Abstract
Case series
Patients: Male, 50-year-old • Male, 37-year-old • Male, 65-year-old • Male, 72-year-old
Final Diagnosis: BK nephropathy
Symptoms: Renal disfunction
Medication: —
Clinical Procedure: —
Specialty: Transplantology
Objective:
Unusual or unexpected effect of treatment
Background:
BK virus nephropathy (BKVN) is the major cause of transplant renal dysfunction. However, a specific antiviral agent to treat it does not exist. One therapeutic option is to reduce use of immunosuppression drugs, which can cause allograft rejection. Leflunomide has both antiviral and immunosuppressive effects, and clinical research has demonstrated its clinical efficacy against BKVN. However, a phase II randomized trial did not support this effect. Therefore, the efficacy of leflunomide remains controversial.
Case Reports:
We examined 4 BKVN patients whose Cr levels stabilized with leflunomide therapy. BKVN was confirmed by a kidney biopsy 7–16 months after transplantation. The Cr levels in 3 cases continued to increase after the reduction of immunosuppression drugs, then leflunomide was administered. In 1 case, leflunomide was administered when the immunosuppression drugs were reduced. In all of the cases, mycophenolate mofetil was replaced with everolimus, and tacrolimus was replaced with cyclosporine A. The maintenance doses of leflunomide were 20 mg/day, and leflunomide was used as an antiviral agent for 3 months. In all of the cases, Cr levels and plasma BKV-PCR loads improved after the administration of leflunomide. Renal function was stable without BKVN recurrence or allograft rejection over 3 years after transplantation.
Conclusions:
Our 4 cases show that short-term use of leflunomide during the active phase of BKVN and a combination of leflunomide and everolimus may be effective against BKVN.
MeSH Keywords: BK Virus, Immunosuppressive Agents, Kidney Transplantation
Background
BK virus nephropathy (BKVN) is a major cause of renal allograft failure. BKVN occurs in 1–15% of renal transplant patients, and the graft survival rate of BKVN patients within 5 years is approximately 50% [1,2]. However, despite the devastating effect on renal allograft function, a specific antiviral agent for BKVN does not exist. One therapeutic option is to reduce use of immunosuppression drugs [3], but this can cause allograft rejection. Scaub et al. reported that 8.6% of BKVN patients experienced subsequent clinical rejection after the reduction of immunosuppression drugs [4]. Therefore, a specific antiviral therapeutic option is strongly desired.
Leflunomide is a pyrimidine synthesis inhibitor and inhibits DNA and RNA synthesis [5]. It suppresses the proliferation of lymphocytes and viruses by this suppression of DNA and RNA synthesis. It has both immunosuppressive and antiviral effects. Reports about the use of leflunomide as a therapeutic option for BKVN have recently increased. Josephson et al. reported that leflunomide with immunosuppression reduction decreased blood and urine BKV viral load levels in 22/26 BKVN patients [6]. Teschner et al. reported that 12/13 BKVN patients who received leflunomide with immunosuppression reduction were cured of BKV and their Cr levels stabilized or improved [7]. However, a phase II randomized trial showed that FK778, the derivative of the active metabolite of leflunomide, decreased plasma BKV load, but did not decrease allograft survival rates, biopsy-confirmed rejection, or Cr levels when compared with the control treatment (only immunosuppression reduction) [8]. Therefore, the efficacy of leflunomide for BKVN is controversial.
Everolimus is a mammalian target of rapamycin (mTOR) inhibitor and, like leflunomide, has immunosuppressive and antiviral effects by suppressing cell proliferation of activated T cells, enhancing immune memory and inhibiting the Akt pathway [9]. In a study of the efficacy of everolimus against BKVN, a randomized controlled trial (RCT) showed its efficacy after liver transplant in patients with BKVN [10]. However, with respect to post-renal transplant patients, although retrospective trials and small prospective studies showed the efficacy of everolimus against BKVN [11,12], there are no sufficient RCTs and insufficient evidence at present. Therefore, like leflunomide, everolimus also has potential as a treatment for BKVN, although the evidence is not as strong as it should be.
