Abstract
Objectives
To study the prevalence of exercise‐induced bronchoconstriction (EIB) and exercise‐induced laryngeal obstruction (EILO) in adolescent athletes.
Methods
All adolescents (n = 549) attending first year at a sports high school in 2016 and 2017, were invited to answer a questionnaire on respiratory symptoms. The 367 responding participants were divided into two groups based on whether they reported exercise‐induced dyspnea (dyspnea group) or not (nondyspnea group). Randomly selected participants in each group were invited to undergo two standardized exercise tests, an EIB test and a continuous laryngoscopy exercise (CLE) test, to investigate EILO.
Results
In total, 98 participants completed an EIB test, 75 of whom also completed a CLE test. Positive EIB tests: eight of 41 in the dyspnea group and 16 of 57 in the nondyspnea group. Positive CLE tests: 5 of 34 in the dyspnea group and three of 41 in the nondyspnea group. The estimated prevalence of EIB was 23.1% (95% confidence interval [CI]: 14.5–33.8) and of EILO 8.1% (95% CI: 2.5–18.5) in the whole study population. No differences in prevalence of EIB or EILO were found between the dyspnea and the nondyspnea groups.
Conclusion
EIB was highly prevalent in this cohort of adolescent athletes. EILO was less prevalent, but represents an important differential diagnosis to EIB. Self‐reported exercise‐induced dyspnea is a weak indicator for both EIB and EILO and standardized testing should be provided.
Keywords: adolescents, dyspnea, exercise tests, high school athletes
1. INTRODUCTION
Exercise‐induced respiratory symptoms are common among adult elite athletes, 1 , 2 but knowledge about their occurrence among adolescent athletes is limited. In this group, the prevalence of exercise‐induced respiratory symptoms, such as shortness of breath, wheezing, chest tightness or cough has been reported to vary between 15% and 28%. 3 , 4 This is higher than the prevalence reported in adolescents in general populations. 5 , 6
There are several different conditions that may cause exercise‐induced respiratory symptoms. 7 The most widely studied condition is exercise‐induced bronchoconstriction (EIB). This refers to a transient airway narrowing that occurs in association with physical activity. 8 EIB is present in 35%–39% of adult athletes, 1 , 9 even higher among swimmers and athletes performing cold weather sports. 10 , 11 In comparison, one study reported EIB in 13% of recreationally active adults in the general population. 12 In contrast to adult athletes, EIB in adolescent athletes is less studied but prevalence figures ranging from 10% to 20% have been reported. 4 For adolescents in the general population, EIB prevalence has been estimated at approximately 5%–20%. 8 , 13 , 14 The wide span of reported EIB prevalence rates can be explained by variation of study designs and diagnostic methods.
Exercise‐induced laryngeal obstruction (EILO) is a condition where breathing is hampered by narrowing of the larynx upon exercise. This may occur either in the form of vocal fold adduction (glottic EILO) or due to medial movement of the cuneiform tubercles and the aryepiglottic folds obstructing the laryngeal inlet (supraglottic EILO). 15 , 16 For adolescents in the general population the prevalence of EILO has been estimated to 5.7%–7.5%. 13 , 17 There are no cross‐sectional studies investigating prevalence of EILO in athletes, though EILO is believed to be more prevalent in young athletes than in the general population. 18 Knowledge of respiratory symptoms in connection to EILO is scarce, but EILO may be misdiagnosed as EIB due to respiratory symptoms occurring during exercise. Thus, correct diagnosis cannot rely on history alone. 19 , 20 The continuous laryngoscopy exercise (CLE) test is the recommended diagnostic test for EILO. 16
EIB and EILO have been described to coexist in some individuals, demonstrating that a positive test for EIB does not exclude the possibility of a positive CLE test and vice versa. 13 , 21 Though these conditions can have similar clinical manifestations, EIB and EILO are physiologically different and require different clinical management. For EIB there is efficient pharmacological treatment, 22 whereas for EILO pharmacological treatment is not recommended. For selected cases of EILO surgical treatment with supraglottoplasty may be successful. 23 Given the potential negative impact of these conditions on sports performance, investigating EIB and EILO in athletes is important. 24
The aim of this study was to investigate the prevalence of exercise‐induced dyspnea, EIB and EILO among adolescent athletes.
2. METHODS
In early autumn of the years 2016 and 2017, all students starting their first year at a sports high school (2016: n = 270, 2017: n = 279, age 15–17 years) in Uppsala, Sweden, were asked to participate and fill out a questionnaire. The questionnaire comprised questions on respiratory symptoms, asthma and allergy. It was based on the International Study of Asthma and Allergy in Childhood Questionnaire 25 and European Community Respiratory Health Survey, 26 and has been previously used in adolescent populations. 5
In total, 367 participants consented to participate and filled out the questionnaire (2016: n = 172, 2017: n = 195), equaling 66.8% of all eligible adolescents. One female with previously diagnosed EILO was not included in the study and 181 adolescents declined all participation.
