] [Google Scholar]
- 55.Lanz J, Wyss D, Räber L, Stortecky S, Hunziker L, Blöchlinger S, Reineke D, Englberger L, Zanchin T, Valgimigli M, Heg D, Windecker S, Pilgrim T. Mechanical complications in patients with ST-segment elevation myocardial infarction: a single centre experience. PLoS One 14: e0209502, 2019. doi: 10.1371/journal.pone.0209502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lewis AJ, Burchell HB, Titus JL. Clinical and pathologic features of postinfarction cardiac rupture. Am J Cardiol 23: 43–53, 1969. doi: 10.1016/0002-9149(69)90240-9. [DOI] [PubMed] [Google Scholar]
- 57.Lindsey ML, Bolli R, Canty JM Jr, Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Schwartz Longacre L, Ripplinger CM, Van Eyk JE, Heusch G. Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 314: H812–H838, 2018. doi: 10.1152/ajpheart.00335.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu FY, Fan D, Yang Z, Tang N, Guo Z, Ma SQ, Ma ZG, Wu HM, Deng W, Tang QZ. TLR9 is essential for HMGB1-mediated post-myocardial infarction tissue repair through affecting apoptosis, cardiac healing, and angiogenesis. Cell Death Dis 10: 480, 2019. doi: 10.1038/s41419-019-1718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu S, Du P, Shan C, Wang Y, Ma C, Dong J. Haploinsufficiency of Hand1 improves mice survival after acute myocardial infarction through preventing cardiac rupture. Biochem Biophys Res Commun 478: 1726–1731, 2016. doi: 10.1016/j.bbrc.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 60.Luo M, Guan X, Luczak ED, Lang D, Kutschke W, Gao Z, Yang J, Glynn P, Sossalla S, Swaminathan PD, Weiss RM, Yang B, Rokita AG, Maier LS, Efimov IR, Hund TJ, Anderson ME. Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J Clin Invest 123: 1262–1274, 2013. doi: 10.1172/JCI65268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma Y, Halade GV, Zhang J, Ramirez TA, Levin D, Voorhees A, Jin YF, Han HC, Manicone AM, Lindsey ML. Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circ Res 112: 675–688, 2013. doi: 10.1161/CIRCRESAHA.111.300502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mann JM, Roberts WC. Rupture of the left ventricular free wall during acute myocardial infarction: analysis of 138 necropsy patients and comparison with 50 necropsy patients with acute myocardial infarction without rupture. Am J Cardiol 62: 847–859, 1988. doi: 10.1016/0002-9149(88)90881-8. [DOI] [PubMed] [Google Scholar]
- 63.Maruyama S, Nakamura K, Papanicolaou KN, Sano S, Shimizu I, Asaumi Y, van den Hoff MJ, Ouchi N, Recchia FA, Walsh K. Follistatin-like 1 promotes cardiac fibroblast activation and protects the heart from rupture. EMBO Mol Med 8: 949–966, 2016. doi: 10.15252/emmm.201506151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsui Y, Ikesue M, Danzaki K, Morimoto J, Sato M, Tanaka S, Kojima T, Tsutsui H, Uede T. Syndecan-4 prevents cardiac rupture and dysfunction after myocardial infarction. Circ Res 108: 1328–1339, 2011. doi: 10.1161/CIRCRESAHA.110.235689. [DOI] [PubMed] [Google Scholar]
- 65.Matsumura S, Iwanaga S, Mochizuki S, Okamoto H, Ogawa S, Okada Y. Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. J Clin Invest 115: 599–609, 2005. doi: 10.1172/JCI22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Michael LH, Entman ML, Hartley CJ, Youker KA, Zhu J, Hall SR, Hawkins HK, Berens K, Ballantyne CM. Myocardial ischemia and reperfusion: a murine model. Am J Physiol 269: H2147–H2154, 1995. doi: 10.1152/ajpheart.1995.269.6.H2147. [DOI] [PubMed] [Google Scholar]
