Abstract
Background & Aims
To explore whether sarcopenia, diagnosed by an abbreviated magnetic resonance imaging (MRI) protocol is a risk factor for hepatic decompensation and mortality in patients with chronic liver disease (CLD).
Methods
In this retrospective single‐centre study we included 265 patients (164 men, mean age 54 ± 16 years) with CLD who had undergone MRI of the liver between 2010 and 2015. Transverse psoas muscle thickness (TPMT) was measured on unenhanced and contrast‐enhanced T1‐weighted and T2‐weighted axial images. Sarcopenia was defined by height‐adjusted and gender‐specific cut‐offs in women as TPMT < 8 mm/m and in men as TPMT < 12 mm/m respectively. Patients were further stratified into three prognostic stages according to the absence of advanced fibrosis (FIB‐4 < 1.45, non‐advanced CLD), compensated‐advanced CLD (cACLD) and decompensated‐advanced CLD (dACLD).
Results
The inter‐observer agreement for the TPMT measurements (κ = 0.98; 95% confidence interval [95% CI]:0.96‐0.98), as well as the intra‐observer agreement between the three image sequences (κ = 0.99; 95% CI: 0.99‐1.00) were excellent. Sarcopenia was not predictive of first or further hepatic decompensation. In patients with cACLD and dACLD, sarcopenia was a risk factor for mortality (cACLD: hazard ratio (HR):3.13, 95% CI: 1.33‐7.41, P = .009; dACLD:HR:2.45, 95% CI: 1.32‐4.57, P = .005) on univariate analysis. After adjusting for the model of end‐stage liver disease (MELD) score, albumin and evidence of clinical significant portal hypertension, sarcopenia (adjusted HR: 2.76, 95% CI: 1.02‐7.42, P = .045) remained an independent risk factor for mortality in patients with cACLD.
Conclusion
Sarcopenia can be easily evaluated by a short MRI exam without the need for contrast injection. Sarcopenia is a risk factor for mortality, especially in patients with cACLD.
Keywords: cirrhosis, liver, magnetic resonance imaging, sarcopenia
Abbreviations
- cACLD
compensated advanced chronic liver disease
- CLD
chronic liver disease
- CTP
Child‐Turcotte‐Pugh
- dACLD
decompensated advanced chronic liver disease
- ICC
interclass correlation
- INR
international normalized ratio
- IQR
interquartile range
- MELD
Model for End‐Stage Liver Disease
- TFS
transplant‐free survival
Lay summary.
MRI‐based evaluation of transverse psoas muscle thickness (TPMT) measurements on unenhanced T1‐, contrast‐enhanced T1‐ and T2‐weighted images is highly reproducible with excellent intra‐ and inter‐reader agreement. Sarcopenia as evaluated by MRI is a risk factor for mortality in patients with advanced chronic liver disease. MRI‐based TPMT can easily be calculated. Thus, the presence or absence of sarcopenia may be reported in every MRI done on patients with advanced chronic liver disease.
1. INTRODUCTION
Sarcopenia is defined as the generalized loss of muscle mass, muscle strength and muscle function. 1 It is prevalent in as many as 78% of patients with advanced chronic liver disease (ACLD). 2 Importantly, sarcopenia is also associated with adverse clinical outcome in patients with ACLD. 2 Current guidelines recommend screening for the presence of sarcopenia in routine computed tomography (CT) imaging. 3 Even though magnetic resonance imaging (MRI) has also been used to diagnose sarcopenia, 4 its role in patients with chronic liver disease (CLD) has not been established. Thus, MRI‐based sarcopenia has not been incorporated into current guidelines. 3
However, MRI is increasingly used in the diagnostic workup of patients with CLD. With multiparametric MRI, including T1 and T2, chemical shift imaging (CSI) as well as diffusion weighted imaging (DWI) and dynamic contrast‐enhanced imaging, the anatomy and pathology of the liver and the biliary tree can be accurately assessed. 5 , 6 , 7 Furthermore, because of its high soft tissue contrast, MRI can even be performed without administration of contrast media in those patients with contraindications.
Several methods have been described that quantify sarcopenia on cross‐sectional imaging with the skeletal muscle index (SMI) being the most commonly used one. 2 However, this metric requires specialized software which is not available on most workstations and therefore, limits its clinical applicability in routine practice. In contrast, the transverse psoas muscle thickness (TPMT) – defined as the largest transverse diameter of the right psoas muscle perpendicular to its long axis – normalized to body height can be easily assessed. In addition, the TPMT has been shown to accurately assess sarcopenia in patients with liver disease. 4 , 8 Paternostro et al recently proposed specific TPMT cut‐off values for CT‐based measurements at the level of the third lumbar vertebrae which were even more accurate than the SMI in predicting patient survival. 8
Furthermore, it is clinically important to predict hepatic decompensation. Therefore, we wondered if sarcopenia might be a risk factor for hepatic decompensation, although this has not been systematically assessed. Finally, in patients undergoing transjugular intrahepatic portosystemic shunt, sarcopenia was identified as an independent risk factor for the development of acute‐on‐chronic liver failure. 9
Thus, the aims of this study were to assess (i) the feasibility of MRI‐based sarcopenia assessment by TPMT‐L3 using an abbreviated MRI protocol, and (ii) to investigate the impact of sarcopenia on the development of hepatic decompensation and mortality in patients with compensated and decompensated ACLD.
2. METHODS
2.1. Patients
This study represents a retrospective single‐centre analysis of chronic liver disease (CLD) patients who underwent routine clinical MRI. The study was approved by the institutional review board (EK Nr 2027/2017) and the requirement for individual patient informed consent waived. The inclusion criteria were (i) MRI with gadoxetic acid using a standard examination protocol, (ii) histological or clinical evidence of CLD and (iii) available information on platelet count, albumin levels, prothrombin time (PT), international normalized ratio (INR), sodium, alanine amino transferase (ALT), aspartate amino transferase (AST), bilirubin levels, alkaline phosphatase (AP) and creatinine levels, all of which were acquired within 2 weeks of the MRI examination. Exclusion criteria were (i) current or prior malignancy including hepatocellular carcinoma (HCC), (ii) mechanical cholestasis, (iii) history of liver transplantation and (iv) a follow‐up time <90 days.
Note that this same cohort has been used previously 10 , 11 when we evaluated the prognostic value of the functional liver imaging score (FLIS) evaluated on gadoxetic acid‐enhanced MRI. In the present study, we aimed to assess the ability to diagnose sarcopenia on non‐contrast– and contrast‐enhanced MRI sequences and to evaluate whether sarcopenia is a risk factor for hepatic decompensation and mortality.
2.2. Clinical data
Patients' medical records were systematically reviewed by MD/MD PhD students and a MD under the supervision of internal medicine and gastroenterology/hepatology specialists with 7 and 13 years of experience respectively. These reviewers were blinded to any imaging information. The body surface area (BSA) was calculated according to the following formula: BSA = (weight(kg)0.425 × height(m)0.725) × 0.007184.
