Abstract
Dantrolene, an FDA approved drug to treat malignant hyperthermia and muscle spasm, has been demonstrated to inhibit severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mediated toxicity of host cells. Ryanodine receptor overactivation and associated disruption of intracellular Ca2+ homeostasis play important roles in SARS-CoV-2 infection and replication of host cells. Dantrolene, as an inhibitor of RyRs, is expected to ameliorate these detrimental effects of SARS-CoV-2 in host cells. Additionally, dantrolene has also been shown to inhibit multiple cell or organ damage induced by hypoxia/ischemia, mitochondria damage, oxidative stresses, inflammation, impairment of autophagy and apoptosis, etc., which are often the causes of severity and mortality of COVID-19 patients. We have repurposed that dantrolene has a high potential at treating COVID-19 patients and reducing its morbidity and mortality.
Keywords: SARS-CoV-2, COVID-19, Infection, Replication, Dantrolene
Introduction
The epidemic of coronavirus disease 2019 (COVID-19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has lasted more than half a year with varied population-level case fatality ratio ranged between 2–8%1, though much lower after adjusting for demography and under-ascertainment, which is still higher in aged groups (≥60 years: 6·4%, ≥80 years: 13·4%)1. Nevertheless, there is still a lack of powerful drugs to treat COVID-19 patients, even with the promising drug Remdesivir, a nucleotide analog with broad-spectrum antiviral activity2. Furthermore, randomized clinical trials have also shown disappointing findings of other drugs, including hydroxychloroquine3 and lopinavir-ritonavir4. In the setting of the absence of robust drug and vaccine, it may be beneficial to develop drugs that can reduce the infection and replication of SARS-CoV-2 and severity of the symptoms5, protect the organs, ameliorate the deterioration6 and reduce mortality in the critically ill COVID-19 patients7. Considering its plausible ability to inhibit SARS-CoV-2 virus cytotoxicity of host cells8, cytoprotection9, and organ protection10 in a wide variety of models of stress and disease, we propose that dantrolene, an FDA approved drug to treat malignant hyperthermia and muscle spasm, could be repurposed as a potential adjuvant drug for the treatment of COVID-19 patients.
1. Potential and Proposed Mechanisms of dantrolene to inhibit SARS-CoV-2 Infection and/or Replication in the Host Cells
Infection and replication of SARS-CoV-2 (Figure 1) in the host cells initially require binding of the S1 domain of the virus spike protein (S protein) to angiotensin-converting enzyme 2 (ACE2) on the plasma membrane, followed by fusion with the plasma membrane mediated by S2 domain of S protein to make its entry11,12.
Figure 1.
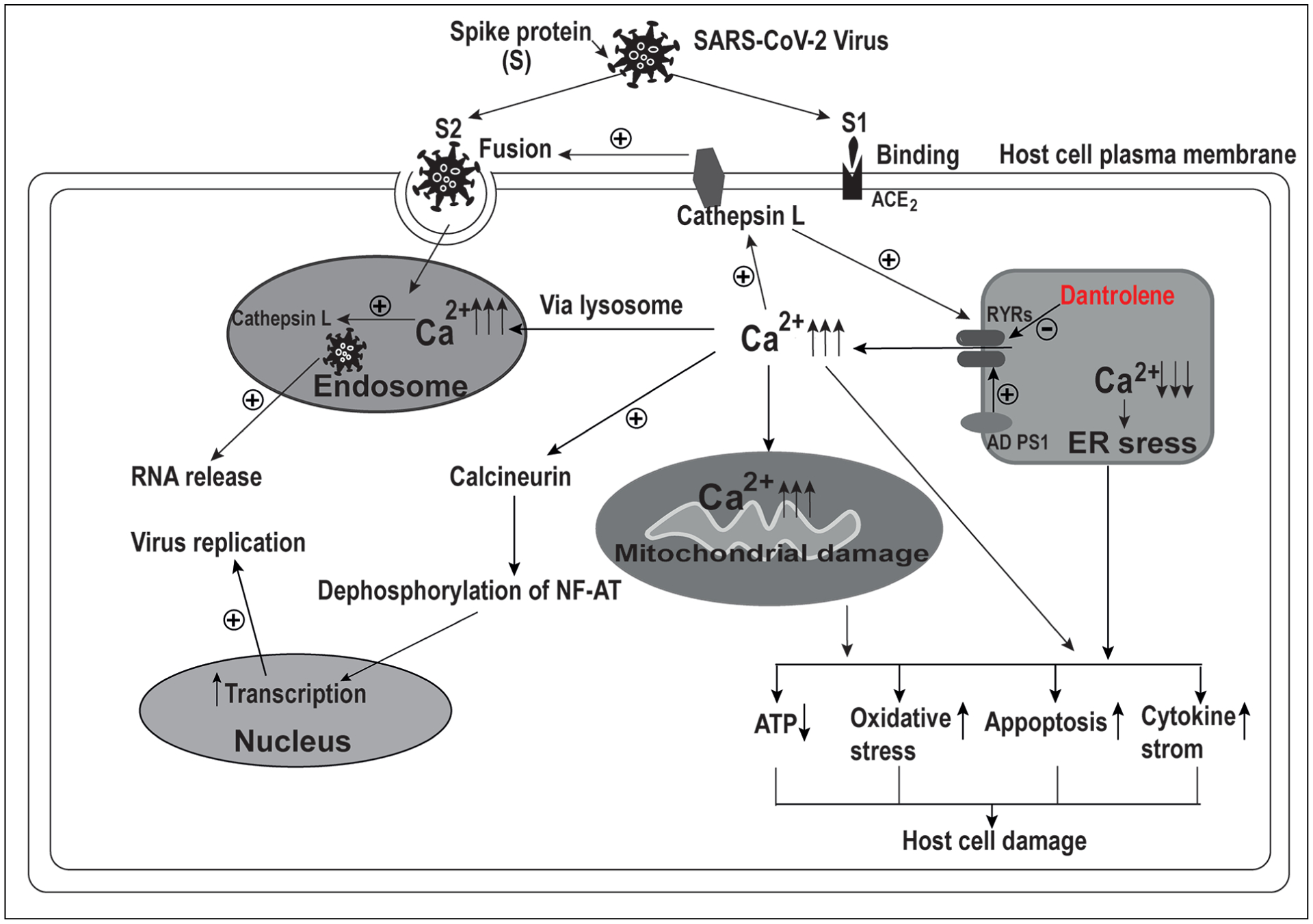
Dantrolene might inhibit infection and replication of SARS-CoV-2 and associated pathology. Cathepsin L, a protease on the plasma membrane of host cells, increases Ca2+ release from the endoplasmic reticulum (ER) via the ryanodine receptors (RyRs). The associated elevation of cytosolic Ca2+ concentration, in turn, increases cathepsin L activity. Cathepsin L promotes virus fusion with host cells by cleaving and activating the spike (S) protein. High levels of extracellular and cytosolic Ca2+ concentrations are also necessary for virus fusion and endocytosis. Cathepsin L in the endosome, under the condition of a high level of Ca2+ concentration, promotes virus RNA release into the cytosol. On the other hand, the increased cytosolic Ca2+ concentration due to the overactivation of RyRs activates calcineurin, which dephosphorylates NF-AT and translocates into the nucleus for promoting transcription and virus replication. Excess Ca2+ release from ER via overactivation of RyRs in AD cells results in depletion of ER Ca2+ and associated ER stress, as well as the overloading of mitochondria with Ca2+ and associated mitochondria damage. All of the above pathologies eventually result in impaired ATP production, oxidative stress damage, apoptosis, and cytokine storm, leading to final host cell damage. Dantrolene inhibits the infection and replication of the SARS-CoV-2 virus and host cell damage by inhibiting abnormal and excessive activation of RyRs and restoring the intracellular Ca2+ homeostasis.
