Purpose and appropriate samples
This 27‐color panel has been validated and optimized to comprehensively profile natural killer (NK) cells isolated from human tumors using a collagenase Type II‐based digestion protocol. We confirmed that detection of protein expression by antibodies used in our final panel was not affected during tissue digestion. During this evaluation process, we found that detection of CD56, a biomarker typically used to identify NK cells, was affected substantially by collagenase‐based digestion. Thus, our panel is centered around expression of NKp46, which is sufficient to identify NK cells and not affected by the tissue collagenase digestion step. Our panel further includes biomarkers used to extrapolate NK‐cell maturation, differentiation, migration, homing potential, and functional state. Our panel is intended to provide in‐depth characterization of human NK cells isolated from tissues, which we specifically tested using oral squamous cell carcinomas tissues, but it is compatible with other tissues that can be dissociated with a collagenase Type II‐based protocol. © 2020 The Authors. Cytometry Part A published by Wiley Periodicals LLC on behalf of International Society for Advancement of Cytometry.
Keywords: high‐dimensional cytometry, natural killer cells, human tumor, immunophenotyping, human tissue, human PBMCs
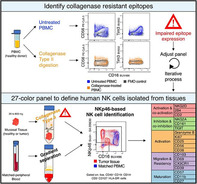
This OMIP describes a 27‐color flow cytometry panel suited to characterize NK cells isolated from human tissues. This panel was optimized for compatibility with collagenase type II‐based digestion.
Background
Natural killer (NK) cells were first identified in the 1970s for their spontaneous cytotoxicity against target cells (1, 2). Based on this ability to eliminate tumor cells without the need of prior immunization, it has been proposed that NK cells possess an ongoing preventive role to eliminate malignantly transformed cells and contribute to the control of hematologic malignancies or tumor metastasis (3, 4). In addition to exerting direct cytotoxicity, NK cells are also able to produce cytokines and chemokines that modulate the local microenvironment and recruit additional immune cells (5, 6, 7, 8). Together, these data suggest that NK cells could make significant contributions to antitumor immune responses in solid tumors (9). However, a more in‐depth analysis of intra‐tumoral NK cells is needed to further decipher how NK cells affect tumor growth. Of note, multiple studies have shown that the tumor progression and outcomes in humans correlate with the presence of NK cells at the tumor site (10, 11, 12, 13, 14) highlighting that these cells are of interest for the development of immunotherapeutic approaches in solid tumors (15, 16, 17, 18).
One roadblock to study human NK cells isolated from tumors is the limited amount of tissue that is typically available from biopsies or tumor resections. It is thus of utmost importance to extract as much information as possible from the tumor sample on a single‐cell level while minimizing further sample loss (Table 1).
Table 1.
Summary table for application of this OMIP
| Purpose | Immunophenotyping of human NK cells in tumor tissues |
|---|---|
| Species | Human |
| Cell types | Tumor tissues and peripheral blood. Oral squamous cell carcinoma tissues with donor‐matched blood were used to profile NK cells with this 27‐color panel |
| Cross‐references | OMIP‐007, OMIP‐027, OMIP‐029, OMIP‐039, OMIP‐056, OMIP‐058, OMIP‐064 |
The first critical step to profile infiltrating NK cells is to obtain a single‐cell suspension yielding a high viability with minimal cell debris and preserved ability to detect critical cell surface antigens. Here, we used a well‐established digestion method combining collagenase Type II and DNase. This collagenase Type II‐based protocol has been used for nearly 20 years and yields an excellent percentage of viable mucosal mononuclear cells when processing human mucosal tissues (19, 20, 21).
The second critical step is to extract as much information as possible from this cell suspension. To that end, high parameter flow cytometry is particularly well suited. Indeed, while cell loss can occur during staining and washing procedures, it is minimal during acquisition, with a current cell transmission efficiency greater than 95% for a standard fluorescence‐based flow cytometer (22).
Here, we report the development and validation of a 27‐parameter flow cytometry panel suitable to define the phenotype and activation status of NK cells isolated from human tumor tissues. We use a type II collagenase‐based isolation protocol, which ensures that the panel described here is useful and applicable for a range of human mucosal tissues and possibly others (20, 23, 24). All reagents included in the final panel are listed in Table 2.