2.3. Classification of disease severity
Based on the Fibrosis‐4 score (FIB‐4; cut‐off 1.45) 12 and history of or current hepatic decompensation, patients were classified as having non‐advanced (non‐ACLD; FIB‐4 ≤ 1.45), compensated (cACLD; FIB‐4 > 1.45) or decompensated (dACLD; history of or current hepatic decompensation) ACLD. The FIB‐4 score was calculated using the formula: FIB‐4 = age(years) × AST(U/L)/[PLT(109/L) × ALT(U/L)0.5]. 13
We created a composite variable coding for evidence of clinically significant portal hypertension (CSPH) consisting of (i) varices on endoscopy and (ii) hepatic venous pressure gradient (HVPG) ≥10 mmHg (within 365 days before or after MRI).
2.4. Definition of hepatic decompensation and transplant‐free survival
Event‐free survival time was defined from the time of MRI to the development of first or further hepatic decompensation. We applied a commonly used definition of hepatic decompensation, including: requirement of paracentesis, grade 3/4 hepatic encephalopathy, variceal bleeding or liver‐related death. 14 , 15 , 16 , 17 Transplant‐free survival (TFS) time was defined as the time from MRI to death or end of follow‐up, while patients undergoing liver transplantation were censored at the day of surgery. Patients treated with transjugular intrahepatic portosystemic shunt (TIPS) during the study period were censored at the day of intervention. In an additional sensitivity analysis, we also censored patients with viral hepatitis who started antiviral medication and patients with alcoholic liver disease who stopped alcohol consumption after MRI.
2.5. MRI protocol
All exams (n = 265) were performed using a 3T MRI scanner (Magnetom Trio, A Tim; Siemens Healthcare, Erlangen, Germany) with a combined six‐element, phased‐array abdominal coil and a fixed spine coil. A standard protocol including T1‐ and T2‐weighted images with fat saturation in axial and coronal view, chemical shift imaging (CSI), diffusion weighted imaging (DWI) and dynamic imaging after injection of gadoxetic acid (0.025 mmol/kg; Primovist; Bayer Healthcare, Berlin, Germany) intravenously at a rate of 1.0 mL/s, immediately followed by a 20 mL saline flush. The contrast‐enhanced sequence consisted of three‐dimensional, T1‐weighted, volume‐interpolated, breath‐hold examination (VIBE) sequences obtained before and after contrast injection using test bolus for the arterial phase, and then 70 seconds for the portal venous phase and 3 minutes for the transitional phase. The parameters of the T1 VIBE sequence were as follows: section thickness, 1.7 mm; TR, 2.67 msec; TE, 0.92 msec; FOV, 430 and flip angle, 13. The parameters of the axial T2‐weighted images were as follows: section thickness, 5 mm; TR, 1800 msec; TE, 150 msec; FOV, 400 and flip angle, 150.
2.6. Image analysis
Two radiologist, one board certified with 9 years of experience in abdominal imaging (radiologist 1, blinded for clinical data) one in the 3rd year of training (radiologist 2, blinded for clinical data), independently measured TPMT on the axial unenhanced and portal‐venous phase‐enhanced T1 weighted as well as axial T2‐weighted images on a picture archiving and communication system (PACS, workstation, Impax; Agfa, Mortsel, Belgium). TPMT was measured at the third lumbar vertebral body. 8 TPMT was defined as the greatest transverse diameter of the right psoas muscle perpendicular to the long axis (anterior‐posterior oblique) of the psoas muscle diameter at the cranial L3 vertebra endplate (Figure 1). Results were normalized to body height and shown as millimetre (mm) psoas muscle thickness per meter (m) body height (mm/m). The presence of sarcopenia was defined at a TPMT‐L3 <12 mm/m in men and <8 mm/m in women. 8 Furthermore, 50 randomly selected cases we re‐assessed by radiologist 1 after a 4‐week interval to evaluate intra‐reader repeatability. The radiologists were blinded to all clinical, histological and laboratory data.
Figure 1.
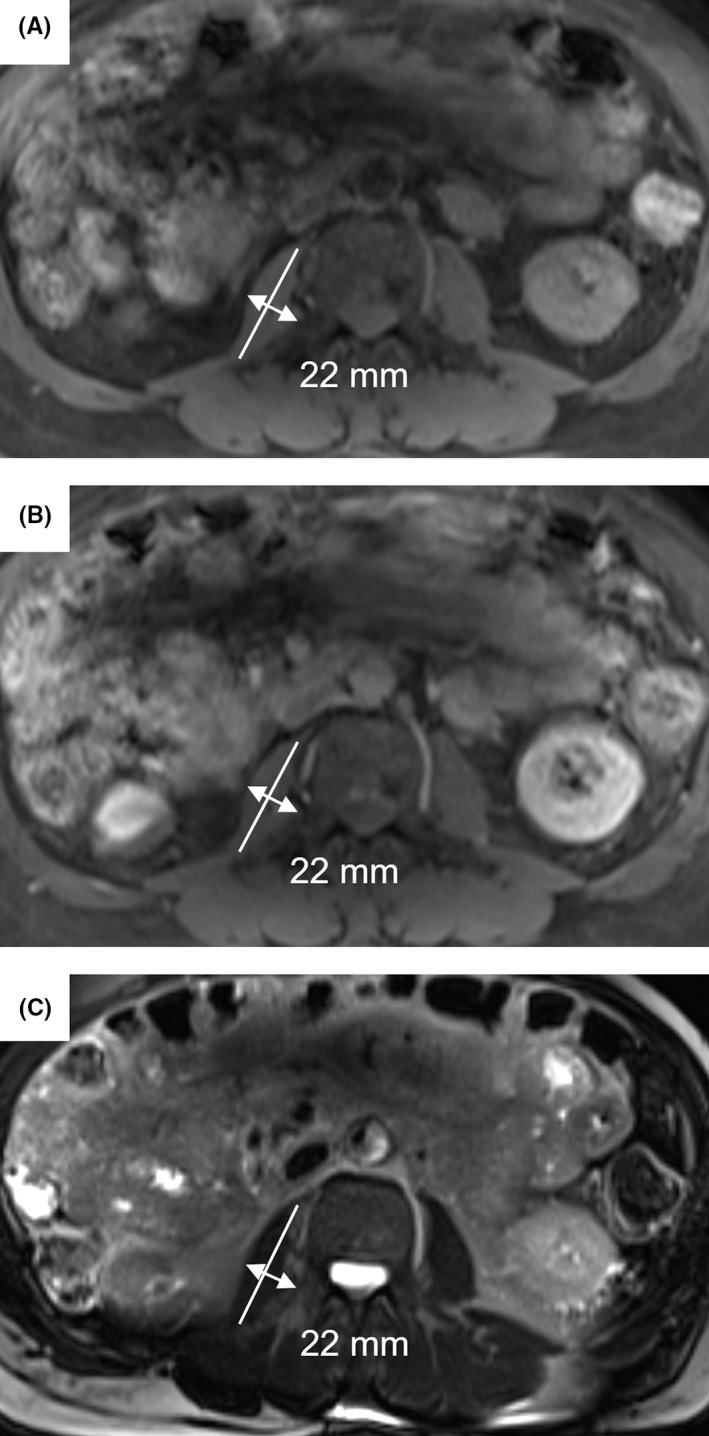
Image illustration for measuring the transversal psoas muscle thickness (TPMT). TPMT was defined as the greatest transverse diameter of the right psoas muscle perpendicular to the long axis (anterior‐posterior oblique) of the psoas muscle diameter at the cranial L3 vertebra endplate. A, T1‐weighed non‐contrast–enhanced sequence. B, T1‐weighed contrast–enhanced sequence. C, T2‐weighted sequence
2.7. Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics Version 24 (IBM, Armonk, NY) and GraphPad Prism Version 5.01 (GraphPad Software, La Jolla, CA). Continuous variables were reported as mean and standard deviation (SD) given parametric distribution of data or median (interquartile range (IQR)) in case of non‐parametric data distribution, while categorical variables were reported as number and percentage of patients with the specific characteristics. Student's t test was used for group comparisons of parametric data, while Mann‐Whitney U test was applied for non‐parametric data. Group comparisons of categorical variables were performed using the chi‐squared test.
