Abstract
Background
The values of the skin prick test (SPT) and allergen-specific IgE (sIgE) measurement in predicting dog and cat allergies remain unclear. We aimed to evaluate the usefulness of SPT and sIgE measurement in predicting self-reported allergic symptoms during exposure to dogs and cats in Korean adults.
Methods
A total of 552 participants in a pet exhibition in Korea completed questionnaires regarding exposure to dog or cat and the development of allergic symptoms during exposure. Study participants also underwent SPT using 3 different commercially available reagents, and had their blood drawn for measurement of serum total IgE and dog/cat-dander-IgE using ImmunoCAP®.
Results
Measurement of sIgE for dog and cat dander allergens provided the highest positive and negative predictive values and sensitivity, but not specificity (58%, 87.2%, 67.9%, and 93.1% for allergic symptoms on dog exposure; 64.7%, 83.2%, 74.8%, and 88.9% for those on cat exposure, respectively), in predicting self-reported allergic symptoms on dog and cat exposure. The sIgE level consistently exhibited the highest area under the receiver operating characteristic curve (0.749 and 0.719 for allergic symptoms on dog and cat exposure, respectively). Careful interpretation of SPT and sIgE measurements maximized the positive and negative predictive values, sensitivity, and specificity for predicting allergic symptoms on dog exposure (71.4%, 87.3%, 75.3%, and 99.3%) and those on cat exposure (71.4%, 85.3%, 79.3%, and 98.9%).
Conclusions
The measurement of dog and cat dander sIgE levels may be useful for the exclusion of allergic symptoms related to pet exposure. Collective interpretation of SPT and sIgE tests facilitates identification of allergic symptoms on dog or cat exposure, giving a better rule-in test result.
Keywords: Allergy, Cats, Dogs, Skin prick test, Specific IgE
Abbreviations: SPT, Skin prick test; sIgE, Allergen-specific IgE; MWD, Mean wheal diameter; A/H ratio, Allergen-to-histamine ratio; PPV, Positive predictive value; NPV, Negative predictive value; SN, Sensitivity; SP, Specificity; ROC, Receiver-operating characteristic; AUC, Area under the curve
Introduction
Domestic animals are common sources of allergens worldwide, and exposure to animal allergens is a major risk factor for allergic sensitization and subsequent development of allergic diseases.1,2 The number of pets per household, including dogs and cats, has also increased over the past decade. Furthermore, sensitization to pet allergens has also increased among patients with various allergic diseases.3 Animal allergens are small particles that can be easily spread and inhaled by humans; hence, the possibility of direct or indirect exposure to animal allergens is high, particularly among pet owners.4 Although allergen avoidance is recommended whenever possible, sufficient symptom control cannot be achieved with allergen avoidance alone; appropriate drugs or immunotherapeutic interventions for symptom relief are often required for effective control of allergic diseases.5, 6, 7 In addition to proper management of allergic diseases, the accurate diagnosis of allergies is crucial.
Skin prick test (SPT) and measurement of allergen-specific IgE (sIgE) level have been widely performed for allergy diagnosis and monitoring.8, 9, 10, 11 SPT is a rapid method that provides information on the sensitivity to individual allergens and therefore is used to diagnose respiratory allergic diseases. However, the accuracy of SPT can be influenced by various factors, including age, sex, race, concomitant drug treatments, and the SPT technique used.12, 13, 14 Allergy diagnosis can also be achieved by in vitro measurement of the sIgE level. Measurement of the sIgE level allows quantification and is not influenced by the operator technique, antihistamine intake, or underlying medical conditions.15
Since the introduction of these 2 methods into clinical practice in the late 20th century, their diagnostic performance has been evaluated and compared extensively.8,16, 17, 18, 19, 20 Nevertheless, their diagnostic value has not been established yet, especially for allergies to dogs and cats. In a previous study, only 38.8% of dog owners who suffered from allergic symptoms during exposure to their dog showed positive results for dog allergy in SPT, so did 31.1% of cat owners for cat allergy.21 In this study, using all 3 allergen extracts for SPT commercially available in Korea, we next planned to evaluate comprehensively the usefulness of SPT, as well as that of sIgE measurement, in predicting self-reported allergic symptoms on dog and cat exposure.
Methods
Study subjects
The study cohort included individuals who attended the “Korea Pet (KOPET) Show” pet exhibition from September 7–9, 2018. Individuals who were exposed to dogs and/or cats completed questionnaires regarding their exposure to these animals and the development of any allergic symptoms during exposure. Participants who had dermographism or who had administered medications that could influence the SPT results such as systemic glucocorticosteroids and tricyclic antidepressants, as well as antihistamines within a week were excluded from this study. These individuals underwent SPT for dog and cat allergens, and blood samples were collected to measure total IgE and sIgE levels induced by dog and cat dander allergens. Informed consent was obtained from all study participants.
Questionnaires regarding demographics, pet ownership, and allergic symptoms on pet exposure
Study participants completed questionnaires regarding their age, sex, presence of allergic diseases, family history of allergic diseases, development of allergic symptoms during exposure to pets, and location of pet exposure (their own home, home of relatives or friends, workplace, or public places such as pet shops, pet café, and pet hospitals). The questionnaires were modified versions of the questionnaires administered to subjects in a previous study.21 Individuals who experienced any allergic symptoms during exposure to dogs or cats were considered as symptomatic group to dogs or cats. For this study, we classified allergic symptoms on dog and cat exposure into several categories, including allergic conjunctivitis, allergic rhinitis, asthma, skin allergy, and cough. We defined allergic symptoms as follows, with a temporal relationship between the appearance of those symptoms and pet exposure: 1) those who had allergic conjunctivitis, when the subjects suffered from red, itchy eyes, edema and profuse watery discharge; 2) those who had allergic rhinitis, when the subjects suffered from one or more of watery rhinorrhea, sneezing, nasal congestion, postnasal drip, and itchy nose apart from common cold or flu; 3) those who had asthma, when the subjects suffered from dyspnea, chest discomfort and wheezing that fluctuated with nocturnal aggravation; 4) those who had skin allergy, when the subjects suffered from one or more itchy hives and rashes on their skin; and 5) those who had cough, when the subjects suffered from recurrent or long standing cough.
