Summary
The mammalian vocal pattern generator is situated in the brainstem but its exact structure is debated. We mapped these circuits in rats by cooling and microstimulation. Local cooling disrupted call production above an anterior and a posterior brainstem position. Anterior cooling affected predominantly high-frequency calls, whereas posterior cooling affected low-frequency calls. Electrical microstimulation of the anterior part led to modulated high-frequency calls, whereas microstimulation of the posterior part led to flat, low-frequency calls. At intermediate positions cooling did not affect calls and stimulation did not elicit calls. The anterior region corresponds to a subsection of the parvicellular reticular formation that we term the vocalization parvicellular reticular formation (VoPaRt). The posterior vocalization sites coincide with the nucleus retroambiguus (NRA). VoPaRt and NRA neurons were very small and the VoPaRt was highly myelinated, suggestive of high-speed processing. Our data suggest an anatomically and functionally bipartite vocal pattern generator.
Subject Areas: Biological Sciences, Neuroscience, Behavioral Neuroscience, Neuroanatomy
Graphical Abstract
Highlights
-
•
Cooling and stimulation reveal two brainstem regions involved in vocal patterning
-
•
Both regions consist of very small cells and are highly myelinated
-
•
The anterior region (VoPaRt) controls frequency-modulated, high-frequency calls
-
•
The posterior region (NRA) mostly controls flat, low-frequency calls
Biological Sciences; Neuroscience; Behavioral Neuroscience; Neuroanatomy
Introduction
Vocalizations arise from some of the most complex motor patterns produced by vertebrates. The sheer complexity of the vocalization process makes understanding its neural underpinnings very challenging. It is therefore not surprising that a great number of brain structures are involved in vocalization control. The role of the forebrain in vocalizations has been highlighted early by Broca's discovery (Broca, 1861) of left hemispheric speech localization, and numerous studies on songbirds have clarified the role of avian forebrain circuits in song learning (Sakata et al., 2020). Downstream of the forebrain, an important structure for vocalizations is the periaqueductal gray (PAG). Its involvement in vocalizations is long known (Jürgens, 1994), and a recent study manipulated neuronal firing in the PAG evoking or inhibiting vocalizations, underlining its gateway functions for call production (Tschida et al., 2019). In mammals, there is also a consensus that brainstem circuits coordinate the patterning of vocalizations. This conclusion rests on the following observations: (1) Electrical (Bennett et al., 2019; Jürgens and Ploog, 1970; Kyuhou and Gemba, 1998) and chemical (Lu and Jürgens, 1993; Zhang et al., 1995) stimulation of forebrain and midbrain circuits upstream of the brainstem often evokes species-specific, complex, and complete vocalizations; (2) electrical (Dressnandt and Jürgens, 1992) and chemical (Jürgens and Richter, 1986) stimulation of brainstem sites often evokes artificial and elementary vocalizations; (3) several different structures in the brainstem project to all involved phonatory motor neuron pools (Fay and Norgren, 1997; Hannig and Jürgens, 2005; Thoms and Jürgens, 1987).
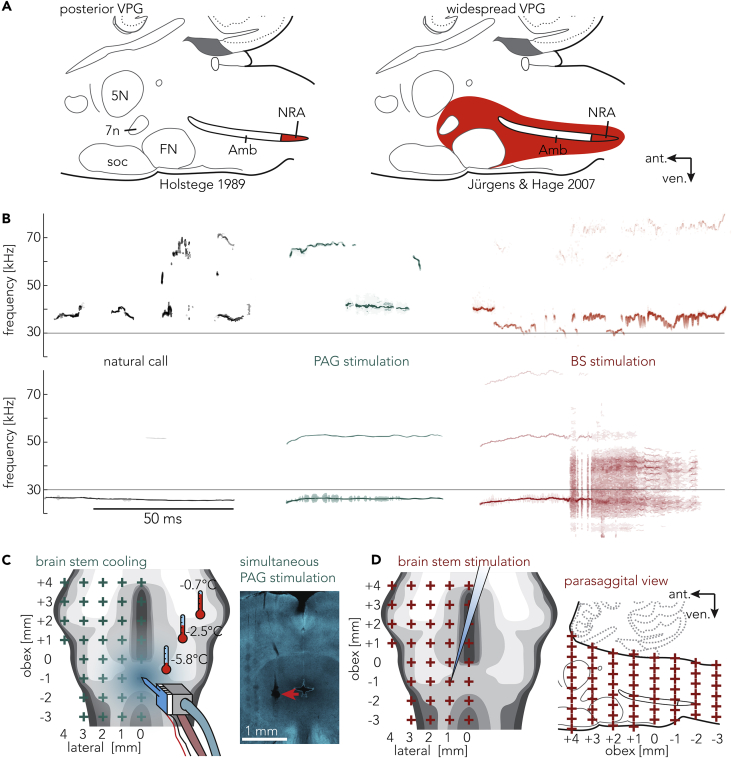
These observations have led to the idea of a vocal patterning generator localized in the brainstem, but there is no clear picture yet of the identity of the putative vocal pattern generator (VPG), especially in the rodent. In fact, as shown in Figure 1A, there are competing accounts of the mammalian VPG. Based on anatomical evidence from the cat, Holstege (1989) suggested that the VPG is situated in the nucleus retroambiguus (NRA, Figure 1A, left). In line with this idea, lesions and transections of the NRA result in an abolishment of calls (Holstege, 1989; Zhang et al., 1995) and the NRA projects to the relevant motor neuron pools. Competing with this model is the suggestion that the VPG is widely spread through the reticular formation (Figure 1A right) (Jürgens and Hage, 2007; Lüthe et al., 2000). Neuronal activity close to the superior olive in squirrel monkeys correlated with vocalizations, especially frequency modulated ones (Hage and Jürgens, 2006a, 2006b). Although neuronal recording data contribute important correlative data, causation and necessity studies spanning across the brain stem could contribute valuable information to the organization of the VPG. We therefore made a new attempt for mapping the mammalian VPG in rats.
Figure 1.
Vocal Pattern Generator Hypotheses, Rat Vocalizations, Stimulation-Evoked Vocalizations, and Local Brainstem Cooling
(A) Competing hypotheses about the mammalian vocal pattern generator superimposed to a schematic mammalian (rat) brainstem. Red areas depict the assumed location of the vocal pattern generator. Left: nucleus retroambiguus as the VPG (adapted from Holstege 1989). Right: dedicated VPG across the ventrolateral pontine brainstem according to Jürgens and Hage (2007). 5N, motortrigeminal nucleus; 7n, facial nerve; Amb, nucleus ambiguus; FN, facial nucleus; NRA, nucleus retroambiguus; soc, superior olivary complex.
(B) Vocalizations of a rat. Top: high-frequency calls, e.g., >30 kHz. Bottom: low-frequency calls, e.g., <30 kHz. Black: species-specific calls of an awake, behaving animal. Teal: vocalizations triggered by electrical microstimulation of the PAG in an anesthetized rat. Red: vocalizations triggered by electrical microstimulation in the brainstem in an anesthetized rat. Note the unnatural shaped vocalizations evoked by direct brainstem stimulation, reflecting the stimulation pattern in the vocalization itself. High- and low-frequency calls evoked by PAG/brainstem stimulation stem from different stimulation sites in the respective areas. High- and low-frequency calls could be evoked at the same PAG location, as shown in example calls of Figure 4.
(C) Left: PAG stimulation and simultaneous, local cooling of the brainstem. Thermistors measuring the temperature on the contralateral side of the brainstem. Blue haze indicates cooling effect. Red crosses indicate the 36 positions arranged in a 1 × 1-mm grid above the brainstem. Scaling and orientation are identical with maps in Figures 2 and 4. Right: coronal section of the PAG with the red arrow indicating the site of stimulation and lesion.
