Abstract
Liver steatosis is associated with increased ischaemia reperfusion (I/R) injury. Our previous studies have shown that irisin, an exercise‐induced hormone, mitigates I/R injury via binding to αVβ5 integrin. However, the effect of irisin on I/R injury in steatotic liver remains unknown. Kindlin‐2 directly interacts with β integrin. We therefore suggest that irisin protects against I/R injury in steatotic liver via a kindlin‐2 dependent mechanism. To study this, hepatic steatosis was induced in male adult mice by feeding them with a 60% high‐fat diet (HFD). At 12 weeks after HFD feeding, the mice were subjected to liver ischaemia by occluding partial (70%) hepatic arterial/portal venous blood for 60 minutes, which was followed by 24 hours reperfusion. Our results showed HFD exaggerated I/R‐induced liver injury. Irisin (250 μg/kg) administration at the beginning of reperfusion attenuated liver injury, improved mitochondrial function, and reduced oxidative and endoplasmic reticulum stress in HFD‐fed mice. However, kindlin‐2 inhibition by RNAi eliminated irisin's direct effects on cultured hepatocytes. In conclusion, irisin attenuates I/R injury in steatotic liver via a kindlin‐2 dependent mechanism.
Keywords: hepatic I/R, high‐fat diet, irisin, kindlin‐2, steatotic liver
1. INTRODUCTION
The incidence of non‐alcoholic fatty liver disease (NAFLD) is increasing rapidly worldwide. 1 , 2 , 3 In China, the national prevalence of NAFLD is estimated at 29.2% and the burden of NAFLD is expected to increase dramatically. 4 Hepatic ischaemia reperfusion (I/R) injury is an inevitable complication associated with liver transplantation, partial hepatectomy and hypovolemic shock. 5 Steatotic liver appears to be more sensitive to I/R injury. 6 Fat‐laden hepatocytes are damaged by chronic oxidative/nitrosative stress, which is further increased during I/R, leading to extensive parenchymal damage. 7
Irisin, an exercise‐induced and muscle‐secreted myokine, was first discovered for driving browning of white fat and thermogenesis in 2012. 8 As then, many studies have revealed that irisin is also a potent antioxidant. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 Our previous studies have also shown irisin improves mitochondrial function and decreases oxidative stress via binding to αVβ5 integrin in I/R injury. 17 , 18 However, the effect of irisin on I/R injury in steatotic liver remains unknown.
Kindlin‐2 is a focal adhesion protein that regulates integrin signalling and cell‐matrix adhesion. 19 It directly interacts with the cytoplasmic tail of β integrin, 20 which is recognized as a part of the irisin receptor. 21 A recent study has shown kindlin‐2 expression is up‐regulated in human and mouse fibrotic livers and depletion of kindlin‐2 reduces CCL4‐induced liver injury in mice. However, the role of kindlin‐2 in irisin's biological function is currently unclear.
We therefore suggested that irisin attenuates hepatic I/R injury via a kindlin‐2 dependent mechanism in steatotic liver. The aim of the present study was to explore the effects and likely mechanisms of irisin on hepatic I/R injury in high‐fat diet (HFD)‐fed mice.
2. MATERIALS AND METHODS
2.1. Experimental animals and diets
Male wild‐type C57BL/6J mice (18 ± 3 g; 6‐8 weeks) were purchased from the Experimental Animal Center of Xi'an Jiaotong University and bred in a pathogen‐free environment under 12‐hour light‐dark cycle at a temperature of 23‐25°C. Standard chaw (Control diet; CD) or 60% high‐fat diet (D12492, Research Diets Inc) were provided for 12 weeks. All mice were treated according to the guidelines of the China Council on Animal Care and Use. This project was approved by the Institutional Animal Care and Use Committee of the Ethics Committee of Xi'an Jiaotong University Health Science Center, China.
2.2. Mouse model of hepatic I/R and experimental design
After 12 weeks on a control diet or high‐fat diet, the mouse hepatic I/R model was established as we described before. 17 Briefly, mice were anaesthetized with isoflurane inhalation and maintained at a concentration of 1.5%‐2%. Liver ischaemia was induced by occluding partial (70%) hepatic arterial/portal venous blood for 60 minutes by a microvascular clip. Then, clip was removed and reperfusion began. Sham operation underwent all the procedures except hepatic ischaemia. There were five groups involved in present study: (a) CD‐Sham: CD‐fed mice underwent sham operation, and 0.5 mL saline was administrated intraperitoneally; (b) CD‐I/R: CD‐fed mice underwent hepatic I/R, and 0.5 mL saline was administrated intraperitoneally immediately after the initiation of reperfusion; (c) HFD‐Sham: also showed as sham group, HFD‐fed mice underwent sham operation and 0.5 mL saline was administrated intraperitoneally; (d) HFD‐I/R: also showed as vehicle group, HFD‐fed mice underwent hepatic I/R, and 0.5 mL saline was administrated intraperitoneally immediately after the initiation of reperfusion; (e) HFD‐irisin: also showed as irisin group, HFD‐fed mice underwent hepatic I/R, and irisin (250 μg/kg, 0.5 mL; 067‐29A, Phoenix Pharmaceuticals, Inc) 17 was administrated intraperitoneally immediately after the initiation of reperfusion.
2.3. Haematoxylin and eosin (H&E) staining and oil red O staining
The liver sections fixed in 4% paraformaldehyde were embedded in paraffin. Then cut the paraffin blocks into 5 mm‐slices and stained with haematoxylin and Eosin. Liver injury score was evaluated as we described before. 17 The frozen liver sections were stained with Oil Red O to evaluate hepatic fat content.
2.4. Measurement of serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and glutathione peroxidase activity (GSH‐Px)
The alanine aminotransferase (ALT) assay Kit (C009‐2, NanJing JianCheng Bioengineering Institute, Nanjing, China), aspartate aminotransferase (AST) assay Kit (C010‐2, NanJing JianCheng Bioengineering Institute, Nanjing, China) and glutathione peroxidase activity (GSH‐Px) assay Kit (A005, NanJing JianCheng Bioengineering Institute, Nanjing, China) were used for measuring the level of serum ALT, AST and liver GSH‐Px according to the instructions of the manufacturer.
2.5. Western blot analysis
The protein extraction and Western blot analysis were performed as previous described. 22 The antibody information was as following: kindlin‐2 antibody (13562s, Cell Signaling Technology), Bax antibody (14796, Cell Signaling Technology), Bcl‐2 antibody (ab194583, Abcam), GRP78 antibody (GRP78, 3183, Cell Signaling Technology), CHOP antibody (2895, Cell Signaling Technology), PDI antibody (3501, Cell Signaling Technology), Ero1‐Lα antibody (ab81959, Abcam), Drp‐1 antibody (ab184247, Abcam), Tfam antibody (ab131607, Abcam), ND3 (ab192306, Abcam), Mfn‐2 (9482, Cell Signaling Technology), Fis‐1 (ab71498, Abcam), ATPB (ab170947, Abcam), β actin Antibody (HRP‐60008, Proteintech), Goat anti‐rabbit IgG antibody (SA00001‐2, Proteintech) and Goat anti‐mouse IgG antibody (SA00001‐1, Proteintech). The Image J software was used for quantitative analysing, and relative protein levels were expressed as the intensity ratio of target protein and β actin.