Based on their answers to the question “Have you had an attack of shortness of breath that came during or following strenuous activity at any time, in the last 12 months?”, 27 the participants were stratified into two groups. Participants who responded positively to this question were assigned to the dyspnea group and participants who responded negatively were assigned to the nondyspnea group.
The stratification was based on the hypothesis that the prevalence rates of EIB and EILO would be higher in the dyspnea group than in the nondyspnea group. The same method has been applied in a previous epidemiological study of EIB and EILO. 13 The intention was to perform exercise tests in 100 participants, evenly distributed between the dyspnea and the nondyspnea groups. To obtain a representative sample for the whole population, participants within the dyspnea and nondyspnea groups were sequenced by computer randomization. Participants from both groups were invited in order of sequence number, to undergo a standardized EIB test. For all participants undergoing the EIB test, data regarding weight, height and average weekly training hours were obtained. All participants who completed an EIB test were invited to undergo a CLE test at a second visit.
All investigators (KE, HJ for EIB tests, EM, KN, LN for CLE tests) were blinded to whether the participants belonged to the dyspnea or nondyspnea group. CLE test investigators were also blinded to the EIB test results.
In total, 98 participants (41 in the dyspnea group and 57 in the nondyspnea group) completed an EIB test, 75 of whom (34 in the dyspnea group and 41 in the nondyspnea group) also completed a CLE test. All tests took place at Uppsala University Hospital during September–November 2016 and 2017.
2.1. Exercise‐induced bronchoconstriction test
Before EIB testing, the participants were instructed to withdraw any short‐acting β2‐agonists 8 h before the test, long‐acting β2‐agonists 24 h before the test and leukotriene receptor agonists 72 h before the test. The participants were instructed not to use inhaled corticosteroids on the day of their test. They were also asked to avoid vigorous exercise, heavy meals, nicotine and caffeine 4 h before their EIB test. 28
Baseline spirometry was conducted in accordance with the American Thoracic Society standards. 29 The baseline value of forced expiratory volume in one second (FEV1) was documented as the best of three reproducible measurements (Cardio Perfect dynamic spirometry; Welch Allyn). The EIB tests were performed on a treadmill (Tunturi T50). Dry air (H2O < 5 mg/L, 18–22°C) was administered through a breathing tube (Aiolos Asthmatest; Aiolos Medical). The EIB test comprised 7–8 min of running. Heart rate was monitored continuously using a heart rate monitor (Polar RCX5; Polar Electro OY). The protocol required reaching a heart rate of 90% of the predicted maximum ((220 − age) × 0.9) within the first 2 min and maintaining this level for the remaining 5–6 min. The heart rate level was maintained by continuous adjustment of the treadmill's speed and slope. FEV1 was measured 5, 10, 15, 30, and 60 min after the test. The best FEV1 value of two measurements at each time point was documented. EIB was defined as a decrease of FEV1 ≥ 10% from baseline. 28
During EIB testing, two participants in the nondyspnea group stopped running in minute four, that is, not completing the test in accordance with the test protocol; one stopped due to throat tightness and one due to nausea. However, lung function measurements were performed according to protocol on these participants and they have been included in the analyses of the study.
2.2. Continuous laryngoscopy exercise test
The CLE tests were performed 3–22 (median 14) days after the EIB tests. Participants who regularly used asthma medication before physical exercise were instructed to do so before the CLE test. The participants were asked to avoid vigorous exercise the day before the test.
The CLE tests were performed using bicycle ergometry (model 828E, Monark).
The bicycle seat was adjusted for each subject. Naphazoline‐lidocaine was sprayed in the nostrils to achieve local anesthetic and decongestive effect in the nasal cavity. A fiber optic laryngoscope (Olympus ENF‐P3) was inserted in one nostril until its tip was just above the epiglottis, providing a detailed view of the larynx. The laryngoscope was fixed to the nose, fastened in a helmet to secure position and connected to a camera (Ubicam; Sopro imaging). Normal ad‐ and abduction ability of the vocal folds was confirmed.
The pedal frequency was 80 rotations per minute and the load started at 80 watts and was increased by 40 watts every second minute until exhaustion. The participants were instructed to continue pedaling until they experienced disabling shortness of breath or until complete exhaustion. The larynx was filmed during the entire test and continuously for two minutes after cessation. Laryngeal obstruction was graded 0–3, at glottic and supraglottic levels according with the criteria described by Maat et al. 30 Obstruction of grade two or higher at the supraglottic and/or the glottic level was defined as a positive CLE test, consistent with EILO. All CLE test investigators were medical doctors in the field of otorhinolaryngology (EM, KN, LN). The laryngeal obstruction was graded during the test by the attending investigator. For uncertain cases and cases graded as 2 or higher, the recordings from the tests were also evaluated by one additional investigator.