Intra‐observer intraclass correlation coefficients (ICCs) and their 95% confident intervals were calculated based on a single‐measurement, absolute‐agreement, two‐way mixed‐effects model. Inter‐observer ICC variability and 95% confident intervals were calculated based on a single‐reader, absolute‐agreement, two‐way random‐effects model.
The association of sarcopenia and clinical/laboratory data with first/further hepatic decompensation and mortality was investigated using Kaplan‐Meier analysis and Cox regression analysis. A two‐sided P value ≤.05 was considered statistically significant.
3. RESULTS
3.1. Patients characteristics
We included 265 patients (164/61.9% men) who had an established diagnosis of CLD with a mean age of 53 (SD ± 14) years in this study (Table 1, Figure 2, Table S1). Overall, the most common indications for the MRI were the evaluation of hepatic nodules (186/265 (70%)) followed by the query cholangiocellular carcinoma/obstruction (29/265 (10.9%)). As outlined in the methods section, patients with current or prior malignancy including hepatocellular carcinoma (HCC) were excluded. The most common causes of CLD were viral hepatitis (HBV: n = 23 (9%), HCV: 55 (21%)) and ALD (n = 51 (19%)). In patients with dACLD, the types of previous (first) hepatic decompensation were as follows: ascites 65/99 (66%), SBP 8/99 (8%), HE 8/99 (8%) and variceal bleeding 18/99 (18%).
Table 1.
Patient characteristics
| Patient characteristics | A, cACLD (n = 110) | B, dACLD (n = 99) | ||||
|---|---|---|---|---|---|---|
| Sarcopenia | Sarcopenia | |||||
| No, n = 92 (83.6%) | Yes, n = 18 (16.4%) | P value | No, n = 59 (59.6%) | Yes, n = 40 (40.4%) | P value | |
| Indication for MRI | ||||||
| HCC? | 78 (85%) | 13 (72%) | .28 | 48 (81%) | 36 (90%) | .63 |
| PSC/PBC? | 3 (3%) | 0 (0%) | 4 (7%) | 0 (0%) | ||
| CCC/obstruction? | 4 (4%) | 3 (17%) | 1 (2%) | 1 (3%) | ||
| Diffuse liver disease? | 2 (2%) | 1 (6%) | 1 (2%) | 0 (0%) | ||
| Other | 5 (5%) | 1 (6%) | 5 (9%) | 3 (6%) | ||
| Age, years | 59 ± 12 | 58 ± 14 | .63 | 56 ± 13 | 56 ± 13 | .82 |
| Gender | ||||||
| Male | 48 (52%) | 17 (94%) | .001 | 36 (61%) | 36 (90%) | .001 |
| Female | 44 (48%) | 1 (6%) | 23 (39%) | 4 (10%) | ||
| Body weight, kg | 75(72‐79) | 78 (74‐90) | .13 | 76 (72‐82) | 77 (71‐81) | .99 |
| Height, m | 1.7 (1.67‐1.72) | 1.75 (1.72‐1.79) | .04 | 1.7 (1.67‐1.73) | 1.75 (1.73‐1.79) | .01 |
| BMI, kg/m2 | 25 (25‐27) | 25 (24‐29) | .56 | 25.8 (24.9‐27.8) | 24.7 (23.2‐25.9) | .17 |
| BSA, m2 | 1.85 (1.81‐1.91) | 1.91 (1.89‐2.06) | .046 | 1.92 (1.8‐1.95) | 1.92 (1.84‐1.98) | .42 |
| Smoking | 27 (29%) | 5 (28%) | 1.0 | 28 (48%) | 19 (47%) | 1.0 |
| Diabetes | ||||||
| NIDDM | 10 (11%) | 1 (6%) | .78 | 4//%) | 2 (5%) | .72 |
| IDDM | 11 (12%) | 2 (11%) | 12 (20%) | 6 (15%) | ||
| Aetiology of CLD | ||||||
| HCV | 28 (30%) | 4(17%) | .53 | 13 (22%) | 3 (8%) | .14 |
| HBV | 9 (10%) | 1 (6%) | 4 (7%) | 4 (10%) | ||
| ALD | 8 (9%) | 4 (22%) | 17 (29%) | 19 (47%) | ||
| Cholestatic | 5 (5%) | 1 (19%) | 1 (1%) | 1 (1%) | ||
| NAFLD | 10 (11%) | 1 (6%) | 2 (3%) | 0 (0%) | ||
| AIH | 5 (5%) | 0 (0%) | 5 (9%) | 1 (3%) | ||
| Genetic | 3 (3%) | 0 (0%) | 1 (1%) | 0 (0%) | ||
| Cryptogenic | 13 (14%) | 6 (33%) | 9 (15%) | 9 (23%) | ||
| Other | 5 (6%) | 1 (6%) | 8 (13%) | 3 (8%) | ||
| Antiviral therapy during follow‐up | 20 (72%) | 3 (100%) | .53 | 7 (54%) | 3 (100%) | .43 |
| Alcohol consumption | ||||||
| Below threshold | 8 (9%) | 4 (22%) | .11 | 8 (14%) | 2 (5%) | .38 |
| Above threshold | 8 (9%) | 3 (17%) | 8 (14% | 6 (15%) | ||
| Alcohol abstinence during follow‐up | 1 (6%) | 0 (0%) | 1 (6%) | 1 (14%) | .53 | |
| Varices | ||||||
| Small | 11 (34%) | 2 (25%) | .61 | 7 (16%) | 8 (33%) | .10 |
| Large | 21 (66%) | 6 75%) | 37 (84%) | 16 (67%) | ||
| Evidence of CSPH | 41 (43%) | 9 (56%) | .42 | 47 (80%) | 29 (73%) | .41 |
| NSBB | 17 (53%) | 6 (75%) | .43 | 27 (61%) | 12 (50%) | .45 |
| CTP stage | ||||||
| A | 79 (86%) | 14 (78%) | .39 | 8 (14%) | 2 (5%) | .03 |
| B | 13 (14%) | 4 (22%) | 41 (70%) | 22 (55%) | ||
| C | 10 (17%) | 16 (40%) | ||||
| Ascites present | 37 (64%) | 29 (73%) | .39 | |||
| MELD, points | 7 (3%) | 8 (7) | .22 | 13 (9) | 17 (9) | .002 |
| Platelet count, G/L | 135 (84) | 133 (85) | .61 | 105 (70) | 110 (87) | .41 |
