Summary
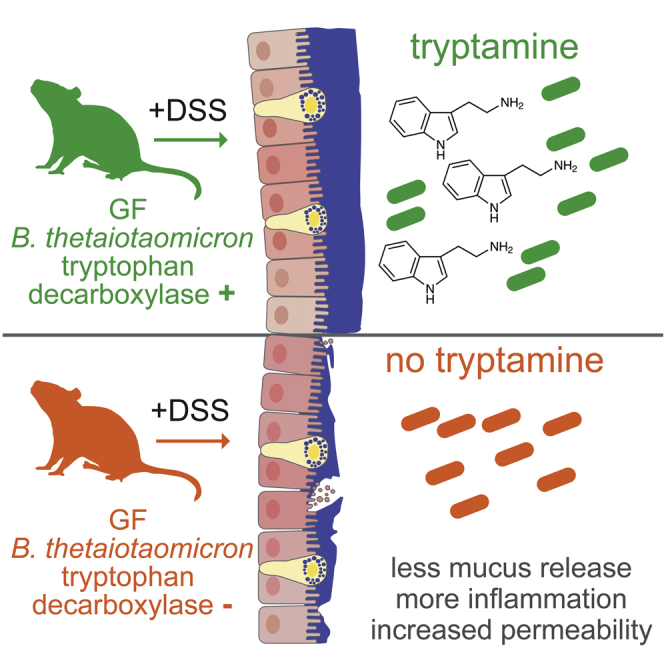
Recent studies emphasize the role of microbial metabolites in regulating gastrointestinal (GI) physiology through activation of host receptors, highlighting the potential for inter-kingdom signaling in treating GI disorders. In this study, we show that tryptamine, a tryptophan-derived bacterial metabolite, stimulates mucus release from goblet cells via activation of G-protein-coupled receptor (GPCR) 5-HT4R. Germ-free mice colonized with engineered Bacteroides thetaiotaomicron optimized to produce tryptamine (Trp D+) exhibit decreased weight loss and increased mucus release following dextran sodium sulfate treatment when compared with mice colonized with control B. thetaiotaomicron (Trp D-). Additional beneficial effects in preventing barrier disruption and lower disease activity index were seen only in female mice, highlighting sex-specific effects of the bacterial metabolite. This study demonstrates potential for the precise modulation of mucus release by microbially produced 5-HT4 GPCR agonist as a therapeutic strategy to treat inflammatory conditions of the GI tract.
Subject Areas: Rodent Gastroenterology, Microbiology
Graphical Abstract
Highlights
-
•
Tryptamine increases serotonin-receptor4-dependent colonic mucus release
-
•
Bacterially derived tryptamine attenuates weight loss in DSS colitis mouse model
-
•
Protective effect of tryptamine in DSS colitis is more pronounced in female mice
-
•
Tryptamine reduces colitis severity and barrier disruption specifically in female mice
Rodent Gastroenterology; Microbiology
Introduction
The gastrointestinal (GI) tract harbors a diverse microbial community which plays an important role in regulating intestinal physiology and human health (Bhattarai, 2018). These microbial communities are unique among individuals and can in part explain the inter-individual variability in GI physiology. Gut bacteria can communicate with the host via an array of bioactive metabolites in order to influence GI function. We recently described a role for tryptamine produced by bacterial metabolism of dietary tryptophan in increasing intestinal secretion by activating host serotonin receptor 4 (5-HT4R) (Bhattarai et al., 2018). 5-HT4R is a G-protein-coupled receptor (GPCR), which is expressed along the intestinal epithelium and plays an important role in GI physiology beyond regulating intestinal secretion. In this regard, a previous study in transgenic mice has also shown that 5-HT4R is expressed in epithelial goblet cells and pharmacological activation of epithelial 5-HT4R causes goblet cell cavitation suggesting a role of 5-HT4R in luminal mucus release (Hoffman et al., 2012). In addition, epithelial 5-HT4R agonists have also been reported to reduce colitis severity in dextran sodium sulfate (DSS)-treated mice (Spohn et al., 2016) signifying a potential link between epithelial 5-HT4R-induced mucus release and attenuation of colitis severity in mice.
