Abstract
Malnutrition is highly prevalent in liver cirrhosis and its presence carries important prognostic implications. The clinical conditions and pathophysiological mechanisms that cause malnutrition in cirrhosis are multiple and interrelated. Anorexia and liver decompensation symptoms lead to poor dietary intake; metabolic changes characterised by elevated energy expenditure, reduced glycogen storage, an accelerated starvation response and protein catabolism result in muscle and fat wasting; and, malabsorption renders the cirrhotic patient unable to fully absorb or utilise food that has been consumed. Malnutrition is therefore a considerable challenge to manage effectively, particularly as liver disease progresses. A high energy, high protein diet is recognised as standard of care, yet patients struggle to follow this recommendation and there is limited evidence to guide malnutrition interventions in cirrhosis and liver transplantation. In this review, we seek to detail the factors which contribute to poor nutritional status in liver disease, and highlight complexities far greater than “poor appetite” or “reduced oral intake” leading to malnutrition. We also discuss management strategies to optimise nutritional status in this patient group, which target the inter-related mechanisms unique to advanced liver disease. Finally, future research requirements are suggested, to develop effective treatments for one of the most common and debilitating complications afflicting cirrhotic patients.
Keywords: Malnutrition, Cirrhosis, Liver transplantation, Chronic liver disease, Nutrition, Sarcopenia
Core Tip: Malnutrition is widespread in liver cirrhosis. This paper highlights the multifactorial aetiology of liver-related malnutrition, and details the complex challenges cirrhotic patients face in achieving nutritional targets. Although potentially modifiable, there are a scarcity of successful treatments hence the evidence base pertaining to nutritional interventions is surprisingly weak. Further research is required to bridge the gap between actual and ideal nutritional status in cirrhosis. If this goal can be realised, the potential impact on patient and clinical outcomes is immense.
INTRODUCTION
Malnutrition is common in chronic liver disease and increases as the severity of liver disease progresses. It affects up to 80% of patients with decompensated cirrhosis[1-3], and is more widespread than the traditionally recognised sequalae of advanced liver disease, namely hepatic encephalopathy (40%)[4], bleeding oesophageal varices (5%-15%)[5,6], refractory ascites (5%-10%)[7], spontaneous bacterial peritonitis (1.5%-3%)[8] and hepatocellular carcinoma (3%-5%)[9,10]. Compromised nutritional status occurs regardless of the cause of liver disease[11], though is reported most commonly in those with alcoholic cirrhosis and cholestatic liver disease[12,13].
The impact of malnutrition on patient outcomes is increasingly recognized. Malnutrition increases the incidence and severity of decompensation symptoms, contributes to compromised immune function, reduces muscle mass, decreases functional status and quality of life, delays wound healing and is associated with increased mortality[14-16]. Malnutrition is particularly associated with the development and severity of hepatic encephalopathy[17]. Malnourished patients also require prolonged mechanical ventilation and have longer length of stay in both the intensive care unit and hospital following liver transplant[18,19]; all of which translate to significantly increased healthcare costs. Optimising nutritional status in this patient population is therefore of critical importance.
Despite the known deleterious effects of malnutrition, successful strategies to counter its impact in cirrhosis are lacking. In theory, malnutrition is a modifiable element to target to improve the course of liver disease and patient outcomes, though is rarely achieved in practice. So why can’t patients identified with malnutrition simply eat more to improve their nutritional state? And why has food failed to provide the therapy desperately needed to treat malnutrition in cirrhosis? In this review, we aim to describe the factors contributing to liver-related malnutrition to highlight the challenges cirrhotic patients face in attaining nutritional targets. We also emphasize areas where data to support clinical recommendations are limited, with the goal of encouraging further research in the area.
MALNUTRITION IN CIRRHOSIS-ETIOLOGY
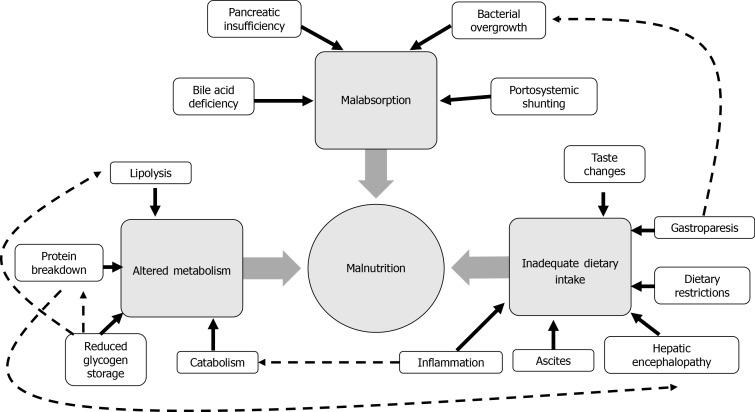
The development of malnutrition in cirrhosis is multifactorial, and primarily stems from inadequate dietary intake, altered metabolism and malabsorption (Figure 1). Patients may be afflicted with any or all of these etiological factors, which present unique barriers to effective nutritional support and management.
Figure 1.
Multifactorial aetiology of malnutrition in cirrhosis. Direct contributing factors represented by solid black arrows, inter-related factors represented by dashed black arrows.
Inadequate dietary intake
Reduced dietary intake plays a central role in the pathogenesis of malnutrition in cirrhosis. Whilst poor appetite may simply be ascribed to generalized ill-health; factors specific to liver disease play a significant role. Inflammation, early satiety from ascites, hepatic encephalopathy, adverse gastrointestinal symptoms, taste changes and unpalatable dietary restrictions all influence food consumption and their contribution to a negative energy balance needs to be appreciated to identify appropriate interventions to improve dietary intake.
Inflammation: Cirrhosis is a pro-inflammatory state primarily triggered by bacterial translocation from the gut to the circulation due to portal hypertension and increased intestinal permeability[20]. Systematic inflammation induces a range of brain-mediated responses including fever, anorexia and taste changes, with particular aversion to sweet flavours[21]. An increased production of cytokines ensues, which have demonstrated anorexigenic effects[20] and elevate energy expenditure[22].
Pro-inflammatory cytokines such as interleukin-1b, and stimulants that release cytokines (lipopolysaccharides), have been shown to reduce both the quantity and frequency of spontaneous food intake in humans and animals[23]. The precise mechanism by which pro-inflammatory factors act on the neural system to inhibit appetite is highly complex, involving different cell groups and neurotransmitters[24]. The function of inflammation-associated anorexia is to help sustain bodily functions in the face of infection and injury, and is observed during both acute and chronic inflammatory disease. Ultimately, energy is redistributed away from activities that are considered superfluous (i.e. food seeking and digestion), towards that required for mounting the immune response[24].
Ascites: Ascites is a common symptom of hepatic decompensation that directly impacts oral intake by limiting physical capacity of the stomach, and indirectly by contributing to post-prandial discomfort. Patients with refractory ascites have a high prevalence of malnutrition and characteristically demonstrate the lowest calorie intake of all patients with liver disease[25]. In addition to the effect of ascites on gastric reserve, depressed appetite and early satiety; repeated paracentesis causes significant nutrient losses. Not only does the body expend considerable energy to heat a large body of ascitic fluid, but ascitic fluid has considerable calorie content (in the form of proteins, carbohydrates and fats), and removal of this via large-volume paracentesis results in calorie debt[26]. Failure to replace this energy loss exacerbates the catabolic state already seen in advanced cirrhosis. Infusion of serum albumin after paracentesis is necessary to promote plasma volume expansion and prevent hyponatremia[1], though albumin replacement after ascitic drainage has no effect on nutritional status or repletion of protein stores[27].
Following paracentesis, patients generally report improvement in early satiety and an ability to consume larger meals and more calories[28,29]; changes which obviously correspond to increased gastric reserve. However, an increased calorie intake may be short-lived in refractory ascites as fluid reaccumulates, and translation to improved patient outcomes are not always realised[30,31]. Nutrition intervention trials in patients with refractory ascites that have successfully demonstrated enhancements in nutritional and clinical parameters have shared features of long duration of intervention, intensive dietetic input, and the requirement of artificial nutrition support (enteral nutrition, parenteral nutrition) to meet nutritional requirements[26,32].