Here, we examined 4 BKVN patients whose Cr level did not improve with only immunosuppression reduction but was stabilized by leflunomide therapy with everolimus.
Case Reports
Case 1
The patient was a 50-year-old man who began dialysis because of chronic glomerular nephritis when he was 25 years old. He received a kidney transplant at age 46 years from a 53-year-old male donor who died of a cerebral hemorrhage. The immunological data of the patient and donor showed A(+) blood types, 3 mismatches in histocompatibility locus antigen class I (Donor: A11, A31, B39, B51; Recipient: A26, A31, B62, B54), and 0 mismatches in class II (Donor: DR8, DR8; Recipient: DR8, DR14). The initial immunosuppression therapy included administration of tacrolimus (TAC), mycophenolate mofetil (MMF), methylprednisolone (MP), and basiliximab (BXM). The patient did not experience delayed graft function.
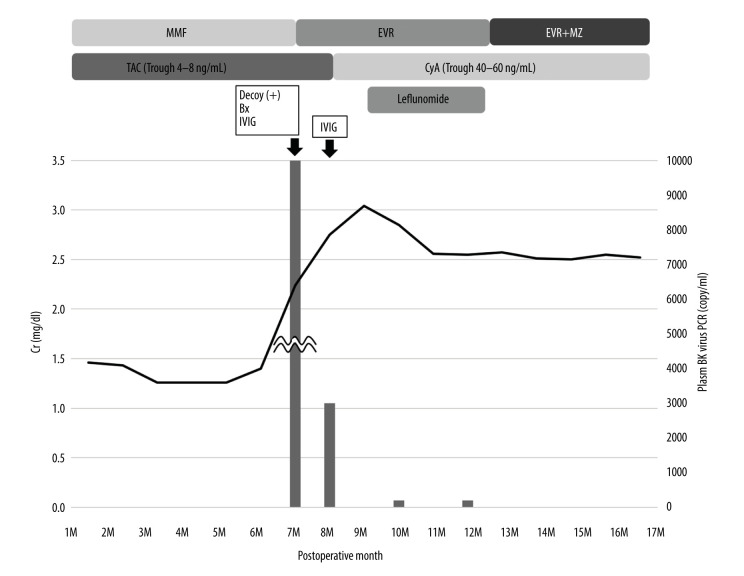
The clinical course after kidney transplantation is shown in Figure 1. His Cr level was 1.3–1.4 mg/dl with TAC and MMF at 6 months after surgery. However, at 7 months after surgery, his Cr level increased to 2.06 mg/dl. Decoy cells were observed in his urine and the plasma BKV-PCR load was 1×107 copy/ml. A renal biopsy diagnosed BKVN stage B. MMF was replaced with EVR, and intravenous immunoglobulin (IVIG) was administered. At months after surgery, although BK viremia decreased to 3×103 copy/ml, his Cr level increased to 2.75 mg/dl. Therefore, TAC was replaced with cyclosporine A (CyA) and IVIG was administered again. However, his Cr level continued to increase to 3.05 mg/dl, and we began the administration of leflunomide at 9 months after surgery. We administered leflunomide 40 mg/day for the first 5 days and continued with 20 mg/day for 3 months. The maintenance dose of leflunomide was in accordance with the Japanese dosage of 20 mg/day for rheumatoid arthritis. Use of a loading dose was dependent on the case and was approximately 0.8 mg/kg/day. After the administration of leflunomide, his Cr level improved to 2.56 mg/dl and his plasma and urine BKV-PCR became negative (<1×104 copy/ml). After this episode, his renal function was stable with no BKVN recurrence or allograft rejection and his Cr level improved to 2.08 mg/dl at 50 months after surgery.
