Abstract
The neocortex generates spontaneous slow oscillations that consist of up and down states during quiescence. Up states are short epochs of persistent activity that resemble the state of cortical activation during arousal and cognition. The excitability of cortical cells and synaptic networks is impacted by up states. This review describes the characteristics and putative functional role of up states and their similarity with activated states.
Keywords: slow oscillation, thalamocortical slice, thalamus, cortex, down state
The thalamus and neocortex form reciprocal and recurrent networks (Sherman and Guillery 1996; Steriade and others 1997) that generate a variety of rhythmic electrical activities during different behavioral states (Castro-Alamancos and Connors 1997a). The different states of thalamocortical networks have profound consequences on signals flowing through them (Castro-Alamancos 2004a). Two distinct states can be easily distinguished in behaving animals: arousal and quiescence.
Arousal consisting of vigilance and alertness is accompanied by an electrographic sign called activation (Fig. 1A), which consists of a low-amplitude, fast field potential activity recorded from the forebrain (Moruzzi and Magoun 1949; Castro-Alamancos 2004a). This activity contrasts with the large-amplitude, slow oscillatory activity typical of quiescent or nonactivated states (Fig. 1A). Activated states can be produced in anesthetized animals by stimulating the brain stem reticular formation, which is a convenient way to test the impact of activated states on cell excitability (Rudolph and others 2005) and sensory responsiveness (Castro-Alamancos and Oldford 2002). The thalamus is particularly susceptible to changes in activation. For example, during activation, thalamo cortical cells relay more effectively high-frequency sensory inputs to the neocortex (Castro-Alamancos 2002a), have larger receptive fields (Aguilar and Castro-Alamancos 2005), and increase or decrease spontaneous firing depending on the activating neuromodulator (Hirata and others 2006).
Figure 1.
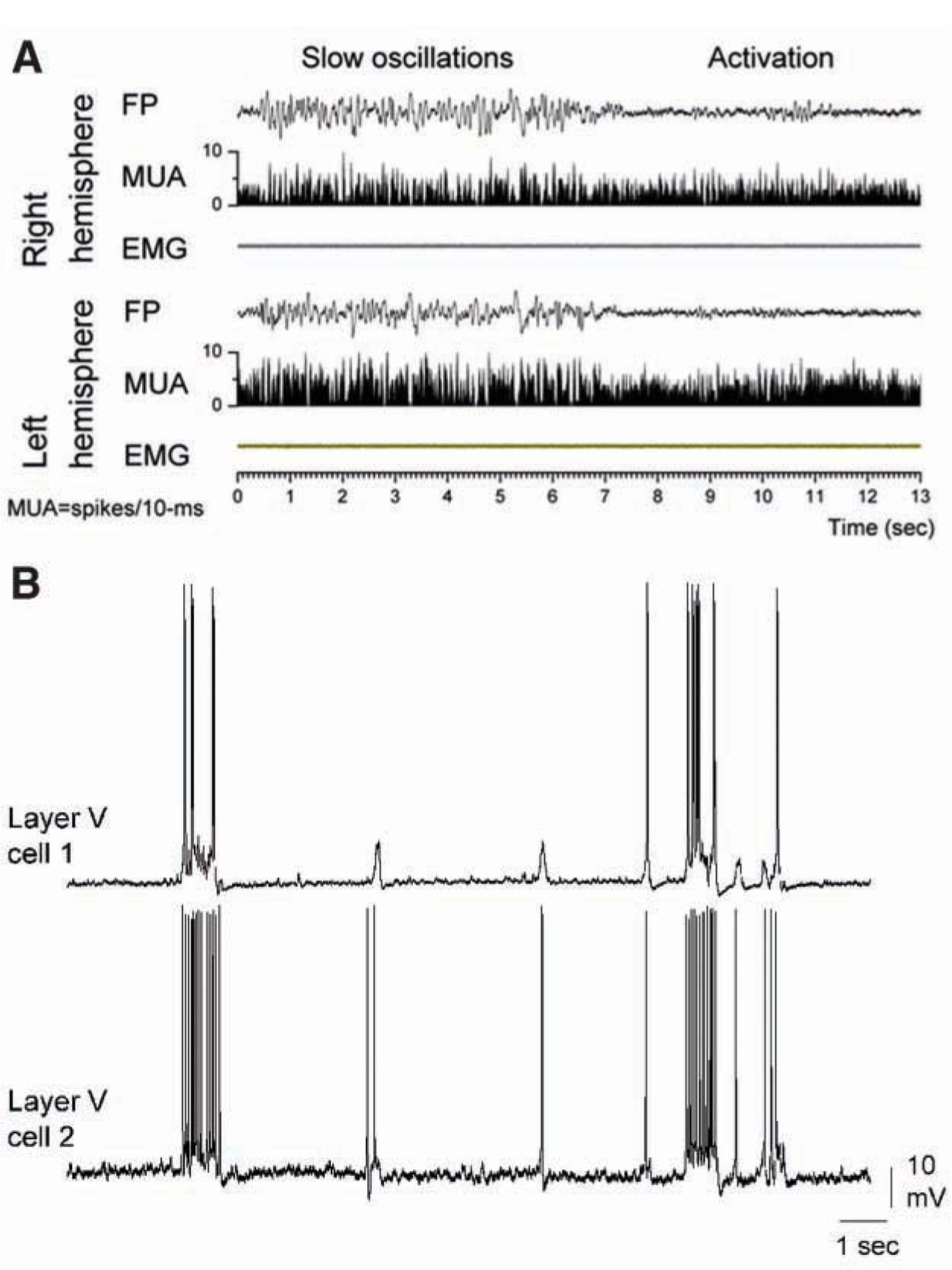
Cortical slow oscillations in vivo and in vitro. (A) Transition between slow oscillations and activation recorded from the cortex of a freely moving rat. Continuous bilateral field potential (FP), multi-unit activity (MUA), and whisker electromyographic (EMG) recordings in the motor cortex, showing slow oscillations during slow-wave sleep and activation during spontaneous waking. Figure based on Castro-Alamancos 2006. (B) Dual intracellular recordings from a pair of layer 5 cells located in a slice of the mouse somatosensory cortex in vitro. Recordings reveal synchronized slow oscillations consisting of up and down states.
