Abstract
Despite the fact that proton pump inhibitors (PPIs) are one of the most prescribed medications, several epidemiological studies have reported many adverse effects related to their long-term usage. Nevertheless, there were inconsistent findings in the literature with regard to PPI use and bone mineral density (BMD) change. The aim of this systematic review and meta-analysis is to evaluate the association between the use of PPIs and change in BMD. The PubMed/MEDLINE, EMBASE, Cochrane and CINAHL databases were searched up to March 2019. Ten studies fulfilled the eligibility criteria (4761 cases and 30,809 controls), from which the mean difference and mean annualized percent change in BMD were pooled using RevMan 5.3.5 The results showed no statistically significant association between PPI users and non-users in mean annualized percent change in BMD (0.06; 95% CI −0.07, 0.18) with moderate heterogeneity (I2: 63%). There was a statistically significant reduction in the mean BMD difference among PPI users (−0.03; 95% CI −0.04, −0.01) with no substantial heterogeneity (I2: 26%). This meta-analysis reported inconsistent results regarding the use of PPIs and BMD loss. Thus, the effect of PPIs on BMD needs to be elucidated by other studies, and healthcare providers should prescribe PPIs with caution considering their unfavorable consequences on bone health.
Keywords: Bone mineral density, Proton pump inhibitor, Systematic review
1. Introduction
Proton pump inhibitors (PPIs) were first introduced in 1989; since then, they have been commonly used for treating acid-related disorders such as gastroesophageal reflux disease (GERD) and peptic ulcer disease (PUD) (Strand et al., 2017; Hershcovici and Fass, 2010; Sandhu and Fass, 2018). PPIs are used worldwide as prescribed medications, and in many countries, they are available as over-the-counter (OTC) medications due to their efficacy and known safety profile (Strand et al., 2017; Johnson et al., 2017). Recently, several studies reported many adverse effects related to the long-term use of PPIs, such as an increased risk of osteoporotic-related fractures, Clostridium difficile infection, pneumonia, and vitamin B12 and magnesium deficiencies (Heidelbaugh, 2013; Nehra et al., 2018; Wang et al., 2019; Savarino et al., 2018; Pezeshkian and Conway, 2018).
Many systematic reviews and observational studies have shown an increased risk of osteoporotic fractures after the long-term use of PPIs (Zhou et al., 2016; Nassar and Richter, 2018; Hussain et al., 2018; Islam et al., 2018; Abramowitz et al., 2016). However, the precise mechanism remains unclear, and the causality of the association is inconclusive. Studies that assessed the relationship between PPI use and increased osteoporotic fracture risk suggested several mechanisms, such as a reduction in intestinal calcium absorption, an interruption in osteoclast function in bone remolding and repair, and a decrease in bone mineral density (BMD) (Ito and Jensen, 2010; Arj et al., 2016; Maléth and Hegyi, 2013).
Although many epidemiological studies reported that PPI treatment reduces BMD (Heidelbaugh, 2013; Lau and Ahmed, 2012), others failed to find a significant association (Lau and Ahmed, 2012). Moreover, two systematic reviews and meta-analyses with different inclusion criteria reported no significant difference in the mean values of BMD between PPI users and controls (Zhou et al., 2016; Nassar and Richter, 2018). These results attract our attention to conduct a systematic review and meta-analysis of existing observational studies to evaluate the association between the use of PPIs and changes in BMD.
2. Methodology
This systematic review and meta-analysis was conducted following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (Moher et al., 2009) to explore the association of PPI use and BMD change.
2.1. Eligibility criteria
All studies that fulfilled the following criteria were included: (a) cohort or case-control study design; (b) study population above 18 years of age in both sexes; (c) the use of PPI was defined as an exposure; (d) the change in BMD was reported as an outcome by using dual-energy X-ray absorptiometry (DXA or DEXA); and (e) the means ± standard deviations (SDs) were provided for the PPI users and the control group or adequate information was provided to calculate them. Studies were excluded if they have the following criteria: (a) the study examined the association between PPI use and change in BMD in combination with histamine2-receptor antagonists or other drugs that affect bone metabolism such as bisphosphonate or glucocorticoids and/or (b) use of peripheral quantitative computed tomography scans (pQCT) or other methods rather than DXA or DEXA for the measurement of BMD.