(D) Left: direct stimulation of the brainstem using a tungsten electrode in the same locations as the cooling. Right: depth stimulation of the brainstem was done in 500-μm steps throughout the brainstem.
Recent focus on rodent vocalizations (Neff, 2019; Okobi et al., 2019; Tschida et al., 2019) underlines the need of a better understanding of the rodent VPG. Rats are highly vocal animals and emit two broad categories of ultrasonic vocalizations (USVs) (Burgdorf et al., 2008): higher-frequency (>30 kHz) calls often associated with positive emotional content and lower-frequency (<30 kHz) calls often associated with fear and alarm (Figure 1B) (Brudzynski, 2005; Knutson et al., 2002).
We took the following measures for a maximally controlled mapping of this structure: (1) We studied calls evoked by midbrain (periaqueductal gray, PAG) stimulation in anesthetized animals, which allowed us to study many hours of uninterrupted stimulation-evoked calling in numerous preparations (Figure 1B). (2) We removed the cerebellum and visualized the brainstem under a surgical microscope, which provided reproducible sub-millimeter precision access to the brainstem. (3) We employed localized cooling as pioneered by Long and Fee (2008). Local cooling powerfully affects vocalization generation and allows for a high-resolution mapping (Figure 1C). (4) We applied a systematic microstimulation mapping of brain stem circuits (Figure 1D).
We find an excellent agreement of localized cooling and stimulation effects. Although the data support the idea that both posterior structures (NRA) and anterior structures (parvicellular reticular formation) contribute to pattern generation in rat vocalizations, the data refute distributed VPG model. Lastly, we reveal major functional differences between the anterior and posterior part of the VPG.
Results
Electrical Stimulation in the Periaqueductal Gray and Brain Stem Evokes Vocalizations
We investigated the localization of the vocal pattern generator (VPG) in the mammalian brain. To do so, we evoked species-specific calls under urethane anesthesia by periaqueductal gray (PAG) stimulation (Figure 1B) and simultaneously applied local cooling (Figure 1C). Single PAG stimulations evoked bouts of 3.5 ± 1.3 (mean ± SD) calls. As far as we could tell, removal of the cerebellum had no major effect on call production (Figure S1). Calls could be in the high- (>30 kHz) or low-frequency range (<30 kHz, Figure 1B); in a subset of experiments PAG stimulation evoked a series of both high- and low-frequency calls. Call type and number stayed constant for minutes to hours, and only trials with constant calling patterns were used for further analysis. We included an average of 20 ± 7 (mean ± SD) sites of 5 experiments resulting in 98 total sites. The spatial distribution of these cooling sites is shown in Figure 1C.
In a second set of experiments, we applied electrical microstimulation (Figure 1D) directly to the brainstem, in the same 1 × 1-mm grid as the cooling. We stimulated in total 246 locations on the frontal plane in 8 male rats, on average 30.8 ± 1.7 sites per rat and evoked calls in 98 sites, averaging 12.3 ± 1.3 (mean ± SEM) sites per rat. Stimulation was performed at the same locations as the cooling (Figure 1D) and additionally at different depths (500 μm steps) at each location. Including the depth z axis, we evoked calls in 195 locations, on average 24.4 ± 3.3 per animal (mean ± SEM). Contrary to the bouts of calls evoked by PAG stimulation, brain stem stimulation only evoked single vocalizations with durations different from PAG stimulations (Figure S1).
Cooling Effects on PAG-Evoked Calls
Cooling of the brainstem had a multitude of effects on PAG stimulation-triggered vocalizations. In the cooled condition, the number, amplitude, and duration of calls were reduced (Figure 2A). Often, calls got fragmented and interrupted, and in a subset of cases, they were completely abolished. In most instances calls recovered after the offset of cooling along with a rewarming of the brain stem. Maps corresponding to Figure 2A for maximum amplitude and call duration can be found in Figure S2. Across all cooling experiments and all locations, we found that PAG stimulation evoked fewer calls during cooling (1.1 ± 1.1 calls less, mean ± SD).
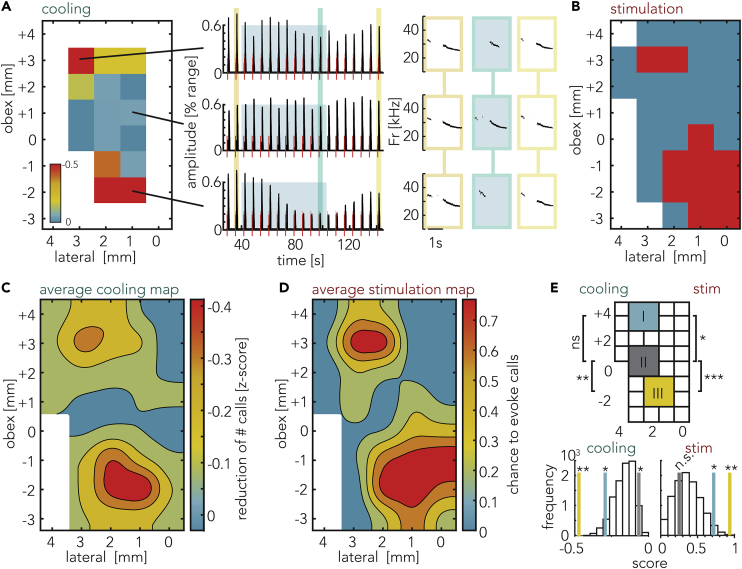
Figure 2.
Two Discrete Locations in the Brainstem Affect Vocalizations
(A) Cooling effects on evoked calls. Left: map of the left brainstem surface presenting cooling effects on call numbers. The measures are taken relative to obex (obex: −13.5 mm [posterior] to Bregma and 0 mm lateral). In the sagittal axis, positive coordinates indicate location anterior to obex and negative coordinates indicate location posterior to obex. The heatmap shows the Z score of the effect of cooling on the call numbers following a stimulation, negative values (red) represent a reduction of call numbers. The white areas have not been sampled in this animal. Middle: amplitude of three cooling locations (indicated by the lines) in the same experiment, y axis depicts relative signal strength as a fraction of the detection range of the microphone settings. The observed reduction of amplitude corresponds to a drop of 3.8 dB for the top trace, 0.2 dB for the center trace, and 11.3 dB for the bottom trace, each calculated between the last three trials before cooling and the last three trials during cooling. The red bars indicate onsets and durations of electrical PAG stimulations. The blue backdrop indicates onset and duration of cooling. The yellow and blue bars indicate calls depicted on the right plot. Right: spectrograms of calls before cooling, late cooling, and rewarming as indicated by the respective colors in the waveform plot to the left. Note the strong reduction of call amplitude and duration during cooling of the anterior (top trace) and posterior (bottom trace) locations, but not during cooling of the intermediate location (middle trace).
(B) Map of brainstem stimulation effects from an example animal. Two distinct areas evoked calls upon stimulation. Orientation and scale as in (A). Red indicates locations where calls could be triggered, blue where no calls were triggered. White areas have not been stimulated in this animal.
(C) Average map of the effect of localized brainstem cooling. Depicted is the reduction of evoked calls during the last three stimulation trials compared against the last three trials prior to cooling onset. For each experiment the Z score was calculated and then averaged across experiments. Orientation and scale as in (A).
(D) Mean response to direct brainstem stimulation. Orientation and scale as in (A). Depth data not taken into account. Unsmoothed version of Figures 2C and 2D are depicted in Figure S2.