2.6. RNA extraction, reverse transcription and quantitative PCR (q‐PCR)
RNA was extracted from liver samples using TRIzol Reagent (9108, TAKARA BIO INC). One thousand nanograms of RNA was reverse transcribed to cDNA using PrimeScript™ RT Master Mix (RR036A, TAKARA BIO INC) and amplified by q‐PCR using SYBR green PCR Master Mix (RR820A, TAKARA BIO INC). The following primer sets (TAKARA BIO INC) were used: mouse IL‐1β (forward 5′ TCC AGG ATG AGG ACA TGA GCA C 3′; reverse, 5′ GAA CGT CAC ACA CCA GCA GGT TA 3′), mouse IL‐6 (forward 5′ CCA CTT CAC AAG TCG GAG GCT TA 3′; reverse, 5′ TGC AAG TGC ATC ATC GTT GTT C 3′), mouse MCP‐1 (forward 5′ AGC AGC AGG TGT CCC AAA GA 3′; reverse, 5′ GTG CTG AAG ACC TTA GGG CAG A 3′), mouse CXCL‐1 (forward 5′ TGC ACC CAA ACC GAA GTC 3′; reverse, 5′ GTC AGA AGC CAG CGT TCA CC 3′), mouse Fis‐1 (forward 5′ TGG GCA ACT ACC GGC TCA A 3′; reverse, 5′ TTA TCA ATC AGG CGT TCC AGC TC 3′), mouse PGC‐1α (forward 5′ AAC AGG AAC AGC AGC AGA GAC A 3′; reverse, 5′ GAG GAG TTG TGG GAG GAG TTA GG 3′) and mouse β actin (endogenous control; forward 5′ GTG ACG TTG ACA TCC GTA AAG A 3′; reverse, 5′ GTA ACA GTC CGC CTA GAA GCA C 3′).
2.7. Immunohistochemistry and immunofluorescence staining
Immunohistochemistry and immunofluorescence staining were performed as previous described. 17 The MPO antibody (Santa Cruz Biotechnology, Inc) was used for detection of liver MPO expression by immunohistochemistry staining. Liver Terminal Deoxynucleotidyl Transferase‐Mediated dUTP Nick End Labelling Assay (TUNEL, Roche), CD11b antibody (GB11058, Servicebio) and Dihydroethidium (DHE) dye (D7008, Sigma‐aldrich) were used for detection of liver apoptosis cells, CD11b positive inflammatory cells and reactive oxygen species (ROS) levels via immunofluorescence staining. Image J software was used to quantitative analysis.
2.8. Cell culture, lipotoxic induction and hypoxia/reoxygenation (H/R)
HL‐7702 cells (Normal human hepatocytes) were cultured in RPMI‐1640 medium with 10% foetal bovine serum (FBS) and 100 units/ml penicillin/ streptomycin mixture (Gibco) at 37°C with 100% humidity and 5% CO2 in vitro. HL‐7702 cells were treated with palmitic acid (PA, 0.2 mmol/L) and oleic acid (OA, 0.1 mmol/L) to induce lipotoxicity injury of hepatocytes. Cells were deprived of oxygen (94% N2, 5% CO2, 1% O2) in a serum‐free and deoxyglucose‐rich (5 mmol/L) medium to simulate hepatic ischaemia and hypoxia of mice for 1 hour; then, the cells were changed to 5% CO2 condition with RPMI‐1640 medium containing 10% FBS for 6 hours.
2.9. Transfection of small interfering RNA (siRNA)
The siRNA was constructed by GenePharma Corporation and transfected according to the experimental instructions. The siRNA‐kindlin‐2:5′‐GCU UCC CAA CAU GAA GUA UTT‐3′ and 5′‐AUA CUU CAU GUU GGG AAG CTT‐3′ (GenePharma) was used to deplete the expression of kindlin‐2 cells and a siRNA‐negative control: 5′‐UUC UCC GAA CGU GUC ACG UTT‐3′ and 5′‐ACG UGA CAC GUU CGG AGA ATT‐3′ (GenePharma) was used as negative control in HL‐7702 cells.
2.10. Statistical analysis
The data were expressed as mean ± standard error (SE). The t test or one‐way ANOVA was used to analyse the differences between groups. SPSS version 18.0 (IBM) was used for statistical analysis, and P value < .05 was considered statistically significant.
3. RESULTS
3.1. HFD exaggerates I/R‐induced liver injury
To study the effects of HFD on hepatic I/R injury, mice were fed either a control diet (CD) or a high‐fat diet (HFD) for 12 weeks. As showed in Figure S1A, HFD‐fed mice were heavier than CD‐fed mice (P < .05). HFD‐fed mice were associated with more prominent liver histological damage and more fat content (Figures S1B‐D, P < .05). Then, HFD‐fed and CD‐fed mice underwent hepatic I/R (CD‐I/R or HFD‐I/R) or sham operation (CD‐Sham or HFD‐Sham). Figure S2A showed that hepatic I/R increased serum irisin level in HFD‐fed mice (P < .05). As showed in Figure 1A,B, HFD induced significant liver injury characterized by hepatic steatosis (P < .05). After I/R, HFD‐fed mice showed more severe liver injury and larger necrosis area than CD‐fed mice (Figure 1A‐C, P < .05). The changes of serum AST and ALT were consistent with histological lesions (Figure 1D,E, P < .05). In addition, HFD exaggerated I/R‐induced ROS production (Figure 1F,G, P < .05).
Figure 1.