The cleavage and activation of S protein by protease, especially cathepsin L13, provides a preliminary priming step of these enveloped viruses14. Meanwhile, cathepsin L promotes activation of the ryanodine receptors (RyRs)15, which results in an abnormal increase in cytosolic Calcium ions (Ca2+) concentration heightening the activity of Ca2+-dependent cathepsin L16. Further, the endosomes containing the virus enter cytosol via endocytosis12, and the high Ca2+ concentration in the mature endosome activates cathepsin L14,17. These processes finally release the virus RNA into the cytosol. Dantrolene inhibits the abnormal and excessive activation of RyRs and restores the intracellular Ca2+ homeostasis9, which breaks the pathological feedback between the cathepsin L and the Ca2+ and prevents the entry of the virus.
It was suggested that S-mediated membrane fusion was Ca2+-dependent (Figure 1)18. The Ca2+ binding to fusion peptides via conserved negatively charged residues are required to trigger the fusion process18. To promote fusion, the virus needs additional Ca2+ which is imported from the ER via RyRs (Figure 1) to the endosomes. So it is intriguing to note that amiodarone, a drug that blocks endosomal/lysosomal Ca2+ channels, inhibits SARS-CoV entry after endosomal uptake19. Commonly used Ca2+ channel blockers showed therapeutic effects in COVID-19 patients20. Dantrolene may inhibit calcium influx from extracellular space and elevation of cytosolic Ca2+ primarily by reducing the capacitive calcium entry (CCE). The ability of dantrolene to inhibit L-type Ca2+ channel or NMDA glutamate receptor is not fully clear. Therefore, it is not surprising to demonstrate that SARS-CoV entry was inhibited by Ca2+ chelators such as BAPTA-AM at the cytosol and endosomes18. The critical initiation of infection and subsequent virus replication depends on the presence of Ca2+, especially the intracellular Ca2+ concentration14,18. Dantrolene is expected to ameliorate SARS-CoV-2 mediated over activation of RyRs and associated disruption of intracellular Ca2+ homeostasis (Figure 1)9. These effects, in turn, are expected to inhibit the SARS-CoV-2 virus infection of the host cells. Likewise, inspiring news demonstrated the in vitro antiviral cytotoxicity activity of dantrolene against SARS-CoV-2, in clinically relevant concentrations and duration, with minimal cytotoxicity of dantrolene itself8. Furthermore, the abnormal increase in cytosolic Ca2+ concentration via over activation of RyRs on the ER membrane enhances the calcineurin’s activity, which promotes NF-AT nucleus translocation and transcription, leading to the subsequent promotion of virus replication in the cytosol (Figure 1)16. So, as an antagonist of RyRs, dantrolene is theoretically expected to inhibit the replication of SARS-CoV-2, although it needs to be investigated in future studies.
2. Proposed Mechanisms of Dantrolene to Reduce Cell Stress and Damage
1). Dantrolene Reduces Pathological Inflammation
Both SARS-CoV-2 and SARS-CoV are characterized by a pathological inflammatory response. The host inflammatory response is a major cause of tissue damage and subsequent mortality. Increased inflammatory response and elevated levels of cytokines (IL-1β, IL-6, IL-8, MCP-1, IP-10, TNFα, IFNγ, et al) have been observed in patients with COVID-19, which implied potential of a cytokine storm21–24. In an animal study, the cytokine and IFNγ were also detected in the lungs of the SARS-CoV-2-infected animals, which suggested that SARS-CoV-2 triggered the innate immune response and the activation of inflammation25. Furthermore, the SARS-CoV E protein forms a Ca2+ permeable channel in ERGIC/Golgi membranes. The channel activity alters Ca2+ homeostasis within cells and boosts the activation of the NLRP3 inflammasome, which leads to the overproduction of IL-1β26. The development of an uncontrolled inflammatory response can thus lead to detrimental outcomes such as diffused alveolar damage and fibrosis, progressive respiratory failure, and multiple organ damage and dysfunction. Additionally, inflammation and SARS-CoV proteins cause ER stress, which consequently leads to dysregulation of Ca2+ homeostasis27,28.
Intracellular Ca2+ signalling is essential in the release of pro-inflammatory cytokines and the elevation of the intracellular Ca2+ has been suggested to be a critical event in sepsis29. Calcium influx may play a partial role in promoting the plasma levels of cytokines, because the calcium channel blockers have been demonstrated to ameliorate excessive inflammation30. Subsequently, calcium channel blockers have been proposed to treat COVID-19 patients31. With its ability to ameliorate Ca2+ dysregulation by inhibiting over activation of RyRs (Figure 1), dantrolene has been demonstrated to suppress plasma and tissue concentration of IL-632, IL-833, IL-1β, TNF-α34,35, and IFN-γ36 in vivo and in vitro. Consequently, dantrolene inhibited ER-mediated Ca2+ release and ameliorated ER stress37.
2). Dantrolene Reduces Pathological Oxidative Stress
Oxidative stress generated from SARS-CoV-2, might further exacerbate the pro-inflammatory epigenetic changes and result in a vicious circle of cytokine response. At the same time, response to SARS-CoV-2 infection, DNA methylation defect exacerbated by oxidative stress will further enhance viral entry through epigenetic de-repression of ACE2 and increased ACE2 expression38. As for SARS-CoV, oxidative stress-sensitive genes were upregulated in peripheral blood mononuclear cells of patients39. Alterations of reactive oxygen species (ROS) production that are caused by respiratory viral infections are implicated in inflammation, lung epithelial disruption, tissue damage, and even pulmonary fibrosis40.