2.2. Search strategy
A comprehensive literature search was performed up to March 2019 in electronic databases including the PubMed/MEDLINE (national center for biotechnology information), EMBASE (Elsevier), Cochrane (Wiley online library) and Cumulative Index to Nursing and Allied Health Literature (CINAHL) databases without restriction to language using the following keywords: (a) proton pump inhibitors, lansoprazole, pantoprazole, rabeprazole, esomeprazole, omeprazole, dexlansoprazole, gastric acid-suppressive agents, gastric acid inhibitors, antacid, OR antiulcer agents; (b) osteoporosis, bone mineral density OR osteopenia. Moreover, a manual search of the retrieved articles' references was conducted.
2.3. Study selection and data extraction
Studies that fulfilled the inclusion criteria were selected by two independent reviewers (SE and SH), and conflicts were resolved by a third investigator (MF). The two reviewers abstracted the qualitative and quantitative data from the included articles by using a designed data extraction template including the following: study author, year of publication, study country/setting, study design, study period, study population sex/mean age/number of controls/numbers of PPI users, exposure type/dose/duration, outcome mean ± SD and P value.
2.4. Risk of bias and quality assessment
An assessment of the quality of the included cohort studies was performed by two independent reviewers (SE and SH) using the Newcastle–Ottawa scale (NOS) (Wells et al., 2015) for evaluating the quality of nonrandomized studies in meta-analyses. Three factors were considered for scoring the quality of the studies: (1) selection, (2) comparability, and (3) outcome (Wells et al., 2015). The quality of the studies was rated by awarding stars in each subset with a total maximum score of 9 (Wells et al., 2015). Studies that scored ≥7 were considered high-quality, while those that scored <7 were considered low-quality.
2.5. Statistical analysis
Statistical analysis was performed using Review Manager version 5.3.5 (RevMan 5.3.5) (Review Manager (RevMan), 2014) to assess the association between PPI use and change in mean or annualized mean percent change in BMD. A fixed effects model was used for the meta-analysis in the absence of substantial heterogeneity, and a random effects model was used for the meta-analysis when heterogeneity existed. Furthermore, the I2 level was calculated to evaluate the heterogeneity across studies. The I2 value lies between 0 and 100%, and an I2 value of 75% represents a high level of heterogeneity; likewise, 50% represents a substantial level of heterogeneity. Subgroup analysis was performed based on body site (hip, femoral neck, and spine). However, subgrouping was not performed based on PPI types due to insufficient data for comparison in the selected studies.
2.6. Dealing with missing data
The SDs of the mean difference before and after treatment were missing in some of the selected studies. Therefore, the authors of these studies were contacted, but the data were not retrieved; thus, the missing data were recalculated by a statistician (IA). The formula for calculation of standard deviation is: SD = {√[N∑fx2 − (∑fx)2]} ÷ N (Wan et al., 2014).
2.7. Ethical approval
The present study was approved by the King Abdullah International Medical Research Center IRB committee on Feb 25, 2018 (research number RC18/048/R) (Mustafa, n.d.).
3. Results
3.1. Literature search
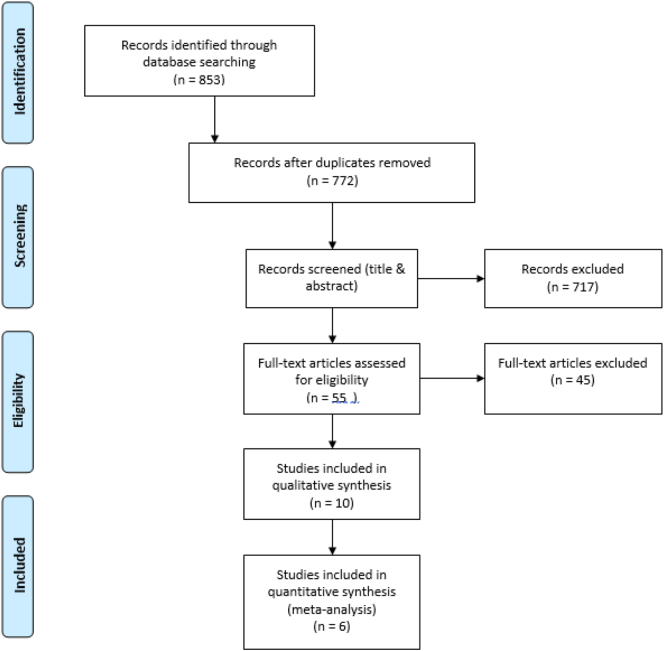
The search strategy identified a total of 853 citations. After removing duplicates, 772 relevant studies remained. Based on the title and abstract screening, 717 articles were excluded. Out of 55 full-text articles, 10 observational studies met the inclusion criteria for qualitative and quantitative analyses (Gray et al., 2010; Solomon et al., 2015; Bahtiri et al., 2016; Targownik et al., 2017; Shin et al., 2019; Xuan et al., 2014; Ozdil et al., 2013; Roux et al., 2012; Targownik et al., 2012; Elaine et al., 2008; Elaine et al., 2011) (Fig. 1).