| Albumin, g/L | 40.1 (7.9) | 35.6 (10.2) | .047 | 34.4 (7.2) | 30.8 (10) | .08 |
| Bilirubin, mg/dL | 0.87 (0.87) | 1.11 (1.05) | .17 | 1.9 (2.4) | 3.2 (7.4) | .03 |
| INR | 1.2 (0.1) | 1.3 (0.1) | .74 | 1.3 (0.4) | 1.4 (0.2) | .45 |
| Creatinine, mg/dL | 0.81 (0.27) | 0.83 (0.22) | .52 | 0.87 (0.53) | 1.13 (1.15) | .04 |
| Sodium, mmol/L | 140 (4) | 140 (5) | .48 | 137 (7) | 135 (6) | .06 |
| ALP, U/L | 98 (80) | 94 (30) | .46 | 112 (70) | 131 (95) | .48 |
| GGT, U/L | 73 (145) | 122 (144) | .65 | 98 (146) | 64 (135) | .39 |
| AST, U/L | 45 (36) | 29 (51) | .26 | 47 (37) | 47 (71) | .96 |
| ALT, U/L | 35 (44) | 26 (43) | .14 | 28 (28) | 33 (27) | .66 |
Abbreviations: ACLD, advanced chronic liver disease; AIH, autoimmune hepatitis; ALD alcoholic liver disease; ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; body surface area; BSA; CCC, cholangiocellular carcinoma; CLD, chronic liver disease; CSPH, clinical significant portal hypertension; CTP, Child‐Turcotte‐Pugh; GGT, gamma‐glutamyltransferase; IDDM, insulin‐dependent diabetes mellitus; INR, international normalized ratio; MELD, model for end‐stage liver disease; N/A not applicable; NAFLD, non‐alcoholic fatty liver disease; NIDDM, non‐insulin–dependent diabetes mellitus; NSBB, non‐selective beta blockers; SBP, spontaneous bacterial peritonitis; STI, soft tissue infection; UTI, urinary tract infection.
Figure 2.

Study flow chart
Sarcopenia was present in 14/56 (25%) of patients in the non‐ACLD group, in 18/110 (16.4%) of patients in the cACLD group and in 40/99 (40%) of patients in the dACLD group. Nine (3.4%) of patients developed an HCC during the follow‐up period and seven (2.6%) patients were treated with a transjugular intrahepatic portosystemic shunt, on which day follow‐up ceased.
3.2. Reader agreement for TPMT and sarcopenia
There was a strong correlation between the various TPMT measurements performed on all sequences for both readers (Spearman's rho > 0.95, P < .001).
Intra‐observer ICCs for TPMT measurements on the three different sequences were 0.98 (95% confidence interval (CI): 0.98‐99) for R1 and 0.99 (95% CI: 0.99‐0.99) for R2. Inter‐observer ICC for the TPMT measurement were 0.98 (95% CI: 0.96‐0.98) for the unenhanced T1‐weighted sequences, 0.98 (95% CI: 0.97‐0.98) for the contrast‐enhanced T1 weighted sequences and 0.98 (95% CI: 0.97‐0.99) for the T2‐weighted sequences. Intra‐observer ICC for a repeated evaluation of 50 scans using all three sequences was 0.99 (95% CI: 0.99‐1.00).
3.3. Sarcopenia is not a risk factor for the development of first or further hepatic decompensation
The median follow‐up time was 30.2 months (range 0‐99) (Table 2, Figure 3). None of the patients in the non‐ACLD group developed hepatic decompensation during follow‐up. Twenty‐one patients (19%) in the cACLD group developed hepatic decompensation: 14 patients (12.7%) ascites, four patients (3.6%) hepatic encephalopathy, two patients (1.8%) variceal bleeding and one patient (0.9%) a liver‐related death. Sixty patients (61%) in the dACLD group developed further hepatic decompensations: 35 patients (35%) first occurrence or worsening of ascites, 14 patients (14%) first occurrence or worsening of hepatic encephalopathy, two patients (2%) SBP, two patients (2%) variceal bleeding and seven patients (7%) died for a liver‐related cause.
Table 2.
Risk factors for first (cACLD) or further (dACLD) hepatic decompensation
| Patient characteristics | cACLD, n = 110 | dACLD, n = 99 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | aHR | 95% CI | P value | HR | 95% CI | P value | aHR | 95% CI | P value | |
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| Sarcopenia | 1.46 | 0.49‐4.35 | .5 | 0.76 | 0.24‐2.4 | .64 | 1.36 | 0.82‐2.27 | .24 | 1.06 | 0.6‐1.85 | .85 |
| MELD, per point | 1.03 | 0.94‐1.12 | .56 | 0.96 | 0.87‐1.06 | .44 | 1.11 | 1.07‐1.15 | <.001 | 1.09 | 1.05‐1.13 | <.001 |
| Albumin, per g/L | 0.9 | 0.84‐0.96 | .02 | 0.88 | 0.8‐0.93 | .006 | 0.95 | 0.03‐0.98 | .002 | 0.94 | 0.92‐0.99 | .008 |
| Varices | 2.77 | 1.05‐7.31 | .04 | N/A | N/A | N/A | N/A | N/A | ||||
| Evidence of CSPH | 3.2 | 1.24‐8.26 | .02 | 2.96 | 1.1‐7.96 | .03 | N/A | N/A | ||||
Abbreviations: 95% CI, 95% confidence interval; CSPH, clinical significant portal hypertension; HR, hazard ratio; MELD, model for end‐stage liver disease.
Figure 3.