(E) Top: comparison of different regions on the brainstem with (I) located anterior, (II) medial, and (III) posterior. The grid aligns with the map of (C) and (D). There is a significant difference in the effect of cooling between the three regions (Friedmann, p = 0.0156). The results of the Tukey-Kramer test are detailed in the text. During stimulation, regions differed in the likelihood to evoke calls (Friedmann, p = 0.0006). Bottom: random shuffling of data and comparison against the measured values for each region. Bottom left: the anterior (yellow) and posterior regions (blue) showed a stronger reduction of calls (random shuffling, p = 0.0475 and p = 0.001, respectively). The central (gray) region showed less call number reduction than the randomly shuffled data (random shuffling, p = 0.0406). Bottom right: for stimulation, both the anterior region as well as the posterior region showed a significantly higher rate of evoked calls (random shuffling, p = 0.03 and p = 0.00005, respectively). The center region did not differ from the shuffled data.
Two Discrete Brain Stem Regions Affect Rat Vocalizations
We noticed an inhomogeneous distribution of call reduction upon cooling (Figure 2A). When stimulating the brainstem directly, a similar, bipartite distribution of call evoking sites can be observed (Figure 2B). Average maps of all experiments of both the cooling (Figure 2C) as well as stimulation (Figure 2D) experiments reaffirm this bipartite distribution. The anterior region affecting calls centered on +3.5 mm anterior and 2.5 mm lateral from obex. The posterior region affecting calls was located 1.5 mm posterior and 1.5 mm lateral from the obex.
In order to assess the spatial heterogeneity of the responses to cooling, we analyzed and compared cooling and stimulation effects between three 2 × 2-mm regions defined along the antero-posterior axis. The anterior and posterior regions were centered on the coordinates mentioned above, and a third region was chosen just in between (Figure 2E top).
There were on average fewer calls per stimulation during cooling the anterior region (1.75 ± 0.23 calls less) and the posterior region (2.37 ± 0.27 calls less). Cooling the center region only led to a marginal reduction of 0.3 ± 0.07 calls. The reduction of calls differed between the regions (Friedmann, p = 0.0183) and was more pronounced for cooling the posterior region than for cooling the central region (Tukey-Kramer, p = 0.0130, Figure 2E top). There was no significant difference between the anterior and the posterior region (Tukey-Kramer, p = 0.33) or between the anterior and the central region (Tukey-Kramer, p = 0.33). To test if the three regions differ from the average response to cooling and if the observed spatial distribution is different from a random distribution, we compared the measured values in our regions against shuffled data. The observed distribution for all regions was significantly different from a random distribution (see Figure 2E bottom left and Methods for details).
In agreement with the distribution of cooling effects, we found that direct stimulation of the anterior region evoked calls in 72% of trials and in the posterior region in 94% of trials (23 and 30 trials of 32 trials, respectively). In contrast, the central region only evoked calls in 17% (5 of 30 trials). The difference between these three regions was significant (Friedmann, p = 0.0006). Anterior and posterior regions evoked more calls than the central region (Tukey-Kramer, p = 0.0206 and p = 0.0005, respectively, Figure 2E top); the difference between anterior and posterior was not significant (Tukey-Kramer, p = 0.53). Compared against randomly distributed data, the anterior and posterior data were significantly different (see Figure 2E bottom right).
The spatial distribution of call reduction induced by cooling and that of the evoked vocalizations by direct electrical stimulations were correlated (Pearson correlation, R = 0.605, p = 0.00012). The excellent spatial correspondence of those maps suggests a highly reproducible fine grain spatial organization of call production in the rat brain stem.
The Two Brain Stem Vocalization Regions Map to Part of the Parvicellular Reticular Formation and the Nucleus Retroambiguus
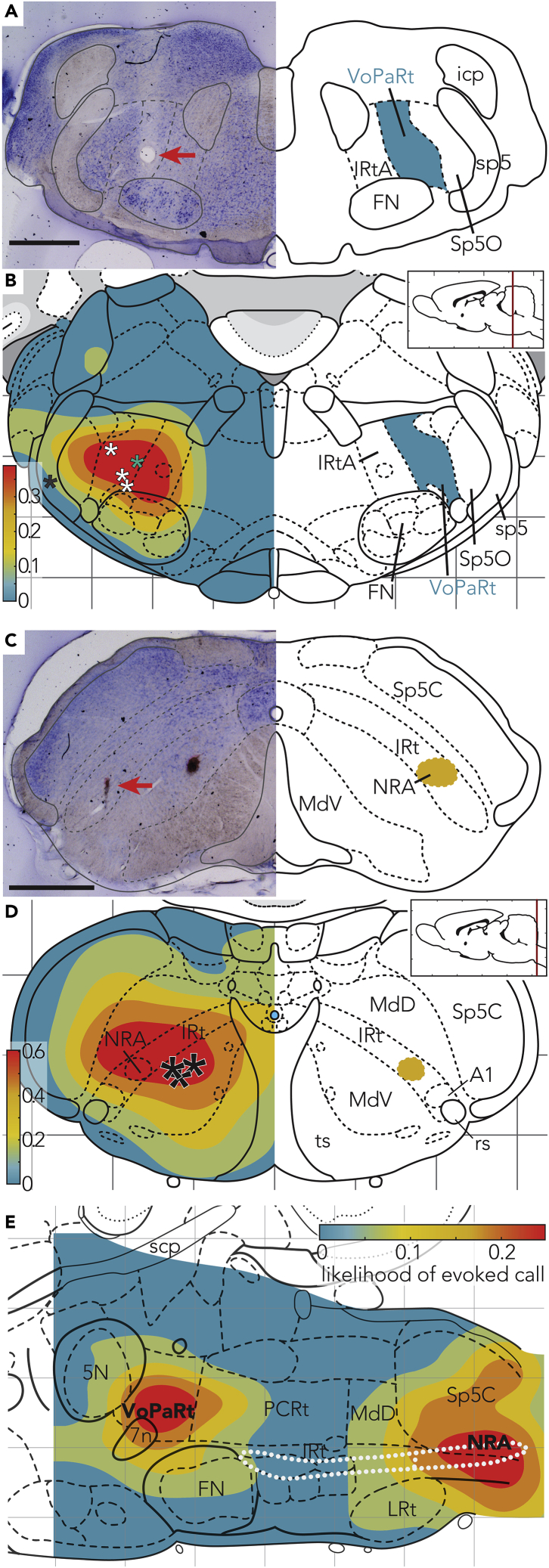
To assess the anatomical identity of the anterior and the posterior vocalization region we combined stimulation experiments and electrolytic lesions. To this end, we obtained depth data by stimulating from the dorsal surface down to the ventral side of the brainstem in steps of 500 μm. At the site where we could trigger calls with the lowest threshold, we placed a lesion at the end of the experiment. In Figure 3A, a brain section at bregma +10.8 mm is depicted with a red arrow marking the lesion. In total we recovered five lesions, of which four were placed within the posterior subsection of the parvicellular reticular formation alpha part (posterior PCRtA). Figure 3B also shows the response rate to stimulations at different depths at obex +3 superimposed on the Paxinos Brain Atlas. The peak response was around the posterior half of the parvicellular reticular formation alpha part (bregma −10.5 to −11.1 mm; lateral 1.5 to 3.3 mm). We therefore suggest the term VoPaRt (vocalization region of the parvicellular reticular formation) for this anterior part of the VPG.
Figure 3.
The Anterior Region of the VPG Corresponds to the Parvicellular Reticular Formation that We Term the VoPaRt, whereas the Posterior Region Maps to the NRA and Its Close Surrounding
(A) Coronal section with Nissl staining. Lesion (red arrow) in the anterior part, −10.80 mm from bregma. Scale bar, 1 mm. On the right, identified regions are drawn.