During quiescence, such as slow-wave sleep or certain types of anesthesia, cortical networks are not silent but engage in different types of spontaneous synchronized activity (Castro-Alamancos and Connors 1997a; Steriade and others 1997). Among these activities, slow oscillations are characterized by rhythmic cycles of synaptically mediated depolarization and action potentials (up states), followed by a decrease of synaptic inputs, leading to membrane hyperpolarization and cessation of firing (down states). Up states are generated intrinsically in the neocortex (Steriade, Nunez, and Amzica 1993; Sanchez-Vives and McCormick 2000) and can recruit cortical targets such as, for example, the thalamus (Steriade, Contreras, and others 1993; Rigas and Castro-Alamancos 2007) and the striatum (Cowan and Wilson 1994). The activity of cortical networks during up states resembles activity during activation, and this has raised the hypothesis that up states may be equivalent to activated states typical of arousal.
Slow oscillations consisting of up and down states occur spontaneously in anesthetized or sleeping animals but are also observed, albeit at lower frequencies, in isolated cortical slices (Figs. 1B and 2A) that are bathed in the appropriate medium (Sanchez-Vives and McCormick 2000; Castro-Alamancos and Rigas 2002; Shu, Hasenstaub, and McCormick 2003; Rigas and Castro-Alamancos 2007). This in vitro model has proven useful to investigate the cellular and network mechanisms responsible for slow oscillations. Here, the cellular and network properties of up states and their putative functional role are discussed.
Figure 2.
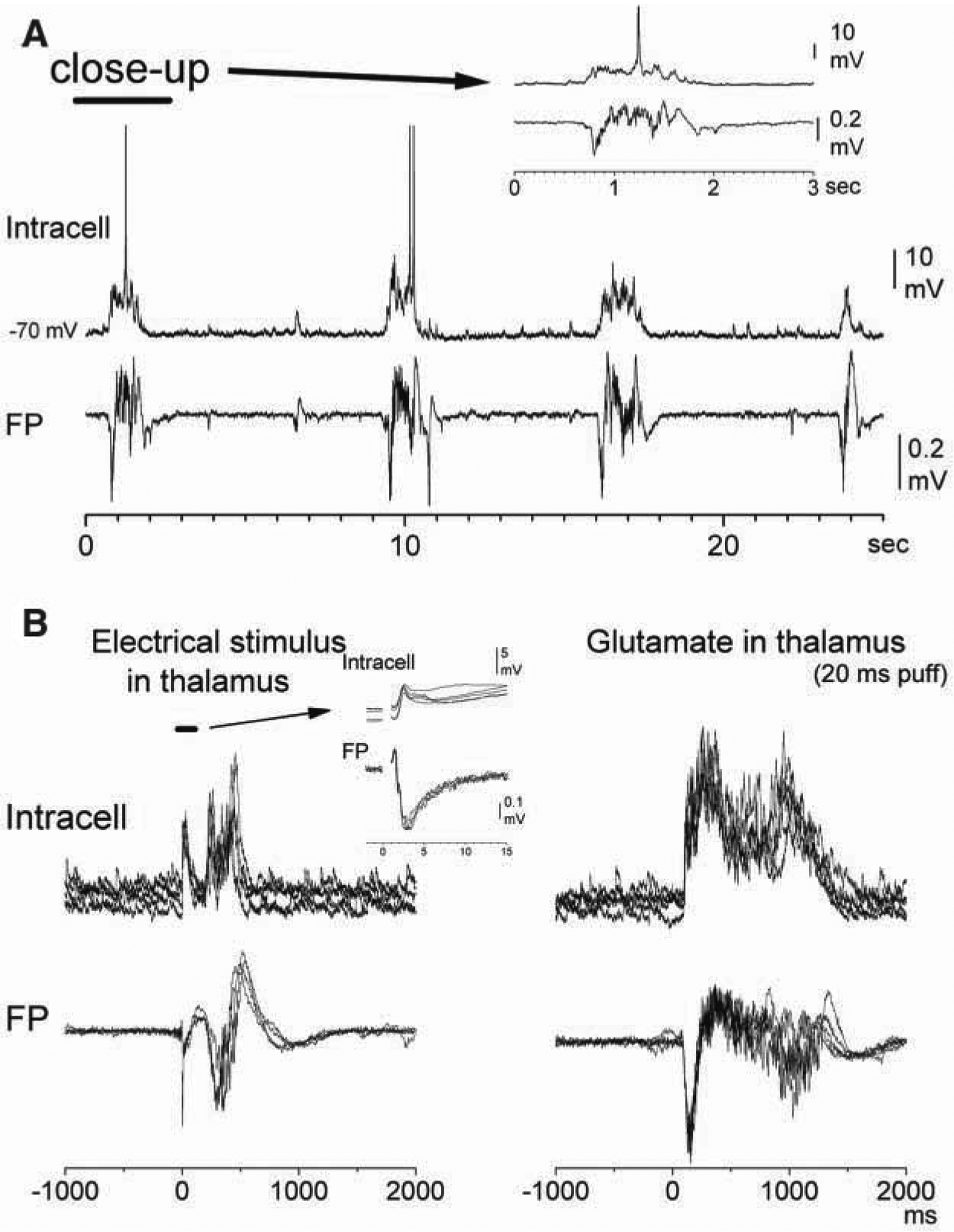
Thalamocortical activity triggers cortical up states in slices. (A) Spontaneous up/down states recorded from a cell in layer 3/4 and from a field potential (FP) electrode located close by in the somatosensory cortex of a mouse thalamocortical slice. (B) Electrical stimulation or glutamate puffs applied in the ventrobasal (VB) thalamus trigger up states recorded in layer 3/4 of the somatosensory cortex in a mouse thalamocortical slice. Note that up states are distinct from the short-latency responses evoked by the electrical stimulus (see inset). Figure based on Rigas and Castro-Alamancos 2007.
