Abstract
Objective:
Concern exists regarding surgery after thoracic radiation (RT). We aim to assess early results of anatomic resection following induction therapy with platinum-based chemotherapy (C) and full dose RT for resectable N2 + stage IIIA NSCLC.
Methods:
Two prospective trials were recently conducted by NRG Oncology in patients with resectable N2 + IIIA NSCLC with primary end point of mediastinal node sterilization following concurrent full-dose CRT (RTOG 0229 and 0839). All surgeons demonstrated post-induction resection expertise. Induction consisted of weekly carboplatin (AUC =2.0) and paclitaxel (50 mg/m2) and concurrent RT 60 Gy (0839)/ 61.2 Gy (0229) in 30 fractions. Patients in 0839 were randomized 2:1 to weekly panitumumab + CRT or CRT alone during induction. Primary results were similar in all treatment arms and reported previously. Short-term surgical outcomes are reported here.
Results:
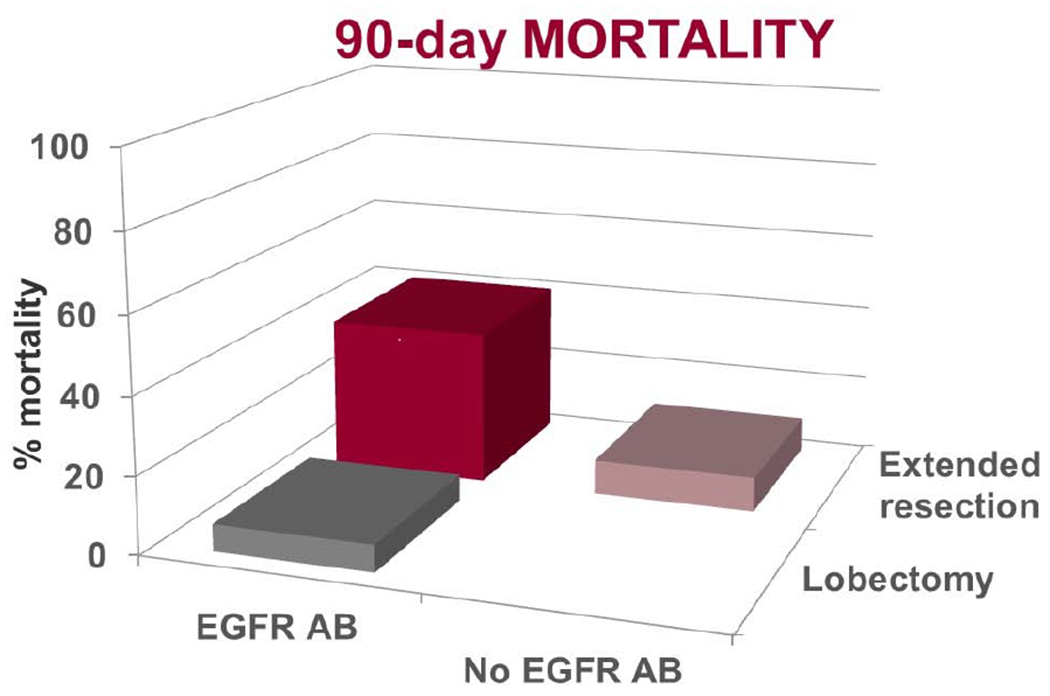
126 patients enrolled; 93 (74%) had anatomic resection, 77 lobectomies and 16 extended resections. R0 resections occurred in 85 (91%). Fourteen (15%) resections were attempted minimally invasively including 2 converted without event. Grade 3/4 surgical adverse events (AEs) were reported in 26 (28%), 30-day mortality in 4 (4%) and 90-day mortality in 5 (5%). Patients undergoing extended resections suffered similar rates of Grade 3/4 AEs [OR 0.95, CI: 0.42-3.8] but higher 30-day (1.3% vs. 18.8%) [OR 17.54 95%CI: 1.75-181.8] and 90-day mortality (2.6% vs. 18.8%) [OR8.65, 95%CI: 1.3-56.9].
Conclusion:
Lobectomy was performed safely following full-dose concurrent CRT in these multi-institutional prospective trials, increased mortality was noted with extended resections.
Background
Stage III non-small cell lung cancer (NSCLC) presents a treatment challenge. Patients are treated for cure, but only 20-30% achieve that goal, and acceptable treatment plans vary dramatically.1 Chemotherapy-based multimodality therapy is standard of care, but ideal local therapy is unclear. Concurrent chemoradiotherapy (CRT) without surgery is commonly accepted as the standard of care,2 but local relapse is the first site of failure in >30%.3 Therefore, adding surgery to improve outcomes is attractive. Lung resection following induction CRT to 45 Gy was shown to be safe in Southwest Oncology Group 88054 and the Pancoast Intergroup study 0160.5 The Intergroup trial 0139 evaluated adding surgery to CRT for the treatment for N2+ IIIA NSCLC.6 The primary endpoint was improved overall survival (OS), but was not met despite a significant improvement in progression-free survival (PFS). Some of this discrepancy was due to an unacceptably high number of patients undergoing pneumonectomy and excessive mortality following pneumonectomy.6 Preoperative radiotherapy (RT) was limited to 45 Gy in these trials, but multiple single institution series report the safety of lung resection following CRT to 60Gy.7,8 NRG Oncology RTOG 0229 trial (0229) and RTOG 0839 trial (0839) evaluated the feasibility of a multicenter trial of trimodality therapy in which surgical resection followed CRT with full dose (60 Gy) RT. Surgical certification was required in both trials to ensure expertise in post-induction lung resections. (Figure 1) Mediastinal lymph node (LN) sterilization was the primary endpoint of both trials; as a potential surrogate for long-term benefit because of its strong association with OS following induction therapy.6 RTOG 0839 (0839) also tested the hypothesis that adding an epidermal growth factor receptor (EGFR) antibody to concurrent induction CRT could improve mediastinal LN sterilization and outcomes in operable N2+IIIA NSCLC. EGFR antibodies potentiate radiation effects in head and neck cancers,9 and RTOG trial 0324 combined cetuximab with CRT in inoperable stage III NSCLC patients and demonstrated excellent 2-year OS with minimal toxicity increase.10,11 Panitumumab is a fully human monoclonal EGFR antibody.
Figure 1.
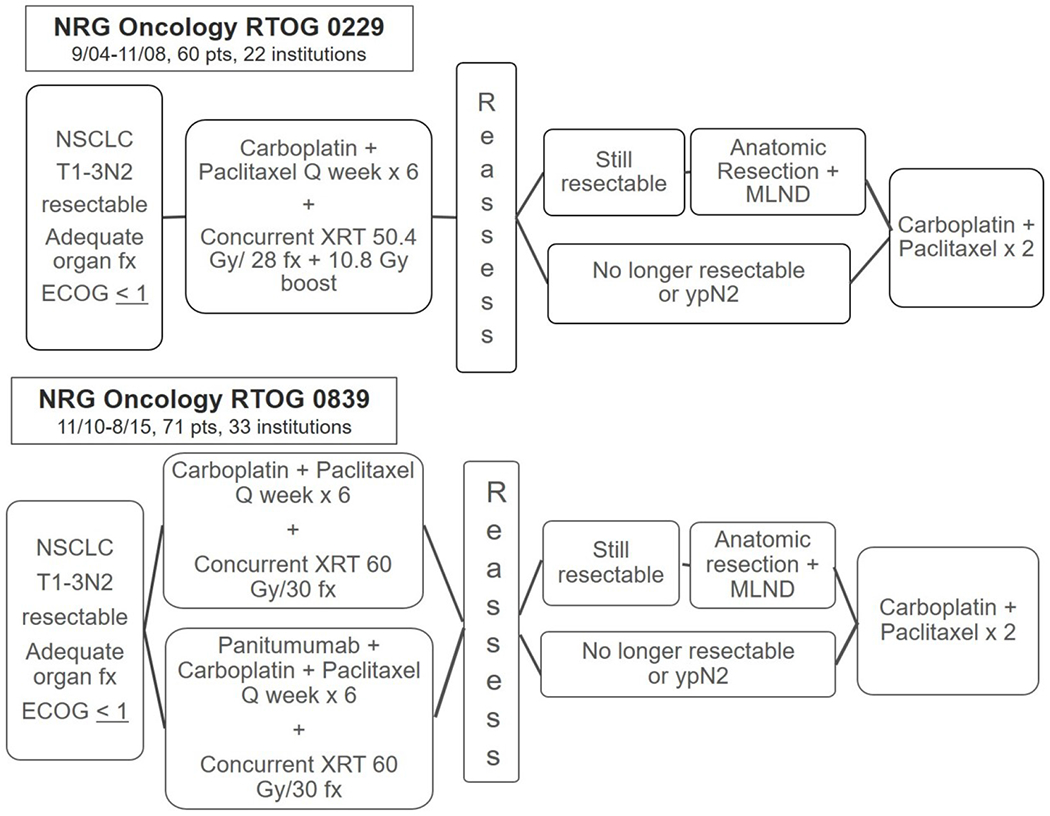
Schema for NRG Oncology trial 0229 (A) and NRG Oncology trial 0839 (B): both were prospective, multi-institutional phase II trials evaluating the efficacy of concurrent neoadjuvant chemotherapy and high-dose chest radiation prior to resection for stage III non-small cell lung cancer, with the primary endpoint of mediastinal nodal clearance. Randomization in NRG Oncology 0839 was 2:1 in favor of research arm.