Table 2.
Reagents used for OMIP‐070
| Specificity | Alternative name | Clone | Fluorochome | Purpose |
|---|---|---|---|---|
| Lineage | ||||
| Dead cells | N.A. | N.A. | Blue | Viability |
| CD45 | Protein tyrosine phosphatase receptor type C | HI30 | BUV805 | Lymphocytes |
| CD14 | N.A. | M5E2 | BV570 | Monocytes |
| CD19 | N.A. | SJ25‐C1 | BUV395 | B cells |
| CD3 | N.A. | UCHT1 | BUV661 | T cells |
| CD127 | IL‐7Rα | HIL‐7R‐M21 | BV786 | ILC |
| HLA‐DR | MHC class II molecule | G46.6 | APC‐H7 | Exclusion / activation marker |
| NK cell identification | ||||
| NKp46 | CD335, NCR1 | 9E2 | Biotin | NK cell marker and activating receptor |
| Streptavidin | N.A. | N.A. | BB630 | N.A. |
| CD16 | FcγRIII | 3G8 | BUV496 | NK cell marker and ADCC |
| NK cell markers | ||||
| NKG2D | CD314, KLRK1 | 1D11 | PE‐Cy7 | Activating receptor |
| CD244 | 2B4 | 2–69 | BV421 | Activating receptor |
| CD2 | Cluster of differentiation 2 | RPA‐2.10 | BV605 | Co‐stimulatory receptor |
| NKG2A | CD159a | REA110 | PE | Inhibitory receptor |
| CD161 | NKR‐P1 | DX12 | BB700 | Inhibitory receptor |
| TIGIT | T‐cell immunoreceptor with Ig and ITIM domains | 741182 | BV711 | Co‐inhibitory receptor |
| CD69 | Cluster of differentiation 69 | FN50 | BV650 | Residency and activation |
| CD39 | Cluster of differentiation 39 | TU66 | PE‐CF594 | Residency and activation |
| CD103 | Integrin alpha E | Ber‐ACT8 | BV750 | Residency |
| CX3CR1 | Fractalkine receptor | 2A9‐1 | BUV737 | Migration |
| CD57 | HNK‐1, Leu‐7 | NK‐1 | FITC | Maturation and differentiation |
| CD27 | Tumor necrosis factor receptor superfamily 7 | M‐T271 | BB660 | Maturation |
| CD11b | Integrin alpha M | ICRF44 | PE‐Cy5 | Maturation and adhesion |
| CD38 | Cyclic ADP ribose hydrolase | HIT2 | BB790 | Activation |
| Ki67 | N.A. | Sola15 | eFluor660 | Proliferation |
| Granzyme B | N.A. | QA16A2 | AF700 | Cytolytic abilities |
| CD25 | IL‐2Rα | 2A3 | BUV563 | Activation |
| CD56 | Neural cell adhesion molecule (NCAM) | CMSSB | PECy5.5 | Activation marker and NK‐cell marker for peripheral blood |
In order to identify NK cells in tumor tissue, we applied the gating strategy depicted in Figure 1A. This gating strategy was designed to ensure that other immune populations such as B cells, T cells, and monocytes, could also be enumerated. NK cells were gated as live hematopoietic cells (Dead−, CD45+), non B cells (CD19−), non‐monocytes (CD14−), non T cells (CD3−), non‐innate lymphoid cells (ILC) (CD127−), and HLA‐DR− cells (Fig. 1A). Of note, HLA‐DR is a common surrogate for immune activation on lymphocytes (25) that has been reported to be expressed by a fraction of NK cells (26, 27, 28). However, CD16 expression by HLA‐DR+ cells could also point to the identification of non‐classical CD14− monocytes (29). We observed that a small fraction of NKp46+ cells expressed HLA‐DR in the blood and the tumor tissues but kept HLA‐DR as a lineage marker. Future single‐cell RNA sequencing studies will help determine the nature of these cells and dictate if HLA‐DR should be included in the gating strategy or as an activation marker.