2.3. Statistical analyses
The data were analyzed using STATA 14.2 (Stata Corp) and SAS 9.4 (SAS Institute Inc.). Anthropometric data, lung function, asthma medication and exercise test results were summarized as arithmetic means, standard deviations, and minimum and maximum for continuous variables, and as numbers and percentages for categorical variables. Age, body mass index, and FEV1 were compared between groups using unpaired Student′s t tests. For all categorical variables, a cross‐tabulation versus groups was performed and groups were compared using the chi square test or the Fisher exact test (if group size <6). The results were considered to be statistically significant at p < .05. The participants who underwent EIB and CLE tests were assumed to be random samples from the two groups, dyspnea and nondyspnea. Considering the larger drop‐out rate among males compared with females, post‐stratification by sex was applied to assign statistical weight to the participants. 31 Calculations were based on four strata: dyspnea females, dyspnea males, nondyspnea females, and nondyspnea males. The prevalence rates of EIB and EILO were estimated by multiplying the proportions of positive tests within the strata with their relative proportion of the study population. Wald based confidence limits were calculated for estimates >25% and modified Clopper–Pearson confidence limits were calculated for estimates <25%. 32
2.4. Ethics
All participants signed a written informed consent form at inclusion in the study. The ethical review board in Uppsala, Sweden, provided ethics approval for this study (Dnr 2016/169).
3. RESULTS
The characteristics of all participants and the dyspnea and nondyspnea groups are presented in Table 1.
Table 1.
Characteristics of all participants and participants in the dyspnea group and nondyspnea group
| All participants (n = 367) | Dyspnea (n = 75) | Nondyspnea (n = 292) | p value | |
|---|---|---|---|---|
| Female, n (%) | 150 (40.8) | 53 (70.7) | 97 (33.2) | < 0.001 |
| Age (years), mean (SD) | 15.8 (± 0.4) | 15.7 (±0.48) | 15.8 (±0.4) | 0.45 |
| BMI, mean (SD) | 21.7 (±2.9) | 21.5 (±3.1) | 21.8 (±2.8) | 0.46 |
| Overweight and obesitya, n (%) | 59 (17.3) | 10 (14.2) | 49 (18) | 0.58 |
| Current asthmab, n (%) | 50 (14) | 33 (44) | 17 (6) | < 0.001 |
| Rhinitis, n (%) | 107 (29.9) | 24 (33.3) | 83 (29.2) | 0.56 |
| Wheeze, n (%) | 72 (20.1) | 35 (48.6) | 37 (12.9) | < 0.001 |
| Day time Dyspnea, n (%) | 28 (7.8) | 13 (20.3) | 15 (4.6) | < 0.001 |
| Nocturnal Dyspnea, n (%) | 9 (2.5) | 6 (8.1) | 3 (1) | 0.003 |
| ICSc, n (%) | 28 (7.8) | 16 (22.3) | 12 (4.4) | < 0.001 |
| SABAc, n (%) | 63 (18.3) | 36 (50) | 27 (9.9) | < 0.001 |
| LABAc, n (%) | 10 (2.9) | 4 (6.2) | 6 (2.2) | 0.010 |
| LTRAc, n (%) | 5 (1.5) | 3 (4.5) | 2 (0.7) | 0.054 |
P‐value: Dyspnea versus Non‐dyspnea. BMI, body mass index (kg/m2); ICS, inhaled corticosteroids; SABA, short‐acting β2‐agonists; LABA, long‐acting β2‐agonists; LTRA, leukotriene receptor antagonist.
Available data: All 367 subjects responded to all the questions in the questionnaire except BMI n = 342, physician‐diagnosed asthma n = 357, rhinitis, wheeze, day‐time dyspnea, nocturnal dyspnea and ICS n = 358, SABA n = 345, LABA and LTRA n = 340.
Overweight and obesity defined as BMI ≥ 85th percentile.
Self‐reported physician‐diagnosed with symptoms and/or medication excluding SABA only.
Any use in last three months.
3.1. Dyspnea group
Exercise‐induced dyspnea was reported by 20.4% of all participants and similar proportions were reported in 2016 and in 2017 (20.3% and 20.5%, respectively, p > .99). EIB tests were completed by 41 participants in the dyspnea group, eight of which were positive. Subsequently 34 of these participants also completed a CLE tests, of which five were positive for EILO. One participant was positive in both tests.
3.2. Nondyspnea group
The nondyspnea group comprised 292 of 367 participants (97 females, 195 males). EIB tests were completed by 57 participants in the nondyspnea group, 16 of which were positive. Subsequently 41 of these participants also completed a CLE test, of which three were positive for EILO.
