Abstract
Vascular cognitive impairment and dementia (VCID) is defined as a progressive dementia disease related to cerebrovascular injury and often occurs in aged populations. Despite decades of research, effective treatment for VCID is still absent. The pathological processes of VCID are mediated by the molecular mechanisms that are partly modulated at the post‐transcriptional level. As small endogenous non‐coding RNAs, microRNAs (miRs) can regulate target gene expression through post‐transcriptional gene silencing. miRs have been reported to play an important role in the pathology of VCID and have recently been suggested as potential novel pharmacological targets for the development of new diagnosis and treatment strategies in VCID. In this review, we summarize the current understanding of VCID, the possible role of miRs in the regulation of VCID and attempt to envision future therapeutic strategies. Since manipulation of miR levels by either pharmacological or genetic approaches has shown therapeutic effects in experimental VCID models, we also emphasize the potential therapeutic value of miRs in clinical settings.
Keywords: aging, dementia, microRNAs, regulatory mechanisms, vascular cognitive impairment
miR regulatory mechanisms in experimental VCID.
![]()
1. INTRODUCTION
Vascular cognitive impairment and dementia (VCID) is currently the second most common type of dementia just after Alzheimer's disease (AD). 1 In 2015, approximately 47.5 million people were affected by dementia, which is expected to increase to 75.6 million by 2030. The annual global social cost for VCID and vascular dementia (VaD) is $604 billion, accounting for 1.0% of the global gross domestic product. 2
Vascular cognitive impairment and dementia occurs when cerebral blood flow is compromised. It is a comprehensive brain disorder that comprises mild cognitive impairment (MCI), VaD, and mixed dementia, such as mixed vascular and AD‐type cognitive impairment. 3 VCID presents a significant decline in cognitive function due to cerebral vascular damage, including clinical stroke, asymptomatic infarcts and microinfarcts, leukoaraiosis, cerebral amyloid angiopathy (CAA), 4 transient ischemic attack (TIA), and micro hemorrhage. 5 Diagnosis is further defined according to whether there is a causal relationship between cognitive impairment syndrome and vascular disease. 6 , 7
Up to now, treatment for VCID is still limited to relief and therapy of symptoms. 8 For example, Donepezil was found to enhance the cognitive ability of VaD patients. 3 Administration of Galantamine is beneficial for patients with mixed AD and VaD. 9
Non‐coding RNAs, especially miRs, are one of the many biological factors that cause functional changes during VCID. Because of the redundancy of targets, miRs can target multiple signal pathways; one miR can target multiple messenger RNAs (mRNAs), whereas numerous miRs can act on one mRNA at the same time. 10 VCID can trigger altered miR expression in the blood and brain of rodents and humans. 11 , 12 , 13 , 14 Besides, miRs can be regulated by external agents to improve symptoms caused by VCID. 15 , 16 , 17 , 18
In this review, we summarize the current advances on the pathogenesis and treatment of VCID, with a focus on the possible role of miRs in disease regulation and attempt to explore future therapeutic strategies.
2. OVERVIEW OF VASCULAR COGNITIVE IMPAIRMENT AND DEMENTIA (VCID)
Vascular cognitive impairment and dementia includes any degree of cognitive impairment resulting from vascular brain pathology, from MCI to dementia, regardless of its specific mechanism. 19
2.1. White matter injury in VCID
Given that blood flow in white matter (WM) is supplied by long, penetrating arterioles that lack anastomotic branches, WM is more susceptible to reduced CBF. 20 , 21 WM is composed of neuronal axons, the surrounding myelin sheath, and glial cells such as astrocytes, oligodendrocytes, pericytes, and microglia. 22 Myelinated WM tracts are responsible for long‐range connectivity through axonal transport, and their lesions can lead to neuronal circuits processing speed deficits and corticocortical disconnections. 23 The pathological changes in WM can predict VCID according to neuropathology guidelines, and WM injury is a significant contributor to dementia. 24 , 25 , 26
In animal models of VCID, permanent occlusion of the common carotid arteries (CCAs) is the most frequently used large vessel occlusion model that leads to BBB disruption and significant WM impairment. 27 This model shares several common pathological consequences with small vessel disease, including microinfarcts and WM changes. 28 , 29 WM lesions and rarefaction mainly occur in the corpus callosum with remarkable myelin loss, axonal damage, microglia, and astrocyte activation, without causing neuronal damage. 30 , 31 , 32 , 33 In the model of small vessel occlusion, microglia/macrophage polarization was also found strongly linked to VCID. 34 Except for the rodent models, the accumulation of myelin defects such as myelination, swelling, and complete axonal degeneration has also been found to be correlated with cognitive decline in rhesus monkeys. 35 Besides, aging exacerbates the degeneration of WM, and the consequences of chronic cerebral hypoperfusion (CCH) are more severe in aged animals. 36
2.2. Gray matter injury in VCID
The neurovascular unit consists of neurons, glia, perivascular, and vascular cells. Together, they maintain the normal physiological function of neurons, repair damaged neurons, and play an essential role in keeping the homeostasis of the cerebral microenvironment. 37 The pathogenesis of VCID mainly appears in the neurovascular unit. Among these neurovascular components, the neuron is the basic structural and functional unit of the nervous system. Global neuronal loss that is produced by persistent cerebral hypoperfusion in specific brain regions, such as the hippocampus, can lead to severe learning and memory impairment. 38
Neurons are electrically active cells that require a continuous supply of oxygen and glucose to produce a tremendous amount of energy, which is needed to maintain membrane potential. Therefore, neurons are vulnerable to ischemic injury. Different from stroke, which is induced by a sudden and complete disruption of blood supply to different regions of the brain, non‐stroke causes of VCID is often caused by a moderate but sustained decrease in blood supply by CBF. The mild but continuous reduction of blood supply could cause a reduction in oxygen and glucose supply to the brain, leading to cell death, memory impairment, and dementia. 39
2.3. Molecular mechanism of VCID
Although animal models cannot entirely present the complex clinical symptoms of VCID in humans, they aid in understanding the molecular changes in the brain during cerebrovascular injury to a certain extent, which eventually leads to cognitive impairment of VCID. 40 Several mechanisms can be used to generalize the pathological causes of the VCID at the molecular level (Figure 1).
Figure 1.
