Abstract
The amplitude of the H-reflex during the development and progression of fatigue reflects a complex interplay between central and peripheral factors. The purpose of this study is to characterize H-reflex homosynaptic post-activation depression (PAD) in an online fashion during a sustained submaximal fatigue task. The task required a high motor output in order to increase the likelihood of creating partial muscle ischemia with accumulation of fatigue metabolites, an important potential inhibitory influence upon the H-reflex during the progression of fatigue. Eleven subjects without neurologic impairment maintained volitional, isometric plantar flexion at 60% of maximal voluntary contraction until exhaustion. A paired-pulse stimulus (2 Hz) was delivered to the tibial nerve to elicit paired H-reflexes before, during, and after the fatigue protocol. The normalized amplitude of the second H-reflex (depression ratio) served as an estimate of PAD. Depression ratio increased during the first half of the fatigue protocol (P < 0.001), indicating a diminution of PAD, and then returned as exhaustion approached. The biphasic behavior of homosynaptic H-reflex depression during fatigue to exhaustion suggests a role for metabolic mediators of post-activation depression during fatigue.
Keywords: fatigue, homosynaptic depression, H-reflex, ischemia
1 |. INTRODUCTION
During motor tasks, the amplitude of the Hoffmann reflex (H-reflex) reveals a complex interplay between depressive and facilitatory influences upon spinal neural circuitry. The H-reflex therefore serves as a useful probe into a number of afferent and efferent processes that help mediate motor output. In pathologies such as spinal cord injury (SCI), changes in H-reflex behavior signify reorganization of spinal circuitry1 and may be germane to clinical syndromes such as spasticity.2 The normalization of H-reflex behavior to a pre-SCI pattern is a potential target for post-SCI rehabilitation interventions.3,4 Understanding the conditions that modulate H-reflex excitability in neurologically intact humans is an important foundation for the development of therapies for patients with neurologic pathology.
Descending afferent drive strongly influences H-reflex excitability in humans without neurologic impairment. H-reflex amplitude increases in conjunction with voluntary muscular contraction, then plateaus or declines at activation levels exceeding ~30% to 60% of maximum voluntary contraction (MVC).5,6 With tasks that elicit fatigue, the amplitude of the H-reflex has been observed to remain constant7,8 or to increase9 for protocols that use intermittent contractions. Among other potential mediating factors, this likely reflects a net enhancement of excitatory drive to the alpha motoneuronal pool during the progression of fatigue.10 On the other hand, H-reflex amplitude has been observed to decrease during tasks that involve sustained submaximal contraction7,11 or maximal contraction punctuated by very brief pauses.12 A key physiologic difference between intermittent and steady contraction protocols is that sustained contractions of >25% MVC may yield ischemic conditions within the muscle that prevent the removal of fatigue metabolites.13 Rising metabolite levels trigger activation of Group III and IV afferents, which in turn exert an inhibitory effect upon the H-reflex.14 Studies that maintained ischemia after fatigue lent additional support for the role of muscle metabolites in the diminution of the H-reflex during fatigue.15 More recently, spectrographic studies more directly illustrated the key role played by metabolite accumulation during fatigue, suggesting that this factor also may influence the H-reflex.16,17 The behavior of the H-reflex during fatigue may therefore be conceptualized as an interplay between competing central and peripheral mechanisms whose net excitatory and inhibitory influences are strongly linked to task conditions.
H-reflex amplitude is modifiable by preceding homosynaptic and heterosynaptic influences.8 Thus, a more complete understanding of the H-reflex response to fatigue can be gained by examining of the effects of conditioning stimuli that modify the amplitude of subsequent H-reflex responses. With repetitive stimulation of a muscle such as the soleus, H-reflex depression is attributed to homosynaptic depression at the pre-synaptic terminal of the Ia afferent fiber.18,19 Changes in H-reflex depression after fatigue would indicate whether homosynaptic post-activation depression (PAD) is responsive to the many central and peripheral physiologic alterations that occur during fatigue. Moreover, alterations of PAD are a key adaptation to neurologic insult in humans2,20 and can be altered via long-term training with fatigue-inducing contractions.4 Examination of PAD has therefore become a key tool for understanding the integration of excitatory and inhibitory central and peripheral processes that regulate muscle output during fatiguing contractions, with direct applicability to the understanding of adaptations to neurologic injury and disease.
Traditionally, PAD has been examined by measuring the H-reflex response to a train of ~10 stimuli, a time-consuming process that is disadvantageous for fatigue protocols that require rapid assessment of sensorimotor conditions. We recently demonstrated the reliability of evoking H-reflexes via paired-pulse (doublet) stimulation, showing that PAD exhibited by the second H-reflex response (H2) is consistent with PAD demonstrated by the average of all subsequent pulses.21 This more rapid, less-cumbersome approach could be especially useful for fatigue protocols that employ a constant, sustained contraction, and aim to characterize PAD in real-time during the progression of fatigue. Several previous reports elicited fatigue via a sustained contraction and evoked H-reflexes in an online fashion, rather than after cessation of the fatiguing contraction.7,10,22,23 However, none of these reports examined homosynaptic post-activation depression. Other reports which elicited fatigue via a sustained contraction neither included online characterization of H-reflexes nor examined homosynaptic PAD.24 We are not aware of previous studies that examined the real-time effects of sustained, sub-maximal, fatiguing contractions on PAD. Some insights may be gained by fatigue studies that employed intermittent contractions and very brief pauses. Two such studies indicated that PAD may be expected to decline during the development of fatigue.12,25 However, the introduction of brief pauses into a strong contraction could allow for resolution of ischemia and removal of fatigue metabolites, making the role of Group III/IV afferent input uncertain.