Skin prick test
Participants underwent SPT using dog and cat dander allergen extracts from 3 different companies (HollisterStier, Spokane, WA, USA; Lofarma, Milan, Italy; and Allergy Therapeutics, Worthing, West Sussex, UK). SPT was performed on the forearms by trained investigators using sharp-pointed lancets. Histamine solutions (10 mg/mL, HollisterStier; 1%, Lofarma; 0.1%, Allergy Therapeutics) and glycerinated saline were used as positive and negative controls, respectively. The skin test results were interpreted after 15 min by measuring the mean wheal diameter (MWD) induced by each allergen. The SPT results were regarded as positive according to 1 of 3 different conditions: a wheal of any size was induced by an allergen, the MWD was ≥3 mm, or the allergen-to-histamine ratio (A/H ratio) of MWD was ≥1.
Serologic analysis
Blood samples were collected, and plasma was isolated for sIgE analysis. IgE antibodies against dog dander extract (e5) and cat dander extract (e1) were analyzed using the ImmunoCAP® system (Thermo Fisher Scientific, Uppsala, Sweden) according to the manufacturer's instructions.22,23 Results were presented as kilounits of allergen per liter (kUA/L); the cut-off values used to determine the presence of allergen-specific IgE were 0.10, 0.35, and 3.5 kUA/L as described in previous studies.24, 25, 26
Statistical analysis
Continuous variables are expressed as means ± standard deviation or as medians (interquartile ranges), depending on their distribution. Categorical variables are expressed as absolute numbers and percentages. For continuous data, comparisons between groups were performed using Student's t-test (parametric data) or the Mann–Whitney U test (non-parametric data). For categorical data, comparisons between groups were performed using the chi-squared test. The positive predictive value (PPV), negative predictive value (NPV), sensitivity (SN), specificity (SP), and receiver operating characteristics (ROC) curve analysis, summarized by area under the curve (AUC) with 95% confidence intervals (CIs), were obtained to assess the diagnostic performance of the tests. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Two-sided p values less than 0.05 were considered statistically significant.
Results
Study population
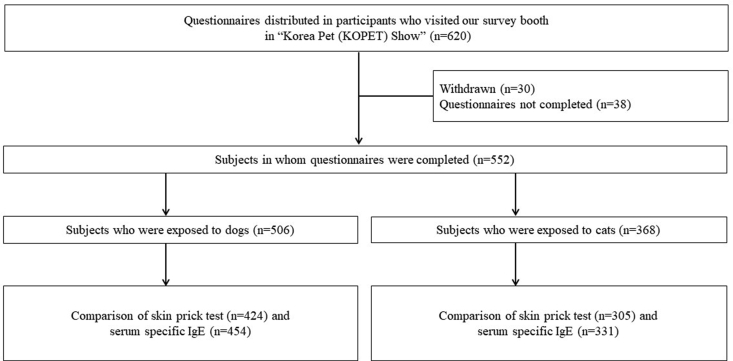
Initially, 620 KOPET exhibition participants were enrolled in our study. Of these participants, 30 (4.8%) eventually withdrew from the study, and 38 (6.1%) were excluded due to incomplete questionnaires. Thus, our analyses included data from 552 (89.1%) individuals (Fig. 1). The demographic characteristics of the study participants are shown in Table 1.
Fig. 1.
Flowchart of the study population.
Table 1.
Demographic characteristics of the study subjects.
| All subjects (n = 552) | Subjects with exposure to dogs (n = 506) |
Subjects with exposure to cats (n = 368) |
|||||
|---|---|---|---|---|---|---|---|
| Symptomatic groupa (n = 112) | Non-symptomatic group (n = 394) | P-value∗ | Symptomatic groupa (n = 125) | Non-symptomatic group (n = 243) | P-value∗ | ||
| Female | 439 (79.5) | 88 (78.6) | 317 (80.5) | 0.660 | 104 (83.2) | 192 (79.0) | 0.338 |
| Age, years | 30.2 ± 9.2 | 30.5 ± 8.7 | 29.8 ± 9.3 | 0.447 | 29.1 ± 8.1 | 28.5 ± 8.2 | 0.535 |
| Underlying allergic diseases | |||||||
| Allergic rhinitis | 245 (44.4) | 80 (71.4) | 144 (36.5) | < 0.001 | 73 (58.4) | 84 (34.6) | < 0.001 |
| Allergic conjunctivitis | 110 (19.9) | 37 (33.0) | 61 (15.5) | < 0.001 | 39 (31.2) | 42 (17.3) | 0.002 |
| Atopic dermatitis | 73 (13.2) | 23 (20.5) | 43 (10.9) | 0.008 | 24 (19.2) | 25 (10.3) | 0.017 |
| Food allergy | 59 (10.7) | 16 (14.3) | 37 (9.4) | 0.136 | 16 (12.8) | 26 (10.7) | 0.548 |
| Asthma | 42 (7.6) | 19 (17.0) | 18 (4.6) | < 0.001 | 17 (13.6) | 15 (6.2) | 0.017 |
| Chronic urticaria | 39 (7.1) | 11 (9.8) | 23 (5.8) | 0.137 | 10 (8.0) | 19 (7.8) | 0.951 |
| Drug allergy | 29 (5.3) | 11 (9.8) | 14 (3.6) | 0.007 | 9 (7.2) | 13 (5.3) | 0.478 |
| Family history of allergy | 238 (43.1) | 62 (55.4) | 157 (39.8) | 0.003 | 65 (52.0) | 87 (35.8) | 0.003 |
| Pet exposure place | |||||||
| Home | 492 (89.1) | 98 (87.5) | 307 (77.9) | 0.025 | 73 (58.4) | 93 (38.3) | < 0.001 |
| Relatives' or friends' house | 37 (6.7) | 18 (16.1) | 75 (19.0) | 0.475 | 40 (32.0) | 72 (29.6) | 0.640 |
| Workplace | 17 (3.1) | 11 (9.8) | 42 (10.7) | 0.798 | 6 (4.8) | 26 (10.7) | 0.057 |
| Pet shop, Pet café, Pet hospital | 5 (0.9) | 11 (9.8) | 57 (14.5) | 0.203 | 18 (14.4) | 74 (30.5) | 0.001 |
Data are shown as mean ± SD or frequency (%).