Figure 1.
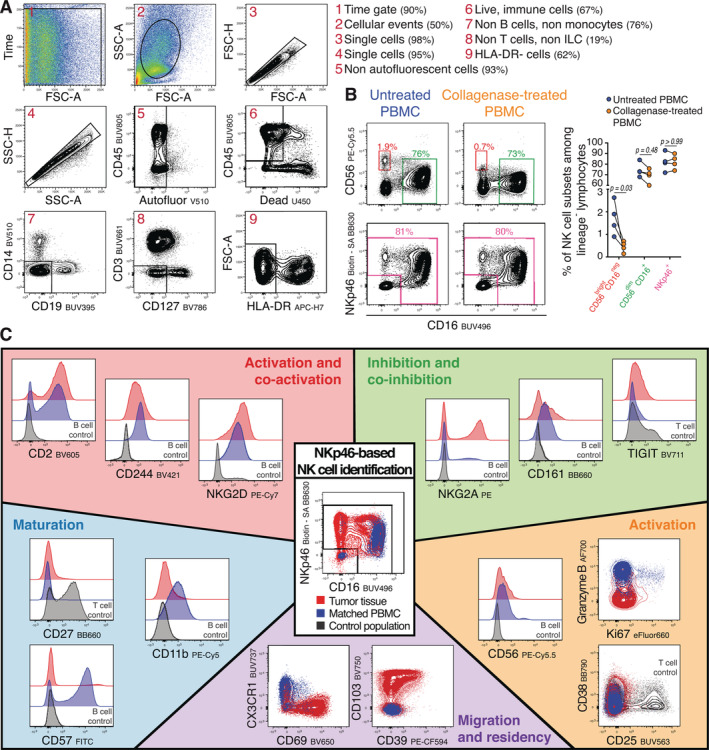
Gating strategy, NKp46‐based approach and panel performance. (A) Gating strategy to identify NK cells in a single‐cell suspension from a representative tumor tissue. Stable acquisition over time is monitored (1), followed by exclusion of debris (2), doublets (3, 4), and autofluorescent cells (5). NK cells were identified as live lymphocytes (6), non‐CD14+ monocytes (7), non‐CD19+ B cells (7), non‐CD3+ T cells (8), non‐CD127+ innate lymphoid cells 1 (ILC1) (8), and non‐HLA‐DR+ cells (9). (B) To determine whether protein detection is affected by the collagenase Type II‐based tissue digestion protocol, peripheral blood mononuclear cells (PBMC) from healthy donors were treated with 350 U/ml of collagenase Type II for 30 min at 37°C. The bivariate dot plots are representing the expression of CD56 and CD16 or NKp46 and CD16 from one healthy donor before or after collagenase digestion. The right graph shows the frequencies of each different highlighted population within the HLA‐DR− cells as depicted in A (n = 4, Mann Whitney Test). (C) Overview of the 18 phenotypic molecules analyzed within the NKp46+ cells of the tumor tissue (red) of a representative donor and its matched‐peripheral blood (blue). Central bivariate dot plot shows the gate used to identify NKp46‐expressing cells for both samples. Surrounding histograms or dot plots are showing the expression of the markers for both samples classified in the different categories: activation and co‐activation markers, inhibition and co‐inhibition markers, maturation markers, activation markers and migration and residency markers. As controls, both fluorescence minus one staining (FMOs) (Supporting Information Online Fig. S7), as well as reference populations, which were either CD19+ B cells (serving as negative expression control) or CD3+ T cells (serving as positive expression control) from the same tumor tissue, were used.