Fig. 1.
PRISMA flow diagram showing the study selection process.
3.2. Study characteristics
In the literature search, 10 longitudinal cohort design studies reported the association between PPI use and change in BMD. The retrieved studies were published between 2008 and 2018. Nine studies were in English, and one was in Chinese. The studies were conducted in the USA, the Republic of Kosovo, Canada, China, Turkey and South Korea (Gray et al., 2010; Solomon et al., 2015; Bahtiri et al., 2016; Targownik et al., 2017; Shin et al., 2019; Xuan et al., 2014; Ozdil et al., 2013; Roux et al., 2012; Targownik et al., 2012; Elaine et al., 2008). Overall, the data for 4761 cases and 30,809 controls including males and females with ages ranging from 28.9 to 83.1 years were used in this meta-analysis. Different PPI classes were used with a range of duration of 30 days to 10 years. In the included studies, BMD change was measured by DXA using Hologic instruments. Most of the studies' results were adjusted for age, sex, and BMI (Table 1).
Table 1.
Characteristics of the included studies.
| Study | Study design | Study population | Mean age | No. of cases | No. of controls | PPI use | Outcome |
|---|---|---|---|---|---|---|---|
| Ozdil et al. (2013) | Prospective cohort | Males & females | 37.7 ± 8.8 | 114 | 110 | PPI doses & type: Lansoprazole 30 mg/day, Pantoprazole 40 mg/day, or Esomeprazole 40 mg/day.The duration was 8.5 ± 2.3 months | PPI results in a statistically significant reduction in BMD of the vertebra and femur. |
| Gray et al. (2010) | Prospective cohort | Females | PPI users 64.8 ± 7.1, PPI non-users 63.1 ± 7.3 |
3396 | 148,394 | PPI classes include: Omeprazole, Esomeprazole, Lansoprazole, Pantoprazole, Rabeprazole. The duration (<1Y, 1–3Y, >3Y) | PPI use was associated with only a marginal effect on 3-year BMD change at the hip but not at other site & this association was not present in longer follow up 6 years |
| Elaine et al. (2008) | Prospective cohort | Females | 79.3 | 234 | 4574 | The type and dose of PPI were not recorded. The average follows up 4.6 years. | No significant BMD differences were observed |
| Solomon et al. (2015) | Prospective cohort | Females | PPI users 50.7 ± 4.2, PPI non-users 50.2 ± 3.9 |
207 | 1605 | The PPI type, dose was not recorded. The duration was 9.9 years. | No difference in the adjusted model in the annualized BMD change at the lumbar spine, femoral neck or total hip in the PPI users compared with non-users. |
| Bahtiri et al. (2016) | Prospective cohort | Males & females | 50.59 ± 10.61 | 167 | 42 | PPI doses & type: Omeprazole at 20 mg/day, Esomeprazole at 20 mg/day, Lansoprazole at 30 mg/day, and Pantoprazole at 40 mg/day. The duration was 1 year | PPI treatment for 12 months resulted in lower femur neck & total hip BMD T scores |