The luminal mucus layer provides a niche for commensal microbial colonization and serves as a carbon source for some gut microbes. In addition to providing a niche for microbial colonization, the mucus layer also serves as a crucial line of defense against invasion and disruption of epithelial layer by pathogens and toxic metabolites. Perturbation of the mucosal barrier as a result of genetic factors or by gut microbiota could therefore allow increased permeability for luminal microbes and their products causing innate immune activation and exacerbation of inflammatory processes such as those observed in patients with inflammatory bowel disease (IBD) (Pullan et al., 1994; Sicard et al., 2017).
Given the importance of mucosal barrier in regulating GI health and preventing inflammatory condition such as IBD (Heazlewood et al., 2008; Johansson et al., 2014; Swidsinski et al., 2007; Van der Sluis et al., 2006), in this study, we tested the effect of tryptamine, a recently identified bacterially derived 5-HT4R agonist, on mucus release and inflammation in a mouse model of IBD.
Results
Tryptamine Induces Goblet Cell Cavitation by Activating 5-HT4R
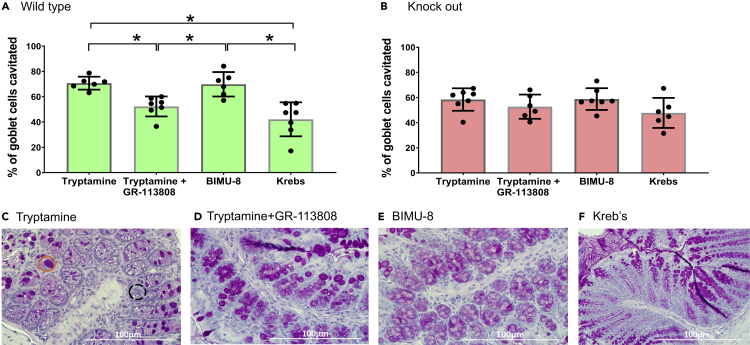
The mucosal bilayer within the colon is constantly replenished and maintained through “basal” steady-state release of mucins from epithelial goblet cells and is controlled by several factors such as luminal contents (e.g. polysaccharides), bioactive metabolites (neuropeptides, short chain fatty acids [acetate and butyrate]), neurotransmitters (e.g. 5-HT), and immune factors (inflammatory cytokines) (Barcelo et al., 2000; Burger-van Paassen et al., 2009; Lindén et al., 2008; Mawe and Hoffman, 2013). Besides basal release, a recent study showed that goblet cells can also be stimulated to release mucus through activation of 5-HT4R, a GPCR which is expressed in enterocytes and goblet cells (Hoffman et al., 2012). Therefore, to test if tryptamine, a bacterially derived 5-HT4R-specific agonist (Bhattarai et al., 2018) induces mucus release, we quantified ex vivo mucus release from colonic tissues following treatment with 3 mM tryptamine, a concentration that is comparable to the physiologic concentration of other bacterial metabolites such as short-chain fatty acids (Williams et al., 2014), and compared its efficacy with a known pharmacologic 5-HT4R-agonist, BIMU-8 (10 μM). We found a significant increase in the percentage of cavitated goblet cells following treatment of proximal colonic tissue from conventionally raised 129/Sv wild-type (WT) mice with tryptamine when compared to control (Krebs solution; Figures 1A, 1C–1F, and S1). Tryptamine-induced goblet cell cavitation was significantly reduced by pre-incubating proximal colon tissue with 5-HT4R-antagonist GR-13808 (30 nM) for 10 min to levels similar to the Krebs-only control (Figure 1A). Goblet cell cavitation following treatment with BIMU-8 was comparable to the cavitation induced by tryptamine in WT mice (Figures 1C–1F). Next, to conclusively determine that the tryptamine-induced mucus release was mediated through 5-HT4R, we repeated our ex vivo experiments in 5-HT4R knockout (KO) mice (Figure 1B). Tryptamine and BIMU-8 did not significantly increase goblet cell cavitation compared to control (Krebs) in KO mice unlike the effect seen in WT mice. Together, our data suggest that tryptamine causes goblet cell cavitation and stimulates mucus release in the mouse proximal colon specifically through activation of 5-HT4R. Mucus serves as the first line of defense against luminal factors driving inflammatory responses in the colonic epithelium. Hence, we next determined the physiological relevance of increased mucus release in response to tryptamine in a murine model of IBD.