Primary endpoints for each trial were previously reported and similar in all treatment arms, mediastinal LN sterilization rate was 63% in 0229,12 68% in the control arm of 0839 and 50% in experimental arm.13 Since inclusion criteria, induction strategies, and surgical requirements were similar in the trials, short-term surgical results are combined to increase power and reported here. The primary goal of this analysis examination of short-term surgical outcome following full-dose concurrent CRT induction and determine factors associated with Grade 3/4 adverse events (Gr3/4AEs), and 30-day and 90-day mortality.
Materials and Methods
Patients
Patients with histologically documented NSCLC, stage III N2+, ECOG performance status of 0-1 and normal organ function (creatinine < 1.5, normal liver function tests) were potentially eligible. Mediastinal LN involvement had to be proven pathologically, and with the goal of reducing extended resections LN had to be < 3cm in greatest diameter, and could not be in direct continuation with the primary tumor. Multimodality evaluation with the operating surgeon was required prior to enrollment to determine suitability for tri-modality therapy and resectability. Each institution was required to have local Institutional Review Board approval, and patients to provide written informed consent. This analysis is limited to those patients who completed CRT and anatomic resection.
Induction and Consolidative Therapy
In both trials, chemotherapy consisted of weekly carboplatin (AUC =2.0), paclitaxel (50 mg/m2) for 6 weeks. Weekly panitumumab at 2.5 mg/kg was added during CRT in the experimental arm of 0839. Concurrent thoracic RT in 0229 was 61.2 Gy in 1.8-Gy daily fractions (50.4 Gy to the gross disease, ipsilateral hilar and mediastinal nodes, and 10.8 Gy boost to gross disease and involved nodes). In 0839 a total 60 Gy was delivered in 2 Gy daily fractions to gross disease only without irradiation of clinically uninvolved LNs. Consolidation chemotherapy in both trials consisted of carboplatin (AUC =6) and paclitaxel (200 mg/m2) every 21 days for two courses.
Surgery
All surgeons required authorization from RTOG. Surgeons were all board certified in thoracic surgery, and needed to 1) be familiar with the American Thoracic Society LN map, 2) adhere to intra-operative requirements for mediastinal staging and bronchial buttressing, and 3) perform a minimal of 10 lobectomies or pneumonectomies per year, with 5 after induction therapy. Required pre-surgical evaluation following CRT included repeat history and physical, pulmonary function tests, and restaging PET/CT to identify changes in functional status and rule out disease progression. The mediastinum was pathologically reassessed pre-operatively in a separate procedure or at the time of resection at each institution’s discretion. Resections were performed 4-8 weeks following CRT by lobectomy or greater to achieve complete resection. Minimally invasive resections were permitted in 0839. Systematic mediastinal LN evaluation was required at resection including stations 2R, 4R, 7, 9R, and 10R for right sided tumors and stations 5, 6, 7, 9L, and 10L for the left side. If persistently positive mediastinal LNs were found at pre-operative staging or thoracotomy, resection of the primary was at the surgeon’s discretion. Bronchial stump buttressing with autologous vascularized soft-tissue was required. Patients were preferentially extubated in the operating room and postoperative fluids kept to a minimum. Major surgical deviations included resection of patients with FEV1 < 800 cc, inadequate LN sampling, lack of bronchial stump coverage, surgery > 10 weeks after CRT, and surgery deferred for other than medical reasons. Surgical Quality Assurance was performed using operative notes and pathology reports at the midpoint of 0229 and continuously during 0839. Adverse events were recorded by each institution per the NCI Common Terminology Criteria for Adverse Events (CTCAE) v3 in 0229 and v4 in 0839. No significant differences exist between versions for AEs reported here.
Dosimetric Assessment
Correlations between induction RT dose to specific cardiothoracic structures and short-term surgical outcomes was evaluated in the 0839 cohort (0229 radiation plans were not accessible). The lungs were delineated using automatic thresholding and excluding gross tumor volume (GTV). The heart was contoured from base to apex beginning at the origin of the ascending aorta. Dose volume histograms were generated for heart, combined lungs, ipsilateral and contralateral lungs, and esophagus.
Statistical Considerations
Univariate logistic regression methods were used to differentiate between grade 3-5 and less serious morbidity. When logistic regression modeling was not possible due to insufficient events (< 10 events), Fisher’s exact testing was used to compare categorical outcomes and Wilcoxon rank sum was used for continuous data comparisons.. For multiplicity adjustment, the Benjamini-Hochberg method was used to control the false discovery rate less than 0.25 for univariate analysis of morbidity.
Role of the funding source
The sponsors were not involved in the data interpretation.
Results
The trials were open sequentially, 0229 from 9/04-11/08 and enrolled 60 patients from 22 institutions. 0839 was open 11/10- 8/15; it accrued 71 patients from 33 institutions prior to closure by the Data Monitoring Committee after a planned interim analysis. A total of 93 (74.4%) patients underwent anatomic resection. Reasons for not undergoing resection were similar between trials and are outlined in CONSORT diagram (Figure 2).
Figure 2.