∗P-value < 0.05 is shown as boldface in comparing variables between subjects with allergic symptoms on dog exposure and those without it or between subjects with allergic symptoms on cat exposure and those without it.
Subjects who experienced symptoms of allergic rhinitis, allergic conjunctivitis, skin allergy, asthma and cough during exposure to dog or cat.
The study participants were aged 30.2 ± 9.2 years, and most were female (79.5%). Allergic rhinitis (44.4%) was the most common underlying allergic disease, followed by allergic conjunctivitis (19.9%), atopic dermatitis (13.2%), food allergy (10.7%), asthma (7.6%), chronic urticaria (7.1%), and drug allergy (5.3%). In total, 238 (43.1%) participants had a family history of allergic diseases. Among 506 subjects who were exposed to dogs, 112 (22.1%) experienced allergic symptoms during exposure. Among 368 individuals who were exposed to cats, 125 (33.9%) experienced allergic symptoms during exposure. There were no significant differences in sex or age between individuals with and those without allergic symptoms on dog or cat exposure. Among the study subjects who were exposed to dogs, allergic rhinitis, allergic conjunctivitis, atopic dermatitis, and asthma were more frequent among those with allergic symptoms on dog exposure than those without (71.4% vs. 36.5%, p < 0.001; 33.0% vs. 15.5%, p < 0.001; 20.5% vs. 10.9%, p = 0.008; 17.0% vs. 4.6%, p < 0.001). Similarly, among the individuals who were exposed to cats, allergic rhinitis, allergic conjunctivitis, atopic dermatitis, and asthma were more frequent among those with than those without allergic symptoms on cat exposure (58.4% vs. 34.6%, p < 0.001; 31.2% vs. 17.3%, p = 0.002; 19.2% vs. 10.3%, p = 0.017; 13.6% vs. 6.2%, p = 0.017).
Among the study participants who were exposed to dogs, drug allergy was more frequent among those with allergic symptoms on dog exposure than those without (9.8% vs. 3.6%, p = 0.007). The most common location of pet exposure was the participant's home (89.1%), followed by the home of relatives or friends (6.7%), workplaces (3.1%), and public places such as pet shops, pet cafés, and pet hospitals (0.9%). Among the subjects who were exposed to dogs, their own home was more often the place of pet exposure among those with allergic symptoms on dog exposure than those without (87.5% vs. 77.9%, p = 0.025). Similar findings were obtained among patients who were exposed to cats (58.4% vs. 38.3%, p < 0.001). Moreover, individuals with allergic symptoms on cat exposure were exposed to cats at public places less frequently compared to those without symptoms (14.4% vs. 30.5%, p = 0.001).
Allergic symptoms during exposure to dogs and cats
The allergic symptoms experienced by the study participants during exposure to dogs or cats are summarized in supplemental material (Table S1). Among 112 and 125 individuals with allergic symptoms on dog or cat exposure, the most frequent allergy that occurred during exposure to the animals was allergic rhinitis (81.3% and 80.0%, respectively), followed by allergic conjunctivitis (65.2% and 73.6%), skin allergy (55.4% and 56.0%), cough (30.4% and 29.6%), and asthma (15.2% and 16.8%).
Results of skin prick test using dog and cat dander allergen extracts
Among the 424 and 305 study participants who were exposed to dogs and cats, respectively, SPT using 3 commercially available dog and cat dander allergen extracts was conducted. As shown in Table 2, subjects with allergic symptoms on dog or cat exposure showed a significantly larger MWD and A/H ratio compared with those without symptoms, except for product B, which showed no significant difference in the MWD of dog dander allergen. The SPT positivity with 3 reagents of dog dander allergen was more frequently observed in individuals with allergic symptoms on dog exposure according to a criterion of any wheal, while the SPT positivity did not show consistent results by products according to other 2 criteria of MHD ≥3 mm, and A/H ratio ≥1. We found that the SPT positivity with all 3 reagents of cat dander allergen was more frequently observed in individuals with allergic symptoms on cat exposure according to all 3 criteria for positivity (any wheal, MHD ≥3 mm, and A/H ratio ≥1).
Table 2.
Results of SPT using dog or cat dander allergen extracts according to allergic symptoms on dog and cat exposure.
| SPT with dog allergen (n = 424) |
P-value∗ | SPT with cat allergen (n = 305) |
P-value∗ | |||
|---|---|---|---|---|---|---|
| Symptomatic groupa (n = 89) | Non-symptomatic group (n = 335) | Symptomatic groupa (n = 205) | Non-Symptomatic group (n = 100) | |||
| MWD, mm | ||||||
| Product (A) | 1.1 ± 1.6 | 0.6 ± 1.3 | 0.010 | 2.7 ± 2.8 | 1.0 ± 1.9 | < 0.001 |
| Product (B) | 0.9 ± 1.7 | 0.5 ± 1.3 | 0.061 | 1.4 ± 1.9 | 0.6 ± 1.4 | < 0.001 |
| Product (C) | 1.7 ± 2.2 | 1.2 ± 1.8 | 0.043 | 3.1 ± 3.1 | 1.4 ± 2.3 | < 0.001 |
| A/H ratio | ||||||
| Product (A) | 0.3 ± 0.6 | 0.1 ± 0.3 | 0.008 | 0.6 ± 0.7 | 0.2 ± 0.6 | < 0.001 |
| Product (B) | 0.2 ± 0.6 | 0.1 ± 0.3 | 0.018 | 0.2 ± 0.4 | 0.1 ± 0.3 | 0.004 |
| Product (C) | 0.6 ± 0.9 | 0.3 ± 0.5 | 0.003 | 0.8 ± 0.9 | 0.3 ± 0.6 | < 0.001 |
| Positivity, % | ||||||
| Positive if any wheal observed | ||||||
| Product (A) | 29 (32.6) | 60 (17.9) | 0.003 | 57 (57.0) | 52 (25.4) | < 0.001 |
| Product (B) | 24 (27.0) | 54 (16.1) | 0.019 | 41 (41.0) | 34 (16.6) | < 0.001 |
| Product (C) | 41 (46.1) | 123 (36.7) | 0.107 | 60 (60.0) | 72 (35.1) | < 0.001 |
| Positive if MHD ≥3 mm | ||||||
| Product (A) | 23 (25.8) | 42 (12.5) | 0.002 | 48 (48.0) | 44 (21.5) | < 0.001 |
| Product (B) | 15 (16.9) | 39 (11.6) | 0.190 | 30 (30.0) | 26 (12.7) | < 0.001 |
| Product (C) | 29 (32.6) | 82 (24.5) | 0.122 | 55 (55.0) | 55 (26.8) | < 0.001 |
| Positive if A/H ratio ≥1 | ||||||
| Product (A) | 8 (9.0) | 13 (3.9) | 0.057 | 26 (26.0) | 16 (7.8) | < 0.001 |
| Product (B) | 8 (9.0) | 12 (3.6) | 0.046 | 10 (10.0) | 6 (2.9) | 0.009 |
| Product (C) | 25 (28.1) | 43 (12.8) | <0.001 | 34 (34.0) | 27 (13.2) | < 0.001 |
Data are shown as mean ± SD or frequency (%).