Given SARS-CoV induced oxidative stress cell damage, anti-oxidative treatment may play a role in the SARS-CoV treatment. Dantrolene was reported to protect cells against oxidative stress by elevating the levels of GSH and GSH/GSSG41,42. Calcium release from the ER was associated with the generation of ROS43, which was inhibited by dantrolene via lowering mitochondrial superoxide, ROS44.
3). Dantrolene Inhibits Cell Death By Apoptosis
Apoptosis is induced as one of the host antiviral responses to limit virus replication and production during viral infections. Lymphopenia was common in SARS-CoV-2 infected patients, probably due to lymphocyte apoptosis21,24,45. Also, laboratory research in peripheral blood mononuclear cells demonstrated that TP53, an important gene in the process of apoptosis, showed an increasing trend in patients infected with SARS-CoV-224. In SARS-CoV-2 infected animals, apoptosis has been found in the respiratory tract and TUNEL staining showed the diffused signals in the lungs, bronchiolar lumen cell debris, and collapsed alveolar walls25. The release of Ca2+ from ER has been proposed to be involved in the induction of apoptosis by oxidative stress, which is also a pathological process induced by SARS-CoV-243.
Apoptosis contributes to SARS-CoV-2 virus pathogenesis, and inhibition of apoptosis may protect host cells against damage. Abnormal Ca2+ release from the ER and consequent increase in cytosolic and mitochondria Ca2+ levels play pivotal roles in inducing cell apoptosis in a variety of cell types46. Thus, dantrolene can suppress apoptosis through inhibiting RyR-mediated abnormal and excessive Ca2+ release47,48. Moreover, dantrolene can ameliorate apoptosis by directly inhibiting nuclear condensation and fragmentation49,50.
4). Dantrolene Ameliorates Impairment of Autophagy
SARS-CoV has the potential to inhibit the autophagy process. An analysis of a relatively wide database of SARS-CoV-2 genomes of worldwide isolates representative of COVID-19 has revealed two synonymous mutations, of which one is non-structural viral proteins 6 (NSP6)51. NSP6 is a common component of both α and β-coronaviruses, which locates to the ER and generates autophagosomes52. It has been shown that NSP6 and ER binding may favor coronavirus infection by compromising the ability of autophagosomes to deliver viral components to lysosomes for degradation53,54. Thus, this would limit autophagosome expansion and activity55. Moreover, overexpression of membrane-associated papain-like protease PLP2 of SARS-CoV and MERS-CoV led to blockage of autophagosomes-lysosomes fusion and suppression of the autophagic flux56. It has been shown that high cytosolic Ca2+ concentration suppressed vesicle fusion, and calcium channel blockers can promote autophagosome-lysosome fusion57. Dantrolene, as a calcium channel blocker, through inhibition of the RyRs in ER, has been reported to promote autophagy activity by inducing autophagy induction58,59 and, therefore, potentially ameliorating the impaired autophagy function mediated by SARS-CoV-2 viruses.
3. Dantrolene Potentially Ameliorates the Multiple Organ Damages in COVID-19 Patients
COVID-19 typically demonstrates severe progressive lung injury, multi-organ failure, and death3,60,61. Although SARS-CoV-2 initially infects the lungs and causes lung damage, the virus eventually reaches many organs, resulting in multiple organ damage62. Critically ill patients are typically found to have systemic multiple-organ damage and dysfunction63,64.
1). Lung
Acute respiratory distress syndrome (ARDS) is often seen in critically ill COVID-19 patients, which is usually life-threatening because it is associated with progressive hypoxia and associated multiple organ damage3,60,65. Pulmonary hypertension (PH) is a recognized consequence of AR-DS and a severe condition with a very poor survival rate66,67, which was presented in COVID-19 patients68. Pulmonary vasoconstriction due to hypoxia and inflammation constitutes the majority of the underlying mechanisms of PH69. It has been proposed that the correction of abdominal pH by reducing hypoxic pulmonary vasoconstriction could benefit COVID-19 patients.
RyRs play an important role in hypoxia-induced Ca2+ release and contraction70, which contributes significantly to the development of pulmonary hypertension71. Chronic hypoxia increases RyR2 expression and further induces pulmonary hypertension72. Dantrolene can inhibit hypoxia-induced Ca2+ release in the pulmonary arterial smooth muscle cell and vasoconstriction of the pulmonary artery70,73,74, which reverses the hypoxic vasoconstriction75. In light of this beneficial effect, dantrolene may be a potential adjunctive countermeasure.
Moreover, in the airway smooth muscle, RyRs also mediate the Ca2+ response and thus bronchoconstriction, which can be attenuated by dantrolene76. This potentially mitigates the high airway pressure, which might result in the pneumothorax of COVID-19 patients77.
2). Cardiovascular System
Cardiac injury in COVID-19 patients was more likely related to multiple stress factors rather than direct damage by the virus68. Therefore, the goal is to minimize the myocardial ischemia and ischemia-reperfusion injury (IRI) in these patients.
Cytosolic Ca2+ overload plays a major role in the development of irreversible injury during myocardial ischemia, while the abnormal Ca2+ release from the sarcoplasmic reticulum contributes to this damage significantly78. Dantrolene reduced ischemic injury even at concentrations that did not affect contractile performance in the heart79. In vitro studies showed that dantrolene attenuated the lethal cellular injury80, reduced infarct damage79–81, protected cardiac function79,82,83, and was even antiarrhythmic83 under IRI.
Cardiac arrhythmia and associated cardiac arrest are often seen in COVID-19 patients3. In heart failure, arrhythmogenic Ca2+ release and chronic Ca2+ depletion arise due to the altered function of the RyR Ca2+ release channel84. Dantrolene has been demonstrated to have antiarrhythmic effects against Ca2+ overload mediated arrhythmias85,86, while at the same time preserving inotropy84. Dantrolene can also improve survival after ventricular fibrillation by mitigating impaired Ca2+ handling in animal models87, and prevent catecholaminergic polymorphic ventricular tachycardia88.
3). Brain
The expression and distribution of ACE2 in the brain89 suggest that the SARS-CoV-2 may cause some neurologic manifestations through direct90 or indirect mechanisms91. The infection itself has also been described as a risk factor for stroke92. The ischemia of the brain seems to be a severe threat to COVID-19 patients.
One approach to protect the brain against ischemia is to reduce the tissue’s functional activity to preserve energy for the metabolic processes that are essential to viability93. The neuroprotective effect of dantrolene, which inhibits abnormal Ca2+ release from ER, and then contributes to the large reversible reductions in O2 consumption, glycolysis, and electrophysiological function93, appears rather consistent across multiple cells and animal models of neurological injury that include excitotoxicity94–98, oxygen-glucose deprivation (OGD), forebrain ischemia104–107, focal ischemia108, global ischemia109,100, and traumatic injury111. In humans, dantrolene is capable of attenuating cerebral vasospasm112 and providing neuroprotection113.