(B) Coronal map of the average vocalization rate as response of brainstem stimulation. Asterisks indicate lesions placed at sites of strongest responses across animals (single black asterisk indicates a lesion placed 1 mm lateral of the strongest response). In the anterior-posterior axis, lesions ranged from bregma −10.50 mm to bregma −11.16 mm. Lesions have been overlayed with the coronal section of bregma −10.80 from Paxinos & Watson Rat Brain Atlas for illustration purposes.
(C) Coronal section with Nissl staining. Lesion (red arrow) in the posterior part −14.52 mm from bregma. Scale bar, 1 mm.
(D) Average depth response rate to brainstem stimulation superimposed with the Paxinos brain atlas −15.00 mm posterior of bregma. Lesions ranged from −14.52 to −15.72 mm posterior of bregma.
(E) Parasagittal view of the brainstem with a heatmap showing sites of calls evoked by electrical stimulation. The white, dotted structure shows the nucleus ambiguous and the nucleus retroambiguus (subsection in posterior brainstem).
5N, motor trigeminal nucleus; 7n, facial nerve; A1, A1 noadrenaline cells; FN, facial nucleus; icp, inferior cerebellar pedunculus; IRtA, intermediate reticular nucleus alpha part; IRt, intermediate reticular nucleus; LRt, Lateral reticular nucleus; MdD, medullary reticular nucleus dorsal part; MdV, medullary reticular nucleus ventral part; rs, rubospinal tract; Sp5C, Spinal trigeminal tract caudal part; Sp5O,Spinal trigeminal tract oral part; sp5, spinal trigeminal tract; soc, superior olivary complex; ts, tectospinal tract.
We also placed lesions at the posterior region (Figure 3C). We recovered three lesions, all close to the NRA. Lesions ranged from −14.52 to −15.72 mm posterior of bregma. Superimposing the likelihood of calls at the obex −2 position, we found the center of the heatmap overlapping with the NRA, further confirming the role of this region in the call production (Figure 3D). We visualized the brain stem stimulation response also in a parasagittal section with the Paxinos brain atlas (Figure 3E).
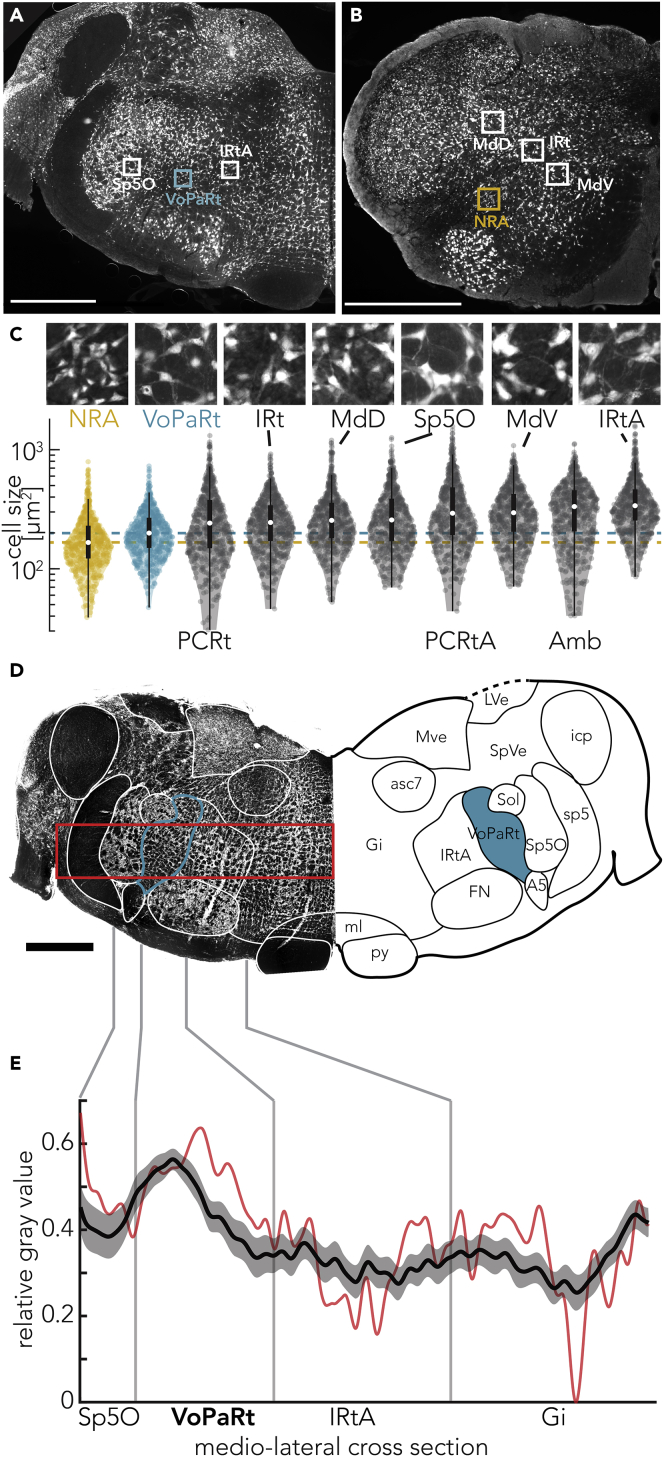
To further characterize the target structures, we quantified the cross-sectional area of somata from ten chosen regions of the brainstem and found that they were different (Figures 4A–4C, Kruskal-Wallis, p = 5.767∗10−151). Specifically, neurons in both VoPaRt and NRA are significantly smaller than neurons in any other regions that we sampled (post hoc Tukey-Kramer, Figure S3A and Table S1). Interestingly, neurons in the anterior PCRtA, not part of the VoPaRt, cell body sizes were significantly larger. Also, neurons in the rest of the parvicellular nucleus were larger. In a similar fashion, the NRA neurons were smaller than any other areas we sampled and even smaller than neurons in the directly anterior neighboring nucleus ambiguus. NRA neurons were smaller than neurons in the VoPaRt (Tukey-Kramer test, p = 2.7∗10−4). Furthermore, we found the VoPaRt to be highly myelinated (Figure 4D). Comparing the myelinization against surrounding areas (Figure 4E), we find the myelinization to be significantly higher than that of both the intermediate reticular formation alpha part and the gigantocellular reticular nucleus (Tukey-Kramer, p = 0.0004 and p = 0.0017, respectively). The difference to the Sp5O was not significant (Tukey-Kramer, p = 0.12).
Figure 4.
VoPaRt and NRA Consist of Exceptionally Small Cells and the VoPaRt Is Highly Myelinated
(A) NeuN staining of a coronal section of the brainstem around the target area of the anterior VPG region, bregma −11.2 mm. The boxes indicate the locations of magnifications in (C). Scale bar, 1 mm.
(B) NeuN staining of a coronal section of the brainstem around the target area of the posterior VPG region, bregma −14.6 mm. The boxes indicate the locations of magnifications in (C). Scale bar, 1 mm.
(C) Comparison of the cell cross-sectional area (nneurons = 600 per region, nanimals = 2). Magnified sections correspond to numbered rectangles in the overview of (F) and (G). Magnified sections are 150× 150 μm. Soma in VoPaRt and NRA are significantly smaller than any other brainstem region measured here; statistical details are summarized in Figure S3 and Table S1. Boxplots overlayed with violin plots depict the soma size for each region. PCRtA lies anterior of the coronal section in (E) and PCRt and Amb are between the sections of (E) and (F); therefore, all three regions are not shown in (E) or (F).