Synaptic Conductance Increases During Up States
Up states are associated with increased cortical cell firing, which implies that synaptic activity is pervasive during up states. Although the absolute estimates of conductance changes can vary considerably, there is agreement that up states are associated with an enhancement of synaptic conductance (Destexhe and others 2003; McCormick and others 2003; Shu, Hasenstaub, and McCormick 2003; Waters and Helmchen 2006). However, there is less agreement about the nature of the synaptic conductance change. One group reports that up states consist of balanced inhibition and excitation (McCormick and others 2003; Shu, Hasenstaub, and McCormick 2003). Others argue that the ratio favors excitation and that inhibition is sparse (Waters and Helmchen 2006). Furthermore, others argue that the ratio favors synaptic inhibition during up states so that up states can be defined as high-conductance states dominated by inhibition (Rudolph and others 2005).
One possibility to explain these differences is that there may be different types of up states under different experimental conditions that favor excitation or inhibition. Another possibility is that different cell types react differentially during up states so that the weights of excitation and inhibition vary between cell types. Regardless, future studies should clarify these discrepancies.
Cell Excitability Increases During Up States
There is good agreement that up states increase the excitability of cortical cells (Steriade and others 2001; McCormick and others 2003). Cells produce more action potentials in response to an equivalent somatic current pulse injection during up states than during down states. However, the increased excitability appears counterintuitive because, as mentioned above, up states are considered high-conductance states, which implies a more leaky, less excitable, neuron.
Apart from the enhanced synaptic conductance, up states are associated with several changes in cortical cells, most notably, depolarization by 5 to 15 mV and an increase in membrane potential variance or synaptic noise. Depolarization per se increases the excitability of cortical cells, making cells more responsive to current pulses. Also, injection of a small balanced excitatory and inhibitory synaptic conductance that increases membrane potential variance results in a sharp increase in cell excitability that mimics the change observed during up states (Chance and others 2002; McCormick and others 2003). The effect of increasing synaptic variance is complex because the excitability increase depends on the duration and amplitude of the test stimulus (Shu, Hasenstaub, Badoual, and others 2003). For example, synaptic noise may increase the response to small artificial EPSPs but can decrease the response to medium and large EPSPs, which can be viewed as a change in gain (Chance and others 2002).
The mechanism underlying the effect of synaptic noise and depolarization on cell excitability is unclear. A recent study has proposed that the reason why increases in synaptic conductance and depolarization lead on balance to enhanced excitability is because these changes are opposed by inward rectification (Connors and others 1982; Stafstrom and others 1982), resulting in a net increase in input resistance during up states in vivo (Waters and Helmchen 2006) or only a small decrease in slices (Rigas and Castro-Alamancos, unpublished observations). Basically, in many cortical pyramidal cells, slope conductance is not linear but negative because of the activation of voltage-dependent, noninactivating inward currents during subthreshold depolarization that sum with outward currents. The resulting increased apparent input resistance triggered by the depolarization during up states makes these cells more compact and counteracts synaptically induced conductance increases. Apparently, up states mainly increase input resistance variance and reduce only slightly the average input resistance compared to down states (Rigas and Castro-Alamancos, unpublished observations). These effects occur because decreases in input resistance caused by synaptic noise are opposed by increases caused by depolarization. In conclusion, cell excitability may increase during up states due to depolarization and because intrinsic voltage-dependent cellular mechanisms counteract the shunt produced by enhanced synaptic conductance.
Up States Affect Synaptic Inputs
The increased intrinsic excitability and increased synaptic conductance of cortical cells during up states may affect incoming synaptic inputs in opposite directions. So, the impact of up states on synaptic inputs is not obvious. When studying synaptic inputs, it is important to clearly distinguish between evoked short-latency (primary) responses and evoked longer-latency up states, which are clearly differentiated in slices (Rigas and Castro-Alamancos 2007). Using thalamocortical slices, we recently compared the effect of up states on thalamocortical and intracortical short-latency synaptic responses and found that they are differentially impacted by up states (Rigas and Castro-Alamancos, unpublished observations). Three main effects were observed. First, depolarization during up states makes EPSPs reach threshold faster and more successfully, which leads to faster onset spikes for intracortical responses and increased spike probability for thalamocortical responses. Second, evoked IPSPs are enhanced by up states because depolarization moves the membrane away from the reversal potential for IPSPs, increasing their driving force. The enhanced IPSPs inhibit excitatory responses that occur within the IPSP time window, such as long-latency intracortical spikes and intracortical EPSPs that have longer onset latencies. Thalamocortical EPSPs are less vulnerable to this inhibitory effect because of their faster onsets (Gabernet and others 2005). Also, intracortical IPSPs are stronger than thalamocortical IPSPs, which lead to stronger suppression of intracortical EPSPs. The cause of the stronger intracortical IPSPs is currently unclear. It could be due to the possibility that the intracortical electrical stimulus directly discharges inhibitory interneurons close to the electrode. Finally, when the voltage was kept constant in voltage-clamp recordings, we found that intracortical EPSCs were depressed whereas thalamocortical EPSCs were unaffected by up states. Both thalamocortical and intracortical synaptic responses depress with activity (Castro-Alamancos 1997; Castro-Alamancos and Connors 1997b; Gil and others 1997; Thomson and Deuchars 1997; Markram and others 1998). Therefore, we reason that enhanced presynaptic intracortical activity during up states depresses intracortical synaptic strength, and this does not occur for thalamocortical responses because of the lack of major reciprocal connections between the cortex and thalamus in the slice. Thus, presynaptic thalamocortical activity driven by up states is mostly mute in the slice, particularly compared to the situation in vivo. Presynaptic intracortical activity during up states is obviously enhanced in the slice, as this is what allows up states to ensue. In conclusion, activity-dependent synaptic depression and enhanced evoked inhibition appear to be the main culprits of synaptic suppression during up states. However, synaptic depression and enhanced inhibition are counteracted by depolarization during up states, which can result in faster onset and more successful evoked spikes compared to the down state. Depending on the conditions, the overall balance between these factors should determine neuronal output in response to synaptic inputs.