4). Liver
Many patients with COVID-19 range from differing degrees of liver damage and function abnormality61. Pneumonia-associated hypoxia and immune-mediated inflammation, such as cytokine storm, might contribute to liver injury or even develop into liver failure in patients who are critically ill114.
It was reported that dantrolene offered significant functional and structural protection of the ischemic liver, by decreasing TNF-α but increasing IL-10 and was also associated with better liver function tests and less necrosis during ischemia in rat livers115.
5). Kidney
Kidney failure may be part of whole-body events in COVID-19 patients62. Renal ischemia/reperfusion injury is a common cause of acute renal failure116 and induces renal tubule apoptosis, which is associated with the elevation of the cytosolic calcium concentration117. The renal tubular cell injury can be attenuated by dantrolene118.
6). Pathological Inflammation and Cytokine Storm
In COVID-19, higher plasma levels of cytokines including IL-6, IL-2, IL-7, IL-10, granulocyte-colony stimulating factor, interferon-γ–inducible protein, monocyte chemoattractant protein, macrophage inflammatory protein 1α, and TNF-α were found in ICU patients, which implied that a cytokine storm occurred21,61. For COVID-19 patients, cytokine storms are a major reason that some require intensive care and ventilation. Dantrolene has been shown to inhibit various cytokine release and inflammation in various animal models34–36. It was reported that dantrolene decreased TNF-α in the lung (26.1%), liver (29.4%), and spleen (35.4%) and IL-1α in the lung (30.0%) and liver (25.4%)34. These beneficial effects of dantrolene make it potentially effective at ameliorating cytokine-mediated pathological inflammatory reaction and associated cytokine storm in COVID-19 patients.
Conclusions
In such a global pandemic, little is known for certain. Besides direct antivirus treatment, attention should also be paid to reducing the severity of the symptoms, protecting the organs, and ameliorating the deterioration. Based on previous studies illustrating the dantrolene protective effects against SARS-CoV-2 virus cytotoxicity in host cells, cell or organ damage induced by hypoxia/ischemia, mitochondrial damage, oxidative stresses, inflammation, impaired autophagy function, etc., we propose that dantrolene might be a potential repurposed drug for the treatment of COVID-19 patients (Figure 2), with an expectation to assist in reducing mortality. Further studies at the varied molecular, cellular, animal, and patient levels are important and recommended.
Figure 2.

Dantrolene is expected to protect cell and organ damage induced by multiple pathological stresses in COVID-19 patients.
Acknowledgements
We appreciate the English editing by Matan Ben-Abou from Drexel University, Philadelphia, PA, USA.
Funding
This work was supported by grants to HW from the National Institute on Aging (R01AG061447). The funding paid a partial salary to HW and GL and full salary to SL.
Footnotes
Conflict of Interest
Drs. Huafeng Wei and Ge Liang are listed as inventors of a US provisional patent application entitled “Intranasal Administration of Dantrolene for Treatment of Alzheimer’s Disease” filed on June 28, 2019 (Serial number 62/868,820) by the University of Pennsylvania Trustee. The provisional patent application is also part of the research collaboration agreement between the University of Pennsylvania and Eagle Pharmaceutical Company, which produces and sells a new formula of dantrolene (Ryanodex) for the treatment of malignant hyperthermia. Other authors declare no conflict of interest.
References
- 1).Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, Cuomo-Dannenburg G, Thompson H, Walker PGT, Fu H, Dighe A, Griffin JT, Baguelin M, Bhatia S, Boonyasiri A, Cori A, Cucunuba Z, FitzJohn R, Gaythorpe K, Green W, Hamlet A, Hinsley W, Laydon D, Nedjati-Gilani G, Riley S, Van Elsland S, Volz E, Wang H, Wang Y, Xi X, Donnelly CA, Ghani AC, Ferguson NM. Estimates of the severity of Coronavirus disease 2019: a model-based analysis. Lancet Infect Dis 2020; 20: 669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395: 1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, Wu Y, Xiao W, Liu S, Chen E, Chen W, Wang X, Yang J, Lin J, Zhao Q, Yan Y, Xie Z, Li D, Yang Y, Liu L, Qu J, Ning G, Shi G, Xie Q. Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial. medRxiv 2020: 2020.04.10.20060558. [Google Scholar]
- 4).Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020; 382: 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Shneider A, Kudriavtsev A. Can melatonin reduce the severity of COVID-19 pandemic? Int Rev Immunol 2020; 39: 153–162. [DOI] [PubMed] [Google Scholar]
- 6).Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020; 117: 10970–10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Eagle Pharmaceuticals, Inc. Eagle pharmaceuticals announces laboratory test results demonstrating in vitro antiviral activity of RYANODEX® (Dantrolene Sodium) against coronavirus SARS-CoV-2. Available from, https://businesswire.com/news/home/20200416005156/en. [Accessed 10 May 2020].