(D) Myelin-stained coronal section at the height of the VoPaRt, where calls were affected by cooling/evoked by stimulation. The VoPaRt (blue) shows a stronger staining (darker) response than surrounding areas. The red rectangle marks the area used for analysis in (E). Additional abbreviations: A5, A5 noradrenaline cells; asc7,ascending fibers of the facial nerve; Gi, gigantocellular reticular nucleus; LVe, lateral vestibular nucleus; ml, medial lemniscus; Mve, medial vestibular nucleus; py, pyramidal tract; SpVe, spinal vestibular nucleus; Sol, nucleus of the solitary tract.
(E) Quantification of myelinization for VoPaRt and surrounding areas. The black line shows the average myelinization from Sp5O to the midline of the brainstem, normalized to the maximum myelinization of each brain section (nsections = 18, nhemispheres = 6, nbrains = 3) analyzed. Gray shading depicts the SEM. The red line is the measurement of the example section presented in (D).
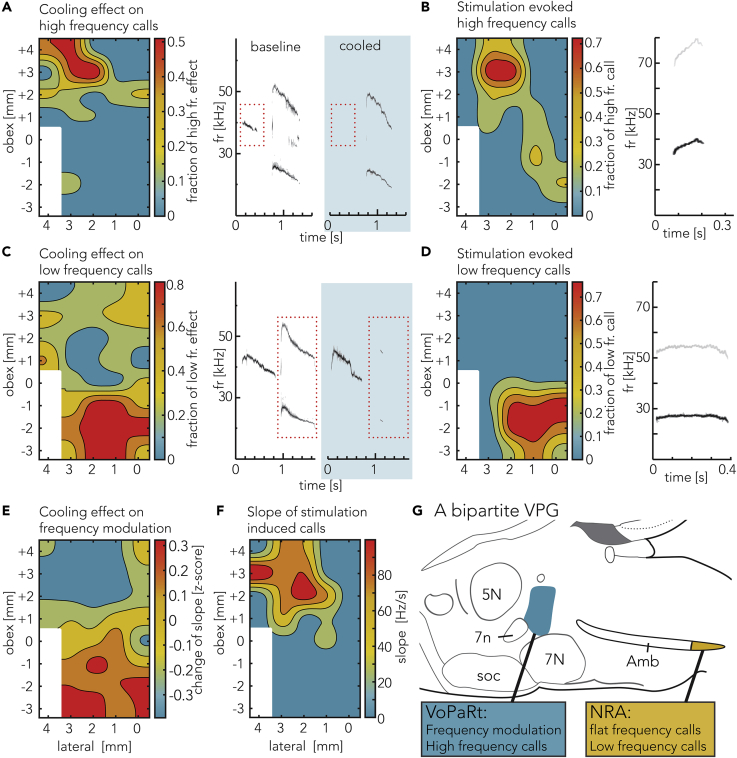
The Anterior VoPaRt Affects High-Frequency Calls and Frequency Modulation, whereas the NRA Affects Flat, Low-Frequency Calls
Having identified two subparts of the VPG, we asked if the VoPaRt and NRA were functionally different. We found that VoPaRt cooling led to a loss of high-frequency calls, i.e., calls with a fundamental frequency higher than 30 kHz (Figure 5A) in 53% of the trials (10 of 19 trials). Cooling in the central region only affected these high-frequency calls in 10% (2 of 21 trials) and cooling the NRA never affected the high-frequency calls selectively (0 of 16 trials). The differences on high-frequency calls between the VoPaRt, center, and NRA region were significant (Friedmann, p = 0.0152), and cooling the VoPaRt showed a significantly stronger reduction in high-frequency calls than the NRA region (Tukey-Kramer, p = 0.013). Cooling reduced the number of high-frequency calls in the VoPaRt more often than it would be expected from a random distribution of the effect (Random shuffling, p = 0.0002, Figure S4A).
Figure 5.
The Identified Anterior Region Selectively Affects High-Frequency and Frequency-Modulated Calls, whereas the Posterior Region Selectively Affects Low-Frequency Calls
(A) Left: average effect on high-frequency calls (>30 kHz) of brainstem cooling. Right: example calls before and during late cooling. High-frequency calls vanish, whereas the low-frequency calls and their harmonics (vertically shifted) are unaffected.
(B) Probability map of locations where stimulation triggered high-frequency calls. High-frequency calls are predominantly evoked in the anterior brainstem (VoPaRt region). Right: example call.
(C) Left: average effect on low-frequency calls (<30 kHz) of brainstem cooling. Right: example calls before and during cooling. More example calls are shown in Figure S5. Low-frequency calls and their respective harmonics are strongly affected, whereas high-frequency calls are unaffected.
(D) Probability map of locations where stimulation triggered low-frequency calls. Low-frequency calls are predominantly evoked in the posterior brainstem (NRA region). Right: example call.
(E) Average change of slopes during cooling trials depicted as the Z score. Red indicates an increase of slope due to cooling, whereas blue indicates a decrease of slope. Example calls can be found in Figure S5.
(F) Average, absolute slope of calls triggered by brainstem stimulation. Red indicates a call with a steep slope, whereas blue indicates a flat call. For example calls, view example calls from (B) and (D) in this figure.
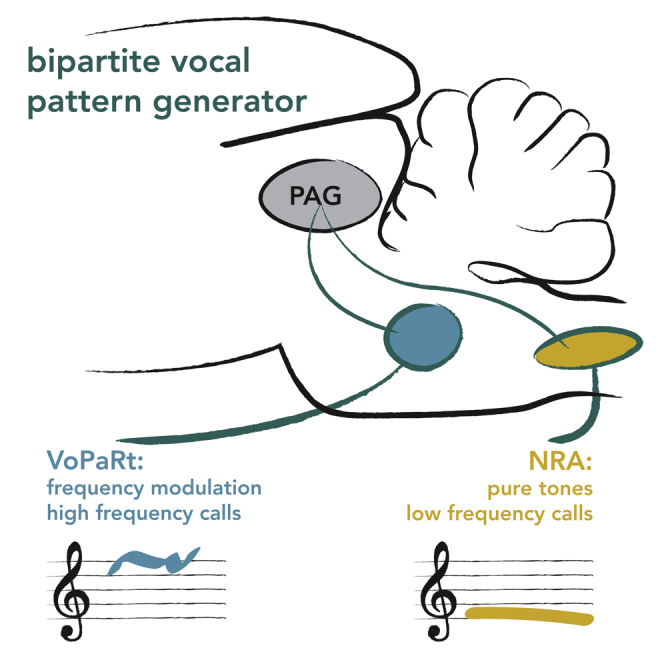
(G) Our suggested model of the bipartite vocal pattern generator in the rat brainstem. Two regions are involved. The VoPaRt is located in the alpha part of the parvicellular reticular formation and has a range of about 700 μm anterior-posterior centered at bregma −10.80 mm posterior of bregma. The second region is the NRA and possibly its direct surroundings at bregma −15.00 mm. Furthermore, the VoPaRt is involved with modulated, high-frequency calls and the NRA with flat and low-frequency calls.
In line with these results, we found that stimulating the VoPaRt led to high-frequency calls in 72% of trials (23 of 32 trials, Figure 5B). In contrast, only 16% (5 of 32) of NRA stimulation trials evoked high-frequency calls and 13% in the center region (4 of 30 trials), resulting in a significant difference between the three regions Friedmann, p = 0.00098). VoPaRt stimulation led to more high-frequency calls than stimulation in the NRA or center region (Tukey-Kramer, p = 0.0011 and p = 0.0161, respectively). The distribution of high-frequency call-evoking sites was further different from a random distribution (Random shuffling, p = 0.0002, see Figure S4B). The spatial distribution of cooling and stimulation effects correlate significantly (Pearson’s correlation, R = 0.553, p = 0.00057). We conclude that the VoPaRt is involved specifically in the production of high-frequency vocalizations.