∗P-value < 0.05 is shown as boldface in comparing variables between subjects with allergic symptoms on dog exposure and those without it or between subjects with allergic symptoms on cat exposure and those without it. SPT, SPT, skin prick test; MWD, mean wheal diameter provoked by allergen in skin prick test; A/H ratio, allergen-to-histamine mean wheal diameter ratio.
Subjects who experienced symptoms of allergic rhinitis, allergic conjunctivitis, skin allergy, asthma, and cough during exposure to dog or cat
Serum levels of total IgE and dog/cat-dander-specific IgE
To measure serum levels of total IgE and sIgE, we collected blood samples from 454 to 331 study participants who were exposed to dogs and cats, respectively. Serum levels of total IgE were higher in individuals with allergic symptoms on dog exposure (146.60 [57.58–368.43] (median, [quartile]) vs. 71.40 [27.90–157.43] kUA/L, p < 0.001) and those on cat exposure (120.40 [33.40–281.70] vs. 79.85 [28.35–184.75] kUA/L, p = 0.043) compared with those without symptoms. Furthermore, serum levels of dog-dander-specific IgE were higher in subjects with than in those without symptoms (1.33 [0.05–7.06] vs. 0.02 [0.01–0.22] kUA/L, p < 0.001). Similarly, subjects with allergic symptoms on cat exposure had higher cat-dander-specific IgE levels compared with those without symptoms (1.58 [0.09–8.89] vs. 0.01 [0.00–0.42] kUA/L, p < 0.001; Table 3). Moreover, the number of individuals positive for dog- or cat-dander-specific IgE was higher among those with than those without allergic symptoms on dog or cat exposure; this was true for all 3 cut-off values (0.1, 0.35, or 3.5 kUA/L; p < 0.001 for all).
Table 3.
Serum levels of dog- and cat-specific IgE according to allergic symptoms on dog and cat exposure.
| Dog-specific IgE (n = 454) |
P-value∗ | Cat-specific IgE (n = 331) |
P-value∗ | |||
|---|---|---|---|---|---|---|
| Symptomatic groupa (n = 106) | Non-symptomatic group (n = 348) | Symptomatic groupa (n = 115) | Non-symptomatic group (n = 216) | |||
| Dog or cat dander-specific IgE | ||||||
| Concentration, kUA/L | 1.33 [0.05–7.06] | 0.02 [0.01–0.22] | < 0.001 | 1.58 [0.09–8.89] | 0.01 [0.00–0.42] | < 0.001 |
| Positivity, number (%) | ||||||
| Positive, if sIgE ≥0.1 kUA/L | 72 (67.9) | 116 (33.3) | < 0.001 | 86 (74.8) | 72 (33.3) | < 0.001 |
| Positive, if sIgE ≥0.35 kUA/L | 66 (62.3) | 76 (21.8) | < 0.001 | 74 (64.3) | 57 (26.4) | < 0.001 |
| Positive, if sIgE ≥3.5 kUA/L | 34 (32.1) | 24 (6.9) | < 0.001 | 44 (38.3) | 24 (11.1) | < 0.001 |
Data are shown as median [interquartile range] or frequency (%).
∗P-value < 0.05 is shown as boldface in comparing variables between subjects with allergic symptoms on dog exposure and those without it or between subjects with allergic symptoms on cat exposure and those without it.
Subjects who experienced symptoms of allergic rhinitis, allergic conjunctivitis, skin allergy, asthma, and cough during exposure to dog or cat
Diagnostic values of SPT and the sIgE level for allergic symptoms on pet exposure
The diagnostic values of SPT (dog [n = 424] and cat [n = 305]) and the sIgE level (n = 454 and n = 331) for allergic symptoms on pet exposure are shown in Table 4. The highest NPV, PPV, and SN were presented in specific IgE measurements (87.2%, 58.6%, and 67.9% for allergic symptoms on dog exposure; 83.2%, 64.7%, and 74.8% for those on cat exposure), while the highest SP were noted in SPT (96.4% for allergic symptoms on dog exposure; 97.1% for those on cat exposure). Combined sIgE and SPT results showed increased NPV and SN when at least 1 of the following 2 conditions were fulfilled: (1) a wheal of any size induced by dog and cat allergen in SPT, (2) dog and cat-dander-specific IgE level ≥ 0.01 kUA/L. They also showed increased PPV and SP when both of the following 2 conditions were fulfilled: (1) A/H ratio ≥ 1 in SPT using dog and cat allergen, (2) dog and cat-dander-specific IgE level ≥ 3.5 kUA/L.
Table 4.