- 9).Inan S, Wei H. The cytoprotective effects of dantrolene: a ryanodine receptor antagonist. Anesth Analg 2010; 111: 1400–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Boys JA, Toledo AH, Anaya-Prado R, Lopez-Neblina F, Toledo-Pereyra LH. Effects of dantrolene on ischemia-reperfusion injury in animal models: a review of outcomes in heart, brain, liver, and kidney. J Investig Med 2010; 58: 875–882. [DOI] [PubMed] [Google Scholar]
- 11).Hoffmann M, Kleine-Weber H, Pöhlmann S. A multi-basic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell 2020; 78: 779–784.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J, Xiang Z, Mu Z, Chen X, Chen J, Hu K, Jin Q, Wang J. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 2020; 11: 1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Liu T, Luo S, Libby P, Shi GP. Cathepsin L-selective inhibitors: A potentially promising treatment for COVID-19 patients. Pharmacol Ther 2020; 213: 107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Millet JK, Whittaker GR. Physiological and molecular triggers for SARS-CoV membrane fusion and entry into host cells. Virology 2018; 517: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Elliott EB, McCarroll D, Hasumi H, Welsh CE, Panissidi AA, Jones NG, Rossor CL, Tait A, Smith GL, Mottram JC, Morrison LJ, Loughrey CM. Trypanosoma brucei cathepsin-L increases arrhythmogenic sarcoplasmic reticulum-mediated calcium release in rat cardiomyocytes. Cardiovasc Res 2013; 100: 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Tanaka Y, Sato Y, Sasaki T. Suppression of coronavirus replication by cyclophilin inhibitors. Viruses 2013; 5: 1250–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Straus MR, Tang T, Lai AL, Flegel A, Bidon M, Freed JH, Daniel S, Whittaker GR. Ca2+ ions promote fusion of middle east respiratory syndrome coronavirus with host cells and increase infectivity. J Virol 2020; 94: e00426–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Lai AL, Millet JK, Daniel S, Freed JH, Whittaker GR. The SARS-CoV fusion peptide forms an extended bipartite fusion platform that perturbs membrane order in a calcium-dependent manner. J Mol Biol 2017; 429: 3875–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Stadler K, Ha HR, Ciminale V, Spirli C, Saletti G, Schiavon M, Bruttomesso D, Bigler L, Follath F, Pettenazzo A, Baritussio A. Amiodarone alters late endosomes and inhibits SARS coronavirus infection at a post-endosomal level. Am J Respir Cell Mol Biol 2008; 39: 142–149. [DOI] [PubMed] [Google Scholar]
- 20).Solaimanzadeh I. Nifedipine and Amlodipine are associated with improved mortality and decreased risk for intubation and mechanical ventilation in elderly patients hospitalized for COVID-19. Cureus 2020; 12: e8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21).Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect 2020; 80: e14–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, Lit LC, Hui DS, Chan MH, Chung SS, Sung JJ. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 2004; 136: 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Xiong Y, Liu Y, Cao L, Wang D, Guo M, Jiang A, Guo D, Hu W, Yang J, Tang Z, Wu H, Lin Y, Zhang M, Zhang Q, Shi M, Liu Y, Zhou Y, Lan K, Chen Y. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect 2020; 9: 761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Chan JF, Zhang AJ, Yuan S, Poon VK, Chan CC, Lee AC, Chan WM, Fan Z, Tsoi HW, Wen L, Liang R, Cao J, Chen Y, Tang K, Luo C, Cai JP, Kok KH, Chu H, Chan KH, Sridhar S, Chen Z, Chen H, To KK, Yuen KY. Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Infect Dis. 2020. March 26:ciaa325. doi: 10.1093/cid/ciaa325. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Nieto-Torres JL, Verdia-Baguena C, Jimenez-Guardeno JM, Regla-Nava JA, Castano-Rodriguez C, Fernandez-Delgado R, Torres J, Aguilella VM, Enjuanes L. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology 2015; 485: 330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Shi CS, Nabar NR, Huang NN, Kehrl JH. SARS-Coronavirus Open Reading Frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov 2019; 5: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Fung TS, Liu DX. Human Coronavirus: host-pathogen interaction. Annu Rev Microbiol 2019; 73: 529–557. [DOI] [PubMed] [Google Scholar]
- 29).Delano MJ, Ward PA. The immune system’s role in sepsis progression, resolution, and long-term outcome. Immunol Rev 2016; 274: 330–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Kurum T, Tatli E, Yuksel M. Effects of carvedilol on plasma levels of pro-inflammatory cytokines in patients with ischemic and nonischemic dilated cardiomyopathy. Tex Heart Inst J. 2007; 34: 52–59. [PMC free article] [PubMed] [Google Scholar]
- 31).Skayem C, Ayoub N. Carvedilol and COVID-19: a potential role in reducing infectivity and infection severity of SARS-CoV-2. Am J Med Sci 2020; 360: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Ducreux S, Zorzato F, Müller C, Sewry C, Muntoni F, Quinlivan R, Restagno G, Girard T, Treves S. Effect of ryanodine receptor mutations on interleukin-6 release and intracellular calcium homeostasis in human myotubes from malignant hyperthermia-susceptible individuals and patients affected by central core disease. J Biol Chem 2004; 279: 43838–43846. [DOI] [PubMed] [Google Scholar]
- 33).Hisatsune J, Nakayama M, Isomoto H, Kurazono H, Mukaida N, Mukhopadhyay AK, Azuma T, Yamaoka Y, Sap J, Yamasaki E, Yahiro K, Moss J, Hirayama T. Molecular characterization of Helicobacter pylori VacA induction of IL-8 in U937 cells reveals a prominent role for p38MAPK in activating transcription factor-2, cAMP response element binding protein, and NF-kappaB activation. J Immunol 2008; 180: 5017–5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Hotchkiss RS, Osborne DF, Lappas GD, Karl IE. Calcium antagonists decrease plasma and tissue concentrations of tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-1 alpha in a mouse model of endotoxin. Shock 1995; 3: 337–342. [PubMed] [Google Scholar]
- 35).Fischer DR, Sun X, Williams AB, Gang G, Pritts TA, James JH, Molloy M, Fischer JE, Paul RJ, Hasselgren PO. Dantrolene reduces serum TNFalpha and corticosterone levels and muscle calcium, calpain gene expression, and protein breakdown in septic rats. Shock 2001; 15: 200–207. [DOI] [PubMed] [Google Scholar]
- 36).Németh ZH, Haskó G, Szabó C, Salzman AL, Vizi ES. Calcium channel blockers and dantrolene differentially regulate the production of interleukin-12 and interferon-gamma in endotoxemic mice. Brain Res Bull 1998; 46: 257–261. [DOI] [PubMed] [Google Scholar]
