Abstract
BACKGROUND & AIMS:
The incidence and mortality of early-onset colorectal cancer (CRC) are increasing. Adenoma detection, removal, and subsequent endoscopic surveillance might modify risk of CRC diagnosed before age 50 years (early-onset CRC). We conducted a systematic review of young-onset adenoma (YOA) prevalence, associated risk factors, and rate of metachronous advanced neoplasia after YOA diagnosis.
METHODS:
We performed a systematic search of multiple electronic databases through February 12, 2019 and identified studies of individuals 18 to 49 years old that reported prevalence of adenoma, risk factors for adenoma, and/or risk for metachronous advanced neoplasia. Summary estimates were derived using random effects meta-analysis, when feasible.
RESULTS:
The pooled overall prevalence of YOA was 9.0% (95% CI, 7.1%–11.4%), based on 24 studies comprising 23,142 individuals. On subgroup analysis, the pooled prevalence of YOA from autopsy studies was 3.9% (95% CI, 1.9%–7.6%), whereas the prevalence from colonoscopy studies was 10.7% (95% CI, 8.5%–13.5). Only advancing age was identified as a consistent risk factor for YOA, based on 4 studies comprising 78,880 individuals. Pooled rate of metachronous advanced neoplasia after baseline YOA diagnosis was 6.0% (95% CI, 4.1%–8.6%), based on 3 studies comprising 1493 individuals undergoing follow-up colonoscopy, with only 1 CRC case reported. Overall, few studies reported metachronous advanced neoplasia and no studies evaluated whether routine surveillance colonoscopy decreases risk of CRC.
CONCLUSIONS:
In a systematic review, we estimated the prevalence of YOA to be 9% and to increase with age. Risk for metachronous advanced neoplasia after YOA diagnosis is estimated to be 6%. More research is needed to understand the prevalence, risk factors, and risk of CRC associated with YOA.
Keywords: Young Adult, Colon, Tumor, Neoplasm
Colorectal cancer (CRC) is the third most common cancer worldwide and the second most common cause of cancer mortality, with an incidence of 1.8 million and 881,000 deaths in 2018.1 In the United States, CRC incidence and mortality have been declining among older adults.2 In contrast, CRC incidence and mortality have increased among adults younger than age 50. Between 1975 and 1980, the overall incidence rate of early-onset CRC was 9.9 per 100,000, with an increase to 11.7 per 100,000 between 2010 and 2014.3 Although factors responsible are not well-understood, postulated risk factors include male sex, obesity, smoking, alcohol intake, antibiotic exposure, and dietary changes such as exposure to more processed foods.4–9
Adenomas are the precursors of most CRCs, and adenoma removal can reduce CRC incidence and mortality.10–14 On the basis of observation of the impact of polypectomy and surveillance outcomes among older individuals, systematic detection and removal of adenomas with subsequent surveillance may have the ability to improve early detection and prevention of early-onset CRC.11–15 Clinical experience suggests that adenomas are detected among individuals younger than age 50 (young-onset adenoma [YOA]). However, YOA prevalence, risk factors associated with YOA, rates of metachronous advanced neoplasia and CRC after polypectomy, and whether surveillance has potential to reduce risk for advanced adenoma or CRC on follow-up have not been well-characterized. Clarifying these issues will help determine whether detection, removal, and surveillance of adenomas have potential to address rising early-onset CRC incidence and mortality.
To address this literature gap, we conducted a systematic review of the prevalence, risk factors, and risk of metachronous advanced neoplasia and CRC in individuals with YOA to specifically address the following key questions:
Among individuals age 18–49, what is the prevalence of YOA?
Among individuals age 18–49, what are potential risk factors associated with YOA?
Among individuals with YOA, what is the risk for metachronous advanced neoplasia on follow-up colonoscopy?
Among individuals with YOA, what is the risk for subsequent CRC on follow-up colonoscopy?
Among individuals with YOA, does exposure to surveillance colonoscopy, versus no surveillance, reduce risk for CRC on follow-up?
Methods
Study Design
We conducted and reported a systematic review following the recommendation of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.16 Details of the protocol for this systematic review were registered on PROSPERO (registration #CRD42019125508).17
Search Strategy
We searched the Embase (Elsevier) and PubMed from inception until February 12, 2019. The search was developed with the help of an experienced librarian (KMH) (Supplementary Material). Additional records were identified through review of reference sections of included studies and reviewed in full text if they met title and abstract review criteria (Supplementary Material).
Selection Criteria
Two individuals (NE, MYC) independently reviewed identified abstracts for eligibility. All abstracts reporting on outcomes related to our key questions for individuals aged 18–49 years were selected for full-text review. Disagreements were resolved by involving a third author (SG). The same 2 reviewers then conducted a full-text review of articles meeting inclusion criteria and of articles for which there was some uncertainty as to eligibility (Supplementary Material). Articles focused on patients with inflammatory bowel disease, hereditary CRC syndromes, or family history of CRC were excluded.
Data Abstraction and Risk of Bias/Quality Assessment
Two individuals (NE, MYC) conducted data abstraction, including study characteristics such as author, year of publication, study design/setting, time period of colonoscopy, and the total sample size. Outcome data abstracted included risk factors for YOA and their respective odds ratios (ORs) from multivariate analysis, number of patients with YOA receiving follow-up colonoscopy, proportion of individuals with baseline adenoma with advanced neoplasia on follow-up, and proportion of individuals with baseline adenoma with CRC on follow-up. Risk of bias/quality was assessed by both reviewers for each study by using a structured approach (Supplementary Material).
Data Synthesis and Statistical Analyses
Key Question 1 on the prevalence of YOA and Key Question 3 on the rate of metachronous neoplasia on follow-up had sufficient data for pooled estimates. For these 2 questions, we pooled corresponding data using the random-effects model described by DerSimonian and Laird.18
For adenoma prevalence, the outcome was expressed as a pooled proportion, with 95% confidence intervals (CIs). Pre-planned subgroup analyses were based on study type (colonoscopy vs autopsy studies) and publication date (before vs after 1995). The year 1995 was used as the cutoff for these publication date analyses because it was the year after which early-onset CRC began to rise.3 For rate of metachronous neoplasia on follow-up, the outcome was expressed as a proportion, with 95% CIs. We assessed statistical heterogeneity by using I2 statistic.19 All analyses were performed by using Comprehensive Meta-Analysis version 2 (Biostat, Englewood, NJ). Small study effects were assessed by examining funnel plot asymmetry (Supplementary Material).
Results
Literature Review
Figure 1 summarizes the literature review process. Out of 2063 unique references, 830 were selected for abstract review on the basis of title assessment, 451 were selected for full-text review after abstract assessment, and an additional 11 studies were identified after reviewing the reference sections of included studies. Ultimately, 28 studies were included in the systematic review on the basis of prespecified criteria.20–44 Our search strategy did not identify any article that addressed the impact of surveillance colonoscopy in patients with YOA on incidence and mortality from CRC.