Characteristics and exercise test results for the participants who underwent EIB test are presented in Table 2.
Table 2.
Characteristics of participants who underwent EIB tests
| All tested (n = 98) | Dyspnea (n = 41) | Nondyspnea (n = 57) | p‐value | |
|---|---|---|---|---|
| Age (years), mean (SD) | 15.8 (± 0.1) | 16 (±0.2) | 15.8 (±0.1) (15,17) | >0.99 |
| Females, n (%) | 57 (58.2) | 29 (70.7) | 28 (49.1) | 0.053 |
| Weekly training hours, median | 12.5 | 12.5 | 12.5 | >0.99 |
| BMI, mean (SD) | 22.1 (±6.0) | 21.1 (±6.0) | 22.2 (±6.1) | 0.52 |
| Overweight and obesitya (%) | 18 (19.4) | 7 (18.4) | 11 (20) | 0.85 |
| FEV1 % predictedb, mean (SD) | 95.8 (±9.9) | 97.5 (±10.8) (±10.8) | 94.6 (±9.2) | 0.92 |
| Current asthmac, n (%) | 21 (21.4) | 18 (43.9) | 3 (5.2) | <0.001 |
| Rhinitis, n (%) | 26 (27.6) | 13 (33.3) | 13 (23.6) | 0.30 |
| Wheeze, n (%) | 34 (35.4) | 21 (52.5) | 13 (23.6) | 0.002 |
| Day time Dyspnea, n (%) | 11 (11.2) | 6 (15) | 5 (8.6) | 0.34 |
| Nocturnal Dyspnea, n (%) | 2 (2) | 0 | 2 (3.4) | 0.51 |
| ICSd, n (%) | 13 (13.5) | 9 (23.1) | 4 (7.1) | 0.035 |
| SABAd, n (%) | 28 (29.2) | 18 (46.2) | 10 (17.5) | 0.002 |
| LABAd, n (%) | 4 (4.3) | 2 (5.4) | 2 (3.6) | >0.99 |
| LTRAd, n (%) | 1 (1.1) | 0 (0) | 1 (1.8) | >0.99 |
| Exercise tests, positive/all tested, n (%) | ||||
| EIB test | 24/98 (24.5) | 8/41 (19.5) | 16/57 (28.1) | 0.46 |
| CLE test | 8/75 (10.7) | 5/34 (14.7) | 3/41 (7.3) | 0.46 |
P‐value: Dyspnea versus Nondyspnea. BMI, body mass index (kg/m2); ICS, inhaled corticosteroids; SABA, short‐acting β2‐agonists; LABA, long‐acting β2‐agonists; LTRA, leukotriene receptor antagonist; EIB test, exercise‐induced bronchoconstriction test; CLE test, continuous laryngoscopy exercise test.
Available data: all 98 subjects responded to all the questions in the questionnaire except wheeze, ICS and SABA n = 96, rhinitis n = 94, BMI, overweight and obesity, LABA and LTRA n = 93.
Overweight and obesity defined as BMI ≥ 85th percentile.
Refolded before exercise‐induced bronchoconstriction test.
Self‐reported physician‐diagnosed with symptoms and/or medication excluding SABA only.
Any use in last three months.
3.3. Current asthma
Current asthma was defined as self‐reported physician‐diagnosed asthma with concomitant symptoms and/or medication (excluding treatment with short‐acting β2‐agonists only). This was reported more often by participants in the dyspnea group than in the nondyspnea group. Self‐reported wheeze, daytime‐ and nocturnal dyspnea and use of asthma medication were also reported more often by participants in the dyspnea group (Table 1).
3.4. Sports participation
The participants were competing at national or international level (68%) or regional level (32%). Twenty‐one different sports were represented among the participants, with the most common being soccer (21%), floorball (11%), American football (8%), basketball (7%), and orienteering (6%). Cold weather sports, such as ice hockey and alpine skiing, were practiced by 9% of the participants. Swimming was performed by less than 3% of the participants.
The types of sports were evenly distributed among the participants in the dyspnea group and the nondyspnea groups, apart from orienteering; ten of 21 (48%) orienteers reported exercise‐induced dyspnea (p = .032). The distributions of types of sports were similar in the sample who underwent exercise tests and in all participants.
3.5. Participants declining or not completing exercise tests
In total, 152 participants declined all testing. In the dyspnea group 25 of the 66 (37.9%) invited to testing declined: 14 of 45 (31%) females and 11 of 21 (52.4%) males. In the nondyspnea group, 127 of 184 (69%) invited to testing declined: 32 of 58 (55.2%) females and 95 of 126 (75.4%) males. The number of participants declining tests were significantly higher among males compared with females (p < .0001) and in the nondyspnea group compared with the dyspnea group (p < .0001). The group that declined testing did not differ from the tested sample with regard to self‐reported current asthma or types of sports participation.