Thalamocortical Activity Triggers Up States and Vice Versa
Cortical up states can drive thalamocortical cells in vivo (Steriade, Contreras, and others 1993) and in slices (Rigas and Castro-Alamancos 2007). The existence of a massive corticothalamic pathway from the cortex to the VB thalamus, which is preserved in thalamocortical slices, is well documented (Sherman and Guillery 1996; Castro-Alamancos 2004a), and cortical afferents are the most abundant input to the thalamus (Guillery 1971, 1995). In fact, it is estimated that for every thalamocortical axon traversing to the cortex, there are about 10 returning to the thalamus (Sherman and Guillery 1996). We confirmed the existence of this massive pathway in thalamocortical slices by applying neurobiotin in the VB thalamus and found that large numbers of layer VI corticothalamic cells are labeled retrogradely (Rigas and Castro-Alamancos 2007). Therefore, it is logical that the synchronous discharge of cortical cells during up states drives thalamocortical cells.
In addition, thalamocortical activity can trigger cortical up states quite effectively, via thalamocortical connections (MacLean and others 2005; Rigas and Castro-Alamancos 2007). Cutting the connections between the thalamus and cortex in thalamocortical slices reduces the incidence of cortical up states, indicating that thalamocortical activity facilitates the occurrence of up states (Rigas and Castro-Alamancos 2007). Moreover, electrical or chemical stimulation of the thalamus is very effective at triggering and enhancing up states (Rigas and Castro-Alamancos 2007) (Fig. 2B). The observation that chemical stimulation of the thalamus triggers up states is important because electrical stimulation may inevitably lead to antidromic stimulation of corticothalamic fibers whose intracortical collaterals recurrently activate cortical cells, which, as mentioned above, are present in thalamocortical slices (Rigas and Castro-Alamancos 2007). Although it is clear that cortical up states do not require the thalamus (Steriade, Nunez, and Amzica 1993; Sanchez-Vives and McCormick 2000; Rigas and Castro-Alamancos 2007), these results indicate that thalamocortical cell activity can affect the induction and duration of cortical up states. In essence, thalamic activity may have a critical role in setting the state of the cortex.
Suppressing Inhibitory Currents Transforms Up States into Epileptiform Discharges
Up states are generated through a balance between excitation and inhibition (Shu, Hasenstaub, and McCormick 2003). When the balance is broken, aberrant activities result (Castro-Alamancos 2000; Castro-Alamancos and Rigas 2002). For example (Fig. 3), suppression of synaptic inhibition, by blocking GABA receptors, transforms spontaneous up states into paroxysmal depolarizing shifts (interictal discharges) that in some cortical areas, such as the motor cortex, trigger faster (~10 Hz) oscillations riding on the depolarization (Castro-Alamancos 2000; Castro-Alamancos and Rigas 2002). A similar transformation occurs when intrinsic outward currents are suppressed by blocking K+ channels (Castro-Alamancos and others 2007; Castro-Alamancos and Tawara-Hirata 2007). Interestingly, the motor cortex oscillations driven by the interictal discharges trigger involuntary jerks in the represented body parts, which resembles a cortical myoclonus (Castro-Alamancos 2006). Importantly, motor output driven by spontaneous up states in the motor cortex of sleeping animals is rare (Castro-Alamancos 2006), which indicates that the level of synchronization and neuronal discharge during normal up states is safely below the threshold for motor output.
Figure 3.
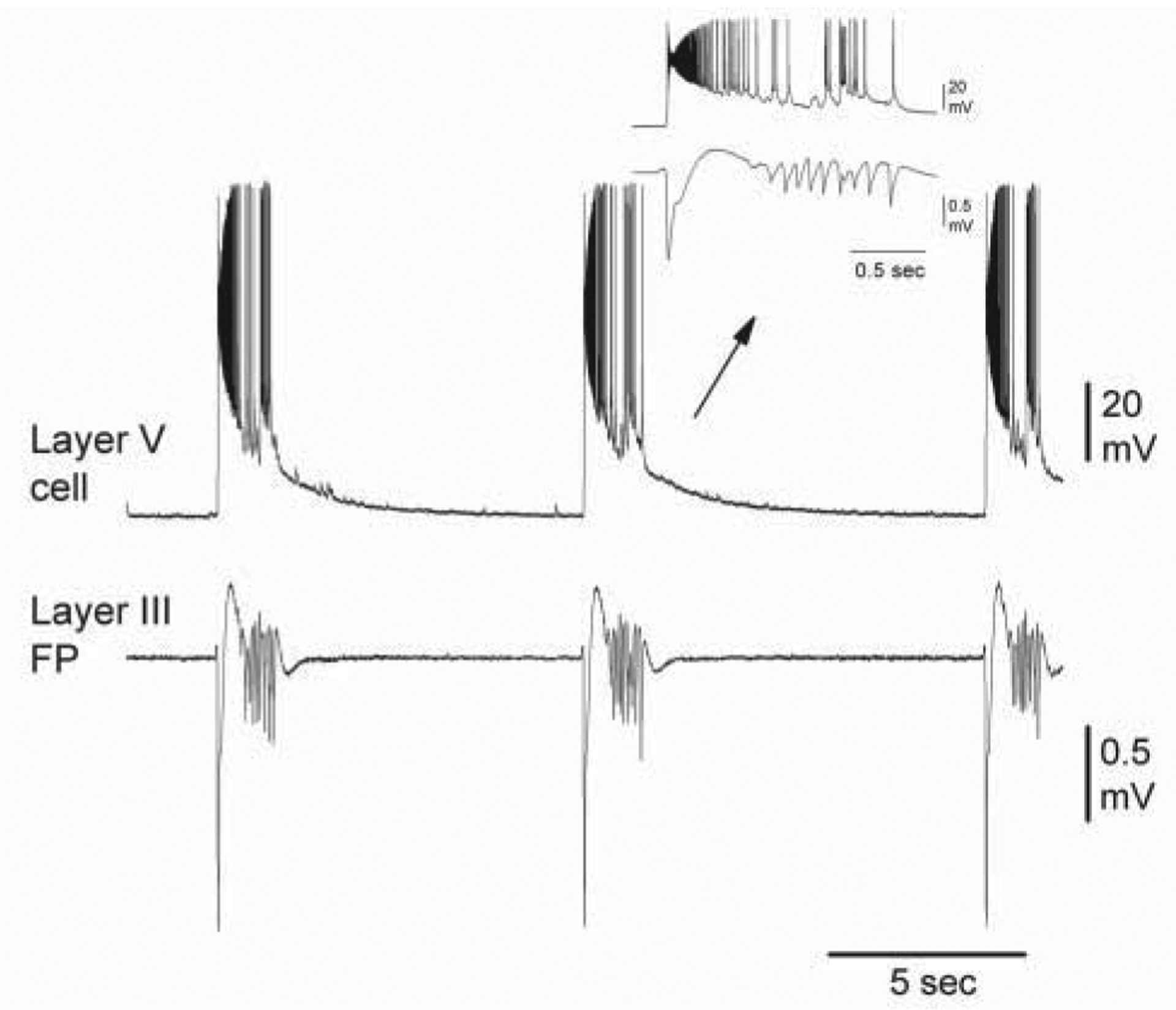
Block of GABA receptors transforms up states into paroxysmal depolarizing shifts. Intracellular recording from a layer 5 cell and field potential (FP) recording from layer 3 during application of GABAA and GABAB receptor antagonists in a mouse motor cortex slice. Note the occurrence of depolarizing shifts and ~10-Hz oscillations riding on the depolarization. Figure based on Castro-Alamancos and Rigas 2002.