- 67.Mishina H, Watanabe K, Tamaru S, Watanabe Y, Fujioka D, Takahashi S, Suzuki K, Nakamura T, Obata JE, Kawabata K, Yokota Y, Inoue O, Murakami M, Hanasaki K, Kugiyama K. Lack of phospholipase A2 receptor increases susceptibility to cardiac rupture after myocardial infarction. Circ Res 114: 493–504, 2014. doi: 10.1161/CIRCRESAHA.114.302319. [DOI] [PubMed] [Google Scholar]
- 68.Nahrendorf M, Hu K, Frantz S, Jaffer FA, Tung CH, Hiller KH, Voll S, Nordbeck P, Sosnovik D, Gattenlöhner S, Novikov M, Dickneite G, Reed GL, Jakob P, Rosenzweig A, Bauer WR, Weissleder R, Ertl G. Factor XIII deficiency causes cardiac rupture, impairs wound healing, and aggravates cardiac remodeling in mice with myocardial infarction. Circulation 113: 1196–1202, 2006. doi: 10.1161/CIRCULATIONAHA.105.602094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW 2nd, Conway SJ, Aronow BJ, Robbins J, Molkentin JD. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res 101: 313–321, 2007. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olsen MB, Hildrestrand GA, Scheffler K, Vinge LE, Alfsnes K, Palibrk V, Wang J, Neurauter CG, Luna L, Johansen J, Øgaard JDS, Ohm IK, Slupphaug G, Kuśnierczyk A, Fiane AE, Brorson SH, Zhang L, Gullestad L, Louch WE, Iversen PO, Østlie I, Klungland A, Christensen G, Sjaastad I, Sætrom P, Yndestad A, Aukrust P, Bjørås M, Finsen AV. NEIL3-dependent regulation of cardiac fibroblast proliferation prevents myocardial rupture. Cell Reports 18: 82–92, 2017. doi: 10.1016/j.celrep.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 71.Omiya S, Omori Y, Taneike M, Protti A, Yamaguchi O, Akira S, Shah AM, Nishida K, Otsu K. Toll-like receptor 9 prevents cardiac rupture after myocardial infarction in mice independently of inflammation. Am J Physiol Heart Circ Physiol 311: H1485–H1497, 2016. doi: 10.1152/ajpheart.00481.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pohjola-Sintonen S, Muller JE, Stone PH, Willich SN, Antman EM, Davis VG, Parker CB, Braunwald E. Ventricular septal and free wall rupture complicating acute myocardial infarction: experience in the multicenter investigation of limitation of infarct size. Am Heart J 117: 809–818, 1989. doi: 10.1016/0002-8703(89)90617-0. [DOI] [PubMed] [Google Scholar]
- 73.Puerto E, Viana-Tejedor A, Martínez-Sellés M, Domínguez-Pérez L, Moreno G, Martín-Asenjo R, Bueno H. Temporal trends in mechanical complications of acute myocardial infarction in the elderly. J Am Coll Cardiol 72: 959–966, 2018. doi: 10.1016/j.jacc.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 74.Pullen AB, Kain V, Serhan CN, Halade GV. Molecular and cellular differences in cardiac repair of male and female mice. J Am Heart Assoc 9: e015672, 2020. doi: 10.1161/JAHA.119.015672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rainer PP, Hao S, Vanhoutte D, Lee DI, Koitabashi N, Molkentin JD, Kass DA. Cardiomyocyte-specific transforming growth factor β suppression blocks neutrophil infiltration, augments multiple cytoprotective cascades, and reduces early mortality after myocardial infarction. Circ Res 114: 1246–1257, 2014. doi: 10.1161/CIRCRESAHA.114.302653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Samnegård A, Hulthe J, Silveira A, Ericsson CG, Hamsten A, Eriksson P. Gender specific associations between matrix metalloproteinases and inflammatory markers in post myocardial infarction patients. Atherosclerosis 202: 550–556, 2009. doi: 10.1016/j.atherosclerosis.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 77.Savvatis K, Pappritz K, Becher PM, Lindner D, Zietsch C, Volk HD, Westermann D, Schultheiss HP, Tschöpe C. Interleukin-23 deficiency leads to impaired wound healing and adverse prognosis after myocardial infarction. Circ Heart Fail 7: 161–171, 2014. doi: 10.1161/CIRCHEARTFAILURE.113.000604. [DOI] [PubMed] [Google Scholar]
