Abstract
Physical exercise is an important component in the management of type 1 diabetes across the lifespan. Yet, acute exercise increases the risk of dysglycaemia, and the direction of glycaemic excursions depends, to some extent, on the intensity and duration of the type of exercise. Understandably, fear of hypoglycaemia is one of the strongest barriers to incorporating exercise into daily life. Risk of hypoglycaemia during and after exercise can be lowered when insulin‐dose adjustments are made and/or additional carbohydrates are consumed. Glycaemic management during exercise has been made easier with continuous glucose monitoring (CGM) and intermittently scanned continuous glucose monitoring (isCGM) systems; however, because of the complexity of CGM and isCGM systems, both individuals with type 1 diabetes and their healthcare professionals may struggle with the interpretation of given information to maximise the technological potential for effective use around exercise (ie, before, during and after). This position statement highlights the recent advancements in CGM and isCGM technology, with a focus on the evidence base for their efficacy to sense glucose around exercise and adaptations in the use of these emerging tools, and updates the guidance for exercise in adults, children and adolescents with type 1 diabetes.
Keywords: Adolescents, Adults, CGM, Children, Continuous glucose monitoring, Exercise, Physical activity, Position statement, Type 1 diabetes
Abbreviations
- ADL
Activities of daily living
- CGM
Continuous glucose monitoring
- IAH
Impaired awareness of hypoglycaemia
- isCGM
Intermittently scanned continuous glucose monitoring
- ISPAD
International Society for Pediatric and Adolescent Diabetes
- MARD
Mean absolute relative difference
- SMBG
Self‐monitored blood glucose
1. INTRODUCTION
Most common continuous glucose monitoring (CGM) systems measure glucose in the interstitial fluid, providing real‐time sensor glucose data, while intermittently scanned CGM (isCGM) systems measure interstitial glucose levels at the time of scanning via a reader device. CGM improves the time spent in euglycaemia and reduces (severe) hypoglycaemia in people with impaired awareness of hypoglycaemia (IAH), decreases HbA1c levels and ameliorates general measures of glycaemic control, as shown in different randomised (crossover) studies [1, 2, 3, 4]. isCGM reduces the time spent in hypoglycaemia, as shown by randomised controlled studies [5, 6], and decreases HbA1c levels, as shown by a meta‐analysis [7] of largely observational studies. Interestingly, switching from isCGM to CGM may have a beneficial impact on hypoglycaemia outcomes in people with a high risk of hypoglycaemia, perhaps because the latter displays data without the need to scan and is augmented by alerts for when the sensor glucose approaches hypoglycaemia, as detailed in a randomised parallel‐group study [8].
Nonetheless, CGM technology is also accompanied by limitations, like skin irritation and pain, as assessed in children and pregnant women [9, 10, 11, 12], sleep disruption due to discomfort associated with the position of the device [13], a constant reminder of having diabetes, overload of information and that the device is visible to others [11, 13, 14]. Furthermore, it is important to note that if hypo‐ and hyperglycaemic symptoms are not in line with the sensor glucose value, a confirmatory self‐monitored blood glucose (SMBG) should be performed.
A lag time between the glucose value in the vasculature and interstitial fluid does exist and, thus, influences sensor glucose measurement accuracy against blood glucose values [15]. Moreover, other physiological changes during exercise, such as alterations in blood flow rate, body temperature and body acidity, can theoretically have an impact on interstitial glucose‐sensing accuracy [15], though the degree of impact of these factors is unknown on an individual basis. Thus, when assessing the accuracy of different interstitial glucose monitoring systems vs reference glucose, which is usually assessed by a median or mean absolute relative difference (MARD), a part of the difference should be interpreted as lag time rather than inaccuracy, as shown in a secondary outcome analysis of an experimental study [16]. Additionally, other factors, like local metabolic rate, sensor site [17], exercise type [18, 19], vasoconstriction [20], potential interference with medications [21], the direction of glucose rate of change [22, 23] and baseline glucose level may influence lag time [15]. Also, the glucose concentration, per se, might have an impact on the sensor accuracy, as seen for isCGM, detailing a lower MARD for hyperglycaemia and higher MARD for hypoglycaemia, as shown by two experimental studies [24, 25]. At rest, a lag time of ~5 min is seen in healthy individuals [26], while, in situations of rapid glucose changes, it can increase to up to 12‐24 min or even longer during exercise, as seen in people with type 1 diabetes [22, 23, 27]. Depending on the CGM and isCGM device, the overall mean of all MARDs during different types of exercise in people with type 1 diabetes is ~13.63% (95% CI 11.41%, 15.84%), as detailed in Figure 1 [16, 17, 18, 22, 24, 25, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37]. However, more recent CGM and isCGM devices, not yet investigated for exercise, might have a lower MARD and enhanced performance.
FIGURE 1.
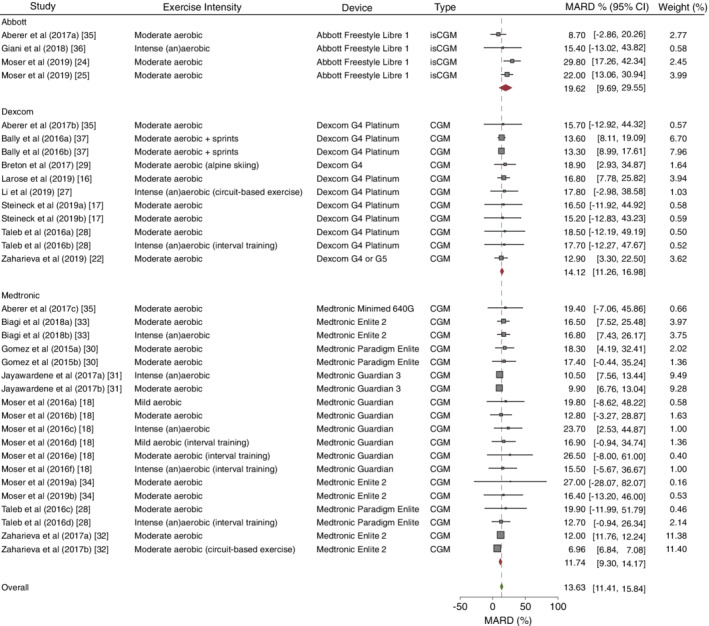
MARD (%) of current CGM and isCGM devices during exercise. MARD data are weighted for the number of participants and SD of MARD for different manufacturers of all CGM and isCGM devices. The dashed line and the green diamond represent the MARD of all CGM and isCGM devices. Red diamonds represent the MARD for each specific company. Horizontal bars represent the 95% CIs for the specific studies. All types of studies using CGM and/or isCGM during exercise in people with type 1 diabetes were included (the studies by Giani et al [36] and Breton et al [29] were performed in children and adolescents). This figure is available as part of a downloadable slideset
2. METHODS USED FOR GROUP CONSENSUS
Due to the growing popularity of CGM and isCGM technologies, this writing group produced modified and novel recommendations based on current evidence, consensus statements and position statements for people with type 1 diabetes around exercise (ie, before, during and after). This writing group consists of exercise physiologists, sports scientists, diabetologists, endocrinologists, paediatric diabetologists, bioengineers and nutritionists. After performing one‐on‐one meetings with all members of the writing group, a first outline was written based on the members' recommendations, including defined work packages for the authors. Subsequently, two lead authors (OM and JKM) produced a manuscript and circulated it within the writing group for further discussions. A consensus meeting was held during the Advanced Technologies & Treatments for Diabetes (ATTD) 2020 conference in Madrid, Spain, and consensus was obtained. A final version of the position statement was then sent to the writing group for additional discussion, comments and final edits. All authors approved the final manuscript.
3. DATA SOURCES, SEARCHES AND STUDY SELECTION
The consensus group accepted the position statement on physical activity/exercise and diabetes, of the American Diabetes Association (ADA) [38], the consensus statement for exercise management in type 1 diabetes [39] and the International Society for Pediatric and Adolescent Diabetes (ISPAD) clinical practice consensus guidelines for exercise in children and adolescents with diabetes [40] as a starting point. Additionally, a systematic literature search was conducted by three independent researchers, using PubMed, EMBASE and the Cochrane Library for publications, on CGM and isCGM systems around exercise in people with type 1 diabetes between November and December 2019. Details on the keywords and the search strategy are available in electronic supplementary material (ESM) Tables 1, 2, 3. Original data investigating the performance of CGM and isCGM systems during exercise were used to produce a forest plot detailing the overall MARD between sensor glucose and reference glucose. Systematic reviews and meta‐analyses were included as additional information for the use of CGM and isCGM around exercise. In addition, reference lists from each relevant publication were screened to identify additional articles pertinent to the topic. Papers were grouped per theme and the authors reviewed the evidence. Nevertheless, though evidence‐based, the recommendations presented within this position statement are the opinions of the authors.
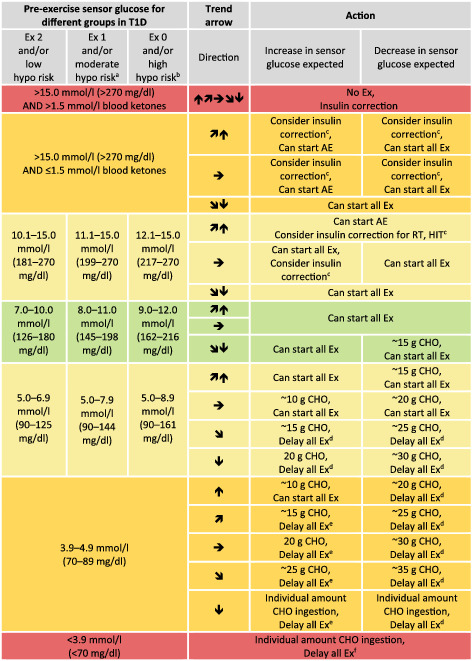
TABLE 1.
Sensor glucose targets in advance of exercise in regard to different groups of people with type 1 diabetes
|
Note: Sensor glucose targets are detailed for the following groups in type 1 diabetes (T1D): intensively exercising and/or low risk of hypoglycaemia (Ex 2); moderately exercising and/or moderate risk of hypoglycaemia (Ex 1); minimally exercising and/or high risk of hypoglycaemia (Ex 0).
When reaching the required sensor glucose level to start exercise, only consume carbohydrates again when trend arrow is starting to decrease.
These recommendations are not applicable to hybrid closed‐loop systems.
Green shading, no/minimal action required; light‐yellow shading, minimal/moderate action required; dark‐yellow shading, moderate/intense action required; red shading, no/delay exercise.
AE, mild‐to‐moderate intensity aerobic exercise; CHO, carbohydrate; Ex, exercise; HIT, high‐intensity training; hypo, hypoglycaemia; RT, resistance training.
Recommendation for older adults with coexisting chronic illnesses and intact cognitive and functional status.