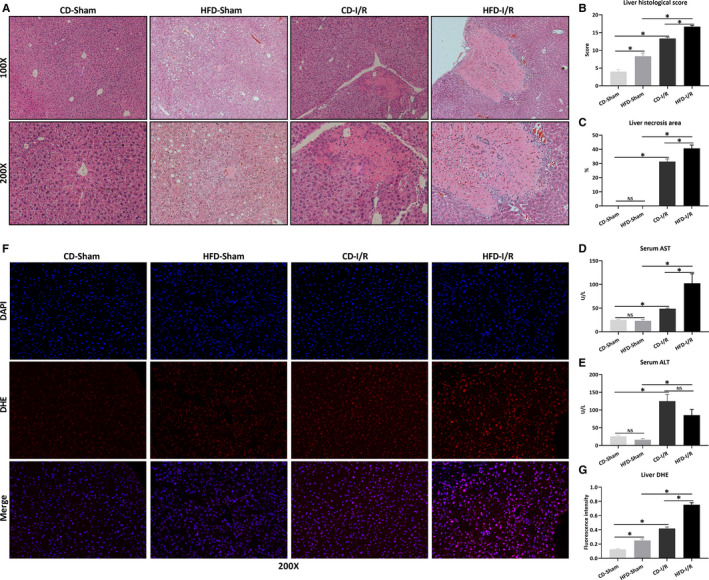
HFD exaggerates I/R‐induced liver injury. Hepatic ischaemia was induced by occluding partial (70%) hepatic arterial/portal venous blood for 60 min, followed by 24 h of reperfusion. Sham mice underwent all the procedures except hepatic ischaemia. After 12 wk of control diet (CD) or high‐fat diet (HFD), mice underwent sham operation (CD‐Sham, HFD‐Sham) or hepatic I/R (CD‐I/R, HFD‐I/R). Liver H&E staining (A), histological score (B) and necrosis area (C). Original magnification, 100× and 200×. The levels of serum AST (D) and ALT (E). F, Liver DHE staining (red) and counterstained with DAPI (blue). Original magnification, 200×. G, The quantitative analysis of liver DHE fluorescence intensity. Results are expressed as mean ± SE (n = 4‐5/group) and compared by t test or one‐way ANOVA. *P < .05, NS P > .05
3.2. Irisin attenuates hepatic I/R injury in HFD‐fed mice
Next, the effect of irisin on hepatic I/R injury in HFD‐fed mice was explored. Figure 2A,B demonstrated that irisin administration reduced I/R‐induced hepatic necrosis in HFD‐fed mice (P < .05). Similarly, serum ALT levels were also significantly decreased by irisin treatment (118.6 ± 17.0 U/L vs 76.1 ± 6.2 U/L, Figure 2C, P < .05). Figure 2D,E indicated that TUNEL positive cells increased markedly at 24 hours after I/R in HFD‐fed mice. Irisin treatment significantly decreased liver TUNEL positive cells after I/R in HFD‐fed mice (P < .05). Consistently, irisin treatment reduced the expression of cleaved‐caspase 3 at the protein level (Figure 2H,I) and decreased the expression of Bax in mRNA (Figure 2F) and protein levels (Figure 2H,J), while increased the expression of Bcl‐2 in mRNA (Figure 2G) and protein levels (Figure 2H,K) after hepatic I/R in HFD‐fed mice (all P < .05).
Figure 2.
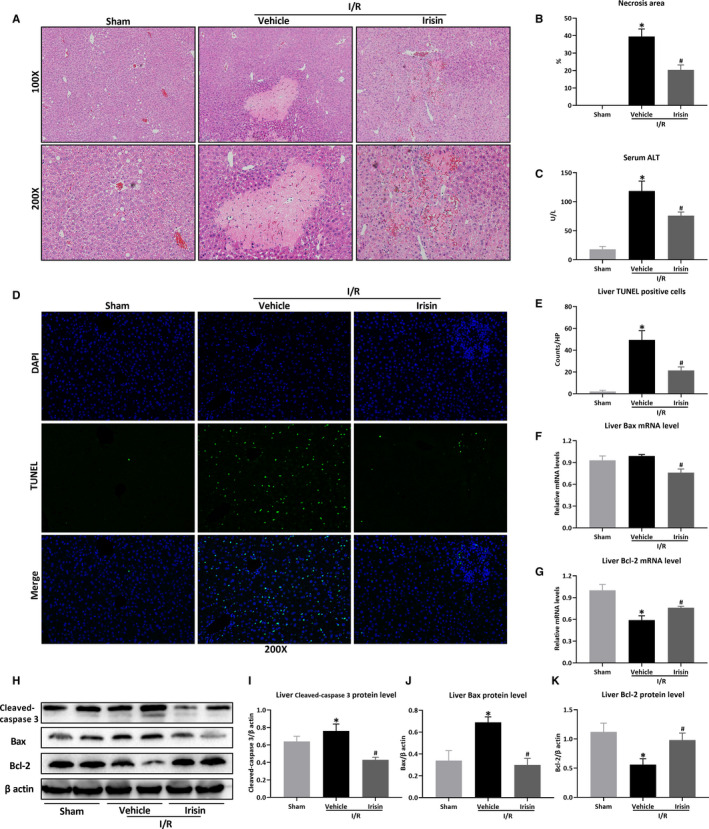
Irisin attenuates hepatic I/R injury in HFD‐fed mice. Hepatic ischaemia was induced by occluding partial (70%) hepatic arterial/portal venous blood for 60 min, followed by 24 h of reperfusion. Sham mice underwent all the procedures except hepatic ischaemia. After 12 wk of high‐fat diet (HFD), HFD‐fed mice underwent sham operation (Sham) or hepatic I/R treated with 0.5 mL saline (Vehicle) or irisin (250 μg/kg, 0.5 mL). Liver H&E staining (A) and necrosis area (B). Original magnification, 100× and 200×. C, The level of serum ALT. D, Liver TUNEL staining (green) and counterstained with DAPI (blue). Original magnification, 200×. E, The quantitative analysis of liver TUNEL positive cells. Liver relative mRNA levels of Bax (F) and Bcl‐2 (G). Western blot analysis of Bax and Bcl‐2 (H) and the quantitative analysis of Bax (I) and Bcl‐2 (J). Results are expressed as mean ± SE (n = 4‐5/group) and compared by t test or one‐way ANOVA. *P < .05 vs sham group, # P < .05 vs vehicle group
3.3. Irisin alleviates oxidative stress after hepatic I/R in HFD‐fed mice
Then, the effects of irisin on oxidative stress were evaluated. As shown by liver DHE staining in Figure 3A,B, irisin administration inhibited the production of ROS compared to vehicle‐treated mice (P < .05). Antioxidant GSH‐Px decreased in hepatic I/R and increased after irisin administration in HFD‐fed mice (Figure 3C, P < .05).
Figure 3.