Figure 1.
Clinical course of case 1. Bx – biopsy; CyA – cyclosporin A; EVR – everolimus; IVIG –intravenous immunoglobulin; MMF – mycophenolate mofetil; MZ – mizoribine; TAC – tacrolimus.
Case 2
This patient was a 37-year-old man who developed end-stage renal disease for unknown reasons at age 33 years, and was treated at our department for preemptive kidney transplantation. He received a kidney transplant from his wife, a 28-year-old woman. The immunological data showed blood type from O(+) to AB(+), 4 mismatches in histocompatibility locus antigen class I (Donor: A24, A24, B35, B54; Recipient: A11, A26, B48, B62), and 1 mismatch in class II (Donor: DR9, DR4; Recipient: DR14, DR4). The initial immunosuppression therapy included the administration of TAC, MMF, MP, and BXM. He did not experience delayed graft function.
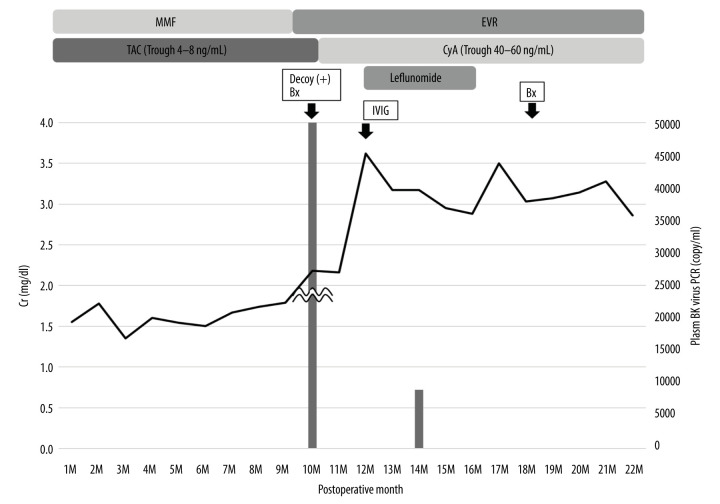
The clinical course after kidney transplantation is shown in Figure 2. His Cr level was 1.3–1.5 mg/dl with TAC and MMF at 8M PO. However, at 9 months after surgery, his Cr level increased to 1.84 mg/dl. Decoy cells were observed in his urine, and the plasma BKV-PCR load was 2×103 copy/ml. A renal biopsy diagnosed BKVN stage B. MMF was replaced with EVR+Mizoribine (MZ), IVIG was administered, and the administration of leflunomide began. We administered leflunomide 60 mg/day for the first 5 days and continued with 20 mg/day for 3 months. Although his Cr level continued to elevate to 2.78 mg/dl at 12 months after surgery, it began to decrease 12 months after surgery, and his plasma BKV-PCR was under 2×102 copy/ml. After the BKVN was cured, his renal function was stable with no BKVN recurrence or allograft rejection and his Cr level improved to 2.13 mg/dl at 36 months after surgery.
Figure 2.
Clinical course of case 2. Bx – biopsy; CyA – cyclosporin A; EVR – everolimus; IVIG –intravenous immunoglobulin; MMF – mycophenolate mofetil; MZ – mizoribine; TAC – tacrolimus.
Case 3
This patient was a 65-year-old man who began dialysis because of diabetic kidney disease at age 61 years. He received a kidney transplant from his wife at age 62 years. The donor was a 58-year-old woman. The immunological data showed blood type from O(+) to A(+), 4 mismatches in histocompatibility locus antigen class I (Donor: A2, A24, B44, B52; Recipient: A11, A33, B13, B48), and 2 mismatches in class II (Donor: DR13, DR15; Recipient: DR8, DR14). The initial immunosuppression therapy included the administration of TAC, MMF, MP, and BXM. Although he received dialysis therapy 2 times after the kidney transplant due to delayed graft function, his urine output became adequate 1 week after the transplant.