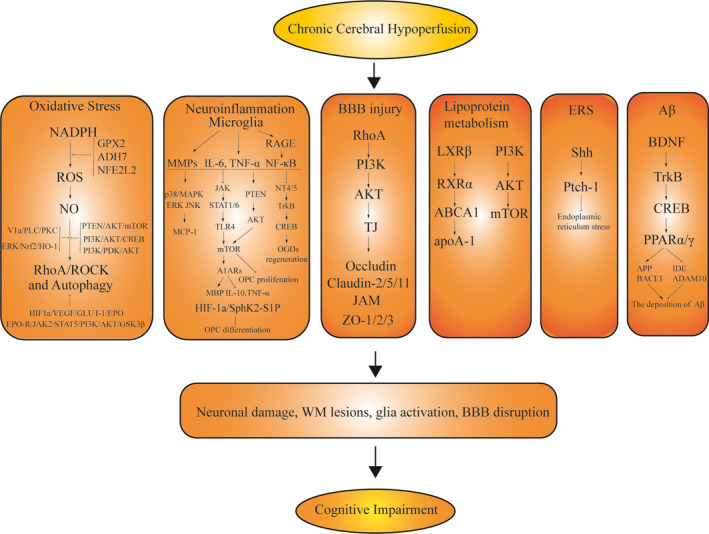
Potential molecular mechanisms in VCID. CCH can cause a cascade of pathological changes: neuronal damage, WM lesions, glial activation, and BBB disruption, resulting in cognitive impairment and dementia in experimental studies. The following cellular hemostasis abnormalities mainly contribute to the above pathological changes in VCID: oxidative stress, neuroinflammation, BBB disruption, abnormal lipoprotein metabolism, endoplasmic reticulum stress, and the deposition of Aβ
2.3.1. Oxidative stress
The molecular mechanisms of oxidative stress‐induced cognitive impairments can be studied by using animal models, such as bilateral carotid artery occlusion (BCAO) rat models. In the BCAO model, oxidative stress is characterized by an increase in ROS production, 41 which is one of the triggers leading to cardiovascular pathophysiology and neurodegeneration. 42 The accumulation of ROS and the decrease in antioxidant enzymes may directly affect the synaptic activity and excitatory transmission of neurons, leading to cognitive impairment. 43 The enzyme that produces excessive ROS production during VCID is nicotinamide adenine dinucleotide phosphate (NADPH). 43 ROS produced by NADPH co‐enzymes are the critical contributors to cerebrovascular dysregulation and might lead to cognitive impairment through cell dysfunction and cell death. 43 Inhibition of NADPH oxidase activity reduced the cognitive impairment induced by BCAO models in rodents. 44 Similar alterations are also observed in VCID patients. Several studies have reported that the level of antioxidant enzymes in blood samples of VaD patients is decreased. 45 , 46
There are many signal pathways involved in the regulation of oxidative stress in VCID. Some researchers reported that activation of the PI3K/PDK1/AKT pathway could inhibit the apoptosis of neurons in VCID through antioxidative stress effects. 47 The activated ERK‐Nrf2‐HO‐1 signaling pathway is also related to the antioxidant protection in response to hypoperfusion injury. 48 L‐carnitine is an antioxidant agent, which can regulate PTEN/the mammalian target of rapamycin (mTOR) signaling pathway in the rat CCH model, thereby enhancing axonal plasticity and oligodendrocyte expression. 49
2.3.2. Neuroinflammation
Neuroinflammation is closely involved in the pathophysiology of VCID. 50 Inflammatory‐related microglia causes cognitive impairment by activating receptor for advanced glycation end products (RAGE), which is present on both microglia and neurons. RAGE can further stimulate the expression of nuclear factor kappa B (NF‐κB), a transcription factor that regulates the expression of several pro‐inflammatory genes. 51 Besides, microglia releases cytokines, such as interleukin (IL) and tumor necrosis factor‐α (TNF‐α), that play essential roles in the pathogenesis of dementia. 52 , 53 Increased IL‐6 is associated with VaD in patients. 54 Accordingly, the serum IL‐6 level of VaD patients also increased significantly. 55
2.3.3. BBB integrity and injury in VCID
Vascular cognitive impairment and dementia is a cerebrovascular injury‐related disease associated with BBB disruption. Endothelial tight junction (TJ) proteins are major components of the BBB and are responsible for sealing gaps between adjacent endothelial cells. 56 Altered distribution or loss of TJ proteins is frequently seen in ischemic‐induced cerebral microvessel injuries, resulting in compromised BBB integrity and dementia. 57 TJ proteins include transmembrane proteins, cytoplasmic attachment proteins, and cytoskeletal proteins. 58 Transmembrane proteins include three complete membrane proteins, occludins, claudins, and junctional adhesive molecules (JAMs). Cytoplasmic attachment proteins, which are also named as closed small loop proteins, contain ZO‐1, ZO‐2, and ZO‐3. Prolonged hypoperfusion in white matter and gray matter eventually leads to TJ disruption and BBB leakage, which come into appearance before cognitive impairments. 59 As the most common model in VCID studies, the BCAS model shows BBB leakage not only in the corpus callosum and external capsule but also in the gray matter. 60 A meta‐analysis from 31 studies counted 1953 individuals of normal aging or cerebral microvascular disease. In 693 healthy human, increasing age was associated with the increase in BBB permeability. BBB permeability was increased further in 510 patients with either VCID or AD presented compared with 547 aged‐matched controls. 59 , 61 In the post‐mortem brains of VCID patients, there are higher levels of claudin‐2, claudin‐5, and claudin‐11. 62 Besides, claudin‐1 genetic polymorphisms were found to be highly associated with VCID. 63 Besides TJ disruption and subsequent paracellular leakage, the reverse transcytosis across BBB is also involved in the clearance of Aβ from brain. 64 , 65 Failure or reduction in Aβ brain clearance through endothelial transcytosis may lead to the accumulation of Aβ in brain and finally result in dementia. 66
2.3.4. Other mechanisms
In recent years, cumulative evidences have shown that the pathogenesis of VCID is closely related to the destruction of cholesterol homeostasis and lipoprotein disturbances. 67 , 68 Changes in cholesterol homeostasis lead to abnormal cholesterol uptake from plasma to brain. 69 Apolipoproteins (such as ApoA, ApoE) and the cholesterol efflux transporter, ABCA1 (ATP‐binding cassette transporter A1), are involved in the cholesterol conversion between astrocytes and neurons in the brain. 70 Liver X receptor‐β/retinoid X receptor‐α (RXR‐α)/ABCA1 signaling cascade plays a vital role in lipoprotein metabolism. 71 , 72 , 73 ApoA1 and cholesterol, the downstream mediators of this signaling pathway, may provide a protective role in cerebral hypoperfusion. 70 Fatty acid amide hydrolase (FAAH) inhibitor UBR597 blocks the PI3K‐AKT‐mTOR pathway and autophagy to attenuate CCH‐induced neuronal damage and improve cognitive function. 74 Curcumin can reduce the discharge of excess cholesterol and prevent further brain injury by activating the LXR/RXR‐ABCA1/apoA‐1 pathway. 75
Endoplasmic reticulum stress (ERS) is a process by which unfolded/misfolded proteins accumulate in the ER after an ER homeostasis disorder. 76 Specific stress conditions such as hypoxia, nutrient deprivation, calcium consumption, and hyperglycemia can trigger ERS. 77 Sustained ERS eventually leads to cell apoptosis. N‐Butylphthalide (NBP) can reduce ERS and treat VCID by activating the Shh/Ptch1 pathway in the hippocampus. 78
It is estimated that 40% of AD patients also have some forms of VCID. 79 , 80 , 81 , 82 The most common hypothesis for the progression of AD is the amyloid cascade hypothesis, stating that beta‐amyloid (Aβ) aggregation leads to hyperphosphorylation of tau and tangle formation, which then leads to neurodegeneration. 83 , 84 CCH can induce the deposition of Aβ in the hippocampus, 85 along with neuronal morphological damage and cognitive deficits. 86 , 87 , 88 , 89 Icariin can downregulate the level of insoluble Aβ fragments in the hippocampus by decreasing the expression levels of Aβ‐protein precursor (APP) and β‐site APP‐cleaving enzyme 1 (BACE1), while increasing the expression levels of insulin‐degrading enzyme (IDE) and ADAM metallopeptidase domain 10 (ADAM10). The effect of Icariin on Aβ reduction is mediated by upregulation of peroxisome proliferator‐activated receptor α (PPARα) and γ (PPARγ), and the activation of BDNF/TrkB/CREB signaling pathway. 90
3. MICRORNAS AND VCID
miRs function as a novel class of small non‐coding RNAs (~21‐25 nt) that negatively regulate gene expression. 91 , 92 By hybridizing to the 3’‐untranslated regions (3’‐UTR) of one or more mRNAs, miRs negatively regulate gene expression. 91 , 92 miRs involve almost all cellular processes, including cell proliferation, differentiation, metabolism, apoptosis, and immune responses in various pathophysiological conditions. 93
3.1. The biomarker functions of miRs in VCID
miRs are very stable in various biofluids, including blood, plasma, CSF, and saliva, 93 , 94 , 95 and thus, circulating miRs can serve as potentially informative biomarkers for a range of neurological diseases (Table 1). By performing plasma miR profiling in small‐vessel VaD patients and also in age‐ and sex‐matched healthy controls, Prabhakar et al demonstrated that among the 44 differentially expressed miRs, miR‐409‐3p decreased more than 4‐fold whereas miR‐451a, miR‐486‐5p, and miR‐502‐3p increased more than 3.6‐fold compared with healthy controls. 96 A validation study further suggested these miRs as potential biomarkers for identifying small‐vessel VaD. 96 Sheinerman et al also found that two sets of circulating brain‐enriched miRs, the miR‐132 family (miR‐128, miR‐132, and miR‐874) and the miR‐134 family (miR‐134, miR‐323‐3p, and miR‐382), were significantly different in MCI patients from age‐matched controls with very high sensitivity and specificity. 97 In a recent study, Marchegiani et al also reported that compared with both healthy controls and dementia patients, the level of miR‐222 was significantly increased in VaD patients, suggesting miRs are novel and promising biomarkers to diagnose VaD. 98
Table 1.