Gastroparesis and autonomic dysfunction: Abdominal pain, nausea and bloating symptoms are not exclusive to those with ascites and are a frequent complaint in many cirrhotic patients[33,34]. Symptoms can sometimes be explained by the presence of organic disorders, however in many cirrhotic patients, no clear cause is apparent[35].
Distorted metabolic, hormonal and neural function may account for this global gut dysfunction in the absence of a specific diagnosis. Insulin resistance and subsequent elevated postprandial glucose have been shown to delay gastric emptying and lower spontaneous dietary intake in cirrhotic patients compared to healthy controls[36-38]. In addition to a high prevalence of gastroparesis in cirrhosis, small bowel motility may also be abnormal, and has been shown to be worse in those with portal hypertension manifesting in symptoms of diarrhoea and abdominal pain[39,40].
Autonomic dysfunction is also implicated in gastrointestinal symptom development, in a similar fashion to long-term autonomic and peripheral neuropathies observed in diabetes mellitus. Autonomic neuropathy encompassing both sympathetic upregulation and parasympathetic downregulation has been reported in 30%-70% of patients with cirrhosis, leading to “an effective vagotomy” and possibly accounting for gastric and intestinal dysmotility in these patients[35,41-43].
Despite the known association of gastrointestinal dysfunction with cirrhosis, the role of adverse gastrointestinal symptoms in directly limiting energy intake and thus contributing to weight loss and malnutrition has not been explored extensively. In a single study comparing the oral intake of 40 cirrhotic patients to controls, patients with significant gastrointestinal symptoms reached satiation earlier compared to patients without symptoms and healthy controls, resulting in significantly lower energy intake[44]. Another study by the same researchers demonstrated that severity of gastrointestinal symptoms were associated with recent weight loss and impaired health-related quality of life, which correlated with severity of liver disease[45].
Hunger hormones: Disrupted glucose and insulin metabolism also contribute to abnormal hormone levels that help control appetite and food intake. Leptin and ghrelin influence energy intake and expenditure[46], with the normal role of leptin involved in suppression of energy intake and accelerating energy expenditure; whilst ghrelin (the “hunger hormone”) increases before a meal to stimulate appetite and dietary intake.
In cirrhosis, levels of leptin and ghrelin are abnormal. Leptin is significantly elevated[36,47-49], translating to reduced food intake and increased resting energy expenditure. Although baseline ghrelin has not shown to be significantly different between cirrhotic subjects and controls[36,50], cirrhotic patients have an irregular pattern of ghrelin secretion compared to healthy controls[36]. In liver disease, ghrelin levels fail to rise pre-prandially, so the expected effect of ghrelin on increasing appetite and meal initiation is lost. This blunted ghrelin level is likely related to a combination of insulin resistance, elevated postprandial glucose, and overexpression of serum leptin[36,51,52].
Hepatic encephalopathy: Hepatic encephalopathy and sarcopenia are closely related to malnutrition, sharing common etiological factors and pathophysiological pathways all intrinsically linked to muscle health in liver disease. Skeletal muscle tissue plays a central role in removing ammonia from the circulation when its clearance by the liver is impaired. Thus in situations of muscle wasting, commonly precipitated by inadequate dietary intake and hyperammonemia itself in cirrhosis, the neuropsychiatric symptoms of encephalopathy are worsened[17,53].
The spectrum of neurocognitive impairment in cirrhosis ranges from minimal to overt hepatic encephalopathy, which manifest in variable degrees of impaired cognition, alertness and attentiveness[17]. This altered cognitive state, coupled with periods of increased (daytime) somnolence, limit the opportunity for cirrhotic patients with even low-grade encephalopathy to achieve an adequate dietary intake[54]. Forgetfulness, sleeping through meal and snack periods, and difficulty with meal preparation are significant barriers encountered in clinical nutrition practice with this patient group. Compliance with dietary therapies is also problematic[53] and patient management requires a multidisciplinary approach with reliance on patient supports and caregivers. The cycle of poor nutritional intake, leading to muscle loss and sarcopenia that worsens encephalopathy, which in turn exacerbates reduced dietary intake and malnutrition, is difficult to break.
Inappropriate dietary recommendations due to incorrect beliefs that protein restriction is necessary to improve encephalopathy have the potential to worsen malnutrition in this high-risk population. This strategy has no scientific merit though remains broadly practiced[55]. In a randomized trial by Córdoba et al[56], patients hospitalised with encephalopathy demonstrated no benefit of protein restriction in resolution of encephalopathy when compared to normal protein diet[56]. The study also showed even short-term protein restriction to 0.5 g/kg/d resulted in elevated muscle tissue breakdown. Recommendations have since evolved to promote a higher protein intake of 1.2-1.5 g/kg/d to prevent muscle wasting and reverse muscle loss in those who are sarcopenic[57].
Unpalatable diets: The recommendation to implement a sodium restriction is often the first dietary advice provided to patients with liver disease, due to the effect of sodium on fluid retention and subsequent development of peripheral oedema and ascites. International consensus guidelines recommend a dietary sodium restriction of < 2000 mg/d for management of ascites[27]. Unfortunately, limiting dietary sodium can negatively impact nutritional status. Many low-sodium foods are unpalatable, and advice to “remove all salt and processed foods” from the diet, without providing appropriate dietary education regarding nutritional adequacy has the potential to worsen malnutrition due to additional dietary constraints.
Sodium restriction alone will only eliminate ascites in approximately 10%-15% of patients[58], and some authors have shown no benefit to a sodium restricted diet when compared to an unrestricted diet in reducing ascites when diuretics were also administered[59]. A recent systematic review concluded increased calorie intake in conjunction with a low-sodium diet resulted in significantly improved outcomes[31]. Thus, advice to follow a low-salt diet should be provided by a dietitian experienced in the management of liver disease, to ensure overly restrictive diets are avoided.
Taste changes: Taste changes are another common complaint in cirrhosis, and generally manifest as a reduction in taste acuity for detection and recognition of some or all of the basic tastes of bitter, salt, sweet, and sour[60-64]. Reduced calorie intake has been demonstrated in patients suffering dysgeusia both with and without liver disease[60]. In addition to exacerbation of protein energy malnutrition in cirrhosis, impaired gustatory function has shown to adversely affect general wellbeing and quality of life by reducing pleasure associated with food intake[65].
Zinc and vitamin A are important nutrients involved in maintaining taste integrity, and are commonly deficient in cirrhosis[65]. Zinc is involved in the synthesis and activity of the salivary protein gustin, which plays a role at taste bud receptor sites[66] as well as being critical to a number of enzymatic processes and formation of structural proteins related to taste[67]. Vitamin A is required for the production of mucopolysaccharides in the epithelial cells of taste buds[65]. The literature regarding the association between zinc and vitamin A status with taste acuity in liver disease is variable though. Several authors failed to show any association between low serum zinc[61,62,65] or vitamin A[65] concentration and taste acuity. In contrast, two intervention trials observing the effect of long term zinc supplementation in cirrhosis demonstrated improved taste perception after supplementation, with a subsequent increase in serum zinc concentration[68,69]; whilst another group studying deficient patients supplemented with vitamin A resulted in both enhanced taste function and repletion of serum vitamin A levels[64].
Altered metabolism in cirrhosis
Increased energy expenditure, reduced synthesis of endogenous substrates, insulin resistance and low respiratory quotient characterize the metabolic disturbances common in patients with chronic liver disease.
Increased energy expenditure: Hypermetabolism, defined as measured resting energy expenditure (REE) > 20% above predicted REE, is often encountered in cirrhosis. Hypermetabolic patients are more often malnourished, have reduced lean body mass, and have reduced survival after liver transplant compared to those with normal metabolic rate[70,71]. Hypermetabolism in cirrhosis makes nutritional targets even more unattainable for this patient group, causing a negative impact on nutritional status. The prevalence of hypermetabolism ranges from 15% in a recent study of 268 New Zealand patients awaiting liver transplant[72], up to 34% in an early German study, which remains the largest to date with 473 cirrhotic patients considered for transplant[73].