Human peripheral blood NK cells are typically defined by the relative expression of two different surface markers: CD56 (NCAM), an adhesion molecule that has also been shown to be involved in NK cell activation (30), and CD16, the low‐affinity Fc receptor known as FcyRIII that binds to the Fc domain of IgG antibodies and induces antibody‐dependent cellular cytotoxicity (ADCC) upon binding (31). We found that the detected level of CD56 was decreased during the isolation process required to fully dissociate tumor tissue and obtain a single cell suspension (Fig. 1B). This sensitivity to a commonly used collagenase Type II‐based digestion protocol (20) highlighted that CD56 was not an ideal marker to clearly define NK‐cell subsets in tissues. Likewise, it might also explain the increased frequency of CD56dim CD16− NK cells that has been observed in human tissues (32, 33, 34). As an alternative we have decided to use NKp46, a member of the highly conserved natural cytotoxicity receptor (NCR) family of NK‐activating receptors, which has been proposed to be a unique marker to define NK cells across species (35) in context of other lineage markers.
We then compiled a set of 18 biomarkers, which are highly relevant for assessing NK‐cell phenotype and function. First, as NK‐cell activation is regulated by the integration of signals from a wide array of receptors (36), we included the activating receptors NKG2D and CD244, and the inhibitory receptors CD161 and NKG2A. We also included CD2, a cell adhesion molecule that has been shown to be relevant for activation of NK cells and memory formation (37, 38).
We then tested several proteins with inhibitory properties such as PD‐1, Tim3, and TIGIT (39), but only included TIGIT in the final panel (40). We found that Tim3 expression was sensitive to collagenase treatment, and, in line with a recent publication we did not observe PD‐1 expression by NK cells (41).
We also included markers used to define NK‐cell differentiation (CD57, CD11b, CD27) (42, 43, 44), activation (CD38, CD25) (45, 46), and cytotoxic potential (Granzyme B). Ki67 was included as an indicator of cell proliferation. Finally, to assess migratory potential and tissue residence, CD69, CD103, and CX3CR1 were included in this panel along with CD39, a marker of chronic antigen stimulation (18).
The performance of the NK panel is displayed in Figure 1C. We found that this combination of markers enabled a thorough phenotypic characterization of NK cells within tumor tissues limited in both size and cell number. Of note, although our panel was developed primarily to assess NK cell phenotype, the expression of most markers could be assessed for CD3+ T cells, providing additional valuable biological insight (Supporting Information Online Fig. S9).
Human Samples
Following approval from the ethical committee of the Institutional Review Board (IRB) of the Fred Hutchinson Cancer Research Center (Seattle USA), fragments of fresh tumor tissue as well as peripheral blood from the same donor were obtained from patients undergoing surgery for a head and neck squamous cell carcinoma in the Department of Otolaryngology, Head and Neck Surgery and Oncology, University of Washington, Seattle, USA.
Similarity to Other Published OMIPs
While there are other OMIPs that allow for a broad immunophenotyping of NK cells, to date, there is no OMIP specifically designed to deeply profile NK cells isolated from tumor tissues. We included the necessary concomitant assessment of how the tissue digestion protocol affects detection of surface proteins. OMIP‐007 (47), OMIP‐029 (48), OMIP‐056 (49), OMIP‐058 (50), and OMIP‐064 (51) focus on phenotypic analysis of human NK cells from peripheral blood, OMIP‐027 (52) is optimized to assess functional responses of NK cells, and OMIP‐039 (53) allows distinguishing adaptive from conventional NK cells.
This 27‐parameter OMIP is optimized for the identification and in‐depth characterization of the NK cell infiltrate within collagenase‐digested human tumor tissue and will be a helpful tool for the study of immune cell function in human tumors as well as other human mucosal tissues.
Author Contributions
Marie Frutoso: Conceptualization; data curation; formal analysis; investigation; methodology; visualization; writing‐original draft. Florian Mair: Conceptualization; formal analysis; investigation; methodology; visualization; writing‐original draft; writing‐review and editing.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Appendix S1 MIFlowCyt: MIFlowCyt‐Compliant Items
Appendix S2 Supporting Information.
Acknowledgments
The authors would like to thank the HIV Vaccine Trial Network for access to their instruments. The authors thank Aaron Tyznik (BD Biosciences) for providing reagents and advice. F.M. is an ISAC Marylou scholar and was supported through The American Association of Immunologists (AAI) Intersect Fellowship Program for Computational Scientists and Immunologists. Grant sponsor: NIH R01Al123323 and U19Al128914.