Figure 1.
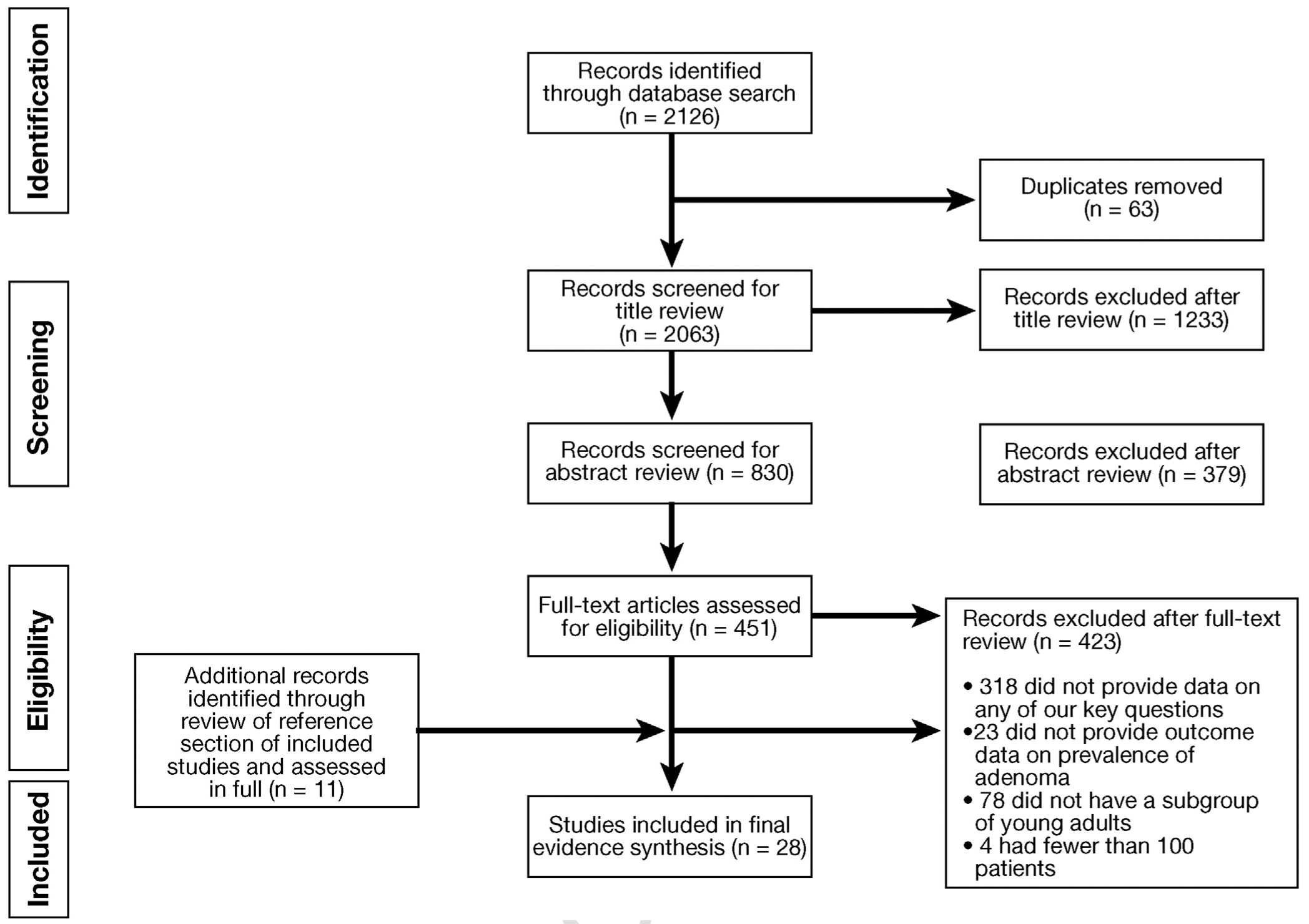
Study selection PRISMA flow diagram.
Supplementary Table 1 describes the characteristics and quality of each included study, assessed as low, moderate, or high quality. Overall, 89% of included studies (n = 25/28) were judged to be of at least mod erate quality, with the remaining 11% (n = 3/28) judged to be of low quality.
Key Question 1: Among Individuals Ages 18–49, What Is the Prevalence of Young-Onset Adenoma?
The 24 studies addressing YOA prevalence included 5 autopsy studies (n = 1638)36–40 and 19 studies of patients undergoing colonoscopy (n = 19,295; Supplementary Table 2).20–26,28–32,34,35,41–43,45,46 Of the 19 studies of patients undergoing colonoscopy, 7 studies were performed on symptomatic patients alone,20,21,23,30,32,41,43 6 on asymptomatic patients,22,28,31,34,43,47 and 6 did not specify whether patients were symptomatic or not.24,26,29,35,42,45 The time period for assessment of our outcome data ranged from 1972 to 2017, with 19 of them conducted in patients undergoing colonoscopy after 1995, 3 studies before 1995, and 2 that began before 1995 but continued past this time. All the autopsy studies were performed before 1995. Of the studies providing prevalence data, 7 studies were performed in North America (all in the United States), 1 study in South America (Brazil), 6 studies in Europe (2 Italy, 1 Poland, 1 Greece, 1 Sweden, 1 Norway), 6 studies in the Middle East (4 Iran, 1 Pakistan, 1 Lebanon), and 4 studies in East Asia (South Korea). These were grouped into Western studies (14 studies) and Afro-Asian studies (10 studies) (Supplementary Table 3).
Pooled prevalence of YOA was estimated to be 9.0% (95% CI, 7.1%–11.4%), with a range of 1.2%–25.4% across studies (Figure 2). Substantial heterogeneity was noted (I2 = 96%). On subgroup analysis, the pooled prevalence of YOA among autopsy studies was 3.9% (95% CI, 1.9%–7.6%), whereas prevalence among colonoscopy studies was 10.7% (95% CI, 8.5%–13.5%); P value for difference between subgroups was <.01 (Figure 3). Pooled prevalence of YOA based on colonoscopies performed before 1995 was 4.2% (95% CI, 7.4%–12.0%), whereas prevalence was 10.0% (95% CI, 7.8%–12.8%) on the basis of studies performed after 1995 (Figure 4). Pooled prevalence based on colonoscopies performed on asymptomatic patients was 13.9% (95% CI, 9.5%–20.1%), whereas pooled prevalence based on colonoscopies performed on symptomatic patients was 8.6% (95% CI, 6.2%–11.7%; P value for differences between subgroups = .05). Pooled prevalence based on Western studies was 9.0% (95% CI, 6.6%–12.1%) versus 9.2% for Afro-Asian studies (95% CI, 6.5%–12.9%; P = .919; Supplementary Figure 1). To assess whether any one study had a dominant effect on the pooled prevalence estimate, each study was individually excluded, and its effect on the main summary estimate and I2 test for heterogeneity was evaluated. No study markedly influenced the overall prevalence of YOA or degree of heterogeneity. Because considerable heterogeneity was observed across all studies, evaluation of publication by bias using funnel plot was not conducted.
