Abstract
The current threats of climate change are driving attention away from the petrochemical industry towards more sustainable and bio‐based production processes for fuels and speciality chemicals. These processes require suitable low‐cost starting material. One potential material assessed here is the oat hull. Its overall chemical composition has so far not been fully characterized. Furthermore, it is not known how it is affected by extreme weather events.
Oat hulls (Kerstin and Galant varieties) grown during ‘normal’ weather years (2016 and 2017) are compared to the harvest of the warmer and drier year (2018). Standard methods for determination of plant chemical composition, with focus on carbohydrate composition, are utilized.
Oat hulls grown in ‘normal’ weather conditions (2017) are rich in lignocellulose (84%), consisting of 35% hemicellulose, 25% lignin and 23% cellulose. Arabinoxylan was found to be the major biopolymer (32%). However, this composition is greatly influenced by weather variations during the oat growth phase. A lignocellulose reduction of 25% was recorded in the warmer and drier 2018 harvest. Additionally, a 6.6‐fold increase in starch content, a four‐fold increase in protein content and a 60% decrease in phenolic content was noted.
Due to its high lignocellulose composition, with an exceptionally large hemicellulose fraction, the chemical composition of oat hulls is unique among agricultural by‐products. However, this characteristic is significantly reduced when grown in warmer and drier weather, which could compromise its suitability for use in a successful biorefinery.
Keywords: biorefinery, chemical composition, climate effects, lignocellulosic biomass, oat hull
The chemical composition of oat hulls is unique among agricultural side streams and significantly altered by warmer and drier weather.

Introduction
Oat (Avena sativa L.) is a crop grown worldwide and is now attracting more attention due to the discovery of its many health benefits as well as an increased interest in plant‐based diets. Its increasing popularity is reflected in global production quantities, which have risen by nearly 30%, from 19 million tons in 2010 to 26 million tons in 2017, and is expected to increase further (FAOSTAT Database, 2019). Hence, the handling of low value side streams will also become more important. One large quantity by‐product of oat production is the grain's outer shell, i.e. the hull (or husk), which makes up 25% to 35% of the entire grain (Redaelli & Berardo 2007). Currently, its value is rather low as it is seen as a waste product, which is often burned for energy production. Due to its lignocellulosic composition (i.e. cellulose, hemicellulose and lignin), however, it has great potential as starting material in a biorefinery process, which aims to produce conventional and novel fuels as well as specialty chemicals from biomass. Potential products include biobased films for packaging (Sousa et al. 2016) and composites for construction and building material (Vo & Navard 2016). Compared to traditional petroleum‐based refineries, biorefineries are attractive alternatives as they are based on the conversion of renewable material and emit zero net CO2 into the atmosphere (Amoah et al. 2019). In order to assess the oat hull's suitability to act as a biorefinery starting material, as well as to design the most value‐adding process, an in‐depth understanding of its complex chemical composition is crucial. All characterizations performed so far have only assessed the quantity of its main components and have not further analysed its lignocellulose composition (Welch et al. 1983; Crosbie et al. 1985; Thompson et al. 2000).
Particularly large quantities of oats (up to 2.6 million tons per year) are grown in the Nordic countries due to the ability of oats to thrive in the short seasons of cool and wet climates with long periods of daylight (Buerstmayr et al. 2007; FAOSTAT Database, 2019). Previous studies have shown that oat growth is greatly influenced by changes in climate conditions. Elevated temperatures (especially during the early growth season) as well as reduced precipitation significantly impact agricultural yield of many oat varieties at diverse growth locations (Buerstmayr et al. 2007; Peltonen‐Sainio et al. 2011; Klink et al. 2014). Even more recently bred oat cultivars, which were expected to be better adapted to current weather conditions, have suffered from significant yield losses in warmer and drier years (Klink et al. 2014). The oat hull chemical content has been shown to remain the same at elevated temperatures, indicating that it is similarly affected as the grain (Peltonen‐Sainio et al. 2011). As oat growth is so sensitive to altered weather conditions, it is likely that not only macroscopic factors such as grain yield, plant height and lodging severity are influenced, but also its chemical composition. This must be taken into consideration when constructing a biorefinery process to produce speciality chemicals from oat hulls. Adaptive process engineering and construction flexibility will become increasingly more important to allow successful production during years with different weather profiles.