- 37).Clark AL, Kanekura K, Lavagnino Z, Spears LD, Abreu D, Mahadevan J, Yagi T, Semenkovich CF, Piston DW, Urano F. Targeting cellular calcium homeostasis to prevent cytokine-mediated beta cell death. Sci Rep 2017; 7: 5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Sawalha AH, Zhao M, Coit P, Lu Q. Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clin Immunol 2020; 215: 108410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Shao H, Lan D, Duan Z, Liu Z, Min J, Zhang L, Huang J, Su J, Chen S, Xu A. Upregulation of mitochondrial gene expression in PBMC from convalescent SARS patients. J Clin Immunol 2006; 26: 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Khomich OA, Kochetkov SN, Bartosch B, Ivanov AV. Redox biology of respiratory viral infections. Viruses 2018; 10: 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Todorova VK, Siegel ER, Kaufmann Y, Kumarapeli A, Owen A, Wei JY, Makhoul I, Klimberg VS. Dantrolene attenuates cardiotoxicity of doxorubicin without reducing its antitumor efficacy in a breast cancer model. Transl Oncol 2020; 13: 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Keles I, Bozkurt MF, Aglamis E, Fidan AF, Ceylan C, Karalar M, Coban S, Denk B, Buyukokuroglu ME. Protective effects of dantrolene and methylprednisolone against spinal cord injury-induced early oxidative damage in rabbit bladder: a comparative experimental study. Adv Clin Exp Med 2019; 28: 1697–1704. [DOI] [PubMed] [Google Scholar]
- 43).Lu Y-C, Lin M-L, Su H-L, Chen S-SJAr. ER-dependent Ca++-mediated cytosolic ROS as an effector for induction of mitochondrial apoptotic and ATM-JNK signal pathways in gallic acid-treated human oral cancer cells. Anticancer Res 2016; 36: 697–705. [PubMed] [Google Scholar]
- 44).Godai K, Takahashi K, Kashiwagi Y, Liu CH, Yi H, Liu S, Dong C, Lubarsky DA, Hao S. Ryanodine receptor to mitochondrial reactive oxygen species pathway plays an important role in chronic human immunodeficiency virus gp120MN-induced neuropathic pain in rats. Anesth Analg 2019; 129: 276–286. [DOI] [PubMed] [Google Scholar]
- 45).Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, Tsoi HW, Lo SK, Chan KH, Poon VK, Chan WM, Ip JD, Cai JP, Cheng VC, Chen H, Hui CK, Yuen KY. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020; 395: 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46).Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 1999; 13: 1211–1233. [DOI] [PubMed] [Google Scholar]
- 47).Shin DH, Leem DG, Shin JS, Kim JI, Kim KT, Choi SY, Lee MH, Choi JH, Lee KT. Compound K induced apoptosis via endoplasmic reticulum Ca2+ release through ryanodine receptor in human lung cancer cells. J Ginseng Res 2018; 42: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48).Martins BC, Torres BBJ, de Oliveira KM, Lavor MS, Osório CM, Fukushima FB, Rosado IR, de Melo EG. Association of riluzole and dantrolene improves significant recovery after acute spinal cord injury in rats. Spine J 2018; 18: 532–539. [DOI] [PubMed] [Google Scholar]
- 49).Xu SY, Hu FY, Ren LJ, Chen L, Zhou ZQ, Zhang XJ, Li WP. Dantrolene enhances the protective effect of hypothermia on cerebral cortex neurons. Neural Regen Res 2015; 10: 1279–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50).Wei H, Kang B, Wei W, Liang G, Meng QC, Li Y, Eckenhoff RG. Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res 2005; 1037: 139–147. [DOI] [PubMed] [Google Scholar]
- 51).Benvenuto D, Angeletti S, Giovanetti M, Bianchi M, Pascarella S, Cauda R, Ciccozzi M, Cassone A. Evolutionary analysis of SARS-CoV-2: how mutation of non-structural protein 6 (NSP6) could affect viral autophagy. J Infect 2020; 81: e24–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52).Forni D, Cagliani R, Clerici M, Sironi M. Molecular evolution of human coronavirus genomes. Trends Microbiol 2017; 25: 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Zhou A, Li S, Khan FA, Zhang S. Autophagy postpones apoptotic cell death in PRRSV infection through Bad-Beclin1 interaction. Virulence 2016; 7: 98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Cottam EM, Whelband MC, Wileman T. Coronavirus NSP6 restricts autophagosome expansion. Autophagy 2014; 10: 1426–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Tang JW, Cheung JL, Chu IM, Sung JJ, Peiris M, Chan PK. The large 386-nt deletion in SARS-associated coronavirus: evidence for quasispecies? J Infect Dis 2006; 194: 808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Chen X, Wang K, Xing Y, Tu J, Yang X, Zhao Q, Li K, Chen Z. Coronavirus membrane-associated papain-like proteases induce autophagy through interacting with Beclin1 to negatively regulate antiviral innate immunity. Protein Cell 2014; 5: 912–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Park HW, Park H, Semple IA, Jang I, Ro SH, Kim M, Cazares VA, Stuenkel EL, Kim JJ, Kim JS, Lee JH. Pharmacological correction of obesity-induced autophagy arrest using calcium channel blockers. Nat Commun 2014; 5: 4834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Wang Y, Liang G, Liang S, Mund R, Shi Y, Wei H. Dantrolene ameliorates impaired neurogenesis and synaptogenesis in induced pluripotent stem cell lines derived from patients with Alzheimer’s disease. Anesthesiology 2020; 132: 1062–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59).Chung KM, Jeong EJ, Park H, An HK, Yu SW. Mediation of autophagic cell death by type 3 ryanodine receptor (RyR3) in adult hippocampal neural stem cells. Front Cell Neurosci 2016; 10: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS, China Medical Treatment Expert Group for C. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Wadman M, Couzin-Frankel J, Kaiser J, Matacic C. A rampage through the body. Science 2020; 368: 356–360. [DOI] [PubMed] [Google Scholar]
- 63).Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8: 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64).Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020; 5: 802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65).Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020; 323: 1612–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66).Bull TM, Clark B, McFann K, Moss M. Pulmonary vascular dysfunction is associated with poor outcomes in patients with acute lung injury. Am J Respir Crit Care Med 2010; 182: 1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67).Boissier F, Katsahian S, Razazi K, Thille AW, Roc he-Campo F, Leon R, Vivier E, Brochard L, Vieillard-Baron A, Brun-Buisson C, Mekontso Dessap A. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med 2013; 39: 1725–1733. [DOI] [PubMed] [Google Scholar]