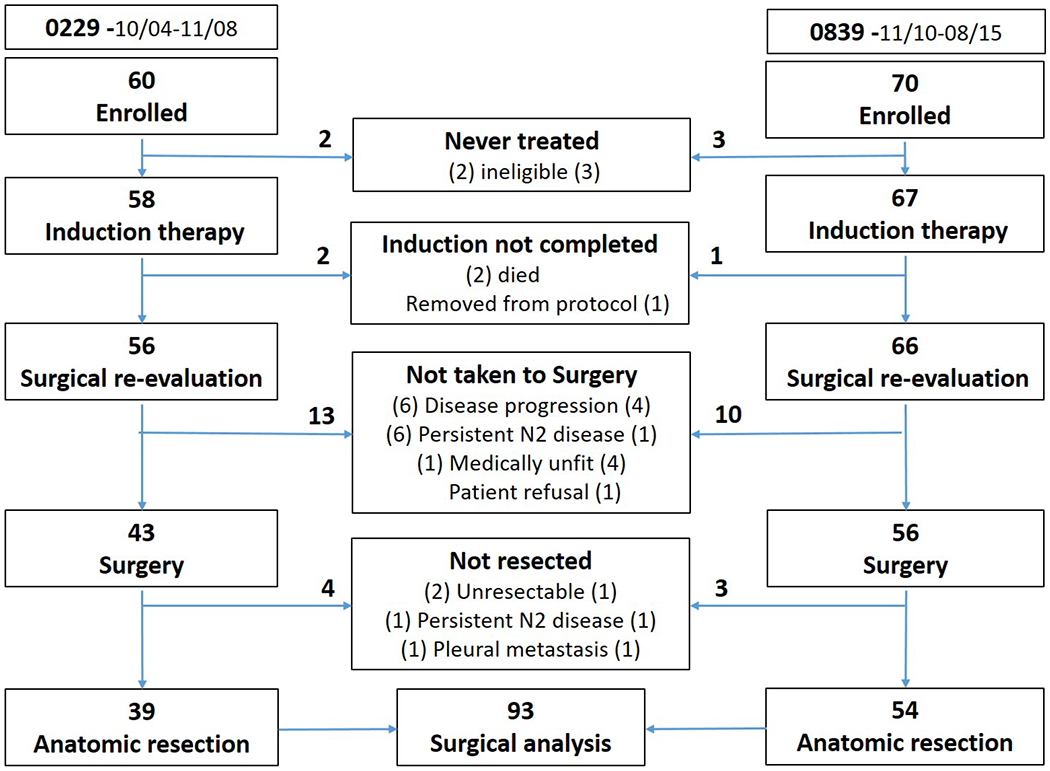
CONSORT (CONsolidated Standards of Reporting Trials) Diagram for the inclusion of patients in the pooled analysis of short-term surgical outcomes form NRG Oncology trial 0229 and NRG NRG Oncology trial 0839. Surgical outcomes analysis was limited to the 93 patients that underwent anatomic resection.
The distribution of patient and tumor characteristics is presented in Table 1. The median age is 60, 57% of the patients were male, and the majority were white (85%). Most patients had T1 (37%) or T2 (51%) tumors. All patients had at least one N2+ node either clinically or pathologically. More tumors were situated in the right upper lobe (47%) and were adenocarcinomas (58%). The median FEV1 at enrollment was 2.34 L. Only 25% of patients undergoing resection had a pathologic complete response (pCR) to CRT, but 72% achieved mediastinal LN clearance.
Table 1.
Patient and Tumor Characteristics
| 0229 (n=39) | 0839 (n=54) | Total (n=93) | |
|---|---|---|---|
| Age | |||
| Median | 58 | 60.5 | 60 |
| Min - Max | 41 - 75 | 32 - 78 | 32 - 78 |
| Q1 - Q3 | 53 - 64 | 54 - 67 | 53 - 65 |
| Gender | |||
| Male | 23 (59.%) | 30 (56%) | 53 (57%) |
| Female | 16 (41.%) | 24 (44%) | 40 (43%) |
| Race | |||
| White | 30 (77%) | 49 (91%) | 79 (85%) |
| Non-white | 9 (23%) | 5 (9%) | 14 (15%) |
| T-stage at enrollment | |||
| T1 | 14 (36%) | 20 (37%) | 34 (37%) |
| T2 | 19 (49%) | 28 (52%) | 47 (50%) |
| T3 | 6 (15%) | 6 (11%) | 12 (13%) |
| Smoking History | |||
| Never | 2 (5%) | 4 (7%) | 6 (6%) |
| Former | 25 (64%) | 39 (72%) | 64 (69%) |
| Current | 8 (21%) | 8 (15%) | 16 (17%) |
| Unknown | 4 (10%) | 3 (6%) | 7 (8%) |
| Pack Years (including non-smokers as 0) | |||
| Median | 39 | 34 | 35 |
| Min - Max | 0 - 110 | 0 - 136 | 0 - 136 |
| Q1 - Q3 | 30 - 60 | 8.5 - 53 | 15 - 54 |
| ECOG performance status | |||
| 0 | 31 (84%) | 42 (78%) | 73 (80%) |
| 1 | 6 (16%) | 12 (22%) | 18 (20%) |
| # Clinically or Pathologically + N2 Nodes @ Diagnosis | |||
| Median | 1 | 1 | 1 |
| Min - Max | 1 - 4 | 1 - 4 | 1 - 4 |
| Q1 - Q3 | 1 - 2 | 1 - 2 | 1 - 2 |
| Tumor Location | |||
| RUL | 18 (46%) | 26 (48%) | 44 (47%) |
| RML | 4 (10%) | 4 (7%) | 8 (9%) |
| RLL | 6 (15%) | 11 (20%) | 17 (18%) |
| LUL | 7 (18%) | 11 (20%) | 18 (19%) |
| LLL | 4 (10%) | 2 (4%) | 6 (6%) |
| Histology | |||
| Adenocarcinoma | 19 (49%) | 35 (65%) | 54 (58%) |
| Squamous | 7 (18%) | 13 (24%) | 20 (21%) |
| Other | 13 (33%) | 6 (11%) | 19 (20%) |
| FEV1 at Baseline | |||
| Median | 2.3 | 2.385 | 2.34 |
| Min - Max | 1.28 - 4.13 | 1.09 - 3.82 | 1.09 - 4.13 |
| Q1 - Q3 | 1.98 - 2.93 | 2.06 - 2.91 | 2.06 - 2.92 |
| Institution Type | |||
| Community | 12 (31%) | 15 (28%) | 27 (29%) |
| Academic | 25 (64%) | 34 (63%) | 59 (63%) |
| VA | 2 (5%) | 5 (9%) | 7 (8%) |
| Panitumumab | |||
| No | 39 (100.0%) | 19 (35%) | 58 (62%) |
| Yes | 0 (0.0%) | 35 (65%) | 35 (38%) |
| pCR | |||
| No | 29 (74%) | 41 (76%) | 70 (75%) |
| Yes | 10 (26%) | 13 (24%) | 23 (25%) |
| Mediastinal Nodal Clearance | |||
| No | 9 (23%) | 17 (31%) | 26 (28%) |
| Yes | 30 (77%) | 37 (69%) | 67 (72%) |
Q1 = first quartile; Q3 = third quartile; ECOG PS, Eastern Cooperative Group performance status; pCR, Complete Response to Chemoradiation
Distribution of surgical data is presented in Table 2. The median time from the end of CRT to surgery was 42 days. Most patients had lobectomies (83%), but lobectomy rate was higher in 0839 (87%) than in 0229 (77%). In 0839, 14 resections were attempted minimally invasively, two converted to open. RO resections occurred in 91% of surgical patients and 68% of all eligible patients. Median duration of surgery was 240 minutes. The number of mediastinal and hilar LN stations sampled was similar in both studies, with a median of 3 mediastinal and 2 hilar. Bronchial stump coverage was used in 84% of surgeries, most frequently with an intercostal muscle (71%). Estimated blood loss was also lower in 0839, and fewer patients required a transfusion than in 0229 (4% vs. 21%). The median length of stay was 7 days for 0229, 5 days for 0839.
Table 2.