Diagnostic values of SPTs and sIgE in prediction of allergic symptoms on dog and cat exposure.
| Dog allergy |
PPV% |
NPV% |
SN% |
SP% |
| SPT (n = 424) | ||||
| Positive if allergen provoked any wheal | ||||
| (A) | 32.6 | 82.1 | 32.6 | 82.1 |
| (B) | 30.8 | 81.2 | 27.0 | 83.9 |
| (C) | 25.0 | 81.5 | 46.1 | 63.3 |
| Positive if MWD ≥ 3 mm | ||||
| (A) | 35.4 | 81.6 | 25.8 | 87.5 |
| (B) | 27.8 | 80.0 | 16.9 | 88.4 |
| (C) | 26.1 | 80.8 | 32.6 | 75.5 |
| Positive if A/H ratio ≥ 1 | ||||
| (A) | 38.1 | 79.9 | 9.0 | 96.1 |
| (B) | 40.0 | 80.0 | 9.0 | 96.4 |
| (C) | 36.8 | 82.0 | 28.1 | 87.2 |
| sIgE (n = 454) | ||||
| Positive if sIgE for dog dander ≥ 0.1 kUA/L | 38.3 | 87.2 | 67.9 | 66.7 |
| Positive if sIgE for dog dander ≥ 0.35 kUA/L | 46.5 | 87.2 | 62.3 | 78.2 |
| Positive if sIgE for dog dander ≥ 3.5 kUA/L | 58.6 | 81.8 | 32.1 | 93.1 |
| SPT or sIgE (n = 380) | ||||
| Positive if sIgE for dog dander ≥ 0.1 kUA/L | ||||
| or any wheal in SPT with Product A | 34.7 | 87.3 | 68.2 | 63.1 |
| or any wheal in SPT with Product B | 34.5 | 87.0 | 67.1 | 63.4 |
| or any wheal in SPT with Product C | 29.8 | 87.3 | 75.3 | 48.8 |
| Positive if sIgE for dog dander ≥ 3.5 kUA/L | ||||
| and A/H ratio ≥ 1 with Product A | 71.4 | 78.6 | 5.9 | 99.3 |
| and A/H ratio ≥ 1 with Product B | 40 | 77.9 | 2.4 | 99.0 |
| and A/H ratio ≥ 1 with Product C |
52.9 |
79.1 |
10.6 |
97.3 |
| Cat allergy |
PPV% |
NPV% |
SN% |
SP% |
| SPT (n = 305) | ||||
| Positive if allergen provoked any wheal | ||||
| (A) | 52.3 | 78.1 | 57.0 | 74.6 |
| (B) | 54.7 | 74.3 | 41.0 | 83.4 |
| (C) | 45.5 | 76.9 | 60.0 | 64.9 |
| Positive if MWD ≥ 3 mm | ||||
| (A) | 52.2 | 75.6 | 48.0 | 78.5 |
| (B) | 53.6 | 71.9 | 30.0 | 87.3 |
| (C) | 50.0 | 76.9 | 55.0 | 73.2 |
| Positive if A/H ratio ≥ 1 | ||||
| (A) | 61.9 | 71.9 | 26.0 | 92.2 |
| (B) | 62.5 | 68.9 | 10.0 | 97.1 |
| (C) | 55.7 | 73.0 | 34.0 | 86.8 |
| sIgE (n = 331) | ||||
| Positive if sIgE for cat dander ≥0.1 kUA/L | 54.4 | 83.2 | 74.8 | 66.7 |
| Positive if sIgE for cat dander ≥0.35 kUA/L | 56.5 | 79.5 | 64.3 | 73.6 |
| Positive if sIgE for cat dander ≥3.5 kUA/L | 64.7 | 73.0 | 38.3 | 88.9 |
| Skin prick test and sIgE (n = 274) | ||||
| Positive if sIgE for cat dander ≥ 0.1 kUA/L | ||||
| or any wheal in SPT with Product A | 50.3 | 85.3 | 79.3 | 60.4 |
| or any wheal in SPT with Product B | 51.5 | 84.1 | 76.1 | 63.7 |
| or any wheal in SPT with Product C | 45.3 | 83.2 | 79.3 | 51.6 |
| Positive if sIgE for cat dander ≥ 3.5 kUA/L | ||||
| and A/H ratio ≥ 1 with Product A | 56.3 | 67.8 | 9.8 | 96.2 |
| and A/H ratio ≥ 1 with Product B | 71.4 | 67.4 | 5.4 | 98.9 |
| and A/H ratio ≥ 1 with Product C | 63.0 | 69.6 | 18.5 | 94.5 |
The highest values of PPV, NPV, SN and SP in SPT, sIgE measurement and their combination are shown as boldfaces. SPT, skin prick test; PPV, Positive predictive values; NPV, negative predictive values; SN, sensitivity; SP, specificity; MWD, mean wheal diameter provoked by allergen
Abilities of total IgE and sIgE levels and skin prick test to predict allergic symptoms on dog or cat exposure
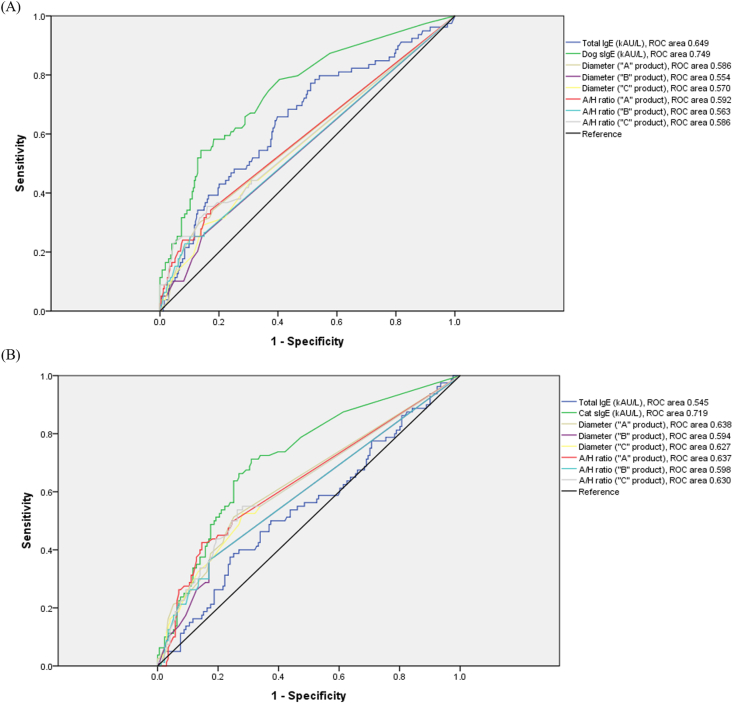
The abilities of total IgE and sIgE levels and SPT to predict allergic symptoms on dog or cat exposure were assessed by ROC analysis (Fig. 2). Dog-dander-specific IgE measurements resulted in the highest AUC (0.749; 95% CI, 0.687–0.812; p < 0.001). When cutoff value was optimized to 0.590 kUA/L, SN and SP were 54.4% and 86.1%, respectively. Meanwhile, total IgE measurements resulted in an AUC of 0.649 (95% CI, 0.578–0.719; p < 0.001). Moreover, the AUC values for MWD and A/H ratio in SPT were lower than those for total IgE measurements, regardless of the allergen extract used (product A, B, or C). Similarly, cat-dander-specific IgE measurements also resulted in the highest AUC (0.719; 95% CI, 0.652–0.787; p < 0.001). When cutoff value was optimized to 0.085 kUA/L, SN and SP were 71.3% and 69.0%. Meanwhile, total IgE resulted in an AUC of 0.545 (95% CI, 0.467–0.622; p = 0.254). AUC values for MWD and A/H ratio were between those for cat-dander-specific sIgE and total IgE.