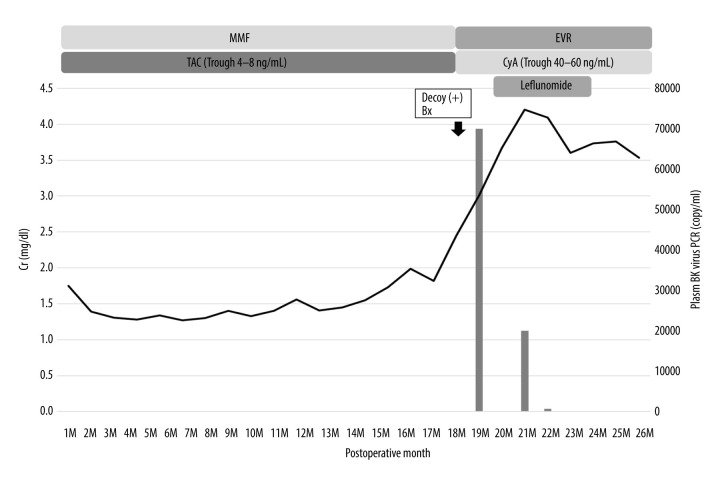
The clinical course after kidney transplantation is shown in Figure 3. His Cr level was 1.3–1.5 mg/dl with TAC and MMF at 15 months after surgery. However, at 16 months after surgery, his Cr level began to increase gradually and became 2.45 mg/dl at 18 months after surgery. Decoy cells were observed in his urine, and a renal biopsy diagnosed BKVN stage B. TAC+MMF replaced CyA+EVR; however, his Cr level continued to rise and the plasma BKV-PCR load was 2×105 copy/ml at 19 months after surgery. Therefore, we began the administration of leflunomide 20 mg/day for 3 months. Although his Cr level continued to increase to 4.20 mg/dl at 21 months after surgery, it began to decrease and his plasma BKV-PCR became negative at 22 months after surgery. After the BKVN was cured, his renal function was stable with no BKVN recurrence or allograft rejection, and his Cr level improved to 2.94 mg/dl at 36 months after surgery.
Figure 3.
Clinical course of case 3. Bx – biopsy; CyA – cyclosporin A; EVR – everolimus; IVIG –intravenous immunoglobulin; MMF – mycophenolate mofetil; MZ – mizoribine; TAC – tacrolimus.
Case 4
This patient was a 72-year-old man who developed end-stage kidney disease because of nephrosclerosis, and was treated at our department for preemptive kidney transplantation at age 69 years. He received a kidney transplant from his wife at age 69 years. The donor was a 68-year-old woman. The immunological data showed blood type from A(+) to O(+), 2 mismatches in histocompatibility locus antigen class I (Donor: A11, A24, B61, B48; Recipient: A2, A24, B61, B51), and 1 mismatch in class II (Donor: DR9, DR15; Recipient: DR13, DR15). His anti-A antibody IgG was 64 times, and his IgM was 128 times. A plasma exchange was performed 4 times, and rituximab was administered as a desensitization therapy before the kidney transplant. The initial immunosuppression therapy included the administration of TAC, MMF, MP, and BXM. He did not experience delayed graft function.