Literature Cited
- 1. Herberman RB, Nunn ME, Holden HT, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer 1975;16:230–239. [DOI] [PubMed] [Google Scholar]
- 2. Kiessling R, Klein E, Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol 1975;5:112–117. [DOI] [PubMed] [Google Scholar]
- 3. Sathe P, Delconte RB, Souza‐Fonseca‐Guimaraes F, Seillet C, Chopin M, Vandenberg CJ, Rankin LC, Mielke LA, Vikstrom I, Kolesnik TB, et al. Innate immunodeficiency following genetic ablation of Mcl1 in natural killer cells. Nat Commun 2014;5:4539. [DOI] [PubMed] [Google Scholar]
- 4. Chockley PJ, Chen J, Chen G, Beer DG, Standiford TJ, Keshamouni VG. Epithelial‐mesenchymal transition leads to NK cell‐mediated metastasis‐specific immunosurveillance in lung cancer. J Clin Invest 2018;128:1384–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martin‐Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN‐gamma for T(H)1 priming. Nat Immunol 2004;5:1260–1265. [DOI] [PubMed] [Google Scholar]
- 6. Krebs P, Barnes MJ, Lampe K, Whitley K, Bahjat KS, Beutler B, Janssen E, Hoebe K. NK‐cell‐mediated killing of target cells triggers robust antigen‐specific T‐cell‐mediated and humoral responses. Blood 2009;113:6593–6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, Nelson AE, Loo K, Kumar R, Rosenblum MD, et al. A natural killer‐dendritic cell axis defines checkpoint therapy‐responsive tumor microenvironments. Nat Med 2018;24:1178–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bottcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza‐Cabrerizo M, Sammicheli S, Rogers NC, Sahai E, Zelenay S, Reis e Sousa C. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 2018;172:1022–1037. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol 2008;9:503–510. [DOI] [PubMed] [Google Scholar]
- 10. Stevens A, Kloter I, Roggendorf W. Inflammatory infiltrates and natural killer cell presence in human brain tumors. Cancer 1988;61:738–743. [DOI] [PubMed] [Google Scholar]
- 11. Coca S, Perez‐Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, Martos JA, Moreno M. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer 1997;79:2320–2328. [DOI] [PubMed] [Google Scholar]
- 12. Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, Aridome K, Hokita S, Aikou T. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer 2000;88:577–583. [PubMed] [Google Scholar]
- 13. Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, Zuil M, Callol L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer 2002;35:23–28. [DOI] [PubMed] [Google Scholar]
- 14. Jin S, Deng Y, Hao JW, Li Y, Liu B, Yu Y, Shi FD, Zhou QH. NK cell phenotypic modulation in lung cancer environment. PLoS One 2014;9:e109976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morvan MG, Lanier LL. NK cells and cancer: You can teach innate cells new tricks. Nat Rev Cancer 2016;16:7–19. [DOI] [PubMed] [Google Scholar]
- 16. Barrow AD, Colonna M. Exploiting NK cell surveillance pathways for cancer therapy. Cancers (Basel) 2019;11(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Habif G, Crinier A, Andre P, Vivier E, Narni‐Mancinelli E. Targeting natural killer cells in solid tumors. Cell Mol Immunol 2019;16:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Souza‐Fonseca‐Guimaraes F, Cursons J, Huntington ND. The emergence of natural killer cells as a major target in cancer immunotherapy. Trends Immunol 2019;40:142–158. [DOI] [PubMed] [Google Scholar]
- 19. Shacklett BL, Yang O, Hausner MA, Elliott J, Hultin L, Price C, Fuerst M, Matud J, Hultin P, Cox C, et al. Optimization of methods to assess human mucosal T‐cell responses to HIV infection. J Immunol Methods 2003;279:17–31. [DOI] [PubMed] [Google Scholar]