The purpose of this study is to characterize H-reflex homosynaptic PAD in an online fashion during a sustained sub-maximal fatigue task. The task required a high motor output (60% MVC) in order to increase the likelihood of creating partial muscle ischemia13 and limit the removal of fatigue metabolites. Based on previous evidence for the inhibitory effect of Group III/IV afferent activation,14 we expected that the magnitude of the un-conditioned H-reflex would decline during the progression of fatigue. And based on the available evidence from fatigue studies that employed strong, intermittent contractions,12,25 we expected to observe a diminution of PAD during the development of fatigue.
2 |. METHODS
2.1 |. Subjects
Eleven healthy adults (eight men, three women; height = 181.3 ± 7.6 cm; weight = 76.7 ± 10.9 kg; age = 25.2 ± 3.7 years) volunteered to participate in the study. The subjects were free from any orthopedic, neuromuscular, and medical problems. Subjects signed an informed consent document approved by The University of Iowa Human Subjects Institutional Review Board.
2.2 |. Mechanical recordings
Subjects sat on a KinCom isokinetic dynamometer (Kin-Com 125 E Plus; Chattecx Corporation). The left hip was flexed to 125°, the knee was flexed to 90°, and the leg was blocked with bolsters to prevent hip rotation. The ankle was placed in neutral position. The center of the ankle joint was aligned with the axis of rotation of the KinCom footplate. The foot was secured to the footplate by hook-and-loop straps, and the subject’s trunk was secured to the table with a belt.
2.3 |. Electrical recordings
Soleus M-waves and H-reflexes were elicited by transcutaneous electrical stimulation of the tibial nerve in the popliteal fossa. The tibial nerve was stimulated with a square pulse of 1000 μs delivered by a constant current stimulator (Digitimer model DS7A). The stimulator output was triggered by a digital pulse from a computer controlled by a custom program. The double-pronged surface stimulating electrode was positioned in the popliteal fossa with the cathode proximal to the anode. The electrode was then secured to the knee with a plastic splint and straps.
The skin over the soleus was abraded and cleaned with rubbing alcohol. A bipolar silver-silver chloride surface electrode (1-cm diameter, fixed 2 cm inter-electrode distance) was placed over the soleus muscle at the mid-dorsal line, distal to the insertion of the gastrocnemius on the Achilles tendon. A reference electrode was positioned on the anterior surface of the distal tibia. All the electrodes were secured with an elastic bandage.
EMG signals were pre-amplified on-site by a factor of 35 before being differentially amplified. The differential amplifier had an input impedance of 15MΏ at 100 Hz, a frequency response of 15–1000 Hz, a common mode rejection ratio of 87 dB at 60 Hz, and gain of 500–10 K times. EMG signals were monitored on an oscilloscope throughout the experiment.
2.4 |. Experimental protocol
Three 2–3 seconds plantar flexion maximum voluntary contractions (MVCs) were obtained. 60–90 seconds of rest separated each attempt, and strong verbal encouragement was given. The greatest force from the three trials was taken as the MVC and used to calculate the target force for the fatigue protocol (60% of MVC).
Two maximum M-waves (Mmax) were obtained via supramaximal electrical stimulation. The stimulus intensity was reduced until H-reflexes could be elicited at the target amplitude (15%–20% Mmax) on the ascending limb of the H-reflex recruitment curve. Paired pulses (doublets) separated by 500 ms (2 Hz) were delivered every 15 seconds to elicit paired H-reflexes (H1 and H2). Two Hz stimulation has been shown to elicit homosynaptic depression of the H-reflex in healthy participants.6 An interval greater than 8 seconds between doublets is recommended to avoid the inhibitory effect of PAD upon subsequent H1 responses.26 Ten paired H-reflexes were measured prior to the fatigue protocol (2.25 minutes). These H-reflexes were normalized to Mmax.
The fatigue protocol consisted of isometric contraction of the left plantar flexors at 60% MVC. Subjects received visual feedback of the plantar flexor force and the target force via an oscilloscope. Subjects were required to hold the target force until exhaustion. Time of exhaustion was noted when the subjects failed to match the target force on more than three attempts even after strong verbal encouragement. The time for torque decline below 60% MVC showed considerable individual variation (range = 5–12 minutes).
Maximum M-waves and paired H-reflexes were elicited at 15-second intervals during the fatigue protocol. The stimulator output was adjusted as needed to ensure uniform size of the small m-waves preceding the H-reflexes. Each H-reflex was normalized to its immediately preceding Mmax: This served to mitigate possible H-reflex fluctuation due to mechanical factors (eg, degree of muscle shortening of the portion of the muscle underlying the EMG electrode).27,28 Immediately after the subjects reached exhaustion, Mmax and five more paired H-reflexes were elicited at 15-second intervals (1.25 minutes post-fatigue).