Figure 2.
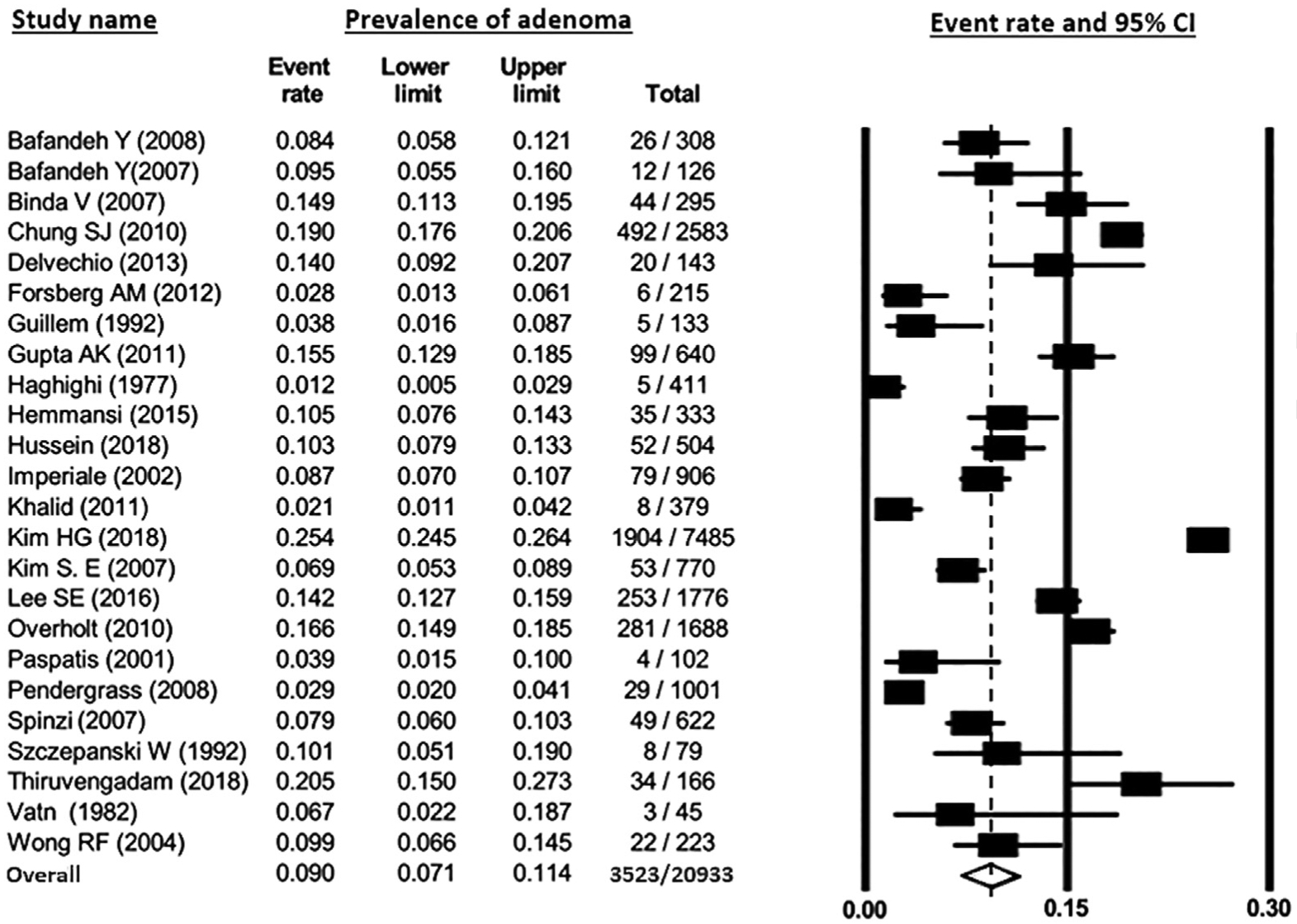
Pooled prevalence of young-onset adenoma. Rectangles denote pooled estimate for each study; open diamond denotes overall pooled estimate for all studies. CI, confidence interval.
Figure 3.
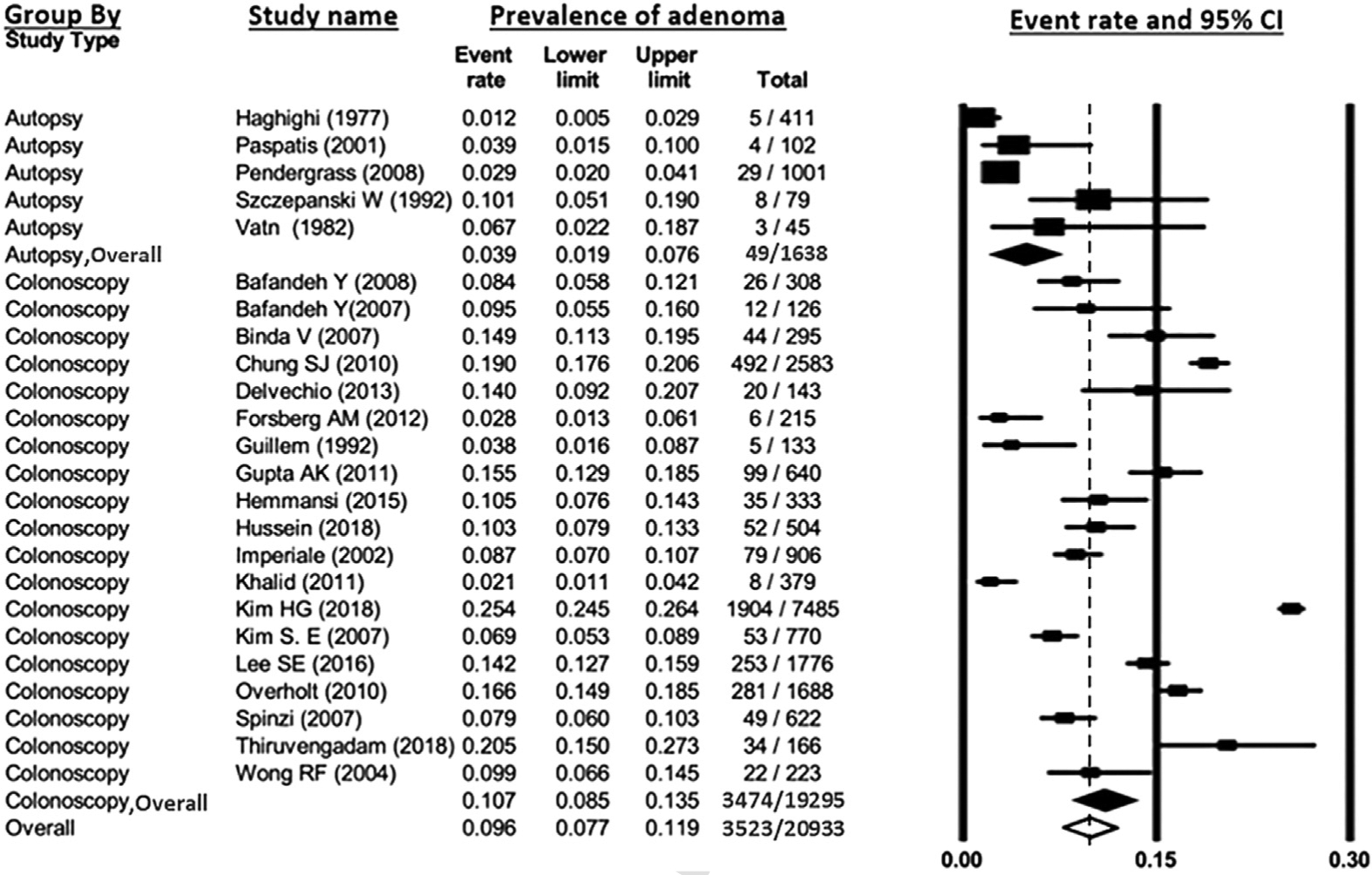
Pooled prevalence of young-onset adenoma, grouped by autopsy versus colonoscopy based-studies. Rectangles denote pooled estimate for each study; filled diamonds denote pooled estimates for the 2 subgroups; unfilled diamond denotes overall pooled estimate for all studies. CI, confidence interval.