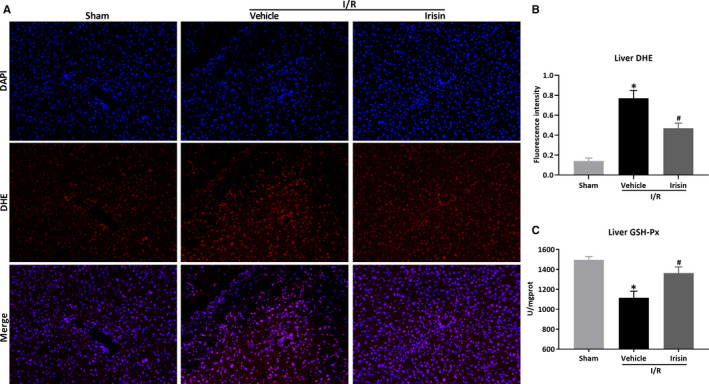
Irisin alleviates oxidative stress after hepatic I/R in HFD‐fed mice. Hepatic ischaemia was induced by occluding partial (70%) hepatic arterial/portal venous blood for 60 min, followed by 24 h of reperfusion. Sham mice underwent all the procedures except hepatic ischaemia. After 12 wk of high‐fat diet (HFD), HFD‐fed mice underwent sham operation (Sham) or hepatic I/R treated with 0.5 mL saline (Vehicle) or irisin (250 μg/kg, 0.5 mL). A, Liver DHE staining (red) and counterstained with DAPI (blue). Original magnification, 200×. B, The quantitative analysis of liver DHE fluorescence intensity. C, The level of liver GSH‐Px. Results are expressed as mean ± SE (n = 4‐5/group) and compared by t test or one‐way ANOVA. *P < .05 vs sham group, # P < .05 vs vehicle group
3.4. Irisin improves mitochondrial function and reduces ER stress after hepatic I/R in HFD‐fed mice
Figure 4A‐C showed that irisin inhibited the excessive expression of mitochondrial fission proteins Drp‐1 and Fis‐1 at mRNA or protein levels (P < .05). Moreover, irisin treatment also restored the reduced expression of mitochondrial biogenesis proteins PGC‐1α and Tfam (Figure 4D‐F, P < .05). As showed in Figure 4G‐K, the expressions of liver endoplasmic reticulum (ER) stress‐related proteins (GRP78, CHOP, PDI and Ero1‐Lα) were significantly up‐regulated after hepatic I/R in HFD‐fed mice but reduced after irisin administration (P < .05).
Figure 4.
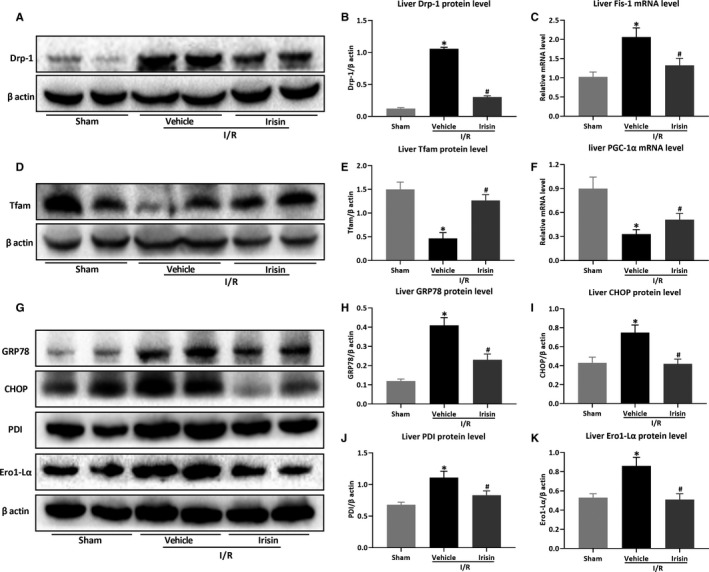
Irisin improves mitochondrial function and reduces ER stress after hepatic I/R in HFD‐fed mice. Hepatic ischaemia was induced by occluding partial (70%) hepatic arterial/portal venous blood for 60 min, followed by 24 h of reperfusion. Sham mice underwent all the procedures except hepatic ischaemia. After 12 wk of high‐fat diet (HFD), HFD‐fed mice underwent sham operation (Sham) or hepatic I/R treated with 0.5 mL saline (Vehicle) or irisin (250 μg/kg, 0.5 mL). Western blot analysis of Drp‐1 (A) and its quantitative analysis (B). C, Liver relative mRNA level of Fis‐1. Western blot analysis of Tfam (D) and its quantitative analysis (E). F, Liver relative mRNA level of PGC‐1α. Western blot analysis of ER stress‐related proteins (G) and their quantitative analysis of liver GRP78 (H), CHOP (I), PDI (J) and Ero1‐Lα (K). Results are expressed as mean ± SE (n = 4‐5/group) and compared by t test or one‐way ANOVA. *P < .05 vs sham group, # P < .05 vs vehicle group
3.5. Irisin inhibits inflammatory response after hepatic I/R in HFD‐fed mice
Inflammatory response was evaluated after hepatic I/R in HFD‐fed mice. The recruitment of neutrophils and macrophages was detected by liver MPO and CD11b immunostaining, while the release of inflammatory factors was detected by q‐PCR. In Figure 5A‐D, hepatic I/R increased liver MPO and CD11b positive cells in HFD‐fed mice, which were reversed after irisin administration (P < .05). Consistently, compared to HFD‐fed mice of hepatic I/R, the mRNA levels of liver inflammatory factors (IL‐1β, IL‐6, MCP‐1 and CXCL‐1) were also reduced after irisin administration in HFD‐fed mice (Figure 5E‐H, P < .05).
Figure 5.
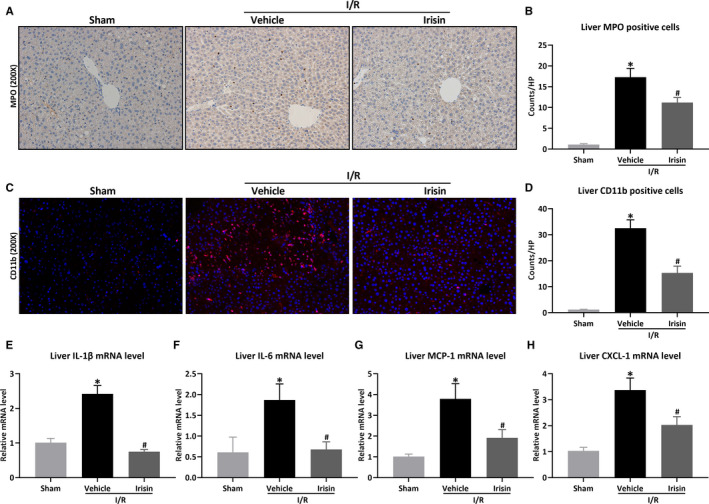
Irisin inhibits inflammatory response after hepatic I/R in HFD‐fed mice. Hepatic ischaemia was induced by occluding partial (70%) hepatic arterial/portal venous blood for 60 min, followed by 24 h of reperfusion. Sham mice underwent all the procedures except hepatic ischaemia. After 12 wk of high‐fat diet (HFD), HFD‐fed mice underwent sham operation (Sham) or hepatic I/R treated with 0.5 mL saline (Vehicle) or irisin (250 μg/kg, 0.5 mL). Liver MPO staining (A) and its quantitative analysis of liver MPO positive cells (B). Original magnification, 200×. Liver CD11b staining (red) (C) and its quantitative analysis of liver CD11b positive cells (D). Original magnification, 200×. Liver relative mRNA levels of IL‐1β (E), IL‐6 (F), MCP‐1 (G), CXCL‐1 (H). Results are expressed as mean ± SE (n = 4‐5/group) and compared by t test or one‐way ANOVA. *P < .05 vs sham group, # P < .05 vs vehicle group
3.6. Kindlin‐2 inhibition by RNAi eliminates irisin's direct effects on cultured hepatocytes
To investigate the role of kindlin‐2 in irisin's biological function, we knocked down kindlin‐2 expression in HL‐7702 cells (Figure S3A,B, P < .05). The cells were exposed to palmitic acid (PA, 0.2 mmol/L) and oleic acid (OA, 0.1 mmol/L) to induce lipotoxicity injury and then subjected to hypoxia and reoxygenation treatment with/without the presence of irisin. As shown in Figure 6, kindlin‐2 knockdown eliminated the effects of irisin on cell apoptosis (Figure 6A‐C), ROS production (Figure 6D,E), mitochondrial function (Figure 6F‐L) and ER stress (Figure 6M‐Q) (all P < .05). Meanwhile, the effect of irisin on kindlin‐2 was detected after irisin treatment in HFD‐fed mice and irisin did not change the expression of kindlin‐2 (Figure S4A,B, P > .05).