The objective of this study was to analyse the chemical composition of oat hulls and assess their suitability as starting material in a biorefinery. Furthermore, changes in the composition due to extreme weather conditions at comparable locations were analysed and evaluated in the context of the productivity of a new oat hull‐based biorefinery. As years with altered weather conditions are expected to occur more frequently in the future, this information is essential to consider during biorefinery process design.
Material and Methods
Raw material and chemicals
Four different oat hull batches were supplied by Lantmännen ek. för (Stockholm, Sweden). The hulls in the three batches from Sweden (one batch per investigated year) were pooled from several fields in the region of Mälardalen in central Sweden. A minority of hulls also came from the regions Östergötland and Västergötland. One additional batch, grown in Denmark in 2018, was also investigated. More detailed information about batch characteristics are summarized in Table 1. All hulls were separated from the grains utilizing a Bühler BSSA stratopact HKE50HP‐Ex peeler (Höflinger Millingsystems, Neustadt an der Weinstraße, Germany).
Table 1.
Description of oat hull batches used in this study. Weather data were retrieved from the archives of the Swedish Meteorological and Hydrological Institute (SMHI Database, 2019) and the Danish Meteorological Institute (DMI Database, 2019). Summer is defined as the months of June, July and August.
| Batch |
Oat variety (Seed origin) |
Harvest year | Growth location | Average temperature summer [ºC] | Average precipitation summer [mm] |
|---|---|---|---|---|---|
| Swe16 |
Kerstin and Galant (SW‐Seed, Sweden) |
2016 | Sweden | 15.0–16.5 | 49–130 |
| Swe17 |
Kerstin and Galant (SW‐Seed, Sweden) |
2017 | Sweden | 13.5–15.0 | 49–98 |
| Swe18 |
Kerstin and Galant (SW‐Seed, Sweden) |
2018 | Sweden | 16.5–18.5 | 33–65 |
| Den18 |
Symphony and Poseidon (Saaten Union, Germany) |
2018 | Denmark | 17.7 | 47.2 |
All chemicals were purchased from Merck (Sweden) unless otherwise specified.
Moisture and ash content analysis
The moisture and ash content were determined by drying at 105 ºC overnight followed by incineration at 575 ºC for 24 h, respectively.
Total starch analysis
Analysis of total starch content was performed using the Total Starch kit from Megazyme. Protocol K‐TSTA 09/14 method a was followed.
For visual confirmation of the presence of starch, whole hulls from batches Swe17 and Swe18 were taken. A drop of undiluted Lugol reagent (Merck) was added to either the interior or exterior side of the hull for 3 min. Imaging was performed using a Nikon OPTIPHOT‐2 microscope equipped with a Plan 20/0.50 DIC 160/0.17 objective and a Nikon Digital Sight DS‐2 Mv camera. The software used for image analysis was NIS‐Elements D 3.1 (Nikon, Tokyo, Japan).
Protein content analysis
The protein content was measured according to the Dumas technique by applying 25 mg of sample to a FlashEA 1112 series N/Protein Analyzer (Thermo Scientific, Waltham, MA, USA) using aspartic acid as the standard. For nitrogen to protein content conversion, a factor of 5.83 was used.
Lipid content analysis
Total lipid content was analysed by lipid extraction in a 2:1 chloroform:methanol (v/v) solution based on the methods of Folch et al. (1957) and Lee et al. (1995). To aid extraction, the samples were homogenized for 60 s using an Ultra Turrax T 25 digital (IKA, Staufen im Breisgau, Germany) blender at 12,000 rpm. The organic phases were separated by mixing with 0.5% sodium chloride solution at a 2:5 ratio, followed by centrifugation for 5 min at 3,893xg. Subsequently, the bottom chloroform layer was isolated and the lipids retrieved and weighed after evaporation of the solvent.
Structural carbohydrates and lignin content analysis
Determination and characterization of lignin, cellulose and hemicellulose content was performed according to the NREL Laboratory Analytical Procedure (NREL/TP‐510‐42618, 2012). Extracted monosaccharides and uronic acids were identified and quantified using an HPAEC‐PAD (ICS‐5000, Thermo Scientific, Sunnyvale, CA, USA) equipped with a CarboPac PA20 analytical column (150 mm × 3 mm; 6 µm) as well as a guard column (30 mm × 3 mm), as previously described in Falck et al. (2014). For the separation of arabinose and rhamnose, the mobile phase concentration was increased to 10 mM sodium hydroxide. Uronic acids were analysed according to the same method, with a mobile phase consisting of 90 mM sodium hydroxide and 150 mM sodium acetate.