Figure 4.

Pooled prevalence of young-onset adenoma, grouped by studies conducted before versus after 1995. Rectangles denote pooled estimate for each study; filled diamonds denote pooled estimates for the 2 subgroups; unfilled diamond denotes overall pooled estimate for all studies. CI, confidence interval.
Key Question 2: Among Individuals Ages 18 to 49, What Are Potential Risk Factors Associated With Young-Onset Adenoma?
Risk factors for YOA were addressed by 4 studies including 78,880 individuals (Supplementary Table C).22,29,35,48 There were 2 studies conducted in South Korea, 1 study in China, and 1 study in the United States. There was 1 multicenter study and 3 single center studies.
The most consistent significant risk factor across all studies was increasing age. Three of 4 studies assessed age as a continuous variable and observed that the likelihood of YOA significantly increased with each unit increase in age (Supplementary Table 4).29,35,48 One study assessed age as a categorical variable and observed a significant increase in the prevalence of YOA as the age category increased: 10.4% in 30–39 years group versus 22.2% in the 40–49 years group (P < .001).22 Male sex was the second most consistently assessed risk factor and was significantly associated with YOA in 2 of 4 studies: Chen et al49 (OR, 2.18; 95% CI, 1.02–4.63) and Gupta et al29 (OR, 1.16; 95% CI, 1.03–1.31).’ Body mass index (BMI) was assessed in 3 of 4 studies, and only 1 of the studies found that the odds of YOA increased with each unit increase in BMI (OR, 1.05; 95% CI, 1.01–1.08).48 Current smoking status was assessed in 2 studies, with 1 observing a significant association with YOA (OR, 2.05; 95% CI, 1.16–3.65).22 Family history of CRC was assessed in 2 studies but was not significantly associated with YOA in either study.
Key Question 3: Among Patients With Young-Onset Adenoma, What Is the Risk for Metachronous Advanced Neoplasia on Follow-up?
The risk of metachronous advanced neoplasia on subsequent follow-up of patients with YOA was reported in 4 articles including 78,880 individuals (Supplementary Table 5). Three of the 4 articles were conducted in South Korea, and 1 was conducted in the United States. Two of the studies were single center studies,50,51 and the other 2 were multicenter studies.33,46 Follow-up times ranged from 33.6 to 49.0 months. One study estimated cumulative incidence (rate) of metachronous advanced neoplasia; this report only provided cumulative incidence for the low and high risk adenoma groups separately and did not provide an overall incidence rate for all adenoma patients combined.46 Three studies reported risk of metachronous advanced neoplasia, defined as the proportion of individuals with baseline adenoma with advanced neoplasia on follow-up.33,52,53 Pooled analysis of metachronous advanced neoplasia was limited to these 3 studies, because the study by Kim et al54 did not provide sufficient data regarding number with adenoma at baseline and number with advanced neoplasia at follow-up to allow for pooling. Pooled risk of metachronous advanced neoplasia was estimated to be 6.0% (95% CI, 4.1%–8.6%; Figure 5). Substantial heterogeneity was noted (I2 = 56%).
Figure 5.
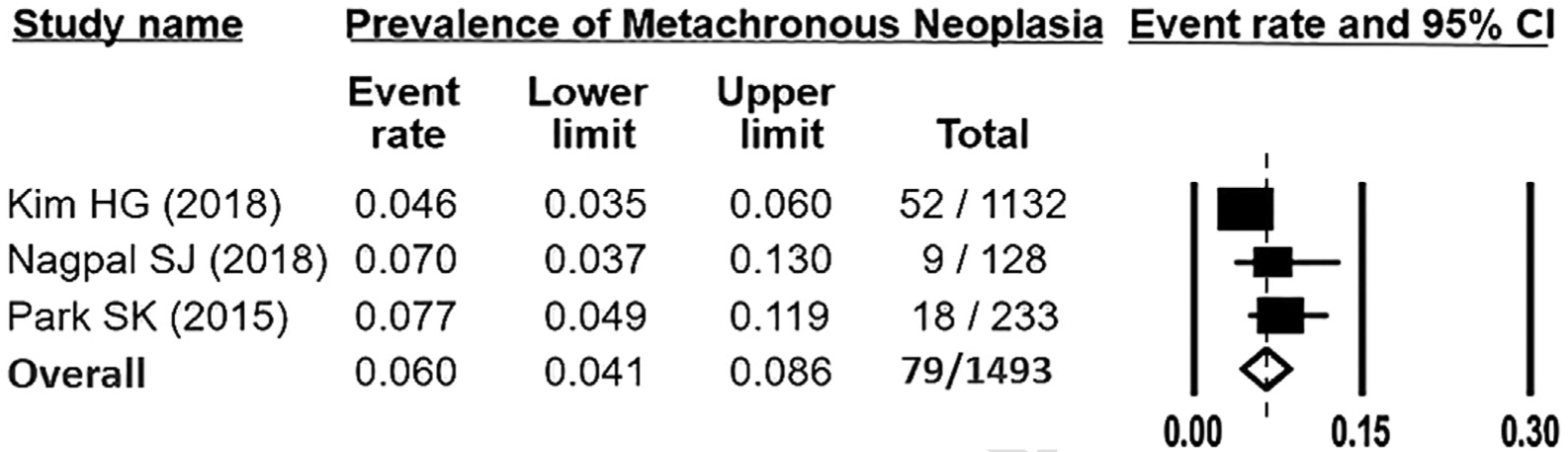
Pooled rate of metachronous advanced neoplasia after baseline young-onset adenoma diagnosis. Rectangles denote pooled estimate for each study; unfilled diamond denotes overall pooled estimate for all studies. CI, confidence interval.