MicroRNAs as potential biomarker in VCID
| MicroRNAs | Biomarkers | Sensitive (%) | Specificity (%) | Reference |
|---|---|---|---|---|
| miR‐409‐3p | Diagnosis | 76 | 89 | Langa et al 81 |
| miR‐451a | Diagnosis | 70 | 75 | Langa et al 81 |
| miR‐486‐5p | Diagnosis | 75 | 83 | Langa et al 81 |
| miR‐502‐3p | Diagnosis | 75 | 89 | Langa et al 81 |
| miR‐128 | Diagnosis | 84 | 96 | Sheinerman et al 97 |
| miR‐132 | Diagnosis | 88 | 93 | Sheinerman et al 97 |
| miR‐874 | Diagnosis | 94 | 96 | Sheinerman et al 97 |
| miR‐134 | Diagnosis | 86 | 82 | Sheinerman et al 97 |
| miR‐323‐3p | Diagnosis | 88 | 80 | Sheinerman et al 97 |
| miR‐382 | Diagnosis | 76 | 90 | Sheinerman et al 97 |
| miR‐222 | Diagnosis | N/A | N/A | Marchegiani et al 98 |
3.2. The role of microRNAs in the pathogenesis of VCID
Apart from the biomarker functions of miRs, accumulating evidence also revealed the critical role of miRs in the pathogenesis of VCID in animal models (Table 2). miR‐195 was the first systematically investigated miR in CCH‐induced cognitive impairment. Ai et al demonstrated that miR‐195 repressed amyloidogenesis via regulating the expression of APP and BACE1 at the post‐transcriptional level. Furthermore, lentivirus‐mediated miR‐195 knockdown induced dementia, whereas overexpression of miR‐195 reduced dementia vulnerability triggered by two‐vessel occlusion (2VO) in rats. 85 Similar to miR‐195, the miR‐132 level was also downregulated in the hippocampus and cerebral cortex of CCH rats. 16 , 99
Table 2.
VCID‐associated microRNAs
| MicroRNAs | Functions | Targets | Reference |
|---|---|---|---|
| miR‐195 | Reduces dementia vulnerability and prevents VCID | APP, BACE1 | Ai et al 85 |
| miR‐132 | Protects against CCH‐induced learning and memory impairments and ameliorates dementia in VCID | Nav1.1, Nav1.2 | Hu et al 16 |
| miR‐9 | Induces cognitive impairment and promotes dementia in VCID | Nav1.1, Nav1.2 and BACE1 | Sun et al 13 , Xie et al 100 |
| miR‐27a | Inhibits the process of autophagy and induces dementia in VCID | LAMP2 | Che et al 11 |
| miR‐124 | Inhibits the formation of Aβ and improves dementia in VCID | BACE1 | Zhang et al 18 |
| miR‐126 | Ameliorates vascular function and inhibits VCID | MMP‐9 | Yu et al 101 |
| miR‐93 | Aggravates inflammatory response and promote dementia in VCID | TLR4 | Shang et al 102 , Liu et al 103 |
| miR‐96 | Inhibits the process of autophagy and induces dementia in VCID | mTOR | Liu et al 12 |
| miR‐501‐3p | Aggravates BBB damage and increases the possibility of dementia in VCID | ZO‐1 | Toyama et al 104 |
| miR‐210‐5p | Decreases synapse number and aggravates dementia in VCID | Snap25 | Ren et al 105 |
| miR‐134‐5p | Promotes cortical neuron injury and dementia in VCID | Foxp2 | Liu et al 106 |
| miR‐26b | Alleviates microglial inflammatory response and protects brain from dementia in VCID | IL‐6 | Kang et al 107 |
| miR‐181c | Increases cellular adaptation to long‐term ischemia and reduces e the possibility of dementia in VCID | TRIM2,NR1 | Fang et al 108 |
| miR‐153 | Promotes synaptic plasticity damage and dysfunction and aggravates dementia in VCID | Snap25,Vamp2, Stx1a and Syt1 | Zhang et al 109 , Yan et al 110 |
miR‐9 is another miR involved in the pathogenesis of CCH. Sun et al discovered that the miR‐9 level was increased in the hippocampus and cerebral cortex of CCH rats after 2VO. 13 Besides, miR‐9 knockdown reduced the symptoms of dementia triggered by 2VO in rat models. 100 Similarly, Wei et al found that miR‐9‐5p was significantly elevated in serum and CSF in VaD patients, as well as in 2VO‐induced CCH rats. Further, the authors discovered that administration of the miR‐9‐5p antagomir significantly attenuated memory impairment, rescued the cholinergic neuronal function, and lowered oxidative stress and neuronal loss in CCH rats. 17 CCH also enhanced the expression of miR‐27a, but inhibited miR‐124 expression. 11 , 18
By using endothelial cell‐specific miR‐126 conditional knockout mice, it was found that EC‐targeted deletion of miR‐126 aggravated cognitive impairment, decreased CBF, myelin density, and axon density, increased inflammation, and exacerbated water channel and glymphatic dysfunction compared with control mice in multiple microinfarction‐induced vascular dementia. 101 Recently, several groups have demonstrated that miR‐93 aggravates inflammatory response by modulating TLR signaling pathways. 102 , 103
Other miRs were also reported to play a regulatory role in the pathogenesis of VaD, and modulation of these miR levels provided therapeutic potential against VCID in animal models. For example, suppression of miR‐96 expression alleviated cognitive impairment. 12 Besides, CCH‐induced TNF‐α could upregulate miR‐501‐3p. 104 miR‐501‐3p inhibitor effectively suppressed CCH‐induced ZO‐1 reduction and BBB destruction in cerebral white matter and significantly improved working memory deficits in the mouse model of CCH. 104 Moreover, there was a significant increase in miR‐210‐5p in the hippocampus of rats after VCID. miR‐210‐5p antagomir effectively attenuated these VCID‐induced phenotypes. 105 In addition, miR‐134‐5p antagomir can relieve cognitive dysfunction in VCID. 106 On the other hand, miR‐26b overexpression significantly attenuated microglial activation, inflammatory responses, neurotoxicity, and cognitive impairments in 2VO‐generated CCH. 107 CCH can inhibit miR‐181c expression in the hippocampus, which is closely associated with the reduction in dendrite spine density and dendritic branching of hippocampal neurons. 108 Virus‐mediated delivery of miR‐181c partially rescued cognitive impairment in rat CCH. 108 Direct knockdown of miR‐153 or overexpression of the antisense molecule (lenti‐AMO‐153) may be a new strategy for alleviating the synaptic pathology and cognitive decline of VaD. 109 , 110
3.3. The mechanism of microRNAs in the regulation of VCID
Constantly reduced blood supply and resultant neuronal hypoxia contribute to oxidative stress, which promotes neuroinflammation and BBB injury, thereby further increasing the susceptibility of the affected tissue to neurodegeneration. 111 Recently, specific miRs have been shown to act on proteins in intrinsic and extrinsic signaling pathways through post‐translation modification, thus regulating cognitive injury after VCID (Figure 2). This section discusses the possible molecular mechanisms and signaling pathways of those miRNAs in VCID pathophysiology.
Figure 2.