Figure 1.
Tryptamine Evokes Mucus Release Ex Vivo, Which Is Blocked by 5-HT4R Antagonist and Absent in 5-HT4R KO Mice
(A–F) Change in goblet cell cavitation in response to tryptamine, tryptamine + GR-113808, BIMU-8, and Krebs in (A) WT sv/129 and (B) 5-HT4R KO sv/129 mice proximal colon. Representative 40X PAS-H images of WT proximal colon incubated in (C) tryptamine (3 mM), (D) tryptamine (3 mM) + GR (30 nM), (E) BIMU-8 (10 μM), or Kreb's solution (F) for 30 min. Orange circle represents intact goblet cells and while dotted black circle represents cavitated goblet cells. n = 6–7. Data are mean ± SD. ∗p < 0.05; one-way ANOVA
Bacterially Derived Tryptamine Attenuates Colitis Severity in a Murine Model of Inflammatory Bowel Disease
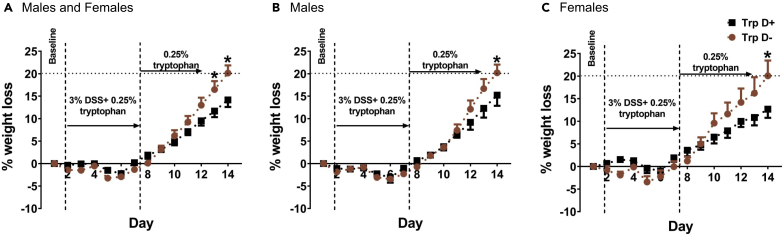
We have previously engineered a B. thetaiotaomicron strain to include tryptophan decarboxylase under control of a phage promoter (B. thetaiotaomicron Trp D+) to produce tryptamine and show that monocolonization of germ-free (GF) mice with tryptamine producing B. thetaiotaomicron Trp D+ results in significantly higher levels of tryptamine and significantly lower levels of tryptophan in the stool when compared to empty vector control (B. thetaiotaomicron Trp D) (Bhattarai et al., 2018). We used our previously engineered strains to test if in vivo tryptamine production by B. thetaiotaomicron Trp D+ strain increased mucus release and was protective against colitis following DSS administration. The experimental outline and study design for bacterial colonization and DSS treatment are highlighted in Figure S2. We used percentage weight loss as the primary surrogate of colitis given that it has been shown to be an objective measure of severity of DSS-induced colitis in mice (Lanka Britto et al., 2019). The optimal concentration of DSS to trigger an inflammatory response has not been well studied in monocolonized mice, and there are scant data in GF mice. Hence, we performed pilot experiments to determine the optimal concentration of DSS that resulted in weight loss in our control B. thetaiotaomicron Trp D- monocolonized mice. We found that 1%, 2%, or 2.5% of DSS administration did not result in a significant decrease in body weight (Figure S3). We however found that administration of 3% DSS for 6 days resulted in weight loss in B. thetaiotaomicron Trp D- monocolonized control mice (Figure 2A, Table S1). Hence, we used 3% DSS for our experiments. We found that B. thetaiotaomicron Trp D+ monocolonized mice showed significantly lower percentage weight loss compared to Trp D- monocolonized mice following treatment with 3% DSS, and this effect was seen in both male (Figures 2B and Table S1A) and female (Figures 2C and Table S1B) monocolonized mice suggesting a protective role for bacterially derived tryptamine in the DSS colitis model. We also performed control experiments to assess the effect of DSS administration in GF mice. However, due to high mortality (≥55% within 3 days of 3% DSS administration) in GF mice compared to monocolonized mice (Figure S4), these experiments had to be discontinued.
Figure 2.