Surgical Information
| 0229 (n=39) | 0839 (n=54) | Total (n=93) | |
|---|---|---|---|
| Days from End of RT to Surgery | |||
| Median | 47 | 41.5 | 42 |
| Min - Max | 30 - 78 | 27 - 119 | 27 - 119 |
| Q1 - Q3 | 42 - 52 | 38 - 48 | 38 - 50 |
| Extent of Resection | |||
| Lobectomy | 30 (77%) | 47 (87%) | 77 (83%) |
| Pneumonectomy | 5 (13%) | 3 (6%) | 8 (9%) |
| Bilobectomy | 3 (8%) | 3 (6%) | 6 (6%) |
| Sleeve | 1 (3%) | 1 (2%) | 2 (2%) |
| Approach | |||
| Open | 38 (97%) | 41 (76%) | 79 (85%) |
| Minimally invasive | 1 (3%) | 13 (24%) | 14 (15%) |
| RO | |||
| No | 5 (13%) | 3 (6%) | 8 (9%) |
| Yes | 34 (87%) | 51 (94%) | 85 (91%) |
| Surgery Duration (minutes) | |||
| Median | 236.5 | 246 | 240 |
| Min - Max | 69 - 570 | 120 - 2503 | 69 - 2503 |
| Q1 - Q3 | 199 - 292 | 184 - 321 | 189 - 318 |
| Number of Mediastinal Node Stations Sampled | |||
| Median | 3 | 3 | 3 |
| Min - Max | 0 - 7 | 1 - 7 | 0 - 7 |
| Q1 - Q3 | 2 - 5 | 3 - 4 | 2 - 4 |
| Number of Hilar Node Stations Sampled | |||
| Median | 2 | 2 | 2 |
| Min - Max | 0 - 5 | 0 - 5 | 0 - 5 |
| Q1 - Q3 | 1 - 3 | 1 - 3 | 1 - 3 |
| Bronchial Coverage | |||
| No | 7 (18%) | 8 (15%) | 15 (16%) |
| Intercostal | 26 (67%) | 40 (74%) | 66 (71%) |
| Other | 6 (15%) | 4 (7%) | 10 (11%) |
| Intraoperative Transfusion | |||
| No | 31 (79%) | 52 (96%) | 83 (89%) |
| Yes | 8 (21%) | 2 (4%) | 10 (11%) |
| Estimated Blood Loss (cc) | |||
| Median | 200 | 150 | 175 |
| Min - Max | 100 - 1400 | 0 - 2000 | 0 - 2000 |
| Q1 - Q3 | 150 - 350 | 62.5 - 259.5 | 100 - 300 |
| Length of Stay (days) | |||
| Median | 7 | 5 | 5 |
| Min - Max | 2 - 60 | 2 - 29 | 2 - 60 |
| Q1 - Q3 | 5 - 9 | 4 - 7 | 4 - 8 |
Q1 = first quartile; Q3 = third quartile.
The distribution of dosimetric data obtained from 0839 is in Supplemental Table 1. The median lung V5 was 56.5%, indicating that over half of the lung excluding GTV received at least 5 Gy in half of the patients. The median mean dose to the lungs excluding GTV was 16.1 Gy. Ipsilateral lung V5 was 68.2% and contralateral lung V5 44.3 %. The ipsilateral and contralateral mean lung doses were 24.5Gy and 6.15 Gy respectively. Heart V10 was 25.3%, but the median heart V60 was low at 0.7%. The mean and maximum esophageal doses were 23.3 Gy, and 63.1 Gy.
Non-fatal post-operative AEs were reported in 50 (53%) patients and rates were similar between trials. Most common AEs were Grade 1-2 atrial fibrillation (20), atelectasis (18) and pneumothorax (17). All AEs are outlined in Table 3. Grade 3/4AEs occurred 21(23.6%). Post-operative 30-day mortality occurred in 4 (4%) and 90-day mortality in 5 (5%). For all resections the rates for any AE, Gr 3/4AE, 30-day mortality, and 90-day mortality were 59%, 28%, 4%, and 5%; for the 77 lobectomy patients the rates were 57%, 26%, 1.3% and 2.6%. In a comparison of outcomes between patients who received panitumumab or not, rates of any AE (40% vs. 71%, p=0.003) and Gr 3/4AE (9% vs. 31%, p=0.02) were lower with the EGFR antibody, but there was trend to higher 30-day (9% vs. 2%, p=0.15) and 90-day mortality (11% vs. 2%, p=0.06). The only death in the cohort that did not receive panitumumab, was a patient with post-operative pulmonary edema following pneumonectomy.
Table 3.
Adverse events entire cohort (N=93)
| Grade 1/2 AEs | Grade 3/4 AEs | |||
|---|---|---|---|---|
| Atrial fibrillation (20) Atelectasis (18) Pneumothorax (17) Effusion (16) Pain (5) Pneumonia (4) Chylothorax (3) Hypotension (3) Hypocalcemia (3) Rash (2) Constipation (2) Anemia (2) ACS, arterial injury, atrial flutter, confusion, diarrhea, esophagitis, hypoglycemia, ileus, narcotic OD (1) |
Pneumonitis (5) Pneumonia (4) Respiratory failure (3) RLN injury (3) Dyspnea (3) Pulmonary edema (3) Atelectasis (2) Pulmonary embolus (2) AKI, anemia, aspiration, bronchial obstruction, chylothorax, DTs, effusion, empyema, esophagitis, hemorrhage, hypocalcemia, hyponatremia, hypotension, hypoxia, lymphopenia, necrotic muscle flap, sepsis, ventricular arrhythmia, UTI, wound infection (1) |
|||
| Grade 5 AEs | ||||
| Demographic | Resection | EGFR AB | Gr 5AE | POD |
| 43 M | R pneumonectomy | No | PPE | 6 |
| 52 M | Bilobectomy | Yes | Hemorrhage | 23 |
| 61 M | Lobectomy | Yes | BPF | 28 |
| 63 F | L pneumonectomy | Yes | Aspiration | 29 |
| 71 F | Lobectomy | Yes | Pneumonitis | 45 |
AE, adverse event; CT, cardiothoracic; ACS, acute coronary syndrome, OD, overdose; AKI, acute kidney injury; DTs, delirium tremors; UTI, urinary tract infection; M male: F, female: EGFR AB, panitumab; R, right; L, left; PPE, post-pneumonectomy pulmonary edema; BPF, broncho-pleural fistula; POD, post-opertative day. Events were reported by each institution per the NCI common terminology criteria for adverse events (CTCAE) v3 in 0229 and v4 in 0839.
The distribution and univariate analysis for morbidity are outlined in Table 4. Panitumumab and higher T-stage were associated with decreased risk of morbidity in the full cohort, while extended resections were associated with an increased risk of fatal morbidity. No RT dose to heart or lung was associated with Gr3/4 AEs in these models, but there was a trend toward increased risk of fatal AEs with higher lung V5, ipsilateral lung V10, and contralateral lung V5. Details for each of mortality are outline in Table 3 and distribution for 90-day mortality is outlined in Table 5. Use of panitumumab and extended resections were associated with an increased risk for operative mortality. While there was a trend toward increased 90-day mortality with higher ipsilateral and contralateral lung V5 and higher total lung V10, that effect was lost after taking into account panitumumab and extent of resection. These models should be viewed with caution given the low number of events.
Table 4.