Phenolic acid content analysis
Phenolic acids were extracted and quantified according to the method described in Sajib et al. (2018). As not all phenolics were identified, the total phenolics content of the different batches is evaluated by comparing the total area beneath all peaks.
Mineral content analysis
For the detection and quantification of minerals, the oat hulls were first dissolved in nitric acid with wet combustion in a microwave system. Subsequently, the samples were analysed via inductively coupled plasma optical emission spectroscopy using an iCap 7400 ICP‐OES (Thermo Scientific). The report limits for the individual minerals in µg g−1 were as follows: Al: 1.0; Ba: 0.2; Ca: 20; Cr: 0.1; Cu: 0.1; Fe: 0.5; K: 10; Mg: 20; Mn: 0.1; Na: 5.0; P: 10; S: 20.
Results and Discussion
Chemical composition
The chemical composition of the four different oat hull batches grown in similar locations, but under different weather conditions (see Table 1), was analysed. Batch Swe17 was chosen as model batch to describe the general composition of oat hulls as it contains a mixture of two common oat varieties grown in Sweden (i.e. Kerstin and Galant) under ‘normal’ weather conditions. The quantity of its main components is summarized in Table 2. The composition analysis shows that oat hulls are a highly lignocellulosic material, comprising 83.9% of the hull dry weight, which is about 8% more than commonly found in oat straw; this places hulls among the most lignocellulose rich agricultural wastes, comparable to wheat straw (Isikgor & Becer 2015). The largest fraction of the hull lignocellulose is hemicellulose (35.1%), followed by lignin with 25.4%. The majority of the lignin is acid insoluble, corresponding to 91.7% of the lignin fraction. The cellulose content at 23.4% is slightly lower than the lignin content. This composition is unique to oat hulls and differentiates them from many other agricultural waste products, as these are typically richest in cellulose and poorest in lignin (Isikgor & Becer 2015). After lignocellulose, the most abundant component is ash, i.e. inorganic materials content, with 5.2%. Minor components include starch (2.5%), proteins (1.4%) and lipids (0.9%). The low lipid content, which is in line with previous findings (Bryngelsson et al. 2002), most likely describes the waxy coating on the outer surface of the hull and does not penetrate into the deeper layers.
Table 2.
Chemical composition of four oat hull batches grown under different weather conditions (see Table 1 for description). All numbers represent triplicate percentages based on dry weight.
| Component | Swe16 | Swe17 | Swe18 | Den18 |
|---|---|---|---|---|
| Lignin | 25.4 ± 1.7 | 25.4 ± 2.3 | 19.7 ± 0.2 | 12.9 ± 0.7 |
| Acid insoluble | 23.1 ± 1.7 | 23.3 ± 2.3 | 17.2 ± 0.2 | 10.5 ± 0.5 |
| Acid soluble | 2.3 ± 0.2 | 2.1 ± 0.1 | 2.5 ± 0.2 | 2.4 ± 0.2 |
| Cellulose | 17.2 ± 1.6 | 23.4 ± 2.6 | 16.0 ± 0.5 | 25.7 ± 0.2 |
| Hemicellulose | 33.1 ± 0.9 | 35.1 ± 0.1 | 27.9 ± 0.3 | 24.0 ± 0.1 |
| Starch | 8.5 ± 0.4 | 2.5 ± 0.6 | 16.3 ± 1.9 | 12.9 ± 0.4 |
| Ash | 6.0 ± 0.3 | 5.2 ± 0.1 | 5.7 ± 0.1 | 6.3 ± 0.1 |
| Protein | 1.7 ± 0.4 | 1.4 ± 0.2 | 5.4 ± 0.5 | 7.4 ± 0.4 |
| Lipid | 0.5 ± 0.2 | 0.9 ± 0.4 | 1.5 ± 0.1 | 1.4 ± 0.3 |
When comparing the composition of all batches analysed in this study, large variability becomes evident (see Table 2). This also holds true for the two batches grown under ‘normal’ weather conditions, i.e. Swe16 and Swe17. A lower cellulose composition was found in batch Swe16, although the starch content was increased. This shows that the chemical composition of oat hulls is influenced by more than just climate changes. However, the remaining components remain very similar among batches. Hence, the general composition of batch Swe17 described above will be used as representative of a ‘normal’ growth year for further comparison with the batches grown in the climatically irregular year 2018. These latter two batches showed very large differences compared to Swe16 and Swe17. The largest variability was found in the starch composition, which substantially increased in both 2018 batches by factors of 6.6 (Swe18) and 5.2 (Den18) in comparison with Swe17. The starch content measured in this study is likely not part of the hull itself but represents a minor fraction of the grain that remains attached to the hull during the dehulling process (observation at the industrial dehulling site; CG Pettersson, Lantmännen ek. för., personal communication). The hulls were removed industrially with a Bühler peeler, which is not as thorough as peeling by hand. Therefore, the presence of minor contaminants from the grain in the hull fraction is likely. To confirm this hypothesis, un‐milled hulls from batches Swe17 and Swe18 were stained on either the interior or exterior side of the hull with Lugol reagent, which selectively colours starch. The results clearly show that starch is only present on the interior side of the hull (see Fig. 1). The starch content results for batches Swe18 and Den18 indicate that the hull is more tightly bound to the grain when grown in warmer seasons with lower precipitation. This development would greatly impact a biorefinery process. Generally, it is challenging to process starch‐containing materials due to the starch gelling nature. Processes that do not account for this might become clogged and unable to process the material; therefore, starch might have to be degraded first when it exceeds a certain process‐dependent limit.