In contrast to the effect on high-frequency calls, we found that the NRA cooling predominantly affected low-frequency calls (Figure 5C). NRA cooling reduced low-frequency calls in 94% trials (15 of 16 trials), whereas VoPaRt cooling only reduced low frequencies in 26% of trials (5 of 19 trials). Cooling the center region affected low-frequency calls in 14% (3 of 21 trials). These differences were significant (Friedmann, p = 0.022) with the NRA cooling inhibiting significantly more than the center region (Tukey-Kramer, p = 0.017, see Figure S4C for random shuffling tests).
Accordingly, NRA stimulation led to low-frequency calls in 91% of cases (29 of 32 trials) but only in 6% and 10% when stimulating in the VoPaRt or central region, respectively (2 of 32 trials for VoPaRt and 3 of 30 trials in center region) (Figure 5D). The difference was significant (Friedmann, p = 0.00079), with NRA stimulation evoking more low-frequency calls than stimulation in either the VoPaRt or the center region (Tukey-Kramer, p = 0.005 and p = 0.0019, respectively). Random shuffling test confirmed the non-random distribution of these effects and is depicted in Figure S4D). The distribution of cooling and stimulation effects were correlated (Pearson's correlation, R = 0.677, p = 0.000008), supporting the interpretation that the two methods, cooling and stimulation, indeed affect the same circuitry in the brain stem.
To quantify the specific regional effects on high and low frequencies in a combined, robust analysis, we sampled a subset of experiments, where PAG stimulation reliably evoked both high- and low-frequency calls after each stimulation. This analysis was done for the VoPaRt, NRA, and center regions (see Methods). A comparison of Z scores for the cooling effect in the three regions confirmed the results obtained by the manual scoring and are summarized in Figure S6. Summarizing, we found cooling the VoPaRt significantly more strongly reduced high-frequency than low-frequency calls (t test, p = 0.0022). In contrast, cooling of the NRA reduced low-frequency calls significantly more strongly than high-frequency calls (t test, p = 0.005). The center region did not show a difference.
To analyze cooling and stimulation effects on the frequency modulation of calls we analyzed the start and end frequency of five calls before cooling and five calls at the end of cooling and calculated the absolute, average Hz per second change (slope) of each call. Therefore, slope is a simplified measure of the frequency modulation of a call, quantifying the absolute change of frequency per second. The difference between the mean slope before and at the end of cooling is shown as a map in Figure 5E as the Z score. In the VoPaRt area, we find that the slope of calls decreased by 6.09 ± 0.96 Hz.s−1 (mean ± SEM) when cooling is applied (example in Figure S5C). By cooling the NRA, the slope of calls was increased by 5.48 ± 0.74 Hz.s−1 and was significantly different from the VoPaRt (Friedman, p = 0.0183, Tukey-Kramer, p = 0.013) (example in Figure S5D). Compared against randomly distributed data, the VoPaRt showed a significant decrease of call slope, whereas cooling the NRA region showed a significant increase in slope (random shuffling, p = 0.0002 and p = 0.0123, respectively, see Figure S4E). Those results indicate that cooling the anterior region (VoPaRt) reduced call frequency modulation, whereas cooling the posterior region (NRA) enhanced call frequency modulation.
This result is confirmed by measuring the average slope of stimulation-evoked calls. Calls evoked by stimulation of the VoPaRt region had slopes of 90.9 ± 8.5 Hz.s−1 (mean ± SEM, Figure 5F) and were distinct from the calls evoked by NRA stimulation, which had average slopes of 9.49 ± 0.95 Hz.s−1 (Wilcoxon sign rank, p = 0.0078). Compared with randomly distributed data, calls evoked by stimulating the VoPaRt showed a higher modulation and calls evoked by stimulating the NRA region showed less modulation (random shuffling, p = 0.021 and p = 0.0111, respectively, see Figure S4F). The results from the cooling and stimulation were negatively correlated (Pearson's correlation, R = −0.7564, p = 0.000019), confirming the spatial similarity of the effects on call modulation.
A Functionally and Anatomically Bipartite Vocal Pattern Generator
Summarizing our findings, we propose the model for the vocal pattern generation in the rat brainstem in Figure 5G. Thus, the rodent VPG consists of a bipartite system including the vocalization region of the parvicellular reticular formation (VoPaRt) and the NRA. The VoPaRt is predominantly involved in the production of high-frequency, frequency-modulated calls. The NRA on the other hand is involved in the production of low-frequency flat calls.
Discussion
We studied the rat's brainstem vocal pattern generation either by looking at the influence of local brainstem cooling on calls evoked by electrical stimulations of the periaqueductal gray or directly via brainstem electrical stimulations. We find a fine-grain, highly reproducible organization of vocalization involved areas in the brainstem. Our data suggest the rat vocal pattern generator is bipartite. The anterior part of the vocal pattern generator localizes to the posterior parvicellular reticular formation alpha part and appears to be predominantly involved in frequency-modulated high-frequency calls. The posterior part of the vocal pattern generator localizes to the NRA and appears to be predominantly involved in low-frequency, non-modulated calls.
The Rat Brainstem Contains a Vocal Pattern Generator
Our results agree with a huge body of work from numerous species that points to the existence of vocal pattern generator circuits in the mammalian brain stem. (1) Thus, as others before, we find that the brain stem is necessary for call production and that cooling of various brain stem sites aborts calls. This result agrees with chemical inactivation (Shiba et al., 1997) and dissection studies (Zhang et al., 1995) that suggested that the brain stem is necessary for mammalian vocalizations. (2) We confirm that the brainstem affects a wide variety of calls (Dressnandt and Jürgens, 1992) and various aspects of call production (Sugiyama et al., 2010). (3) We find that the brainstem is involved in elementary aspects of call production. Accordingly, brainstem stimulation-evoked vocalizations, in sharp contrast to the upstream periaqueductal gray, do not match up with natural calls but consist of elementary non-natural vocalization. (4) Finally, the brainstem structures identified here are not simply laryngeal or respiratory vocalization output neurons, but instead we identified structures upstream of such motoneuron pools (Cunningham and Sawchenko, 2000; Hannig and Jürgens, 2005; Thoms and Jürgens, 1987).
A Bipartite Model of the Vocal Pattern Generator
A variety of models of the vocal pattern generator have been suggested (Figure 1A), and our data support certain aspects as well as reject others. We suggest a new model organization of the vocal pattern generator circuits (Figure 5G). In agreement with Holstege (1989) we find a critical contribution of NRA (Figure 1A, left) to vocal pattern generation in the rat. At the same time our data suggest that the NRA is not the sole VPG in rats. In agreement with data from squirrel monkeys (Hage and Jürgens, 2006b) we also observed a critical contribution of an anterior region (Figure 1A right) to vocal pattern generation in the rat; we identify this region as a subsection of the parvicellular region of the reticular formation (VoPaRt). Our data do not support the idea of a spatially extended VPG as proposed before (Jürgens and Hage, 2007; Sugiyama et al., 2010) (Figure 1A right, red), however. Both our stimulation and our cooling data are incompatible with the critical prediction of this model, namely, a continuous distribution of vocalization effects along the anterior-posterior axis. A major insight from our work is that the anterior VoPaRt region and the posterior NRA parts of the VPG do not seem to be functionally equivalent.