| Roux et al. (2012) | Prospective cohort | Females | PPI users 65.9 ± 6.3, PPI non-users 65.8 ± 6.6 | 61 | 1150 | Omeprazole recorded as a type and no dose recorded. The duration 6 years | Patient using omeprazole had lower lumbar spine and hip BMD T scores at baseline but after follow up there was no difference in hip BMD between users & non-users |
| Targownik et al. (2012) | Prospective cohort | Males & females | Y0 61.0 ± 13.0, Y5 59.9 ± 12.1, Y10 58.0 ± 11.6 | 228 | 8112 | The dose & indication for PPI use were not recorded followed after baseline at 5 years and 10 years | PPI users had lower BMD at baseline than PPI non-users, but PPI use over 10 years did not appear to be associated with accelerated BMD loss |
| Targownik et al. (2017) | Prospective cohort | Males & females | PPI users 65.1 ± 9.1, PPI non-users 64.9 ± 7.9 |
52 | 52 | The dose & type of PPI were not recorded. The duration was 5 years | Long term PPI use is not associated with any changes in BMD or bone strength that would predispose to an increased risk of fracture. |
| Shin et al. (2019) | Retrospective cohort | Females | 64.3 ± 10.4 | 223 | 223 | The dose & type of PPI were not recorded. Exposure to PPI was 30 D, 31–90 Ds, >90 Ds. | Total hip BMD was significantly lower in the PPI exposure groups regardless of the exposure timing. However, the association of BMD with the PPI exposure timing was inconsistent for the lumbar or femoral neck |
| Xuan et al. (2014) | Retrospective cohort | Males & females | 63 ± 10 | 79 | 47 | The dose & type of PPI were not recorded. The duration was less than one year | Long term use of PPI is associated with decrease in hip joint BMD. |
3.3. Primary outcomes
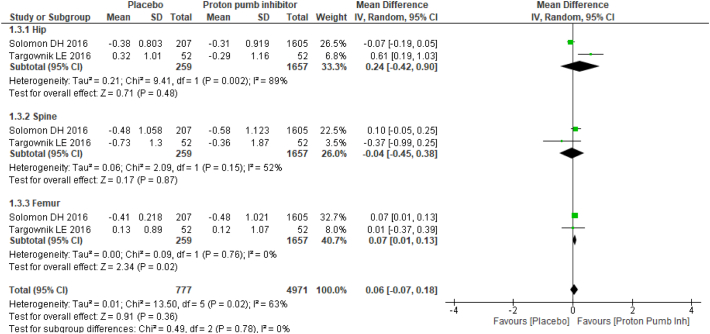
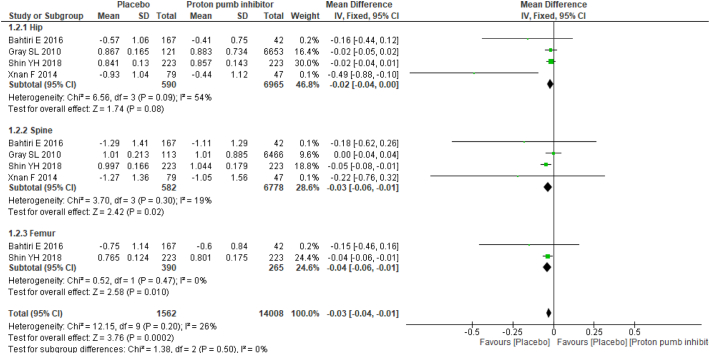
Two studies provided mean annualized percent change in BMD between PPI users and nonusers, and four studies provided mean differences in BMD. Studies with mean difference in BMD results favored no PPI usage with mean difference of −0.03 (95% CI −0.04, 0.01), and no substantial heterogeneity (I2: 26%) was found. Among the studies reported, the mean annualized percent change in BMD results showed no statistically significant association between PPI users and nonusers with a mean difference of 0.06 (95% CI −0.07, 0.18) and moderate heterogeneity (I2: 63%) (Fig. 2).
Fig. 2.
Forest plot showing the meta-analysis of mean annualized percent change in bone mineral density between proton-pump inhibitor (PPI) users and nonusers in the hip, spine & femur.