After completion of EIB tests, 18 participants (3 in the dyspnea group and 15 in the nondyspnea group) declined participation in the CLE test. Another five participants (four in the dyspnea group and one in the nondyspnea group) discontinued their CLE tests after fainting upon insertion of the laryngoscope (Figure 1). These were not included in the analysis of EILO prevalence.
Figure 1.
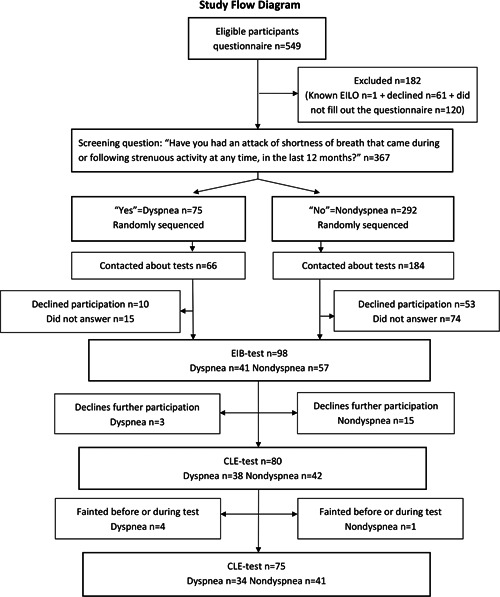
Participants' inclusion and exclusion. CLE test, continuous laryngoscopy exercise test; EIB test, exercise‐induced bronchoconstriction test
3.6. Prevalence of EIB
Twenty‐four (24.5%) participants had positive EIB tests; 8 (19.5%) in the dyspnea group (6 females of whom one also had a positive CLE test) and 16 (28.1%) in the nondyspnea group (11 females) (Table 2). The median fall in FEV1 in the dyspnea group was −13.3% (interquartile range [IQR]: 12.5–18.1) and in the nondyspnea group −15.9% (IQR: 11.9–18.4).
The estimated prevalence of EIB in the whole population was 23.1% (95% CI: 14.5–33.8). The estimated prevalence of EIB among females was 33.4% (95% CI; 19.9–47.0) and among males 16.8% (95% CI: 6.4–33.1) (Table 3).
Table 3.
Estimated prevalence rates of EIB and EILO post‐stratified by sex
| EIB | EILO | |
|---|---|---|
| All, % (95% CI) | 23.1 (14.5 – 33.8)a | 8.1 (2.5 – 18.5)b |
| Females, % (95% CI) | 33.4 (19.9 – 47.0)a | 11.1 (3.8 – 23.7)b |
| Males, % (95% CI) | 16.8 (6.4 – 33.1)b | 6.3 (0.4 – 24.8)b |
EIB, exercise‐induced bronchoconstriction; EILO, exercise‐induced laryngeal obstruction.
Wald confidence limits.
Clopper‐Pearson (exact) confidence limits.
3.7. Prevalence of EILO
Eight participants had positive CLE tests consistent with EILO; five (14.7%) in the dyspnea group (four females of whom one also had a positive EIB test) and three (7.3%) in the nondyspnea group (two females) (Table 2). Two of those with positive CLE‐tests had glottic obstruction, four had supraglottic obstruction and two had both glottic and supraglottic obstruction.
The estimated prevalence of EILO in the whole population was 8.1% (95% CI: 2.5–18.5). The estimated prevalence of EILO among females was 11.1% (95% CI: 3.8–23.7) and among males 6.3% (95% CI: 0.4–24.8) (Table 3).
4. DISCUSSION
In this cross sectional study investigating adolescents attending the first year at a sports high school, 20.4% reported exercise‐induced dyspnea. A positive EIB test was found in 24 of 98 tested participants (24.5%) and a positive CLE test consistent with EILO was found in 8 of 75 tested participants (10.7%). There were no statistically significant differences between the dyspnea and nondyspnea group regarding prevalence rates of EIB and EILO. EIB was numerically more prevalent in the nondyspnea group.