Recommendation for older adults with coexisting chronic illnesses or two or more instrumental ADL impairments or mild‐to‐moderate cognitive impairment.
50% of regular insulin correction factor when sensor glucose is close to the upper threshold.
Delay exercise until reaching at least 5.0 mmoL/L (90 mg/dL) and  ,
,  , or
, or 
Delay exercise until reaching at least 3.9‐4.9 mmoL/L (70‐89 mg/dL) and 
Delay exercise until reaching a sensor glucose of 3.9‐4.9 mmoL/L (70‐89 mg/dL) with an  arrow if an increase in sensor glucose is expected during exercise, or delay exercise until reaching at least 5.0 mmoL/L (90 mg/dL) and
arrow if an increase in sensor glucose is expected during exercise, or delay exercise until reaching at least 5.0 mmoL/L (90 mg/dL) and  ,
,  , or
, or  if a decrease in sensor glucose during exercise is expected.
if a decrease in sensor glucose during exercise is expected.
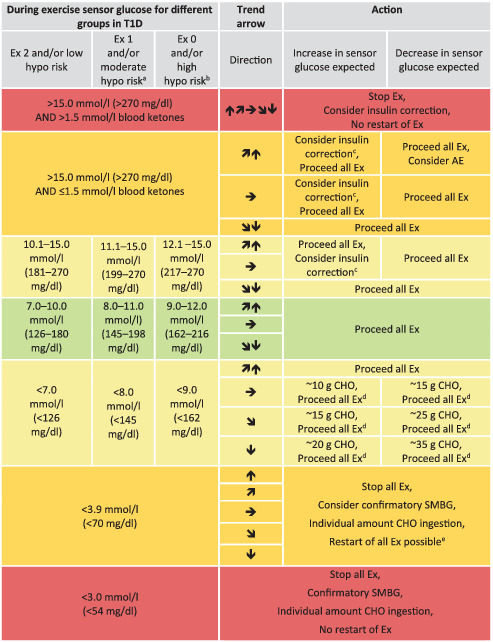
TABLE 2.
Sensor glucose targets during exercise in regard to different groups of people with type 1 diabetes
|
Note: Sensor glucose targets are detailed for the following groups in type 1 diabetes (T1D): intensively exercising and/or low risk of hypoglycaemia (Ex 2); moderately exercising and/or moderate risk of hypoglycaemia (Ex 1); minimally exercising and/or high risk of hypoglycaemia (Ex 0).
When reaching the required sensor glucose level during exercise, only consume carbohydrates again when trend arrow is starting to decrease.
These recommendations are not applicable to hybrid closed‐loop systems.
Green shading, no/minimal action required; light‐yellow shading, minimal/moderate action required; dark‐yellow shading, moderate/intense action required; red shading, stop exercise.
AE, mild‐to‐moderate intensity aerobic exercise; CHO, carbohydrate; Ex, exercise; hypo, hypoglycaemia.
Recommendation for older adults with coexisting chronic illnesses and intact cognitive and functional status.
Recommendation for older adults with coexisting chronic illnesses or two or more instrumental ADL impairments or mild‐to‐moderate cognitive impairment.
50% of the regular insulin correction factor.
Check sensor glucose at least 30 min after carbohydrate consumption and repeat treatment if required.
Restart exercise when reaching sensor glucose levels of at least 4.4 mmoL/L (80 mg/dL) and 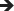 ,
, 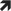 or
or 
TABLE 3.
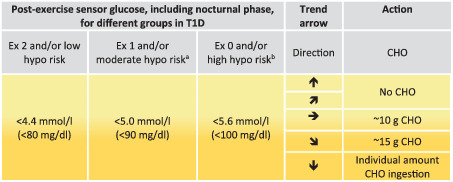
Sensor glucose targets for carbohydrate consumption during the post‐exercise phase, including the nocturnal post‐exercise phase if exercise was performed in the late afternoon/evening
|
Note: Sensor glucose thresholds for treatments are detailed for the following groups in type 1 diabetes(T1D): intensively exercising and/or low risk of hypoglycaemia (Ex 2); moderately exercising and/or moderate risk of hypoglycaemia (Ex 1); minimally exercising and/or high risk of hypoglycaemia (Ex 0).
If an insulin correction is applied due to high sensor glucose levels, then the regular correction factor might be reduced by up to 50%.
Check sensor glucose at least 30 min after carbohydrate consumption and repeat treatment if required.
These recommendations are not applicable to hybrid closed‐loop systems.
The intensity of yellow shading indicates the level of action required: lighter yellow shading indicates that minimal/moderate action is required, while darker yellow shading indicates that moderate/intense action is required.
CHO, carbohydrate; Ex, exercise; hypo, hypoglycaemia.
Recommendation for older adults with coexisting chronic illnesses and intact cognitive and functional status.
Recommendation for older adults with coexisting chronic illnesses or two or more instrumental ADL impairments or mild‐to‐moderate cognitive impairment.
Level of evidence was set according to the Memorandum of Understanding (MOU) between the European Association for the Study of Diabetes (EASD) and the writing group of this position statement. Levels of evidence are, thus, categorised as: (Ia) evidence from meta‐analysis of randomised controlled trials; (Ib) evidence from at least one randomised controlled trial; (IIa) evidence from at least one controlled study without randomisation; (IIb) evidence from at least one other type of quasi‐experimental study; (III) evidence from non‐experimental descriptive studies, such as comparative studies, correlation studies and case‐control studies; or (IV) evidence from expert committee reports or opinions or clinical experience of respected authorities, or both. If recommendations are given within this position statement, the strength of those are expressed as: (A) directly based on category I evidence; (B) directly based on category II evidence or extrapolated recommendation from category I evidence; (C) directly based on category III evidence or extrapolated recommendation from category I or II evidence; (D) directly based on category IV evidence or extrapolated recommendation from category I, II or III.
4. THERAPY DECISIONS FROM SENSOR GLUCOSE VALUES AND TREND ARROWS FOR EXERCISE
Different groups of people with type 1 diabetes may require different glycaemic ranges in preparation for, during and after performing exercise when using CGM and isCGM systems, based on different position and consensus statements [40, 41, 42, 43]. As discussed previously, CGM and isCGM devices may provide a different glucose reading compared with the actual SMBG, especially during exercise, and, thus, the sensor glucose value should be interpreted together with the corresponding trend arrow. Safe glycaemic ranges and specific carbohydrate consumption are recommended below, based on previous position and consensus statements [38](D), [39](D), [40](D), and modified, based on experimental studies [44](D), [45](D) as well as on the experience of this writing group. Groups within the population of type 1 diabetes, based on different characteristics in this position statement, are defined as children and adolescents (6 to <18 years) and adults (≥18 years) with type 1 diabetes. Additionally, further groups were defined based on the risk of hypoglycaemia, exercise experience and health status (older adults aged ≥65 years). Since structured exercise training is less common in pre‐schoolers, as observed in healthy individuals [46], the writing group is unable to provide specific recommendations due to the general lack of scientific evidence for this age group. Sustained hyperglycaemia (hours) and/or frequently occurring hypoglycaemia during exercise should be discussed between the individual and his/her healthcare professional team to develop individualised strategies that improve glycaemic control and support ongoing regular participation in exercise. The recommendations below serve as starting points and should be individualised.
Different glucose responses are evident depending on the type of exercise, as seen in different experimental studies [47, 48, 49, 50]; mild‐to‐moderate aerobic exercise decreases glucose levels [51, 52, 53], whilst intense aerobic and anaerobic exercise and exercises with a load‐profile similar to interval exercise stabilise [54] or increase glucose levels, as seen in various experimental studies [55, 56, 57]. Independent of the aforementioned groups (adults and children/adolescents), individuals who do not routinely exercise may face an increased risk of hypoglycaemia, as partially shown in a prospective observational study [58](C). Additionally, IAH [59](C), preceding episodes of hypoglycaemia [60](B) and advanced age [58](C) potentially increase the risk of hypoglycaemia during and after exercise. Therefore, the writing group recommends that the following groups can be categorised:
Currently minimally exercising and/or high risk of hypoglycaemia
Currently moderately exercising and/or moderate risk of hypoglycaemia
Currently intensively exercising and/or low risk of hypoglycaemia
Assessment of exercise routine and risk of hypoglycaemia is recommended to be performed by a decision tree, modified based on a large‐scale observational study [58](C), and a recent consensus statement [41](D) (Figure 2).
FIGURE 2.
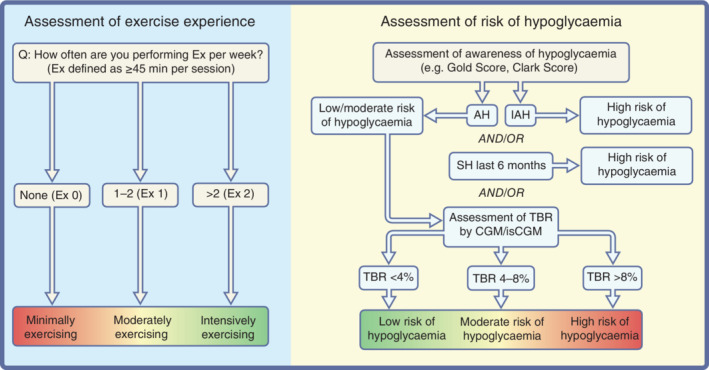
Assessment of exercise experience and risk of hypoglycaemia. Exercise (Ex) represents how often people with type 1 diabetes are exercising with a duration ≥45 min per session per week. Assessment of risk of hypoglycaemia should be based on scoring systems for being aware of hypoglycaemia (AH) or showing IAH. In addition, if the scoring system reveals AH, the time below range (TBR; <3.9 mmoL/L, <70 mg/dL) over the last 3 months should be evaluated to detail the degree of awareness. Furthermore, if an episode of severe hypoglycaemia (SH) occurred within the last 6 months, then there might be a high risk of hypoglycaemia during exercise. This figure is available as part of a downloadable slideset
Older and/or frail adults with type 1 diabetes (aged ≥65 years) may have a different health status that is reflected by different glycaemic goals based on HbA1c and time in range (TIR), as discussed by recent consensus and position statements [41, 43]. In general, for this group, exercise is recommended to be performed 2‐3 times per week as discussed by a position statement [38](D). However, older people with type 1 diabetes are at an increased risk of severe hypoglycaemia around physical activity and exercise [58](C). Considering this, higher glycaemic targets can be recommended for exercise based on patient characteristics and health status, to lower the risk of hypoglycaemia. Therefore, exercise can also be recommended for older adults with few coexisting chronic illnesses and intact cognitive and functional status, and for older adults with coexisting chronic illnesses or two or more instrumental activities‐of‐daily‐living (ADL) impairments or mild‐to‐moderate cognitive impairment [61](D). For older adults with very complex and poor health status, the possibility to perform exercise should be evaluated on a case‐by‐case basis [61](D).