3.4. Subgroup analysis
Subgroup analysis was performed based on anatomic sites. The mean difference in BMD was statistically significant in both the spine and femur BMD results: hip −0.02 (95% CI −0.04, 0.00) with moderate heterogeneity (I2: 54%); spine −0.03 (95% CI −0.06, −0.01) with no substantial heterogeneity (I2: 26%); and femur −0.04 (95% CI −0.06, −0.01) with no heterogeneity (I2: 0%). For the mean annualized percent change in BMD, there was no statistical significance as follows: hip 0.24 (95% CI −0.42, 0.90) with substantial heterogeneity (I2: 89%); spine −0.04 (95% CI −0.45, 0.38) with moderate heterogeneity (I2: 52%); and femur 0.07 (95% CI 0.01, 0.13) with no heterogeneity (I2: 0%) (Fig. 3).
Fig. 3.
Forest plot showing the meta-analysis of mean differences in bone mineral density between proton-pump inhibitor (PPI) users and nonusers in the hip, spine & femur.
4. Discussion
This systematic review and meta-analysis of cohort studies that aimed to explore the association between PPI usage and the change in BMD revealed a reduction in BMD among PPI users regarding the mean difference in BMD. In contrast, there was no statistically significant association between PPI users and nonusers regarding the mean annualized percent change in BMD.
Many systematic reviews have reported an association between PPI use and an increased risk of fractures (Zhou et al., 2016; Elaine et al., 2011). However, the exact underlying mechanism remains unclear (Zhou et al., 2016; Elaine et al., 2011). One of the potential mechanisms that has been suggested in theory is that the role of PPIs in blocking gastric acid secretion could reduce intestinal calcium absorption and lead to a decrease in BMD (Ito and Jensen, 2010). Two recently published systematic reviews that addressed this question reported no significant difference in BMD between PPI users and nonusers (Zhou et al., 2016; Nassar and Richter, 2018), supporting the results of the mean annualized percent change in BMD in this study. Liu et al. failed to find a correlation between PPI use and BMD loss in the femur (SMD: −0.27; 95% CI −0.62, 0.09) and spine (SMD: −0.06; 95% CI −0.54, 0.41), but the results were from only three studies (Zhou et al., 2016). Additionally, Nassar et al. showed no significant difference in the standardized mean differences in BMD between PPI users and controls in cross-sectional (SMD: 0.00; 95% CI −0.18, 0.19) and longitudinal BMD values (SMD: 0.07; 95% CI −0.06, 0.20) (Nassar and Richter, 2018). Several articles included in the abovementioned systematic reviews and meta-analyses are not included in this study for two reasons. The first is the difficulty of inferring the temporal association between PPI use and change in BMD in cross-sectional studies. The second is the inability to mask the effect of bisphosphonates on bone health among the population using bisphosphonates simultaneously with PPIs.
Several observational studies showed conflicting results with regard to the PPI risk of low BMD. A prospective study by Yu et al. in men and women over the age of 65 demonstrated no statistically significant differences in the effect of the long-term use of PPI on BMD after adjusting for potential confounding factors, including age, race, BMI, alcohol use, exercise, oral or inhaled corticosteroid use, NSAID use, calcium supplement use, osteoporosis medication use, and self-reported health and concurrent weight change (Elaine et al., 2008). Furthermore, Targownik et al. observed no association between continuous PPI use and the rate of change in BMD at different measurement sites (including the total hip, femoral neck, and lumbar spine) between PPI users and nonusers at the 5-year and 10-year follow-ups (Targownik et al., 2012). Nevertheless, Ozdil et al. revealed statistically significant reductions in densitometric T-scores of the vertebra and femur in patients who used PPIs (Ozdil et al., 2013). Considering that the mean age group was 37.7 ± 8.8 and the duration of exposure was 8.5 ± 2.3 months, this population was the youngest and had the shortest duration compared to the studies mentioned above.