Distribution of Surgical Morbidity and Univariate Models of Grade 3+ Morbidity
| Variable | Grade 3-4 (n=21) | Grade 5 (n=5) | < Grade 3 Morbidity (n=67) | Grade 3+ Morbidity (n=26) | OR (95% CI) | FDR-adjusted p-value* |
|---|---|---|---|---|---|---|
| Study | ||||||
| 0229 (RL) | 10 (26%) | 1 (2%) | 28 (72%) | 11 (28%) | ||
| 0839 | 11 (20%) | 4 (7%) | 39 (72%) | 15 (28%) | 0.98 (0.39, 2.45) | 0.96 |
| Age | ||||||
| Median | 62 | 61 | 60 | 61.5 | 1.01 (0.96, 1.07) | 0.64 |
| Min-Max | 41-72 | 44-71 | 32-78 | 41-72 | ||
| Q1-Q3 | 56-65 | 52-69 | 53-65 | 54-66 | ||
| Gender | ||||||
| Male (RL) | 9 (17%) | 3 (6%) | 41 (77%) | 12 (23%) | ||
| Female | 12 (30%) | 2 (5%) | 26 (65%) | 14 (35%) | 1.84 (0.74, 4.59) | 0.33 |
| Race | ||||||
| White (RL) | 19 (24%) | 5 (6%) | 55 (70%) | 24 (30%) | ||
| Non-white | 2 (14%) | 0 (0%) | 12 (86%) | 2 (14%) | 0.38 (0.08, 1.84) | 0.33 |
| T-Stage | ||||||
| T1 (RL) | 12 (35%) | 3 (9%) | 19 (56%) | 15 (44%) | ||
| T2/T3 | 9 (15%) | 2 (3%) | 48 (81%) | 11 (19%) | 0.29 (0.11, 0.74) | 0.17 |
| cN2 positive stations | ||||||
| 1 (RL) | 16 (26%) | 4 (7%) | 41 (67%) | 20 (33%) | ||
| 2-4 | 5 (16%) | 1 (3%) | 26 (81%) | 6 (19%) | 0.47 (0.17, 1.33) | 0.33 |
| Tumor location - right vs left | ||||||
| Right-sided (RL) | 17 (25%) | 4 (6%) | 48 (70%) | 21 (30%) | ||
| Left-sided | 4 (17%) | 1 (4 %) | 19 (79%) | 5 (21%) | 0.60 (0.20, 1.83) | 0.48 |
| Tumor location - upper vs lower | ||||||
| Upper lobe (RL) | 13 (21%) | 3 (5%) | 45 (74%) | 16 (26%) | ||
| Lower/Middle lobe | 8 (25%) | 2 (6%) | 22 (69%) | 10 (31%) | 1.28 (0.50, 3.27) | 0.61 |
| Histology | ||||||
| Adenocarcinoma/other (RL) | 14 (19%) | 4 (6%) | 55 (75%) | 18 (25%) | ||
| Squamous | 7 (35%) | 1 (5%) | 12 (60%) | 8 (40%) | 2.04 (0.72, 5.77) | 0.33 |
| FEV1 | ||||||
| Median | 2.16 | 2.24 | 2.41 | 2.20 | 0.75 (0.36, 1.53) | 0.49 |
| Min-Max | 1.28-3.82 | 1.65-3.36 | 1.09-4.13 | 1.28-3.82 | ||
| Q1-Q3 | 1.97-2.85 | 2.16-2.39 | 2.09-2.93 | 1.97-2.85 | ||
| Institution type | ||||||
| Community/VA (RL) | 9 (26%) | 2 (6%) | 23 (68%) | 11 (32%) | ||
| Academic | 12 (20%) | 3 (5%) | 44 (75%) | 15 (25%) | 0.71 (0.28, 1.80) | 0.52 |
| Received Panitumumab | ||||||
| No (RL) | 18 (31%) | 1 (2%) | 39 (67%) | 19 (33%) | ||
| Yes | 3 (9%) | 4 (11%) | 28 (80%) | 7 (20%) | 0.51 (0.19, 1.39) | 0.33 |
| pCR | ||||||
| No (RL) | 15 (21%) | 3 (4%) | 52 (74%) | 18 (26%) | ||
| Yes | 6 (26%) | 2 (9%) | 15 (65%) | 8 (35%) | 1.54 (0.56, 4.24) | 0.49 |
| Mediastinal LN clearance | ||||||
| No (RL) | 6 (23%) | 2 (8%) | 18 (69%) | 8 (31%) | ||
| Yes | 15 (22%) | 3 (5%) | 49 (73%) | 18 (27%) | 0.83 (0.31, 2.23) | 0.71 |
| Days from end of RT to surgery | ||||||
| Median | 48 | 38 | 42 | 44 | 1.02 (0.99, 1.05) | 0.44 |
| Min-Max | 33-119 | 38-47 | 27-106 | 33-119 | ||
| Q1-Q3 | 42-54 | 38-39 | 38-49 | 39-51 | ||
| Extended resection | ||||||
| Lobectomy (RL) | 18 (23%) | 2 (3%) | 57 (74%) | 20 (26%) | ||
| Extended resection | 3 (19%) | 3 (19%) | 10 (62%) | 6 (38%) | 1.71 (0.55, 5.31) | 0.48 |
| Surgical approach | ||||||
| Open (RL) | 18 (23%) | 4 (5%) | 57 (72%) | 22 (28%) | ||
| Minimally invasive | 3 (21%) | 1 (7%) | 10 (71%) | 4 (29%) | 1.04 (0.29, 3.65) | 0.96 |
| RO resection | ||||||
| No (RL) | 0 (0%) | 0 (0%) | 8 (100%) | 0 (0%) | N/A | 0.10† |
| Yes | 21 (25%) | 5 (6%) | 59 (69%) | 26 (31%) | ||
| Surgery duration | ||||||
| Median | 243 | 305 | 233 | 248 | 1.00 (1.00, 1.00) | 0.94 |
| Min-Max | 180-561 | 180-397 | 69-2503 | 180-5641 | ||
| Q1-Q3 | 218-297 | 248-362 | 184-312 | 218-326 | ||
| # mediastinal LN sampled | ||||||
| Median | 3 | 4 | 3 | 3.5 | 1.09 (0.82, 1.44) | 0.58 |
| Min-Max | 1-7 | 1-7 | 0-7 | 1-7 | ||
| # hilar LN sampled | ||||||
| Median | 2 | 2 | 2 | 2 | 0.66 (0.42, 1.03) | 0.25 |
| Min-Max | 0-4 | 1-3 | 0-5 | 0-4 | ||
| Bronchial coverage | ||||||
| No (RL) | 4 (24%) | 0 (0%) | 13 (76%) | 4 (24%) | ||
| Yes | 17 (22%) | 5 (6%) | 54 (71%) | 22 (29%) | 1.32 (0.39, 4.51) | 0.65 |
| Intraoperative transfusion | ||||||
| No (RL) | 18 (22%) | 5 (6%) | 60 (72%) | 23 (28%) | ||
| Yes | 3 (30%) | 0 (0%) | 7 (70%) | 3 (30%) | 1.12 (0.27, 4.70) | 0.88 |
| Estimated blood loss | ||||||
| Median | 175 | 88 | 200 | 150 | 1.00 (1.00, 1.00) | 0.33 |
| Min-Max | 0-500 | 50-300 | 10-2000 | 0-500 | ||
| Q1-Q3 | 100-300 | 50-213 | 100-300 | 87.5-275 | ||
| RTOG 0839 RT Data | Grade 3-4 (n=11) | Grade 5 (n=4) | < Grade 3 Morbidity (n=39) | Grade 3+ Morbidity (n=15) | OR (95% CI) | p-value |
| Lung V5 | ||||||
| Median | 55.9 | 75.0 | 52.9 | 57.7 | 1.03 (1.00, 1.07) | 0.25 |
| Min-Max | 44.0-86.0 | 57.7-88.2 | 19.0-85.1 | 44.0-63.8 | ||
| Q1-Q3 | 49.0-72.9 | 60.8-87.1 | 41.2-67.4 | 34.4-50.6 | ||
| Ipsilateral Lung V10 | ||||||
| Median | 60.9 | 76.2 | 61.1 | 62.9 | 1.03 (1.00, 1.07) | 0.25 |
| Min-Max | 46.1-79.6 | 56.3-95.5 | 26.5-87.0 | 46.1-95.5 | ||
| Q1-Q3 | 50.7-72.2 | 61.1-91.0 | 44.2-72.0 | 51.7-77.5 | ||
| Contralateral Lung V5 | ||||||
| Median | 45.1 | 64.5 | 42.4 | 55.6 | 1.03 (1.00, 1.06) | 0.25 |
| Min-Max | 29.8-85.6 | 49.6-83.0 | 0.5-83.4 | 29.8-85.6 | ||
| Q1-Q3 | 37.4-64.0 | 52.6-78.2 | 24.2-58.2 | 39.8-67.7 | ||
OR, Odds Ratio modeling the risk of developing Grade 3 + Morbidity; RL, Reference level; Q1, first quartile; Q3, third quartile; pCR, pathologic complete response, LN, lymph nodes;
FDR, false discovery rate; p<0.25 considered significantly different
Fisher’s exact test
Table 5.