Recommendations for exercise based on habitual physical activity, risk of hypoglycaemia and health status (older and/or frail people with type 1 diabetes) should follow a more conservative strategy, which enables the avoidance of exercise‐induced glycaemic excursions. If a person is exercising more routinely and/or has a lower risk of hypoglycaemia over a period of 3 months, then the glycaemic upper and lower thresholds for carbohydrate consumption might be adjusted. Furthermore, in the following sections, generic trend arrows are given independently of the device used.
5. ADULTS WITH TYPE 1 DIABETES
CGM and isCGM systems can be used as effective tools to help indicate when carbohydrate intake should be initiated to prevent or treat hypoglycaemia during prolonged exercise, as seen in experimental studies in children and adolescents with type 1 diabetes [44, 45]. However, the significant lag time in these technologies should be taken into consideration when using them to determine when to consume carbohydrates [27]. Furthermore, isCGM requires frequent scanning since no automatic alerts are available for the first‐generation device, as discussed in a narrative review [62]. For adults with type 1 diabetes, the following recommendations are given regarding the glycaemic thresholds below which carbohydrates might be consumed [38](D), [39](D), [40](D), [44](D), [45](D):
<7.0 mmoL/L (<126 mg/dL) for adults with type 1 diabetes, intensively exercising and/or with low risk of hypoglycaemia
<8.0 mmoL/L (<145 mg/dL) for adults with type 1 diabetes, moderately exercising and/or with moderate risk of hypoglycaemia, or for older adults with coexisting chronic illnesses and intact cognitive and functional status
<9.0 mmoL/L (<161 mg/dL) for adults with type 1 diabetes, minimally exercising and/or with high risk of hypoglycaemia, or for older adults with coexisting chronic illnesses or two or more instrumental ADL impairments or mild‐to‐moderate cognitive impairment.
To lower the risk of hypoglycaemia during prolonged exercise, exercise should be initiated when mealtime insulin levels are low or about 90 min after the last carbohydrate‐rich meal with a reduction in mealtime insulin [38](D), [39](D). However, to achieve beneficial effects of exercise on overall glycaemic control, exaggerated bolus insulin dose reductions should be avoided, as discussed in a meta‐analysis [63](D).
5.1. Preparation in advance to exercise
If a CGM or a second‐generation isCGM device is used, hypoglycaemic alerts might be set at the highest possible alarm lower threshold at the onset of exercise, which is currently 5.6 mmoL/L (100 mg/dL) [22](D). This elevated hypoglycaemia alert setting is in line with the expected delay between interstitial glucose and blood glucose when levels are dropping during prolonged exercise [22]. Importantly, for the second‐generation isCGM device, an alert is only shown once and not repeated. Hyperglycaemic alerts can be set to 10.0 mmoL/L (180 mg/dL) or higher, to avoid alarm fatigue [64](D). The rate‐of‐glucose‐change alerts (dropping or rising) can be used to initiate an earlier action, such as a decrease or increase in basal insulin rate for those on continuous subcutaneous insulin infusion (CSII), or a change to glucose‐rich or glucose‐free fluids, depending on the direction of change. Thirty to 60 min prior to the start of prolonged aerobic exercise (>30 min), to reduce hypoglycaemia risk, low glycaemic index carbohydrates can be consumed in those who do not reduce insulin dose, aiming to achieve pre‐exercise sensor glucose targets. A detailed description of pre‐exercise sensor glucose levels, trend arrows and consumption of carbohydrates is shown in Table 1 based on position and consensus statements and experimental studies [38](D), [39](D), [40](D), [44](D), [45](D).
5.2. During exercise
Independent of the type of exercise, the target sensor glucose ranges should be between 5.0 mmoL/L and 10.0 mmoL/L (90 mg/dL and180 mg/dL) and, ideally, between 7.0 mmoL/L and 10.0 mmoL/L (126 mg/dL and 180 mg/dL) for prolonged aerobic exercise for the majority of adults with type 1 diabetes, and slightly higher for those with an increased risk of hypoglycaemia (Table 2) [38](D), [39](D). If sensor glucose is expected to increase, as often seen in people performing fasted high‐intensity interval training [55, 56], resistance training [49, 65, 66] and, also, in training above the anaerobic threshold [67], then an insulin correction can be administered at the onset of, as well as during exercise (50% of typical correction factor) [68](D), [69](D).
For safety reasons, the exercise should be suspended, at least temporarily, if the sensor glucose level reaches <3.9 mmoL/L (<70 mg/dL) and oral carbohydrates should be consumed [70](D). Furthermore, at this lower threshold, a confirmatory SMBG might be performed to confirm the sensor glucose level. After reaching a sensor glucose level close to 4.4 mmoL/L (80 mg/dL), accompanied by a horizontal/upward trend arrow, exercise can be restarted. Many CGM systems provide a hypoglycaemia prediction alert. If the sensor glucose level is predicted to reach <3.0 mmoL/L (<54 mg/dL), then fast‐acting oral carbohydrates should be consumed immediately. If sensor glucose drops below 3.0 mmoL/L (54 mg/dL) then exercise should not be restarted. During exercise, fast‐acting liquid carbohydrates should be consumed consisting primarily of glucose (dextrose) or a mixture of glucose/fructose, as discussed in narrative reviews [71](D), [72](D). A read or scan from the CGM or isCGM system might be performed every 15‐30 min, if feasible, during exercise to ensure early and appropriate treatment against hypo‐ or hyperglycaemia, and to assess the appropriateness of strategies to prevent dysglycaemia. With CGM, alerts could also be activated regarding the rate of change in glucose, providing the user with valuable information. For the majority of adults with type 1 diabetes who are at a low risk of hypoglycaemia, at a glycaemic threshold of 7.0 mmoL/L (126 mg/dL) accompanied by a horizontal trend arrow, 10‐15 g of carbohydrates should be consumed; 15‐25 g carbohydrates should be consumed immediately if accompanied by a (slight) downward trend arrow; 20‐35 g of carbohydrates should be consumed if accompanied with a downward trending arrow (Table 2) [38](D), [39](D), [40](D), [44](D), [45](D). Carbohydrates should be consumed regularly (eg, every 15 to 20 min) when reaching the lower glycaemic threshold, and carbohydrate supplementation should be repeated if sensor glucose is not rising according to the trend arrows within 30 min. These carbohydrate recommendations should be used as a guidance and personalised based on individual glucose responses to exercise. Carbohydrate consumption for endurance performance in individuals with type 1 diabetes is similar to the carbohydrate needs in healthy individuals (30‐80 g carbohydrates per h), typically reflecting the physiological and/or performance demands of exercise [73](A). A lower carbohydrate intake can be achieved during prolonged exercise if desired, but aggressive insulin dose adjustments are likely to be needed [51](B), [74](B), [75](D).
5.3. Post‐exercise period
During the first 90 min following exercise, an interstitial glycaemic range of 4.4 mmoL/L to 10.0 mmoL/L (80 mg/dL to 180 mg/dL) might be targeted in the majority of people with type 1 diabetes who are at a low risk of hypoglycaemia, reflecting the clinical targets for CGM and isCGM [41](D) with a slightly increased lower glycaemic limit, as recently recommended [76](D). People with an elevated risk of hypoglycaemia are recommended to increase the lower limit of sensor glucose to 5.0 mmoL/L (90 mg/dL) or 5.6 mmoL/L (100 mg/dL) during the post‐exercise period [58](D). Sensor glucose may be monitored regularly via CGM, or every 15‐30 min in the case of isCGM, during the 90 min post‐exercise period, and the hypoglycaemia alert can be set at 4.4 mmoL/L (80 mg/dL), 5.0 mmoL/L (90 mg/dL) or 5.6 mmoL/L (100 mg/dL) based on the risk of hypoglycaemia. If sensor glucose is rapidly increasing in the post‐exercise phase (detected by CGM when using the rate‐of‐change alert), then an insulin correction can be considered (50% of typical correction dose) [68](D), [69](D). If exercise was performed at a moderate‐to‐high intensity and/or for a long duration, then glucose may decrease during the acute post‐exercise period, as seen in experimental studies [74, 77]. At a glycaemic threshold of 4.4 mmoL/L (80 mg/dL) or slightly higher, based on the risk of hypoglycaemia [76], accompanied by a horizontal trend arrow, ~10 g of carbohydrates are recommended to be consumed; 15 g of carbohydrates should be consumed if accompanied by a (slight) downward trend arrow; an individual amount of carbohydrates should be consumed if accompanied by rapidly falling downward trend arrows (Table 3) [38](D), [39](D), [40](D), [44](D), [45](D). Treatment should be repeated if sensor glucose is not rising within 30 min as reflected by trend arrows. If an interstitial level <3.0 mmoL/L (<54 mg/dL) is displayed, rapidly acting carbohydrates should be given and a confirmatory SMBG may be performed. Exogenous glucagon is recommended if the person is unable to self‐treat [78](B).
5.4. Nocturnal post‐exercise period
Following an evening or late afternoon exercise session, or an exercise session of high intensity and long duration, people with type 1 diabetes are at an elevated risk of nocturnal hypoglycaemia, as shown in experimental and observational studies [79, 80]. Hypoglycaemia typically occurs within 6 to 15 h after exercise, although the risk may remain longer, as seen in children and adolescents, as well as in adults [81, 82]. People using a CGM system should, therefore, set the hypoglycaemia alert at 4.4 mmoL/L (80 mg/dL) during the night‐time period, or higher for groups with an elevated risk of hypoglycaemia to allow for earlier proactive treatments [76](D). When reaching glucose levels of 4.4 mmoL/L (80 mg/dL), or higher if deemed necessary, the following carbohydrate guidance can be applied [38](D), [39](D), [40](D), [44](D), [45](D): with a horizontal trend arrow, ~10 g of carbohydrates are recommended to be consumed; 15 g of carbohydrates should be consumed if accompanied by a (slight) downward trend arrow; and an individual amount of carbohydrates should be consumed if accompanied by downward trend arrows. These recommendations might also be applied for the second generation of isCGM systems where a real‐time alert is given when reaching hypo‐ and hyperglycaemia threshold.
People using an isCGM system should perform at least one scan during the night‐time period, preferably between midnight and 03:00 hours, since this is typically the nocturnal nadir after exercise [83](D). Carbohydrates should be given at a sensor glucose level of 4.4 mmoL/L (80 mg/dL) or earlier in people with an elevated risk of hypoglycaemia, following the same strategy as given for CGM [76](D) (Table 3). When carbohydrates were consumed after reaching this lower threshold, a scan should be performed no later than 2 h afterwards. If considered necessary, this procedure should be repeated frequently based on the lower glycaemic threshold and trend arrow.