Most of the existing epidemiological evidence investigated the relationship between PPI use and BMD using DXA, which is a two-dimensional technique that provides measurements of areal (mg/cm2) rather than volumetric (mg/cm3) BMD (Gray et al., 2010; Bahtiri et al., 2016; Stathopoulos et al., 2016). As a result, these studies by using DXA failed to assess the independent changes of PPI on trabecular and cortical bone or the important component of bone microarchitecture. In contrast, quantitative computed tomography (QCT) provides a thorough understanding of the bone changes at central and peripheral sites and measures bone mineral mass and the volumetric BMD of the trabecular and cortical compartment (Targownik et al., 2017; Stathopoulos et al., 2016; Lauretani et al., 2006; Russo et al., 2003). A study by Maggio et al. investigated the relationship between the chronic use of PPIs and the cortical and trabecular BMD (vBMDc and vBMDt, respectively) in older individuals by using Tibial pQCT scans; their data showed a negative association between PPI use and vBMDt in a small sample of community-dwelling older persons (Marcello Maggio et al., 2013). These findings support the hypothesis of a possible direct effect of PPIs on bone mineral metabolism despite the no significant association between the use of PPIs and bone geometry.
Regardless of the contradictory results concerning the effect of PPIs on BMD, many systematic reviews revealed an association between PPI use and increased fracture incidence (Zhou et al., 2016; Elaine et al., 2011). Thus, taking PPIs for inappropriate indications or receiving PPI therapy without prescription should be discouraged. Additionally, the negative influence of PPIs on bone quality and bone mineral metabolism should be explored by further studies with larger sample sizes and longer durations, considering other possible confounding factors and different settings to resolve the dispute.
This meta-analysis has a number of remarkable strengths, such as the strict inclusion criteria that focused on only the relationship between PPI use and BMD change. Another strength is the inclusion of observational studies that were published with no language restrictions. Nevertheless, there are several limitations, some of which are the involvement of few observational studies due to the paucity of randomized controlled trials. Therefore, the possibility of residual confounding from various unmeasured variables cannot be excluded. Additionally, significant heterogeneity was present in the outcomes of the studies that provided the mean annualized percent change in BMD. This heterogeneity might be explained by a variety of exposure durations, types of PPI use, and the sex, mean age and sample size of the study populations. Accordingly, the results should be interpreted with caution to better explain the significant statistical and clinical heterogeneity among the studies, and the inability of observational studies to clarify whether the observed association is a causal effect or a result of unmeasured variables should be considered.
5. Conclusion
The literature reviewed that evaluated the risk of PPI use and BMD loss convey inconsistent results with BMD reduction among studies with mean differences and no BMD change among studies with annualized means. Therefore, well-conducted observational and randomized controlled trials are recommended to resolve the conflict. Moreover, healthcare providers should consider the potentially unfavorable effect of PPI use on bone health.
CRediT authorship contribution statement
Research idea by SA and SH. Comprehensive search, abstraction, selection of articles, and manuscript writing by SA and SH. Supervision and resolution of any conflicts in each step by MF.
Critical review and editing by SA and SH.
Funding
There was no funding source for this study.
Declaration of competing interest
We declare that there are no conflicts of interest, and we confirm that this manuscript has not been published elsewhere and is not under consideration by another journal.
References