Comorbidities typically associated with cirrhosis give rise to a theoretical explanation for elevated total energy expenditure in liver disease. These include hyperdynamic circulation (related to increased sympathetic nervous system activity), inflammation, frequent infections and ascites[74]. Pro-inflammatory cytokines induce lipolysis and protein breakdown to mobilize fatty acids and release amino acids, respectively, which are used in production of glucose by gluconeogenic organs (liver, kidney and intestine). The conversion of fat and protein to provide an available energy source in the form of glucose is an inefficient process and known to be expensive in terms of energy utilisation[57]. Compromised gut barrier function also promotes inflammation and development of cirrhotic complications, in particular bacterial infections, with sepsis a known contributor to increased energy expenditure[39]. Extracellular fluid (including ascites), is generally considered metabolically inactive as it does not consume oxygen or produce carbon dioxide. However in liver cirrhosis, some researchers have demonstrated a small but significant increase in metabolic rate prior to removal of ascitic fluid, compared with post-paracentesis energy requirement, potentially attributable to hemodynamic changes and the energy required to heat ascites[75].
The precise reason for hypermetabolism in cirrhosis is uncertain though, with conflicting etiology proposed by researchers. The New Zealand group found no relationship between hypermetabolism and patient gender, disease origin, protein depletion, ascites or severity of liver disease[72]. Conversely, other authors purport an inverse association between the severity of liver disease and resting energy expenditure[76], and presence of ascites with metabolic rate[75]. Given the inconsistency in clinical factors correlating with hypermetabolism in cirrhosis, simple identification of such patients is not feasible, and estimation of nutritional requirements via traditional methods (predictive equations) is known to be inaccurate in advanced cirrhosis[77,78]. Current international guidelines recommend measurement of resting metabolic rate via indirect calorimetry wherever possible[57,79].
Altered macronutrient metabolism: Cirrhosis also affects macronutrient metabolism and is a key factor in contributing to malnutrition in liver disease. The liver’s ability to store glycogen is markedly reduced. Hypoglycaemia occurs readily in the fasted state, and compromised glucose utilization due to insulin resistance is common. Impaired glycogen storage leads to early onset of gluconeogenesis; increasing the use of muscle glycogen, amino acid deamination, free fatty acid oxidation, and hepatic production of ketone bodies as an energy source[28,73,80,81].
This accelerated starvation response has been quantified by several authors. An early study by Schneeweiss et al[76] demonstrated the percentage of total calories derived from fat (86%), carbohydrate (2%) and protein (12%) were significantly different after an overnight 10-hour fast compared to healthy controls who metabolized 45%, 38% and 17% of fat, carbohydrates and protein, respectively[76]. This reduced storage capacity for glycogen means the fuel sources being used by cirrhotic patients after a 10-h fast are similar to that used after three days in an individual with a healthy liver[76,82]. A low respiratory quotient, indicating increased lipid and decreased glucose oxygenation, confirms this change in macronutrient utilisation[73]. The cascade effect of these metabolic alterations combined with poor dietary intake leads to loss of lean body mass and subcutaneous fat wasting. Thus, the prevention of long periods of fasting may reduce the muscle and fat loss commonly seen in cirrhosis. Intervention trials providing a late evening snack to individuals with liver disease support this rationale, with improved nitrogen balance and increased muscle mass demonstrated[82,83].
The metabolic switch of primary fuel source from glucose to amino acids and fatty acids is a prime characteristic of liver disease, and occurs even in the setting of only mild to moderate cholestasis. Increased protein requirements ensue, not only due to this increased rate of amino acid oxidation for gluconeogenesis, but also subsequent to decreased synthesis of protein, as well as protein losses in ascitic fluid and from the gastrointestinal tract[80,84,85]. The minimum protein required to maintain nitrogen homeostasis in healthy individuals is 0.8 g/kg/d[86]. Studies indicate that patients with cirrhosis only achieve positive nitrogen balance at a level of 1.23 g/kg/d, and are able to utilise protein up to 1.8 g/kg/d[87]. Normal to high protein intake does not precipitate hepatic encephalopathy, and thus a protein intake of 1.2-1.5 g/kg/d in cirrhosis is recommended by expert consensus[57,79] to prevent and/or reverse loss of muscle mass common in liver disease.
Lipid metabolism in cirrhosis is characterised by rapid oxidation in the fasted state, peripheral lipolysis and fat malabsorption. During fasting, lipids are oxidised as the preferred substrate as fat stores are mobilised, so plasma free fatty acids as well as glycerol and ketone bodies are increased. After a meal, suppression of lipid oxidation is not uniformly impaired, as would normally be the case with insulin release following a meal in healthy controls[88]. Lipolysis may be secondary to this insulin resistance but might also occur in an effort to provide cells with substrates for oxidation in the setting of dietary fat malabsorption.
Malabsorption in cirrhosis
Malabsorption is the final key contributor to negative energy balance and malnutrition in cirrhosis. Reduced bile flow in cholestatic patients decreases intestinal luminal bile salt availability and micelle formation, with subsequent malabsorption of fat and fat-soluble vitamins[89]. Pancreatic insufficiency may also be present and cause macronutrient maldigestion. Medications that alter the intestinal microbiota (e.g., antibiotics leading to decreased bacterial synthesis of short-chain fatty acids) or decrease bile acid availability (e.g., cholestyramine for pruritis) will also cause malabsorption of luminal nutrition, decreasing the amount of calories available for the body to use.
Malabsorption is further exacerbated by portal hypertensive enteropathy and subsequent changes in the gut microbiota that impair absorption and utilisation of nutrients[90,91]. Increased intestinal permeability and dysbiosis are common features linking the liver to a number of nutritional and gastrointestinal diseases[92]. Mucosal changes are frequently encountered upon endoscopic examination of the GI tract[93,94], where the prevalence of portal hypertensive gastropathy has been reported in 20%-98% of cirrhotic patients[95]. Major predictors of portal hypertensive gastropathy are the presence of oesophageal varices and increased severity of cirrhosis[96,97]. Gastroparesis and delayed gut transit mean that small bowel bacterial overgrowth is also common[98], and appears to be related to liver disease severity[99]. This further compounds dysbiosis and associated malabsorption.
NUTRITIONAL INTERVENTIONS IN CIRRHOSIS
Despite the high prevalence of malnutrition in decompensated liver disease and it’s known deleterious effects, successful strategies to counter malnutrition in cirrhosis are lacking. In theory, malnutrition is a modifiable syndrome to target that may impact the course of liver disease and would theoretically be expected to improve patient outcomes, although evidence to support this remains limited. Indeed, recent international guidelines counter whether malnutrition can be reversed at all in the face of deteriorating liver function[57].
Oral supplementation
A small number of studies have demonstrated dietary counselling and oral nutritional support can improve nutritional intake, nitrogen balance and selected patient outcomes[80,100-102]. Supplementation with high calorie and protein oral nutrition supplements in addition to dietary counselling improved anthropometric parameters and muscle function measured via handgrip strength[101,102], with reduction in hospitalisations also seen in one study[101]. No significant differences were observed with regards to liver function, clinical outcome (e.g., decompensation symptoms, infection rate) or mortality between intervention and control groups in any of the aforementioned studies. Recent meta-analysis on nutrition therapy in liver cirrhosis also failed to show any survival benefit with intervention, though studies included were heterogeneous in terms of severity of liver disease and duration of intervention[103,104].
A possible reason for the lack of demonstrated benefit observed in the literature relates to the marked discrepancy in patients nutritional intake vs that recommended by current international guidelines[57,79], with up to 75% of patients not achieving calorie targets[105], generally agreed to be around 32-35 kcal/kg/d. Dietary protein intake in cirrhotic patients is also found lacking. In a Canadian study of 631 patients awaiting transplantation, only 24% achieved the recommended target of > 1.2 g/kg/d, with 26% consuming a very low protein intake of < 0.8 g/kg/d, which resulted in a 2-fold increase in wait-list mortality[106].