6. CHILDREN AND ADOLESCENTS WITH TYPE 1 DIABETES
Exercise may improve glycaemic control, blood lipid profiles, physical fitness and quality of life and can decrease the total daily dose of insulin in children and adolescents with type 1 diabetes, as shown in randomised controlled trials and in a systematic review [84, 85, 86, 87]. Despite the positive effects of exercise, deterioration of diabetes control, fear of hypoglycaemia and other exercise‐related fears similar to the ones experienced by children without diabetes are major barriers to an active lifestyle in children and adolescents with type 1 diabetes, as assessed via questionnaire [88]. Furthermore, parents of children and adolescents with type 1 diabetes reported a conflict between the need for planned activity to control glucose levels and the spontaneous nature of children's usual activity [89]. Therefore, children and adolescents, as well as their parents, should actively participate in consultations with the healthcare professional team, as parental support appears to be the key to an active lifestyle [88](C).
Hypo‐ and hyperglycaemic alerts should be set at 5.6 mmoL/L (100 mg/dL) and 10.0 mmoL/L (180 mg/dL) or individualised if required [40](D), [44](D), [45](D). The rate‐of‐glucose‐change alerts should be used to initiate an earlier action [40](D), [44](D), [45](D). The use of remote monitors (eg, via a mobile phone application to remotely follow sensor glucose in real‐time) should be used to assess and react to glycaemic excursions during exercise [40](D). Both parents and most caregivers report a decreased overall worry and stress when using remote monitoring [90, 91]. Carbohydrate supplementation might be performed in relation to the individual body weight or as an absolute amount [40](D); however, it was shown that weight‐adjusted carbohydrate supplementation is more effective to treat hypoglycaemia in children with type 1 diabetes in a randomised crossover study [92]. The guidelines in Table 4 can be followed for exercise, which are in alignment with the ISPAD Clinical Practice Consensus Guidelines 2018: exercise in children and adolescents with diabetes, and other consensus and position statements [38](D), [39](D), [40](D).
TABLE 4.
General insulin therapy and carbohydrate recommendations for exercise in children and adolescents with type 1 diabetes
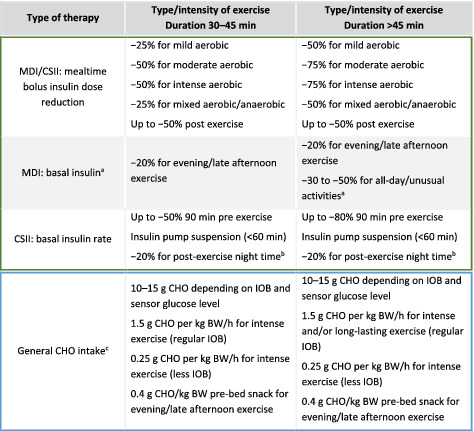
|
Note: BW, body weight; CHO, carbohydrates; CSII, continuous subcutaneous insulin infusion; IOB, insulin on board; MDI, multiple daily injections.
Basal insulin dose might be reduced the day prior and on the day of all‐day exercise.
Basal insulin rate might be reduced by 20% before bedtime if late afternoon/evening exercise was performed, depending on the duration and intensity of exercise.
Regular IOB, no/little insulin reduction has been performed; less IOB, moderate/high insulin reduction has been performed.
6.1. Preparation in advance to exercise
For the immediate pre‐exercise phase, the target sensor glucose range should be between 7.0 mmoL/L and 10.0 mmoL/L (126 mg/dL and 180 mg/dL), or between 8.0 mmoL/L and 11.0 mmoL/L (145 mg/dL and 198 mg/dL) for children and adolescents moderately exercising and/or with a moderate risk of hypoglycaemia, and 9.0 mmoL/L and 12.0 mmoL/L (162 mg/dL and 216 mg/dL) for children and adolescents minimally exercising and/or with a high risk of hypoglycaemia [40](D), [93](D). These glucose targets can be achieved by mealtime insulin dose reduction ranging from 25% to 75% (Table 4) [38](D), [39](D), [40](D). Bolus insulin dose reduction can be based on the individual glucose response to the type, intensity and duration of exercise [94](D). If sensor glucose concentration is below these glycaemic targets, then small amounts of oral carbohydrates should be consumed (eg, 10‐15 g of carbohydrates) [40](D), [44](D), [45](D). Exercise may be started when reaching 5.0 mmoL/L (90 mg/dL) glucose and, ideally, from 7.0 mmoL/L to 10.0 mmoL/L (126 mg/dL to 180 mg/dL) or higher in those with an increased risk of hypoglycaemia (Table 5) [40](D). Depending on the trend arrows on the CGM or isCGM, 5 g, 10 g, 15 g or more carbohydrates may be consumed when reaching the predefined lower glycaemic thresholds [40](D), [44](D), [45](D). At an upper limit of >15.0 mmoL/L (>270 mg/dL) and blood ketone levels >1.5 mmoL/L, exercise is contraindicated and blood ketone levels of 0.6‐1.4 mmoL/L should be addressed before exercise [40](D), [95](D), [96](D). In the case of extreme hyperglycaemia, an insulin correction might be applied (50% of a typical correction dose) [97](D). When using a CGM or a second‐generation isCGM, the hypoglycaemia alert threshold should be set at 5.6 mmoL/L (100 mg/dL) and the hyperglycaemia alert threshold should be set at 10.0 mmoL/L (180 mg/dL) or higher in those with an elevated risk of hypoglycaemia [40](D), [44](D), [45](D). Predictive hypoglycaemia thresholds, as well as rate‐of‐change‐in‐glucose alerts, should be switched on for CGM [40](D), [44](D), [45](D). Remote devices can be used by parents and caregivers to facilitate supportive action during exercise in children and adolescents with type 1 diabetes [90](D). The strategies given in Table 5 should be applied to achieve the recommended glycaemic targets [40](D), [44](D), [45](D).
TABLE 5.
Sensor glucose targets in advance to exercise in regard to different groups of children and adolescents with type 1 diabetes
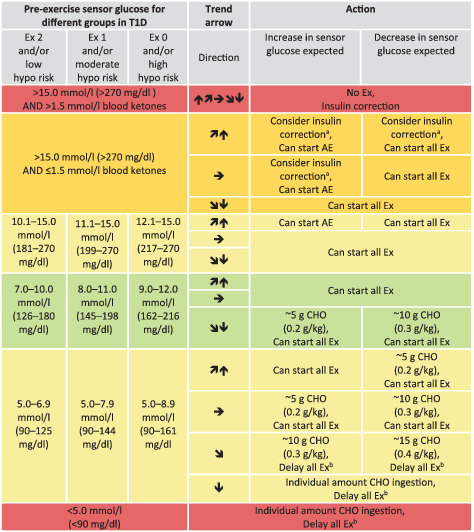
|
Note: Sensor glucose targets are detailed for the following groups in type 1 diabetes (T1D): intensively exercising and/or low risk of hypoglycaemia (Ex 2); moderately exercising and/or moderate risk of hypoglycaemia (Ex 1), minimally exercising and/or high risk of hypoglycaemia (Ex 0).
When reaching the required sensor glucose level to start exercise, only consume carbohydrates again when the trend arrow is starting to decrease.
These recommendations are not applicable to hybrid closed‐loop systems.
Green shading, no/minimal action required; light‐yellow shading, minimal/moderate action required; dark‐yellow shading, moderate/intense action required; red shading, no/delay exercise.
AE, mild‐to‐moderate intensity aerobic exercise; CHO, carbohydrates; Ex, exercise; hypo, hypoglycaemia.
50% of regular insulin correction factor when sensor glucose is close to the upper glycaemic threshold.
Delay exercise until reaching at least 5.0 mmoL/L (90 mg/dL) and, ideally, from 7.0 mmoL/L to 10.0 mmoL/L (126 mg/dL to 180 mg/dL) or higher in those with an increased risk of hypoglycaemia accompanied by 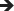 ,
, 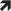 , or
, or 
6.2. During exercise
Sensor glucose ranges of 5.0 mmoL/L to 10.0 mmoL/L (90 mg/dL to 180 mg/dL) and, ideally, of 7.0 mmoL/L to 10.0 mmoL/L (126 mg/dL to 180 mg/dL) should be targeted for exercise [40](D), [44](D), [45](D).These ranges should be higher for children and adolescents who are minimally exercising and/or with a higher risk of hypoglycaemia (Table 6) [40](D), [44](D), [45](D). Carbohydrate consumption at a lower threshold of 7.0 mmoL/L (126 mg/dL l), 8.0 mmoL/L (145 mg/dL) or 9.0 mmoL/L (162 mg/dL), based on the risk of hypoglycaemia, with respect to trend arrows has been shown to avoid significant hypoglycaemia in children with type 1 diabetes [40](D), [44](D), [45](D). If sensor glucose is >15.0 mmoL/L (>270 mg/dL), blood ketones should be measured [40](D), [95](D), [96](D). If blood ketones are >1.5 mmoL/L, exercise should be stopped, the source of hyperglycaemia should be assessed and an insulin correction might be applied (50% of typical correction dose) [97](D). Elevated blood ketone levels should lead to repeated glucose and blood ketone measurements after exercise to ensure that ketosis (blood ketones >1.5 mmoL/L) or diabetic ketoacidosis is not developed. If sensor glucose is >15.0 mmoL/L (>270 mg/dL) and blood ketones are ≤1.5 mmoL/L, then only mild aerobic exercise may be performed to avoid a further increase in glucose levels by sympathoadrenal responses to intense (an)aerobic exercise [98](D). Exercise may be stopped at a sensor glucose level of <5.0 mmoL/L (<90 mg/dL), SMBG may be performed and carbohydrates should be consumed [40](D), [95](D), [96](D). Exercise may be restarted when reaching a sensor glucose level of 5.0 mmoL/L (90 mg/dL) accompanied by horizontal or upward trend arrows. Exercise should not be commenced when reaching a sensor glucose level of <3.0 mmoL/L (<54 mg/dL). Sensor glucose may be checked every 15 min during exercise and parents/caregivers are recommended to observe sensor glucose levels via a remote device [99](D). With respect to the specific trend arrow, a certain amount of carbohydrates may be consumed at a lower glycaemic threshold of 7.0 mmoL/L (126 mg/dL) or higher (Table 6), which should be further personalised in line with individual characteristics [40](D), [44](D), [45](D).
TABLE 6.