- Abramowitz J., Thakkar P., Isa A., Truong A., Park C., Rosenfeld R.M. Adverse event reporting for proton pump inhibitor therapy: an overview of systematic reviews. Otolaryngol. Head Neck Surg. 2016;155(4):547–554. doi: 10.1177/0194599816648298. [DOI] [PubMed] [Google Scholar]
- Arj A., Razavi Zade M., Yavari M., Akbari H., Zamani B., Asemi Z. Proton pump inhibitors use and change in bone mineral density. Int. J. Rheum. Dis. 2016;19(9):864–868. doi: 10.1111/1756-185X.12866. [DOI] [PubMed] [Google Scholar]
- Bahtiri E., Islami H., Hoxha R., Qorraj-Bytyqi H., Rexhepi S., Hoti K. Esomeprazole use is independently associated with significant reduction of BMD: 1-year prospective comparative safety study of four proton pump inhibitors. J. Bone Miner. Metab. 2016;34(5):571–579. doi: 10.1007/s00774-015-0699-6. [DOI] [PubMed] [Google Scholar]
- Elaine W.Y., Blackwell T., Ensrud K.E., Hillier T.A., Lane N.E., Orwoll E. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif. Tissue Int. 2008;83(4):251–259. doi: 10.1007/s00223-008-9170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elaine W.Y., Bauer S.R., Bain P.A., Bauer D.C. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am. J. Med. 2011;124(6):519–526. doi: 10.1016/j.amjmed.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray S.L., LaCroix A.Z., Larson J., Robbins J., Cauley J.A., Manson J.E. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch. Intern. Med. 2010;170(9):765–771. doi: 10.1001/archinternmed.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelbaugh J.J. Proton pump inhibitors and risk of vitamin and mineral deficiency: evidence and clinical implications. Ther. Adv. Drug Saf. 2013;4(3):125–133. doi: 10.1177/2042098613482484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershcovici T., Fass R. An algorithm for diagnosis and treatment of refractory GERD. Best Pract. Res. Clin. Gastroenterol. 2010;24(6):923–936. doi: 10.1016/j.bpg.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Hussain S., Siddiqui A.N., Habib A., Hussain M.S., Najmi A.K. Proton pump inhibitors’ use and risk of hip fracture: a systematic review and meta-analysis. Rheumatol. Int. 2018;38(11):1999–2014. doi: 10.1007/s00296-018-4142-x. [DOI] [PubMed] [Google Scholar]
- Islam M., Poly T.N., Walther B.A., Dubey N.K., Anggraini Ningrum D.N., Shabbir S.-A. Adverse outcomes of long-term use of proton pump inhibitors: a systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018;30(12):1395–1405. doi: 10.1097/MEG.0000000000001198. [DOI] [PubMed] [Google Scholar]
- Ito T., Jensen R.T. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B 12, iron, and magnesium. Curr. Gastroenterol. Rep. 2010;12(6):448–457. doi: 10.1007/s11894-010-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D.A., Katz P.O., Armstrong D., Cohen H., Delaney B.C., Howden C.W. The safety of appropriate use of over-the-counter proton pump inhibitors: an evidence-based review and Delphi consensus. Drugs. 2017;77(5):547–561. doi: 10.1007/s40265-017-0712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Y.T., Ahmed N.N. Fracture risk and bone mineral density reduction associated with proton pump inhibitors. Pharmacotherapy. 2012;32(1):67–79. doi: 10.1002/PHAR.1007. [DOI] [PubMed] [Google Scholar]
- Lauretani F., Bandinelli S., Russo C., Maggio M., Di Iorio A., Cherubini A. Correlates of bone quality in older persons. Bone. 2006;39(4):915–921. doi: 10.1016/j.bone.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maléth J., Hegyi P. Long-term proton pump inhibitor therapy and osteoporosis. Is there a real danger? Orv. Hetil. 2013;154(26):1005–1009. doi: 10.1556/OH.2013.29656. [DOI] [PubMed] [Google Scholar]
- Marcello Maggio F.L., Ceda Gian Paolo, De Vita Francesca, Bondi Giuliana, Corsonello Andrea, Cattabiani Chiara, Lattanzio Fabrizia, Ruggiero Carmelinda, Nouvenne Antonio, Meschi Tiziana, Bandinelli Stefania, Ferrucci Luigi. Use of proton pump inhibitors is associated with lower trabecular bone density in older individuals. Bone. 2013;57:437–442. doi: 10.1016/j.bone.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Prisma Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa S., n.d. King Abdullah International Medical Research Center (KAIMRC). Saudi Arabia.