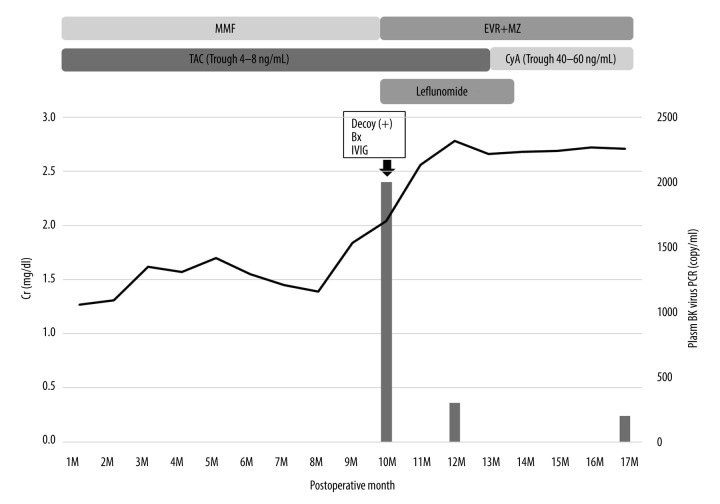
The clinical course after kidney transplantation is shown in Figure 4. His Cr level was 1.3–1.6 mg/dl with TAC and MMF at 7 months after surgery. However, at 8 months after surgery, his Cr level began to gradually increase and reached 2.18 mg/dl at 10 months after surgery. Decoy cells were observed in his urine and the plasma BKV-PCR load was 3×106 copy/ml at 10 months after surgery. A renal biopsy diagnosed BKVN stage B. MMF was replaced with EVR, and although TAC was replaced with CyA at 11M, his Cr level continued to rise and reached 3.62 mg/dl. Therefore, we began IVIG and leflunomide administration at 12 months after surgery. We administered leflunomide 20 mg/day for 3 months. After we began the administration of leflunomide, his Cr level improved and became stable.
Figure 4.
Clinical course of case 4. Bx – biopsy; CyA – cyclosporin A; EVR – everolimus; IVIG –intravenous immunoglobulin; MMF – mycophenolate mofetil; MZ – mizoribine; TAC – tacrolimus.
His plasma BKV-PCR also became negative at 14 months after surgery, and a kidney biopsy confirmed that the BKVN was cured at 18 months after surgery (no peritubular capillaritis, no tubulitis, and SV-40 negative). After the BKVN treatment, his renal function was stable with no BKVN recurrence or allograft rejection, and his Cr level improved to 2.69 mg/dl at 40 months after surgery.
Discussion
In our 4 cases, the administration of leflunomide attenuated the increase of Cr in BKVN. In case 1, although the plasma BKV-PCR load decreased before the administration of leflunomide, the Cr level continued to increase for 3 months after the change of immunosuppression drugs and IVIG and started to decrease after the initiation of leflunomide. In case 2, leflunomide, IVIG and the change of immunosuppression drugs began simultaneously, and the Cr and BKV-PCR load improved after these treatments. Therefore, it is impossible to determine which treatment was effective. In cases 3 and 4, the Cr level continued to increase for 2 months after the change of immunosuppression drugs, and the Cr level and plasma BKVPCR load began to decrease after the initiation of leflunomide. These results indicate that the administration of leflunomide has a therapeutic effect on BKVN.
A771726, the active metabolite of leflunomide, inhibits mitochondrial dihydroorotate dehydrogenase and pyrimidine synthesis, leading to inhibition of DNA and RNA synthesis [5]. Leflunomide inhibits cell proliferation by cell cycle arrest at G1 phase in the activated lymphocytes [13], has immunosuppressive effects, and is now approved for the treatment of rheumatoid arthritis. In renal tubular epithelial cells, leflunomide was reported to reduce BK virus replication by its DNA synthesis inhibitory effects [14]. Therefore, leflunomide has immunosuppressive and antiviral effects by inhibiting DNA and RNA synthesis, making it possible to treat BK virus without increasing the risk of rejection.