- 78.Schellings MW, Vanhoutte D, Swinnen M, Cleutjens JP, Debets J, van Leeuwen RE, d’Hooge J, Van de Werf F, Carmeliet P, Pinto YM, Sage EH, Heymans S. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med 206: 113–123, 2009. doi: 10.1084/jem.20081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, Saito M, Fukuda K, Nishiyama T, Kitajima S, Saga Y, Fukayama M, Sata M, Kudo A. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med 205: 295–303, 2008. doi: 10.1084/jem.20071297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shinde AV, Humeres C, Frangogiannis NG. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim Biophys Acta Mol Basis Dis 1863: 298–309, 2017. doi: 10.1016/j.bbadis.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun M, Dawood F, Wen WH, Chen M, Dixon I, Kirshenbaum LA, Liu PP. Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation 110: 3221–3228, 2004. doi: 10.1161/01.CIR.0000147233.10318.23. [DOI] [PubMed] [Google Scholar]
- 82.Sutton NR, Hayasaki T, Hyman MC, Anyanwu AC, Liao H, Petrovic-Djergovic D, Badri L, Baek AE, Walker N, Fukase K, Kanthi Y, Visovatti SH, Horste EL, Ray JJ, Goonewardena SN, Pinsky DJ. Ectonucleotidase CD39-driven control of postinfarction myocardial repair and rupture. JCI Insight 2: e89504, 2017. doi: 10.1172/jci.insight.89504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tomczyk M, Kraszewska I, Szade K, Bukowska-Strakova K, Meloni M, Jozkowicz A, Dulak J, Jazwa A. Splenic Ly6Chi monocytes contribute to adverse late post-ischemic left ventricular remodeling in heme oxygenase-1 deficient mice. Basic Res Cardiol 112: 39, 2017. doi: 10.1007/s00395-017-0629-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsuda T, Wu J, Gao E, Joyce J, Markova D, Dong H, Liu Y, Zhang H, Zou Y, Gao F, Miller T, Koch W, Ma X, Chu ML. Loss of fibulin-2 protects against progressive ventricular dysfunction after myocardial infarction. J Mol Cell Cardiol 52: 273–282, 2012. doi: 10.1016/j.yjmcc.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsujita K, Kaikita K, Hayasaki T, Honda T, Kobayashi H, Sakashita N, Suzuki H, Kodama T, Ogawa H, Takeya M. Targeted deletion of class A macrophage scavenger receptor increases the risk of cardiac rupture after experimental myocardial infarction. Circulation 115: 1904–1911, 2007. doi: 10.1161/CIRCULATIONAHA.106.671198. [DOI] [PubMed] [Google Scholar]
- 86.Unsöld B, Kaul A, Sbroggiò M, Schubert C, Regitz-Zagrosek V, Brancaccio M, Damilano F, Hirsch E, Van Bilsen M, Munts C, Sipido K, Bito V, Detre E, Wagner NM, Schäfer K, Seidler T, Vogt J, Neef S, Bleckmann A, Maier LS, Balligand JL, Bouzin C, Ventura-Clapier R, Garnier A, Eschenhagen T, El-Armouche A, Knöll R, Tarone G, Hasenfuß G. Melusin protects from cardiac rupture and improves functional remodelling after myocardial infarction. Cardiovasc Res 101: 97–107, 2014. doi: 10.1093/cvr/cvt235. [DOI] [PubMed] [Google Scholar]