Surgical Mortality
| 30 Day Mortality | 90 Day Mortality | |||
|---|---|---|---|---|
| No | Yes | No | Yes | |
| Received Panitumumab | ||||
| No | 57 (98%) | 1 (2%) | 57 (98%) | 1 (2%) |
| Yes | 32 (91%) | 3 (9%) | 31 (89%) | 4 (11%) |
| p-value† | 0.15 | 0.06 | ||
| Extended Resection | ||||
| Lobectomy | 76 (99%) | 1 (1%) | 75 (97%) | 2 (3%) |
| Extended resection | 13 (81%) | 3 (19%) | 13 (81%) | 3 (19%) |
| p-value† | 0.02 | 0.03 | ||
| RTOG 0839 RT Data | n=51 | n=3* | n=50 | n=4 |
| Lung V5 | ||||
| Median | 55.9 | 57.7, 64.0, 86.0* | 55.0 | 75.0 |
| Min-Max | 19.0-88.2 | 19.0-86.0 | 57.7-88.2 | |
| Q1-Q3 | 46.0-69.2 | 46.0-68.3 | 60.8-87.1 | |
| p-value‡ | 0.17 | 0.04 | ||
| Ipsilateral Lung V10 | ||||
| Median | 61.1 | 56.3, 65.9, 95.9* | 61.0 | 76.2 |
| Min-Max | 26.5-87.0 | 26.5-87.0 | 56.3-95.5 | |
| Q1-Q3 | 48.0-72.2 | 48.0-72.0 | 61.1-91.0 | |
| p-value† | 0.30 | 0.09 | ||
| Contralateral Lung V5 | ||||
| Median | 43.4 | 49.6, 55.6, 73.4* | 42.9 | 64.5 |
| Min-Max | 0.5-85.6 | 0.5-85.6 | 49.6-83.0 | |
| Q1-Q3 | 26.7-64.0 | 26.7-62.9 | 52.6-78.2 | |
| p-value‡ | 0.25 | 0.07 | ||
All three values reported, due to small number of events
Fisher’s exact test
Wilcoxon rank sum test
Discussion
The major finding of this analysis is that lobectomy following full-dose concurrent CRT to 60 Gy was performed safely in a multidisciplinary setting. The addition of induction therapy for patients with N2+ IIIA NSCLC who undergo resection has been standard of care since the early 1990s when a series of small phase III trials showed dramatic survival improvements compared with surgery alone.14,15 Controversy has existed ever since as to the best induction strategy, with considerable debate of safety and efficacy of pre-operative RT. Full-dose concurrent CRT appears to provide the highest rates of mediastinal LN sterilization,16–18 but many are hesitant to use it due to high rates of operative complications reported in early series.4,19 Several single institution studies have reported the feasibility of full-dose concurrent CRT and resection,7,8,20,21 but these two RTOG trials are the first to prospectively evaluate this approach in a multi-institutional setting. These findings provide an important and timely baseline as the field moves into an era where immunotherapy is being added to induction regimes.
Two recent randomized trials from Europe attempted to define the role of induction CRT in resectable N2+ NSCLC. The Clinical Trial Group for the German Cancer Society compared CRT plus resection to CRT alone, similar to the Intergroup 0139 trial, but used a more complex induction protocol, and limited enrollment to high volume thoracic centers.22 RT in the surgery group was limited to 45 Gy, 2/3 of patients had IIIB disease and extended resections were used in 43%. Similar to the surgical arm of the Intergroup, 90-day operative mortality was 7%.
The second trial from the Swiss Cancer Research Group randomized 232 patients to 3 cycles of cisplatin-based chemotherapy alone or with the addition of RT to 44 Gy in a sequential fashion followed by resection in both arms.23 The low RT dose and sequential approach limit the ability to evaluate the trimodality approach. Overall operative morbidity was 23% and mortality was 1.5%, and neither were increased in the RT arm. This trial had more extended resections (34%) and fewer R0 resections (86%) suggesting some difference in inclusion criteria or treatment response to the data presented here. Overall, the 23% Gr3/4AEs and 4% 30-day mortality from the RTOG trials compares favorably to these European prospective trials.
The short-term outcomes for lobectomy in this trial also compare favorably with contemporary outcomes for NSCLC resections from the Society for Thoracic Surgery General Thoracic Surgery Database (STS-GTSDB) and resections following chemotherapy alone in IIIA disease. Morbidity and mortality following NSCLC resection reported in the STS-GTSDB from 2002-2008 were 18.5% and 2.2% respectively,24 and lower between 2012-2014, where 30-day mortality was only 1.4% for all NSCLC resections and 1.3% for lobectomy.25 30-day mortality following lobectomy in this series are the same despite higher stage, fewer minimally invasive procedures, and full-dose induction CRT. Similar to outcomes in the STS –GTSDB, thoracic surgical expertise may explain the short-term outcomes of this analysis compared to previous prospective analysis of tri-modality therapy, most specifically, the Intergroup trial 0139. Participating surgeons in these trials were required to be board certified in thoracic surgery, demonstrate experience with resections following induction therapy, and pre-treatment surgical evaluation was required prior to enrollment.
An equally important finding of this analysis is that extended resections were associated with increased mortality, 19% at 30 and 90 days. Excessive mortality following pneumonectomy6,19 and bilobectomy26 as part of tri-modality therapy is previously reported. Extended resections did not portend an increased rate of Gr3/4AEs, but when an AE occurred patients were significantly more likely to die as a result compared to those with AEs following lobectomy. “Failure to rescue” is an important concept in systems related to peri-operative care; induction therapy27 and extended resections28 have independently identified as risks factors for mortality following lung resection, and the combination may expose weaknesses in the biological system. The decreased tolerance of complications increases the importance of appropriate patient selection and intraoperative skill to avoid extended resections in this setting. Excluding patients with central tumors and bulky nodes, decreased, but did not eliminate use of extended resections in these trials.
The complications related to the panitumumab in 0839 limit some of the applicability of this work, but are also a meaningful finding. The addition of an EGFR antibody did not improve the primary outcome and was associated with increased mortality following resection. The etiology of this toxicity is unclear, as similar toxicity is not reported with use of EGFR antibodies prior to resections for other histologies.9,29 It is significant to note the toxicity of EGFR antibodies with RT is related to local inflammation,30 suggesting pneumonitis as a potential cause. This increased toxicity is in line with the results from RTOG 0617, the large 4-arm phase III trial in unresectable stage III NSCLC, where neither the addition of can EGFR antibody nor the increase in RT to 74 Gy improved survival over standard concurrent CRT to 60 Gy.1 More severe toxicity and treatment-related deaths occured in the cetuximab arms of this trial. The results of RTOG 0617 were not yet known when 0839 was designed and implemented, but had been identified in 2015 at the time of the interim analysis which resulted in the trial’s early closure. This represents the second NSCLC trial to report increased toxicity with an EGFR antibody and full dose RT.
A rarely discussed benefit of high dose concurrent CRT over other induction strategies is that concurrent CRT to 60 Gy is standard of care for unresectable IIIA NSCLC,31 and a significant proportion of patients (20.8% in this trial) who start a tri-modality treatment plan, do not undergo resection due to progression or medical decline. This group is not included retrospective series. The reasons for not undergoing surgery in this analysis were progression outside of RT field, persistent N2 on pre-resection biopsy, and medical decline. In this approach, these patients are provided definitive therapy without delays, gaps or sequential RT, and now have the potential for consolidative immunotherapy. The response induction treatment serves as an important in-vivo test of tumor biology. These results also provide evidence for resection of patients referred with residual or persistent disease after definitive CRT, although the majority of resections in these series were all performed within 8 weeks of CRT completion and may not be applicable for resections performed after a prolonged interval.
Recent data on definitive CRT in unresectable stage III NSCLC patients indicate heart dose may be responsible to increased toxicity and poor OS.32,33 Correlations between induction RT dose to specific cardiothoracic structures (heart, esophagus, ipsilateral and contralateral lung) and short-term surgical outcomes has not been previously investigated, but was queried here in the 0839 cohort. While there was a trend toward increased severe toxicity and 90-day mortality with increasing ipsilateral and contralateral V5 and lung V10, the number of events was small and the effect was not seen after controlling for panitumumab and extent of resection.