Up States Resemble Activated States
Cortical up states and activated states course with depolarization and enhanced firing. This raises the obvious question of whether these 2 states are equivalent. Activated states can be produced in anesthetized animals by RF stimulation, which is a convenient way to test the impact of activated states on cell excitability (Rudolph and others 2005) and sensory responsiveness (Castro-Alamancos and Oldford 2002). Moreover, the impact of naturally occurring activation during arousal (or paradoxical sleep) can also be tested in restrained, unanesthetized animals (Steriade and others 2001) and in freely behaving animals (Castro-Alamancos 2004b).
When synaptic conductance is considered, up states are similar but not identical to activated states. It appears that inhibitory conductances are stronger during activated states than during up states (Rudolph and others 2005). When input resistance is considered, up states are similar, but not identical, to activated states caused by RF stimulation (Destexhe and others 2003; Rudolph and others 2005) or natural waking (Steriade and others 2001); cortical cells have higher input resistance during natural or RF-induced activated states than during up states. The main argument that has been given to explain this difference is that during activated states, cortical cells are affected by the release of neuromodulators. Cholinergic activation within the cortex suppresses spontaneous slow oscillations in vivo and makes responses less variable (Oldford and Castro-Alamancos 2003). Preliminary results indicate that both acetylcholine and norepinephrine abolish up and down fluctuations in cortical slices (unpublished data). Future work will need to determine if the state of cortical cells set by these neuromodulators is similar to up states, down states, or a different state altogether.
Sensory (Whisker) Responses Are Suppressed During Up States
The fact that up states enhance the excitability of cortical cells implies that sensory responses in the cortex should be facilitated by up states. Indeed, this appears to be the case in the cat visual cortex but not in the rodent somatosensory cortex. In the cat visual cortex, up states are associated with stronger responses to visual stimuli under a variety of stimulus characteristics (Arieli and others 1996; Azouz and Gray 1999; Haider and others 2007). In contrast, up states reduce barrel cortex responses to brief whisker deflections in rodents (Petersen and others 2003; Sachdev and others 2004). Interestingly, natural or artificially induced activated states also lead to the suppression of barrel cortex responses to brief whisker deflections (Castro-Alamancos and Oldford 2002; Castro-Alamancos 2004b) (Fig. 4A).
Figure 4.
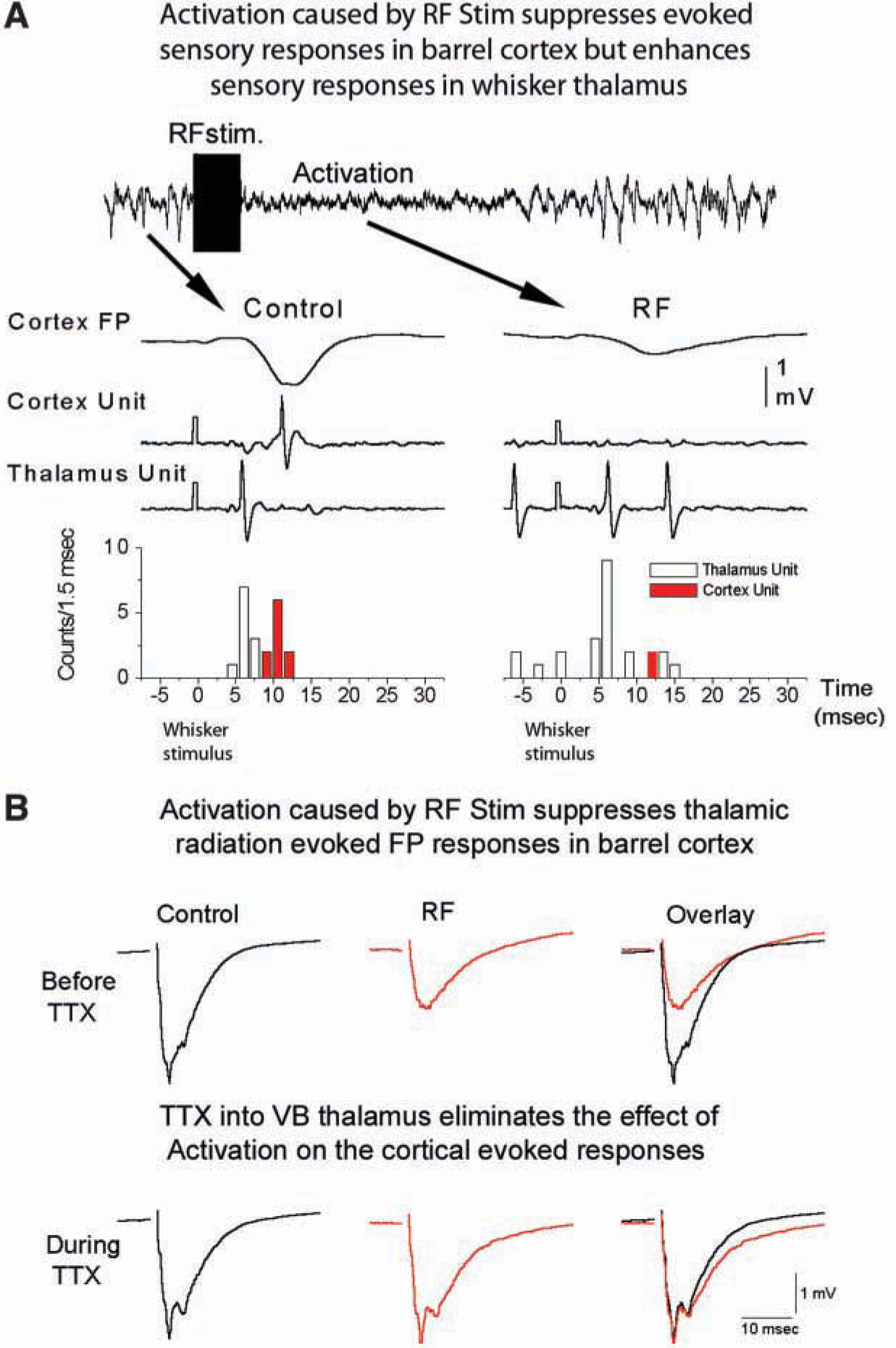
Activation suppresses sensory and thalamic radiation–evoked responses in the barrel cortex. (A) Field potential (FP) and singleunit recordings obtained in the barrel cortex through the same electrode, and a simultaneously recorded single unit in the whisker thalamus of a urethane-anesthetized rat. RF stimulation delivered for 1 second (100 Hz) produced a robust, activating effect consisting of lowamplitude irregular activity in the cortical FP. The cortical FP and unit responses are suppressed during activation caused by the RF stimulation, while the thalamic unit response is enhanced. (B) Similar to the effect on sensory responses, activation caused by RF stimulation also suppresses thalamocortical FP responses evoked in the barrel cortex by stimulating thalamic radiation. Block of thalamic activity by applying TTX into the ventrobasal (VB) thalamus eliminates the suppressing effect of activation on thalamic radiation–evoked responses. Figure based on Castro-Alamancos and Oldford 2002.
An obvious question is what causes the suppression of sensory responses in the rodent barrel cortex during up states and activated states? A variety of factors have been proposed, and several have already been mostly ruled out by a recent study (Hasenstaub and others 2007), such as a change in spike threshold during up states (Sachdev and others 2004) or a decrease in excitatory driving force during up states (Petersen and others 2003). Importantly, up states were found to increase, rather than decrease, the responsiveness of barrel cortex cells in vivo to artificial EPSPs, which indicates that barrel cortex cells are indeed more excitable during up states. All the while, the sensory stimulus recruits less excitatory conductance and proportionally more inhibitory conductance in cortical cells during up states (Hasenstaub and others 2007).