Sensor glucose targets during exercise in regard to different groups of children and adolescents with type 1 diabetes
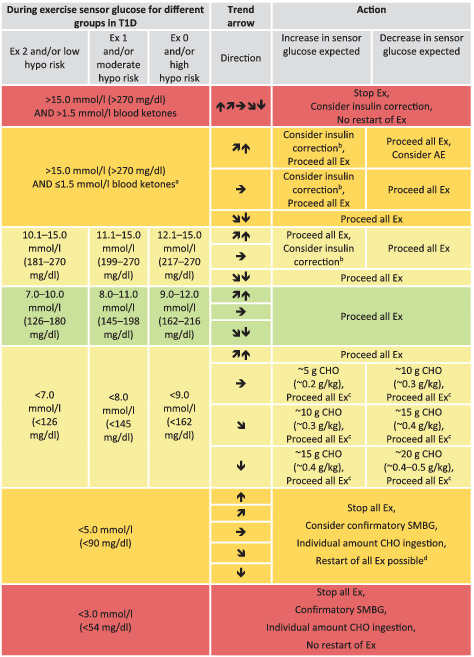
|
Note: Sensor glucose targets are detailed for the following groups in type 1 diabetes (T1D): intensively exercising and/or low risk of hypoglycaemia (Ex 2); moderately exercising and/or moderate risk of hypoglycaemia (Ex 1); minimally exercising and/or high risk of hypoglycaemia (Ex 0).
When reaching the required sensor glucose level during exercise, only consume carbohydrates again when the trend arrow is starting to decrease.
These recommendations are not applicable to hybrid closed‐loop systems.
Green shading, no/minimal action required; light‐yellow shading, minimal/moderate action required; dark‐yellow shading, moderate/intense action required; red shading, stop exercise.
AE, mild‐to‐moderate intensity aerobic exercise; CHO, carbohydrates; Ex, exercise; hypo, hypoglycaemia.
Elevated blood ketone levels should lead to repeated controls after exercise to ensure that ketosis (blood ketones >1.5 mmoL/L) or diabetic ketoacidosis is not developed. If sensor glucose is >15.0 mmoL/L (>270 mg/dL) and blood ketones are ≤1.5 mmoL/L, then only mild aerobic exercise may be performed.
50% of regular insulin correction factor when sensor glucose is close to the upper glycaemic threshold.
Restart exercise when reaching at least sensor glucose levels of 5.0 mmoL/L (90 mg/dL) and 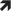 or
or 
Check sensor glucose at least 30 min after carbohydrate consumption and repeat treatment if required.
6.3. Post‐exercise period
Up to 2 h after exercise, children and adolescents may refill the intramuscular and hepatic glycogen storages via carbohydrates and protein, similar to recommendations for children and adolescents without diabetes [100]. After finishing exercise, the sensor glucose target should be between 4.4 mmoL/L and 10.0 mmoL/L (80 mg/dL and 180 mg/dL) or higher, based on the risk of hypoglycaemia, in the 90 min post‐exercise period [40](D), [76](D) (Table 7). If sensor glucose levels increase rapidly post exercise, then an insulin correction can be considered (50% of typical correction dose), based on the individual’s insulin sensitivity factor and sensor glucose level [97](D). However, correctional insulin dose close to bedtime should be avoided since it may increase the risk of post‐exercise nocturnal hypoglycaemia. Importantly, frequently checking sensor glucose values should be stressed to help to reduce the likelihood of developing post‐exercise late‐onset hypoglycaemia following bolus insulin correction.
TABLE 7.
Sensor glucose targets for carbohydrates consumption during the post‐exercise phase, including the nocturnal post‐exercise phase if exercise was performed in the late afternoon/evening, in children and adolescents with type 1 diabetes
|
Note: Sensor glucose threshold for treatments is detailed for the following groups with type 1 diabetes (T1D): intensively exercising and/or low risk of hypoglycaemia (Ex 2); moderately exercising and/or moderate risk of hypoglycaemia (Ex 1); minimally exercising and/or high risk of hypoglycaemia (Ex 0).
If an insulin correction is applied due to high sensor glucose levels, then the regular correction factor might be reduced by up to 50%.
These recommendations are not applicable to hybrid closed‐loop systems.
The intensity of yellow shading indicates the level of action required: lighter yellow shading indicates that minimal/moderate action is required, while darker yellow shading indicates that moderate/intense action is required.
CHO, carbohydrates; Ex, exercise; hypo, hypoglycaemia.
If the sensor glucose level falls below 4.4 mmoL/L (below 80 mg/dL) for children and adolescents of typical hypoglycaemia risk, then oral carbohydrates should be given; carbohydrates should be given at a higher glycaemic threshold for individuals at higher risk of hypoglycaemia (Table 7) [38](D), [39](D), [40](D). Oral carbohydrate consumption should be repeated, as required, to stabilise glucose levels. There will be a time delay of up to 20 min following oral carbohydrate consumption before a change in the trend arrow should be expected to be observed.
6.4. Nocturnal post‐exercise period
Children and adolescents may set the hypoglycaemia alert threshold at 4.4 mmoL/L (80 mg/dl), or even higher in those with a higher risk of hypoglycaemia, to be able to prospectively counteract impending hypoglycaemia [76](D). When reaching this lower threshold, the guidance as shown in Table 7 can be followed and further individualised, if required. Children and adolescents using an isCGM should perform a scan at least twice during the nocturnal period (eg, at 01:00 hours and 04:00 hours) due to the increased risk of nocturnal hypoglycaemia [101, 102, 103, 104](D), especially after exercise [81, 104, 105, 106](D). Parents or other care providers can be alerted using the remote monitoring function within CGMs, which can support parents in their effort to avoid nocturnal hypoglycaemia in children [91](D). In addition to the intake of carbohydrates, the insulin strategies mentioned in Table 4 should also be applied to lower the risk of nocturnal hypoglycaemia [38](D), [39](D), [40](D).
7. DISCUSSION
 In this position statement, we detailed the use of sensor glucose values accompanied by trend arrows for CGM and isCGM systems for different groups of people with type 1 diabetes and for different sensor glucose responses to exercise. Of note, in this position statement, recommendations for carbohydrate consumption were stratified with respect to the rate of change in glucose for the pre‐exercise phase, during exercise, post‐exercise and the nocturnal post‐exercise phase. Taking the lag time of CGM and isCGM systems against SMBG around exercise into account, safe sensor glucose thresholds are recommended for people with type 1 diabetes. In general, these recommendations can be used as an initial guidance tool that also needs to be tailored individually. The recommendations in this position statement will need to be updated in future years to provide the best and most robust evidence‐based recommendations for people with type 1 diabetes using CGM and isCGM for glycaemic control during exercise.
In this position statement, we detailed the use of sensor glucose values accompanied by trend arrows for CGM and isCGM systems for different groups of people with type 1 diabetes and for different sensor glucose responses to exercise. Of note, in this position statement, recommendations for carbohydrate consumption were stratified with respect to the rate of change in glucose for the pre‐exercise phase, during exercise, post‐exercise and the nocturnal post‐exercise phase. Taking the lag time of CGM and isCGM systems against SMBG around exercise into account, safe sensor glucose thresholds are recommended for people with type 1 diabetes. In general, these recommendations can be used as an initial guidance tool that also needs to be tailored individually. The recommendations in this position statement will need to be updated in future years to provide the best and most robust evidence‐based recommendations for people with type 1 diabetes using CGM and isCGM for glycaemic control during exercise.
AUTHORS' RELATIONSHIPS AND ACTIVITIES
OM has received lecture fees from Medtronic, travel grants from Novo Nordisk A/S, Novo Nordisk AT, Novo Nordisk UK and Medtronic AT, research grants from Sêr Cymru II COFUND fellowship/European Union, Sanofi‐Aventis, Novo Nordisk A/S, Novo Nordisk AT, Dexcom Inc., as well as material funding from Abbott Diabetes Care. MCR has received speaker’s honorarium from Medtronic and Insulet and has served on advisory boards for Dexcom, Sanofi and Eli Lilly. MLE has received a KESS2/European Social Fund scholarship and travel grants from Novo Nordisk A/S and Sanofi‐Aventis. PA has received research support or advisory board fees from Eli Lilly, Novo Nordisk and Roche, funding from Research and Development, Region Halland, and is an employee of Region Halland. RRL reports having received a consumable gift (in kind) from Medtronic. KN is a shareholder of Novo Nordisk, has received research support from Novo Nordisk, Roche Diagnostics and Zealand Pharma, has received lecture fees from Medtronic, Roche Diagnostics, Rubin Medical, Sanofi, Zealand Pharma, Novo Nordisk and Bayer, and has served on advisory panels for Medtronic, Abbott and Novo Nordisk. NSO has received honoraria for speaking and advisory board participation from Abbott Diabetes, Dexcom, Medtronic Diabetes and Roche Diabetes. DPZ has received speaker’s honoraria from Medtronic Diabetes, Ascensia Diabetes and Insulet Corporation. TB has received honoraria for participation on advisory boards for Novo Nordisk, Sanofi, Eli Lilly, Boehringer, Medtronic and Bayer HealthCare, and as a speaker for AstraZeneca, Eli Lilly, Bayer, Novo Nordisk, Medtronic, Sanofi and Roche, and owns stocks of DreaMed Diabetes. CDB has received speaker honoraria from MiniMed Medtronic and is a member of its European Psychology Advisory Board. RMBe has received research support from, consulted for or has been on a scientific advisory board for Abbott Diabetes Care, Dexcom, Eli Lilly, Johnson & Johnson, Medtronic, Novo Nordisk, Onduo, Roche, Sanofi and United HealthCare. BB received grant support and advisory board fees from Medtronic Diabetes and ConvaTec, grant support and presentation fees from Insulet, advisory board fees from Novo Nordisk and Profusa, grant support from Eli Lilly, grant support and equipment from Dexcom, and is holding patent 61 197 230 on a hypoglycaemia prediction algorithm. EC is a scientific advisory board member/consultant for Novo Nordisk, Adocia, MannKind, Lexicon and Arecor, and a speaker for Novo Nordisk. TH is a shareholder of Profil, which has received research funds from Adocia, Boehringer Ingelheim, Dance Pharmaceuticals, Eli Lilly, Johnson & Johnson, MedImmune, Merck Sharp and Dohme, Mylan, Nordic Bioscience, Novo Nordisk, Poxel, Roche Diagnostics, Saniona, Sanofi, Senseonics and Zealand Pharma. SH has served as a consultant or speaker for Lilly, Novo Nordisk, Takeda, Boehringer Ingelheim, Mannkind, Sanofi‐Aventis, Zealand Pharma and UN‐EEG. LL reports having received speaker honoraria from Animas, Abbott, Insulet, Medtronic, Novo Nordisk, Roche and Sanofi, serving on advisory panels for Animas, Abbott, Novo Nordisk, Dexcom, Medtronic, Sanofi, and Roche, and research support from Novo Nordisk and Dexcom. CM serves or has served on the advisory panel for Novo Nordisk, Sanofi, Merck Sharp and Dohme, Eli Lilly and Company, Novartis, AstraZeneca, Boehringer Ingelheim, Hanmi Pharmaceuticals, Roche, Medtronic, ActoBio Therapeutics, Pfizer, Dianax and UCB. CS reports having received speaker honoraria from Medtronic and Ypsomed, and serving on advisory panels for Novo Nordisk, Medtronic, Roche and Sanofi. MT has received speaker honoraria from MiniMed Medtronic and Novo Nordisk. EGW has received personal fees from Abbott Diabetes Care, Dexcom, Eli Lilly, Medtronic, Novo Nordisk and Sanofi‐Aventis. HS has received honoraria, travel support or unrestricted research grants by Amgen, Astra Zeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novo Nordisk and Sanofi‐Aventis. PGJ has a financial interest in Pacific Diabetes Technologies Inc., a company that may have a commercial interest in the results of this research and technology. RMBr has received honoraria as well as travel and educational grant support from Boehringer Ingelheim, Eli Lilly and Company, Novo Nordisk and Sanofi‐Aventis. JKM is a member of the advisory board of Boehringer Ingelheim, Eli Lilly, Medtronic, Prediktor A/S, Roche Diabetes Care and Sanofi, and received speaker honoraria from Abbott Diabetes Care, AstraZeneca, Dexcom, Eli Lilly, Medtronic, Merk Sharp & Dohme, Novo Nordisk A/S, Roche Diabetes Care, Sanofi, Servier and Takeda. The remaining authors have no relevant conflicts of interest to disclose.