Fortunately, the literature regarding inclusion of a late evening snack in cirrhosis is more conclusive. Cirrhotic patients who consume a late evening snack are able to demonstrate changes in substrate utilisation via increased respiratory quotient, indicating increased use of glucose similar to that of healthy controls, as well as an improvement in nitrogen balance[87,106]. This improvement in the pattern of fuel utilisation from inclusion of a late evening snack has also shown to translate to improved clinical outcomes in longer-term studies. In a comprehensive study by Plank et al[82], which followed 103 patients for 12-mo and compared outcomes from daytime vs nighttime consumption of a high calorie dietary supplement (710 kcal), significant improvements in total body protein accretion equivalent to 2 kg of lean tissue sustained over 12 mo were demonstrated in the nighttime supplementation group[82]. Hence shortening periods without food by including a late evening snack is considered a useful strategy to reverse protein catabolism and sarcopenia of cirrhosis. In addition to improved body composition, a meta-analysis concluded a late evening snack in cirrhosis also confers benefits on quality of life and survival, and reduction in frequency and severity of hepatic encephalopathy[83]. Poor patient compliance is an obstacle that still plagues implementation of a late evening snack however, with only half of the patients in the Plank et al[82] study able to consume the supplement at the prescribed amount and time[82], thus strategies to enhance patient compliance in clinical practice need to be considered.
Several randomized controlled trials have demonstrated the positive effects of oral BCAA supplementation in not only improving hepatic encephalopathy, but also nutritional status, liver function, quality of life and survival in malnourished cirrhotic patients[107-109]. Each of these trials were conducted in the outpatient setting and demonstrated good patient compliance with supplementation ranging between 14-30 g BCAA per day. A prolonged intervention period and follow up (> 12 mo) were also shared features, and likely provided sufficient time for these patients to increase their muscle mass and improve nutritional status, leading to superior ammonia clearance. A recent Cochrane review of 16 randomized trials confirmed the beneficial effect of BCAAs on hepatic encephalopathy symptoms, though found no such gains for survival, quality of life, or nutritional parameters[110].
Enteral nutrition
Consensus guidelines from international societies recommend enteral nutrition for such patients who are unable to achieve adequate dietary intake[57], though only a handful of investigations have actually evaluated enteral nutrition in patients with liver disease[111]. These studies are afflicted by either small sample size or limited duration of therapy. The study with the longest treatment duration was published over 30 years ago and involved a 60 d intervention with NG feeding[112] with subsequent gain of body weight as muscle, bone and fat stores (which only became evident after 30 d), though patient outcome data pertaining to liver disease was not described. The remaining studies all have an intervention period of 4 wk or less; two of which found those treated with EN had improved survival compared to oral diet[113,114], while another study of mean 2.8 wk NG feeding found no survival benefit[115]. However, given the relatively short duration of EN provision in all published literature, it is difficult to make any inferences about the effect of EN on short- to medium-term survival. It is currently unknown whether EN given over a longer period (e.g. > 8 wk) could reduce complications and improve clinical outcomes including mortality. It is also unknown if enteral nutrition support delivered in the pre-transplant period can affect post-liver transplant operative outcomes; as the aforementioned studies all exclude patients awaiting liver transplant.
Parenteral nutrition
The indication for parenteral nutrition (PN) support in cirrhosis is consistent with the recommendation in non-cirrhotic patients; those who cannot be fed orally or where enteral nutrition is either contraindicated (e.g. intestinal ileus, obstruction) or not tolerated, should be considered for parenteral nutrition[79]. ESPEN guidelines recommend PN be initiated immediately in cirrhotic patients with moderate to severe malnutrition who cannot receive adequate nutrition via the enteral route, on the basis of higher rates of complications and reduced survival in malnourished cirrhotic patients[54]. In situations where fasting is prolonged greater than 72 h, PN is also endorsed. Strict adherence to aseptic central line management is paramount in these patients, to minimise risk of infection and sepsis in this high risk group[79] (Table 1).
Table 1.
Strategies to treat malnutrition in cirrhosis
| Nutritional recommendations |
| Small, frequent meals and snacks (5-7 per day) |
| High calorie intake (≥ 32 kcal/kg/d) |
| High protein intake (1.2-1.5 g/kg/d) |
| Late evening snack containing protein and carbohydrate |
| Add oral nutrition supplements when unable to meet energy-protein requirements via ad-libitum dietary intake |
| Low sodium diet (≤ 2000 mg/d) if ascites or oedema present |
| Supplement with branched chain amino acids (25%-30% of total protein requirement) if hepatic encephalopathy or sarcopenia present, whilst ensuring overall protein intake meets requirements |
| Initiate enteral feeds (nasogastric) if unable to meet energy-protein needs via oral diet (polymeric, energy-dense formula). Consider nasojejunal tube if severe gastroparesis or intolerance of nasogastric feeds |
| Initiate parenteral nutrition if malnourished and enteral route either not accessible or unable to tolerate full energy-protein requirements |
Need for future studies
Several meta-analyses have found no convincing evidence that either oral feeding, or enteral or parenteral nutrition improved outcomes in patients with liver disease[103,104,116]. The most recent[104] included 13 randomized trials including 663 patients, and although fixed-effects analysis demonstrated that nutrition intervention prevented hepatic encephalopathy (0.73; 95%CI: 0.55-0.96) and infection (0.66; 95%CI: 0.45-0.98) the results were not confirmed in random-effects analysis. Included studies were particularly heterogeneous with the difference in daily calorie and protein intake ranging 10-fold between studies, and duration of therapy ranging from 3 to 365 d. An earlier meta-analysis of six trials and 470 patients in which the primary outcome measure was survival, found no reduction in mortality [RR: 0.75 (0.42-1.32), P = 0.31][116]. A Cochrane review from 2012 included 37 trials and most analyses failed to find any significant differences, with the conclusion that “data do not compellingly justify the routine use of parenteral, enteral or oral nutrition support in patients with liver disease”[103]. All authors reported a low quality of trials with an urgent need for data from well-designed and implemented randomized studies to inform future clinical practice.
Despite the low grade of evidence supporting nutritional recommendations in cirrhosis, it is well accepted that malnutrition is associated with a host of poor outcomes, and efforts to prevent its occurrence and progression should be prioritised. Given the limitations of current studies, there is an urgent need for large, well-designed trials with appropriate intervention and duration. Most importantly, analysis of clinical outcomes is required to determine whether improving malnutrition can have a lasting impact on pre- and post-liver transplant morbidity and mortality.
CONCLUSION
Malnutrition negatively impacts the course of patients with liver cirrhosis and has a complex, multifactorial etiology. It remains a potentially reversible prognostic marker in cirrhosis, but remains a challenging problem with little evidence to guide intervention. The presence of anorexia and other symptoms leading to poor oral intake, the catabolic nature of the disease process, and underlying metabolic changes leads to significant difficulties in adequately meeting the nutritional needs of cirrhotic patients. Based on the published literature, it appears these barriers are often insurmountable. Many patients cannot achieve a stable anabolic state with a dietary intake far distant from what is recommended. There is an urgent need for further large-scale research to provide evidence for many aspects of the appropriate nutrition management for patients with cirrhosis, and indeed determine whether artificial nutrition support is able to bridge the nutrition gap so often encountered in clinical practice. A focus on patient reported outcomes will also enhance compliance with dietary therapies and analysis of clinical endpoints is critical.
Footnotes
Conflict-of-interest statement: Nothing to disclose.
Manuscript source: Invited manuscript
Peer-review started: June 16, 2020
First decision: September 24, 2020
Article in press: October 20, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kaya M, Kim SE S-Editor: Zhang L L-Editor: A P-Editor: Wang LL
Contributor Information
Brooke Chapman, Nutrition and Dietetics Department, Austin Health, Heidelberg 3084, Australia. brooke.chapman@austin.org.au.
Marie Sinclair, Liver Transplant Unit, Austin Health, Heidelberg 3084, Australia.
Paul J Gow, Liver Transplant Unit, Austin Health, Heidelberg 3084, Australia.
Adam G Testro, Liver Transplant Unit, Austin Health, Heidelberg 3084, Australia.