2.5 |. Data analysis
The peak-to-peak amplitudes of all H-reflexes and M-waves were determined in an automated fashion according to their latencies. H-reflex pairs were rejected from analysis if H1 was not elicited at the target amplitude (15%–20% Mmax). The fatigue period was divided into three phases: start of fatigue (F1), mid-fatigue (F2), and late-fatigue (F3). F1 was obtained from the H-reflex pair elicited closest to 25% of each subject’s total fatigue time. F2 and F3 were similarly obtained at 50% and at 75% of fatigue time, respectively. We opted to sample just one H1/H2 pair at F1, F2, and at F3 because our priority was to estimate the instantaneous state of PAD at these time points. The opposite approach, creating a mean H1/H2 ratio for multiple doublets surrounding each time point, would have yielded a smoothed estimate of PAD that negated potential sources of instantaneous fluctuation (head movement, etc).
H-reflex depression was analyzed before (PRE), during (F1, F2, F3), and immediately after (POST) the fatigue protocol. The H-reflex depression ratio was calculated as the normalized amplitude of H2 divided by the normalized amplitude of H1 for each doublet pair.
The amplitudes of H1 and of the small M-waves accompanying H1 and H2 were analyzed to verify stimulus consistency. Normalized H1 amplitude provides an indicator of the percentage of the soleus motor neuron pool that was recruited during the experiment.
2.6 |. Statistical analysis
Data are reported as mean (±SD) within the text and as mean (±SE) in the figures to enhance visual clarity. A one-way repeated measures analysis of variance (ANOVA) was used to analyze changes across time (PRE, F1, F2, F3, and POST) for each of the five dependent variables: H1, H2, H-reflex depression ratio, Mmax, and the small m-waves accompanying the H-reflexes. Each of these five ANOVAs passed the assumption of equal variance (all P > 0.193), supporting that sources of error remained consistent across the five time points (PRE, F1, F2, F3, and POST). Pairwise comparisons (Tukey) were carried out as indicated. Significance for all tests was set at P < 0.05.
3 |. RESULTS
A representative example of paired reflexes recorded before, during, and after the fatigue protocol for a single subject appears in Figure 1. Figure 2 depicts a second representative example of the entire experiment showing the force, EMG, and H-reflexes recorded during the fatigue protocol.
FIGURE 1.
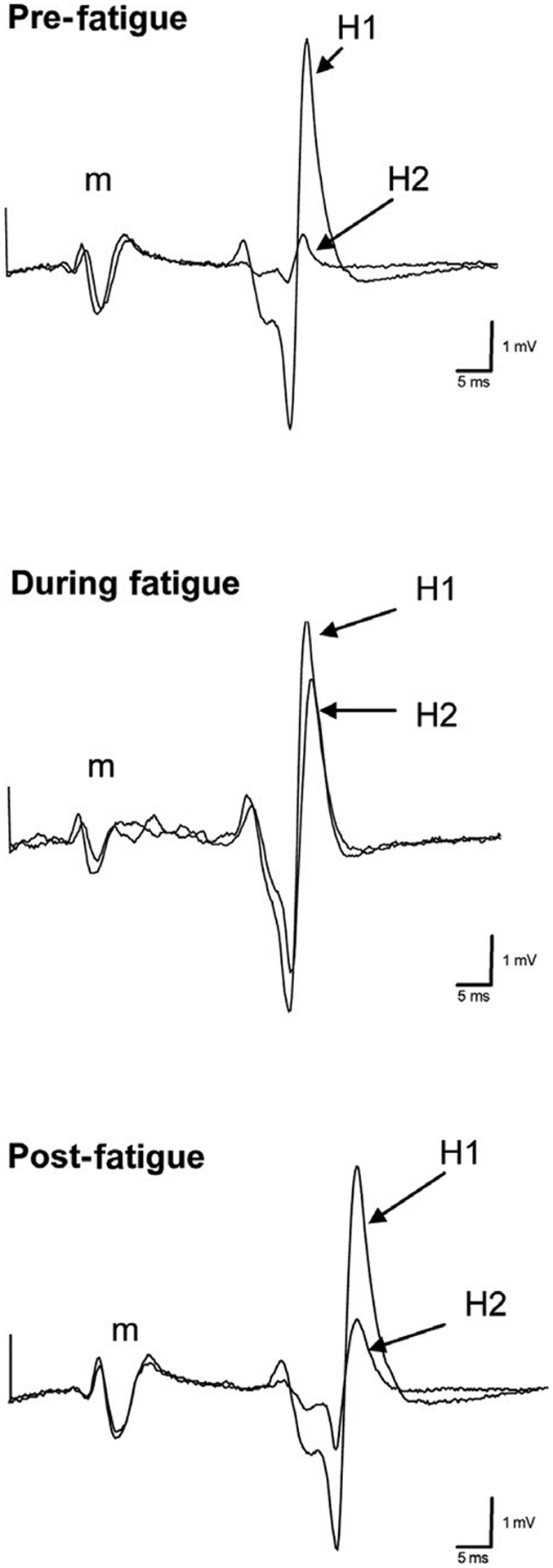
Representative example of H-reflex depression before, during, and after fatigue. Note the consistency of the small m-wave associated with the H-reflex for both H1 and H2 in all conditions
FIGURE 2.