CONTRIBUTION STATEMENT
All authors of this position statement substantially contributed to conception and design, acquisition of data, or analysis and interpretation of data, drafted the article or revised it critically for important intellectual content and approved the final version to be published.
Supporting information
ESM Table 1 Systematic review and meta‐analysis (Pubmed/Medline)
ESM Table 2: Systematic review and meta‐analysis (Embase)
ESM Table 3: Systematic review and meta‐analysis (Cochrane)
ACKNOWLEDGEMENTS
We want to thank P. Choudhary (King’s College Hospital NHS Foundation Trust, London, UK and Department of Diabetes, School of Life Course Sciences, King’s College London, London, UK), S. Gray (Campuslife, Swansea University, Swansea, UK), S. Hofer (Department of Pediatrics, Medical University of Innsbruck, Innsbruck, Austria), M. Sauer (Energie Graz GmbH & Co KG, Graz, Austria) and D. J. West (Population Health Sciences Institute, Newcastle University, Newcastle upon Tyne, UK) for critical assessment of the manuscript before submission. We want to thank F. Aziz, A. Mueller and C. Unteregger (all Cardiovascular Diabetology Research Group, Division of Endocrinology and Diabetology, Medical University of Graz, Graz, Austria) for the support with the production of figures and tables.
Moser O, Riddell MC, Eckstein ML, et al. Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: position statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA). Pediatr Diabetes. 2020;21:1375–1393. 10.1111/pedi.13105
This article is co‐published in the journals Diabetologia and Pediatric Diabetes, available at [https://doi.org/10.1007/s00125-020-05263-9] and [https://onlinelibrary.wiley.com/journal/13995448], respectively.
This is an abridged version of the Position Statement. The full‐length version is available on the EASD website (https://www.easd.org/sites/default/files/Exercise%20CGM%20EASD%20position%20statement_final.pdf).
Funding information Medical University of Graz; Newcastle University; Medical University of Innsbruck; Swansea University; King’s College London; King’s College Hospital NHS Foundation Trust
REFERENCES
- 1. Foster NC, Miller KM, Tamborlane WV, Bergenstal RM, Beck RW. Continuous glucose monitoring in patients with type 1 diabetes using insulin injections. Diabetes Care. 2016;39:e81‐e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beck RW, Riddlesworth T, Ruedy K, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the gold randomized clinical trial. JAMA. 2017;317(4):371 10.1001/jama.2016.19975. [DOI] [PubMed] [Google Scholar]
- 3. van Beers CAJ, DeVries JH, Kleijer SJ, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open‐label, crossover trial. Lancet Diabetes Endocrinol. 2016;4(11):893‐902. 10.1016/S2213-8587(16)30193-0. [DOI] [PubMed] [Google Scholar]
- 4. Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317(4):379‐387. 10.1001/jama.2016.19976. [DOI] [PubMed] [Google Scholar]
- 5. Bolinder J, Antuna R, Geelhoed‐Duijvestijn P, Kröger J, Weitgasser R. Novel glucose‐sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non‐masked, randomised controlled trial. Lancet. 2016;388(10057):2254‐2263. 10.1016/S0140-6736(16)31535-5. [DOI] [PubMed] [Google Scholar]
- 6. Oskarsson P, Antuna R, Geelhoed‐Duijvestijn P, Krӧger J, Weitgasser R, Bolinder J. Impact of flash glucose monitoring on hypoglycaemia in adults with type 1 diabetes managed with multiple daily injection therapy: a pre‐specified subgroup analysis of the IMPACT randomised controlled trial. Diabetologia. 2018;61(3):539‐550. 10.1007/s00125-017-4527-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Evans M, Welsh Z, Ells S, Seibold A. The impact of flash glucose monitoring on glycaemic control as measured by HbA1c: a meta‐analysis of clinical trials and real‐world observational studies. Diabetes Ther. 2020;11(1):83‐95. 10.1007/s13300-019-00720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reddy M, Jugnee N, Anantharaja S, Oliver N. Switching from flash glucose monitoring to continuous glucose monitoring on hypoglycemia in adults with type 1 diabetes at high hypoglycemia risk: the extension phase of the I HART CGM study. Diabetes Technol Ther. 2018;20(11):751‐757. 10.1089/dia.2018.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feig DS, Donovan LE, Corcoy R, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet. 2017;390(10110):2347‐2359. 10.1016/S0140-6736(17)32400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berg AK, Olsen BS, Thyssen JP, et al. High frequencies of dermatological complications in children using insulin pumps or sensors. Pediatr Diabetes. 2018;19(4):733‐740. 10.1111/pedi.12652. [DOI] [PubMed] [Google Scholar]
- 11. Hilliard ME, Levy W, Anderson BJ, et al. Benefits and barriers of continuous glucose monitoring in young children with type 1 diabetes. Diabetes Technol Ther. 2019;21(9):493‐498. 10.1089/dia.2019.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Messer LH, Berget C, Beatson C, Polsky S, Forlenza GP. Preserving skin integrity with chronic device use in diabetes. Diabetes Technol Ther. 2018;20:S254‐S264. 10.1089/dia.2018.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Messer LH, Johnson R, Driscoll KA, Jones J. Best friend or spy: a qualitative meta‐synthesis on the impact of continuous glucose monitoring on life with type 1 diabetes. Diabet Med. 2018;35:409‐418. [DOI] [PubMed] [Google Scholar]
- 14. Larson NS, Pinsker JE. The role of continuous glucose monitoring in the care of children with type 1 diabetes. Int J Pediatr Endocrinol. 2013;2013(1):8 10.1186/1687-9856-2013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moser O, Yardley J, Bracken R. Interstitial glucose and physical exercise in type 1 diabetes: integrative physiology, technology, and the gap in‐between. Nutrients. 2018;10(1):93 10.3390/nu10010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Larose S, Rabasa‐Lhoret R, Roy‐Fleming A, et al. Changes in accuracy of continuous glucose monitoring using Dexcom G4 Platinum over the course of moderate intensity aerobic exercise in type 1 diabetes. Diabetes Technol Ther. 2019;21(6):364‐369. 10.1089/dia.2018.0400. [DOI] [PubMed] [Google Scholar]
- 17. Steineck IIK, Mahmoudi Z, Ranjan A, Schmidt S, Jørgensen JB, Nørgaard K. Comparison of continuous glucose monitoring accuracy between abdominal and upper arm insertion sites. Diabetes Technol Ther. 2019;21(5):295‐302. 10.1089/dia.2019.0014. [DOI] [PubMed] [Google Scholar]
- 18. Moser O, Mader J, Tschakert G, et al. Point accuracy of interstitial continuous glucose monitoring during resistance and aerobic exercise in type 1 diabetes. Nutrients. 2016;8(8):489 10.3390/nu8080489. [DOI] [Google Scholar]
- 19. Yardley JE, Sigal RJ, Kenny GP, Riddell MC, Perkins BA. Point accuracy of interstitial continuous glucose monitoring during resistance and aerobic exercise in type 1 diabetes. Can J Diabetes. 2012;36(5):S14‐S15. 10.1016/j.jcjd.2012.07.063. [DOI] [PubMed] [Google Scholar]
- 20. Gilligan BJ, Shults MC, Rhodes RK, Updike SJ. Evaluation of a subcutaneous glucose sensor out to 3 months in a dog model. Diabetes Care. 1994;17(8):882‐887. 10.2337/diacare.17.8.882. [DOI] [PubMed] [Google Scholar]
- 21. Maahs DM, DeSalvo D, Pyle L, et al. Effect of acetaminophen on CGM glucose in an outpatient setting. Diabetes Care. 2015;38(10):e158‐e159. 10.2337/dc15-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real‐time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21(6):313‐321. 10.1089/dia.2018.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mcclatchey PM, Mcclain ES, Williams IM, Gregory JM, Cliffel D WD (2019) Continuous glucose monitor readings lag interstitial glucose by several minutes. Diabetes 68(Supplement 1):962‐P (Abstract). 10.2337/db19-962-p [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moser O, Eckstein ML, Mueller A, et al. Impact of physical exercise on sensor performance of the FreeStyle Libre intermittently viewed continuous glucose monitoring system in people with type 1 diabetes: a randomized crossover trial. Diabet Med. 2019;36(5):606‐611. 10.1111/dme.13909. [DOI] [PubMed] [Google Scholar]
- 25. Moser O, Eckstein ML, McCarthy O, et al. Performance of the Freestyle Libre flash glucose monitoring (flash GM) system in individuals with type 1 diabetes: a secondary outcome analysis of a randomized crossover trial. Diabetes Obes Metab. 2019;21:2505‐2512. 10.1111/dom.13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Basu A, Dube S, Slama M, et al. Time lag of glucose from intravascular to interstitial compartment in humans. Diabetes. 2013;62(12):4083‐4087. 10.2337/db13-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li A, Riddell MC, Potashner D, Brown RE, Aronson R. Time lag and accuracy of continuous glucose monitoring during high intensity interval training in adults with type 1 diabetes. Diabetes Technol Ther. 2019;21(5):286‐294. 10.1089/dia.2018.0387. [DOI] [PubMed] [Google Scholar]
- 28. Taleb N, Emami A, Suppere C, et al. Comparison of two continuous glucose monitoring systems, Dexcom G4 Platinum and Medtronic Paradigm Veo Enlite system, at rest and during exercise. Diabetes Technol Ther. 2016;18(9):561‐567. 10.1089/dia.2015.0394. [DOI] [PubMed] [Google Scholar]
- 29. Breton MD, Cherñavvsky DR, Forlenza GP, et al. Closed‐loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the artificial pancreas ski study. Diabetes Care. 2017;40(12):1644‐1650. 10.2337/dc17-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gomez AM, Gomez C, Aschner P, et al. Effects of performing morning versus afternoon exercise on glycemic control and hypoglycemia frequency in type 1 diabetes patients on sensor‐augmented insulin pump therapy. J Diabetes Sci Technol. 2015;9(3):619‐624. 10.1177/1932296814566233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jayawardene DC, McAuley SA, Horsburgh JC, et al. Closed‐loop insulin delivery for adults with type 1 diabetes undertaking high‐intensity interval exercise versus moderate‐intensity exercise. Diabetes Technol Ther. 2017;19(6):340‐348. 10.1089/dia.2016.0461. [DOI] [PubMed] [Google Scholar]