- Nassar Y., Richter S. Proton-pump inhibitor use and fracture risk: an updated systematic review and meta-analysis. J. Bone Metab. 2018;25(3):141–151. doi: 10.11005/jbm.2018.25.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehra A.K., Alexander J.A., Loftus C.G., Nehra V., editors. Mayo Clinic Proceedings. Elsevier; 2018. Proton pump inhibitors: review of emerging concerns. [DOI] [PubMed] [Google Scholar]
- Ozdil K., Kahraman R., Sahin A., Calhan T., Gozden E.H., Akyuz U. Bone density in proton pump inhibitors users: a prospective study. Rheumatol. Int. 2013;33(9):2255–2260. doi: 10.1007/s00296-013-2709-0. [DOI] [PubMed] [Google Scholar]
- Pezeshkian S., Conway S.E. Proton pump inhibitor use in older adults: long-term risks and steps for deprescribing. Consult. Pharm. 2018;33(9):497–503. doi: 10.4140/TCP.n.2018.497. [DOI] [PubMed] [Google Scholar]
- Review Manager (RevMan) The Nordic Cochrane Centre TCC; Copenhagen: 2014. [Computer program]. Version 5.3. [Google Scholar]
- Roux C., Goldstein J., Zhou X., Klemes A., Lindsay R. Vertebral fracture efficacy during risedronate therapy in patients using proton pump inhibitors. Osteoporos. Int. 2012;23(1):277–284. doi: 10.1007/s00198-011-1574-5. [DOI] [PubMed] [Google Scholar]
- Russo C., Lauretani F., Bandinelli S., Bartali B., Di Iorio A., Volpato S. Aging bone in men and women: beyond changes in bone mineral density. Osteoporos. Int. 2003;14(7):531–538. doi: 10.1007/s00198-002-1322-y. [DOI] [PubMed] [Google Scholar]
- Sandhu D.S., Fass R. Current trends in the management of gastroesophageal reflux disease. Gut Liver. 2018;12(1):7. doi: 10.5009/gnl16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino E., Marabotto E., Zentilin P., Furnari M., Bodini G., Pellegatta G. A safety review of proton pump inhibitors to treat acid-related digestive diseases. Expert Opin. Drug Saf. 2018;17(8):785–794. doi: 10.1080/14740338.2018.1497155. [DOI] [PubMed] [Google Scholar]
- Shin Y.H., Gong H.S., Baek G.H. Lower trabecular bone score is associated with the use of proton pump inhibitors. J. Clin. Densitom. 2019;22(2):236–242. doi: 10.1016/j.jocd.2018.06.008. [DOI] [PubMed] [Google Scholar]
- Solomon D.H., Diem S.J., Ruppert K., Lian Y.J., Liu C.C., Wohlfart A. Bone mineral density changes among women initiating proton pump inhibitors or H2 receptor antagonists: a SWAN cohort study. J. Bone Miner. Res. 2015;30(2):232–239. doi: 10.1002/jbmr.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos K.D., Zoubos A.B., Papaioannou N.A., Mastrokalos D., Galanos A., Mastrokalos D., Papagelopoulos P.J., Skarantavos G. Differences of bone mineral mass, volumetric bone mineral density, geometrical and structural parameters and derived strength of the tibia between premenopausal and postmenopausal women of different age groups: a peripheral Quantitative Computed Tomography (pQCT) study. J. Musculoskelet. Neuronal Interact. 2016;16(2):113–121. [PMC free article] [PubMed] [Google Scholar]
- Strand D.S., Kim D., Peura D.A. 25 years of proton pump inhibitors: a comprehensive review. Gut Liver. 2017;11(1):27. doi: 10.5009/gnl15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targownik L.E., Leslie W.D., Davison K.S., Goltzman D., Jamal S.A., Kreiger N. The relationship between proton pump inhibitor use and longitudinal change in bone mineral density: a population-based from the Canadian Multicentre Osteoporosis Study (CaMos) Am. J. Gastroenterol. 2012;107(9):1361. doi: 10.1038/ajg.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targownik L.E., Goertzen A.L., Luo Y., Leslie W.D. Long-term proton pump inhibitor use is not associated with changes in bone strength and structure. Am. J. Gastroenterol. 2017;112(1):95. doi: 10.1038/ajg.2016.481. [DOI] [PubMed] [Google Scholar]
- Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14(1):135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.-H., Li C.-H., Hsieh R., Fan C.-Y., Hsu T.-C., Chang W.-C. Proton pump inhibitors therapy and the risk of pneumonia: a systematic review and meta-analysis of randomized controlled trials and observational studies. Expert Opin. Drug Saf. 2019;18(3):163–172. doi: 10.1080/14740338.2019.1577820. [DOI] [PubMed] [Google Scholar]
- Wells G.A., Tugwell P., O’Connell D., Welch V., Peterson J., Shea B. 2015. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-analyses. [Google Scholar]
- Xuan J., Zhang Qingwen, Zhang Zhenyu, Liu Yulan. 2014. Risk Observation of Long-term Application of Proton Pump Inhibitors. [Google Scholar]
- Zhou B., Huang Y., Li H., Sun W., Liu J. Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporos. Int. 2016;27(1):339–347. doi: 10.1007/s00198-015-3365-x. [DOI] [PubMed] [Google Scholar]