Figure 6.
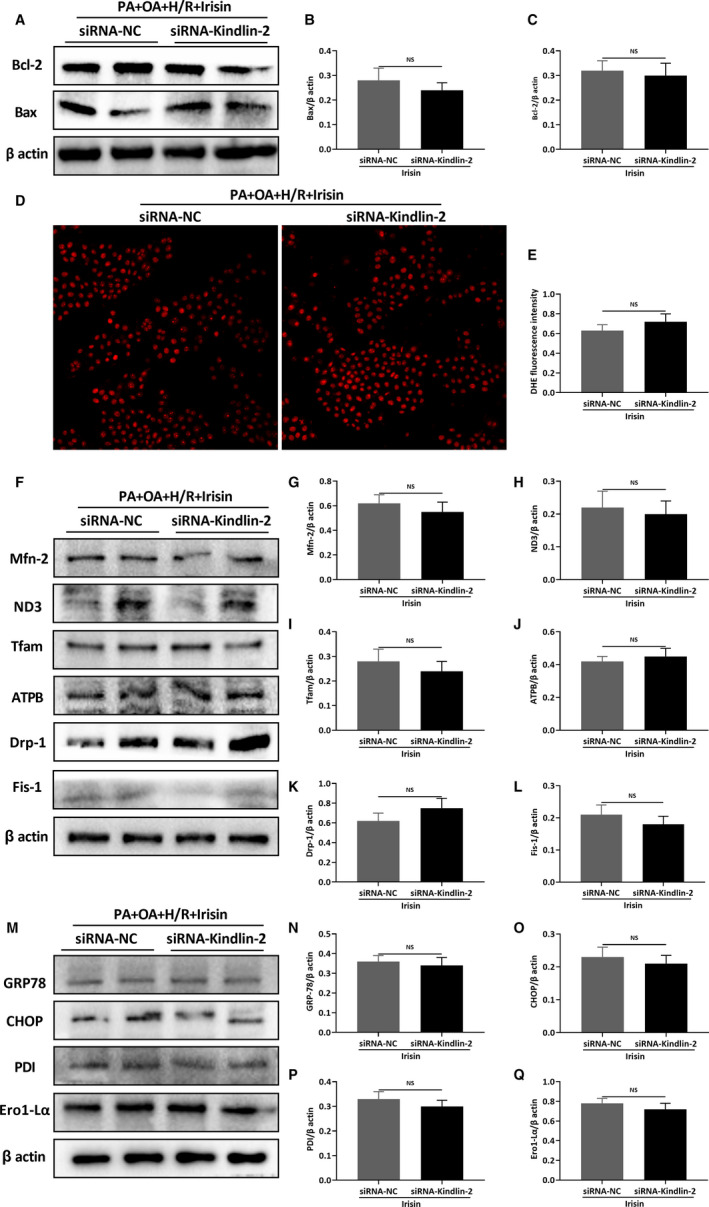
The knockdown of kindlin‐2 eliminates the protective effect of irisin. The HL‐7702 cells were transfected with siRNA of kindlin‐2 (or negative control) for 48 h and treated with palmitic acid (PA, 0.2 mmol/L) and oleic acid (OA, 0.1 mmol/L) to induce lipotoxicity injury of hepatocytes. Cells were deprived of oxygen (94% N2, 5% CO2, 1% O2) in a serum‐free and deoxyglucose‐rich (5 mmol/L) medium to simulate hepatic ischaemia and hypoxia of mice for 1 h; then, the cells were changed to 5% CO2 condition with RPMI‐1640 medium containing 10% FBS for 6 h. Western blot analysis of Bax and Bcl‐2 (A) and their quantitative analysis of Bax (B) and Bcl‐2 (C). DHE staining (red) (D) and its quantitative analysis of DHE fluorescence intensity (E). Western blot analysis of mitochondrial related proteins (F) and their quantitative analysis of Mfn‐2 (G), ND3 (H), Tfam (I), ATPB (J), Drp‐1 (K) and Fis‐1 (L). Western blot analysis of ER stress‐related proteins (M) and their quantitative analysis of GRP78 (N), CHOP (O), PDI (P) and Ero1‐Lα (Q). Results are expressed as mean ± SE (n = 3‐4/group) and compared by t test. NS P > .05
4. DISCUSSIONS
In the present study, using an established model of hepatic I/R in HFD‐fed mice, we found that HFD exaggerated I/R‐induced liver injury and ROS production. And irisin administration attenuated hepatic injury, improved mitochondrial function, and reduced oxidative and ER stress in HFD‐fed hepatic I/R mice. However, in cultured hepatocytes, inhibition of kindlin‐2 by RNAi eliminated irisin's effects on apoptosis, mitochondrial function, oxidative and ER stress (Figure 7).
Figure 7.