References
- 1.Johnson TM, Overgard EB, Cohen AE, DiBaise JK. Nutrition assessment and management in advanced liver disease. Nutr Clin Pract. 2013;28:15–29. doi: 10.1177/0884533612469027. [DOI] [PubMed] [Google Scholar]
- 2.Cheung K, Lee SS, Raman M. Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clin Gastroenterol Hepatol. 2012;10:117–125. doi: 10.1016/j.cgh.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Tsiaousi ET, Hatzitolios AI, Trygonis SK, Savopoulos CG. Malnutrition in end stage liver disease: recommendations and nutritional support. J Gastroenterol Hepatol. 2008;23:527–533. doi: 10.1111/j.1440-1746.2008.05369.x. [DOI] [PubMed] [Google Scholar]
- 4.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–938. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 6.North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319:983–989. doi: 10.1056/NEJM198810133191505. [DOI] [PubMed] [Google Scholar]
- 7.Ginés P, Quintero E, Arroyo V, Terés J, Bruguera M, Rimola A, Caballería J, Rodés J, Rozman C. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7:122–128. doi: 10.1002/hep.1840070124. [DOI] [PubMed] [Google Scholar]
- 8.Evans LT, Kim WR, Poterucha JJ, Kamath PS. Spontaneous bacterial peritonitis in asymptomatic outpatients with cirrhotic ascites. Hepatology. 2003;37:897–901. doi: 10.1053/jhep.2003.50119. [DOI] [PubMed] [Google Scholar]
- 9.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 10.Thein HH, Isaranuwatchai W, Campitelli MA, Feld JJ, Yoshida E, Sherman M, Hoch JS, Peacock S, Krahn MD, Earle CC. Health care costs associated with hepatocellular carcinoma: a population-based study. Hepatology. 2013;58:1375–1384. doi: 10.1002/hep.26231. [DOI] [PubMed] [Google Scholar]
- 11.Montano-Loza AJ. New concepts in liver cirrhosis: clinical significance of sarcopenia in cirrhotic patients. Minerva Gastroenterol Dietol. 2013;59:173–186. [PubMed] [Google Scholar]
- 12.Caly WR, Strauss E, Carrilho FJ, Laudanna AA. Different degrees of malnutrition and immunological alterations according to the aetiology of cirrhosis: a prospective and sequential study. Nutr J. 2003;2:10. doi: 10.1186/1475-2891-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thuluvath PJ, Triger DR. Evaluation of nutritional status by using anthropometry in adults with alcoholic and nonalcoholic liver disease. Am J Clin Nutr. 1994;60:269–273. doi: 10.1093/ajcn/60.2.269. [DOI] [PubMed] [Google Scholar]
- 14.Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16:95–131. doi: 10.1016/j.cld.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maharshi S, Sharma BC, Srivastava S. Malnutrition in cirrhosis increases morbidity and mortality. J Gastroenterol Hepatol. 2015;30:1507–1513. doi: 10.1111/jgh.12999. [DOI] [PubMed] [Google Scholar]
- 16.Møller S, Bendtsen F, Christensen E, Henriksen JH. Prognostic variables in patients with cirrhosis and oesophageal varices without prior bleeding. J Hepatol. 1994;21:940–946. doi: 10.1016/s0168-8278(05)80599-9. [DOI] [PubMed] [Google Scholar]
- 17.Bajaj JS, Lauridsen M, Tapper EB, Duarte-Rojo A, Rahimi RS, Tandon P, Shawcross DL, Thabut D, Dhiman RK, Romero-Gomez M, Sharma BC, Montagnese S. Important Unresolved Questions in the Management of Hepatic Encephalopathy: An ISHEN Consensus. Am J Gastroenterol. :2020. doi: 10.14309/ajg.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 18.Pikul J, Sharpe MD, Lowndes R, Ghent CN. Degree of preoperative malnutrition is predictive of postoperative morbidity and mortality in liver transplant recipients. Transplantation. 1994;57:469–472. doi: 10.1097/00007890-199402150-00030. [DOI] [PubMed] [Google Scholar]
- 19.Harrison J, McKiernan J, Neuberger JM. A prospective study on the effect of recipient nutritional status on outcome in liver transplantation. Transpl Int. 1997;10:369–374. doi: 10.1007/s001470050072. [DOI] [PubMed] [Google Scholar]
- 20.Dirchwolf M, Ruf AE. Role of systemic inflammation in cirrhosis: From pathogenesis to prognosis. World J Hepatol. 2015;7:1974–1981. doi: 10.4254/wjh.v7.i16.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nilsson A, Wilhelms DB, Mirrasekhian E, Jaarola M, Blomqvist A, Engblom D. Inflammation-induced anorexia and fever are elicited by distinct prostaglandin dependent mechanisms, whereas conditioned taste aversion is prostaglandin independent. Brain Behav Immun. 2017;61:236–243. doi: 10.1016/j.bbi.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Ye J. Regulation of energy balance by inflammation: common theme in physiology and pathology. Rev Endocr Metab Disord. 2015;16:47–54. doi: 10.1007/s11154-014-9306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langhans W, Savoldelli D, Weingarten S. Comparison of the feeding responses to bacterial lipopolysaccharide and interleukin-1 beta. Physiol Behav. 1993;53:643–649. doi: 10.1016/0031-9384(93)90168-f. [DOI] [PubMed] [Google Scholar]
- 24.Gautron L, Layé S. Neurobiology of inflammation-associated anorexia. Front Neurosci. 2009;3:59. doi: 10.3389/neuro.23.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campillo B, Richardet JP, Scherman E, Bories PN. Evaluation of nutritional practice in hospitalized cirrhotic patients: results of a prospective study. Nutrition. 2003;19:515–521. doi: 10.1016/s0899-9007(02)01071-7. [DOI] [PubMed] [Google Scholar]
- 26.Sorrentino P, Castaldo G, Tarantino L, Bracigliano A, Perrella A, Perrella O, Fiorentino F, Vecchione R, D' Angelo S. Preservation of nutritional-status in patients with refractory ascites due to hepatic cirrhosis who are undergoing repeated paracentesis. J Gastroenterol Hepatol. 2012;27:813–822. doi: 10.1111/j.1440-1746.2011.07043.x. [DOI] [PubMed] [Google Scholar]
- 27.Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut. 2006;55 Suppl 6:vi1–v12. doi: 10.1136/gut.2006.099580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aqel BA, Scolapio JS, Dickson RC, Burton DD, Bouras EP. Contribution of ascites to impaired gastric function and nutritional intake in patients with cirrhosis and ascites. Clin Gastroenterol Hepatol. 2005;3:1095–1100. doi: 10.1016/s1542-3565(05)00531-8. [DOI] [PubMed] [Google Scholar]
- 29.Scolapio JS, Ukleja A, McGreevy K, Burnett OL, O'Brien PC. Nutritional problems in end-stage liver disease: contribution of impaired gastric emptying and ascites. J Clin Gastroenterol. 2002;34:89–93. doi: 10.1097/00004836-200201000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Harper C. How interdisciplinary documentation improves the bottom line. Rehabil Nurs. 2007;32:91–92, 111. doi: 10.1002/j.2048-7940.2007.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 31.Baki J, Brown P, Tapper EB. Do Nutritional Interventions Improve the Outcomes of Patients with Cirrhosis and Ascites: a Systematic Review of Randomized Trials. Curr Hepatol Rep . 2020;19: :71–77. doi: 10.1007/s11901-020-00513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vidot H, Bowen DG, Carey S, McCaughan GW, Allman-Farinelli M, Shackel NA. Aggressive nutrition intervention reduces ascites and frequency of paracentesis in malnourished patients with cirrhosis and ascites. JGH Open. 2017;1:92–97. doi: 10.1002/jgh3.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riley TR 3rd, Chinchilli VM, Shoemaker M, Koch K. Is nausea associated with chronic hepatitis C infection? Am J Gastroenterol. 2001;96:3356–3360. doi: 10.1111/j.1572-0241.2001.05337.x. [DOI] [PubMed] [Google Scholar]