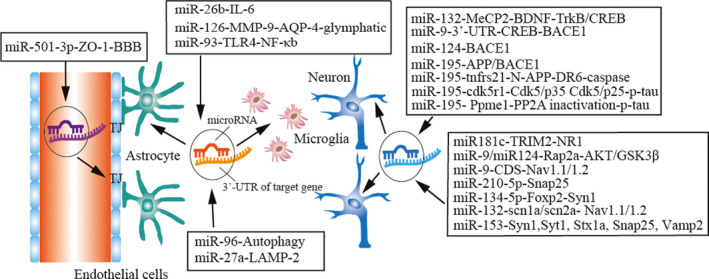
miR regulatory mechanisms in experimental VCID. miR‐132, miR‐9, and miR‐124 can regulate oxidative stress by the CREB pathway, and miR‐195 can inhibit the deposition of Aβ in neurons. miR‐181c, miR‐9, miR‐124, miR‐210‐5p, miR‐134‐5p, miR‐132, and miR‐153 can regulate synaptic loss by AKT/GSK3β and other signaling pathways in neuron dendrites. miR‐26b, miR‐126, and miR‐93 can regulate neuroinflammation. miR‐96 and miR‐27a can suppress autophagy in neurovascular units. miR‐501‐3p can decrease tight junction expression in white matter and the blood‐brain barrier
miR‐9 can target the 3’‐UTR domain of the creb gene to directly inhibit the expression of CREB, which suppresses BACE1 expression. 100 miR‐9 could also regulate the process of Nav1.1/Nav1.2 trafficking in 2VO rats by binding to the coding sequence domain of Navβ2. 13 Contrary to miR‐9, the expression of miR‐124 was continuously inhibited in the 2VO rat model. Aβ might upregulate the expression of BACE1 by inhibiting miR‐124 expression. 18 In addition, miR‐9 and miR‐124 presented the synergistic effect in the regulation of dendritic branching by binding to the Rap GTP‐binding protein Rap2a and regulating the AKT/GSK3β pathway. 112 By binding to the 3’‐UTR of snap25 gene mRNA, miR‐210‐5p exacerbated cognitive impairment. The dysfunction of the miR‐210‐5p‐snap25 signaling pathway might be relevant to synaptic loss in VCID. 105 Like miR‐210‐5p, by binding to the 3’‐UTR of foxp2 (forkhead box p2), the miR‐134‐5p/foxp2/Syn1 pathway was found to contribute to cognitive impairment in chronic ischemia‐induced VCID through loss of cortical neurons and synaptic proteins. 106
The reduced miR‐132 level increased tau phosphorylation at Ser396. Nimodipine alleviated cell apoptosis and reduced hyperphosphorylation of the Tau protein by activating the miR132/GSK3β pathway. 99 miR‐132 may participate in the downregulation of methyl CpG binding protein 2 (MeCP2) after CCH, and MeCP2 downregulation was possibly involved in cognitive deficit through BDNF and its downstream pathways (TrkB and CREB) after 2VO. 14 miR‐132 ameliorates CCH‐induced learning and memory impairments by targeting the scn1a and scn2a genes to downregulate the expression of Nav1.1 and Nav1.2. 16
miR‐96 can suppress autophagy by regulating the PTEN‐Akt‐mTOR signaling pathway. 12 miR‐27a affects autophagosome clearance through post‐transcriptionally regulating lysosomal‐associated membrane protein‐2 (LAMP‐2) expression. 11 miR‐181c might improve cognitive impairment, promote hippocampal neuronal remodeling, and increase N‐methyl‐D‐aspartate receptor 1 (NR1) subunit expression through the effect of TRIM2 on neurofilament light (NF‐L) ubiquitination. 108
miR‐195 improved dementia susceptibility in 2VO rats by inhibiting the expression of APP and BACE1 at the post‐transcriptional level via targeting different genes. 85 In a later study, Sun et al discovered that miR‐195 could also bind to the 3’‐UTR of the cdk5r1 gene, thereby regulating tau hyperphosphorylation. Knockdown of miR‐195 increased tau phosphorylation at Ser202/Thr205, Ser262, Thr231, and Ser422 and activated the Cdk5/p25 pathway. Overexpression of miR‐195 reversed these effects in 2VO‐induced CCH in rats. 113 Moreover, researchers from the same group further found that miR‐195 can increase phosphatase methylesterase‐1 (PME‐1) expression by binding to the 3’‐UTR domains of the Ppme1 gene. Downregulation of miR‐195 in CCH reversed this effect and reduced phosphatase‐2A (PP2A) protein and activity. 114 Overexpression of miR‐195 rescued CCH‐induced dendritic degeneration and neuron apoptosis by targeting the tnfrs21 gene to downregulate the expression of DR6 and suppress the N‐APP/DR6/caspase pathway. 15 Considering the critical role of miR‐195 in VCID, the complement of exogenous miR‐195 may be a potentially anti‐dementia approach. A recent report showed that miR‐153 is involved in the CCH‐impaired hippocampal glutamatergic synaptic vesicle trafficking by binding site in the 3′ untranslated region (3’UTR) of the SYN1, Snap25, Vamp2, Stx1a, and Syt1 genes, which may be a new drug target for prevention or treatment of AD and VaD. 109 , 110
Overexpression of miR‐26b ameliorates inflammation, neurotoxicity, and cognitive impairment by decreasing the number of activated microglia and targeting IL‐6. 107 As an angiogenic miR, miR‐126 regulates various vascular functions. 115 , 116 miR‐126 not only regulates angiogenesis and WM remodeling but can also regulate glymphatic function to affect innate immune response and inflammation. 117 , 118 Regulation of the miR‐93‐mediated TLR signal pathway is probably a potential mechanism for alleviating the inflammatory response of VCID. 119
Given the importance of BBB integrity in VCID progression, manipulating target genes that regulate the BBB or TJ integrity may protect against VCID. Our previous data show that miR‐15a/16‐1 inhibition alleviates ischemia‐induced BBB disruption in mice. 21 , 104 Other miRs such as miR‐212, miR‐132, miR‐150, miR‐181a, miR‐155, miR‐501‐3p, and miR‐128‐1‐5p are also associated with the regulation of ZO‐1, occludin, and claudin‐5 expression, therefore contributing to the alterations in BBB stability. 120 For example, TNF‐α combined with miR‐501‐3p downregulated ZO‐1 and lowered cell‐cell resistance, which plays an essential role in the pathogenesis of cerebral hypoperfusion, especially in BBB disruption. 104
4. TARGETING REGULATORY MICRORNAS IS A NOVEL THERAPEUTIC APPROACH FOR VCID
miRs are key mediators in the pathogenesis of VCID. Understanding their functional significance and molecular mechanisms will provide new insights in developing novel miR‐based therapeutics to delay or rescue cognitive impairments and dementia.
4.1. Current methods
The current approaches for targeting miRNAs in animals include transgene or gene knockout of miRs of interest, and exogenous injection of miR mimic or antagomir/inhibitor into the vein, the lateral cerebral ventricle, parenchymal infarct area, and intranasal cavity. For instance, some researchers injected miR antagomirs into lateral cerebral ventricles or hippocampus by stereotactic technology to examine the role of specific miRNA in VCID, showing a significant neuroprotective role in the rodent experimental CCH model. 15 , 105 , 107 However, intraventricular delivery of antagomir in the treatment of VCID has limitations in clinical application. A few research groups have reported that intravenous administration of specific miRNA antagomirs may be an effective therapeutic approach in experimental stroke. 21 , 121 , 122 , 123 One of the significant limitations in this field is the selection of suitable gene targeting vectors. 124 So far, this administration method has not been reported in the clinical treatment of VCID.