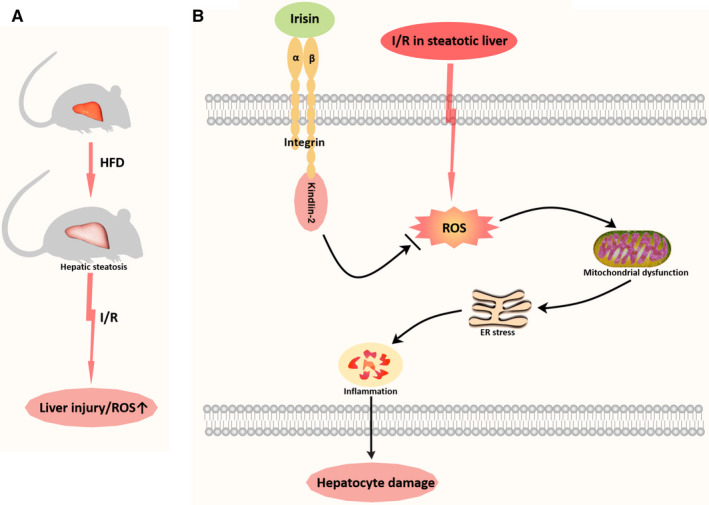
Irisin attenuates hepatic I/R injury via a kindlin‐2 dependent mechanism in steatotic liver. HFD exaggerated I/R‐induced liver injury and ROS production. And irisin administration attenuated liver injury, improved mitochondrial function, and reduced oxidative and ER stress in HFD‐fed hepatic I/R mice. However, inhibition of kindlin‐2 by RNAi eliminated irisin's effects on apoptosis, mitochondrial function, oxidative and ER stress in cultured hepatocytes
Hepatic I/R is a life‐threaten complication in liver surgery and associated with significant morbidity and mortality, especially in hepatic I/R of steatotic liver. 6 , 23 , 24 , 25 , 26 , 27 Research has shown that about 20% of patients undergoing hepatectomy have various degrees of hepatic steatosis. 28 Moreover, steatotic liver (about 20%‐30% of liver donors) has been introduced as the most common type of ‘extended criteria’ organs due to organ shortage. 29 , 30 , 31 However, hepatic steatosis exaggerates I/R‐induced liver injury, which has been proven in clinical and experimental studies. 6 , 23 , 24 , 25 , 26 , 27 Although the exact mechanism remains unclear, ROS is believed to play an important role in both hepatic steatosis 32 , 33 and I/R injury. 34 , 35 , 36 In NAFLD, fat‐laden hepatocytes are damaged by chronic oxidative/nitrosative stress (ONS). And ONS is acutely exacerbated during hepatic I/R, leading to extensive parenchymal damage. 7 In the present study, we also showed more severe liver injury and ROS production after hepatic I/R in HFD‐fed mice, suggesting inhibition of ROS production may be an effective therapeutic target.
Irisin, an exercise‐induced hormone, has emerged as a key regular of energy homeostasis in obesity, diabetes and NAFLD. 37 , 38 , 39 , 40 A recent study has shown that irisin expression increased in non‐parenchymal cells of fatty liver and was associated with the increase in innate immune cells (ie CD11b positive cells). 41 In the present study, liver CD11b positive cells were increased significantly in HFD‐hepatic I/R mice, which may be the reason for the increase of serum irisin levels under such conditions. We and others have shown irisin plays a protective role in I/R of multiple organs and improvement of mitochondrial function and oxidative stress are the most common mechanisms. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 However, the effect of irisin on I/R in steatotic liver remained unknown. The present study is the first one to reveal that irisin attenuated liver injury, improved mitochondrial function, and reduced oxidative and ER stress after I/R in steatotic liver. The dosage of irisin was based on our previous study. 17 As shown in the present study, it was also protective in HFD‐hepatic I/R mice. However, the optimal dosage and the dose‐dependent effect of irisin in HFD‐hepatic I/R warrants further investigation. Hepatic I/R is accompanied by increased production of ROS. Mitochondria are a main source of ROS and ROS impairs mitochondrial function. 42 , 43 , 44 , 45 Endoplasmic reticulum (ER) stress is closely related to mitochondrial dysfunction. ER stress inhibition protects steatotic and non‐steatotic liver from hepatic I/R. 46 , 47 , 48 ER‐stressed steatotic hepatocytes activate apoptotic and inflammatory pathways in hepatic I/R and NAFLD, 49 , 50 leading to liver injury.
Kindlin‐2, a member of kindlins, directly interacts with the cytoplasmic tail of β integrin to mediate cell adhesion, cell motility, cytoskeletal organization, cell survival, gene transcription and cell proliferation. 51 , 52 , 53 Kindlin‐2 has been shown to promote tumour invasion and metastasis. 54 , 55 It is also essential for preserving integrity of the heart, vascular permeability in angiogenesis, chondrogenesis, regulation of podocyte structure and function, control of adipogenesis and lipid metabolism as well as bone homeostasis. 56 , 57 , 58 , 59 , 60 , 61 Kindlins protect cells against oxidative damage. 62 , 63 , 64 , 65 Ling Guo et al have discovered that depletion of kindlin‐2 increased ROS production. 66 αVβ5 integrin was reported to be the receptor of irisin, 21 and our recent studies have shown irisin mitigated I/R injury via binding to αVβ5 integrin. 18 However, the effect of irisin on kindlin‐2, an important regulator of αVβ5 integrin function, remained unknown. In the present study, we found irisin did not change the expression of kindlin‐2 after hepatic I/R in HFD‐fed mice. However, kindlin‐2 knockdown by RNAi eliminated the beneficial effects of irisin in hypoxia/reoxygenation‐treated hepatocytes, suggesting kindlin‐2 is involved in irisin's biological function. However, whether depletion of Kindlin‐2 inhibits irisin induced protection in hepatic IR in the HFD‐mice warrants further investigation. And the detailed mechanism of kindlin‐2 after hepatic I/R in the HFD‐mice will be further explored in our future studies.
In summary, using a model of hepatic I/R in HFD‐fed mice, we demonstrated that irisin attenuates I/R injury in steatotic liver. The protective effect of irisin under such conditions requires kindlin‐2. Irisin may be a novel effective treatment for NAFLD patients with hepatic I/R.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Jia Zhang: Conceptualization (equal); Data curation (lead); Formal analysis (equal); Methodology (equal); Project administration (equal). Yifan Ren: Data curation (equal). Jianbin Bi: Data curation (equal). Mengzhou Wang: Data curation (equal). Lin Zhang: Data curation (equal). Tao Wang: Data curation (equal). Shasha Wei: Data curation (equal); Funding acquisition (equal). Xingyi Mou: Data curation (equal). Yi Lv: Conceptualization (supporting); Project administration (supporting). Rongqian Wu: Conceptualization (lead); Funding acquisition (lead); Project administration (lead).
Supporting information
Fig S1‐S4
ACKNOWLEDGEMENTS
This work was supported by grants from the National Natural Science Foundation of China (No. 81770491 and No. 81701960) and Ministry of Education Innovation Team Development Program of China (No. IRT16R57).