- 34.Fritz E, Hammer J. Gastrointestinal symptoms in patients with liver cirrhosis are linked to impaired quality of life and psychological distress. Eur J Gastroenterol Hepatol. 2009;21:370–375. doi: 10.1097/MEG.0b013e328318ed19. [DOI] [PubMed] [Google Scholar]
- 35.Verne GN, Soldevia-Pico C, Robinson ME, Spicer KM, Reuben A. Autonomic dysfunction and gastroparesis in cirrhosis. J Clin Gastroenterol. 2004;38:72–76. doi: 10.1097/00004836-200401000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Kalaitzakis E, Bosaeus I, Ohman L, Björnsson E. Altered postprandial glucose, insulin, leptin, and ghrelin in liver cirrhosis: correlations with energy intake and resting energy expenditure. Am J Clin Nutr. 2007;85:808–815. doi: 10.1093/ajcn/85.3.808. [DOI] [PubMed] [Google Scholar]
- 37.Greco AV, Mingrone G, Benedetti G, Capristo E, Tataranni PA, Gasbarrini G. Daily energy and substrate metabolism in patients with cirrhosis. Hepatology. 1998;27:346–350. doi: 10.1002/hep.510270205. [DOI] [PubMed] [Google Scholar]
- 38.Richardson RA, Davidson HI, Hinds A, Cowan S, Rae P, Garden OJ. Influence of the metabolic sequelae of liver cirrhosis on nutritional intake. Am J Clin Nutr. 1999;69:331–337. doi: 10.1093/ajcn/69.2.331. [DOI] [PubMed] [Google Scholar]
- 39.Kalaitzakis E. Gastrointestinal dysfunction in liver cirrhosis. World J Gastroenterol. 2014;20:14686–14695. doi: 10.3748/wjg.v20.i40.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalaitzakis E, Sadik R, Holst JJ, Ohman L, Björnsson E. Gut transit is associated with gastrointestinal symptoms and gut hormone profile in patients with cirrhosis. Clin Gastroenterol Hepatol. 2009;7:346–352. doi: 10.1016/j.cgh.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 41.Hendrickse MT, Thuluvath PJ, Triger DR. Natural history of autonomic neuropathy in chronic liver disease. Lancet. 1992;339:1462–1464. doi: 10.1016/0140-6736(92)92042-e. [DOI] [PubMed] [Google Scholar]
- 42.Trevisani F, Sica G, Bernardi M. Autonomic neuropathy in advanced liver disease. Hepatology. 1996;24:1549. doi: 10.1053/jhep.1996.v24.ajhep0241549. [DOI] [PubMed] [Google Scholar]
- 43.Gumurdulu Y, Yapar Z, Canataroglu A, Serin E, Gumurdulu D, Kibar M, Colakoglu S. Gastric emptying time and the effect of cisapride in cirrhotic patients with autonomic neuropathy. J Clin Gastroenterol. 2003;36:175–178. doi: 10.1097/00004836-200302000-00017. [DOI] [PubMed] [Google Scholar]
- 44.Hartshorn KL, Karnad AB, Tauber AI. Influenza A virus and the neutrophil: a model of natural immunity. J Leukoc Biol. 1990;47:176–186. doi: 10.1002/jlb.47.2.176. [DOI] [PubMed] [Google Scholar]
- 45.Kalaitzakis E, Simrén M, Olsson R, Henfridsson P, Hugosson I, Bengtsson M, Björnsson E. Gastrointestinal symptoms in patients with liver cirrhosis: associations with nutritional status and health-related quality of life. Scand J Gastroenterol. 2006;41:1464–1472. doi: 10.1080/00365520600825117. [DOI] [PubMed] [Google Scholar]
- 46.Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50:1511–1525. doi: 10.1373/clinchem.2004.032482. [DOI] [PubMed] [Google Scholar]
- 47.Ockenga J, Bischoff SC, Tillmann HL, Rifai K, Widjaja A, Böker KH, Manns MP, Brabant G. Elevated bound leptin correlates with energy expenditure in cirrhotics. Gastroenterology. 2000;119:1656–1662. doi: 10.1053/gast.2000.20256. [DOI] [PubMed] [Google Scholar]
- 48.Testa R, Franceschini R, Giannini E, Cataldi A, Botta F, Fasoli A, Tenerelli P, Rolandi E, Barreca T. Serum leptin levels in patients with viral chronic hepatitis or liver cirrhosis. J Hepatol. 2000;33:33–37. doi: 10.1016/s0168-8278(00)80156-7. [DOI] [PubMed] [Google Scholar]
- 49.Ben-Ari Z, Schafer Z, Sulkes J, Manhaim V, Tur-Kaspa R, Fainaru M. Alterations in serum leptin in chronic liver disease. Dig Dis Sci. 2002;47:183–189. doi: 10.1023/a:1013248427783. [DOI] [PubMed] [Google Scholar]
- 50.Marchesini G, Bianchi G, Lucidi P, Villanova N, Zoli M, De Feo P. Plasma ghrelin concentrations, food intake, and anorexia in liver failure. J Clin Endocrinol Metab. 2004;89:2136–2141. doi: 10.1210/jc.2003-031771. [DOI] [PubMed] [Google Scholar]
- 51.Murdolo G, Lucidi P, Di Loreto C, Parlanti N, De Cicco A, Fatone C, Fanelli CG, Bolli GB, Santeusanio F, De Feo P. Insulin is required for prandial ghrelin suppression in humans. Diabetes. 2003;52:2923–2927. doi: 10.2337/diabetes.52.12.2923. [DOI] [PubMed] [Google Scholar]
- 52.Blom WA, Stafleu A, de Graaf C, Kok FJ, Schaafsma G, Hendriks HF. Ghrelin response to carbohydrate-enriched breakfast is related to insulin. Am J Clin Nutr. 2005;81:367–375. doi: 10.1093/ajcn.81.2.367. [DOI] [PubMed] [Google Scholar]
- 53.Amodio P, Bemeur C, Butterworth R, Cordoba J, Kato A, Montagnese S, Uribe M, Vilstrup H, Morgan MY. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology. 2013;58:325–336. doi: 10.1002/hep.26370. [DOI] [PubMed] [Google Scholar]
- 54.Plauth M, Cabré E, Campillo B, Kondrup J, Marchesini G, Schütz T, Shenkin A, Wendon J ESPEN. ESPEN Guidelines on Parenteral Nutrition: hepatology. Clin Nutr. 2009;28:436–444. doi: 10.1016/j.clnu.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 55.Kachaamy T, Bajaj JS. Diet and cognition in chronic liver disease. Curr Opin Gastroenterol. 2011;27:174–179. doi: 10.1097/MOG.0b013e3283409c25. [DOI] [PubMed] [Google Scholar]
- 56.Córdoba J, López-Hellín J, Planas M, Sabín P, Sanpedro F, Castro F, Esteban R, Guardia J. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41:38–43. doi: 10.1016/j.jhep.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 57.European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172–193. doi: 10.1016/j.jhep.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eghtesad S, Poustchi H, Malekzadeh R. Malnutrition in liver cirrhosis:the influence of protein and sodium. Middle East J Dig Dis. 2013;5:65–75. [PMC free article] [PubMed] [Google Scholar]
- 59.Reynolds TB, Lieberman FL, Goodman AR. Advantages of treatment of ascites without sodium restriction and without complete removal of excess fluid. Gut. 1978;19:549–553. doi: 10.1136/gut.19.6.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mattes-Kulig DA, Henkin RI. Energy and nutrient consumption of patients with dysgeusia. J Am Diet Assoc. 1985;85:822–826. [PubMed] [Google Scholar]
- 61.Smith FR, Henkin RI, Dell RB. Disordered gustatory acuity in liver disease. Gastroenterology. 1976;70:568–571. [PubMed] [Google Scholar]
- 62.Sturniolo GC, D'Incà R, Parisi G, Giacomazzi F, Montino MC, D'Odorico A, Soranzo P, Naccarato R. Taste alterations in liver cirrhosis: are they related to zinc deficiency? J Trace Elem Electrolytes Health Dis. 1992;6:15–19. [PubMed] [Google Scholar]