This analysis has several limitations. First, its small size, despite being a combined cohort from two prospective RTOG trials, only 93 patients were evaluable. The subsequent limited number of events (4 deaths within 30 days and 5 at 90 days) makes the toxicity models for mortality exploratory. This was further hindered by the lack of RT planning data from 0229. While a trend toward increased toxicity was noted with increase ipsilateral and contralateral lung V5, and total lung V10, events were too few for definitive conclusions. This topic deserves greater investigation, and could provide RT planning guidance to reduce surgical morbidity without decreasing the benefit of mediastinal LN sterilization. Second, the trials permitted surgeons to defer resection in patients with persistent nodal disease on post-induction invasive staging. This design allowed for evaluation of the primary outcome of mediastinal nodal clearance, but may have skewed the population in this analysis. A third limitation is the lacks of diffusion data from pulmonary function tests, which has strong correlation with short-term lobectomy outcomes.
Additionally, these trials employed the low dose carboplatin/paclitaxel regimen with concurrent RT. Some argue this approach results in lower chemotherapy doses and decreased efficacy outside of the RT field. The same regime was used in the control arm of RTOG 0617, and resulted in 58% OS at 2 years, the best results for unresectable stage III reported prior to use of immune therapies.1 Two recent meta-analyses also demonstrate comparable outcomes with less toxicity for this approach compared with a “full dose” cisplatin based regimen.34,35 Therefore, the low dose carboplatin/paclitaxel regimen seems uniquely suited for the tri-modality approach. It allows for delivery of full dose induction RT, and chemotherapy in the consolidation setting.
Finally, this analysis examines only short-term surgical outcomes and does not look at survival or toxicity past 90 days. While it is recognized that full-dose induction CRT increases rates of pCR and mediastinal LN sterilization, it is less clear if that translates to improved OS survival compared to lower doses or bimodality approaches. A major question regarding aggressive CRT induction strategies is the potential for increased long-term non-cancer mortality.18 Others hypothesize that the response to systemic therapy is the driver of primary long-term survival and local control is secondary and should be achieved by which ever therapy can provide it with the least toxicity.17 Unfortunately data from retrospective analyses are affected by inherent treatment bias and of limited value when comparing such divergent treatments. Some of these issues could be addressed in longer-term follow up of this cohort.
In conclusion, tri-modality therapy with concurrent high dose CRT remains a viable option for well-selected stage IIIA NSCLC patients, but was associated with excessive mortality when combined with extended resections. This approach is known to increase sterilize mediastinal LN disease, which is strongly associated with OS benefit. We have demonstrated the ability of thoracic surgeons at multiple institutions, including community and VA hospitals to perform these resections safely in the context of a clinical trial. Additional follow-up is required to determine long-term benefit of the tri-modality approach.
Supplementary Material
Central Picture.
NRG Oncology 0229 and 0839 trials assessed full dose concurrent chemoradiotherapy and resection.
Central Message.
Lobectomy was performed safely following full-dose concurrent chemoradiotherapy in these multi-institutional prospective trials.
Perspective Statement.
Controversy exists over ideal induction strategy for resectable N2+ IIIA NSCLC. Full-dose concurrent chemoradiotherapy results in high rates of mediastinal lymph node sterilization, but there is fear of operating after full-dose radiation. This multi-institutional prospective analysis suggests no excess in morbidity or mortality for lobectomy following full-dose concurrent chemoradiotherapy.
Acknowledgements:
We would like to thank the following doctors for accruing patients to the studies associated with this paper, Steven J Feigenberg, MD, University of Maryland School of Medicine, Elizabeth M Gore, MD, Medical College of Wisconsin, Vita V McCabe, MD, Michigan Cancer Research Consortiums, Cliff G Robinson, MD, Washington University School of Medicine, Gregory M Videtic, MD, Case Western Reserve/University Hospital Seidman Cancer Center, Nathaniel R Evans, MD, Thomas Jefferson University, Sidney Kimmel Medical College, Paul J Thurmes, MD, Metro-Minnesota CCOP, Maximilian Diehn, MD PhD, Stanford Cancer Institute, Roy H Decker, MD, Yale School of Medicine.
Funding: This project was supported by grants U10CA180868 (NRG Oncology Operations), U10CA180822 (NRG Oncology SDMC), U24CA180803 (IROC) from the National Cancer Institute (NCI) and Amgen.
Conflicts of interest: Dr. Donington reports honorarium and travel expenses from AstraZeneca, outside the submitted work. Ms. Paulus reports grant from NCI and Amgen for the work under consideration. Dr. Edelman reports grants (to institution) and consulting fees from BMS, Lilly Oncology, Ariad and Genentech, outside the submitted work. Dr. Le reports grants from RedHil, Varian, and Amgen and travel fees from BMS, outside the submitted work. Dr. Loo Jr reports grants from Varian Medical Systems and RaySearch Laboratories, honoraria from Varian Medical Systems and that he is a board member of TibaRay, Inc., outside the submitted work. Dr. Robinson reports grants from Varian and Elekta, and personal fees from Varian and ViewRay, outside the submitted work. Dr. Diehn reports a grant from Varian Medical Systems, outside the submitted work. Dr. Decker reports a grant from Merck & Co and advisory board fees from Regeneron Pharmaceuticals, outside the submitted work. Dr. Hu reports a grant from NCI for the work under consideration, a grant from NCI and personal fees and non-financial support from Varian Medical Systems, outside the submitted work. Dr. Bradley reports a grant (to institution) and travel fees from Mevion Medical Systems, Inc and scientific advisory board fees from ViewRay and Varian Medical, outside the submitted work.