Zhang J, Ren Y, Bi J, et al. Involvement of kindlin‐2 in irisin’s protection against ischaemia reperfusion‐induced liver injury in high‐fat diet‐fed mice. J. Cell. Mol. Med.. 2020;24:13081–13092. 10.1111/jcmm.15910
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Estes C, Anstee QM, Arias‐Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69:896‐904. [DOI] [PubMed] [Google Scholar]
- 2. Rinella M, Charlton M. The globalization of nonalcoholic fatty liver disease: Prevalence and impact on world health. Hepatology. 2016;64:19‐22. [DOI] [PubMed] [Google Scholar]
- 3. Lee HW, Wong VW. Changing NAFLD epidemiology in China. Hepatology. 2019;70(4):1095‐1098. [DOI] [PubMed] [Google Scholar]
- 4. Zhou F, Zhou J, Wang W, et al. Unexpected rapid increase in the burden of NAFLD in China from 2008 to 2018: a systematic review and meta‐analysis. Hepatology. 2019;70:1119‐1133. [DOI] [PubMed] [Google Scholar]
- 5. Papadopoulos D, Siempis T, Theodorakou E, Tsoulfas G. Hepatic ischemia and reperfusion injury and trauma: current concepts. Arch Trauma Res. 2013;2:63‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walsh MJ. Systematic review and meta‐analysis of steatosis as a risk factor in major hepatic resection (Br J Surg 2010: 97: 1331–1339). Br J Surg. 2010;97:1339. [DOI] [PubMed] [Google Scholar]
- 7. Reiniers MJ, van Golen RF, van Gulik TM, Heger M. Reactive oxygen and nitrogen species in steatotic hepatocytes: a molecular perspective on the pathophysiology of ischemia‐reperfusion injury in the fatty liver. Antioxid Redox Signal. 2014;21:1119‐1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bostrom P, Wu J, Jedrychowski MP, et al. A PGC1‐alpha‐dependent myokine that drives brown‐fat‐like development of white fat and thermogenesis. Nature. 2012;481:463‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Z, Chen K, Han Y, et al. Irisin protects heart against ischemia‐reperfusion injury through a SOD2‐dependent mitochondria mechanism. J Cardiovasc Pharmacol. 2018;72:259‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen K, Xu Z, Liu Y, et al. Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. Sci Transl Med. 2017;9(418):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Du J, Fan X, Yang B, Chen Y, Liu KX, Zhou J. Irisin pretreatment ameliorates intestinal ischemia/reperfusion injury in mice through activation of the Nrf2 pathway. Int Immunopharmacol. 2019;73:225‐235. [DOI] [PubMed] [Google Scholar]
- 12. Wang H, Zhao YT, Zhang S, et al. Irisin plays a pivotal role to protect the heart against ischemia and reperfusion injury. J Cell Physiol. 2017;232:3775‐3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li DJ, Li YH, Yuan HB, Qu LF, Wang P. The novel exercise‐induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism. 2017;68:31‐42. [DOI] [PubMed] [Google Scholar]
- 14. Zhao G, Zhang X, Xu P, Mi JY, Rui YJ. The protective effect of Irisin against ischemia‐reperfusion injury after perforator flap grafting in rats. Injury. 2018;49:2147‐2153. [DOI] [PubMed] [Google Scholar]
- 15. Jin Z, Guo P, Li X, Ke J, Wang Y, Wu H. Neuroprotective effects of irisin against cerebral ischemia/reperfusion injury via Notch signaling pathway. Biomed Pharmacother. 2019;120:109452. [DOI] [PubMed] [Google Scholar]
- 16. Fan X, Du J, Wang MH, et al. Irisin contributes to the hepatoprotection of dexmedetomidine during intestinal ischemia/reperfusion. Oxid Med Cell Longev. 2019;2019:7857082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bi J, Zhang J, Ren Y, et al. Irisin alleviates liver ischemia‐reperfusion injury by inhibiting excessive mitochondrial fission, promoting mitochondrial biogenesis and decreasing oxidative stress. Redox Biol. 2019;20:296‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bi J, Zhang J, Ren Y, et al. Irisin reverses intestinal epithelial barrier dysfunction during intestinal injury via binding to the integrin alphaVbeta5 receptor. J Cell Mol Med. 2020;24:996‐1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Larjava H, Plow EF, Wu C. Kindlins: essential regulators of integrin signalling and cell‐matrix adhesion. EMBO Rep. 2008;9:1203‐1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li H, Deng Y, Sun K, et al. Structural basis of kindlin‐mediated integrin recognition and activation. Proc Natl Acad Sci USA. 2017;114:9349‐9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim H, Wrann CD, Jedrychowski M, et al. Irisin mediates effects on bone and fat via alphaV integrin receptors. Cell. 2018;175:1756‐1768 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang J, Bi J, Ren Y, et al. Natural killer T cell ligand alpha‐galactosylceramide protects against gut ischemia reperfusion‐induced organ injury in mice. Cytokine. 2018;111:237‐245. [DOI] [PubMed] [Google Scholar]
- 23. Gehrau RC, Mas VR, Dumur CI, et al. Donor hepatic steatosis induce exacerbated ischemia‐reperfusion injury through activation of innate immune response molecular pathways. Transplantation. 2015;99:2523‐2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reddy SK, Marsh JW, Varley PR, et al. Underlying steatohepatitis, but not simple hepatic steatosis, increases morbidity after liver resection: a case‐control study. Hepatology. 2012;56:2221‐2230. [DOI] [PubMed] [Google Scholar]
- 25. McCormack L, Petrowsky H, Jochum W, Furrer K, Clavien PA. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: a matched case‐control study. Ann Surg. 2007;245:923‐930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Selzner N, Selzner M, Jochum W, Amann‐Vesti B, Graf R, Clavien PA. Mouse livers with macrosteatosis are more susceptible to normothermic ischemic injury than those with microsteatosis. J Hepatol. 2006;44:694‐701. [DOI] [PubMed] [Google Scholar]
- 27. Tevar AD, Clarke C, Wang J, et al. Clinical review of nonalcoholic steatohepatitis in liver surgery and transplantation. J Am Coll Surg. 2010;210:515‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vetelainen R, van Vliet A, Gouma DJ, van Gulik TM. Steatosis as a risk factor in liver surgery. Ann Surg. 2007;245:20‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCormack L, Dutkowski P, El‐Badry AM, Clavien PA. Liver transplantation using fatty livers: always feasible? J Hepatol. 2011;54:1055‐1062. [DOI] [PubMed] [Google Scholar]
- 30. Nocito A, El‐Badry AM, Clavien PA. When is steatosis too much for transplantation? J Hepatol. 2006;45:494‐499. [DOI] [PubMed] [Google Scholar]
- 31. Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105‐113. [DOI] [PubMed] [Google Scholar]
- 32. Spahis S, Delvin E, Borys JM, Levy E. Oxidative stress as a critical factor in nonalcoholic fatty liver disease pathogenesis. Antioxid Redox Signal. 2017;26:519‐541. [DOI] [PubMed] [Google Scholar]