Bacterially Derived Tryptamine Attenuates Weight Loss Following DSS Administration
(A–C) Figures show DSS induced percentage weight loss in both male and female Trp D+ and Trp D monocolonized mice. Dotted line denotes 20% decrease in body weight. n = 12–14 (7–8 males; 5–6 females). Data are mean ± SEM. ∗p < 0.05; two-way ANOVA with Bonferroni post hoc.
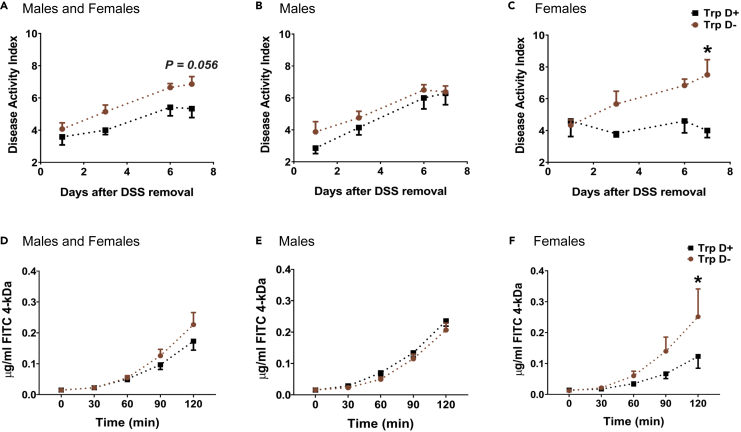
The Disease Activity Index (DAI, Table S2) is a severity index often used to assess severity of colitis in DSS-treated mice. In order to determine the severity of colitis, we measured the DAI in B. thetaiotaomicron Trp D+ and Trp D- monocolonized mice. We found that while B. thetaiotaomicron Trp D+ monocolonized mice had a lower DAI, this was significant only in female mice and not in male mice (Figures 3A–3C) when compared with B. thetaiotaomicron Trp D- monocolonized mice. Interestingly, we did not observe any significant difference in histopathological damage score between male or female Trp D+ and Trp D- mice (Figure S5, Table S3). As tryptamine predominantly increases mucus release but does not modify the underlying inflammatory response, the findings above on weight loss, DAI, and histology suggest that tryptamine either helps contain the spread of colitis or blocks initiation of lesion in multiple regions within the colon rather than reduce the severity in individual sites.
Figure 3.
Bacterially Derived Tryptamine Attenuates Colitis Severity as Measured by Disease Activity Index and Epithelial Permeability in Female Mice Following DSS Administration
(A–C) Graphs show change in the DAI over a 7-day period post-DSS administration in both male and female Trp D+ and Trp D monocolonized mice. n = 12–14 (7–8 males; 5–6 females).
(D–F) Graphs highlight change in 4-kDa FITC flux across proximal colon tissue over two hours in both male and female DSS-treated Trp D+ and Trp D monocolonized mice. n = 9 (4–5 males; 4–5 females). Data are mean ± SEM. ∗p < 0.05; two-way ANOVA with Bonferroni post hoc.
Bacterially Derived Tryptamine Reduces DSS-Induced Epithelial Barrier Disruption in Female Mice
The intestinal epithelial layer serves as a physical barrier that prevents inflammation by restricting the unwanted passage of luminal contents, pathogens, and toxic metabolites, which can trigger the intestinal immune system. Previous studies have shown that patients with IBD often display disrupted paracellular permeability which may be the initial pathogenic event in IBD (Chang et al., 2017; Lechuga and Ivanov, 2017; McCole, 2014; Söderholm et al., 1999). We therefore tested changes in epithelial permeability following development of DSS colitis in colonic tissues from mice colonized with tryptamine producing B. thetaiotaomicron Trp D+ bacteria and control B. thetaiotaomicron Trp D- monocolonized. Again, similar to our finding in the DAI, we found that while B. thetaiotaomicron Trp D+ monocolonized mice exhibit decreased colonic permeability as indicated by lower 4KDa FITC flux (measure of paracellular permeability), this difference was significant only in female mice but not in male mice (Figures 3D–3F).