- 63.Deems RO, Friedman MI, Friedman LS, Munoz SJ, Maddrey WC. Chemosensory function, food preferences and appetite in human liver disease. Appetite. 1993;20:209–216. doi: 10.1006/appe.1993.1021. [DOI] [PubMed] [Google Scholar]
- 64.Garrett-Laster M, Russell RM, Jacques PF. Impairment of taste and olfaction in patients with cirrhosis: the role of vitamin A. Hum Nutr Clin Nutr. 1984;38:203–214. [PubMed] [Google Scholar]
- 65.Madden AM, Bradbury W, Morgan MY. Taste perception in cirrhosis: its relationship to circulating micronutrients and food preferences. Hepatology. 1997;26:40–48. doi: 10.1002/hep.510260106. [DOI] [PubMed] [Google Scholar]
- 66.Henkin RI. Drug-induced taste and smell disorders. Incidence, mechanisms and management related primarily to treatment of sensory receptor dysfunction. Drug Saf. 1994;11:318–377. doi: 10.2165/00002018-199411050-00004. [DOI] [PubMed] [Google Scholar]
- 67.Fernandes SA, Bona S, Cerski CT, Marroni NP, Marroni CA. ALTERATION OF TASTE BUDS IN EXPERIMENTAL CIRRHOSIS. Is there correlation with human hypogeusia? Arq Gastroenterol. 2016;53:278–284. doi: 10.1590/S0004-28032016000400013. [DOI] [PubMed] [Google Scholar]
- 68.Weismann K, Christensen E, Dreyer V. Zinc supplementation in alcoholic cirrhosis. A double-blind clinical trial. Acta Med Scand. 1979;205:361–366. doi: 10.1111/j.0954-6820.1979.tb06065.x. [DOI] [PubMed] [Google Scholar]
- 69.Scholmerich J, Langenberger G, Steiert B, Lohle E, Wokalat H, Tauber R, Kottgen E, Gerok W. Long term zinc substitution in patients with zinc deficiency in liver cirrhosis - results of a placebo controlled trial [Abstract] Gastroenterology. 1986;90:1765. [Google Scholar]
- 70.Selberg O, Böttcher J, Tusch G, Pichlmayr R, Henkel E, Müller MJ. Identification of high- and low-risk patients before liver transplantation: a prospective cohort study of nutritional and metabolic parameters in 150 patients. Hepatology. 1997;25:652–657. doi: 10.1002/hep.510250327. [DOI] [PubMed] [Google Scholar]
- 71.Mathur S, Peng S, Gane EJ, McCall JL, Plank LD. Hypermetabolism predicts reduced transplant-free survival independent of MELD and Child-Pugh scores in liver cirrhosis. Nutrition. 2007;23:398–403. doi: 10.1016/j.nut.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 72.Peng S, Plank LD, McCall JL, Gillanders LK, McIlroy K, Gane EJ. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr. 2007;85:1257–1266. doi: 10.1093/ajcn/85.5.1257. [DOI] [PubMed] [Google Scholar]
- 73.Müller MJ, Böttcher J, Selberg O, Weselmann S, Böker KH, Schwarze M, von zur Mühlen A, Manns MP. Hypermetabolism in clinically stable patients with liver cirrhosis. Am J Clin Nutr. 1999;69:1194–1201. doi: 10.1093/ajcn/69.6.1194. [DOI] [PubMed] [Google Scholar]
- 74.Cabré E, Abad-Lacruz A, Núñez MC, González-Huix F, Fernández-Bañares F, Gïl A, Esteve-Comas M, Moreno J, Planas R, Guilera M. The relationship of plasma polyunsaturated fatty acid deficiency with survival in advanced liver cirrhosis: multivariate analysis. Am J Gastroenterol. 1993;88:718–722. [PubMed] [Google Scholar]
- 75.Dolz C, Raurich JM, Ibáñez J, Obrador A, Marsé P, Gayá J. Ascites increases the resting energy expenditure in liver cirrhosis. Gastroenterology. 1991;100:738–744. doi: 10.1016/0016-5085(91)80019-6. [DOI] [PubMed] [Google Scholar]
- 76.Schneeweiss B, Graninger W, Ferenci P, Eichinger S, Grimm G, Schneider B, Laggner AN, Lenz K, Kleinberger G. Energy metabolism in patients with acute and chronic liver disease. Hepatology. 1990;11:387–393. doi: 10.1002/hep.1840110309. [DOI] [PubMed] [Google Scholar]
- 77.Glass C, Hipskind P, Cole D, Lopez R, Dasarathy S. Handheld calorimeter is a valid instrument to quantify resting energy expenditure in hospitalized cirrhotic patients: a prospective study. Nutr Clin Pract. 2012;27:677–688. doi: 10.1177/0884533612446195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Madden AM, Morgan MY. Resting energy expenditure should be measured in patients with cirrhosis, not predicted. Hepatology. 1999;30:655–664. doi: 10.1002/hep.510300326. [DOI] [PubMed] [Google Scholar]
- 79.Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Bischoff SC. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38:485–521. doi: 10.1016/j.clnu.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Norman K, Valentini L, Lochs H, Pirlich M. [Protein catabolism and malnutrition in liver cirrhosis - impact of oral nutritional therapy] Z Gastroenterol. 2010;48:763–770. doi: 10.1055/s-0029-1245388. [DOI] [PubMed] [Google Scholar]
- 81.Owen OE, Reichle FA, Mozzoli MA, Kreulen T, Patel MS, Elfenbein IB, Golsorkhi M, Chang KH, Rao NS, Sue HS, Boden G. Hepatic, gut, and renal substrate flux rates in patients with hepatic cirrhosis. J Clin Invest. 1981;68:240–252. doi: 10.1172/JCI110240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Plank LD, Gane EJ, Peng S, Muthu C, Mathur S, Gillanders L, McIlroy K, Donaghy AJ, McCall JL. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology. 2008;48:557–566. doi: 10.1002/hep.22367. [DOI] [PubMed] [Google Scholar]
- 83.Tsien CD, McCullough AJ, Dasarathy S. Late evening snack: exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol. 2012;27:430–441. doi: 10.1111/j.1440-1746.2011.06951.x. [DOI] [PubMed] [Google Scholar]
- 84.McCullough AJ, Tavill AS. Disordered energy and protein metabolism in liver disease. Semin Liver Dis. 1991;11:265–277. doi: 10.1055/s-2008-1040445. [DOI] [PubMed] [Google Scholar]
- 85.Swart GR, van den Berg JW, Wattimena JL, Rietveld T, van Vuure JK, Frenkel M. Elevated protein requirements in cirrhosis of the liver investigated by whole body protein turnover studies. Clin Sci (Lond) 1988;75:101–107. doi: 10.1042/cs0750101. [DOI] [PubMed] [Google Scholar]
- 86.Rand WM, Pellett PL, Young VR. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am J Clin Nutr. 2003;77:109–127. doi: 10.1093/ajcn/77.1.109. [DOI] [PubMed] [Google Scholar]
- 87.Swart GR, van den Berg JW, van Vuure JK, Rietveld T, Wattimena DL, Frenkel M. Minimum protein requirements in liver cirrhosis determined by nitrogen balance measurements at three levels of protein intake. Clin Nutr. 1989;8:329–336. doi: 10.1016/0261-5614(89)90008-3. [DOI] [PubMed] [Google Scholar]
- 88.Petrides AS, Groop LC, Riely CA, DeFronzo RA. Effect of physiologic hyperinsulinemia on glucose and lipid metabolism in cirrhosis. J Clin Invest. 1991;88:561–570. doi: 10.1172/JCI115340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Romiti A, Merli M, Martorano M, Parrilli G, Martino F, Riggio O, Truscelli A, Capocaccia L, Budillon G. Malabsorption and nutritional abnormalities in patients with liver cirrhosis. Ital J Gastroenterol. 1990;22:118–123. [PubMed] [Google Scholar]