- 68).Deng Q, Hu B, Zhang Y, Wang H, Zhou X, Hu W, Cheng Y, Yan J, Ping H, Zhou Q. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol 2020; 311: 116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).Price LC, Wort SJ. Pulmonary hypertension in ARDS: inflammation matters! Thorax 2017; 72: 396–397. [DOI] [PubMed] [Google Scholar]
- 70).Zheng YM, Wang QS, Rathore R, Zhang WH, Mazurkiewicz JE, Sorrentino V, Singer HA, Kotlikoff MI, Wang YX. Type-3 ryanodine receptors mediate hypoxia-, but not neurotransmitter-induced calcium release and contraction in pulmonary artery smooth muscle cells. J Gen Physiol 2005; 125: 427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71).Song T, Zheng YM, Wang YX. Cross talk between mitochondrial reactive oxygen species and sarcoplasmic reticulum calcium in pulmonary arterial smooth muscle cells. Adv Exp Med Biol 2017; 967: 289–298. [DOI] [PubMed] [Google Scholar]
- 72).Dahan D, Ducret T, Quignard JF, Marthan R, Savineau JP, Esteve E. Implication of the ryanodine receptor in TRPV4-induced calcium response in pulmonary arterial smooth muscle cells from normoxic and chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol 2012; 303: L824–833. [DOI] [PubMed] [Google Scholar]
- 73).Pourmahram GE, Snetkov VA, Shaifta Y, Drndarski S, Knock GA, Aaronson PI, Ward JP. Constriction of pulmonary artery by peroxide: role of Ca2+ release and PKC. Free Radic Biol Med 2008; 45: 1468–1476. [DOI] [PubMed] [Google Scholar]
- 74).Becker S, Knock GA, Snetkov V, Ward JP, Aaronson PI. Role of capacitative Ca2+ entry but not Na+/Ca2+ exchange in hypoxic pulmonary vasoconstriction in rat intrapulmonary arteries. Novartis Found Symp 2006; 272: 259–268; discussion 68–79. [PubMed] [Google Scholar]
- 75).Du W, Frazier M, McMahon TJ, Eu JP. Redox activation of intracellular calcium release channels (ryanodine receptors) in the sustained phase of hypoxia-induced pulmonary vasoconstriction. Chest 2005; 128: 556s–558s. [DOI] [PubMed] [Google Scholar]
- 76).Du W, Stiber JA, Rosenberg PB, Meissner G, Eu JP. Ryanodine receptors in muscarinic receptor-mediated bronchoconstriction. J Biol Chem 2005; 280: 26287–26294. [DOI] [PubMed] [Google Scholar]
- 77).Yao W, Wang T, Jiang B, Gao F, Wang L, Zheng H, Xiao W, Yao S, Mei W, Chen X, Luo A, Sun L, Cook T, Behringer E, Huitink JM, Wong DT, Lane-Fall M, McNarry AF, McGuire B, Higgs A, Shah A, Patel A, Zuo M, Ma W, Xue Z, Zhang LM, Li W, Wang Y, Hagberg C, O’Sullivan EP, Fleisher LA, Wei H, collaborators. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: lessons learnt and international expert recommendations. Br J Anaesth 2020; 125: e28–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78).Chen W, London R, Murphy E, Steenbergen C. Regulation of the Ca2+ gradient across the sarcoplasmic reticulum in perfused rabbit heart. A 19F nuclear magnetic resonance study. Circ Res 1998; 83: 898–907. [DOI] [PubMed] [Google Scholar]
- 79).Yu G, Zucchi R, Ronca-Testoni S, Ronca G. Protection of ischemic rat heart by dantrolene, an antagonist of the sarcoplasmic reticulum calcium release channel. Basic Res Cardiol 2000; 95: 137–143. [DOI] [PubMed] [Google Scholar]
- 80).Preckel B, Schlack W, Comfere T, Thamer V. Effect of dantrolene in an in vivo and in vitro model of myocardial reperfusion injury. Acta Anaesthesiol Scand 2000; 44: 194–201. [DOI] [PubMed] [Google Scholar]
- 81).Zucchi R, Yu G, Ghelardoni S, Ronca F, Ronca-Testoni S. A3 adenosine receptor stimulation modulates sarcoplasmic reticulum Ca(2+) release in rat heart. Cardiovasc Res 2001; 50: 56–64. [DOI] [PubMed] [Google Scholar]
- 82).Mitchell MB, Winter CB, Banerjee A, Harken AH. Inhibition of sarcoplasmic reticulum calcium release reduces myocardial stunning. J Surg Res 1993; 54: 411–417. [DOI] [PubMed] [Google Scholar]
- 83).Said M, Becerra R, Palomeque J, Rinaldi G, Kaetzel MA, Diaz-Sylvester PL, Copello JA, Dedman JR, Mundina-Weilenmann C, Vittone L, Mattiazzi A. Increased intracellular Ca2+ and SR Ca2+ load contribute to arrhythmias after acidosis in rat heart. Role of Ca2+/calmodulin-dependent protein kinase II. Am J Physiol Heart Circ Physiol 2008; 295: H1669–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84).Maxwell JT, Domeier TL, Blatter LA. Dantrolene prevents arrhythmogenic Ca2+ release in heart failure. Am J Physiol Heart Circ Physiol 2012; 302: H953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85).Walweel K, Oo YW, Laver DR. The emerging role of calmodulin regulation of RyR2 in controlling heart rhythm, the progression of heart failure and the antiarrhythmic action of dantrolene. Clin Exp Pharmacol Physiol 2017; 44: 135–142. [DOI] [PubMed] [Google Scholar]
- 86).Penttinen K, Swan H, Vanninen S, Paavola J, Lahtinen AM, Kontula K, Aalto-Setala K. Antiarrhythmic effects of dantrolene in patients with catecholaminergic polymorphic ventricular tachycardia and replication of the responses using iPSC models. PLoS One 2015; 10: e0125366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87).Zamiri N, Masse S, Ramadeen A, Kusha M, Hu X, Azam MA, Liu J, Lai PF, Vigmond EJ, Boyle PM, Behradfar E, Al-Hesayen A, Waxman MB, Backx P, Dorian P, Nanthakumar K. Dantrolene improves survival after ventricular fibrillation by mitigating impaired calcium handling in animal models. Circulation 2014; 129: 875–885. [DOI] [PubMed] [Google Scholar]
- 88).Kobayashi S, Yano M, Uchinoumi H, Suetomi T, Susa T, Ono M, Xu X, Tateishi H, Oda T, Okuda S, Doi M, Yamamoto T, Matsuzaki M. Dantrolene, a therapeutic agent for malignant hyperthermia, inhibits catecholaminergic polymorphic ventricular tachycardia in a RyR2(R2474S/+) knock-in mouse model. Circ J 2010; 74: 2579–2584. [DOI] [PubMed] [Google Scholar]
- 89).Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004; 203: 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90).Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, Nakao A, Takeda M, Haro H, Inoue O, Suzuki-Inoue K, Kubokawa K, Ogihara S, Sasaki T, Kinouchi H, Kojin H, Ito M, Onishi H, Shimizu T, Sasaki Y, Enomoto N, Ishihara H, Furuya S, Yamamoto T, Shimada S. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis 2020; 94: 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91).Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92).Markus HS, Brainin M. COVID-19 and stroke-A global World Stroke Organization perspective. Int J Stroke 2020; 15: 361–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93).Zager EL, Ames A 3rd. Reduction of cellular energy requirements. Screening for agents that may protect against CNS ischemia. J Neurosurg 1988; 69: 568–579. [DOI] [PubMed] [Google Scholar]