Two studies stratified the rate or risk of advanced neoplasia on follow-up on the basis of whether low risk (defined as having 1–2 tubular adenomas measuring <10 mm in size) vs high risk (defined as having advanced adenomas or ≥3 adenomas) adenomas were present.33,46 Kim HG33 et al found that the cumulative rate of advanced neoplasia was 4.9% among 798 individuals with low risk adenoma at baseline and 3.9% among 334 individuals with high risk adenoma at baseline. Kim NH et al46 found that the 5-year risk of advanced neoplasia on follow-up among individuals with low risk adenoma at baseline was 2.8% for ages 30–39 and 3.3% for ages 40–49, and that the 3-year risk among individuals with high risk adenoma at baseline was 1.9% for ages 30–39 and 3.6% for ages 40–49.
Key Question 4: Among Patients With Young-Onset Adenoma, What Is the Risk for Subsequent Colorectal Cancer?
The 4 articles (n = 78,880) that addressed Question 3 on the risk of metachronous advanced neoplasia also addressed Question 4 (Supplementary Table 6). Across these studies, only 1 case of CRC was reported among 9341 patients (0.01%).
Key Question 5: Among Patients With Young-Onset Adenoma, Does Exposure to Surveillance Colonoscopy, Versus No Surveillance, Reduce Risk for Colorectal Cancer on Follow-up?
We did not identify any study reporting the impact of surveillance colonoscopy in patients with YOA on incidence and mortality from CRC.
Discussion
Adenomas are found in individuals younger than 50, but prevalence, risk factors, and subsequent management and impact have not been previously well-characterized. In a systematic review focusing on 5 key questions concerning YOA, we observed that the prevalence of YOA was 9%. Estimated risks of metachronous advanced neoplasia and CRC were 6% and 0.01%, respectively, although there is a paucity of data for this outcome. Increasing age was found to be the most consistent risk factor for YOA. We did not identify any studies on the impact of routine colonoscopic surveillance in patients with YOA on incidence and mortality from CRC. Our findings may inform current clinical practice as well as future research on YOA and strategies for reducing incidence and mortality from early-onset CRC.
Young-Onset Adenoma Prevalence
In a meta-analysis of 24 studies contributing data from 20,933 individuals, we found the pooled prevalence of YOA was estimated to be 9.0%. Prevalence was substantially lower among autopsy studies (3.9%) compared with colonoscopy studies (10.7%). The lower prevalence observed in autopsy studies could be because these studies are more representative of the general population or because of variation in the protocols used to assess presence of adenomas.55 Higher prevalence observed in colonoscopy studies could be because colonoscopy is more sensitive for adenoma detection than routine autopsy, or because the group of patients referred for colonoscopy younger than age 50 (most often for specific signs or symptoms of disease or family history of CRC) are not representative of the general population. Indeed, 14 of 19 studies included in this evidence synthesis reported findings from patients undergoing colonoscopy for signs or symptoms of possible disease.
In a subgroup analysis, pooled adenoma prevalence was estimated to be 4.2% before and 10.0% after 1995, the year around which early-onset CRC incidence began to increase. This observation could be due to actual increases in YOA prevalence driven by risk factors overlapping with risk factors for early-onset CRC or temporal trends such as changes in attention to adenoma detection as a quality measure56 and introduction of high definition colonoscopes. Indeed, in 1 study, observed prevalence of YOA increased from 11.2% in the period between 1999 and 2006 to 18.8% in the 2007–2009 period after high definition colonoscopes were adopted in their institution.29
Taken together, our study suggests that prevalence of YOA may be as high as 11.4% (the upper bound of the 95% CI around our estimated prevalence of 9%), but those data are insufficient to determine whether there has been an increase in prevalence of YOA over time. We acknowledge that conventional statistical measures of heterogeneity (I2 value) suggest high heterogeneity. However, these measures were designed for comparative studies in which summary estimates were OR, relative risk, etc. Interpreting these measures for prevalence studies is challenging, and all meta-analyses of prevalence studies have high I2 value.57–59 Sources of heterogeneity in our study include the long time span of our included studies (from 1977 to 2018), the limited number of studies addressing this key question, and the diverse patient population (spanning different continents). We sought to minimize heterogeneity at a conceptual level by limiting analyses to studies that were as homogenous as possible, excluding modeling and cost-effectiveness studies, and by using a rigorous protocol. We also evaluated potential sources of heterogeneity by examining pooled prevalence in specific predefined subgroups. Our findings are similar to a recent narrative review, which concluded that colorectal adenomas are increasingly detected in young people,60 and extend their conclusions by presentation of evidence from a systematic review and meta-analysis.
Young-Onset Adenoma Risk Factors
Across 4 studies contributing data from 78,880 individuals, we found increasing age, male sex, and increasing BMI reported as risk factors for YOA. Only increasing age, a nonmodifiable risk factor, was consistently identified as a risk factor across all studies.22,29,35,48 Formal meta-analysis was not possible because of study design heterogeneity. Thus, there is a lack of available data to provide significant insights into factors associated with YOA. Future research should use large cohorts of individuals with YOA (such as those identified through colonoscopy) to further understand risk factors associated with YOA diagnosis and assess overlap with risk factors for early-onset CRC.