- 20. McKinnon LR, Hughes SM, De Rosa SC, Martinson JA, Plants J, Brady KE, Gumbi PP, Adams DJ, Vojtech L, Galloway CG, et al. Optimizing viable leukocyte sampling from the female genital tract for clinical trials: An international multi‐site study. PLoS One 2014;9:e85675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leelatian N, Doxie DB, Greenplate AR, Sinnaeve J, Ihrie RA, Irish JM. Preparing viable single cells from human tissue and tumors for cytomic analysis. Curr Protoc Mol Biol 2017;118:25C 1 1–25C 1 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bendall SC, Nolan GP, Roederer M, Chattopadhyay PK. A deep profiler' guide to cytometry. Trends Immunol 2012;33:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Slichter CK, McDavid A, Miller HW, Finak G, Seymour BJ, McNevin JP, Diaz G, Czartoski JL, McElrath MJ, Gottardo R, et al. Distinct activation thresholds of human conventional and innate‐like memory T cells. JCI Insight 2016;1:e86292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Woodward Davis AS, Roozen HN, Dufort MJ, DeBerg HA, Delaney MA, Mair F, Erickson JR, Slichter CK, Berkson JD, Klock AM, et al. The human tissue‐resident CCR5(+) T cell compartment maintains protective and functional properties during inflammation. Sci Transl Med 2019:11;521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferenczi K, Burack L, Pope M, Krueger JG, Austin LM. CD69, HLA‐DR and the IL‐2R identify persistently activated T cells in psoriasis vulgaris lesional skin: Blood and skin comparisons by flow cytometry. J Autoimmun 2000;14:63–78. [DOI] [PubMed] [Google Scholar]
- 26. Evans JH, Horowitz A, Mehrabi M, Wise EL, Pease JE, Riley EM, Davis DM. A distinct subset of human NK cells expressing HLA‐DR expand in response to IL‐2 and can aid immune responses to BCG. Eur J Immunol 2011;41:1924–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Erokhina SA, Streltsova MA, Kanevskiy LM, Telford WG, Sapozhnikov AM, Kovalenko EI. HLA‐DR(+) NK cells are mostly characterized by less mature phenotype and high functional activity. Immunol Cell Biol 2018;96:212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dutertre CA, Becht E, Irac SE, Khalilnezhad A, Narang V, Khalilnezhad S, Ng PY, van den Hoogen LL, Leong JY, Lee B, et al. Single‐cell analysis of human mononuclear phagocytes reveals subset‐defining markers and identifies circulating inflammatory dendritic cells. Immunity 2019;51:573–589. e8. [DOI] [PubMed] [Google Scholar]
- 29. Mukherjee R, Kanti Barman P, Kumar Thatoi P, Tripathy R, Kumar Das B, Ravindran B. Non‐classical monocytes display inflammatory features: Validation in sepsis and systemic lupus erythematous. Sci Rep 2015;5:13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van Acker HH, Capsomidis A, Smits EL, Van Tendeloo VF. CD56 in the immune system: More than a marker for cytotoxicity? Front Immunol 2017;8:892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Caligiuri MA. Human natural killer cells. Blood 2008;112:461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mamessier E, Pradel LC, Thibult ML, Drevet C, Zouine A, Jacquemier J, Houvenaeghel G, Bertucci F, Birnbaum D, Olive D. Peripheral blood NK cells from breast cancer patients are tumor‐induced composite subsets. J Immunol 2013;190:2424–2436. [DOI] [PubMed] [Google Scholar]
- 33. Marquardt NSM, Mold JE, Hård J, Kekäläinen E, Buggert M, Nguyen S, Wilson JN, Al‐Ameri M, Ljunggren HG, Michaëlsson J. High‐dimensional analysis reveals a distinct population of adaptive‐like tissue‐resident NK cells in human lung. bioRxiv 2019. 10.1101/2019.12.20.883785. [DOI] [Google Scholar]
- 34. Dogra P, Rancan C, Ma W, Toth M, Senda T, Carpenter DJ, Kubota M, Matsumoto R, Thapa P, Szabo PA, et al. Tissue determinants of human NK cell development, function, and residence. Cell 2020;180:749–763. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walzer T, Jaeger S, Chaix J, Vivier E. Natural killer cells: From CD3(−)NKp46(+) to post‐genomics meta‐analyses. Curr Opin Immunol 2007;19:365–372. [DOI] [PubMed] [Google Scholar]