- 94).Duzenli S, Bakuridze K, Gepdiremen A. The effects of ruthenium red, dantrolene and nimodipine, alone or in combination, in NMDA induced neurotoxicity of cerebellar granular cell culture of rats. Toxicol In Vitro 2005; 19: 589–594. [DOI] [PubMed] [Google Scholar]
- 95).Simpson PB, Challiss RA, Nahorski SR. Involvement of intracellular stores in the Ca2+ responses to N-Methyl-D-aspartate and depolarization in cerebellar granule cells. J Neurochem 1993; 61: 760–763. [DOI] [PubMed] [Google Scholar]
- 96).Frandsen A, Schousboe A. Mobilization of dantrolene-sensitive intracellular calcium pools is involved in the cytotoxicity induced by quisqualate and N-methyl-D-aspartate but not by 2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propionate and kainate in cultured cerebral cortical neurons. Proc Natl Acad Sci U S A 1992; 89: 2590–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97).Lei SZ, Zhang D, Abele AE, Lipton SA. Blockade of NMDA receptor-mediated mobilization of intracellular Ca2+ prevents neurotoxicity. Brain Res 1992; 598: 196–202. [DOI] [PubMed] [Google Scholar]
- 98).Segal M, Manor D. Confocal microscopic imaging of [Ca2+]i in cultured rat hippocampal neurons following exposure to N-methyl-D-aspartate. J Physiol 1992; 448: 655–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99).Mitani A, Yanase H, Sakai K, Wake Y, Kataoka K. Origin of intracellular Ca2+ elevation induced by in vitro ischemia-like condition in hippocampal slices. Brain Res 1993; 601: 103–110. [DOI] [PubMed] [Google Scholar]
- 100).Wang C, Nguyen HN, Maguire JL, Perry DC. Role of intracellular calcium stores in cell death from oxygen-glucose deprivation in a neuronal cell line. J Cereb Blood Flow Metab 2002; 22: 206–214. [DOI] [PubMed] [Google Scholar]
- 101).Mattson MP, Zhu H, Yu J, Kindy MS. Presenilin-1 mutation increases neuronal vulnerability to focal ischemia in vivo and to hypoxia and glucose deprivation in cell culture: involvement of perturbed calcium homeostasis. J Neurosci 2000; 20: 1358–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102).Tasker RC, Sahota SK, Cotter FE, Williams SR. Early postischemic dantrolene-induced amelioration of poly(ADP-ribose) polymerase-related bioenergetic failure in neonatal rat brain slices. J Cereb Blood Flow Metab 1998; 18: 1346–1356. [DOI] [PubMed] [Google Scholar]
- 103).Massote PD, Pinheiro AC, Fonseca CG, Prado MA, Guimaraes AL, Massensini AR, Gomez MV. Protective effect of retinal ischemia by blockers of voltage-dependent calcium channels and intracellular calcium stores. Cell Mol Neurobiol 2008; 28: 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104).Nakayama R, Yano T, Ushijima K, Abe E, Terasaki H. Effects of dantrolene on extracellular glutamate concentration and neuronal death in the rat hippocampal CA1 region subjected to transient ischemia. Anesthesiology 2002; 96: 705–710. [DOI] [PubMed] [Google Scholar]
- 105).Zhang L, Andou Y, Masuda S, Mitani A, Kataoka K. Dantrolene protects against ischemic, delayed neuronal death in gerbil brain. Neurosci Lett 1993; 158: 105–108. [DOI] [PubMed] [Google Scholar]
- 106).Phillis JW, Diaz FG, O’Regan MH, Pilitsis JG. Effects of immunosuppressants, calcineurin inhibition, and blockade of endoplasmic reticulum calcium channels on free fatty acid efflux from the ischemic/reperfused rat cerebral cortex. Brain Res 2002; 957: 12–24. [DOI] [PubMed] [Google Scholar]
- 107).Yano T, Nakayama R, Imaizumi T, Terasaki H, Ushijima K. Dantrolene ameliorates delayed cell death and concomitant DNA fragmentation in the rat hippocampal CA1 neurons subjected to mild ischemia. Resuscitation 2001; 50: 117–125. [DOI] [PubMed] [Google Scholar]
- 108).Li F, Hayashi T, Jin G, Deguchi K, Nagotani S, Nagano I, Shoji M, Chan PH, Abe K. The protective effect of dantrolene on ischemic neuronal cell death is associated with reduced expression of endoplasmic reticulum stress markers. Brain Res 2005; 1048: 59–68. [DOI] [PubMed] [Google Scholar]
- 109).Wei H, Perry DC. Dantrolene is cytoprotective in two models of neuronal cell death. J Neurochem 1996; 67: 2390–2398. [DOI] [PubMed] [Google Scholar]
- 110).Gwak M, Park P, Kim K, Lim K, Jeong S, Baek C, Lee J. The effects of dantrolene on hypoxic-ischemic injury in the neonatal rat brain. Anesth Analg 2008; 106: 227–233, table of contents. [DOI] [PubMed] [Google Scholar]
- 111).Yoon KW, Mitchell HL, Broder LD, Brooker RW, Delisle RK. Trauma-induced neurotoxicity in rat hippocampal neurons. Stroke 1996; 27: 122–126. [DOI] [PubMed] [Google Scholar]
- 112).Muehlschlegel S, Carandang R, Hall W, Kini N, Izzy S, Garland B, Ouillette C, van der Bom IM, Flood TF, Gounis MJ, Weaver JP, Barton B, Wakhloo AK. Dantrolene for cerebral vasospasm after subarachnoid haemorrhage: a randomised double blind placebo-controlled safety trial. J Neurol Neurosurg Psychiatry 2015; 86: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 113).Muehlschlegel S, Sims JR. Dantrolene: mechanisms of neuroprotection and possible clinical applications in the neurointensive care unit. Neurocrit Care 2009; 10: 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114).Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol 2020; 5: 428–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115).Lopez-Neblina F, Toledo-Pereyra LH, Toledo AH, Walsh J. Ryanodine receptor antagonism protects the ischemic liver and modulates TNF-alpha and IL-10. J Surg Res 2007; 140: 121–128. [DOI] [PubMed] [Google Scholar]
- 116).Eefting F, Rensing B, Wigman J, Pannekoek WJ, Liu WM, Cramer MJ, Lips DJ, Doevendans PA. Role of apoptosis in reperfusion injury. Cardiovasc Res 2004; 61: 414–426. [DOI] [PubMed] [Google Scholar]
- 117).Wu D, Chen X, Ding R, Qiao X, Shi S, Xie Y, Hong Q, Feng Z. Ischemia/reperfusion induce renal tubule apoptosis by inositol 1,4,5-trisphosphate receptor and L-type Ca2+ channel opening. Am J Nephrol 2008; 28: 487–499. [DOI] [PubMed] [Google Scholar]
- 118).Yano T, Itoh Y, Kawamura E, Maeda A, Egashira N, Nishida M, Kurose H, Oishi R. Amphotericin B-induced renal tubular cell injury is mediated by Na+ Influx through ion-permeable pores and subsequent activation of mitogen-activated protein kinases and elevation of intracellular Ca2+ concentration. Antimicrob Agents Chemother 2009; 53: 1420–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]