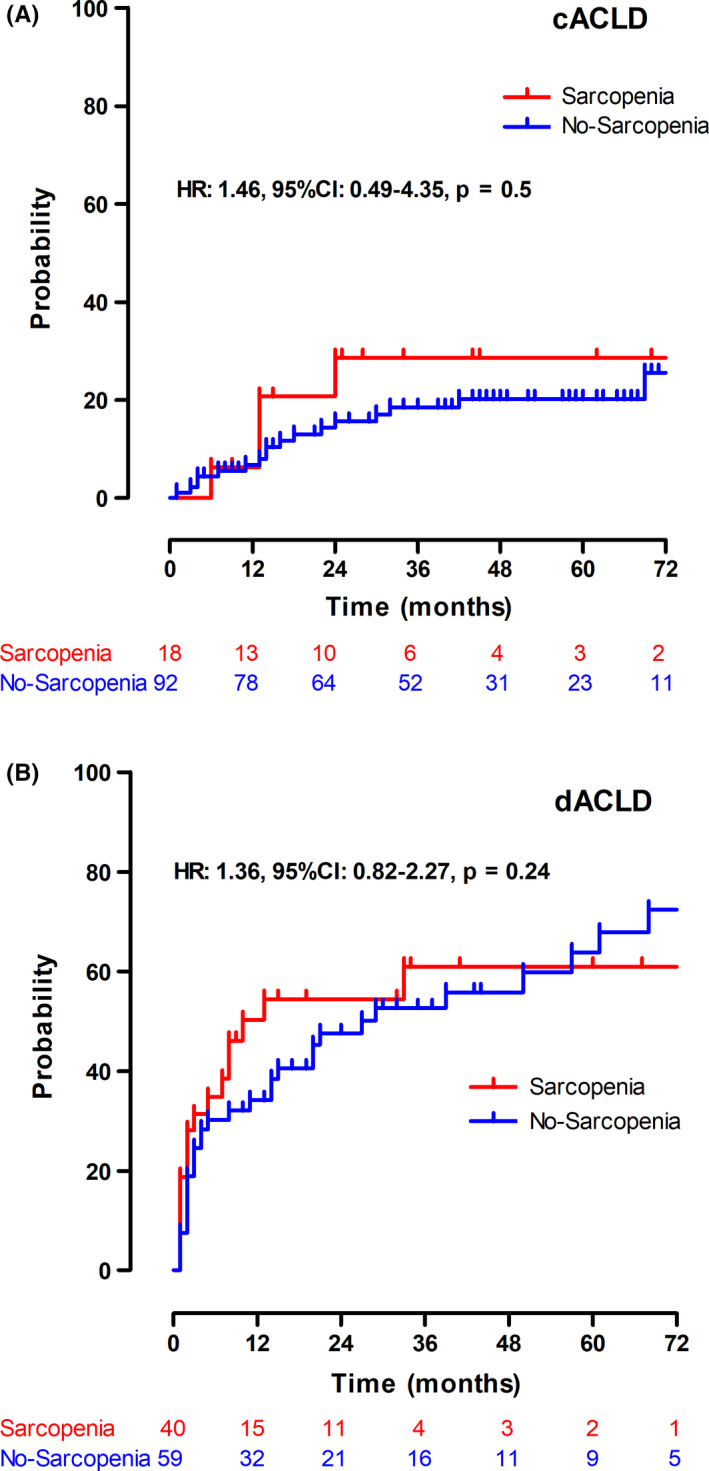
Kaplan‐Meier curves for first and further hepatic decompensation. A, First hepatic decompensation in compensated, advanced chronic liver disease (cACLD) patients and (B) further hepatic decompensation in patients with decompensated advanced chronic liver disease (dACLD). Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval
Sarcopenia impacted neither the risk of first nor further hepatic decompensation. cACLD: hazard ratio (HR): 1.46, 95% CI: 0.49‐4.35, P = .50; dACLD: HR: 1.36, 95% CI: 0.82‐2.27, P = .24. Using a pooled analysis including both patients with cACLD and dACLD, sarcopenia was identified as a risk factor for the development of hepatic decompensation (HR: 1.99, 95% CI: 1.26‐3.13, P = .003) in the univariate analysis. However, after adjusting for MELD and serum albumin levels, sarcopenia was not associated with the development of hepatic decompensation (adjusted HR (aHR): 1.14, 95% CI: 0.87‐2.2, P = .18) (Table S2).
3.4. Sarcopenia is an independent risk factor for mortality in patients with cACLD
Nine patients (8%) in the cACLD group underwent liver transplantation and 23 patients (22%) died (Table 3, Figure 4, Figure S1). Nineteen patients (19%) in the dACLD group underwent liver transplantation, while 44 (44%) died.
Table 3.
Risk factors for mortality in patients with cACLD and dACLD
| Patient characteristics | cACLD, n = 110 | dACLD, n = 99 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | aHR | 95% CI | P value | HR | 95% CI | P value | aHR | 95% CI | P value | |
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||||
| Sarcopenia | 3.13 | 1.33‐7.41 | .009 | 2.76 | 1.02‐7.42 | .045 | 2.45 | 1.32‐4.57 | .005 | 1.46 | 0.74‐2.92 | .28 |
| MELD, per point | 1.06 | 0.99‐1.14 | .09 | 0.99 | 0.91‐1.08 | .83 | 1.16 | 1.12‐1.21 | <.001 | 1.14 | 1.09‐1.2 | <.001 |
| Albumin, per g/L | 0.9 | 0.84‐0.95 | .001 | 0.89 | 0.81‐0.97 | .007 | 0.93 | 0.9‐0.97 | <.001 | 0.94 | 0.9‐0.98 | .003 |
| Varices | 1.52 | 0.6‐3.87 | .38 | N/A | N/A | N/A | N/A | N/A | ||||
| Evidence of CSPH | 1.65 | 0.72‐3.76 | .24 | 1.56 | 0.62‐3.93 | .35 | N/A | N/A | ||||
Abbreviations: 95% CI, 95% confidence interval; CSPH, clinical significant portal hypertension; HR, hazard ratio; MELD, model for end‐stage liver disease.
Figure 4.