The Anterior Vocal Pattern Generator Region in the Parvicellular Reticular Formation Contributes to Frequency-Modulated High-Frequency Calls
Our data agree with several studies indicating an anterior location of the VPG, somewhere in the reticular formation (Dressnandt and Jürgens, 1992; Hage and Jürgens, 2006b; Jürgens, 2000; Siebert and Jürgens, 2003; Zhang et al., 1995). Our results extend the conclusion of these studies in two major ways. First, we clarify the exact histological identity of the region as the posterior part of the alpha-nucleus of the parvicellular reticular formation. Such histological identification also allowed us to show that this vocalization region is made up of very small cells. The small cell size of VoPaRt neuron is somewhat surprising at first sight given that this region probably has diverse and far-reaching connections; we wonder if small cell size and the associated short membrane time constants might contribute to high-speed processing for rat vocalization patterns, which are modulated on the millisecond timescale, with muscle contractions as high as 150 Hz in the vocal tract (Riede, 2011). It has been shown that small cells with their comparable small membrane surfaces have a small membrane capacity and thus short time constants. Thus, cerebellar granular cells, which are among the smallest neurons in the mammalian brain, have very short time constants (Brickley et al., 2001; D'Angelo et al., 1995; Silver et al., 1992). The cellular time constant is a major but not the only factor determining processing speed; for review see Koch et al. (1996). We find it therefore likely that the small size of VoPaRt and NRA neurons has functional implications for processing speed. The small cell sizes are further emphasized by the difference in cell size between the anterior section of the PCRtA and the VoPaRt. Another important feature of processing speed is myelinization, and we found the VoPaRt to be highly myelinated, more so than the intermediate reticular nucleus, directly besides it, further strengthening the hypothesis of high-speed processing in the VPG. The second insight from our work is that VoPaRt does not seem to contribute to all vocalizations equally but according to both cooling and stimulation data seems to be particularly relevant for high-frequency calls and frequency modulation. Differential effects on call types have been indicated in lesion studies in squirrel monkeys. PAG stimulation triggered vocalizations in anesthetized monkeys, comparable with our study. By injection of kynurenic acid in the periolivary region (presumably the region homologous to VoPaRt), calls with a strong frequency modulation were stopped, but other calls were unaffected (Jürgens, 2000). In bats echolocation calls have been inhibited by injection of kynurenic acid into the paralemniscal area, whereas the lower-frequency communication calls were unaffected (Fenzl and Schuller, 2005). Intriguingly, there is also evidence from recording studies in monkeys (Hage and Jürgens, 2006a) that call-related activity in the anterior VPG region is specific to high-frequency, frequency-modulated calls.
The Posterior Vocal Pattern Generator Corresponds to the Nucleus Retroambiguus and Contributes to the Low-Frequency, Flat-Frequency Calls
As already discussed above, our result that the NRA plays a critical role in vocal pattern generation is in full agreement with earlier studies. Our analysis added aspects, however. First, we find that NRA cells, much like neurons in the VoPaRt, are exceptionally small. The concurrence of these two results makes us wonder if small cell size is a signature of VPG structures. Second, we find that the NRA does not contribute to all vocalizations equally. Both cooling effects and stimulation indicate that the NRA is particularly relevant for low-frequency, non-frequency-modulated sounds. Thus, we support that the VPG has a functionally dedicated structure, as suggested by Hage and Jürgens (2006a).
Mechanisms of Call Generation
What do these results tell us about how calls are generated? In a series of elegant experiments Long and Fee (2008) introduced the cooling tools to study the role of avian HVC in song control. In this seminal publication, the authors describe a dilation of the zebra finch song upon cooling of the song production pathway (HVC and RA). Thus, these experiments suggested that the HVC might work like a clock in the millisecond range for song control. In our experiments we did not find a dilation of the vocalizations. Instead, we consistently observed a reduction of call duration and sometimes breaking of calls. Comparable syllable breaking was also observed upon stronger cooling of the birdsong motor pathway (Goldin et al., 2013). In our experiments, cooling of the VPG structures resulted in a breaking of the neural circuits underlying the call production and resulting in an interrupted call production. Thus, we do not think that VoPaRt or NRA functions as “clocks” for call production. If anything, these structures seem to function as “drivers” for call production, a cooling of which results in call shortening, breaking, and call loss.
Another insight from our work seems to be that the circuits forming the bipartite mammalian VPG seem to be highly conserved. The predictive power of vocal pattern generation data from monkeys and cats for the functional organization in the rat is certainly astounding.
A Bipartite Vocal Pattern Generator and the Functional Dichotomy of Rat Ultrasonic Vocalizations
What are the functional implications of our results? The first thing that comes to mind is that the functional division of the rat VPG into an anterior high-frequency/frequency-modulation VoPaRt region and a posterior low-frequency NRA division matches up with a functional dichotomy in rat vocalizations. As a coarse first-order approximation rat vocalizations can be divided into high-frequency, often frequency-modulated, calls (often referred to as 50-kHz ultrasonic vocalizations), which communicate positive affect and into low-frequency, often flat calls (referred to as 22-kHz ultrasonic vocalizations), which communicate negative affect/fear/alarm (Brudzynski, 2005; Knutson et al., 2002; Panksepp and Burgdorf, 2003; Schwarting et al., 2007). Dividing the VPG into two subregions that generate sound of opposite valence could greatly simplify the wiring of the rat nervous system and avoid the need of multiplexing opposing messages through the same vocalization centers.
Limitations of the Study
Neuronal recordings could strengthen the results presented here but go beyond the scope of this publication. Experiments with awake animals could strengthen the results further but are methodologically difficult.
Resource Availability
Lead Contact
Requests for further information should be directed to and will be fulfilled by the Lead Contact, Michael Brecht (michael.brecht@bccn-berlin.de).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
The dataset and code generated during this study will be available upon request from the corresponding author.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Undine Schneeweiß for excellent technical assistance and Jean Simonnet, Eduard Maier, and Wei Tang for comments on the manuscript. K.H. was supported by the German Academic Scholarship Foundation; M.B. was a recipient of a European Research Council Synergy Grant (BrainPlay) grant and the Gottfried Wilhelm Leibniz Prize.
Authors Contribution
Conceptualization, Methodology, Investigation, Formal Analysis, Writing, K.H. and M.B.; Visualization, K.H.; Supervision, M.B.
Declaration of Interests
The authors declare no competing interests.
Published: December 18, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101804.