4.2. Improving drug delivery systems for the treatment of VCID
Exosomes are vesicles (approximately 30‐100nm) derived from endosomes released from all living systems, including cells. 125 Exosomes play an essential role in intercellular communication between the source and target cells by transferring proteomic and genomic materials, as well as proteins, mRNAs, and miRs. 125 Comparing with routine systemic administration, exosomes, which are produced by ΒMSCs (bone marrow mesenchymal cells), due to receptor‐mediated transcytosis and benefit from their lipid bilayer encapsulation, can quickly pass through the BBB and deliver miR molecules into brain cells. 126 , 127 , 128 Cell‐based exosome therapy is used to promote brain remodeling and improve neurological function. For example, after the synthesized double‐stranded miRNA was introduced into BMSCs, miR‐143 carrying exosomes were secreted and quickly transferred into osteosarcoma cells to inhibit their migration. 129 MSC therapy has already been applied in clinical trials of stroke treatment. 130 miR‐133b secreted by exosomes from ΒMSCs can induce neural plasticity and functional recovery in rats after stroke. 131 Through secreting exosomes, neurons can translocate miR‐132 to endothelial cells to maintain central brain vascular integrity. 132 Although there is no report on the treatment of VCID with various exosomal miRs, this method has broad application prospects in the future.
4.3. MicroRNA‐based therapy for VCID
There are some small‐molecule chemical compounds targeting miRs to treat VCID. For example, as a candidate drug to treat tauopathy in CCH, Nimodipine has been shown to inhibit tau phosphorylation at the Ser 396 site via the miR‐132/GSK‐3β pathway. 99 Obviously, manipulation of miRs can affect multiple signal pathways in VCID by different molecular mechanisms (Figure 3).
Figure 3.
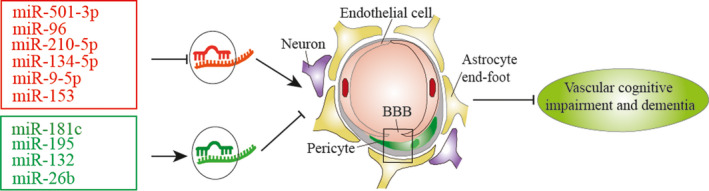
miR‐based therapy in experimental VCID. There are two therapeutic strategies to treat VCID with miR mediators: restoring downregulated miRs by using miR mimics (Green) or blocking upregulated miRs by applying their antagomirs (Red)
To improve the efficiency of miR inhibition in vivo, different chemical modifications have been developed to improve antagomir's bioavailability. 133 , 134 These modifications include anti‐miR oligonucleotides that are synthesized and modified by incorporations of a methyl group (2’‐O‐methyl) together with partial phosphonothioate linkage and cholesterol conjugation at the 3’ end of the strand (which improves tissue distribution and cellular uptake), and the use of locked nucleic acids (LNAs). For example, LNA‐anti‐miR‐501‐3p was intraperitoneally injected to effectively reduce BBB disruption and improve VCID in the mouse BCAS model. 104
5. CONCLUSION AND PROSPECTS
miRs can translationally repress hundreds of proteins in multiple regulatory signaling pathways. Thus, physiological expression of miRs is essential for maintaining healthy development and function of the brain, whereas the imbalance of miR levels in brain cells may increase the susceptibility of VCID and other nervous system diseases. Altered miR expression and activities have been shown to play critical roles in the pathophysiology and progression of VCID.
The levels of hundreds of miRs have been found to be altered in peripheral blood samples of VCID patients, which may provide a new avenue for rapid diagnosis and treatment of VCID. 135 Through studies from experimental animal models, it is suggested that promoting or inhibiting the expression of various VCID‐associated miRs by pharmaceutical and non‐pharmaceutical approaches is beneficial to the improvement or recovery of cognitive function in VCID. 120 However, effective application of miR‐based therapy in VCID may encounter several challenges. A major obstacle to the effective treatment of VCID is the limited understanding of the role of miRs in its pathogenesis. At the present time, miR‐related research in VCID mainly focuses on investigating the relationship between a specific miR of interest and its regulated target genes. In the future, we need to further study miRNA regulatory networks to understand the role of their complex post‐transcriptional regulatory mechanisms. Another major challenge for miR‐based therapy in VCID is the development of pharmacological tools that can aid in effective transport of miR inhibitors and mimics across the BBB to affected brain regions with optimal concentrations.
Currently, miR‐based VCID treatment is only at the beginning stages of testing in experimental VCID models. The application of transgenic animals, especially vascular cell‐specific miR transgenic or knockout mice, 136 can help us to better understand the mechanisms in VCID. Understanding the pathogenesis of VCID is still superficial at the present time, and subsequent research should focus on the key pathogenic mechanisms of VCID.
It is generally accepted that aging is not only a simple physiological process but also accompanied by many related complications, including hypertension, metabolic diseases, and dementia. 137 Since VCID is closely related to aging, it is necessary for us to strengthen our understanding of the relationship between aging and its pathogenesis and clinical manifestations. 138 There are a class of miRs that are associated with the aging process and influence lifespan by targeting components of longevity networks or by regulating stem cell behavior. 139 , 140 , 141 These aging‐related miRs, including miR‐27, miR‐29, miR‐30, and miR‐71, may become the research hot spot for future miR‐based therapy in VCID.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health Grants: NS091175 and NS094930.
Zhang J, Sun P, Zhou C, et al. Regulatory microRNAs and vascular cognitive impairment and dementia. CNS Neurosci Ther. 2020;26:1207–1218. 10.1111/cns.13472
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this review article as no new data were created or analyzed in this review manuscript.
REFERENCES
- 1. Du S‐Q, Wang X‐R, Xiao L‐Y, et al. Molecular mechanisms of vascular dementia: what can be learned from animal models of chronic cerebral hypoperfusion? Mol Neurobiol. 2017;54(5):3670‐3682. [DOI] [PubMed] [Google Scholar]
- 2. Corriveau RA, Bosetti F, Emr M, et al. The science of Vascular Contributions to Cognitive Impairment and Dementia (VCID): a framework for advancing research priorities in the cerebrovascular biology of cognitive decline. Cell Mol Neurobiol. 2016;36(2):281‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672‐2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xiong LI, Boulouis G, Charidimou A, et al. Dementia incidence and predictors in cerebral amyloid angiopathy patients without intracerebral hemorrhage. J Cereb Blood Flow Metab. 2018;38(2):241‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Breteler MM. Vascular involvement in cognitive decline and dementia. Epidemiologic evidence from the Rotterdam Study and the Rotterdam Scan Study. Ann N Y Acad Sci. 2000;903(1):457‐465. [DOI] [PubMed] [Google Scholar]
- 6. Dichgans M, Leys D. Vascular cognitive impairment. Circ Res. 2017;120(3):573‐591. [DOI] [PubMed] [Google Scholar]
- 7. Rosenberg GA, Wallin A, Wardlaw JM, et al. Consensus statement for diagnosis of subcortical small vessel disease. J Cereb Blood Flow Metab. 2016;36(1):6‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ma J, Xiong J‐Y, Hou W‐W, et al. Protective effect of carnosine on subcortical ischemic vascular dementia in mice. CNS Neurosci Ther. 2012;18(9):745‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomised trial. Lancet. 2002;359(9314):1283‐1290. [DOI] [PubMed] [Google Scholar]
- 10. Lim LP, Lau NC, Garrett‐Engele P, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433(7027):769‐773. [DOI] [PubMed] [Google Scholar]