- 32. Zaharieva D, Yavelberg L, Jamnik V, Cinar A, Turksoy K, Riddell MC. The effects of basal insulin suspension at the start of exercise on blood glucose levels during continuous versus circuit‐based exercise in individuals with type 1 diabetes on continuous subcutaneous insulin infusion. Diabetes Technol Ther. 2017;19(6):370‐378. 10.1089/dia.2017.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Biagi L, Bertachi A, Quirós C, et al. Accuracy of continuous glucose monitoring before, during, and after aerobic and anaerobic exercise in patients with type 1 diabetes mellitus. Biosensors. 2018;8(1):22 10.3390/bios8010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moser O, Pandis M, Aberer F, et al. A head‐to‐head comparison of personal and professional continuous glucose monitoring systems in people with type 1 diabetes: hypoglycaemia remains the weak spot. Diabetes Obes Metab. 2019;21:1043‐1048. 10.1111/dom.13598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aberer F, Hajnsek M, Rumpler M, et al. Evaluation of subcutaneous glucose monitoring systems under routine environmental conditions in patients with type 1 diabetes. Diabetes. Obes Metab. 2017;19(7):1051‐1055. 10.1111/dom.12907. [DOI] [PubMed] [Google Scholar]
- 36. Giani E, Macedoni M, Barilli A, et al. Performance of the flash glucose monitoring system during exercise in youth with type 1 diabetes. Diabetes Res Clin Pract. 2018;146:321‐329. 10.1016/j.diabres.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 37. Bally L, Zueger T, Pasi N, Carlos C, Paganini D, Stettler C. Accuracy of continuous glucose monitoring during differing exercise conditions. Diabetes Res Clin Pract. 2016;112:1‐5. 10.1016/j.diabres.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 38. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065‐2079. 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Riddell MC, Gallen IW, Smart CE, et al. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol. 2017;8587(17):1‐14. 10.1016/S2213-8587(17)30014-1. [DOI] [PubMed] [Google Scholar]
- 40. Adolfsson P, Riddell MC, Taplin CE, et al. ISPAD clinical practice consensus guidelines 2018: exercise in children and adolescents with diabetes. Pediatr Diabetes. 2018;19:205‐226. 10.1111/pedi.12755. [DOI] [PubMed] [Google Scholar]
- 41. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593‐1603. 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chiang JL, Kirkman MS, Laffel LMB, Peters AL. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37(7):2034‐2054. 10.2337/dc14-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. American Diabetes Association . Standards of medical care in diabetes—2014. Diabetes Care. 2014;37:14‐80. 10.2337/dc14-S014. [DOI] [Google Scholar]
- 44. Burckhardt MA, Chetty T, Smith GJ, et al. Use of continuous glucose monitoring trends to facilitate exercise in children with type 1 diabetes. Diabetes Technol Ther. 2019;21(1):51‐55. 10.1089/dia.2018.0292. [DOI] [PubMed] [Google Scholar]
- 45. Riddell MC, Milliken J. Preventing exercise‐induced hypoglycemia in type 1 diabetes using real‐time continuous glucose monitoring and a new carbohydrate intake algorithm: an observational field study. Diabetes Technol Ther. 2011;13(8):819‐825. 10.1089/dia.2011.0052. [DOI] [PubMed] [Google Scholar]
- 46. Hinkley T, Salmon J, Okely AD, Crawford D, Hesketh K. Preschoolers' physical activity, screen time, and compliance with recommendations. Med Sci Sports Exerc. 2012;44(3):458‐465. 10.1249/MSS.0b013e318233763b. [DOI] [PubMed] [Google Scholar]
- 47. Bally L, Zueger T, Buehler T, et al. Metabolic and hormonal response to intermittent high‐intensity and continuous moderate intensity exercise in individuals with type 1 diabetes: a randomised crossover study. Diabetologia. 2016;59(4):776‐784. 10.1007/s00125-015-3854-7. [DOI] [PubMed] [Google Scholar]
- 48. Reddy R, Wittenberg A, Castle JR, et al. Effect of aerobic and resistance exercise on glycemic control in adults with type 1 diabetes. Can J Diabetes. 2019;43(6):406‐414. 10.1016/j.jcjd.2018.08.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Turner D, Gray BJ, Luzio S, et al. Similar magnitude of post‐exercise hyperglycemia despite manipulating resistance exercise intensity in type 1 diabetes individuals. Scand J Med Sci Sports. 2016;26(4):404‐412. 10.1111/sms.12472. [DOI] [PubMed] [Google Scholar]
- 50. García‐García F, Kumareswaran K, Hovorka R, Hernando ME. Quantifying the acute changes in glucose with exercise in type 1 diabetes: a systematic review and meta‐analysis. Sport Med. 2015;45(4):587‐599. 10.1007/s40279-015-0302-2. [DOI] [PubMed] [Google Scholar]
- 51. Rabasa‐Lhoret R, Bourque J, Ducros F, Chiasson JL. Guidelines for premeal insulin dose reduction for postprandial exercise of different intensities and durations in type 1 diabetic subjects treated intensively with a basal‐bolus insulin regimen (ultralente‐lispro). Diabetes Care. 2001;24(4):625‐630. 10.2337/diacare.24.4.625. [DOI] [PubMed] [Google Scholar]
- 52. West DJ, Morton RD, Bain SC, Stephens JW, Bracken RM. Blood glucose responses to reductions in pre‐exercise rapid‐acting insulin for 24 h after running in individuals with type 1 diabetes. J Sports Sci. 2010;28(7):781‐788. 10.1080/02640411003734093. [DOI] [PubMed] [Google Scholar]
- 53. Moser O, Eckstein ML, Mueller A, et al. Pre‐exercise blood glucose levels determine the amount of orally administered carbohydrates during physical exercise in individuals with type 1 diabetes—a randomized cross‐over trial. Nutrients. 2019;11(6):1287 10.3390/nu11061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Scott SN, Cocks M, Andrews RC, et al. Fasted high‐intensity interval and moderate‐intensity exercise do not lead to detrimental 24‐hour blood glucose profiles. J Clin Endocrinol Metab. 2019;104(1):111‐117. 10.1210/jc.2018-01308. [DOI] [PubMed] [Google Scholar]
- 55. Riddell MC, Pooni R, Yavelberg L, et al. Reproducibility in the cardiometabolic responses to high‐intensity interval exercise in adults with type 1 diabetes. Diabetes Res Clin Pract. 2019;148:137‐143. 10.1016/j.diabres.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 56. Harmer AR, Chisholm DJ, McKenna MJ, et al. High‐intensity training improves plasma glucose and acid‐base regulation during intermittent maximal exercise in type 1 diabetes. Diabetes Care. 2007;30(5):1269‐1271. 10.2337/dc06-1790. [DOI] [PubMed] [Google Scholar]
- 57. Farinha JB, Boff W, dos Santos GC, et al. Acute glycemic responses along 10‐week high‐intensity training protocols in type 1 diabetes patients. Diabetes Res Clin Pract. 2019;153:111‐113. 10.1016/j.diabres.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 58. Bohn B, Herbst A, Pfeifer M, et al. Impact of physical activity on glycemic control and prevalence of cardiovascular risk factors in adults with type 1 diabetes: a cross‐sectional multicenter study of 18,028 patients. Diabetes Care. 2015;38(8):1536‐1543. 10.2337/dc15-0030. [DOI] [PubMed] [Google Scholar]
- 59. Lin YK, Hung M, Sharma A, et al. Impaired awareness of hypoglycemia continues to be a risk factor for severe hypoglycemia despite the use of continuous glucose monitoring system in type 1 diabetes. Endocr Pract. 2019;25(6):517‐525. 10.4158/EP-2018-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Galassetti P, Tate D, Neill RA, Richardson A, Leu SY, Davis SN. Effect of differing antecedent hypoglycemia on counterregulatory responses to exercise in type 1 diabetes. Am J Physiol Endocrinol Metab. 2006;290(6):E1109‐E1117. 10.1152/ajpendo.00244.2005. [DOI] [PubMed] [Google Scholar]
- 61. American Diabetes Association . Older adults: standards of medical care in diabetes‐2020. Diabetes Care. 2020;43(Suppl. 1):S152‐S162. 10.2337/dc20-S012. [DOI] [PubMed] [Google Scholar]
- 62. Kudva YC, Ahmann AJ, Bergenstal RM, et al. Approach to using trend arrows in the freestyle libre flash glucose monitoring systems in adults. J Endocr Soc. 2018;2(12):1320‐1337. 10.1210/js.2018-00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kennedy A, Nirantharakumar K, Chimen M, et al. Does exercise improve glycaemic control in type 1 diabetes? a systematic review and meta‐analysis. PLoS One. 2013;8(3):e58861 10.1371/journal.pone.0058861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shivers JP, Mackowiak L, Anhalt H, Zisser H. “Turn it off!”: diabetes device alarm fatigue considerations for the present and the future. J Diabetes Sci Technol. 2013;7(3):789‐794. 10.1177/193229681300700324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Eshghi SRT, Yardley JE. Morning (fasting) vs. afternoon resistance exercise in individuals with type 1 diabetes: a randomized cross‐over study. J Clin Endocrinol Metab. 2019;104(11):5217‐5224. 10.1210/jc.2018-02384. [DOI] [PubMed] [Google Scholar]
- 66. McCarthy O, Moser O, Eckstein ML, et al. Resistance isn’t futile: the physiological basis of the health effects of resistance exercise in individuals with type 1 diabetes. Front Endocrinol. 2019;10:507 10.3389/fendo.2019.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Peter Adams O. The impact of brief high‐intensity exercise on blood glucose levels. Diabetes, Metab Syndr Obes Targets Ther. 2013;6:113‐122. 10.2147/DMSO.S29222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aronson R, Brown RE, Li A, Riddell MC. Optimal insulin correction factor in post–high‐intensity exercise hyperglycemia in adults with type 1 diabetes: the FIT study. Diabetes Care. 2019;42(1):10‐16. 10.2337/dc18-1475. [DOI] [PubMed] [Google Scholar]