Several papers have reported that leflunomide is effective against BKVN. Williams et al. and Josephson et al. reported that the administration of leflunomide 100 mg/day for the first 5 days and then 20–60 mg/day to maintain a concentration of A771726 at 40 μg/ml or more was effective [6,15]. On the other hand, Leca et al. reported that leflunomide remained effective regardless of the concentration of A771726 and that higher levels of A771726 (>40 μg/ml) were associated with hemolysis and thrombotic microangiopathy [16]. Leca et al. administered leflunomide 60 mg/day for 3 days and the medium maintenance dose in the low-concentration group was 33.3 mg/day. In our 4 cases, the maintenance dose of leflunomide was 20 mg/day, which was lower than that reported by Leca et al. Furthermore, in all previous reports, leflunomide replaced MMF as an immunosuppression drug, but in our 4 cases, leflunomide was used as an antiviral drug in addition to a change in immunosuppression drugs for only 3 months, which is the active phase of BKVN. The novelty of our cases was that the short-term use of low-dose leflunomide as an antiviral drug was effective against BKVN.
In a phase II randomized controlled trial, FK-778, the derivative of the active metabolite of leflunomide, was reported to not be effective against BKVN [8]. Moreover, a recent systematic review also reported that the use of leflunomide is ineffective against BKVN [17]. In previous reports, the protocol for a reduction of immunosuppressive drugs was mainly replacing MMF with leflunomide, reducing the dose of TAC, and continuing the MP dose. In a randomized trial of FK-778, the reduction of immunosuppressive drugs used the same protocol. In our 4 cases, the immunosuppressive drugs were changed from MMF to EVR and then from TAC to CyA, and leflunomide was used for 3 months because the Cr levels remained elevated despite these drug changes. Similar to leflunomide, EVR is expected to have an antiviral and immunosuppressive effect. In vitro experiments have shown that the combination of leflunomide and sirolimus, an mTOR inhibitor like EVR, effectively reduced BK viral proliferation [18]. Previous case series have also reported that the administration of leflunomide with EVR was effective in treating BKVN [19]. Therefore, the combination of leflunomide and EVR may enhance the antiviral effect of leflunomide against BKVN.
Since the present report is only a case series and not a case-control study, there are many biases in our assessment of the therapeutic efficacy of leflunomide. Also, in our cases, various treatments other than leflunomide, such as EVR and IVIG, were used in addition to the reduction of immunosuppressive drugs. Therefore, it cannot be ruled out that the improvement of Cr that was observed after the administration of leflunomide was a result of these other therapies.
Conclusions
In our 4 cases, the combination therapy of leflunomide and everolimus during the active phase of BKVN was assessed. Leflunomide has an immunosuppressive effect as well as an antiviral effect and can therefore be used as an antiviral agent while reducing the possibility of transplant rejection.
Acknowledgments
We thank Yukiko Kanetsuna, who is the pathologist of Atami Hospital, International University of Health and Welfare, for helping with the diagnosis of BK virus nephropathy from biopsy samples.
Footnotes
Conflict of interests
None.
References:
- 1.Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyoma-virus type BK Replication and Nephropathy in Renal-Transplant Recipients. N Engl J Med. 2002;347(7):488–96. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 2.Ramos E, Drachenberg CB, Papadimitriou JC, et al. Clinical course of polyoma virus nephropathy in 67 renal transplant patients. Am Soc Nephrol. 2002;13(8):2145–51. doi: 10.1097/01.asn.0000023435.07320.81. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch HH, Randhawa P, AST Infectious Diseases Community of Practice BK Polyomavirus in solid organ transplantation. Am J Transplant. 2013;13(Suppl. 4):179–88. doi: 10.1111/ajt.12110. [DOI] [PubMed] [Google Scholar]