- 36. Lanier LL. Up on the tightrope: Natural killer cell activation and inhibition. Nat Immunol 2008;9:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu LL, Landskron J, Ask EH, Enqvist M, Sohlberg E, Traherne JA, Hammer Q, Goodridge JP, Larsson S, Jayaraman J, et al. Critical role of CD2 co‐stimulation in adaptive natural killer cell responses revealed in NKG2C‐deficient humans. Cell Rep 2016;15:1088–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Geary CD, Sun JC. Memory responses of natural killer cells. Semin Immunol 2017;31:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sun H, Sun C. The rise of NK cell checkpoints as promising therapeutic targets in cancer immunotherapy. Front Immunol 2019;10:2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A 2009;106:17858–17863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Judge SJ, Dunai C, Aguilar EG, Vick SC, Sturgill IR, Khuat LT, Stoffel KM, Van Dyke J, Longo DL, Darrow MA, et al. Minimal PD‐1 expression in mouse and human NK cells under diverse conditions. J Clin Invest 2020;130:3051–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, Caligiuri MA. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med 2006;203:1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol 2006;176:1517–1524. [DOI] [PubMed] [Google Scholar]
- 44. Bjorkstrom NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, Bjorklund AT, Flodstrom‐Tullberg M, Michaelsson J, Rottenberg ME, et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK‐cell differentiation uncoupled from NK‐cell education. Blood 2010;116:3853–3864. [DOI] [PubMed] [Google Scholar]
- 45. Pahl JHW, Koch J, Gotz JJ, Arnold A, Reusch U, Gantke T, Rajkovic E, Treder M, Cerwenka A. CD16A activation of NK cells promotes NK cell proliferation and memory‐like cytotoxicity against cancer cells. Cancer Immunol Res 2018;6:517–527. [DOI] [PubMed] [Google Scholar]
- 46. Le Gars M, Seiler C, Kay AW, Bayless NL, Sola E, Starosvetsky E, Moore L, Shen‐Orr SS, Aziz N, Khatri P, et al. CD38 contributes to human natural killer cell responses through a role in immune synapse formation. bioRxiv 2019. 10.1101/349084. [DOI] [Google Scholar]
- 47. Eller MA, Currier JR. OMIP‐007: Phenotypic analysis of human natural killer cells. Cytom Part A 2012;81A:447–449. [DOI] [PubMed] [Google Scholar]
- 48. Mahnke YD, Beddall MH, Roederer M. OMIP‐029: Human NK‐cell phenotypization. Cytom Part A 2015;87A:986–988. [DOI] [PubMed] [Google Scholar]
- 49. Dintwe O, Rohith S, Schwedhelm KV, McElrath MJ, Andersen‐Nissen E, De Rosa SC. OMIP‐056: Evaluation of human conventional T cells, donor‐unrestricted T cells, and NK cells including memory phenotype by intracellular cytokine staining. Cytom Part A 2019;95A:722–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liechti T, Roederer M. OMIP‐058: 30‐parameter flow cytometry panel to characterize iNKT, NK, unconventional and conventional T cells. Cytom Part A 2019;95A:946–951. [DOI] [PubMed] [Google Scholar]
- 51. Hertoghs N, Schwedhelm KV, Stuart KD, McElrath MJ, De Rosa SC. OMIP‐064: A 27‐color flow Cytometry panel to detect and characterize human NK cells and other innate lymphoid cell subsets, MAIT cells, and gammadelta T cells. Cytom Part A 2020. 10.1002/cyto.a.24031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Costanzo MC, Creegan M, Lal KG, Eller MA. OMIP‐027: Functional analysis of human natural killer cells. Cytom Part A 2015;87:803–805. [DOI] [PubMed] [Google Scholar]
- 53. Hammer Q, Romagnani C. OMIP‐039: Detection and analysis of human adaptive NKG2C(+) natural killer cells. Cytom Part A 2017;91:997–1000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 MIFlowCyt: MIFlowCyt‐Compliant Items
Appendix S2 Supporting Information.