Fig. 2.
ROC curve assessments of the sensitivity and specificity of total IgE, specific IgE and SPT in predicting allergic symptoms during exposure to dog (A) and cat (B). ROC curve, receiver operating characteristic curve; SPT, skin prick test; MWD, mean wheal diameter provoked by allergen in skin prick test; A/H ratio, allergen-to-histamine mean wheal diameter ratio
Discussion
In this study, we performed a cross-sectional survey and evaluated the usefulness of in vitro assays as diagnostic tools for predicting allergic symptoms on dog and cat exposure. In Korea, pet ownership has increased drastically in recent years. In 2018, more than a quarter of all households owned pets, with dogs and cats being the most common companion animals.27 The increased popularity of pets could lead to an increase in allergen exposure and subsequent development of allergic diseases.
We showed that individuals with allergic symptoms during pet exposure suffered from underlying allergic conditions and frequently had a family history of allergic diseases. These results are consistent with previous studies reporting an association between pet allergies and underlying allergic diseases or atopic inheritance.21,28,29 We found that 22.1% and 34.0% of individuals who were exposed to dogs and cats suffered from allergic symptom on exposure, respectively; these findings are in line with previous studies reporting a 20% incidence rate of dog and cat allergies worldwide.1 The results of our questionnaires revealed a high frequency of self-reported symptoms, such as nasal, ocular, and skin allergic symptoms, which accounted for more than 50% of symptomatic individuals. We found that the results of SPT were variable depending on different manufacturers. Consistently, previous studies reported differences in allergenic potency and major allergen concentrations among different SPT reagents, leading to inconsistent results.30 Different manufacturers use different processes and raw materials to create crude extracts with uncharacterized quality and composition. Therefore, the IgE-binding capacity of these products can vary immensely.17,31 Importantly, a channel-activating protease inhibition study demonstrated that the total allergen potencies per protein content of dog and cat allergen extracts used in SPT differ among companies. In contrast, less variation was observed in other allergens, such as house dust mite, oak, ragweed, and Japanese hop.17 Furthermore, SPT reproducibility is influenced by the technique or prick test device used, as well as the criteria used to interpret skin reaction results.12, 13, 14 Hence, SPT suffers from low reproducibility and uncertain clinical relevance. Based on the history of exposure to pets and clinical symptoms, we found that sIgE measurements provided higher overall SN, PPV, and NPV compared with SPT. Consistently, a previous study reported that the UniCAP® (also known as ImmunoCAP®) system was more sensitive than SPT.18 In line with these findings, our ROC analysis suggested that dog- and cat-dander-specific sIgE levels had higher diagnostic values, with AUC values of 0.75 and 0.72, respectively. Our analyses demonstrated that dog and cat-dander-specific IgE/total IgE ratio are similar to sIgE levels in predicting self-reported allergic symptoms on dog and cat exposure (data not shown). Moreover, the AUCs for dog dander SPT were even lower than those for serum total IgE measurements, irrespective of the allergen product used in SPT. The use of ImmunoCAP® to measure circulating sIgE levels in serum or plasma can reliably identify sIgE antibody levels as low as 0.1 kUA/L. Measurement of the sIgE level using 0.1 kUA/L as the cut-off value showed high SN. Collective interpretation of SPTs (any wheal provoked by dog or cat dander allergen) or sIgE assays (dog or cat dander-specific IgE ≥0.01 kUA/L) further improved SN and NPV (75.3% and 87.3% for allergic symptoms on dog exposure, 85.3% and 85.3% for those on cat exposure). In contrast, another strategy using SPTs (A/H ratio ≥1 in SPT with dog or cat dander-allergen) and sIgE assays (dog or cat dander-specific IgE ≥ 3.5 kUA/L) showed very high SP (99.3% and 98.9% for allergic symptoms on dog and cat exposure, respectively) and PPV (71.4% for allergic symptoms on dog and cat exposure, respectively) but low SN (10.6% and 18.5%) and NPV (79.1% and 69.6%). Hence, with higher NPV and SN compared to SPT, serum sIgE measurements using cutoff value of 0.1 kUA/L may be more useful to exclude patients who do not warrant further investigation and who can reliably be advised that allergen avoidance is neither necessary nor helpful. When we collectively interpret results of SPT and sIgE measurement, enhanced PPV and SP may help us to identify patients with allergic symptom on pet exposure, giving a better rule-in test result.
This study has several limitations. First, our study relied on self-reported pet exposure and allergic symptoms. Thus, the possibility of reporting bias cannot be excluded, particularly for study participants whose allergies were not confirmed by doctors. Second, we could not distinguish false-positive results from asymptomatic sensitization to pet allergens. Since asymptomatic sensitization to pet allergens is a risk factor for later development of allergies, this might have introduced some bias in our results. Hence, longitudinal studies evaluating the development of pet allergies are required to confirm our findings. Third, the results of SPT and sIgE measurements reported herein might have been influenced by contamination by allergens from other sources or by cross-reactivity between dog and cat allergens.32, 33, 34, 35 Additionally, allergic symptoms can be influenced by multiple environmental factors other than exposure to allergens. Therefore, positive results in SPT or sIgE measurements do not necessarily indicate the presence of pet allergies. Provocation tests are required to confirm the presence of pet allergies. However, provocation tests are neither well-validated nor standardized. Additionally, allergen extract heterogeneity exists due to differences in collection site and allergen composition, compromising assay accuracy and reproducibility, even among products from the same company.36,37 Finally, study participants whose allergies were not confirmed by doctors were more likely to overestimate the actual prevalence of pet allergies.