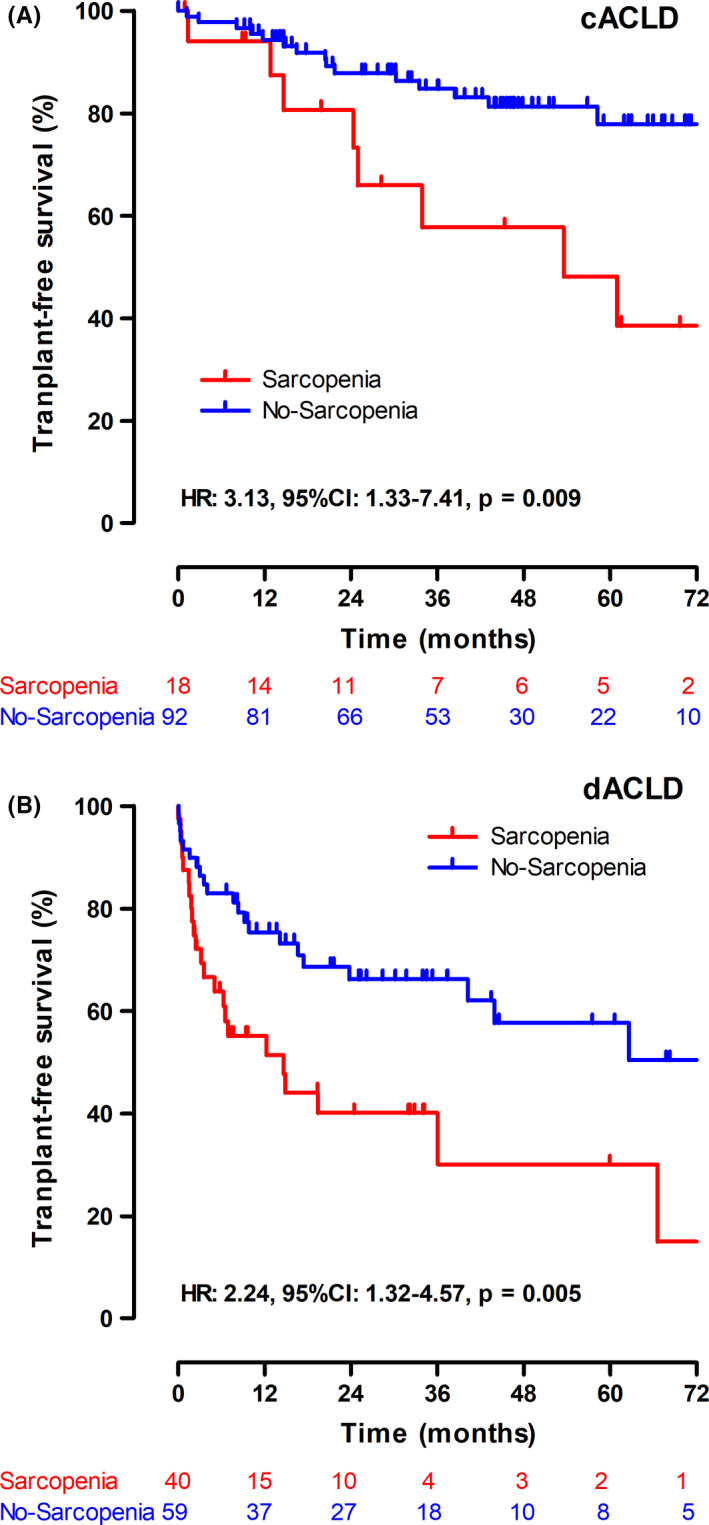
Kaplan‐Meier curves for transplant‐free survival. Transplant‐free survival in patients with (A) compensated advanced chronic liver disease (cACLD) and (B) decompensated advanced chronic liver disease (dACLD). Abbreviations: HR, hazard ratio; 95% CI, 95% confidence interval
Sarcopenia was identified as a risk factor for mortality in both patients with cACLD (HR: 3.13, 95% CI: 1.33‐7.41, P = .009) and patients with dACLD (HR: 2.34, 95% CI: 1.29‐4.3, P = .005) in univariate analysis. After adjusting for MELD, albumin levels and the evidence of CSPH, sarcopenia remained an independent risk factor for mortality in patients with cACLD (aHR: 2.76, 95% CI: 1.02‐7.42, P = .045). In contrast, sarcopenia was only associated with a numerically increased risk in dACLD (aHR: 1.46, 95% CI: 0.74‐2.92, P = .28). Performing an analysis combining patients with cACLD and dACLD, sarcopenia was identified as an independent risk factor for mortality (aHR: 2.16, 95% CI: 1.29‐3.62, P = .0004; Table S3).
3.5. Sarcopenia is a risk factor for development of infections and infection‐related mortality in patients with cACLD
One hundred and twenty‐two (46%) patients developed an infection during the follow‐up period, with urinary tract infection (36 patients; 39.5% of all infections) the most frequent infection site (Tables S4‐S6). There were no significant differences in the type of infection between patients with and without sarcopenia. However, sarcopenia was associated with an increased risk for the development of infections in patients with cACLD (HR: 2.27, 95% CI: 1.07‐4.78, P = .032), while the increase in risk did not attain statistical significance in patients with dACLD (HR: 1.62, 95% CI: 0.88‐2.98; P = .12). After the correction for the MELD, albumin and the evidence of CSPH, there was no significant association between sarcopenia and the development of infections in cACLD (aHR: 1.84, 95% CI: 0.84‐4.02, P = .13).
In addition, we assessed whether sarcopenia was a risk factor for infection‐related death. In the univariate analysis, sarcopenia was associated with increased risk for infection‐related death in patients with cACLD (HR: 7.98, 95% CI: 1.33‐47.78, P = .02), while the association did not attain statistical significance in dACLD (HR: 2.17, 95% CI: 0.8‐5.91; P = .13). In the multivariable analysis in patients with cACLD, sarcopenia was still a risk factor for infection‐related deaths (aHR: 12.41, 95% CI: 1.4‐110.13, P = .02), even after adjusting for MELD, albumin and evidence of CSPH. Details can be found in the Tables S5 and S6.
3.6. Sensitivity analysis: Impact of aetiological treatment
Thirty‐six patients with viral hepatitis began antiviral therapy during follow‐up and three patients with alcoholic liver disease stopped alcohol consumptions during the follow‐up (Tables S7 and S8). We performed a sensitivity analysis and stopped follow‐up at the day of aetiological cure and performed revised time‐to‐event analysis for patients with cACLD and dACLD. In line with our analysis shown above, neither in patients with cACLD (univariate analysis: HR: 1.1, 95% CI: 0.33‐3.76; P = .88) nor in patients with dACLD (univariate analysis: HR: 1.38, 95% CI: 0.82‐2.32; P = .23), sarcopenia was associated with the development of first or further hepatic decompensation after censoring patients who achieved aetiological cure.
In the univariate analysis, sarcopenia was associated with mortality in patients with cACLD and dACLD (see Table S8). However, after adjusting for MELD and albumin (plus ‘evidence of CSPH’ in patients with cACLD), there were trends towards increased mortality in patients with sarcopenia (P = .1 and P = .11 in cACLD and dACLD patients respectively).