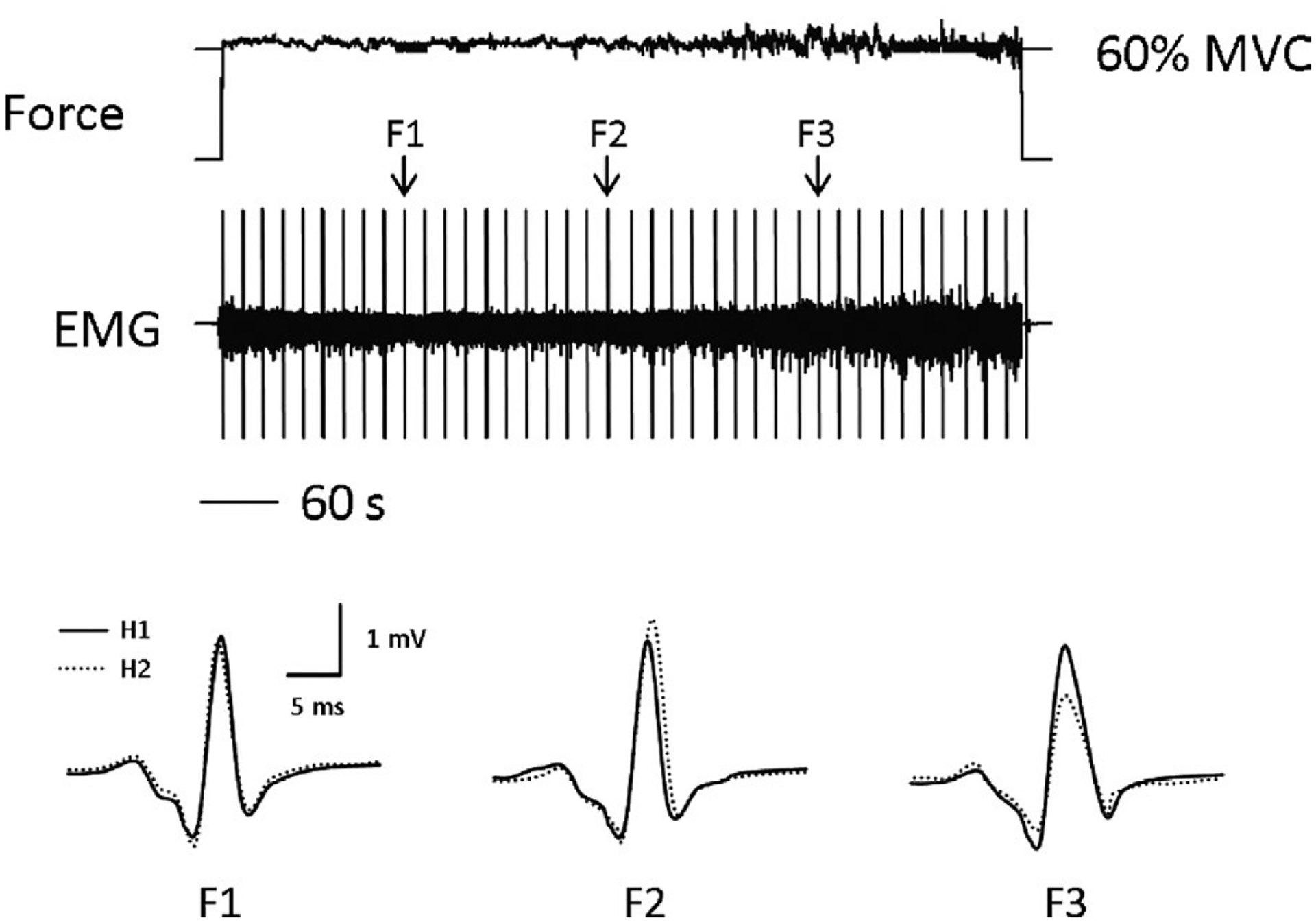
Force, EMG, and H-reflexes measured during the fatigue task. Note that there is more fluctuation in force toward the end of the fatigue task, when peak-to-peak EMG is maximal
Figure 3 depicts H-reflex responses before, during, and after the fatigue protocol. H1 amplitude was 15.9(9.0) %Mmax pre-fatigue and 15.3(9.4) %Mmax post-fatigue, compared to 11.3(8.8), 12.8(9.7), and 12.6(9.8) %Mmax at F1, F2, and F3, respectively (Figure 3A). Despite this trend toward reduced H-reflex excitability during the fatiguing contraction, H1 amplitude did not vary significantly as a function of time (F(4,10) = 2.03, P = 0.109). In contrast, H2 amplitude showed a significant effect of time over the course of the experiment (F(4,10) = 3.86, P = 0.01). H2 amplitude was 6.2 (3.9) %Mmax pre-fatigue, rose to 11.3 (8.7), 12.7 (10.5), and 10.2 (10.5) %Mmax at F1, F2, and F3, and then declined to 7.6 (6.4) %Mmax at the cessation of contraction (Figure 3B). A significant pairwise difference existed between F2 and PRE (P = 0.013).
FIGURE 3.