Fig. 1.
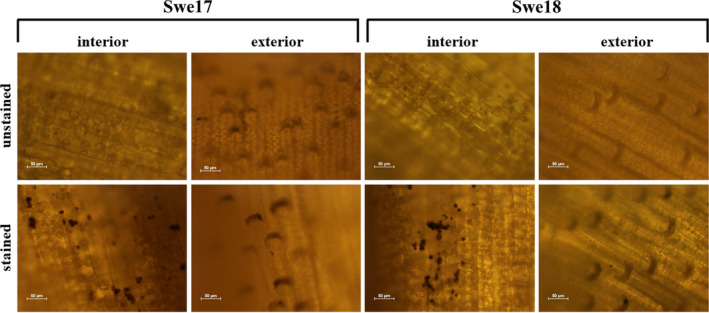
Microscope images depicting the Swe17 and Swe18 oat hull surfaces from the interior and exterior sides with and without staining (Lugol reagent). The stained starch is visible as dark spots.
The lignocellulosic content of the hulls grown in 2018 decreased to 63.6% and 62.6%, respectively, of values in 2017. This represents a loss of approximately 25%, which would also have significant implications for a biorefinery process. Mostly the hemicellulose and lignin fractions of the lignocellulose were reduced. However, in the lignin fraction it is only the acid‐insoluble part that showed a significant reduction. The acid‐soluble part remains the same. The cellulose content differed greatly among all batches, so that no trend related to weather variations could be determined. On the other hand, both protein and lipid content are increased for the 2018 growth season. This increase might also be partially caused by a larger grain content in the hull fraction, as described above for starch. However, an increase in both components when the crops are grown in warmer temperatures has also been observed for soybean seeds (Wolf et al. 1982) and oat grains (only protein measured; Peltonen‐Sainio et al. 2011), suggesting an additional higher production of proteins and lipids by the plant under warmer conditions. Immature hulls are known to contain more protein than mature hulls (Pomeranz et al., 1976). This is an indication that the extreme weather conditions inhibited ripening. Regarding lipid content, an increase in the thickness of the waxy layer on the surface of the hull is most likely the result of a protective mechanism in the plant to prevent evaporation of water from the grain in warmer weather. The only constant content between years was for ash, which is unlikely to be influenced by differences in weather.
Hemicellulose composition
Xylose, at 28.9%, was the most abundant monosaccharide in the hemicellulose of oat hulls, followed by arabinose (3.5%) (see batch Swe17 in Table 3). This indicates that arabinoxylan is the most abundant biopolymer, with an arabinose to xylose (A/X) ratio of 0.1. Compared to the oat grain, in which A/X ratios are 0.5 to 1.0, depending on the extraction method have been measured, this is a rather low ratio indicating the presence of few chemical branches (Virkki et al. 2005). Further minor monosaccharides present are galactose (1.4%), mannose (0.1%) and rhamnose, whose concentration was too low to be quantified. Additionally, minor galacturonic (0.5%) and glucuronic (0.8%) acid substitutions were found. The composition of batch Swe16 was very similar, the only difference being the absence of mannose and rhamnose, which indicates that the hemicellulose composition is rather stable in ‘normal’ years.