Exercise‐induced dyspnea was reported by 20.4%, which is a figure in line with previous research in athletes. 33 Our results also confirm previous findings that this symptom is more prevalent in females than in males. 5 , 34
The estimated prevalence of EIB was 23.1%, which is somewhat higher than in a general adolescent population. 13 No sex difference was found, which is in accordance with previous studies on EIB in the general adolescent population 35 and in college athletes. 9 The method of using both inhaled dry air and exercise in the EIB test may have contributed to higher prevalence of EIB than previously reported. However, this method is recommended in European Respiratory Society technical standard on bronchial challenge testing. 28 Using the threshold for a positive test of ≥10% postexercise fall in FEV1 may also have contributed to a higher prevalence rate of EIB in this study. A threshold of 15%–20% has been reported to be more specific for EIB, 36 but the 10% threshold has been proposed when assessing EIB in athletes. 28
A substantial number of positive EIB tests were found in both the dyspnea and nondyspnea groups. This finding, together with the fact that EIB was numerically more prevalent in the nondyspnea group, confirms that self‐reported exercise‐induced dyspnea is a poor predictor of EIB among young athletes. This is in line with other studies in athlete populations 9 , 24 , 37 but in contrast to a previous study on adolescents from the general population. 13
The dissociation between self‐reported symptoms and results from objective exercise tests demonstrates the need for screening for EIB in young athletes. Considering young athletes' high ventilatory demands and accounting for previous findings of high EIB prevalence in adult athletes, prompt detection and subsequent treatment of EIB could mitigate future airway dysfunction and thus promote both health and performance.
To our knowledge, this is the first cross‐sectional study on prevalence of EILO among athletes. The estimated prevalence of EILO was 8.1%. This figure is slightly higher than that reported in a cross‐sectional study of adolescents from a general population. 13 However, in a retrospective study by Nielsen et al, 21 where athletes were referred to a tertiary clinic for evaluation of respiratory symptoms, 35% had a positive CLE test and females more often had EILO compared with males. Substantial differences in study design may explain the diverging results between the Nielsen study and the present, both regarding the overall number of positive CLE tests and possible sex difference. In the present study numerically more females than males had a positive CLE test, but there was no sex difference regarding estimated prevalence of EILO. This is in concordance with a previous study of prevalence of EILO among adolescents from the general population. 13 It is unlikely that laryngeal immaturity could account for the lack of sex difference in this study, since all participants were at an age where the larynx is expected to have reached adult size in both females and males. However, the small total number of positive CLE tests may have affected the lack of sex difference in our estimates. Furthermore, a selection bias in the present study cannot be ruled out, as there was a non negligible dropout among male participants.
Participants with EILO were found to a similar extent in both the dyspnea and nondyspnea groups, indicating that self‐reported exercise‐induced dyspnea is a poor predictor also for EILO. Certainly, characterization of respiratory symptoms in connection to EILO requires further investigation in larger populations.
This study has several strengths. The cross‐sectional design with a randomized sample provides new information about athletes in an age group where competitive sport is common. The EIB tests were performed in accordance with international guidelines and the CLE test was performed accordance with current recommendations. 15 , 28 To prevent the risk of assessment bias, all investigators were blinded to participants' questionnaire outcomes, including their status as dyspnea or nondyspnea. The response rate to the questionnaire, 66.8%, is fairly good, but a possible selection bias cannot be overlooked, as there is no information on the adolescents who declined all participation. Another limitation is the use of bicycle ergometry during the CLE tests, instead of running on a treadmill. It may be debated if use of bicycle ergometry is less sensitive than running on a treadmill, resulting in false low prevalence of EILO in this study. However on comparison, the CLE score outcome has been reported to be similar regardless of if a bicycle or a treadmill is used. 38 The larger dropout rate among males resulted in a skewed representation of females versus males among participants undergoing exercise tests. To account for this statistically, post‐stratification was used. Due to the relatively small sample size, the confidence intervals of the estimates in this study are wide and overlapping. The lack of sex differences in the present study may also be affected by the small sample size.
5. CONCLUSION
EIB was highly prevalent in this cohort of adolescent athletes. EILO was less prevalent, but represents an important differential diagnosis to EIB. Self‐reported exercise‐induced dyspnea is a weak indicator for both EIB and EILO. We suggest EIB screening in elite athlete programs, regardless of respiratory symptoms. Athletes with disabling exercise‐induced respiratory symptoms, who are either EIB negative or EIB positive and still symptomatic despite successful EIB treatment, should be referred for CLE test to investigate EILO.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGEMENTS
The authors thank the participating students and the faculty, especially Emma Edbom Sjöö and Jörgen Norman, at Celsiusskolan, Uppsala for enabling this study. The authors would also like to express their gratitude to RN Nilla Westöö, Uppsala University Hospital for her key contribution to the project. This study was partially funded by The Swedish Asthma and Allergy Association (award number F2017‐0010, recipient: Henrik Johansson), the Gillbergska foundation and the Bror Hjerpstedt foundation.
Ersson K, Mallmin E, Malinovschi A, Norlander K, Johansson H, Nordang L. Prevalence of exercise‐induced bronchoconstriction and laryngeal obstruction in adolescent athletes. Pediatric Pulmonology. 2020;55:3509–3516. 10.1002/ppul.25104
Karin Ersson, Elisabet Mallmin, Henrik Johansson, and Leif Nordang contributed equally to this work.