Metachronous Advanced Neoplasia and Colorectal Cancer After Young-Onset Adenoma
A common clinical challenge is determining whether individuals with YOA discovered during colonoscopy represent a group at increased risk for metachronous advanced neoplasia, and whether this group requires specialized surveillance. Across 3 studies contributing data from 1493 individuals, we found that the pooled risk for metachronous advanced neoplasia was 6%. A fourth study reporting on data from 7848 individuals with YOA over 40.8 months of follow-up reported a cumulative incidence of metachronous advanced neoplasia of less than 4% among patients with high and low risk adenomas at baseline. Across the 4 included studies, just 1 individual was reported to develop CRC on follow-up. Sparse data were available to inform assessment of outcomes among individuals with baseline low risk versus high risk YOA. One study reported a 5-year metachronous advanced neoplasia rate of 4.9% after baseline low risk adenoma and a 3-year rate of 3.9% after baseline high risk YOA diagnosis.33 Comparisons of risk of advanced metachronous neoplasia for young adults vs adults older than 50 have not been widely reported. One study comparing the risk of metachronous advanced neoplasia on follow-up among patients aged 20–49 vs 50–54 found the 5-year risk of advanced neoplasia on follow-up after baseline low risk adenoma in patients aged 20–49 years was 4.8% vs 5.0% in patients aged 50–54 years. After baseline high risk adenoma, the 3-year risk of metachronous advanced neoplasia was 3.9% in patients aged 20–49 years vs 3.8% in patients aged 50–54 years.33 Another study including 128 young adults <50 years and 123 older adults who underwent baseline colonoscopy found the risk of advanced neoplasia on follow-up did not differ between younger and older adults (7% vs 12.2%; P = .16).61 Taken together, available evidence suggests that individuals with YOA have a relatively low rate of metachronous advanced neoplasia on follow-up at under 8.6% (the upper bound of the 95% CI for our estimated rate of 6%), but available evidence is insufficient to conclude whether rate of metachronous neoplasia in individuals with YOA is lower, similar to, or higher than individuals with adenomas diagnosed older than age 50. A limitation of all studies included was that a substantial fraction of patients with baseline YOA did not receive surveillance colonoscopy for ascertainment of the outcome of metachronous advanced neoplasia. This decreased the sample size of individuals available for outcome ascertainment and potentially could have introduced bias of unknown direction. Larger cohort studies are needed to better characterize the risk for metachronous advanced neoplasia, including risk for CRC, among patients with YOA. Factors that might influence risk for metachronous advanced neoplasia (such as family history of CRC) also merit investigation. In the interim, on the basis of currently available data, evidence suggests that YOA patients should not be recommended surveillance colonoscopy more frequently than individuals with adenomas diagnosed at ages 50 and older. However, we suggest surveillance recommendations be individualized on the basis of factors such as underlying comorbid conditions, family history of CRC, and quality of baseline bowel preparation pending generation of new evidence.
Impact of Colonoscopy Surveillance After Young-Onset Adenoma Diagnosis
We did not identify any studies that specifically addressed the impact of surveillance colonoscopy among patients with YOA. Understanding whether surveillance improves outcomes could clarify whether YOA patients require close surveillance and help reinforce participation in surveillance. Lack of evidence to support importance of surveillance may contribute to recommendations for surveillance, as well as adherence to surveillance (which may be as low as 24.7% among YOA patients62). Future large cohort studies should examine whether surveillance colonoscopy after YOA diagnosis improves outcomes. Randomized trials can also be considered, but feasibility may be a major challenge because of the very large sample size likely required to show differences in outcomes.
Strengths and Limitations
This is a systematic review and comprehensive evidence synthesis of the 5 key questions regarding YOA we posed. We used best practices for our literature review and evidence synthesis, including (1) pre-specifying the key questions of interest; (2) registering the protocol with PROSPERO; (3) using best practices for the protocol, including a comprehensive literature review, having more than 1 reviewer for assessing inclusion/exclusion criteria and abstracting data, and assessing quality of individual studies. For 2 questions, we also were able to perform meta-analyses that have not yet, to our knowledge, been reported.
Several limitations may be considered in interpreting our report. Despite a rigorous, prespecified search strategy, all relevant publications may not have been identified. We chose to focus on published articles and did not include abstracts from scientific meetings. Most of the data available were from retrospective cohort studies, which may be subject to bias in data collected and challenged by presence of unmeasured confounding variables. Most of the data synthesized comes from patients undergoing colonoscopy for signs or symptoms of suspected gastrointestinal disease. As such, the findings are not representative of the general population of individuals younger than age 50. We were unable to stratify our analyses of adenoma prevalence under age 50 by age categories (such as by age decade) because of a lack of granular data on age-specific prevalence. Future research may help to clarify how much adenoma prevalence varies by age categories under age 50. Similarly, our systematic review and meta-analysis did not include a focus on variation in adenoma characteristics (eg, high vs low risk adenoma) by age or over time; these areas may also be targeted for future research.
At the meta-analysis level, we observed significant heterogeneity in the pooled estimates of prevalence of YOA, risk of recurrent advanced neoplasia, and risk of CRC during follow-up of patients with YOA. Prior studies have documented heterogeneity in providing prevalence estimates from meta-analyses.63 The noted heterogeneity could be from a patient level (different levels of comorbidities, different patient ethnicity, presence or absence of various risk factors contributing to polyp formation) or from a study design setting (differences in study design, inclusion/exclusion criteria such as asymptomatic vs symptomatic patient populations, study setting, definition of outcomes such as advanced neoplasia). To minimize this heterogeneity, at the conceptual phase of our study, we used strict inclusion and exclusion criteria. We also performed pre-planned subgroup analyses to explore sources of heterogeneity. Despite these steps, observed heterogeneity contributed to lowering our assessment of the quality of evidence to support answers to our key questions. Another limitation of our study pertaining to our analysis of the risk of advanced neoplasia on follow-up of patients with YOA (Key Question 3) is the that the pooled studies used varying length of surveillance intervals, precluding ability to use a consistent follow-up time point after baseline polypectomy (eg, 3 or 5 years) to estimate proportion with metachronous advanced neoplasia on follow-up. This may have contributed to heterogeneity in our pooled estimates.
Conclusion
In a comprehensive systematic review and meta-analysis, we found that the pooled prevalence of YOA is estimated to be 9%. Evidence is insufficient to determine whether prevalence is increasing over time. Risk factors for YOA reported by currently available literature include age, male sex, increasing BMI, and smoking, with age being the most consistently reported risk factor across studies. More research on risk factors for YOA is needed, particularly to determine whether early-onset CRC and YOA share common risk factors. Pooled risk for metachronous advanced neoplasia on follow-up after YOA diagnosis was estimated to be 6%. Evidence was insufficient to determine whether risk for metachronous advanced neoplasia differs by baseline adenoma characteristics or to precisely estimate risk for CRC on follow-up; both of these areas require further study. Evidence was insufficient to assess the impact of surveillance colonoscopy on outcomes of individuals with YOA. Overall, current evidence suggests that YOA is common and associated with a relatively low for metachronous advanced neoplasia, but that more research is required to determine prevalence, risk factors, and optimal management, including whether detection, removal, and surveillance of YOA have potential to impact early-onset CRC incidence and mortality.
Supplementary Material
What You Need to Know.
Background
Adenoma detection, removal, and endoscopic surveillance might modify risk of CRC diagnosed before age 50 (early-onset CRC). A systematic review of young-onset adenoma (YOA) evaluated prevalence, associated risk factors, and rate of metachronous advanced neoplasia after YOA diagnosis.
Findings
On the basis of a systematic review of the literature, the prevalence of YOA is estimated to be 9%. Risk increases with age. Risk for metachronous advanced neoplasia after YOA diagnosis is estimated to be 6%.
Implications for patient care
More research is needed to understand the prevalence, risk factors, and risk of CRC associated with YOA.