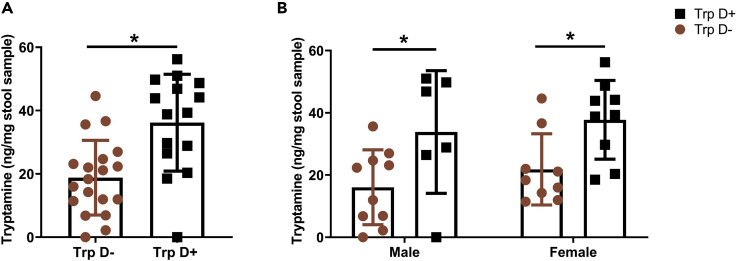
To confirm that the capacity to produce tryptamine by the engineered B. thetaiotaomicron is not affected by DSS challenge or the biological sex of the recipient mice, we measured tryptamine levels in fecal samples collected 7 days after DSS challenge in B. thetaiotaomicron Trp D+ and B. thetaiotaomicron Trp D- monocolonized male and female mice. Indeed, Trp D+ monocolonized mice had significantly higher tryptamine levels compared to Trp D- mice. However, there was no difference in tryptamine levels among Trp D+ monocolonized male and female mice (Figures 4A and 4B). This suggests that sex differences observed in the DAI and colonic permeability between Trp D+ and Trp D- mice following DSS treatment were not driven by differences in tryptamine levels and are likely due to host factors.
Figure 4.
Tryptamine Levels Are Higher in Fecal Samples of DSS Challenged B. thetaiotaomicron Trp D + Colonized Mice Compared to B. thetaiotaomicron Trp D- Colonized Mice
(A and B) Figures show tryptamine concentration in fecal samples of both DSS challenged male and female Trp D+ and Trp D monocolonized mice. n = 15–19 (6–10 males; 9 females). Data are mean ± SD. ∗p < 0.05; unpaired t test and two-way ANOVA with Bonferroni post hoc.
Bacterially Derived Tryptamine Increases In Vivo Mucus Release following DSS Administration
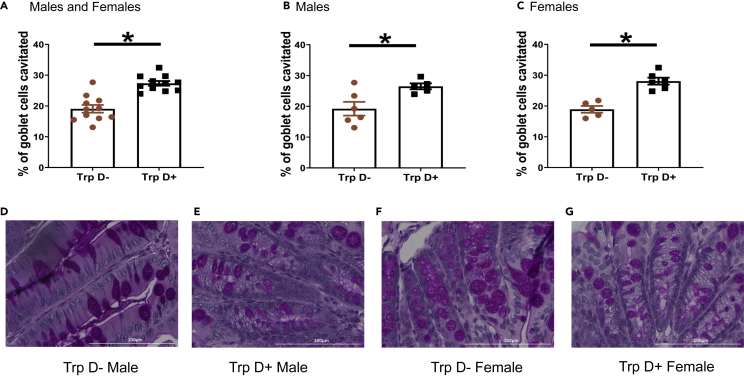
As we observed increased goblet cell cavitation in response to tryptamine ex vivo, we hypothesized that the protective effect of bacterially derived tryptamine to DSS-induced colitis was a result of increased mucus release in vivo. Indeed, we found that B. thetaiotaomicron Trp D+ monocolonized mice exhibit significantly greater goblet cell cavitation following DSS administration, and this effect was seen in both male and female mice (Figures 5A–5C). This suggests that in vivo tryptamine production by gut bacteria increases mucus release, which may play a role in mediating its protective effects in the DSS mouse model of colitis.
Figure 5.
Bacterially Derived Tryptamine Increases In Vivo Mucus Release Following DSS Administration
(A–C) Graphs show difference in goblet cell cavitation post-DSS treatment in both male and female Trp D+ and Trp D monocolonized mice. n = 11 (5–6 males; 5–6 females).
(D–G) Representative 40X PAS-H images of the proximal colon from Trp D- and Trp D+ monocolonized male and female mice subjected to 7 days of DSS challenge. Data are mean ± SD. ∗p < 0.05; unpaired t test.