- 33. Tarantino G, Citro V, Capone D. Nonalcoholic fatty liver disease: a challenge from mechanisms to therapy. Journal of Clinical Medicine. 2020;9(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elias‐Miro M, Jimenez‐Castro MB, Rodes J, Peralta C. Current knowledge on oxidative stress in hepatic ischemia/reperfusion. Free Radic Res. 2013;47:555‐568. [DOI] [PubMed] [Google Scholar]
- 35. Galaris D, Barbouti A, Korantzopoulos P. Oxidative stress in hepatic ischemia‐reperfusion injury: the role of antioxidants and iron chelating compounds. Curr Pharm Des. 2006;12:2875‐2890. [DOI] [PubMed] [Google Scholar]
- 36. Glantzounis GK, Salacinski HJ, Yang W, Davidson BR, Seifalian AM. The contemporary role of antioxidant therapy in attenuating liver ischemia‐reperfusion injury: a review. Liver Transpl. 2005;11:1031‐1047. [DOI] [PubMed] [Google Scholar]
- 37. Polyzos SA, Anastasilakis AD, Efstathiadou ZA, et al. Irisin in metabolic diseases. Endocrine. 2018;59:260‐274. [DOI] [PubMed] [Google Scholar]
- 38. Zhang HJ, Zhang XF, Ma ZM, et al. Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J Hepatol. 2013;59:557‐562. [DOI] [PubMed] [Google Scholar]
- 39. Medicine KDP. Irisin, light my fire. Science. 2012;336:42‐43. [DOI] [PubMed] [Google Scholar]
- 40. Perakakis N, Triantafyllou GA, Fernandez‐Real JM, et al. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol. 2017;13:324‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Canivet CM, Bonnafous S, Rousseau D, et al. Hepatic FNDC5 is a potential local protective factor against non‐alcoholic fatty liver. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165705. [DOI] [PubMed] [Google Scholar]
- 42. Mukhopadhyay P, Horvath B, Zsengeller Z, et al. Mitochondrial reactive oxygen species generation triggers inflammatory response and tissue injury associated with hepatic ischemia‐reperfusion: therapeutic potential of mitochondrially targeted antioxidants. Free Radic Biol Med. 2012;53:1123‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec‐Weglinski JW. Ischaemia‐reperfusion injury in liver transplantation–from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Figueira TR, Barros MH, Camargo AA, et al. Mitochondria as a source of reactive oxygen and nitrogen species: from molecular mechanisms to human health. Antioxid Redox Signal. 2013;18:2029‐2074. [DOI] [PubMed] [Google Scholar]
- 45. Jaeschke H. Role of reactive oxygen species in hepatic ischemia‐reperfusion injury and preconditioning. J Invest Surg. 2003;16:127‐140. [PubMed] [Google Scholar]
- 46. Ben Mosbah I, Alfany‐Fernandez I, Martel C, et al. Endoplasmic reticulum stress inhibition protects steatotic and non‐steatotic livers in partial hepatectomy under ischemia‐reperfusion. Cell Death Dis. 2010;1:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lim JH, Lee HJ, Ho Jung M, Song J. Coupling mitochondrial dysfunction to endoplasmic reticulum stress response: a molecular mechanism leading to hepatic insulin resistance. Cell Signal. 2009;21:169‐177. [DOI] [PubMed] [Google Scholar]
- 48. Zeeshan HM, Lee GH, Kim HR, Chae HJ. Endoplasmic reticulum stress and associated ROS. Int J Mol Sci. 2016;17:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lebeaupin C, Vallee D, Hazari Y, Hetz C, Chevet E, Bailly‐Maitre B. Endoplasmic reticulum stress signalling and the pathogenesis of non‐alcoholic fatty liver disease. J Hepatol. 2018;69:927‐947. [DOI] [PubMed] [Google Scholar]
- 50. Mollica MP, Lionetti L, Putti R, Cavaliere G, Gaita M, Barletta A. From chronic overfeeding to hepatic injury: role of endoplasmic reticulum stress and inflammation. Nutr Metab Cardiovasc Dis. 2011;21:222‐230. [DOI] [PubMed] [Google Scholar]
- 51. Abrams CS. Kindlin ignites a new flame. Blood. 2013;122:2297‐2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rognoni E, Ruppert R, Fassler R. The kindlin family: functions, signaling properties and implications for human disease. J Cell Sci. 2016;129:17‐27. [DOI] [PubMed] [Google Scholar]
- 53. Wang H, Lim D, Rudd CE. Immunopathologies linked to integrin signalling. Semin Immunopathol. 2010;32:173‐182. [DOI] [PubMed] [Google Scholar]
- 54. Shen Z, Ye Y, Dong L, et al. Kindlin‐2: a novel adhesion protein related to tumor invasion, lymph node metastasis, and patient outcome in gastric cancer. Am J Surg. 2012;203:222‐229. [DOI] [PubMed] [Google Scholar]
- 55. An Z, Dobra K, Lock JG, Stromblad S, Hjerpe A, Zhang H. Kindlin‐2 is expressed in malignant mesothelioma and is required for tumor cell adhesion and migration. Int J Cancer. 2010;127:1999‐2008. [DOI] [PubMed] [Google Scholar]
- 56. Qi L, Yu Y, Chi X, et al. Depletion of Kindlin‐2 induces cardiac dysfunction in mice. Sci China Life Sci. 2016;59:1123‐1130. [DOI] [PubMed] [Google Scholar]
- 57. Sun Y, Guo C, Ma P, et al. Kindlin‐2 association with Rho GDP‐dissociation inhibitor alpha suppresses Rac1 activation and podocyte injury. J Am Soc Nephrol. 2017;28:3545‐3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wu C, Jiao H, Lai Y, et al. Kindlin‐2 controls TGF‐beta signalling and Sox9 expression to regulate chondrogenesis. Nat Commun. 2015;6:7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ying J, Luan W, Lu L, Zhang S, Qi F. Knockdown of the KINDLIN‐2 gene and reduced expression of kindlin‐2 affects vascular permeability in angiogenesis in a mouse model of wound healing. Med Sci Monit. 2018;24:5376‐5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang Z, Mu Y, Veevers J, et al. Postnatal loss of kindlin‐2 leads to progressive heart failure. Circ Heart Fail. 2016;9(8):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gao H, Guo Y, Yan Q, et al. Lipoatrophy and metabolic disturbance in mice with adipose‐specific deletion of kindlin‐2. JCI Insight. 2019;4(13):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Emmert H, Culley J, Brunton VG. Inhibition of cyclin‐dependent kinase activity exacerbates H2 O2 ‐induced DNA damage in Kindler syndrome keratinocytes. Exp Dermatol. 2019;28:1074‐1078. [DOI] [PubMed] [Google Scholar]
- 63. Emmert H, Patel H, Brunton VG. Kindlin‐1 protects cells from oxidative damage through activation of ERK signalling. Free Radic Biol Med. 2017;108:896‐903. [DOI] [PubMed] [Google Scholar]
- 64. Maier K, He Y, Wolfle U, et al. UV‐B‐induced cutaneous inflammation and prospects for antioxidant treatment in Kindler syndrome. Hum Mol Genet. 2016;25:5339‐5352. [DOI] [PubMed] [Google Scholar]
- 65. Xu Z, Ni B, Cao Z, et al. Kindlin‐3 negatively regulates the release of neutrophil extracellular traps. J Leukoc Biol. 2018;104:597‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Guo L, Cui C, Zhang K, et al. Kindlin‐2 links mechano‐environment to proline synthesis and tumor growth. Nat Commun. 2019;10:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S4
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