- 11. Che H, Yan Y, Kang X‐H, et al. MicroRNA‐27a promotes inefficient lysosomal clearance in the hippocampi of rats following chronic brain hypoperfusion. Mol Neurobiol. 2017;54(4):2595‐2610. [DOI] [PubMed] [Google Scholar]
- 12. Liu P, Liu P, Wang Z, et al. Inhibition of microRNA‐96 ameliorates cognitive impairment and inactivation autophagy following chronic cerebral hypoperfusion in the rat. Cell Physiol Biochem. 2018;49(1):78‐86. [DOI] [PubMed] [Google Scholar]
- 13. Sun L‐H, Yan M‐L, Hu X‐L, et al. MicroRNA‐9 induces defective trafficking of Nav1.1 and Nav1.2 by targeting Navβ2 protein coding region in rat with chronic brain hypoperfusion. Mol Neurodegener. 2015;10(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yao ZH, Yao XL, Zhang Y, Zhang SF, Hu J. miR‐132 down‐regulates Methyl CpG Binding Protein 2 (MeCP2) during cognitive dysfunction following chronic cerebral hypoperfusion. Curr Neurovasc Res. 2017;14(4):385‐396. [DOI] [PubMed] [Google Scholar]
- 15. Chen X, Jiang X‐M, Zhao L‐J, et al. MicroRNA‐195 prevents dendritic degeneration and neuron death in rats following chronic brain hypoperfusion. Cell Death Dis. 2017;8(6):e2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hu XL, Wang XX, Zhu YM, et al. MicroRNA‐132 regulates total protein of Nav1.1 and Nav1.2 in the hippocampus and cortex of rat with chronic cerebral hypoperfusion. Behav Brain Res. 2019;366(1):118‐125. [DOI] [PubMed] [Google Scholar]
- 17. Wei NA, Zheng K, Xue R, et al. Suppression of microRNA‐9‐5p rescues learning and memory in chronic cerebral hypoperfusion rats model. Oncotarget. 2017;8(64):107920‐107931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang X, Huang X, Fang C, et al. miR‐124 regulates the expression of BACE1 in the hippocampus under chronic cerebral hypoperfusion. Mol Neurobiol. 2017;54(4):2498‐2506. [DOI] [PubMed] [Google Scholar]
- 19. Raz L, Knoefel J, Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. J Cereb Blood Flow Metab. 2016;36(1):172‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80(4):844‐866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang X, Tang X, Sun P, et al. MicroRNA‐15a/16‐1 antagomir ameliorates ischemic brain injury in experimental stroke. Stroke. 2017;48(7):1941‐1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee J, Hamanaka G, Lo EH, Arai K. Heterogeneity of microglia and their differential roles in white matter pathology. CNS Neurosci Ther. 2019;25(12):1290‐1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barker R, Wellington D, Esiri MM, Love S. Assessing white matter ischemic damage in dementia patients by measurement of myelin proteins. J Cereb Blood Flow Metab. 2013;33(7):1050‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skrobot OA, Attems J, Esiri M, et al. Vascular cognitive impairment neuropathology guidelines (VCING): the contribution of cerebrovascular pathology to cognitive impairment. Brain. 2016;139(11):2957‐2969. [DOI] [PubMed] [Google Scholar]
- 25. Snyder HM, Corriveau RA, Craft S, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer's disease. Alzheimers Dement. 2015;11(6):710‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen H‐F, Huang L‐L, Li H‐Y, et al. Microstructural disruption of the right inferior fronto‐occipital and inferior longitudinal fasciculus contributes to WMH‐related cognitive impairment. CNS Neurosci Ther. 2020;26(5):576‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang T, Sun Y, Lu Z, Leak RK, Zhang F. The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Res Rev. 2017;34:15‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hase Y, Craggs L, Hase M, et al. Effects of environmental enrichment on white matter glial responses in a mouse model of chronic cerebral hypoperfusion. J Neuroinflammation. 2017;14(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Holland PR, Searcy JL, Salvadores N, et al. Gliovascular disruption and cognitive deficits in a mouse model with features of small vessel disease. J Cereb Blood Flow Metab. 2015;35(6):1005‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kakae M, Tobori S, Morishima M, Nagayasu K, Shirakawa H, Kaneko S. Depletion of microglia ameliorates white matter injury and cognitive impairment in a mouse chronic cerebral hypoperfusion model. Biochem Biophys Res Commun. 2019;514(4):1040‐1044. [DOI] [PubMed] [Google Scholar]
- 31. Magami S, Miyamoto N, Ueno Y, et al. The effects of astrocyte and oligodendrocyte lineage cell interaction on white matter injury under chronic cerebral hypoperfusion. Neuroscience. 2019;406:167‐175. [DOI] [PubMed] [Google Scholar]
- 32. Mansour A, Niizuma K, Rashad S, et al. A refined model of chronic cerebral hypoperfusion resulting in cognitive impairment and a low mortality rate in rats. J Neurosurg. 2018;131(3):892‐902. [DOI] [PubMed] [Google Scholar]
- 33. Miyanohara J, Kakae M, Nagayasu K, et al. TRPM2 channel aggravates CNS inflammation and cognitive impairment via activation of microglia in chronic cerebral hypoperfusion. J Neurosci. 2018;38(14):3520‐3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suenaga J, Hu X, Pu H, et al. White matter injury and microglia/macrophage polarization are strongly linked with age‐related long‐term deficits in neurological function after stroke. Exp Neurol. 2015;272:109‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kohama SG, Rosene DL, Sherman LS. Age‐related changes in human and non‐human primate white matter: from myelination disturbances to cognitive decline. Age. 2012;34(5):1093‐1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duncombe J, Lennen RJ, Jansen MA, Marshall I, Wardlaw JM, Horsburgh K. Ageing causes prominent neurovascular dysfunction associated with loss of astrocytic contacts and gliosis. Neuropathol Appl Neurobiol. 2017;43(6):477‐491. [DOI] [PubMed] [Google Scholar]
- 37. Sandercock P. Contents of the Cochrane library on the organisation of stroke services. Cerebrovasc Dis. 2003;15(1):2‐4. [DOI] [PubMed] [Google Scholar]
- 38. Langdon KD, Granter‐Button S, Harley CW, Moody‐Corbett F, Peeling J, Corbett D. Cognitive rehabilitation reduces cognitive impairment and normalizes hippocampal CA1 architecture in a rat model of vascular dementia. J Cereb Blood Flow Metab. 2013;33(6):872‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Etherton‐Beer CD. Vascular cognitive impairment in dementia. Maturitas. 2014;79(2):220‐226. [DOI] [PubMed] [Google Scholar]
- 40. Trigiani LJ, Hamel E. An endothelial link between the benefits of physical exercise in dementia. J Cereb Blood Flow Metab. 2017;37(8):2649‐2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li Y, Zhang Z. Gastrodin improves cognitive dysfunction and decreases oxidative stress in vascular dementia rats induced by chronic ischemia. Int J Clin Exp Pathol. 2015;8(11):14099‐14109. [PMC free article] [PubMed] [Google Scholar]
- 42. Csányi G, Miller FJ Jr. Oxidative stress in cardiovascular disease. Int J Mol Sci. 2014;15(4):6002‐6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tönnies E, Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer's disease. J Alzheimers Dis. 2017;57(4):1105‐1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Choi D‐H, Lee K‐H, Kim J‐H, et al. NADPH oxidase 1, a novel molecular source of ROS in hippocampal neuronal death in vascular dementia. Antioxid Redox Signal. 2014;21(4):533‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Famulari AL, Marschoff ER, Llesuy SF, et al. The antioxidant enzymatic blood profile in Alzheimer's and vascular diseases. Their association and a possible assay to differentiate demented subjects and controls. J Neurol Sci. 1996;141(1–2):69‐78. [DOI] [PubMed] [Google Scholar]
- 46. Shi GX, Liu CZ, Wang LP, Guan LP, Li SQ. Biomarkers of oxidative stress in vascular dementia patients. Can J Neurol Sci. 2012;39(1):65‐68. [DOI] [PubMed] [Google Scholar]