Table 3.
Hemicellulose composition of four oat hull batches grown under different weather conditions (see Table 1 for description). ND, not detected; NQ, not quantifiable; A/X ratio, arabinose to xylose ratio. All numbers represent triplicate percentages based on dry weight, except for the A/X ratio, which is not a percentage.
| Component | Swe16 | Swe17 | Swe18 | Den18 |
|---|---|---|---|---|
| Xylose | 27.4 ± 0.9 | 28.9 ± 0.3 | 21.9 ± 0.2 | 17.8 ± 0.1 |
| Arabinose | 3.3 ± 0.0 | 3.5 ± 0.0 | 3.2 ± 0.6 | 3.7 ± 0.0 |
| Galactose | 1.2 ± 0.0 | 1.4 ± 0.2 | 1.3 ± 0.1 | 1.4 ± 0.0 |
| Rhamnose | ND | NQ | NQ | ND |
| Mannose | ND | 0.1 ± 0.1 | 0.2 ± 0.0 | ND |
| Galacturonic acid | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.0 |
| Glucuronic acid | 0.8 ± 0.0 | 0.8 ± 0.1 | 0.7 ± 0.0 | 0.5 ± 0.0 |
| A/X ratio | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 |
However, there were variations in the monosaccharide composition of hemicellulose in the hotter and drier years. These mainly influence the xylose content, which is greatly decreased (24% for Swe18 and 38% for Den18). The arabinose, galactose and uronic acid content remained similar, leading to a doubling of the A/X ratio for Den18 (0.2 compared to 0.1). The content of the minor monosaccharides rhamnose and mannose differed between Swe18 and Den18, indicating that also growth location influences chemical composition. While neither of the monosaccharides could be detected in Den18, both increased in Swe18 (NQ rhamnose and 0.2% mannose). Overall, this analysis shows that not only the hemicellulose amount, but also its composition is altered during differences in weather conditions. This becomes important when a biorefinery aims to produce speciality chemicals based on xylose, or when xylose fermenting microorganisms are used for the production of biofuels.
Phenolic composition
Oat hulls have previously been of interest in the food industry to help prevent lipid oxidation due to their high antioxidant activity originating from their phenolic content (Xing & White, 1997). Several studies have reported the presence of a variety of different phenolic compounds (Xing & White 1997; Emmons & Peterson 1999; Bryngelsson et al. 2002; Varga et al. 2018), which were confirmed in this study. However, the detected quantities are only comparable to the study by Varga et al. (2018). Among previous studies, the largest total phenolic amount reported was 560 µg g‐1 (Xing & White 1997), which is more than 16 times less than the amount found in this study for the oat hull batches grown in a ‘normal’ weather year (Swe16 and Swe17; see Table 4). These large variations are not caused by differences in oat varieties and growth locations, but rather a difference in the effectiveness of the extraction method. While the previous studies which reported lower content of phenolics only extracted the soluble compounds, Varga et al. (2018), as well as this study, utilized a method suitable for the extraction and analysis of bound phenolic compounds. The most abundant phenolic acid found in this study was a mixture of p‐coumaric acid and vanillin (6920 and 6557 µg g−1, respectively), which were not separable in the analysis method established by Sajib et al. (2018). The remaining identified phenolic acids, found in decreasing abundance, were ferulic acid (2596 and 2319 µg g−1) and p‐hydroxybenzaldehyde (215 and 200 µg g−1). Ferulic acid is known to form cross‐links between arabinoxylan chains as well as binding hemicellulose to lignin via ester bonds (Virkki et al. 2005; Amoah et al. 2019). As oat hulls are very rich in arabinoxylan, hemicellulose and lignin (see Tables 2 and 3), the high ferulic acid content is acceptable.
Table 4.