- 90.Quigley EM, Stanton C, Murphy EF. The gut microbiota and the liver. Pathophysiological and clinical implications. J Hepatol. 2013;58:1020–1027. doi: 10.1016/j.jhep.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 91.Quigley EM. Gastrointestinal dysfunction in liver disease and portal hypertension. Gut-liver interactions revisited. Dig Dis Sci. 1996;41:557–561. doi: 10.1007/BF02282341. [DOI] [PubMed] [Google Scholar]
- 92.Pijls KE, Jonkers DM, Elamin EE, Masclee AA, Koek GH. Intestinal epithelial barrier function in liver cirrhosis: an extensive review of the literature. Liver Int. 2013;33:1457–1469. doi: 10.1111/liv.12271. [DOI] [PubMed] [Google Scholar]
- 93.Viggiano TR, Gostout CJ. Portal hypertensive intestinal vasculopathy: a review of the clinical, endoscopic, and histopathologic features. Am J Gastroenterol. 1992;87:944–954. [PubMed] [Google Scholar]
- 94.Hosking SW. Congestive gastropathy in portal hypertension: variations in prevalence. Hepatology. 1989;10:257–258. doi: 10.1002/hep.1840100223. [DOI] [PubMed] [Google Scholar]
- 95.Cubillas R, Rockey DC. Portal hypertensive gastropathy: a review. Liver Int. 2010;30:1094–1102. doi: 10.1111/j.1478-3231.2010.02286.x. [DOI] [PubMed] [Google Scholar]
- 96.Merli M, Nicolini G, Angeloni S, Gentili F, Attili AF, Riggio O. The natural history of portal hypertensive gastropathy in patients with liver cirrhosis and mild portal hypertension. Am J Gastroenterol. 2004;99:1959–1965. doi: 10.1111/j.1572-0241.2004.40246.x. [DOI] [PubMed] [Google Scholar]
- 97.Kim MY, Choi H, Baik SK, Yea CJ, Won CS, Byun JW, Park SY, Kwon YH, Kim JW, Kim HS, Kwon SO, Kim YJ, Cha SH, Chang SJ. Portal hypertensive gastropathy: correlation with portal hypertension and prognosis in cirrhosis. Dig Dis Sci. 2010;55:3561–3567. doi: 10.1007/s10620-010-1221-6. [DOI] [PubMed] [Google Scholar]
- 98.Chang CS, Chen GH, Lien HC, Yeh HZ. Small intestine dysmotility and bacterial overgrowth in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 1998;28:1187–1190. doi: 10.1002/hep.510280504. [DOI] [PubMed] [Google Scholar]
- 99.Pande C, Kumar A, Sarin SK. Small-intestinal bacterial overgrowth in cirrhosis is related to the severity of liver disease. Aliment Pharmacol Ther. 2009;29:1273–1281. doi: 10.1111/j.1365-2036.2009.03994.x. [DOI] [PubMed] [Google Scholar]
- 100.Iwasa M, Iwata K, Hara N, Hattori A, Ishidome M, Sekoguchi-Fujikawa N, Mifuji-Moroka R, Sugimoto R, Fujita N, Kobayashi Y, Takei Y. Nutrition therapy using a multidisciplinary team improves survival rates in patients with liver cirrhosis. Nutrition. 2013;29:1418–1421. doi: 10.1016/j.nut.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 101.Hirsch S, Bunout D, de la Maza P, Iturriaga H, Petermann M, Icazar G, Gattas V, Ugarte G. Controlled trial on nutrition supplementation in outpatients with symptomatic alcoholic cirrhosis. JPEN J Parenter Enteral Nutr. 1993;17:119–124. doi: 10.1177/0148607193017002119. [DOI] [PubMed] [Google Scholar]
- 102.Le Cornu KA, McKiernan FJ, Kapadia SA, Neuberger JM. A prospective randomized study of preoperative nutritional supplementation in patients awaiting elective orthotopic liver transplantation. Transplantation. 2000;69:1364–1369. doi: 10.1097/00007890-200004150-00026. [DOI] [PubMed] [Google Scholar]
- 103.Koretz RL, Avenell A, Lipman TO. Nutritional support for liver disease. Cochrane Database Syst Rev. :2012: CD008344. doi: 10.1002/14651858.CD008344.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fialla AD, Israelsen M, Hamberg O, Krag A, Gluud LL. Nutritional therapy in cirrhosis or alcoholic hepatitis: a systematic review and meta-analysis. Liver Int. 2015;35:2072–2078. doi: 10.1111/liv.12798. [DOI] [PubMed] [Google Scholar]
- 105.Debby BD, Ganor O, Yasmin M, David L, Nathan K, Ilana T, Dalit S, Smollan G, Galia R. Epidemiology of carbapenem resistant Klebsiella pneumoniae colonization in an intensive care unit. Eur J Clin Microbiol Infect Dis. 2012;31:1811–1817. doi: 10.1007/s10096-011-1506-5. [DOI] [PubMed] [Google Scholar]
- 106.Ney M, Abraldes JG, Ma M, Belland D, Harvey A, Robbins S, Den Heyer V, Tandon P. Insufficient Protein Intake Is Associated With Increased Mortality in 630 Patients With Cirrhosis Awaiting Liver Transplantation. Nutr Clin Pract. 2015;30:530–536. doi: 10.1177/0884533614567716. [DOI] [PubMed] [Google Scholar]
- 107. Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C, Rossi Fanelli F, Abbiati R; Italian BCAA Study Group. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124:1792–1801. doi: 10.1016/s0016-5085(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 108. Les I, Doval E, García-Martínez R, Planas M, Cárdenas G, Gómez P, Flavià M, Jacas C, Mínguez B, Vergara M, Soriano G, Vila C, Esteban R, Córdoba J. Effects of branched-chain amino acids supplementation in patients with cirrhosis and a previous episode of hepatic encephalopathy: a randomized study. Am J Gastroenterol. 2011;106:1081–1088. doi: 10.1038/ajg.2011.9. [DOI] [PubMed] [Google Scholar]
- 109. Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S, Kumada H; Long-Term Survival Study Group. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005;3:705–713. doi: 10.1016/s1542-3565(05)00017-0. [DOI] [PubMed] [Google Scholar]
- 110. Gluud LL, Dam G, Les I, Marchesini G, Borre M, Aagaard NK, Vilstrup H. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;5:CD001939. doi: 10.1002/14651858.CD001939.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hasse JM, DiCecco SR. Enteral Nutrition in Chronic Liver Disease: Translating Evidence into Practice. Nutr Clin Pract. 2015;30:474–487. doi: 10.1177/0884533615591058. [DOI] [PubMed] [Google Scholar]
- 112. Smith J, Horowitz J, Henderson JM, Heymsfield S. Enteral hyperalimentation in undernourished patients with cirrhosis and ascites. Am J Clin Nutr. 1982;35:56–72. doi: 10.1093/ajcn/35.1.56. [DOI] [PubMed] [Google Scholar]
- 113. Cabre E, Gonzalez-Huix F, Abad-Lacruz A, Esteve M, Acero D, Fernandez-Bañares F, Xiol X, Gassull MA. Effect of total enteral nutrition on the short-term outcome of severely malnourished cirrhotics. A randomized controlled trial. Gastroenterology. 1990;98:715–720. doi: 10.1016/0016-5085(90)90293-a. [DOI] [PubMed] [Google Scholar]
- 114. Cabré E, Rodríguez-Iglesias P, Caballería J, Quer JC, Sánchez-Lombraña JL, Parés A, Papo M, Planas R, Gassull MA. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology. 2000;32:36–42. doi: 10.1053/jhep.2000.8627. [DOI] [PubMed] [Google Scholar]
- 115. Dupont B, Dao T, Joubert C, Dupont-Lucas C, Gloro R, Nguyen-Khac E, Beaujard E, Mathurin P, Vastel E, Musikas M, Ollivier I, Piquet MA. Randomised clinical trial: enteral nutrition does not improve the long-term outcome of alcoholic cirrhotic patients with jaundice. Aliment Pharmacol Ther. 2012;35:1166–1174. doi: 10.1111/j.1365-2036.2012.05075.x. [DOI] [PubMed] [Google Scholar]
- 116. Ney M, Vandermeer B, van Zanten SJ, Ma MM, Gramlich L, Tandon P. Meta-analysis: oral or enteral nutritional supplementation in cirrhosis. Aliment Pharmacol Ther. 2013;37:672–679. doi: 10.1111/apt.12252. [DOI] [PubMed] [Google Scholar]