- 47. Huang K, Shen L, Niu T, Zhao Y, Fu J, Cao Y. Naomaitai ameliorated brain damage in rats with vascular dementia by PI3K/PDK1/AKT signaling pathway. Evid Based Complement Alternat Med. 2019;2019(1):2702068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mao L, Yang T, Li X, et al. Protective effects of sulforaphane in experimental vascular cognitive impairment: Contribution of the Nrf2 pathway. J Cereb Blood Flow Metab. 2019;39(2):352‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ueno Y, Koike M, Shimada Y, et al. L‐carnitine enhances axonal plasticity and improves white‐matter lesions after chronic hypoperfusion in rat brain. J Cereb Blood Flow Metab. 2015;35(3):382‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hu MY, Lin YY, Zhang BJ, Lu DL, Lu ZQ, Cai W. Update of inflammasome activation in microglia/macrophage in aging and aging‐related disease. CNS Neurosci Ther. 2019;25(12):1299‐1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jin X, Liu MY, Zhang DF, et al. Baicalin mitigates cognitive impairment and protects neurons from microglia‐mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF‐κB signaling pathway. CNS Neurosci Ther. 2019;25(5):575‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Block ML, Zecca L, Hong JS. Microglia‐mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57‐69. [DOI] [PubMed] [Google Scholar]
- 53. Xie D, He M, Hu X. Microglia/macrophage diversities in central nervous system physiology and pathology. CNS Neurosci Ther. 2019;25(12):1287‐1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Miwa K, Okazaki S, Sakaguchi M, Mochizuki H, Kitagawa K. Interleukin‐6, interleukin‐6 receptor gene variant, small‐vessel disease and incident dementia. Eur J Neurol. 2016;23(3):656‐663. [DOI] [PubMed] [Google Scholar]
- 55. Vilar‐Bergua A, Riba‐Llena I, Nafría C, et al. Blood and CSF biomarkers in brain subcortical ischemic vascular disease: Involved pathways and clinical applicability. J Cereb Blood Flow Metab. 2016;36(1):55‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ma F, Zhang X, Yin K‐J. MicroRNAs in central nervous system diseases: a prospective role in regulating blood‐brain barrier integrity. Exp Neurol. 2020;323:113094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang X, Tang X, Liu K, Hamblin MH, Yin KJ. Long noncoding RNA Malat1 regulates cerebrovascular pathologies in ischemic stroke. J Neurosci. 2017;37(7):1797‐1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Furuse M. Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol. 2010;2(1):a002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lin J, Wang D, Lan L, Fan Y. Multiple factors involved in the pathogenesis of white matter lesions. Biomed Res Int. 2017;2017(1):9372050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee ES, Yoon JH, Choi J, Andika FR, Lee T, Jeong Y. A mouse model of subcortical vascular dementia reflecting degeneration of cerebral white matter and microcirculation. J Cereb Blood Flow Metab. 2019;39(1):44‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Farrall AJ, Wardlaw JM. Blood‐brain barrier: ageing and microvascular disease–systematic review and meta‐analysis. Neurobiol Aging. 2009;30(3):337‐352. [DOI] [PubMed] [Google Scholar]
- 62. Romanitan MO, Popescu BO, Spulber S, et al. Altered expression of claudin family proteins in Alzheimer's disease and vascular dementia brains. J Cell Mol Med. 2010;14(5):1088‐1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Srinivasan V, Braidy N, Xu YH, et al. Association of genetic polymorphisms of claudin‐1 with small vessel vascular dementia. Clin Exp Pharmacol Physiol. 2017;44(6):623‐630. [DOI] [PubMed] [Google Scholar]
- 64. Storck SE, Hartz AMS, Bernard J, et al. The concerted amyloid‐beta clearance of LRP1 and ABCB1/P‐gp across the blood‐brain barrier is linked by PICALM. Brain Behav Immun. 2018;73(1):21‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Storck SE, Meister S, Nahrath J, et al. Endothelial LRP1 transports amyloid‐β1–42 across the blood‐brain barrier. J Clin Invest. 2015;126(1):123‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Van Gool BS, Steffen E, Reekmans SM, et al. LRP1 Has a predominant role in production over clearance of Aβ in a mouse model of Alzheimer's disease. Mol Neurobiol. 2019;56(10):7234‐7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62(10):1556‐1560. [DOI] [PubMed] [Google Scholar]
- 68. Raffaitin C, Gin H, Empana J‐P, et al. Metabolic syndrome and risk for incident Alzheimer's disease or vascular dementia: the Three‐City Study. Diabetes Care. 2009;32(1):169‐174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Karasinska JM, Rinninger F, Lutjohann D, et al. Specific loss of brain ABCA1 increases brain cholesterol uptake and influences neuronal structure and function. J Neurosci. 2009;29(11):3579‐3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang L, Zhang X, Lü Y, Tian M, Li Y. Dynamic changes of Apo A1 mediated by LXR/RXR/ABCA1 pathway in brains of the aging rats with cerebral hypoperfusion. Brain Res Bull. 2014;100:84‐92. [DOI] [PubMed] [Google Scholar]
- 71. Buono C, Li Y, Waldo SW, Kruth HS. Liver X receptors inhibit human monocyte‐derived macrophage foam cell formation by inhibiting fluid‐phase pinocytosis of LDL. J Lipid Res. 2007;48(11):2411‐2418. [DOI] [PubMed] [Google Scholar]
- 72. Cheng O, Ostrowski RP, Liu W, Zhang JH. Activation of liver X receptor reduces global ischemic brain injury by reduction of nuclear factor‐kappaB. Neuroscience. 2010;166(4):1101‐1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Crisafulli C, Di Paola R, Mazzon E, et al. Liver X receptor agonist treatment reduced splanchnic ischemia and reperfusion injury. J Leukoc Biol. 2010;87(2):309‐321. [DOI] [PubMed] [Google Scholar]
- 74. Wang D, Lin Q, Su S, Liu K, Wu Y, Hai J. URB597 improves cognitive impairment induced by chronic cerebral hypoperfusion by inhibiting mTOR‐dependent autophagy. Neuroscience. 2017;344:293‐304. [DOI] [PubMed] [Google Scholar]
- 75. Tian M, Zhang X, Wang L, Li Y. Curcumin induces ABCA1 expression and apolipoprotein A‐I‐mediated cholesterol transmembrane in the chronic cerebral hypoperfusion aging rats. Am J Chin Med. 2013;41(5):1027‐1042. [DOI] [PubMed] [Google Scholar]
- 76. Tiwari A, Ngiilmei SD, Tamuli R. The NcZrg‐17 gene of Neurospora crassa encodes a cation diffusion facilitator transporter required for vegetative development, tolerance to endoplasmic reticulum stress and cellulose degradation under low zinc conditions. Curr Genet. 2018;64(4):811‐819. [DOI] [PubMed] [Google Scholar]
- 77. Holczer M, Márton M, Kurucz A, Bánhegyi G, Kapuy O. A Comprehensive systems biological study of autophagy‐apoptosis crosstalk during endoplasmic reticulum stress. Biomed Res Int. 2015;2015:319589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Niu XL, Jiang X, Xu GD, et al. DL‐3‐n‐butylphthalide alleviates vascular cognitive impairment by regulating endoplasmic reticulum stress and the Shh/Ptch1 signaling‐pathway in rats. J Cell Physiol. 2019;234(8):12604‐12614. [DOI] [PubMed] [Google Scholar]
- 79. Bowler JV, Munoz DG, Merskey H, Hachinski V. Fallacies in the pathological confirmation of the diagnosis of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1998;64(1):18‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zekry D, Hauw JJ, Gold G. Mixed dementia: epidemiology, diagnosis, and treatment. J Am Geriatr Soc. 2002;50(8):1431‐1438. [DOI] [PubMed] [Google Scholar]
- 81. Langa KM, Foster NL, Larson EB. Mixed dementia: emerging concepts and therapeutic implications. JAMA. 2004;292(23):2901‐2908. [DOI] [PubMed] [Google Scholar]
- 82. Van Iterson EH, Snyder EM, Joyner MJ, Johnson BD, Olson TP. Intrathecal fentanyl blockade of afferent neural feedback from skeletal muscle during exercise in heart failure patients: Influence on circulatory power and pulmonary vascular capacitance. Int J Cardiol. 2015;201:384‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184‐185. [DOI] [PubMed] [Google Scholar]