REFERENCES
- 1. Dickinson J, McConnell A, Whyte G. Diagnosis of exercise‐induced bronchoconstriction: Eucapnic voluntary hyperpnoea challenges identify previously undiagnosed elite athletes with exercise‐induced bronchoconstriction. Br J Sports Med. 2011;45(14):1126‐1131. [DOI] [PubMed] [Google Scholar]
- 2. Lund TK, Pedersen L, Anderson SD, Sverrild A, Backer V. Are asthma‐like symptoms in elite athletes associated with classical features of asthma? Br J Sports Med. 2009;43(14):1131‐1135. [DOI] [PubMed] [Google Scholar]
- 3. Hallstrand TS, Curtis JR, Koepsell TD, et al. Effectiveness of screening examinations to detect unrecognized exercise‐induced bronchoconstriction. J Pediatr. 2002;141(3):343‐348. [DOI] [PubMed] [Google Scholar]
- 4. de Aguiar KB, Anzolin M, Zhang L. Global prevalence of exercise‐induced bronchoconstriction in childhood: a meta‐analysis. Pediatr Pulmonol. 2018;53(4):412‐425. [DOI] [PubMed] [Google Scholar]
- 5. Johansson H, Norlander K, Hedenstrom H, et al. Exercise‐induced dyspnea is a problem among the general adolescent population. Respir Med. 2014;108(6):852‐858. [DOI] [PubMed] [Google Scholar]
- 6. Pearce N, Ait‐Khaled N, Beasley R, et al. Worldwide trends in the prevalence of asthma symptoms: phase iii of the international study of asthma and allergies in childhood (isaac). Thorax. 2007;62(9):758‐766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weiss P, Rundell KW. Imitators of exercise‐induced bronchoconstriction. Allergy Asthma Clin Immunol. 2009;5(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weilereiler JM, Anderson SD, Randolph C, et al. Pathogenesis, prevalence, diagnosis, and management of exercise‐induced bronchoconstriction: a practice parameter. Ann Allergy Asthma Immunol. 2010;105(6 suppl):S1‐S47. [DOI] [PubMed] [Google Scholar]
- 9. Parsons JP, Kaeding C, Phillips G, Jarjoura D, Wadley G, Mastronarde JG. Prevalence of exercise‐induced bronchospasm in a cohort of varsity college athletes. Med Sci Sports Exerc. 2007;39(9):1487‐1492. [DOI] [PubMed] [Google Scholar]
- 10. Bougault V, Turmel J, Boulet LP. Bronchial challenges and respiratory symptoms in elite swimmers and winter sport athletes: airway hyperresponsiveness in asthma: Its measurement and clinical significance. Chest. 2010;138(2 Suppl):31s‐37s. [DOI] [PubMed] [Google Scholar]
- 11. Parsons JP, Mastronarde JG. Exercise‐induced bronchoconstriction in athletes. Chest. 2005;128(6):3966‐3974. [DOI] [PubMed] [Google Scholar]
- 12. Molphy J, Dickinson J, Hu J, Chester N, Whyte G. Prevalence of bronchoconstriction induced by eucapnic voluntary hyperpnoea in recreationally active individuals. J Asthma. 2014;51(1):44‐50. [DOI] [PubMed] [Google Scholar]
- 13. Johansson H, Norlander K, Berglund L, et al. Prevalence of exercise‐induced bronchoconstriction and exercise‐induced laryngeal obstruction in a general adolescent population. Thorax. 2015;70(1):57‐63. [DOI] [PubMed] [Google Scholar]
- 14. Aggarwal B, Mulgirigama A, Berend N. Exercise‐induced bronchoconstriction: prevalence, pathophysiology, patient impact, diagnosis, and management. NPJ Prim Care Respir Med. 2018;28(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Halvorsen T, Walsted ES, Bucca C, et al. Inducible laryngeal obstruction: an official joint european respiratory society and european laryngological society statement. Eur Respir J. 2017;50(3):1602221. [DOI] [PubMed] [Google Scholar]
- 16. Nordang L, Norlander K, Walsted ES. Exercise‐induced laryngeal obstruction‐an overview. Immunol Allergy Clin North Am. 2018;38(2):271‐280. [DOI] [PubMed] [Google Scholar]
- 17. Christensen PM, Thomsen SF, Rasmussen N, Backer V. Exercise‐induced laryngeal obstructions: prevalence and symptoms in the general public. Eur Arch Otorhinolaryngol. 2011;268(9):1313‐1319. [DOI] [PubMed] [Google Scholar]