Funding
Supported by the Department of Veterans Affairs (I01 HX001574), the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (T32 DK007202, K23 DK117058, and P30 DK120515), the National Cancer Institute of the National Institutes of Health (R37 CA222866, U54 CA132379, U54 CA132384, P30 CA023100), an American College of Gastroenterology Junior Faculty Development Award (Singh), and a Crohn’s and Colitis Foundation Career Development Award (404614). The views expressed in this article are those of the authors and do not necessarily represent the views of the funding agencies.
Abbreviations used in this paper:
- BMI
body mass index
- CI
confidence interval
- CRC
colorectal cancer
- OR
odds ratio
- YOA
young-onset adenoma
Footnotes
Conflicts of interest
The authors disclose no conflicts.
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2020.04.092.
References
- 1.International Agency for Research on Cancer. GLOBOCAN 2018. The Global Cancer Observatory, 2018. [Google Scholar]
- 2.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA: a Cancer Journal for Clinicians 2017; 67:177–193. [DOI] [PubMed] [Google Scholar]
- 3.Murphy CC, Singal AG, Baron JA, et al. Decrease in incidence of young-onset colorectal cancer before recent increase. Gastroenterology 2018;155:1716–1719.e1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mauri G, Sartore-Bianchi A, Russo A-G, et al. Early-onset colorectal cancer in young individuals. Mol Oncol 2019; 13:109–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aleksandrova K, Pischon T, Buijsse B, et al. Adult weight change and risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition. Eur J Cancer 2013; 49:3526–3536. [DOI] [PubMed] [Google Scholar]
- 6.Gausman V, Dornblaser D, Anand S, et al. Risk factors associated with early-onset colorectal cancer. Clin Gastroenterol Hepatol 2019;S1542-3565(19):31108–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venugopal A, Stoffel EM. Colorectal cancer in young adults. Curr Treat Options Gastroenterol 2019;17:89–98. [DOI] [PubMed] [Google Scholar]
- 8.Stoffel EM, Murphy CC. Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology 2020;158:341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Low EE, Demb J, Liu L, et al. Risk factors for early-onset colorectal cancer. Gastroenterology 2020;S0016-5085 (20)30016–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morson B Polyps and cancer of the large bowel. The Western Journal of Medicine 1976;125:93–99. [PMC free article] [PubMed] [Google Scholar]
- 11.Holme Ø, Løberg M, Kalager M, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA 2014;312:606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet 2010; 375:1624–1633. [DOI] [PubMed] [Google Scholar]
- 13.Segnan N, Armaroli P, Bonelli L, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial—SCORE. J Natl Cancer Inst 2011; 103:1310–1322. [DOI] [PubMed] [Google Scholar]
- 14.Hoff G, Grotmol T, Skovlund E, et al. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: randomised controlled trial. BMJ 2009;338:b1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atkin W, Wooldrage K, Brenner A, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol 2017;18:823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–269, w264. [DOI] [PubMed] [Google Scholar]
- 17.Enwerem N, Gupta S, Bortniker E, et al. Risk factors, prevalence and risk of colorectal cancer among patients with young onset colonic adenomas. PROSPERO 2019: CRD42019125508. [Google Scholar]
- 18.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bafandeh Y, Khoshbaten M, Eftekhar Sadat AT, et al. Clinical predictors of colorectal polyps and carcinoma in a low prevalence region: results of a colonoscopy based study. World J Gastroenterol 14:1534–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bafandeh Y, Khoshbaten M, Eftekhar-Sadat AT, et al. Colorectal neoplasms in symptomatic patients without evidence of bleeding: a prospective study in an Iranian population. Asian Pac J Cancer Prev 8:485–488. [PubMed] [Google Scholar]
- 22.Chung SJ, Kim YS, Yang SY, et al. Prevalence and risk of colorectal adenoma in asymptomatic Koreans aged 40–49 years undergoing screening colonoscopy. J Gastroenterol Hepatol 2010;25:519–525. [DOI] [PubMed] [Google Scholar]
- 23.Binda V, Pereira-Lima J, Nunes CA, et al. Is there a role for sigmoidoscopy in symptomatic patients? analysis of a study correlating distal and proximal colonic neoplasias detected by colonoscopy in a symptomatic population. Arq Gastroenterol 2007;44:2–7. [DOI] [PubMed] [Google Scholar]
- 24.Del Vecchio Blanco G, Cretella M, Paoluzi OA, et al. Adenoma, advanced adenoma and colorectal cancer prevalence in asymptomatic 40- to 49-year-old subjects with a first-degree family history of colorectal cancer. Colorectal Dis 2013; 15:1093–1099. [DOI] [PubMed] [Google Scholar]
- 25.Wong RF, Khosla R, Moore JH, et al. Consider colonoscopy for young patients with hematochezia. J Fam Pract 2004; 53:879–884. [PubMed] [Google Scholar]
- 26.Forsberg AM, Kjellstrom L, Agreus L, et al. Prevalence of colonic neoplasia and advanced lesions in the normal population: a prospective population-based colonoscopy study. Scand J Gastroenterol 2012;47:184–190. [DOI] [PubMed] [Google Scholar]
- 27.Guillem JG, Neugut AI, Forde KA, et al. Colonic neoplasms in asymptomatic first-degree relatives of colon cancer patients. Am J Gastroenterol 83:271–273. [PubMed] [Google Scholar]
- 28.Hemmasi G, Sohrabi M, Zamani F, et al. Prevalence of colorectal adenoma in an average-risk population aged 40–50 versus 50–60 years. Eur J Cancer Prev 2015;24:386–390. [DOI] [PubMed] [Google Scholar]
- 29.Gupta AK, Samadder J, Elliott E, et al. Prevalence of any size adenomas and advanced adenomas in 40- to 49-year-old individuals undergoing screening colonoscopy because of a family history of colorectal carcinoma in a first-degree relative. Gastrointest Endosc 2011;74:110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hussein Kamareddine M, Ghosn Y, Karam K, et al. Adenoma detection before and after the age of 50: a retrospective analysis of Lebanese outpatients. BMJ Open Gastroenterol 2018;5: e000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imperiale TF, Wagner DR, Lin CY, et al. Results of screening colonoscopy among persons 40 to 49 years of age. N Engl J Med 2002;346:1781–1785. [DOI] [PubMed] [Google Scholar]
- 32.Khalid AB, Majid S, Salih M, et al. Is full colonoscopic examination necessary in young patients with fresh bleeding per rectum? Endoscopy 2011;43:692–696. [DOI] [PubMed] [Google Scholar]