Discussion
In this study, we show that colonization of the GI tract by engineered B. thetaiotaomicron capable of constitutively producing high levels of tryptamine increases 5-HT4R-dependent mucus release, decreases the severity of colitis, and prevents disruption of the epithelial barrier. The identification of a bacterial metabolite that drives an important host physiologic function can help explain inter-individual variability in susceptibility to inflammatory conditions like IBD, as well as development of a mechanism-based therapeutic approach to treat IBD. Interestingly, tryptamine increases 5-HT4R-dependent mucus release and attenuates weight loss following DSS administration in both sexes. However, the protective effect of bacterially derived tryptamine following exposure to DSS appears more pronounced in female mice as evidenced by significantly lower DAI. This is likely driven by its effect on barrier function and significantly lower intestinal permeability. Our findings highlight the complex interplay between host factors ( e.g. estradiol which is known to play a protective role in DSS colitis) and microbiota-derived bioactive compounds that are important in the pathogenesis of chronic diseases.
Luminal tryptamine is produced through enzymatic decarboxylation of tryptophan which is a bacterial function that is present only in a fraction of human gut microbiomes and is rare among bacteria in general (Facchini et al., 2000; Williams et al., 2014). The few bacterial species that have been shown to express native tryptophan decarboxylase include Clostrodium sporogenes and Ruminococcus gnavus (Williams et al., 2014). Tryptamine production by gut bacteria may represent a selective way for gut microbiota to affect mucus release and utilize mucin as an energy source. For example, the tryptamine producer R. gnavus is also an avid mucin degrader that utilizes sialic acid from mucin glycans as a carbon source (Crost et al., 2016). The dual action of tryptamine-producing commensals might therefore be an evolutionary phenomenon for survival and niche establishment. Given the ability to degrade mucin in addition to producing tryptamine, R. gnavus strains may not be protective and could even lead to worsening of IBD (Henke et al., 2019). The data presented in our study show that engineering non-mucin degrading strains to produce bacterial GPCR agonists could allow for specifically enhancing mucus release and attenuate inflammation as seen in patients with IBD.
In conclusion, our data show that precise control of tryptamine production in the gut by engineering commensal bacteria can help reinforce the mucus barrier and improve overall colitis burden in response to noxious stimuli in mice. This study is an example of how communication between gut bacteria and the host can be exploited for development of novel therapeutics.
Limitations of the Study
Our study does have a few limitations. While we found an interesting sex dependent difference in the DAI and colonic permeability between B. thetaiotaomicron Trp D+ and B. thetaiotaomicronTrp D- colonized mice subjected to DSS treatment and confirmed that this was not due to differences in bacterial tryptamine production, we have not explored all the host mechanisms that may be driving this sex difference. It is also necessary to emphasize that IBD is a complex multifactorial disorder and as such no single animal model can entirely encapsulate the complex pathophysiology of IBD as seen in humans. In this study, we used the DSS model to test the impact of increased mucus release on inflammation, but further studies in additional models are needed to determine the role of bacterially derived tryptamine in IBD. It is too early to directly extrapolate these findings to humans despite encouraging evidence from our study. Future studies can also help verify whether microbial metabolite mimicry through production of synthetic tryptamine analogs (Abiero et al., 2019) could be used as a potential pharmaceutic modality to improve gut inflammatory conditions.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Purna Kashyap (kashyap.purna@mayo.edu).
Materials Availability
This study did not generate new unique reagents or materials. Any materials used in the study will be made available with a material transfer agreement.
Data and Code Availability
This study did not generate novel code, software, or algorithms. No data were generated that required submission in public repositories.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We would like to thank Dr. Valérie Compan for providing 5-HT4R KO mice, Dr. Beverley Greenwood-Van Meerveld for helpful discussions, and Lyndsay Busby for administrative assistance and copyediting. We would like to thank Sarah Becker for technical assistance. We acknowledge the help of Jenny Kirkpatrick and Boyd Palmer from Mayo Clinic Histopathology Core Facility located in Scottsdale, Arizona. We thank Lisa Till and Will Moor with their assistance in the GF facility. This work was made possible by funding from NIH DK114007, Minnesota Partnership for Biotechnology and Medical Genomics and the Center for Individualized Medicine, Mayo Clinic, Rochester, MN.