Despite these limitations, in this study, we evaluated the presence of allergic symptoms on dog and cat exposure by comprehensively evaluating various allergic symptoms during exposure to pets, measuring serum total IgE and sIgE levels and performing SPT using 3 different commercially available allergen products. Moreover, to ensure a more accurate assessment of the diagnostic significance of these tests, we used various criteria to determine positivity in SPT and sIgE measurements and performed a collective interpretation of the two tests.
In conclusion, the measurement of dog and cat dander sIgE is more useful than SPT for the exclusion of allergic symptoms related to pet exposure. Careful interpretation of both sIgE measurements and SPT results can provide a more accurate prediction of allergic symptoms on dog or cat exposure. Considering that pet ownership and animal allergen exposure are continuously on the rise, the establishment of accurate diagnostic tests and interpretation methods can improve the diagnosis and management of dog and cat allergies.
Ethics approval
The protocol of this study was reviewed and approved by the institutional review board (IRB approval number: GAIRB2018-194).
Funding
This research was supported by a grant from the Korean Academy of Asthma, Allergy, and Clinical Immunology (2018), and from Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015R1D1A1A02061943).
Author details
SYK, MSY, SHK and SML designed the study, collected and analyzed the data, and wrote the manuscript. SYP, JHK, HKW, OYK, JHL, YWK, JWJ, WJS and SPL contributed to the design of the study, collection and interpretation of data and revision of the draft critically for important intellectual content. All authors read and approved the final manuscript.
Consent for publication
We hereby declare that we all participated in the study and in the development of the manuscript titled “The role of allergen-specific IgE in predicting self-reported dog and cat allergies among Korean pet exhibition participants”. We have read the final version and give our consent for the article to be published in the World Allergy Organization Journal.
Availability of data and materials
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Declaration of competing interest
The authors have no conflict of interest to declare.
Acknowledgement
We thank Thermo Fisher Scientific for support in measuring serum allergen-specific IgE through ImmunoCAP® system. We are especially grateful to Hyun Ju Im, Jung Won Kim, Hyun Ae Kim, Jung Mi Ryu, Hyun Jung Park, Ji Yeon Suh, Hye Ryun Song, Young Rim Yoo, Jung Cheol Chang, Myung Ja Lee and Yeo Jin Choe for their devotion to our study.
Footnotes
Full list of author information is available at the end of the article https://doi.org/10.1016/j.waojou.2020.100488.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2020.100488.
Contributor Information
Sae-Hoon Kim, Email: shkrins@gmail.com.
Sang Min Lee, Email: sangminlee77@naver.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Chan S.K., Leung D.Y.M. Dog and cat allergies: current state of diagnostic approaches and challenges. Allergy Asthma Immunol Res. 2018;10:97–105. doi: 10.4168/aair.2018.10.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zahradnik E., Raulf M. Respiratory allergens from furred mammals: environmental and occupational exposure. Vet Sci. 2017;4 doi: 10.3390/vetsci4030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park B.W., Park J.Y., Cho E.B., Park E.J., Kim K.H., Kim K.J. Increasing prevalence of the sensitization to cat/dog allergens in Korea. Ann Dermatol. 2018;30:662–667. doi: 10.5021/ad.2018.30.6.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zahradnik E., Raulf M. Animal allergens and their presence in the environment. Front Immunol. 2014;5:76. doi: 10.3389/fimmu.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karlsson A.S., Andersson B., Renstrom A., Svedmyr J., Larsson K., Borres M.P. Airborne cat allergen reduction in classrooms that use special school clothing or ban pet ownership. J Allergy Clin Immunol. 2004;113:1172–1177. doi: 10.1016/j.jaci.2003.12.590. [DOI] [PubMed] [Google Scholar]
- 6.Ling M., Long A.A. Pet dander and difficult-to-control asthma: therapeutic options. Allergy Asthma Proc. 2010;31:385–391. doi: 10.2500/aap.2010.31.3390. [DOI] [PubMed] [Google Scholar]
- 7.Wallace D.V. Pet dander and perennial allergic rhinitis: therapeutic options. Allergy Asthma Proc. 2009;30:573–583. doi: 10.2500/aap.2009.30.3286. [DOI] [PubMed] [Google Scholar]
- 8.Asha'ari Z.A., Suhaimi Y., Yusof R.A., Rushdan I., Maraina C.H. Comparison of serum specific IgE with skin prick test in the diagnosis of allergy in Malaysia. Med J Malaysia. 2011;66:202–206. [PubMed] [Google Scholar]
- 9.de Vos G. Skin testing versus serum-specific IgE testing: which is better for diagnosing aeroallergen sensitization and predicting clinical allergy? Curr Allergy Asthma Rep. 2014;14:430. doi: 10.1007/s11882-014-0430-z. [DOI] [PubMed] [Google Scholar]
- 10.Nam Y.H., Lee S.K. Comparison between skin prick test and serum immunoglobulin E by CAP system to inhalant allergens. Ann Allergy Asthma Immunol. 2017;118:608–613. doi: 10.1016/j.anai.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Visitsunthorn N., Sripramong C., Bunnag C., Jirapongsananuruk O. Comparison between specific IgE levels and skin prick test results of local and imported American cockroach, dog, cat, dust mites and mold allergen extracts. Asian Pac J Allergy Immunol. 2017;35:60–65. doi: 10.12932/AP0745. [DOI] [PubMed] [Google Scholar]
- 12.Bordignon V., Burastero S.E. Age, gender and reactivity to allergens independently influence skin reactivity to histamine. J Investig Allergol Clin Immunol. 2006;16:129–135. [PubMed] [Google Scholar]
- 13.Masse M.S., Granger Vallee A., Chiriac A. Comparison of five techniques of skin prick tests used routinely in Europe. Allergy. 2011;66:1415–1419. doi: 10.1111/j.1398-9995.2011.02679.x. [DOI] [PubMed] [Google Scholar]