- 33.Kim HG, Cho YS, Cha JM, et al. Risk of metachronous neoplasia on surveillance colonoscopy in young patients with colorectal neoplasia. Gastrointest Endosc 2018;87:666–673. [DOI] [PubMed] [Google Scholar]
- 34.Kim SE, Shim KN, Jung SA, et al. An association between obesity and the prevalence of colonic adenoma according to age and gender. J Gastroenterol 2007;42:616–623. [DOI] [PubMed] [Google Scholar]
- 35.Lee SE, Jo HB, Kwack WG, et al. Characteristics of and risk factors for colorectal neoplasms in young adults in a screening population. World J Gastroenterol 2016;22:2981–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haghighi PN, Mohallatee K, Ghassemi E, et al. Colorectal polyps and carcinoma in Southern Iran. Cancer 1977;39:274–278. [DOI] [PubMed] [Google Scholar]
- 37.Paspatis GA, Papanikolaou N, Zois E, et al. Prevalence of polyps and diverticulosis of the large bowel in the Cretan population: an autopsy study. Int J Colorectal Dis 2001;16:257–261. [DOI] [PubMed] [Google Scholar]
- 38.Pendergrass CJ, Edelstein DL, Hylind LM, et al. Occurrence of colorectal adenomas in younger adults: an epidemiologic necropsy study. Clin Gastroenterol Hepatol 2008;6:1011–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szczepanski W, Urban A, Wierzchowski W. Colorectal polyps in autopsy material: part I—adenomatous polyps. Patol Pol 1992; 43:79–85. [PubMed] [Google Scholar]
- 40.Vatn MH, Stalsberg H. The prevalence of polyps of the large intestine in Oslo: an autopsy study. Cancer 1982;49:819–825. [DOI] [PubMed] [Google Scholar]
- 41.Spinzi G, Fante MD, Masci E, et al. Lack of colonic neoplastic lesions in patients under 50 yr of age with hematochezia: a multicenter prospective study. Am J Gastroenterol 2007;102:2011–2015. [DOI] [PubMed] [Google Scholar]
- 42.Overholt BF, Brooks-Belli L, Grace MG, et al. Evaluating screening age for colonoscopy: a quality assurance assessment. J Clin Gastroenterol 2010;44:e147–e153. [DOI] [PubMed] [Google Scholar]
- 43.Thiruvengadam R, Thiruvengadam SS. Pre-cancerous colon polyps in the young: incidental adenoma detection in average-risk persons forty and younger. Scand J Gastroenterol 2018;53:1418–1420. [DOI] [PubMed] [Google Scholar]
- 44.Wong RF, Khosla R, Moore JH, et al. Consider colonoscopy for young patients with hematochezia. J Fam Pract 2004;53:879–884. [PubMed] [Google Scholar]
- 45.Guillem JG, Forde KA, Treat MR, et al. Colonoscopic screening for neoplasms in asymptomatic first-degree relatives of colon cancer patients: a controlled, prospective study. Dis Colon Rectum 1992;35:523–529. [DOI] [PubMed] [Google Scholar]
- 46.Kim NH, Jung YS, Park JH, et al. Risk of developing metachronous advanced colorectal neoplasia after colonoscopic polypectomy in patients aged 30 to 39 and 40 to 49 years. Gastrointest Endosc 2018;88:715–723. [DOI] [PubMed] [Google Scholar]
- 47.Kim KO, Yang H-J, Cha JM, et al. Risks of colorectal advanced neoplasia in young adults versus those of screening colonoscopy in patients aged 50 to 54 years. J Gastroenterol Hepatol 2017;32:1825–1831. [DOI] [PubMed] [Google Scholar]
- 48.Chen HM, Weng YR, Jiang B, et al. Epidemiological study of colorectal adenoma and cancer in symptomatic patients in China between 1990 and 2009. J Dig Dis 2011;12:371–378. [DOI] [PubMed] [Google Scholar]
- 49.Chen KC, Chung CS, Hsu WF, et al. Identification of risk factors for neoplastic colonic polyps in young adults with bloody stool in comparison with those without symptom. J Gastroenterol Hepatol 2018;33:1335–1340. [DOI] [PubMed] [Google Scholar]
- 50.Park SK, Yang HJ, Jung YS, et al. Number of advanced adenomas on index colonoscopy: important risk factor for metachronous advanced colorectal neoplasia. Dig Liver Dis 50:568–572. [DOI] [PubMed] [Google Scholar]
- 51.Nagpal SJS, Mukhija D, Sanaka M, et al. Metachronous colon polyps in younger versus older adults: a case-control study. Gastrointest Endosc 2018;87:657–665. [DOI] [PubMed] [Google Scholar]
- 52.Park SK, Kim NH, Yung YS, et al. Risk of developing advanced colorectal neoplasia after removing high risk adenoma detected at index colonoscopy in young patients: a KASID study. J Gastroenterol Hepatol 2016;31:138–144. [DOI] [PubMed] [Google Scholar]
- 53.Nagpal SJS, Mukhija D, Sanaka M, et al. Metachronous colon polyps in younger versus older adults: a case-control study. Gastrointest Endosc 2018;87:688–694.e682. [DOI] [PubMed] [Google Scholar]
- 54.Kim NH, Jung YS, Park JH, et al. Risk of developing metachronous advanced colorectal neoplasia after colonoscopic polypectomy in patients aged 30 to 39 and 40 to 49 years. Gastrointest Endosc 2018;88:715–723. [DOI] [PubMed] [Google Scholar]
- 55.Rickert RR, Auerbach O, Garfinkel L, et al. Adenomatous lesions of the large bowel: an autopsy survey. Cancer 1979;43:1847–1857. [DOI] [PubMed] [Google Scholar]
- 56.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2002;97:1296–1308. [DOI] [PubMed] [Google Scholar]
- 57.Epstein S, Sparer EH, Tran BN, et al. Prevalence of work-related musculoskeletal disorders among surgeons and interventionalists: a systematic review and meta-analysis. JAMA Surg 2018; 153:e174947–e174947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mata DA, Ramos MA, Bansal N, et al. Prevalence of depression and depressive symptoms among resident physicians: a systematic review and meta-analysis. JAMA 2015;314:2373–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rotenstein LS, Ramos MA, Torre M, et al. Prevalence of depression, depressive symptoms, and suicidal ideation among medical students: a systematic review and meta-analysis. JAMA 2016;316:2214–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bushyhead D, Lin OST, Kozarek RA. A review of the management of sporadic colorectal adenomas in young people: is surveillance wasted on the young? Dig Dis Sci 2019; 64:2107–2112. [DOI] [PubMed] [Google Scholar]
- 61.Nagpal SJS, Mukhija D, Sanaka M, et al. Metachronous colon polyps in younger versus older adults: a case-control study. Gastrointest Endosc 2018;87:657–665. [DOI] [PubMed] [Google Scholar]
- 62.Cha JM, La Selva D, Kozarek RA, et al. Young patients with sporadic colorectal adenomas: current endoscopic surveillance practices and outcomes. Gastrointest Endosc 2018; 88:818–825.e811. [DOI] [PubMed] [Google Scholar]
- 63.Singh S, Singh PP, Murad MH, et al. Prevalence, risk factors, and outcomes of interval colorectal cancers: a systematic review and meta-analysis. Am J Gastroenterol 2014;109:1375–1389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