Total and identified phenolic composition of four oat hull batches grown under different weather conditions (see Table 1 for description). All identified phenolic compounds represent triplicate measurements based on dry weight in µg g‐1. The total phenolics composition is given as percentage of batch Swe16.
| Phenolic compound | Swe16 | Swe17 | Swe18 | Den18 |
|---|---|---|---|---|
| p‐hydroxybenzaldehyde | 214.5 ± 4.3 | 200.3 ± 11.1 | 68.7 ± 1.8 | 60.9 ± 3.3 |
| p‐coumaric acid/vanillin | 6919.6 ± 224.8 | 6557.2 ± 304.8 | 2521.3 ± 86.4 | 3008.6 ± 66.8 |
| Ferulic acid | 2596.2 ± 40.4 | 2318.5 ± 111.3 | 1339.2 ± 41.0 | 1435.6 ± 43.4 |
| Total | 100 | 99.8 | 43.1 | 37.2 |
A large decrease in all phenolics was found in the two batches grown in the hotter and drier year, accumulating to a total decrease of about 57% and 63% for batches Swe18 and Den18, respectively, compared to both Swe16 and Swe17. This finding supports previous results of Menga et al. (2010), who found that environmental factors affect the production of phenolic compounds more than genetic differences in oat variety. Many plants up‐regulate phenolics expression under stress, including high temperatures and drought, to increase the plant's defence capacity (Akula & Ravishankar, 2011). However, this trend could not yet be confirmed for oats. Peterson et al. (2005) found no significant differences in the avenanthramides (phenolic alkaloids unique to oats) content of oats grown in the same location under both dry and irrigated conditions. Instead, differences in phenolics content depending on the maturity stage have been found, with higher content in immature seeds (Alfieri & Redaelli, 2015). This is in contrast to the observation found for the protein content and questions whether maturation is inhibited in warmer weather.
The drastic decrease in phenolic content would not only have implications for biorefinery use, aiming to utilize the antioxidant activity of oat hulls, but could affect any process utilizing oat hulls, as the cinnamic acids among the phenolic acids, i.e. p‐coumaric and ferulic acid, have been shown to be important in antifungal and antimicrobial protection mechanisms of the plant rather than in plant antioxidant activity (Bryngelsson et al. 2002). Therefore, more microbially infected hulls might be harvested in warmer and drier years, which could influence the quality of any product produced from the hulls.
Mineral composition
A mineral composition analysis revealed that with 3100 µg g−1, K is by far the most abundant mineral (see Table 5), followed by Ca (840 µg g−1), Mg (480 µg g−1), P (310 µg g−1) and S (250 µg g−1). All remaining minerals were found in minor concentrations of 31 µg g−1 and below in the Swe17 batch, representing a ‘normal’ growth year. The mineral composition of the second batch grown under ‘normal’ weather conditions (Swe16) is very similar. With only higher amounts of Na, P and S, which corresponds to the slightly higher ash content displayed in Table 2.
Table 5.
Mineral composition of four oat hull batches grown under different weather conditions (see Table 1 for description). All numbers represent duplicate measurements of one hydrolysed sample based on dry weight in μg g−1.
| Minerals | Swe16 | Swe17 | Swe18 | Den18 |
|---|---|---|---|---|
| Aluminium | 1.4 | 2.9 | 13 | 10 |
| Barium | 1.3 | 1.7 | 2.7 | 4 |
| Calcium | 820 | 840 | 1400 | 880 |
| Chromium | <0.1 | <0.1 | 0.1 | 0.5 |
| Copper | 1.3 | 1.4 | 3.3 | 4.7 |
| Iron | 11 | 16 | 76 | 130 |
| Potassium | 2700 | 3100 | 5000 | 6400 |
| Magnesium | 560 | 480 | 950 | 1500 |
| Manganese | 27 | 31 | 52 | 49 |
| Sodium | 89 | 15 | 59 | 14 |
| Phosphorus | 550 | 310 | 2000 | 4400 |
| Sulphur | 380 | 250 | 810 | 1300 |
In contrast, there were large differences in the two batches grown in the climatically more stressful year of 2018. In general, all minerals found had higher concentrations, this trend being more pronounced for batch Den18, and supported by the higher measured ash content (see Table 2). The largest increases were seen for the minerals Fe, K, Mg, P and S. The higher Fe content might be explained by the harsher milling process that was applied on both Swe18 and Den18 and could therefore be contamination introduced by the milling. The largest difference between Swe18 and Den18 is the Ca content, which increased significantly for Swe18, while remaining at approximately the same level as the ‘normal’ year batches for Den18.