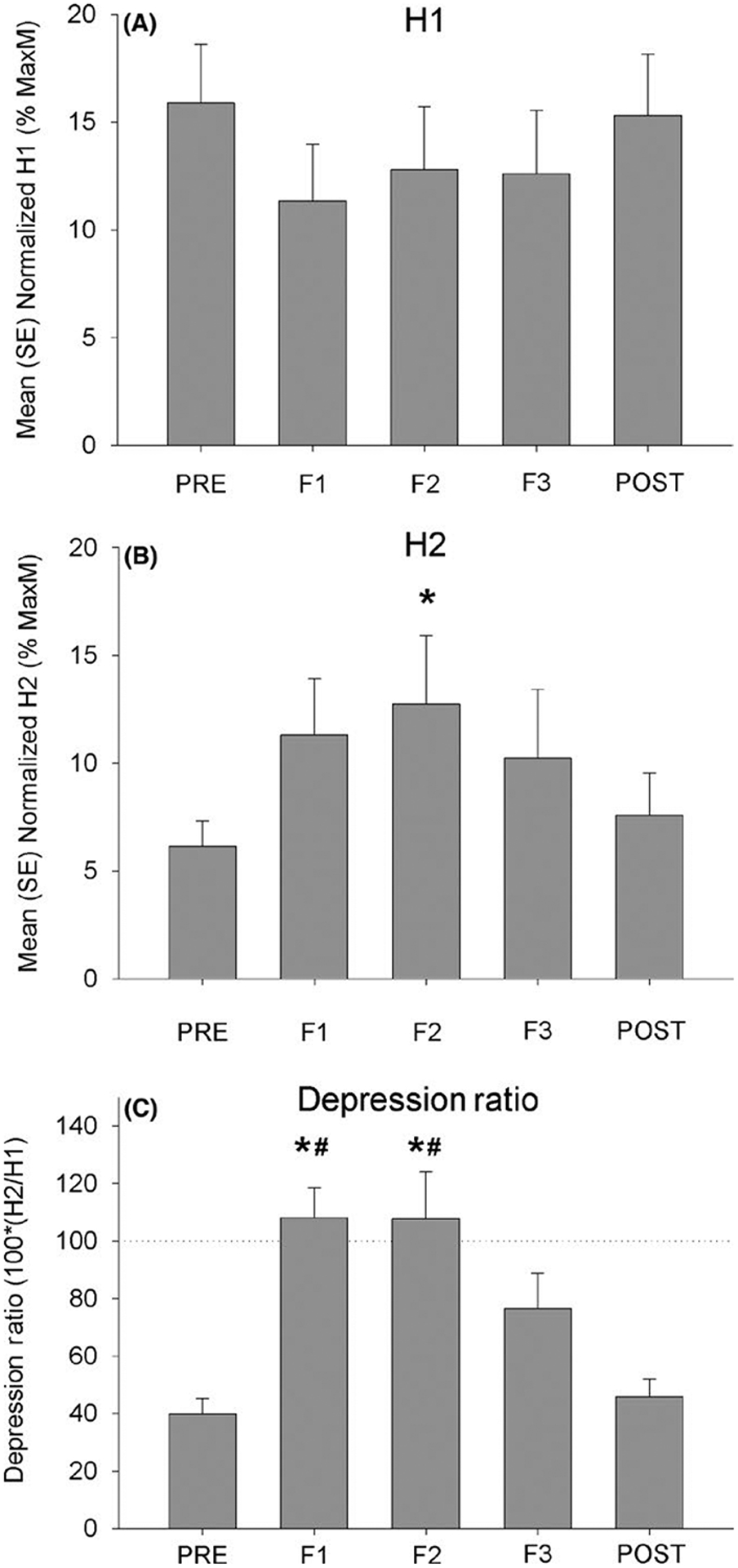
H-reflex amplitude before, during (F1, F2, and F3), and after the fatigue task. *Significantly different from PRE (P < 0.05). #Significantly different from POST (P < 0.05)
The trend toward reduced H1 and the significant enhancement of H2 during the fatiguing contraction combined to create a significant change in the depression ratio over time (F(4,10) = 12.78, P ≤ 0.001). A pre-fatigue depression ratio of 39.9 (18.0)% was followed by relative facilitation of H2 compared to H1 at the first two fatigue intervals (108.1 (34.0)% and 107.7 (54.6)% for F1 and F2, respectively) (Figure 3C). The depression ratio at F3 declined to 76.6 (41.1)%, followed by a further decline to 45.8 (20.3)% post-fatigue. Significant pairwise differences existed between F1 and the PRE- and POST-fatigue conditions (both P < 0.001). Likewise, significant differences existed between F2 and the PRE and POST conditions (both P < 0.001).
3.1 |. Stimulation consistency
A significant effect of time emerged for Mmax amplitude (F(4,10) = 3.08, P = 0.027). However, no significant pairwise differences in Mmax amplitude existed among the experimental time points (all P > 0.054) (Figure 4A). The amplitude of the small m-waves accompanying the H-reflexes did not differ at any time point (F(4,10) = 0.079, P = 0.99) (Figure 4B).
FIGURE 4.