- 87.Usui S, Chikata A, Takatori O, Takashima SI, Inoue O, Kato T, Murai H, Furusho H, Nomura A, Zablocki D, Kaneko S, Sadoshima J, Takamura M. Endogenous muscle atrophy F-box is involved in the development of cardiac rupture after myocardial infarction. J Mol Cell Cardiol 126: 1–12, 2019. doi: 10.1016/j.yjmcc.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 88.Van Aelst LN, Voss S, Carai P, Van Leeuwen R, Vanhoutte D, Sanders-van Wijk S, Eurlings L, Swinnen M, Verheyen FK, Verbeken E, Nef H, Troidl C, Cook SA, Brunner-La Rocca HP, Möllmann H, Papageorgiou AP, Heymans S. Osteoglycin prevents cardiac dilatation and dysfunction after myocardial infarction through infarct collagen strengthening. Circ Res 116: 425–436, 2015. doi: 10.1161/CIRCRESAHA.116.304599. [DOI] [PubMed] [Google Scholar]
- 89.van den Borne SW, van de Schans VA, Strzelecka AE, Vervoort-Peters HT, Lijnen PM, Cleutjens JP, Smits JF, Daemen MJ, Janssen BJ, Blankesteijn WM. Mouse strain determines the outcome of wound healing after myocardial infarction. Cardiovasc Res 84: 273–282, 2009. doi: 10.1093/cvr/cvp207. [DOI] [PubMed] [Google Scholar]
- 90.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2020 update: a report from the american heart association. Circulation 141: e139–e596, 2020. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 91.Wan F, Letavernier E, Le Saux CJ, Houssaini A, Abid S, Czibik G, Sawaki D, Marcos E, Dubois-Rande JL, Baud L, Adnot S, Derumeaux G, Gellen B. Calpastatin overexpression impairs postinfarct scar healing in mice by compromising reparative immune cell recruitment and activation. Am J Physiol Heart Circ Physiol 309: H1883–H1893, 2015. doi: 10.1152/ajpheart.00594.2015. [DOI] [PubMed] [Google Scholar]
- 92.Wang C, Zhang C, Liu L, A X, Chen B, Li Y, Du J. Macrophage-derived mir-155-containing exosomes suppress fibroblast proliferation and promote fibroblast inflammation during cardiac injury. Mol Ther 25: 192–204, 2017. doi: 10.1016/j.ymthe.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Y, Liu J, Kong Q, Cheng H, Tu F, Yu P, Liu Y, Zhang X, Li C, Li Y, Min X, Du S, Ding Z, Liu L. Cardiomyocyte-specific deficiency of HSPB1 worsens cardiac dysfunction by activating NFκB-mediated leucocyte recruitment after myocardial infarction. Cardiovasc Res 115: 154–167, 2019. doi: 10.1093/cvr/cvy163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wehrens XH, Doevendans PA. Cardiac rupture complicating myocardial infarction. Int J Cardiol 95: 285–292, 2004. doi: 10.1016/j.ijcard.2003.06.006. [DOI] [PubMed] [Google Scholar]
- 95.Westermann D, Mersmann J, Melchior A, Freudenberger T, Petrik C, Schaefer L, Lüllmann-Rauch R, Lettau O, Jacoby C, Schrader J, Brand-Herrmann SM, Young MF, Schultheiss HP, Levkau B, Baba HA, Unger T, Zacharowski K, Tschöpe C, Fischer JW. Biglycan is required for adaptive remodeling after myocardial infarction. Circulation 117: 1269–1276, 2008. doi: 10.1161/CIRCULATIONAHA.107.714147. [DOI] [PubMed] [Google Scholar]
- 96.White DA, Su Y, Kanellakis P, Kiriazis H, Morand EF, Bucala R, Dart AM, Gao XM, Du XJ. Differential roles of cardiac and leukocyte derived macrophage migration inhibitory factor in inflammatory responses and cardiac remodelling post myocardial infarction. J Mol Cell Cardiol 69: 32–42, 2014. doi: 10.1016/j.yjmcc.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yan X, Zhang H, Fan Q, Hu J, Tao R, Chen Q, Iwakura Y, Shen W, Lu L, Zhang Q, Zhang R. Dectin-2 deficiency modulates Th1 differentiation and improves wound healing after myocardial infarction. Circ Res 120: 1116–1129, 2017. doi: 10.1161/CIRCRESAHA.116.310260. [DOI] [PubMed] [Google Scholar]