Suitability of oat hulls for biorefineries
Overall, oat hulls are an attractive starting material for lignocellulose‐utilizing biorefineries. However, its lignocellulose composition (determined for Kerstin and Galant varieties grown in Sweden during the ‘normal’ weather year 2017) differed from other lignocellulosic agricultural by‐products, containing an unexpectedly high amount of hemicellulose (35.1%) and lignin (25.4%), but relatively little cellulose (23.4%). The hemicellulose consists exclusively of arabinoxylan with very little galactose, rhamnose, mannose and uronic acid substitutions. Oat hull arabinose to xylose (A/X) ratio at 0.1 is also very low, making it attractive for applications requiring unsubstituted xylan. In addition, a very high content of phenolic compounds was detected, which could be valuable for the food industry due to their antioxidant activity. The remaining components are only present in minor amounts, facilitating its processing. However, this chemical composition is greatly influenced by different weather conditions during the oat growth phase. A loss of about 25% of lignocellulose content was observed for the two batches grown in an average 2 ºC to 5 ºC warmer summer with up to 75% less precipitation compared to a ‘normal’ year. This loss is mostly due to a reduction in the hemicellulose and (acid insoluble) lignin fractions. The cellulose content fluctuates independent of weather trends. Even the hemicellulose monosaccharide composition changed, as characterised by a decrease in the xylose fraction of up to 8%. Furthermore, the starch content increased by a factor of 6.6, which is most likely due to the higher grain content in the hull fraction resulting from difficulties in separating the hull from the grain. Finally, a decrease in the phenolic content of around 60% was noted, which hints at higher susceptibility of the plant to microbial attack. These are very large variations that need to be taken into consideration when designing a new oat hull‐based biorefinery.
Conclusion
Oat hulls have high potential to serve as starting material for a biorefinery due to their extensive lignocellulose content (84%). This lignocellulose is exceptionally rich in hemicellulose (35%), which consists exclusively of arabinoxylan with only a few arabinose substitutions (A/X ratio of 0.1), making it an attractive source for unsubstituted xylan. However, this chemical composition is greatly influenced by weather conditions (25% loss of lignocellulose content in the warmer and drier year). This needs to be considered when designing a new oat hull‐based biorefinery as the trend for warmer and drier seasons in the Nordic regions is increasing.
Acknowledgements
We would like to thank Katarina Gutke and Magnus Paulsson at Nouryon for performing the mineral content analysis and making helpful suggestions. We are furthermore grateful to CG Pettersson at Lantmännen for providing the raw material and for fruitful discussions. This work was supported by the strategic innovation program BioInnovation funded by Vinnova, Formas and the Swedish Energy Agency.
References
- Akula R., Ravishankar G.A. (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signalling & Behavior, 6, 1720–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri M., Redaelli R. (2015) Oat phenolic content and total antioxidant capacity during grain development. Journal of Cereal Science, 65, 39–42. [Google Scholar]
- Amoah J., Kahar P., Ogino C., Kondo A. (2019) Bioenergy and biorefinery: feedstock, biotechnological conversion, and products. Biotechnology Journal, 14, 1800494. [DOI] [PubMed] [Google Scholar]
- Bryngelsson S., Mannerstedt‐Fogelfors B., Kamal‐Eldin A., Andersson R., Dimberg L.H. (2002) Lipids and antioxidants in groats and hulls of Swedish oats (Avena sativa L). Journal of the Science of Food and Agriculture, 82, 606–614. [Google Scholar]
- Buerstmayr H., Krenn N., Stephan U., Grausgruber H., Zechner E. (2007) Agronomic performance and quality of oat (Avena sativa L.) genotypes of worldwide origin produced under Central European growing conditions. Field Crops Research, 101, 343–351. [Google Scholar]
- Crosbie G.B., Tarr A.W., Portmann P.A., Rowe J.B. (1985) Variation in hull composition and digestibility among oat genotypes. Crop Science, 25, 678–680. [Google Scholar]
- Danish Meteorological Institute (2019) DMI Database. Copenhagen, Denmark. Retrieved August 6, 2019 from https://www.dmi.dk/vejrarkiv/.
- Emmons C.L., Peterson D.M. (1999) Antioxidant activity and phenolic content of oat groats and hulls. Cereal Chemistry, 76, 902–906. [Google Scholar]
- Falck P., Aronsson A., Grey C., Stålbrand H., Karlsson E.N., Adlercreutz P. (2014) Production of arabinoxylan‐oligosaccharide mixtures of varying composition from rye bran by a combination of process conditions and type of xylanase. Bioresource Technology, 174, 118–125. [DOI] [PubMed] [Google Scholar]
- Folch J., Lees M., Sloane Stanley G.H. (1957) A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry, 226, 497–509. [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations (2019) FAOSTAT Database. Rome, Italy: FAO. Retrieved July 16, 2019 from http://www.fao.org/faostat/en/#data/QC/visualize.