3.7. Interaction between liver disease severity and the impact of sarcopenia on mortality
We subclassified patients with ACLD according to the MELD and Child‐Turcotte‐Pugh (CTP) score in those who had a MELD < 15 and CTP A/B vs MELD ≥ 15 or CTP C. In the univariate analysis, sarcopenia was identified as a risk factor for mortality in patients with MELD < 15 or CTP A/B (HR: 2.9, 95% CI: 1.39‐6.07, P = .005) and patients with MELD ≥ 15 or CTP C (HR: 2.09, 95% CI: 1.1‐4.0, P = .003) (Table S9). After adjusting for MELD, albumin and the presence of CSPH, sarcopenia remained an independent risk factor for mortality in both groups (aHR: 3.36, 95% CI: 1.05‐7.25, P = .005; aHR: 2.2, 95% CI: 1.03‐3.97, P = .03). Finally, we also included the interaction term sarcopenia*MELD ≥ 15 or CTP C in a multivariate model and the interaction term did not attain statistical significance (P = .37).
4. DISCUSSION
In this study we demonstrate that sarcopenia – as rapidly and reproducibly assessed by TPMT on MRI – represents a risk factor mortality in patients with ACLD. Particularly in cACLD patients, a TPMT‐L3 < 8 mm/m in women and <12 mm/m in men conferred a 2.77‐fold increased risk for death.
Thus, the TPMT evaluated at L3 provides a simple and standardized way of sarcopenia assessment in clinical practice and, importantly, provides critical prognostic information in ACLD patients.
A major strength of sarcopenia assessment by TPMT at L3 by an abbreviated MRI protocol is the lack of need for contrast agent injection. To the best of our knowledge, the imaging techniques that were used to assess sarcopenia either on CT or MRI in previous studies all used contrast agents. In certain clinical scenarios, avoiding contrast may be necessary since a considerable proportion of ACLD patients are suffering from renal impairment and/or are at risk for contrast‐induced nephropathy. 18 In addition, CT is associated with radiation exposure, and CT contrast agents cannot be administered to patients with iodine allergies. Furthermore, MRI contrast agents may accumulate within the body with implications that are not yet fully understood. 19 , 20
Our study used the previously published CT‐based TPMT L3 cut‐off 8 confirming that these gender‐specific and height‐adjusted cut‐offs can be also be applied to non‐contrast–enhanced MRI. Current guidelines only recommend CT as cross‐sectional imaging technique to assess sarcopenia in patients with ACLD, however, we could demonstrate that MRI is a suitable imaging technique to assess sarcopenia, using similar cut‐off values developed and validated on CT‐based studies. Furthermore, the excellent inter‐ and intra‐reader agreement (ICC > 0.98) for TPMT measurement on all MR sequences underlines the reproducibility and robustness of this technique. Our data are in line with previous studies that showed an excellent agreement between CT‐ and MR‐based skeletal muscle index measurements. 21 In contrast to invasive risk assessments, 22 MRI‐based TPMT assessment can be easily performed by both radiologist and clinicians without any expertise in MRI analysis. Moreover, no dedicated software or complex formula is necessary.
Patients with cACLD and sarcopenia showed a considerably increased risk of mortality, even after adjusting for established risk factors such as MELD score, serum albumin levels and the evidence of CSPH, ie the main prognostic factors in this patient group. 14 In contrast, sarcopenia was not associated with the risk for first or further hepatic decompensation. Several studies have reported the association between sarcopenia and increased mortality in patients with ACLD. 4 , 8 , 9 , 23 , 24 , 25 However, studies that consider the complex natural history of CLD progression, ie that hepatic decompensation usually occurs as initial event, followed by further decompensating events and, ultimately, liver‐related mortality, are scare. Our results indicate that the presence of sarcopenia is not associated with an increased risk for first or further hepatic decompensation. These results are in line with recent findings from our group, suggesting that sarcopenia is not linked to the severity of portal hypertension, 8 which is a main driver of hepatic decompensation. 26 , 27 , 28
Conversely, sarcopenia was a strong risk factor for increased mortality, especially in patients with cACLD, in whom it was independently linked to the outcome of interest. While the median TFS for patients with cACLD and dACLD without sarcopenia was 82 and 60 months, respectively, it was only 54 and 30 months in patients with sarcopenia respectively. We also performed a sensitivity analysis and censored all patients with viral hepatitis and ALD at the day they started aetiological therapy during the follow‐up period. After this modification, we still found an association between the presence of sarcopenia and increased mortality in patients with cACLD in the univariate analysis, with a nearly three‐fold increased risk. In contrast, in the multivariable analysis, we only observed a trend towards increased mortality in cACLD patients with sarcopenia (HR: 2.34, P = .10), likely as a result of limited statistical power. Of note, aetiological therapies were uniformly distributed between the sarcopenia subgroups, indicating that they did not confound the association between sarcopenia and mortality.
While sarcopenia was not a risk factor for infections in cACLD/dACLD and did not seem to impact the site of infection, it was associated with increased risk of infection‐related death. This is line with a previous study by Lucidi et al 29 in patients with cirrhosis with sepsis, which reported an association between sarcopenia and the mortality in this setting – CTP A/B patients with sarcopenia were found to have a similar probability of death, as compared to CTP C patients. Thus, the impact of sarcopenia on the course of infections may provide an explanation for the excess mortality observed among patients with sarcopenia in our study.
As previously reported, 30 the prevalence of sarcopenia was higher in patients with dACLD as compared to cACLD patients (40% vs 16%). Accordingly, adopted treatment strategies that target malnutrition and muscle loss may be particularly beneficial in the relatively small group of patients with cACLD and sarcopenia. These interventions should be tested in clinical trials and an abbreviated non‐contrast–enhanced MRI may be used for patient selection and to non‐invasively monitor treatment response.
In addition to its retrospective design, this study might be limited by a selection bias (ie only patients undergoing MRI imaging were included), which may limit the generalizability of our findings. However, since MRI of the liver is the standard‐of‐care imaging in work‐up of focal liver lesions and/or CLD at our institution, a profound selection bias is less likely and we also show that the indication for the MRI was not different between patients with and without sarcopenia. Secondly, the previously described TPMT‐L3 derived cut‐offs to define sarcopenia may not have been the ideal cut‐offs for predicting the clinical outcomes in our study population. However, we decided to use previously defined cut‐offs to validate their prognostic value for MRI‐based assessments.
In conclusion, MRI‐based sarcopenia assessment by a simple MRI TPMT‐L3 measurement represents a quick reproducible tool which is ideal for clinical practice. cACLD patients with low TPMP‐L3 muscle mass should be selected for specific interventions targeting sarcopenia as they are at increased risk for mortality.
CONFLICTS OF INTEREST
The authors report no conflict of interest related to this study. The following conflicts of interests outside of this study exist: LB, NB, SP, KL, YB, DL, JH, BS and GS report no conflict of interests. MM: Speaker and/or consultant and/or advisory board member for AbbVie, Bristol‐Myers Squibb, Collective Acumen, Gilead and W. L. Gore & Associates. TR: Grant support from Abbvie, Boehringer‐Ingelheim, Gilead, MSD, Philips Healthcare, Gore; speaking honoraria from Gilead, Gore, Intercept, Roche, MSD; consulting/advisory board fee from Abbvie, Boehringer‐Ingelheim, Gilead, MSD; and travel support from Boehringer‐Ingelheim, Gilead and Roche. AB: received honoraria for lectures and a consultancy from Bayer.