If cells are more excitable, then what causes sensory suppression during up states?
One possibility is that presynaptic activity is causing the suppression during up states. Indeed, this has been demonstrated to be involved in sensory suppression during activated states (Castro-Alamancos and Oldford 2002). Activated states occur naturally in waking animals or in sleeping animals during paradoxical sleep but can also be induced artificially by RF stimulation (Castro-Alamancos and Oldford 2002) or by applying cholinergic agonists in the thalamus (Castro-Alamancos 2002a). In all of these conditions, activated states are associated with suppression of sensory responses to whisker deflections, and thalamocortical cells (the presynaptic component of the cortical sensory response) increase their firing rates quite significantly. Because we knew that thalamocortical responses in the somatosensory cortex in vivo are sensitive to activity and depress sharply with increased thalamocortical firing rate (Castro-Alamancos and Connors 1996; Castro-Alamancos 1997; Castro-Alamancos and Connors 1997a), we hypothesized that increased thalamocortical activity was suppressing thalamocortical connections and leading to sensory suppression during activated states.
To test this hypothesis, a stimulating electrode was placed in the thalamic radiation to stimulate thalamocortical fibers and monitor thalamocortical responses (Fig. 4B). In addition, a cannula was located in the somatosensory thalamus to affect the firing rate of thalamocortical cells with drug infusions. When we produced cortical activation by RF stimulation, thalamocortical cell firing increased sharply, and both sensory and thalamic radiation–evoked responses were suppressed. However, when we abolished the firing of thalamocortical cells by anesthetizing their cell bodies with TTX in the thalamus (which does not interfere with the ability to stimulate their thalamocortical fibers in the radiation), we found that thalamocortical-evoked responses were no longer suppressed (Castro-Alamancos and Oldford 2002), despite the fact that the cortex showed signs of activation during RF stimulation. This demonstrates that thalamocortical firing is causing the sensory suppression during activation.
Because we know that up states effectively drive thalamocortical firing, it is tempting to propose that increased thalamocortical firing causes suppression of sensory responses during up states. Clearly, future work is needed to test this intriguing possibility in vivo. We recently found that in thalamocortical slices in vitro, a situation where closed thalamocorticothalamic loops are cut (i.e., corticothalamic cells can drive thalamocortical cells during up states, but most of these active thalamocortical cells do not have intact fibers going back to the cortex), up states do not suppress thalamocortical responses (Rigas and Castro-Alamancos, unpublished observations). Instead, up states enhance these responses, consistent with a role of thalamocortical activity in driving the suppression of thalamocortical responses.
Functional Consequences of Cortical Up and Activated States
Up States May Control the Gain of Sensory Responses
There are several major forces at play during up states that will affect sensory inputs. These include postsynaptic depolarization, presynaptic activity, enhanced inhibitory drive, and increased synaptic conductance. Depolarization during up states reduces excitatory drive but has a net facilitating effect because it brings excitatory inputs closer to the firing threshold. This should be particularly helpful for sensory responses evoked by weak stimuli, which could have difficulty reaching threshold during down states but would be boosted by depolarization during up states. Such a facilitating effect is clearly displayed by thalamocortical responses in slices (Rigas and Castro-Alamancos, unpublished observations) and also appears to occur to some degree during continuous stimulation in vivo (Hasenstaub and others 2007). A similar effect has been previously shown to occur in the whisker thalamus. Lemniscal synapses are depressed (made weaker) by high-frequency sensory stimuli, but depolarization of thalamocortical cells during activation compensates by bringing depressed EPSPs to the firing threshold (Castro-Alamancos 2002a, c). Thus, depolarization of thalamocortical cells and synaptic depression at lemniscal synapses work together to control the relay of sensory inputs to the cortex through the thalamus (Castro-Alamancos 2004a).