Authors Contributions
Y.B., S.J., L.S., and P.C.K. designed the experiments and the overall data analysis; S.J., Y.B., and D.R.L. contributed to the mouse experiments; S.G., D.R.L., Y.B., and S.J. contributed to development of novel histochemistry-based digital image analysis algorithm for assessing mucus release; B.B.W., M.A.F., J.S., S.J., and Y.B. contributed to bacterial engineering and determining culture conditions; R.A.T.M. contributed to tryptamine measurement from fecal pellets; D.R.L. and M.P. contributed to histopathological damage assessment from H&E stained colon slides; and Y.B. and P.C.K. wrote the manuscript with input from all co-authors who read, revised, and approved the manuscript.
Declaration of Interests
P.C.K. reports being on the Advisory Board of Novome Biotechnologies and an ad hoc consultant for Pendulum Therapeutics, IP group, and Otsuka Pharmaceuticals. P.C.K. holds patent US20170042860A1 for use of tryptamine producing bacteria (“Transparent Methods for using Ruminococcus gnavus or Clostridium sporogenes to treat gastrointestinal disorders”), and P.C.K. and Mayo Clinic have a financial interest related to this research. These interests have been reviewed and managed in accordance with Mayo Clinic conflict of interest policies. M.A.F. reports being a co-founder and director of Federation Bio. Y.B. is currently a scientist at Takeda Pharmaceuticals. J.L.S. reports being a founder of Novome Biotechnologies, Inc.
Published: December 18, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101798.
Contributor Information
Lei Sha, Email: lei.sha@foxmail.com.
Purna C. Kashyap, Email: kashyap.purna@mayo.edu.
Supplemental Information
References
- Abiero A., Ryu I.S., Jun Botanas C., Custodio R.J.P., Val Sayson L., Kim M., Jun Lee H., Jin Kim H., Seo J.-W., Cho M.C. Four novel synthetic tryptamine analogs induce head-twitch responses and increase 5-HTR2a in the prefrontal cortex of mice. Biomol. Ther. (Seoul) 2019;28:83–91. doi: 10.4062/biomolther.2019.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelo A., Claustre J., Moro F., Chayvialle J.A., Cuber J.C., Plaisancié P. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut. 2000;46:218–224. doi: 10.1136/gut.46.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai Y. Microbiota-gut-brain axis: interaction of gut microbes and their metabolites with host epithelial barriers. Neurogastroenterol Motil. 2018;30:e13366. doi: 10.1111/nmo.13366. [DOI] [PubMed] [Google Scholar]
- Bhattarai Y., Williams B.B., Battaglioli E.J., Whitaker W.R., Till L., Grover M., Linden D.R., Akiba Y., Kandimalla K.K., Zachos N.C. Gut microbiota-produced tryptamine activates an epithelial G-protein-coupled receptor to increase colonic secretion. Cell Host Microbe. 2018;23:775–785.e775. doi: 10.1016/j.chom.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger-van Paassen N., Vincent A., Puiman P.J., van der Sluis M., Bouma J., Boehm G., van Goudoever J.B., van Seuningen I., Renes I.B. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem. J. 2009;420:211–219. doi: 10.1042/BJ20082222. [DOI] [PubMed] [Google Scholar]
- Chang J., Leong R.W., Wasinger V.C., Ip M., Yang M., Phan T.G. Impaired intestinal permeability contributes to ongoing bowel symptoms in patients with inflammatory bowel disease and mucosal healing. Gastroenterology. 2017;153:723–731.e721. doi: 10.1053/j.gastro.2017.05.056. [DOI] [PubMed] [Google Scholar]
- Crost E.H., Tailford L.E., Monestier M., Swarbreck D., Henrissat B., Crossman L.C., Juge N. The mucin-degradation strategy of Ruminococcus gnavus: the importance of intramolecular trans-sialidases. Gut Microbes. 2016;7:302–312. doi: 10.1080/19490976.2016.1186334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchini P.J., Huber-Allanach K.L., Tari L.W. Plant aromatic L-amino acid decarboxylases: evolution, biochemistry, regulation, and metabolic engineering applications. Phytochemistry. 2000;54:121–138. doi: 10.1016/s0031-9422(00)00050-9. [DOI] [PubMed] [Google Scholar]