- Isikgor F.H., Becer C.R. (2015) Lignocellulosic biomass: a sustainable platform for the production of bio‐based chemicals and polymers. Polymer Chemistry, 6, 4497–4559. [Google Scholar]
- Klink K., Wiersma J.J., Crawford C.J., Stuthman D.D. (2014) Impacts of temperature and precipitation variability in the Northern Plains of the United States and Canada on the productivity of spring barley and oat. International Journal of Climatology, 34, 2805–2818. [Google Scholar]
- Lee C., Trevino B., Ma C. (1995) A simple and rapid solvent extraction method for determining total lipids in fish tissue. Journal of AOAC International, 79, 487–492. [PubMed] [Google Scholar]
- Menga V., Fares C., Troccoli A., Cattivelli L., Baiano A. (2010) Effects of genotype, location and baking on the phenolic content and some antioxidant properties of cereal species. International Journal of Food Science and Technology, 45, 7–16. [Google Scholar]
- Peltonen‐Sainio P., Jauhiainen L., Hakala K. (2011) Crop responses to temperature and precipitation according to long‐term multi‐location trials at high‐latitude conditions. Journal of Agricultural Science, 149, 49–62. [Google Scholar]
- Peterson D.M., Wesenberg D.M., Burrup D.E., Erickson C.A. (2005) Relationships among agronomic traits and grain composition in oat genotypes grown in different environments. Crop Science, 45, 1249–1255. [Google Scholar]
- Pomeranz Y., Shands H.L., Robbins G.S., Gilbertson J.T. (1976) Protein content and amino acid composition in groats and hulls of developing oats (Avena sativa). Journal of Food Science, 41, 54–56. [Google Scholar]
- Redaelli R., Berardo N. (2007) Prediction of fibre components in oat hulls by near infrared reflectance spectroscopy. Journal of the Science of Food and Agriculture, 87, 580–585. [Google Scholar]
- Sajib M., Falck P., Sardari R.R.R., Mathew S., Grey C., Nordberg Karlsson E., Adlercreutz P. (2018) Valorization of Brewer's spent grain to prebiotic oligosaccharide: Production, xylanase catalysed hydrolysis, in‐vitro evaluation with probiotic strains and in a batch human fecal fermentation model. Journal of Biotechnology, 268, 61–70. [DOI] [PubMed] [Google Scholar]
- Sousa S., Ramos A., Evtuguin D.V., Gamelas J.A.F. (2016) Xylan and xylan derivatives – Their performance in bio‐based films and effect of glycerol addition. Industrial Crops and Products., 94, 682–689. [Google Scholar]
- Swedish Meteorological and Hydrological Institute (2019) SMHI Database. Norrköping, Sweden. Retrieved August 6, 2019 from https://www.smhi.se/data.
- Thompson R.K., Mustafa A.F., McKinnon J.J., Maenz D., Rossnagel B. (2000) Genotypic differences in chemical composition and ruminal degradability of oat hulls. Canadian Journal of Animal Science, 80, 377–379. [Google Scholar]
- Varga M., Jójárt R., Mihály R., Palágy A. (2018) Phenolic compositions and antioxidant activity of coloured oats. Food Chemistry, 268, 153–161. [DOI] [PubMed] [Google Scholar]
- Virkki L., Johansson L., Ylinen M., Maunu S., Ekholm P. (2005) Structural characterization of water‐insoluble nonstarchy polysaccharides of oats and barley. Carbohydrate Polymers, 59, 357–366. [Google Scholar]
- Vo L.T.T., Navard P. (2016) Treatments of plant biomass for cementitious building materials – A review. Construction and Building Materials, 121, 161–176. [Google Scholar]
- Welch R.W., Hayward M.V., Jones D.I.H. (1983) The composition of oat husk and its variation due to genetic and other factors. Journal of the Science of Food and Agriculture, 34, 417–426. [Google Scholar]
- Wolf R.B., Cavins J.F., Kleiman R., Black L.T. (1982) Effect of temperature on soybean seed constituents: oil, protein, moisture, fatty acids, amino acids and sugars. Journal of the American Oil Chemists’ Society, 59, 230–232. [Google Scholar]
- Xing Y., White P.J. (1997) Identification and function of antioxidants from oat groats and hulls. Journal of the American Oil Chemists’ Society, 74, 303–307. [Google Scholar]