Enhanced presynaptic activity in synaptic pathways that display activity-dependent synaptic depression, such as thalamocortical and most intracortical connections, will tend to suppress sensory responses. Thus, a distinct balance between these opposing forces (postsynaptic depolarization and presynaptic activity) may be reached in different cases to control the gain of sensory inputs in a state-dependent manner.
Up States May Sharpen Sensory Responses
Cortical sensory responses are curtailed by inhibition (Gabernet and others 2005). Up states increase the driving force of inhibitory inputs due to depolarization and may also increase inhibitory strength by facilitating the discharge of inhibitory interneurons. Excitatory responses driven by sensory inputs are fully effective as long as they avoid the inhibitory drive. But evoked long-latency spikes that fall within the time window of inhibition are obliterated during up states (Rigas and Castro-Alamancos, unpublished observations). Therefore, up states may serve to sharpen receptive fields in the barrel cortex because the center of the receptive field drives faster onset responses than the periphery (Hirata and Castro-Alamancos 2008). Similarly, activation spatially focuses the responses of cortical cells (Castro-Alamancos 2002b). Thus, when the responses of single cells to principal and adjacent whiskers are compared during activation, the responses to the adjacent whiskers are more suppressed than those of principal whiskers. Consequently, the receptive fields of cortical neurons become more focused to their principal whiskers during activation, and a similar effect is expected to occur during up states.
Up States May Enhance Response Synchrony
As up states affect the gain and sharpen sensory responses, they may also facilitate the flow of selective sensory inputs by increasing the synchrony of evoked responses. Synchrony in the thalamocortical system is essential to drive cortical responses because of the need for synaptic convergence to trigger cortical responses (Bruno and Sakmann 2006). In addition, attentive processing in behaving animals is associated with increases in cortical synchrony (Steinmetz and others 2000; Fries and others 2001), and there is evidence that cortical response synchrony is enhanced by activation (Munk and others 1996). Response synchrony within the cortex is important because it affects the ability to drive cortical targets.
Evoked Up States During Quiescence May Serve as an Alerting Signal
Sensory stimuli occurring during the down state produce large short-latency responses that can be followed by up states. One possibility is that the large sensory cortical responses in quiescent animals serve as a mechanism of heightened sensitivity for detecting transient stimuli (Castro-Alamancos 2004b). Indeed, the large evoked responses could serve to alert quiescent animals of the presence of significant sensory stimuli during behavior (Castro-Alamancos 2004a). Future work is needed to address these hypotheses. Because thalamocortical activity is effective at triggering and sustaining up states (Rigas and Castro-Alamancos 2007), it is tempting to suggest that repeated sensory inputs that trigger up states serve to drive cortical activity into a sustained activated state.
Corticofugal Output During Up States May Inform Subcortical Targets about the Level of Cortical Activation
A major role of corticofugal output may be to constantly inform cortical targets about the state of cortical activation so that appropriate neural activity and behavioral responses are generated by subcortical structures in accordance with the behavioral state. This may occur for both persistent and evoked activity. For example, during activation, corticotectal (feedback) responses driven by sensory stimuli are suppressed (Cohen and others 2008). Thus, neural output in the superior colliculus may be relatively more robust during quiescence than during activation because of regulation at the level of the sensory cortex. One interpretation of this effect is that if an animal is already alert (i.e., cortical activation), a sensory stimulus does not need to elicit a strong orienting response mediated by the superior colliculus. However, if the animal is quiescent or inattentive, a salient stimulus would robustly drive corticofugal responses that in turn drive collicular cells and presumably appropriate orienting responses toward or away from the stimulus (Cohen and Castro-Alamancos 2007; Cohen and others 2008). In this way, the level of cortical activation may regulate basic behavioral responses that are generated by subcortical structures.
Acknowledgments
This work was supported by the National Institutes of Health (NIH). The author thanks Jeremy Cohen, Akio Hirata, Paul Rigas, Yoshie Tawara-Hirata, and Gladis Thomas.
References
- Aguilar JR, Castro-Alamancos MA. 2005. Spatiotemporal gating of sensory inputs in thalamus during quiescent and activated states. J Neurosci 25:10990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arieli A, Sterkin A, Grinvald A, Aertsen A. 1996. Dynamics of ongoing activity: explanation of the large variability in evoked cortical responses. Science 273:1868–71. [DOI] [PubMed] [Google Scholar]
- Azouz R, Gray CM. 1999. Cellular mechanisms contributing to response variability of cortical neurons in vivo. J Neurosci 19:2209–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno RM, Sakmann B. 2006. Cortex is driven by weak but synchronously active thalamocortical synapses. Science 312:1622–7. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. 1997. Short-term plasticity in thalamocortical pathways: cellular mechanisms and functional roles. Rev Neurosci 8:95–116. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. 2000. Origin of synchronized oscillations induced by neocortical disinhibition in vivo. J Neurosci 20:9195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. 2002a. Different temporal processing of sensory inputs in the rat thalamus during quiescent and information processing states in vivo. J Physiol 539:567–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. 2002b. Role of thalamocortical sensory suppression during arousal: focusing sensory inputs in neocortex. J Neurosci 22:9651–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. 2002c. Properties of primary sensory (lemniscal) synapses in the ventrobasal thalamus and the relay of high-frequency sensory inputs. J Neurophysiol 87:946–53. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. 2004a. Dynamics of sensory thalamocortical synaptic networks during information processing states. Prog Neurobiol 74:213–47. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. 2004b. Absence of rapid sensory adaptation in neocortex during information processing states. Neuron 41:455–64. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. 2006. Vibrissa myoclonus (rhythmic retractions) driven by resonance of excitatory networks in motor cortex. J Neurophysiol 96:1691–8. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Connors BW. 1996. Spatiotemporal properties of short-term plasticity sensorimotor thalamocortical pathways of the rat. J Neurosci 16:2767–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Connors BW. 1997a. Thalamocortical synapses. Prog Neurobiol 51:581–606. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Connors BW. 1997b. Distinct forms of short-term plasticity at excitatory synapses of hippocampus and neocortex. Proc Natl Acad Sci U S A 94:4161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Oldford E. 2002. Cortical sensory suppression during arousal is due to the activity-dependent depression of thalamocortical synapses. J Physiol 541: 319–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Rigas P. 2002. Synchronized oscillations caused by disinhibition in rodent neocortex are generated by recurrent synaptic activity mediated by AMPA receptors. J Physiol 542:567–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Rigas P, Tawara-Hirata Y. 2007. Resonance (approximately 10 Hz) of excitatory networks in motor cortex: effects of voltage-dependent ion channel blockers. J Physiol 578:173–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Tawara-Hirata Y. 2007. Area-specific resonance of excitatory networks in neocortex: control by outward currents. Epilepsia 48:1572–84. [DOI] [PubMed] [Google Scholar]