- Heazlewood C.K., Cook M.C., Eri R., Price G.R., Tauro S.B., Taupin D., Thornton D.J., Png C.W., Crockford T.L., Cornall R.J. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke M.T., Kenny D.J., Cassilly C.D., Vlamakis H., Xavier R.J., Clardy J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn's disease, produces an inflammatory polysaccharide. Proc. Natl. Acad. Sci. U S A. 2019;116:12672–12677. doi: 10.1073/pnas.1904099116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J.M., Tyler K., MacEachern S.J., Balemba O.B., Johnson A.C., Brooks E.M., Zhao H., Swain G.M., Moses P.L., Galligan J.J. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142:844–854.e844. doi: 10.1053/j.gastro.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.E., Gustafsson J.K., Holmén-Larsson J., Jabbar K.S., Xia L., Xu H., Ghishan F.K., Carvalho F.A., Gewirtz A.T., Sjövall H. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2014;63:281–291. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanka Britto S., Krishna M., Kellermayer R. Weight loss is a sufficient and economical single outcome measure of murine dextran sulfate sodium colitis. FASEB Bioadv. 2019;1:493–497. doi: 10.1096/fba.2019-00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechuga S., Ivanov A.I. Disruption of the epithelial barrier during intestinal inflammation: quest for new molecules and mechanisms. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864:1183–1194. doi: 10.1016/j.bbamcr.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindén S.K., Florin T.H., McGuckin M.A. Mucin dynamics in intestinal bacterial infection. PLoS One. 2008;3:e3952. doi: 10.1371/journal.pone.0003952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawe G.M., Hoffman J.M. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 2013;10:473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCole D.F. IBD candidate genes and intestinal barrier regulation. Inflamm. Bowel Dis. 2014;20:1829–1849. doi: 10.1097/MIB.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullan R.D., Thomas G.A., Rhodes M., Newcombe R.G., Williams G.T., Allen A., Rhodes J. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut. 1994;35:353–359. doi: 10.1136/gut.35.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard J.F., Le Bihan G., Vogeleer P., Jacques M., Harel J. Interactions of intestinal bacteria with components of the intestinal mucus. Front. Cell Infect. Microbiol. 2017;7:387. doi: 10.3389/fcimb.2017.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderholm J.D., Peterson K.H., Olaison G., Franzén L.E., Weström B., Magnusson K.E., Sjödahl R. Epithelial permeability to proteins in the noninflamed ileum of Crohn's disease? Gastroenterology. 1999;117:65–72. doi: 10.1016/s0016-5085(99)70551-2. [DOI] [PubMed] [Google Scholar]
- Spohn S.N., Bianco F., Scott R.B., Keenan C.M., Linton A.A., O'Neill C.H., Bonora E., Dicay M., Lavoie B., Wilcox R.L. Protective actions of epithelial 5-hydroxytryptamine 4 receptors in normal and inflamed colon. Gastroenterology. 2016;151:933–944.e3. doi: 10.1053/j.gastro.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidsinski A., Loening-Baucke V., Theissig F., Engelhardt H., Bengmark S., Koch S., Lochs H., Dörffel Y. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut. 2007;56:343–350. doi: 10.1136/gut.2006.098160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Sluis M., De Koning B.A., De Bruijn A.C., Velcich A., Meijerink J.P., Van Goudoever J.B., Büller H.A., Dekker J., Van Seuningen I., Renes I.B. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Williams B.B., Van Benschoten A.H., Cimermancic P., Donia M.S., Zimmermann M., Taketani M., Ishihara A., Kashyap P.C., Fraser J.S., Fischbach M.A. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe. 2014;16:495–503. doi: 10.1016/j.chom.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate novel code, software, or algorithms. No data were generated that required submission in public repositories.