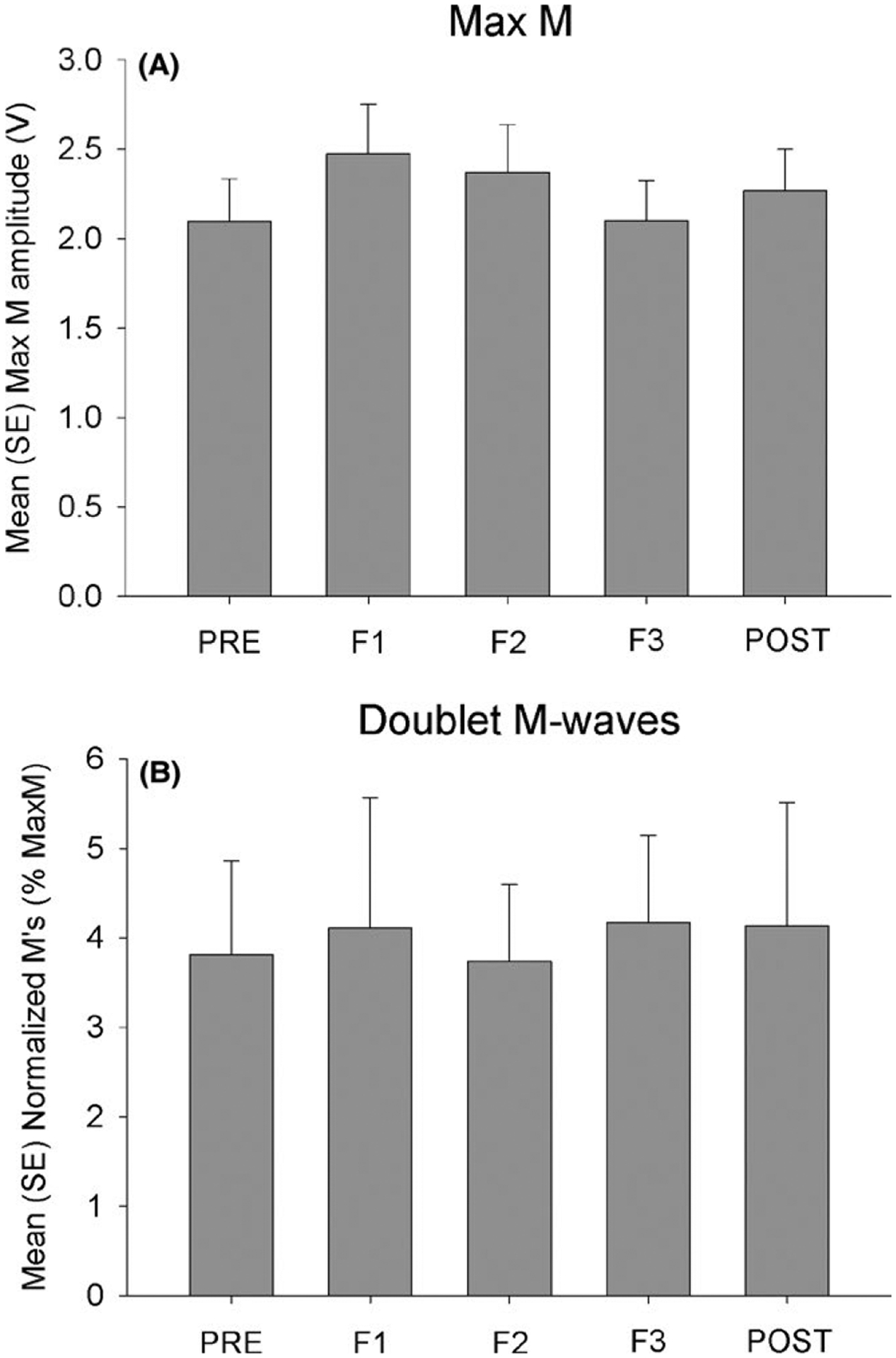
Stimulus consistency before, during (F1, F2, and F3), and after the fatigue task
4 |. DISCUSSION
The purpose of this study was to characterize H-reflex homosynaptic PAD in an online fashion during a sustained sub-maximal fatigue task. Our results indicate that conditioned H-reflex (H2) amplitude increased during fatigue and then declined near the point of exhaustion. Thus during sustained submaximal contraction, PAD demonstrated a biphasic relationship; first decreasing (higher depression ratio) and then normalizing as exhaustion approached.
4.1 |. H-reflex excitability and potential metabolic factors
In the present study, we maintained doublet m-wave amplitude at a consistent level, supporting that the initial stimulation volley recruited a uniform population of soleus motor neurons throughout the experiment. Despite this stimulus consistency, we observed a trend toward reduction of H1 amplitude within the first 25% of the fatigue time, indicating a partial reduction in H-reflex excitability that did not reach statistical significance. H1 amplitude did not change further as exhaustion approached nor did it differ significantly in the rest phase after cessation of contraction (POST). Previous studies that elicited H-reflexes during sustained, fatiguing contractions revealed the importance of contraction intensity in the determination of H-reflex excitability. Low contraction levels (~20% to 30% MVC) led to an increase in H-reflex amplitude,22 in some cases followed by a plateau at about 40% of the total fatigue time.10 Another study observed a progressive decline in H-reflex amplitude during fatigue for both a low (25% MVC) and a higher (50% MVC) level of contraction.7 These authors highlighted the probable role of fatigue metabolites in reducing H-reflex amplitude during sustained contractions, which begs the question of whether 25% MVC and 50% MVC sustained contractions may be sufficient to yield partial muscle ischemia. In the quadriceps muscle, arterial blood flow at 25% and 50% MVC was lower than at <15% MVC.13 Likewise, blood lactate and plasma K+ concentrations were substantially higher at 25% and 50% MVC, supporting that sustained submaximal contractions of this intensity led to the accumulation of fatigue metabolites. It is likely, therefore, that in the sustained 60% MVC fatigue protocol used in the present study, Group III/IV afferent fiber activation exerted an inhibitory influence on H-reflex excitability as fatigue progressed.14 Recent studies that measured muscle metabolism via biopsy and magnetic resonance spectroscopy likewise support a role for metabolite accumulation during fatigue.16,17 The central processes that integrate Group III/IV afferent input to prevent excessive exercise-induced intramuscular metabolic derangement are currently under investigation.29
4.2 |. Biphasic post-activation depression
To examine homosynaptic PAD, we capitalized on a doublet stimulation protocol30 instead of a more traditional 10-pulse protocol.25 This approach enabled us to reliably assess PAD with the fewest pulses possible, reducing an important source of discomfort, distraction, and force variability as the subjects attempted to maintain a relatively strong contraction (60% MVC). During the sustained contraction, we observed an enhancement of H2 that reached statistical significance at the midpoint of the fatigue task (F2). Expressing H2 relative to H1 (depression ratio) facilitated interpretation of H2 amplitude in the context of overall excitability of the H-reflex pathway. Depression ratio was significantly elevated early and midway during the fatigue protocol (F1 and F2), indicating a relative dis-inhibition of H2 (a reduction of PAD). Depression ratio values >100% indicated that H2 actually exceeded H1 amplitude: Inspection of the data revealed that this was the case for 9 of the 11 subjects. This reduction of PAD waned by 75% of fatigue time (F3), revealing a biphasic behavior of PAD as exhaustion approached.