- Chance FS, Abbott LF, Reyes AD. 2002. Gain modulation from background synaptic input. Neuron 35:773–82. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Castro-Alamancos MA. 2007. Early sensory pathways for detection of fearful conditioned stimuli: tectal and thalamic relays. J Neurosci 27:7762–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Hirata A, Castro-Alamancos MA. 2008. Vibrissa sensation in superior colliculus: wide-field sensitivity and state-dependent cortical feedback. J Neurosci 28:11205–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ, Prince DA. 1982. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol 48:1302–20. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Wilson CJ. 1994. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J Neurophysiol 71:17–32. [DOI] [PubMed] [Google Scholar]
- Destexhe A, Rudolph M, Pare D. 2003. The high-conductance state of neocortical neurons in vivo. Nat Rev Neurosci 4:739–51. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. 2001. Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291:1560–3. [DOI] [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. 2005. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron 48: 315–27. [DOI] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. 1997. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron 19:679–86. [DOI] [PubMed] [Google Scholar]
- Guillery RW. 1971. Patterns of synaptic interconnections in the dorsal lateral geniculate nucleus of cat and monkey: a brief review. Vision Res Suppl 3:211–27. [DOI] [PubMed] [Google Scholar]
- Guillery RW. 1995. Anatomical evidence concerning the role of the thalamus in corticocortical communication: a brief review. J Anat 187(Pt 3):583–92. [PMC free article] [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, Yu Y, McCormick DA. 2007. Enhancement of visual responsiveness by spontaneous local network activity in vivo. J Neurophysiol 97:4186–202. [DOI] [PubMed] [Google Scholar]
- Hasenstaub A, Sachdev RN, McCormick DA. 2007. State changes rapidly modulate cortical neuronal responsiveness. J Neurosci 27:9607–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Aguilar J, Castro-Alamancos MA. 2006. Noradrenergic activation amplifies bottom-up and top-down signal-to-noise ratios in sensory thalamus. J Neurosci 26:4426–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Castro-Alamancos MA. 2008. Cortical transformation of wide-field (multiwhisker) sensory responses. J Neurophysiol 100:358–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean JN, Watson BO, Aaron GB, Yuste R. 2005. Internal dynamics determine the cortical response to thalamic stimulation. Neuron 48:811–23. [DOI] [PubMed] [Google Scholar]
- Markram H, Wang Y, Tsodyks M. 1998. Differential signaling via the same axon of neocortical pyramidal neurons. Proc Natl Acad Sci U S A 95:5323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Shu Y, Hasenstaub A, Sanchez-Vives M, Badoual M, Bal T. 2003. Persistent cortical activity: mechanisms of generation and effects on neuronal excitability. Cereb Cortex 13:1219–31. [DOI] [PubMed] [Google Scholar]
- Moruzzi G, Magoun HW. 1949. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol 1:455–73. [PubMed] [Google Scholar]
- Munk MH, Roelfsema PR, Konig P, Engel AK, Singer W. 1996. Role of reticular activation in the modulation of intracortical synchronization [see comments] Science 272:271–4. [DOI] [PubMed] [Google Scholar]
- Oldford E, Castro-Alamancos MA. 2003. Input-specific effects of acetylcholine on sensory and intracortical evoked responses in the “barrel cortex” in vivo. Neuroscience 117:769–78. [DOI] [PubMed] [Google Scholar]
- Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. 2003. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci U S A 100:13638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas P, Castro-Alamancos MA. 2007. Thalamocortical up states: differential effects of intrinsic and extrinsic cortical inputs on persistent activity. J Neurosci 27:4261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph M, Pelletier JG, Pare D, Destexhe A. 2005. Characterization of synaptic conductances and integrative properties during electrically induced EEG-activated states in neocortical neurons in vivo. J Neurophysiol 94:2805–21. [DOI] [PubMed] [Google Scholar]
- Sachdev RN, Ebner FF, Wilson CJ. 2004. Effect of subthreshold up and down states on the whisker-evoked response in somatosensory cortex. J Neurophysiol 92:3511–21. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. 2000. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci 3:1027–34. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. 1996. Functional organization of thalamocortical relays. J Neurophysiol 76:1367–95. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, Badoual M, Bal T, McCormick DA. 2003. Barrages of synaptic activity control the gain and sensitivity of cortical neurons. J Neurosci 23:10388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. 2003. Turning on and off recurrent balanced cortical activity. Nature 423: 288–93. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Schwindt PC, Crill WE. 1982. Negative slope conductance due to a persistent subthreshold sodium current in cat neocortical neurons in vitro. Brain Res 236:221–6. [DOI] [PubMed] [Google Scholar]
- Steinmetz PN, Roy A, Fitzgerald PJ, Hsiao SS, Johnson KO, Niebur E. 2000. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature 404:187–90. [DOI] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Curro DR, Nunez A. 1993. The slow (< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci 13:3284–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Jones EG, McCormick DA. 1997. Thalamus. New York: Elsevier. [Google Scholar]
- Steriade M, Nunez A, Amzica F. 1993. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci 13:3266–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. 2001. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol 85:1969–85. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J. 1997. Synaptic interactions in neocortical local circuits: dual intracellular recordings in vitro. Cereb Cortex 7:510–22. [DOI] [PubMed] [Google Scholar]
- Waters J, Helmchen F. 2006. Background synaptic activity is sparse in neocortex. J Neurosci 26:8267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]