- 18. Liyanagedera S, McLeod R, Elhassan HA. Exercise induced laryngeal obstruction: a review of diagnosis and management. Eur Arch Otorhinolaryngol. 2017;274(4):1781‐1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abu‐Hasan M, Tannous B, Weinberger M. Exercise‐induced dyspnea in children and adolescents: if not asthma then what? Ann Allergy Asthma Immunol. 2005;94(3):366‐371. [DOI] [PubMed] [Google Scholar]
- 20. Buchvald F, Phillipsen LD, Hjuler T, Nielsen KG. Exercise‐induced inspiratory symptoms in school children. Pediatr Pulmonol. 2016;51(11):1200‐1205. [DOI] [PubMed] [Google Scholar]
- 21. Nielsen EW, Hull JH, Backer V. High prevalence of exercise‐induced laryngeal obstruction in athletes. Med Sci Sports Exerc. 2013;45(11):2030‐2035. [DOI] [PubMed] [Google Scholar]
- 22. Reddel HK, FitzGerald JM, Bateman ED, et al. Gina 2019: a fundamental change in asthma management. Eur Respir J. 2019;53(6):1901046. [DOI] [PubMed] [Google Scholar]
- 23. Sandnes A, Hilland M, Vollsæter M, et al. Severe exercise‐induced laryngeal obstruction treated with supraglottoplasty. Front Surg. 2019;6:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boulet LP, O'Byrne PM. Asthma and exercise‐induced bronchoconstriction in athletes. N Engl J Med. 2015;372(7):641‐648. [DOI] [PubMed] [Google Scholar]
- 25. Asher MI, Keil U, Anderson HR, et al. International study of asthma and allergies in childhood (ISAAC): Rationale and methods. Eur Respir J. 1995;8(3):483‐491. [DOI] [PubMed] [Google Scholar]
- 26. Grassi M, Rezzani C, Biino G, Marinoni A. Asthma‐like symptoms assessment through ecrhs screening questionnaire scoring. J Clin Epidemiol. 2003;56(3):238‐247. [DOI] [PubMed] [Google Scholar]
- 27. The European Community Respiratory Health Survey II . Main questionnaire. http://www.ecrhs.org/Quests.htm. Accessed March 24, 2019.
- 28. Hallstrand TS, Leuppi JD, Joos G, et al. ERS technical standard on bronchial challenge testing: pathophysiology and methodology of indirect airway challenge testing. Eur Respir J. 2018;52(5):1801033. [DOI] [PubMed] [Google Scholar]
- 29. Miller MR. Standardisation of spirometry. Eur Respir J. 2005;26(2):319‐338. [DOI] [PubMed] [Google Scholar]
- 30. Maat R, Røksund O, Halvorsen T, et al. Audiovisual assessment of exercise‐induced laryngeal obstruction: reliability and validity of observations. Eur Arch Otorhinolaryngol. 2009;266(12):1929‐1936. [DOI] [PubMed] [Google Scholar]
- 31. Höfler M, Pfister H, Lieb R, Wittchen H‐U. The use of weights to account for non‐response and drop‐out. Soc Psychiatry Psychiatr Epidemiol. 2005;40(4):291‐299. [DOI] [PubMed] [Google Scholar]
- 32. Korn EL, Graubard BI. Confidence intervals for proportions with small expected number of positive counts estimated from survey data. Surv Methodol. 1998;24(1998002):193‐201. [Google Scholar]
- 33. Burnett DM, Vardiman JP, Deckert JA, Ward JL, Sharpe MR. Perception of exercise‐induced bronchoconstriction in college athletes. Respir Care. 2016;61(7):897‐901. [DOI] [PubMed] [Google Scholar]
- 34. Turcotte H, Langdeau J‐B, Thibault G, Boulet L‐P. Prevalence of respiratory symptoms in an athlete population. Respir Med. 2003;97(8):955‐963. [DOI] [PubMed] [Google Scholar]
- 35. Weiler JM, Bonini S, Coifman R, et al. American academy of allergy, asthma & immunology work group report: exercise‐induced asthma. J Allergy Clin Immunol. 2007;119(6):1349‐1358. [DOI] [PubMed] [Google Scholar]
- 36. Weiler JM, Brannan JD, Randolph CC, et al. Exercise‐induced bronchoconstriction update—2016. J Allergy Clin Immunol. 2016;138(5):1292‐1295.e1236. [DOI] [PubMed] [Google Scholar]
- 37. Price OJ, Hull JH, Ansley L, Thomas M, Eyles C. Exercise‐induced bronchoconstriction in athletes—a qualitative assessment of symptom perception. Respir Med. 2016;120:36‐43. [DOI] [PubMed] [Google Scholar]
- 38. Mirza KK, Walsted ES, Backer V. Ergospirometry with concurrent fibre optic laryngoscopy: a randomised crossover study. Eur Clin Respir J. 2017;4(1):1399033. [DOI] [PMC free article] [PubMed] [Google Scholar]