Stein and coauthors highlighted the important differences in PAD that occur between rest and voluntary contraction, concluding that “Measurements at rest do not accurately represent the H-reflex during motor tasks”.6 In their work and in other reports, maintenance of a voluntary contraction yields a reduction in PAD and a concomitant increase in conditioned H-reflex amplitude,9,25,31,32 similar to what we observed at F1 and F2. None of these reports examined fatigue to exhaustion. Of the several previous Groups who have assessed H-reflexes in an online fashion during fatiguing contractions, rather than in an intermittent rest cycle,7,10,22,23 none examined homosynaptic PAD. Two studies examined post-fatigue PAD after an intermittent MVC protocol, with the subjects maintaining a low-level contraction (20%–30% MVC) for acquisition of conditioned H-reflexes.9,25 These studies offered the best prediction of the behavior of PAD that might be expected in the present study. Similar to our findings, Takahashi and coauthors reported a significant reduction in homosynaptic PAD in fatigued muscle.25 Our study extends these findings to reveal an apparent restoration of PAD as exhaustion approaches.
The results of the present study underscore the principle that H-reflex amplitude reflects a complex interplay between central factors, peripheral factors, and task demands. Initially, maintenance of muscle contraction triggers a decline in PAD,6,9,25,31,32 but this process appears to be sensitive to physiologic changes that occur as fatigue intensifies. Given the probable ischemic conditions created by the 60% MVC used in the present study, we believe it is likely that Group III/IV afferent inhibition plays a role in the biphasic behavior of PAD as exhaustion approaches. The manifestation of afferent inhibition is likely to be related to muscle training status, as physical activity yields enhancements in cellular ion buffering capacity and capillary clearance of metabolites.33 The wide range of task failure times observed in the present study (5–12 minutes) suggests that participant training status may have played a role in the changes in PAD observed during fatigue. The interplay between such peripheral influences and fluctuations in central drive34 are an important area for future investigation.
5 |. PERSPECTIVE
H-reflex homosynaptic post-activation depression decreased during the first half of a submaximal fatigue protocol and then returned as exhaustion approached. The fatigue task likely fostered conditions of partial muscle ischemia and accumulation of fatigue metabolites, supporting a role for Group III/IV afferent activation in the biphasic behavior of PAD.
ACKNOWLEDGEMENTS
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development under grants R01-HD084645 and R01-HD082109.
Funding information
Eunice Kennedy Shriver National Institute of Child Health & Human Development, Grant/Award Number: R01-HD084645 and R01-HD082109
REFERENCES
- 1.Calancie B, Broton JG, Klose KJ, Traad M, Difini J, Ayyar DR. Evidence that alterations in presynaptic inhibition contribute to segmental hypo- and hyperexcitability after spinal cord injury in man. Electroencephalogr Clin Neurophysiol. 1993;89:177–186. [DOI] [PubMed] [Google Scholar]
- 2.Grey MJ, Klinge K, Crone C, et al. Post-activation depression of soleus stretch reflexes in healthy and spastic humans. Exp Brain Res. 2008;185:189–197. [DOI] [PubMed] [Google Scholar]
- 3.Yen CL, McHenry CL, Petrie MA, Dudley-Javoroski S, Shields RK. Vibration training after chronic spinal cord injury: Evidence for persistent segmental plasticity. Neurosci Lett. 2017;647:129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shields RK, Dudley-Javoroski S, Oza PD. Low-frequency H-reflex depression in trained human soleus after spinal cord injury. Neurosci Lett. 2011;499:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler AJ, Yue G, Darling WG. Variations in soleus H-reflexes as a function of plantarflexion torque in man. Brain Res. 1993;632:95–104. [DOI] [PubMed] [Google Scholar]
- 6.Stein RB, Estabrooks KL, McGie S, Roth MJ, Jones KE. Quantifying the effects of voluntary contraction and inter-stimulus interval on the human soleus H-reflex. Exp Brain Res. 2007;182:309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duchateau J, Balestra C, Carpentier A, Hainaut K. Reflex regulation during sustained and intermittent submaximal contractions in humans. J Physiol. 2002;541:959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oza PD, Dudley-Javoroski S, Shields RK. Dynamic fatigue does not alter soleus H-reflexes conditioned by homonymous or heteronymous pathways. Motor Control. 2016;21:345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nordlund MM, Thorstensson A, Cresswell AG. Central and peripheral contributions to fatigue in relation to level of activation during repeated maximal voluntary isometric plantar flexions. J Appl Physiol. 2004;96:218–225. [DOI] [PubMed] [Google Scholar]