- 4.Schaub S, Hirsch HH, Dickenmann M, et al. Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am J Transplant. 2010;10(12):2615–23. doi: 10.1111/j.1600-6143.2010.03310.x. [DOI] [PubMed] [Google Scholar]
- 5.Pinto P, Dougados M. Leflunomide in clinical practice. Acta Reumatol Port. 2006;31(3):215–24. [PubMed] [Google Scholar]
- 6.Josephson MA, Billen D, Javaid B, et al. Treatment of renal allograft polyoma BK virus infection with leflunomide. Transplantation. 2006;81:704–10. doi: 10.1097/01.tp.0000181149.76113.50. [DOI] [PubMed] [Google Scholar]
- 7.Teschner S, Gerke P, Geyer M, et al. Leflunomide therapy for polyomavirus-induced allograft nephropathy: Efficient BK virus elimination without increased risk of rejection. Transplant Proc. 2009;41(6):2533–38. doi: 10.1016/j.transproceed.2009.06.099. [DOI] [PubMed] [Google Scholar]
- 8.Guasch A, Roy-Chaudhury P, Woodle E, et al. Assessment of efficacy and safety of FK778 in comparison with standard care in renal transplant recipients with untreated BK nephropathy. Transplantation. 2010;90(8):891–97. doi: 10.1097/TP.0b013e3181f2c94b. [DOI] [PubMed] [Google Scholar]
- 9.Bowman LJ, Brueckner AJ, Doligalski CT. The role of mTOR inhibitors in the management of viral infections: A review of current literature. Transplantation. 2018;102(2S Suppl. 1):S50–59. doi: 10.1097/TP.0000000000001777. [DOI] [PubMed] [Google Scholar]
- 10.Villamil FG, Gadano AC, Zingale F, et al. Fibrosis progression in maintenance liver transplant patients with hepatitis C recurrence: A randomised study of everolimus vs. calcineurin inhibitors. Liver Int. 2014;34:1513–21. doi: 10.1111/liv.12416. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz A, Linnenweber-Held S, Heim A, et al. Factors influencing viral clearing and renal function during polyomavirus BK-associated nephropathy after renal transplantation. Transplantation. 2012;94:396–402. doi: 10.1097/TP.0b013e31825a505d. [DOI] [PubMed] [Google Scholar]
- 12.Polanco N, González Monte E, Folgueira MD, et al. Everolimus-based immunosuppression therapy for BK virus nephropathy. Transplant Proc. 2015;47:57–61. doi: 10.1016/j.transproceed.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Cherwinski HM, McCarley D, Schatzman R, et al. The immunosuppressant leflunomide inhibits lymphocyte progression through cell cycle by a novel mechanism. J Pharmacol Exp Ther. 1995;272(1):460–68. [PubMed] [Google Scholar]
- 14.Bernhoff E, Tylden GD, Kjerpeseth LJ, et al. Leflunomide inhibition of BK virus replication in renal tubular epithelial cells. J Virol. 2010;84(4):2150–56. doi: 10.1128/JVI.01737-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams JW, Javaid B, Kadambi PV, et al. Leflunomide for polyomavirus type BK nephropathy. N Engl J Med. 2005;352(11):1157–58. doi: 10.1056/NEJM200503173521125. [DOI] [PubMed] [Google Scholar]
- 16.Leca N, Muczynski KA, Jefferson JA, et al. Higher levels of leflunomide are associated with hemolysis and are not superior to lower levels for BK virus clearance in renal transplant patients. Clin J Am Soc Nephrol. 2008;3(3):829–35. doi: 10.2215/CJN.03930907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneidewind L, Neumann T, Dräger DL, et al. Leflunomide in the treatment of BK polyomavirus associated nephropathy in kidney transplanted patients – A systematic review. Transplant Rev (Orlando) 2020 doi: 10.1016/j.trre.2020.100565. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Liacini A, Seamone ME, Muruve DA, Tibbles LA. Anti-BK virus mechanisms of Sirolimus and Leflunomide alone and in combination: Toward a new therapy for BK virus infection. Transplantation. 2010;90(12):1450–57. doi: 10.1097/TP.0b013e3182007be2. [DOI] [PubMed] [Google Scholar]
- 19.Jaw J, Hill P, Goodman D. Combination of leflunomide and everolimus for treatment of BK virus nephropathy. Nephrology (Carlton) 2017;22(4):326–29. doi: 10.1111/nep.12948. [DOI] [PubMed] [Google Scholar]