- 14.Wagner N., Rudert M. Sensitivity and specificity of standardised allergen extracts in skin prick test for diagnoses of IgE-mediated respiratory allergies. Clin Transl Allergy. 2019;9:8. doi: 10.1186/s13601-019-0248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton R.G., Franklin Adkinson N., Jr. In vitro assays for the diagnosis of IgE-mediated disorders. J Allergy Clin Immunol. 2004;114:213–225. doi: 10.1016/j.jaci.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 16.Crimi E., Brusasco V., Crimi P., Brancatisano M., Bregante A. The fluoroallergosorbent test: a comparison with rast and skin test in respiratory allergy. Ann Allergy. 1988;61:371–374. [PubMed] [Google Scholar]
- 17.Lee S.C., Sim D.W., Lee J. Comparison between newly developed and commercial inhalant skin prick test reagents using in vivo and in vitro methods. J Kor Med Sci. 2018;33 doi: 10.3346/jkms.2018.33.e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricci G., Capelli M., Miniero R. A comparison of different allergometric tests, skin prick test, Pharmacia UniCAP and ADVIA Centaur, for diagnosis of allergic diseases in children. Allergy. 2003;58:38–45. doi: 10.1034/j.1398-9995.2003.23761.x. [DOI] [PubMed] [Google Scholar]
- 19.Wongpiyabovorn J., Suratannon N., Boonmee S., Chatchatee P. Comparison of specific IgE detection by immunoblotting and fluorescence enzyme assay with in vivo skin prick test. Asian Pac J Allergy Immunol. 2018;36:159–165. doi: 10.12932/AP-270217-0035. [DOI] [PubMed] [Google Scholar]
- 20.Wuthrich B., Arrendal H. RAST in the diagnosis of hypersensitivity to dog and cat allergens. A comparison of different extract preparations with clinical history, skin test and provocation tests. Clin Allergy. 1979;9:191–200. doi: 10.1111/j.1365-2222.1979.tb01541.x. [DOI] [PubMed] [Google Scholar]
- 21.Yang M.S., Lee S.P., Kwon Y.J., Lee S.M. Dog and cat allergies and allergen avoidance measures in Korean adult pet owners who participated in a pet exhibition. Allergy Asthma Immunol Res. 2018;10:155–164. doi: 10.4168/aair.2018.10.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Hage M., Hamsten C., Valenta R. ImmunoCAP assays: pros and cons in allergology. J Allergy Clin Immunol. 2017;140:974–977. doi: 10.1016/j.jaci.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Wang J., Godbold J.H., Sampson H.A. Correlation of serum allergy (IgE) tests performed by different assay systems. J Allergy Clin Immunol. 2008;121:1219–1224. doi: 10.1016/j.jaci.2007.12.1150. [DOI] [PubMed] [Google Scholar]
- 24.Arroyave W.D., Rabito F.A., Carlson J.C. The relationship between a specific IgE level and asthma outcomes: results from the 2005-2006 National Health and Nutrition Examination Survey. J Allergy Clin Immunol Pract. 2013;1:501–508. doi: 10.1016/j.jaip.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Burnett M., Wegienka G., Havstad S. Relationship of dog- and cat-specific IgE and IgG4 levels to allergic symptoms on pet exposure. J Allergy Clin Immunol Pract. 2013;1:350–353. doi: 10.1016/j.jaip.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kack U., Asarnoj A., Gronlund H. Molecular allergy diagnostics refine characterization of children sensitized to dog dander. J Allergy Clin Immunol. 2018;142:1113–1120 e1119. doi: 10.1016/j.jaci.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Apostolovic D., Sanchez-Vidaurre S., Waden K. The cat lipocalin Fel d 7 and its cross-reactivity with the dog lipocalin Can f 1. Allergy. 2016;71:1490–1495. doi: 10.1111/all.12955. [DOI] [PubMed] [Google Scholar]
- 28.Bener A., Mobayed H., Sattar H.A., Al-Mohammed A.A., Ibrahimi A.S., Sabbah A. Pet ownership: its effect on allergy and respiratory symptoms. Eur Ann Allergy Clin Immunol. 2004;36:306–310. [PubMed] [Google Scholar]
- 29.Gergen P.J., Mitchell H.E., Calatroni A. Sensitization and exposure to pets: the effect on asthma morbidity in the US population. J Allergy Clin Immunol Pract. 2018;6:101–107. doi: 10.1016/j.jaip.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pagani M., Antico A., Cilia M. Comparison of different diagnostic products for skin prick testing. Eur Ann Allergy Clin Immunol. 2009;41:23–31. [PubMed] [Google Scholar]
- 31.Calderon M.A., Larenas D., Kleine-Tebbe J. European Academy of Allergy and Clinical Immunology task force report on 'dose-response relationship in allergen-specific immunotherapy. Allergy. 2011;66:1345–1359. doi: 10.1111/j.1398-9995.2011.02669.x. [DOI] [PubMed] [Google Scholar]
- 32.Apostolovic D., Sanchez-Vidaurre S., Waden K. The cat lipocalin Fel d 7 and its cross-reactivity with the dog lipocalin Can f 1. Allergy. 2016;71:1490–1495. doi: 10.1111/all.12955. [DOI] [PubMed] [Google Scholar]
- 33.Madhurantakam C., Nilsson O.B., Uchtenhagen H. Crystal structure of the dog lipocalin allergen Can f 2: implications for cross-reactivity to the cat allergen Fel d 4. J Mol Biol. 2010;401:68–83. doi: 10.1016/j.jmb.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 34.van der Veen M.J., Mulder M., Witteman A.M. False-positive skin prick test responses to commercially available dog dander extracts caused by contamination with house dust mite (Dermatophagoides pteronyssinus) allergens. J Allergy Clin Immunol. 1996;98:1028–1034. doi: 10.1016/s0091-6749(96)80187-4. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto K., Ishibashi O., Sugiura K. Crystal structure of the dog allergen Can f 6 and structure-based implications of its cross-reactivity with the cat allergen Fel d 4. Sci Rep. 2019;9:1503. doi: 10.1038/s41598-018-38134-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agache I., Bilo M., Braunstahl G.J. In vivo diagnosis of allergic diseases--allergen provocation tests. Allergy. 2015;70:355–365. doi: 10.1111/all.12586. [DOI] [PubMed] [Google Scholar]
- 37.Wintersand A., Asplund K., Binnmyr J. Allergens in dog extracts: implication for diagnosis and treatment. Allergy. 2019;74:1472–1479. doi: 10.1111/all.13785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.