Glossary of Abbreviations
- AE
adverse events
- AUC
area under the curve
- C
chemotherapy
- CI
confidence interval
- CRT
chemoradiotherapy
- CTCAE
NCI common terminology criteria for adverse events
- Dmax
Maximum dose
- ECOG
Eastern Cooperative Oncology Group
- EGFR
epidermal growth factor receptor
- FEV1
forced expiratory volume in one second
- GTV
gross tumor volume
- LN
lymph nodes
- NSCLC
non-small cell lung cancer
- OR
odds ratio
- OS
overall survival
- pCR
pathologic complete response
- PET/CT
Positron emission tomography–computed tomography
- PFS
progression free survival
- PTV
primary tumor volume
- RT
radiation therapy
- R0
microscopically margin-negative (resection)
- RTOG
Radiation Therapy Oncology Group
- STS
Society for Thoracic Surgery
- STS-GTSDB
Society for Thoracic Surgery General Thoracic Surgery Database
Biographies







Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trials.gov Identifier: NCT00096226 (RTOG 0229) and NCT00979212 (RTOG 0839)
References
- 1.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. The Lancet Oncology 2015;16:187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bezjak A, Temin S, Franklin G, et al. Definitive and Adjuvant Radiotherapy in Locally Advanced Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Endorsement of the American Society for Radiation Oncology Evidence-Based Clinical Practice Guideline. J Clin Oncol 2015;33:2100–5. [DOI] [PubMed] [Google Scholar]
- 3.Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 1999;17:2692–9. [DOI] [PubMed] [Google Scholar]
- 4.Rusch VW, Albain KS, Crowley JJ, et al. Surgical resection of stage IIIA and stage IIIB non-small-cell lung cancer after concurrent induction chemoradiotherapy. A Southwest Oncology Group trial. J Thorac Cardiovasc Surg 1993;105:97–104; discussion - 6. [PubMed] [Google Scholar]
- 5.Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for non-small cell lung carcinomas of the superior sulcus: Initial results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Thorac Cardiovasc Surg 2001;121:472–83. [DOI] [PubMed] [Google Scholar]
- 6.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerfolio RJ, Bryant AS, Jones VL, Cerfolio RM. Pulmonary resection after concurrent chemotherapy and high dose (60Gy) radiation for non-small cell lung cancer is safe and may provide increased survival. Eur J Cardiothorac Surg 2009;35:718–23; discussion 23. [DOI] [PubMed] [Google Scholar]
- 8.Sonett JR, Suntharalingam M, Edelman MJ, et al. Pulmonary resection after curative intent radiotherapy (>59 Gy) and concurrent chemotherapy in non-small-cell lung cancer. The Annals of thoracic surgery 2004;78:1200–5; discussion 6. [DOI] [PubMed] [Google Scholar]
- 9.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354:567–78. [DOI] [PubMed] [Google Scholar]
- 10.Blumenschein GR Jr., Paulus R, Curran WJ, et al. Phase II study of cetuximab in combination with chemoradiation in patients with stage IIIA/B non-small-cell lung cancer: RTOG 0324. J Clin Oncol 2011;29:2312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Govindan R, Bogart J, Stinchcombe T, et al. Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small-cell lung cancer: Cancer and Leukemia Group B trial 30407. J Clin Oncol 2011;29:3120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suntharalingam M, Paulus R, Edelman MJ, et al. Radiation therapy oncology group protocol 02-29: a phase II trial of neoadjuvant therapy with concurrent chemotherapy and full-dose radiation therapy followed by surgical resection and consolidative therapy for locally advanced non-small cell carcinoma of the lung. Int J Radiat Oncol Biol Phys 2012;84:456–63. [DOI] [PubMed] [Google Scholar]
- 13.Edelman MJ, Hu C, Le Q, et al. Randomized phase II study of preoperative chemoradiotherapy (CRT)+/− Panitumumab (P) followed by consolidation chemotherapy (C) in potentially operabe locally advnced (stage IIIa, N2+) non-smalll cell lung cancer (LANSCLC): NRG oncology /RTOG 0839. ASCO. Chicago: J Clin Oncol; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst 1994;86:673–80. [DOI] [PubMed] [Google Scholar]
- 15.Rosell R, Maestre J, Font A, et al. A randomized trial of mitomycin/ifosfamide/cisplatin preoperative chemotherapy plus surgery versus surgery alone in stage IIIA non-small cell lung cancer. Semin Oncol 1994;21:28–33. [PubMed] [Google Scholar]
- 16.Sher DJ, Fidler MJ, Seder CW, Liptay MJ, Koshy M. Relationship Between Radiation Therapy Dose and Outcome in Patients Treated With Neoadjuvant Chemoradiation Therapy and Surgery for Stage IIIA Non-Small Cell Lung Cancer: A Population-Based, Comparative Effectiveness Analysis. Int J Radiat Oncol Biol Phys 2015;92:307–16. [DOI] [PubMed] [Google Scholar]
- 17.Guo SX, Jian Y, Chen YL, Cai Y, Zhang QY, Tou FF. Neoadjuvant Chemoradiotherapy vesus Chemotherapy alone Followed by Surgery for Resectable Stage III Non-Small-Cell Lung Cancer: a Meta-Analysis. Sci Rep 2016;6:34388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bharadwaj SC, Vallieres E, Wilshire CL, et al. Higher Versus Standard Preoperative Radiation in the Trimodality Treatment of Stage 11 Ia Lung Cancer. Ann Thorac Surg 2015;100:207–13; discussion 13–4. [DOI] [PubMed] [Google Scholar]
- 19.Martin J, Ginsberg RJ, Abolhoda A, et al. Morbidity and mortality after neoadjuvant therapy for lung cancer: the risks of right pneumonectomy. Ann Thorac Surg 2001;72:1149–54. [DOI] [PubMed] [Google Scholar]
- 20.Krasna MJ, Gamliel Z, Burrows WM, et al. Pneumonectomy for lung cancer after preoperative concurrent chemotherapy and high-dose radiation. Ann Thorac Surg 2010;89:200–6; discussion 6. [DOI] [PubMed] [Google Scholar]
- 21.Darling GE, Li F, Patsios D, et al. Neoadjuvant chemoradiation and surgery improves survival outcomes compared with definitive chemoradiation in the treatment of stage IIIA N2 non-small-cell lung cancer. Eur J Cardiothorac Surg 2015;48:684–90; discussion 90. [DOI] [PubMed] [Google Scholar]
- 22.Eberhardt WE, Pottgen C, Gauler TC, et al. Phase III Study of Surgery Versus Definitive Concurrent Chemoradiotherapy Boost in Patients With Resectable Stage IIIA(N2) and Selected IIIB Non-Small-Cell Lung Cancer After Induction Chemotherapy and Concurrent Chemoradiotherapy (ESPATUE). J Clin Oncol 2015;33:4194–201. [DOI] [PubMed] [Google Scholar]
- 23.Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet 2015;386:1049–56. [DOI] [PubMed] [Google Scholar]
- 24.LaPar DJ, Bhamidipati CM, Lau CL, Jones DR, Kozower BD. The Society of Thoracic Surgeons General Thoracic Surgery Database: establishing generalizability to national lung cancer resection outcomes. Ann Thorac Surg 2012;94:216–21; discussion 21. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez FG, Kosinski AS, Burfeind W, et al. The Society of Thoracic Surgeons Lung Cancer Resection Risk Model: Higher Quality Data and Superior Outcomes. Ann Thorac Surg 2016;102:370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho JH, Kim J, Kim K, Shim YM, Kim HK, Choi YS. Risk associated with bilobectomy after neoadjuvant concurrent chemoradiotherapy for stage IIIA-N2 non-small-cell lung cancer. World journal of surgery 2012;36:1199–205. [DOI] [PubMed] [Google Scholar]
- 27.Farjah F, Backhus L, Cheng A, et al. Failure to rescue and pulmonary resection for lung cancer. J Thorac Cardiovasc Surg 2015;149:1365–71; discussion 71,–3 e3. [DOI] [PubMed] [Google Scholar]
- 28.Brunelli A, Xiume F, Al Refai M, Salati M, Marasco R, Sabbatini A. Risk-adjusted morbidity, mortality and failure-to-rescue models for internal provider profiling after major lung resection. Interact Cardiovasc Thorac Surg 2006;5:92–6. [DOI] [PubMed] [Google Scholar]
- 29.Lv W, Zhang GQ, Jiao A, et al. Chemotherapy Plus Cetuximab versus Chemotherapy Alone for Patients with KRAS Wild Type Unresectable Liver-Confined Metastases Colorectal Cancer: An Updated Meta-Analysis of RCTs. Gastroenterology research and practice 2017;2017:8464905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niyazi M, Maihoefer C, Krause M, Rodel C, Budach W, Belka C. Radiotherapy and “new” drugs-new side effects? Radiation oncology 2011;6:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181–90. [DOI] [PubMed] [Google Scholar]
- 32.Gore E, Hu C, Bar-Ad V, et al. Impact of incidental cardiac irradiation on cardiopulmonary toxicity and survival for locally advanced NSCLC: Reanalysis of NRG Oncology RTOG 0617 with centrally contoured cardiac structures. ASTRO; 2016; Bostom, MA. [Google Scholar]
- 33.Wang K, Eblan MJ, Deal AM, et al. Cardiac Toxicity After Radiotherapy for Stage III Non-Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy. J Clin Oncol 2017:JCO2016700229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steuer CE, Behera M, Ernani V, et al. Comparison of Concurrent Use of Thoracic Radiation With Either Carboplatin-Paclitaxel or Cisplatin-Etoposide for Patients With Stage III Non-Small-Cell Lung Cancer: A Systematic Review. JAMA oncology 2016. [DOI] [PubMed] [Google Scholar]
- 35.Santana-Davila R, Devisetty K, Szabo A, et al. Cisplatin and etoposide versus carboplatin and paclitaxel with concurrent radiotherapy for stage III non-small-cell lung cancer: an analysis of Veterans Health Administration data. J Clin Oncol 2015;33:567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