Supporting information
Supplementary Material
ACKNOWLEDGEMENT
We acknowledge the great support from the Department of IT‐Systems & Communications of the Medical University of Vienna who provided clinical and laboratory data.
Beer L, Bastati N, Ba‐Ssalamah A, et al. MRI‐defined sarcopenia predicts mortality in patients with chronic liver disease. Liver Int. 2020;40:2797–2807. 10.1111/liv.14648
Beer and Bastati share first authorship.
Handling Editor: Virginia Hernandez‐Gea
Funding information
No financial support was received for this study.
REFERENCES
- 1. Cruz‐Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2018;48(1):16‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ooi PH, Hager A, Mazurak VC, et al. Sarcopenia in chronic liver disease: impact on outcomes. Liver Transpl. 2019;25(9):1422‐1438. [DOI] [PubMed] [Google Scholar]
- 3. Merli M, Berzigotti A, Zelber‐Sagi S, et al. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70(1):172‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Praktiknjo M, Book M, Luetkens J, et al. Fat‐free muscle mass in magnetic resonance imaging predicts acute‐on‐chronic liver failure and survival in decompensated cirrhosis. Hepatology. 2018;67(3):1014‐1026. [DOI] [PubMed] [Google Scholar]
- 5. Poetter‐Lang S, Staufer K, Baltzer P, et al. The Efficacy of MRI in the diagnostic workup of cystic fibrosis‐associated liver disease: a clinical observational cohort study. Eur Radiol. 2019;29(2):1048‐1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haider L, Mandorfer M, Güngören Z, et al. Noninvasive monitoring of liver disease regression after hepatitis C eradication using gadoxetic acid‐enhanced MRI. Contrast Media Mol Imaging. 2018;2018:8489709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ba‐Ssalamah A, Bastati N, Wibmer A, et al. Hepatic gadoxetic acid uptake as a measure of diffuse liver disease: where are we? J Magn Reson Imaging. 2017;45(3):646‐659. [DOI] [PubMed] [Google Scholar]
- 8. Paternostro R, Lampichler K, Bardach C, et al. The value of different CT‐based methods for diagnosing low muscle mass and predicting mortality in patients with cirrhosis. Liver Int. 2019;39(12):2374‐2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Praktiknjo M, Clees C, Pigliacelli A, et al. Sarcopenia is associated with development of acute‐on‐chronic liver failure in decompensated liver cirrhosis receiving transjugular intrahepatic portosystemic shunt. Clin Transl Gastroenterol. 2019;10(4):e00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beer L, Mandorfer M, Bastati N, et al. Inter‐ and intra‐reader agreement for gadoxetic acid‐enhanced MRI parameter readings in patients with chronic liver diseases. Eur Radiol. 2019;29(12):6600‐6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bastati N, Beer L, Mandorfer M, et al. Does the functional liver imaging score derived from gadoxetic acid–enhanced MRI predict outcomes in chronic liver disease? Radiology. 2020;294(1):98‐107. [DOI] [PubMed] [Google Scholar]
- 12. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317‐1325. [DOI] [PubMed] [Google Scholar]
- 13. De Franchis R. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743‐752. [DOI] [PubMed] [Google Scholar]
- 14. Ripoll C, Groszmann R, Garcia–Tsao G, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133(2):481‐488. [DOI] [PubMed] [Google Scholar]
- 15. Berzigotti A, Garcia‐Tsao G, Bosch J, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54(2):555‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scheiner B, Steininger L, Semmler G, et al. Controlled attenuation parameter does not predict hepatic decompensation in patients with advanced chronic liver disease. Liver Int. 2019;39(1):127‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mandorfer M, Kozbial K, Schwabl P, et al. Changes in HVPG predict hepatic decompensation in patients who achieved SVR to IFN‐free therapy. Hepatology. 2020;71(3):1023‐1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Angeli P, Bernardi M, Villanueva C, et al. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406‐460. [DOI] [PubMed] [Google Scholar]
- 19. Dekkers IA, Roos R, van der Molen AJ. Gadolinium retention after administration of contrast agents based on linear chelators and the recommendations of the European Medicines Agency. Eur Radiol. 2018;28(4):1579‐1584. [DOI] [PubMed] [Google Scholar]
- 20. Raczeck P, Fries P, Bucker A, Schneider G. Gadolinium deposition‐“gadolinium deposition disease”. Der Radiologe. 2019;59(5):435‐443. [DOI] [PubMed] [Google Scholar]
- 21. Sinelnikov A, Qu C, Fetzer DT, et al. Measurement of skeletal muscle area: comparison of CT and MR imaging. Eur J Radiol. 2016;85(10):1716‐1721. [DOI] [PubMed] [Google Scholar]
- 22. Bucsics T, Schoder M, Diermayr M, et al. Transjugular intrahepatic portosystemic shunts (TIPS) for the prevention of variceal re‐bleeding ‐ a two decades experience. PLoS One. 2018;13(1):e0189414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Montano‐Loza AJ, Duarte‐Rojo A, Meza‐Junco J, et al. Inclusion of sarcopenia within MELD (MELD‐Sarcopenia) and the prediction of mortality in patients with cirrhosis. Clin Transl Gastroenterol. 2015;6(7):e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hanai T, Shiraki M, Nishimura K, et al. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31(1):193‐199. [DOI] [PubMed] [Google Scholar]
- 25. van Vugt JLA, Alferink LJM, Buettner S, et al. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: a competing risk analysis in a national cohort. J Hepatol. 2018;68(4):707‐714. [DOI] [PubMed] [Google Scholar]
- 26. Reiberger T, Ulbrich G, Ferlitsch A, et al. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non‐response to propranolol. Gut. 2013;62(11):1634‐1641. [DOI] [PubMed] [Google Scholar]
- 27. Reiberger T, Puspok A, Schoder M, et al. Austrian consensus guidelines on the management and treatment of portal hypertension (Billroth III). Wien Klin Wochenschr. 2017;129(Suppl 3):135‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bucsics T, Hoffman S, Grünberger J, et al. ePTFE‐TIPS vs repetitive LVP plus albumin for the treatment of refractory ascites in patients with cirrhosis. Liver Int. 2018;38(6):1036‐1044. [DOI] [PubMed] [Google Scholar]
- 29. Lucidi C, Lattanzi B, Di Gregorio V, et al. A low muscle mass increases mortality in compensated cirrhotic patients with sepsis. Liver Int. 2018;38(5):851‐857. [DOI] [PubMed] [Google Scholar]
- 30. Lattanzi B, Gioia S, Di Cola S, et al. Prevalence and impact of sarcopenia in non‐cirrhotic portal hypertension. Liver Int. 2019;39(10):1937‐1942. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