Supplemental Information
References
- Bennett P.J.G., Maier E., Brecht M. Involvement of rat posterior prelimbic and cingulate area 2 in vocalization control. Eur. J. Neurosci. 2019;50:3164–3180. doi: 10.1111/ejn.14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley S.G., Revilla V., Cull-Candy S.G., Wisden W., Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Broca P. Remarks on the seat of the faculty of articulated language, following an observation of aphemia (loss of speech) Bull. Soc. Anat. 1861;6:330–357. [Google Scholar]
- Brudzynski S.M. Principles of rat communication: quantitative parameters of ultrasonic calls in rats. Behav. Genet. 2005;35:85–92. doi: 10.1007/s10519-004-0858-3. [DOI] [PubMed] [Google Scholar]
- Burgdorf J., Kroes R.A., Moskal J.R., Pfaus J.G., Brudzynski S.M., Panksepp J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: behavioral concomitants, relationship to reward, and self-administration of playback. J. Comp. Psychol. 2008;122:357–367. doi: 10.1037/a0012889. [DOI] [PubMed] [Google Scholar]
- Cunningham E.T., Sawchenko P.E. Dorsal medullary pathways subserving oromotor reflexes in the rat: implications for the central neural control of swallowing. J. Comp. Neurol. 2000;417:448–466. [PubMed] [Google Scholar]
- D’Angelo E., De Filippi G., Rossi P., Taglietti V. Synaptic excitation of individual rat cerebellar granule cells in situ: evidence for the role of NMDA receptors. J. Physiol. 1995;484:397–413. doi: 10.1113/jphysiol.1995.sp020673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressnandt J., Jürgens U. Brain stimulation-induced changes of phonation in the squirrel monkey. Exp. Brain Res. 1992;89:549–559. doi: 10.1007/BF00229880. [DOI] [PubMed] [Google Scholar]
- Fay R.A., Norgren R. Identification of rat brainstem multisynaptic connections to the oral motor nuclei using pseudorabies virus: III. Lingual muscle motor systems. Brain Res. Rev. 1997;25:291–311. doi: 10.1016/s0165-0173(97)00028-3. [DOI] [PubMed] [Google Scholar]
- Fenzl T., Schuller G. Echolocation calls and communication calls are controlled differentially in the brainstem of the bat Phyllostomus discolor. BMC Biol. 2005;3:17. doi: 10.1186/1741-7007-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin M.A., Alonso L.M., Alliende J.A., Goller F., Mindlin G.B. Temperature induced syllable breaking unveils nonlinearly interacting timescales in birdsong motor pathway. PLoS One. 2013;8:e67814. doi: 10.1371/journal.pone.0067814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage S.R., Jürgens U. On the role of the pontine brainstem in vocal pattern generation: a telemetric single-unit recording study in the squirrel monkey. J. Neurosci. 2006;26:7105–7115. doi: 10.1523/JNEUROSCI.1024-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage S.R., Jürgens U. Localization of a vocal pattern generator in the pontine brainstem of the squirrel monkey. Eur. J. Neurosci. 2006;23:840–844. doi: 10.1111/j.1460-9568.2006.04595.x. [DOI] [PubMed] [Google Scholar]
- Hannig S., Jürgens U. Projections of the ventrolateral pontine vocalization area in the squirrel monkey. Exp. Brain Res. 2005;169:92. doi: 10.1007/s00221-005-0128-5. [DOI] [PubMed] [Google Scholar]
- Holstege G. Anatomical study of the final common pathway for vocalization in the cat. J. Comp. Neurol. 1989;284:242–252. doi: 10.1002/cne.902840208. [DOI] [PubMed] [Google Scholar]
- Jürgens U. The role of the periaqueductal grey in vocal behaviour. Behav. Brain Res. 1994;62:107–117. doi: 10.1016/0166-4328(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Jürgens U. Localization of a pontine vocalization-controlling area. J. Acoust. Soc. Am. 2000;108:1393–1396. doi: 10.1121/1.1289204. [DOI] [PubMed] [Google Scholar]
- Jürgens U., Hage S.R. On the role of the reticular formation in vocal pattern generation. Behav. Brain Res. 2007;182:308–314. doi: 10.1016/j.bbr.2006.11.027. [DOI] [PubMed] [Google Scholar]
- Jürgens U., Ploog D. Cerebral representation of vocalization in the squirrel monkey. Exp. Brain Res. 1970;10:532–554. doi: 10.1007/BF00234269. [DOI] [PubMed] [Google Scholar]
- Jürgens U., Richter K. Glutamate-induced vocalization in the squirrel monkey. Brain Res. 1986;373:349–358. doi: 10.1016/0006-8993(86)90349-5. [DOI] [PubMed] [Google Scholar]
- Knutson B., Burgdorf J., Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol. Bull. 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Koch C., Rapp M., Segev I. A brief history of time (constants) Cereb. Cortex. 1996;6:93–101. doi: 10.1093/cercor/6.2.93. [DOI] [PubMed] [Google Scholar]
- Kyuhou S., Gemba H. Two vocalization-related subregions in the midbrain periaqueductal gray of the Guinea pig. Neuroreport. 1998;9:1607–1610. doi: 10.1097/00001756-199805110-00064. [DOI] [PubMed] [Google Scholar]
- Long M.A., Fee M.S. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature. 2008;456:189–194. doi: 10.1038/nature07448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C.-L., Jürgens U. Effects of chemical stimulation in the periaqueductal gray on vocalization in the squirrel monkey. Brain Res. Bull. 1993;32:143–151. doi: 10.1016/0361-9230(93)90068-m. [DOI] [PubMed] [Google Scholar]
- Lüthe L., Häusler U., Jürgens U. Neuronal activity in the medulla oblongata during vocalization. A single-unit recording study in the squirrel monkey. Behav. Brain Res. 2000;116:197–210. doi: 10.1016/s0166-4328(00)00272-2. [DOI] [PubMed] [Google Scholar]
- Neff E.P. Neural control of duets between Alston’s singing mice, an emerging vocalization model. Lab Anim. 2019;48:137. [Google Scholar]
- Okobi D.E., Banerjee A., Matheson A.M.M., Phelps S.M., Long M.A. Motor cortical control of vocal interaction in neotropical singing mice. Science. 2019;363:983–988. doi: 10.1126/science.aau9480. [DOI] [PubMed] [Google Scholar]
- Panksepp J., Burgdorf J. “Laughing” rats and the evolutionary antecedents of human joy? Physiol. Behav. 2003;79:533–547. doi: 10.1016/s0031-9384(03)00159-8. [DOI] [PubMed] [Google Scholar]
- Riede T. Subglottal pressure, tracheal airflow, and intrinsic laryngeal muscle activity during rat ultrasound vocalization. J. Neurophysiol. 2011;106:2580–2592. doi: 10.1152/jn.00478.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata J.T., Woolley S.C., Fay R.R., Popper A.N. Springer; 2020. The Neuroethology of Birdsong. [Google Scholar]
- Schwarting R.K.W., Jegan N., Wöhr M. Situational factors, conditions and individual variables which can determine ultrasonic vocalizations in male adult Wistar rats. Behav. Brain Res. 2007;182:208–222. doi: 10.1016/j.bbr.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Shiba K., Umezaki T., Zheng Y., Miller A.D. The nucleus retroambigualis controls laryngeal muscle activity during vocalization in the cat. Exp. Brain Res. 1997;115:513–519. doi: 10.1007/pl00005721. [DOI] [PubMed] [Google Scholar]
- Siebert S., Jürgens U. Vocalization after periaqueductal grey inactivation with the GABA agonist muscimol in the squirrel monkey. Neurosci. Lett. 2003;340:111–114. doi: 10.1016/s0304-3940(03)00071-5. [DOI] [PubMed] [Google Scholar]
- Silver R.A., Traynelis S.F., Cull-Candy S.G. Rapid-time-course miniature and evoked excitatory currents at cerebellar synapses in situ. Nature. 1992;355:163–166. doi: 10.1038/355163a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y., Shiba K., Nakazawa K., Suzuki T., Hisa Y. Brainstem vocalization area in Guinea pigs. Neurosci. Res. 2010;66:359–365. doi: 10.1016/j.neures.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Thoms G., Jürgens U. Common input of the cranial motor nuclei involved in phonation in squirrel monkey. Exp. Neurol. 1987;95:85–99. doi: 10.1016/0014-4886(87)90009-4. [DOI] [PubMed] [Google Scholar]
- Tschida K., Michael V., Takatoh J., Han B.-X., Zhao S., Sakurai K., Mooney R., Wang F. A specialized neural circuit gates social vocalizations in the mouse. Neuron. 2019;103:459–472.e4. doi: 10.1016/j.neuron.2019.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.P., Bandler R., Davis P.J. Brain stem integration of vocalization: role of the nucleus retroambigualis. J. Neurophysiol. 1995;74:2500–2512. doi: 10.1152/jn.1995.74.6.2500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset and code generated during this study will be available upon request from the corresponding author.