- 10.Loscher WN, Cresswell AG, Thorstensson A. Excitatory drive to the alpha-motoneuron pool during a fatiguing submaximal contraction in man. J Physiol. 1996;491(Pt 1):271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manca M, Cavazzini L, Cavazza S, Salvadori T, DeGrandis D, Basaglia N. H reflex excitability following voluntary muscle contraction of different duration. Electromyogr Clin Neurophysiol. 1998;38:381–384. [PubMed] [Google Scholar]
- 12.Iguchi M, Shields RK. Cortical and segmental excitability during fatiguing contractions of the soleus muscle in humans. Clin Neurophysiol. 2012;123:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sjogaard G, Savard G, Juel C. Muscle blood flow during isometric activity and its relation to muscle fatigue. Eur J Appl Physiol Occup Physiol. 1988;57:327–335. [DOI] [PubMed] [Google Scholar]
- 14.Pettorossi VE, Della Torre G, Bortolami R, Brunetti O. The role of capsaicin-sensitive muscle afferents in fatigue-induced modulation of the monosynaptic reflex in the rat. J Physiol. 1999;515(Pt 2):599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garland SJ. Role of small diameter afferents in reflex inhibition during human muscle fatigue. J Physiol. 1991;435:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cairns SP, Inman L, MacManus CP, et al. Central activation, metabolites, and calcium handling during fatigue with repeated maximal isometric contractions in human muscle. Eur J Appl Physiol. 2017;117:1557–1571. [DOI] [PubMed] [Google Scholar]
- 17.Cannon DT, Howe FA, Whipp BJ, et al. Muscle metabolism and activation heterogeneity by combined 31P chemical shift and T2 imaging, and pulmonary O2 uptake during incremental knee-extensor exercise. J Appl Physiol. 1985;2013(115):839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohn AF, Floeter MK, Hallett M. Presynaptic inhibition compared with homosynaptic depression as an explanation for soleus H-reflex depression in humans. Exp Brain Res. 1997;116:375–380. [DOI] [PubMed] [Google Scholar]
- 19.Hultborn H, Illert M, Nielsen J, Paul A, Ballegaard M, Wiese H. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res. 1996;108:450–462. [DOI] [PubMed] [Google Scholar]
- 20.Tahayori B, Tahayori B, Koceja D. Characteristics of preceding Ia activity on postactivation depression in health and disease. J Neurophysiol. 2015;113:3751–3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oza PD, Dudley-Javoroski S, Shields RK. Modulation of H-reflex depression with paired-pulse stimulation in healthy active humans. Rehabil Res Pract. 2017;2017:5107097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patikas DA, Bassa H, Kotzamanidis C. Changes in the reflex excitability during and after a sustained, low-intensity muscle contraction. Int J Sports Med. 2006;27:124–130. [DOI] [PubMed] [Google Scholar]
- 23.Levenez M, Kotzamanidis C, Carpentier A, Duchateau J. Spinal reflexes and coactivation of ankle muscles during a submaximal fatiguing contraction. J Appl Physiol. 1985;2005(99):1182–1188. [DOI] [PubMed] [Google Scholar]
- 24.Baudry S, Maerz AH, Gould JR, Enoka RM. Task- and time-dependent modulation of Ia presynaptic inhibition during fatiguing contractions performed by humans. J Neurophysiol. 2011;106:265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi R, Endoh T, Nakajima T, Komiyama T. Modulation of homosynaptic depression during voluntary contraction and muscle fatigue with different test reflex size. J Phys Fitness Sports Med. 2013;2:251–258. [Google Scholar]
- 26.Crone C, Nielsen J. Methodological implications of the post activation depression of the soleus H-reflex in man. Exp Brain Res. 1989;78:28–32. [DOI] [PubMed] [Google Scholar]
- 27.Zehr PE. Considerations for use of the Hoffmann reflex in exercise studies. Eur J Appl Physiol Occup Physiol. 2002;86:455–468. [DOI] [PubMed] [Google Scholar]
- 28.Chang YJ, Shields RK. Within-train neuromuscular propagation varies with torque in paralyzed human muscle. Muscle Nerve. 2002;26:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blain GM, Mangum TS, Sidhu SK, et al. Group III/IV muscle afferents limit the intramuscular metabolic perturbation during whole body exercise in humans. J Physiol. 2016;594:5303–5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oza PD, Dudley-Javorosk S, Shields RK. Modulation of H-reflex depression with paired-pulse stimulation in healthy active humans. Rehabil Res Pract 2017;2017:5107097 In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clair JM, Anderson-Reid JM, Graham CM, Collins DF. Postactivation depression and recovery of reflex transmission during repetitive electrical stimulation of the human tibial nerve. J Neurophysiol. 2011;106:184–192. [DOI] [PubMed] [Google Scholar]
- 32.McNulty PA, Jankelowitz SK, Wiendels TM, Burke D. Postactivation depression of the soleus H reflex measured using threshold tracking. J Neurophysiol. 2008;100:3275–3284. [DOI] [PubMed] [Google Scholar]
- 33.Juel C, Klarskov C, Nielsen JJ, Krustrup P, Mohr M, Bangsbo J. Effect of high-intensity intermittent training on lactate and H+ release from human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E245–251. [DOI] [PubMed] [Google Scholar]
- 34.Sogaard K, Gandevia SC, Todd G, Petersen NT, Taylor JL. The effect of sustained low-intensity contractions on supraspinal fatigue in human elbow flexor muscles. J Physiol. 2006;573:511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]