- 69. Turner D, Luzio S, Gray BJ, et al. Algorithm that delivers an individualized rapid‐acting insulin dose after morning resistance exercise counters post‐exercise hyperglycaemia in people with Type 1 diabetes. Diabet Med. 2016;33(4):506‐510. 10.1111/dme.12870. [DOI] [PubMed] [Google Scholar]
- 70. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631‐1640. 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57(12):3169‐3176. 10.2337/db08-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fuchs CJ, Gonzalez JT, Loon LJC. Fructose co‐ingestion to increase carbohydrate availability in athletes. J Physiol. 2019;597(14):3549‐3560. 10.1113/JP277116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Temesi J, Johnson NA, Raymond J, Burdon CA, O’Connor HT. Carbohydrate ingestion during endurance exercise improves performance in adults. J Nutr. 2011;141(5):890‐897. 10.3945/jn.110.137075. [DOI] [PubMed] [Google Scholar]
- 74. Moser O, Tschakert G, Mueller A, et al. Effects of high‐intensity interval exercise versus moderate continuous exercise on glucose homeostasis and hormone response in patients with type 1 diabetes mellitus using novel ultra‐long‐acting insulin. PLoS One. 2015;10(8):e0136489 10.1371/journal.pone.0136489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Scott SN, Anderson L, Morton JP, Wagenmakers AJM, Riddell MC. Carbohydrate restriction in type 1 diabetes: a realistic therapy for improved glycaemic control and athletic performance? Nutrients. 2019;11(5):1022 10.3390/nu11051022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Castle JR, Rodbard D. How well do continuous glucose monitoring systems perform during exercise. Diabetes Technol Ther. 2019;21:305‐309. [DOI] [PubMed] [Google Scholar]
- 77. Campbell MD, Walker M, Bracken RM, et al. Insulin therapy and dietary adjustments to normalize glycemia and prevent nocturnal hypoglycemia after evening exercise in type 1 diabetes: a randomized controlled trial. BMJ Open Diabetes Res Care. 2015;3(1):e000085 10.1136/bmjdrc-2015-000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Carstens S, Sprehn M. Prehospital treatment of severe hypoglycaemia: a comparison of intramuscular glucagon and intravenous glucose. Prehosp Disaster Med. 1998;13(2–4):44‐50. 10.1017/s1049023x00030132. [DOI] [PubMed] [Google Scholar]
- 79. Maran A, Pavan P, Bonsembiante B, et al. Continuous glucose monitoring reveals delayed nocturnal hypoglycemia after intermittent high‐intensity exercise in nontrained patients with type 1 diabetes. Diabetes Technol Ther. 2010;12(10):763‐768. 10.1089/dia.2010.0038. [DOI] [PubMed] [Google Scholar]
- 80. Moser O, Eckstein ML, McCarthy O, et al (2019) 66‐LB: Greater time spent in hypoglycemia during night compared with day during intensified training in professional cyclists with type 1 diabetes—a prospective observational study. Diabetes 68(Supplement 1):66‐LB (Abstract). 10.2337/db19-66-lb 30305368 [DOI] [Google Scholar]
- 81. Tsalikian E, Mauras N, Beck RW, et al. Impact of exercise on overnight glycemic control in children with type 1 diabetes mellitus. J Pediatr. 2005;147(4):528‐534. 10.1016/j.jpeds.2005.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. MacDonald MJ. Postexercise late‐onset hypoglycemia in insulin‐dependent diabetic patients. Diabetes Care. 1987;10(5):584‐588. 10.2337/diacare.10.5.584. [DOI] [PubMed] [Google Scholar]
- 83. Iscoe KE, Corcoran M, Riddell MC. High rates of nocturnal hypoglycemia in a unique sports camp for athletes with type 1 diabetes: lessons learned from continuous glucose monitoring systems. Can J Diabetes. 2008;32(3):182‐189. 10.1016/S1499-2671(08)23008-X. [DOI] [Google Scholar]
- 84. Salem MA, Aboelasrar MA, Elbarbary NS, Elhilaly RA, Refaat YM. Is exercise a therapeutic tool for improvement of cardiovascular risk factors in adolescents with type 1 diabetes mellitus? A randomised controlled trial. Diabetol Metab Syndr. 2010;2(1):47 10.1186/1758-5996-2-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Absil H, Baudet L, Robert A, Lysy PA. Benefits of physical activity in children and adolescents with type 1 diabetes: a systematic review. Diabetes Res Clin Pract. 2019;156:107810. [DOI] [PubMed] [Google Scholar]
- 86. D’hooge R, Hellinckx T, Van Laethem C, et al. Influence of combined aerobic and resistance training on metabolic control, cardiovascular fitness and quality of life in adolescents with type 1 diabetes: a randomized controlled trial. Clin Rehabil. 2011;25(4):349‐359. 10.1177/0269215510386254. [DOI] [PubMed] [Google Scholar]
- 87. Heyman E, Toutain C, Delamarche P, et al. Exercise training and cardiovascular risk factors in Type 1 diabetic adolescent girls. Pediatr Exerc Sci. 2007;19(4):408‐419. 10.1123/pes.19.4.408. [DOI] [PubMed] [Google Scholar]
- 88. Jabbour G, Henderson M, Mathieu ME. Barriers to active lifestyles in children with type 1 diabetes. Can J Diabetes. 2016;40(2):170‐172. 10.1016/j.jcjd.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 89. Quirk H, Blake H, Dee B, Glazebrook C. “You can’t just jump on a bike and go”: a qualitative study exploring parents’ perceptions of physical activity in children with type 1 diabetes. BMC Pediatr. 2014;14(1):1‐12. 10.1186/s12887-014-0313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Erie C, Van Name MA, Weyman K, et al. Schooling diabetes: use of continuous glucose monitoring and remote monitors in the home and school settings. Pediatr Diabetes. 2018;19(1):92‐97. 10.1111/pedi.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Burckhardt MA, Roberts A, Smith GJ, Abraham MB, Davis EA, Jones TW. The use of continuous glucose monitoring with remote monitoring improves psychosocial measures in parents of children with type 1 diabetes: a randomized crossover trial. Diabetes Care. 2018;41(12):2641‐2643. 10.2337/dc18-0938. [DOI] [PubMed] [Google Scholar]
- 92. McTavish L, Corley B, Weatherall M, Wiltshire E, Krebs JD. Weight‐based carbohydrate treatment of hypoglycaemia in people with type 1 diabetes using insulin pump therapy: a randomized crossover clinical trial. Diabet Med. 2018;35(3):339‐346. 10.1111/dme.13576. [DOI] [PubMed] [Google Scholar]
- 93. Riddell MC, Zaharieva DP, Tansey M, et al. Individual glucose responses to prolonged moderate intensity aerobic exercise in adolescents with type 1 diabetes: the higher they start. the harder they fall Pediatr Diabetes. 2019;20(1):99‐106. 10.1111/pedi.12799. [DOI] [PubMed] [Google Scholar]
- 94. Admon G, Weinstein Y, Falk B, et al. Exercise with and without an insulin pump among children and adolescents with type 1 diabetes mellitus. Pediatrics. 2005;116(3):e348‐e355. 10.1542/peds.2004-2428. [DOI] [PubMed] [Google Scholar]
- 95. Samuelsson U, Ludvigsson J. When should determination of ketonemia be recommended? Diabetes Technol Ther. 2002;4(5):645‐650. 10.1089/152091502320798286. [DOI] [PubMed] [Google Scholar]
- 96. Laffel LMB, Wentzell K, Loughlin C, Tovar A, Moltz K, Brink S. Sick day management using blood 3‐hydroxybutyrate (3‐OHB) compared with urine ketone monitoring reduces hospital visits in young people with T1DM: a randomized clinical trial. Diabet Med. 2006;23(3):278‐284. 10.1111/j.1464-5491.2005.01771.x. [DOI] [PubMed] [Google Scholar]
- 97. Hanas R, Adolfsson P. Bolus calculator settings in well‐controlled prepubertal children using insulin pumps are characterized by low insulin to carbohydrate ratios and short duration of insulin action time. J Diabetes Sci Technol. 2017;11(2):247‐252. 10.1177/1932296816661348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chetty T, Shetty V, Fournier PA, Adolfsson P, Jones TW, Davis EA. Exercise management for young people with type 1 diabetes: a structured approach to the exercise consultation. Front Endocrinol. 2019;10:326 10.3389/fendo.2019.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kaiserman K, Buckingham BA, Prakasam G, et al. Acceptability and utility of the mysentry remote glucose monitoring system. J Diabetes Sci Technol. 2013;7(2):356‐361. 10.1177/193229681300700211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Montfort‐Steiger V, Williams CA. Carbohydrate intake considerations for young athletes. J Sport Sci Med. 2007;6:343‐352. [PMC free article] [PubMed] [Google Scholar]
- 101. Raju B, Arbelaez AM, Breckenridge SM, Cryer PE. Nocturnal hypoglycemia in type 1 diabetes: an assessment of preventive bedtime treatments. J Clin Endocrinol Metab. 2006;91(6):2087‐2092. 10.1210/jc.2005-2798. [DOI] [PubMed] [Google Scholar]
- 102. Mauras N, Xing D, Beck RW, et al. Prolonged nocturnal hypoglycemia is common during 12 months of continuous glucose monitoring in children and adults with type 1 diabetes. Diabetes Care. 2010;33(5):1004‐1008. 10.2337/dc09-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Franchini S, Comegna L, Prezioso G, Blasetti A. Hypoglycemia in children with type 1 diabetes: unawareness is a concrete risk. Curr Med Res Opin. 2016;32(9):1487‐1491. 10.1080/03007995.2016.1185400. [DOI] [PubMed] [Google Scholar]
- 104. Reddy R, El Youssef J, Winters‐Stone K, et al. The effect of exercise on sleep in adults with type 1 diabetes. Diabetes Obes Metab. 2018;20(2):443‐447. 10.1111/dom.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Taplin CE, Cobry E, Messer L, McFann K, Chase HP, Fiallo‐Scharer R. Preventing post‐exercise nocturnal hypoglycemia in children with type 1 diabetes. J Pediatr. 2010;157(5):784‐788.e1. 10.1016/j.jpeds.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bachmann S, Hess M, Martin‐Diener E, Denhaerynck K, Zumsteg U. Nocturnal hypoglycemia and physical activity in children with diabetes: new insights by continuous glucose monitoring and accelerometry. Diabetes Care. 2016;39(7):e95‐e96. 10.2337/dc16-0411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ESM Table 1 Systematic review and meta‐analysis (Pubmed/Medline)
ESM Table 2: Systematic review and meta‐analysis (Embase)
ESM Table 3: Systematic review and meta‐analysis (Cochrane)