- 84. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353‐356. [DOI] [PubMed] [Google Scholar]
- 85. Ai J, Sun L‐H, Che H, et al. MicroRNA‐195 protects against dementia induced by chronic brain hypoperfusion via its anti‐amyloidogenic effect in rats. J Neurosci. 2013;33(9):3989‐4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cechetti F, Pagnussat AS, Worm PV, et al. Chronic brain hypoperfusion causes early glial activation and neuronal death, and subsequent long‐term memory impairment. Brain Res Bull. 2012;87(1):109‐116. [DOI] [PubMed] [Google Scholar]
- 87. de la Torre JC. Cerebral hemodynamics and vascular risk factors: setting the stage for Alzheimer's disease. J Alzheimers Dis. 2012;32(3):553‐567. [DOI] [PubMed] [Google Scholar]
- 88. Li E, Hyun Kim D, Cai M, et al. Hippocampus‐dependent spatial learning and memory are impaired in growth hormone‐deficient spontaneous dwarf rats. Endocr J. 2011;58(4):257‐267. [DOI] [PubMed] [Google Scholar]
- 89. Vicente É, Degerone D, Bohn L, et al. Astroglial and cognitive effects of chronic cerebral hypoperfusion in the rat. Brain Res. 2009;1251:204‐212. [DOI] [PubMed] [Google Scholar]
- 90. Yin C, Deng Y, Liu Y, Gao J, Yan L, Gong Q. Icariside II ameliorates cognitive impairments induced by chronic cerebral hypoperfusion by inhibiting the amyloidogenic pathway: involvement of BDNF/TrkB/creb signaling and up‐regulation of PPARα and PPARγ in rats. Front Pharmacol. 2018;9:1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281‐297. [DOI] [PubMed] [Google Scholar]
- 92. Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6(5):376‐385. [DOI] [PubMed] [Google Scholar]
- 93. Sun P, Liu DZ, Jickling GC, Sharp FR, Yin KJ. MicroRNA‐based therapeutics in central nervous system injuries. J Cereb Blood Flow Metab. 2018;38(7):1125‐1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sun Y, Zhang K, Fan G, Li J. Identification of circulating microRNAs as biomarkers in cancers: what have we got? Clin Chem Lab Med. 2012;50(12):2121‐2126. [DOI] [PubMed] [Google Scholar]
- 95. Barbagallo C, Mostile G, Baglieri G, Giunta F, Nicoletti A. Specific signatures of serum miRNAs as potential biomarkers to discriminate clinically similar neurodegenerative and vascular‐related diseases. Cell Mol Neurobiol. 2019;40(4):531‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Prabhakar P, Chandra SR, Christopher R. Circulating microRNAs as potential biomarkers for the identification of vascular dementia due to cerebral small vessel disease. Age Ageing. 2017;46(5):861‐864. [DOI] [PubMed] [Google Scholar]
- 97. Sheinerman KS, Tsivinsky VG, Abdullah L, Crawford F, Umansky SR. Plasma microRNA biomarkers for detection of mild cognitive impairment: biomarker validation study. Aging (Albany NY). 2013;5(12):925‐938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Marchegiani F, Matacchione G, Ramini D, et al. Diagnostic performance of new and classic CSF biomarkers in age‐related dementias. Aging (Albany NY). 2019;11(8):2420‐2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tan Z, Chen Y, Xie W, Liu X, Zhu Y, Zhu Y. Nimodipine attenuates tau phosphorylation at Ser396 via miR‐132/GSK‐3β pathway in chronic cerebral hypoperfusion rats. Eur J Pharmacol. 2018;819(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 100. Xie H, Zhao Y, Zhou Y, et al. MiR‐9 regulates the expression of BACE1 in dementia induced by chronic brain hypoperfusion in rats. Cell Physiol Biochem. 2017;42(3):1213‐1226. [DOI] [PubMed] [Google Scholar]
- 101. Yu P, Venkat P, Chopp M, et al. Role of microRNA‐126 in vascular cognitive impairment in mice. J Cereb Blood Flow Metab. 2019;39(12):2497‐2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shang Y, Dai S, Chen X, Wen W, Liu X. MicroRNA‐93 regulates the neurological function, cerebral edema and neuronal apoptosis of rats with intracerebral hemorrhage through TLR4/NF‐κB signaling pathway. Cell Cycle. 2019;18(22):3160‐3176. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 103. Liu J, Jiang M, Deng S, et al. miR‐93‐5p‐containing exosomes treatment attenuates acute myocardial infarction‐induced myocardial damage. Mol Ther Nucleic Acids. 2018;11(1):103‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Toyama K, Spin JM, Deng AC, et al. MicroRNA‐mediated therapy modulating blood‐brain barrier disruption improves vascular cognitive impairment. Arterioscler Thromb Vasc Biol. 2018;38(6):1392‐1406. [DOI] [PubMed] [Google Scholar]
- 105. Ren Z, Yu J, Wu Z, et al. MicroRNA‐210‐5p contributes to cognitive impairment in early vascular dementia rat model through targeting Snap25. Front Mol Neurosci. 2018;11(1):388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Liu X, Zhang R, Wu Z, et al. miR‐134‐5p/Foxp2/Syn1 is involved in cognitive impairment in an early vascular dementia rat model. Int J Mol Med. 2019;44(5):1729‐1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kang YC, Zhang L, Su Y, Li Y, Ren WL, Wei WS. MicroRNA‐26b regulates the microglial inflammatory response in hypoxia/ischemia and affects the development of vascular cognitive impairment. Front Cell Neurosci. 2018;12(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Fang C, Li Q, Min G, et al. MicroRNA‐181c ameliorates cognitive impairment induced by chronic cerebral hypoperfusion in rats. Mol Neurobiol. 2017;54(10):8370‐8385. [DOI] [PubMed] [Google Scholar]
- 109. Zhang S, Yan M‐L, Yang L, et al. MicroRNA‐153 impairs hippocampal synaptic vesicle trafficking via downregulation of synapsin I in rats following chronic cerebral hypoperfusion. Exp Neurol. 2020;332(1):113389. [DOI] [PubMed] [Google Scholar]
- 110. Yan M‐L, Zhang S, Zhao H‐M, et al. MicroRNA‐153 impairs presynaptic plasticity by blocking vesicle release following chronic brain hypoperfusion. Cell Commun Signal. 2020;18(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. White BC, Sullivan JM, DeGracia DJ, et al. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179(1‐2):1‐33. [DOI] [PubMed] [Google Scholar]
- 112. Xue Q, Yu C, Wang Y, et al. miR‐9 and miR‐124 synergistically affect regulation of dendritic branching via the AKT/GSK3β pathway by targeting Rap2a. Sci Rep. 2016;6(1):26781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sun L‐H, Ban T, Liu C‐D, et al. Activation of Cdk5/p25 and tau phosphorylation following chronic brain hypoperfusion in rats involves microRNA‐195 down‐regulation. J Neurochem. 2015;134(6):1139‐1151. [DOI] [PubMed] [Google Scholar]
- 114. Liu C‐D, Wang Q, Zong D‐K, et al. Knockdown of microRNA‐195 contributes to protein phosphatase‐2A inactivation in rats with chronic brain hypoperfusion. Neurobiol Aging. 2016;45(1):76‐87. [DOI] [PubMed] [Google Scholar]
- 115. Fish JE, Santoro MM, Morton SU, et al. miR‐126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15(2):272‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wei XJ, Han M, Yang FY, et al. Biological significance of miR‐126 expression in atrial fibrillation and heart failure. Braz J Med Biol Res. 2015;48(11):983‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ferretti C, La Cava A. miR‐126, a new modulator of innate immunity. Cell Mol Immunol. 2014;11(3):215‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hu J, Zeng L, Huang J, Wang G, Lu H. miR‐126 promotes angiogenesis and attenuates inflammation after contusion spinal cord injury in rats. Brain Res. 2015;1608(1):191‐202. [DOI] [PubMed] [Google Scholar]
- 119. Wang L, Yang JW, Lin LT, Huang J, Liu CZ. Acupuncture attenuates inflammation in microglia of vascular dementia rats by inhibiting miR‐93‐mediated TLR4/MyD88/NF‐κB signaling pathway. Oxid Med Cell Longev. 2020;2020(9):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Toyama K, Spin JM, Mogi M, Tsao PS. Therapeutic perspective on vascular cognitive impairment. Pharmacol Res. 2019;146(1):104266. [DOI] [PubMed] [Google Scholar]
- 121. Caballero‐Garrido E, Pena‐Philippides JC, Lordkipanidze T, et al. In vivo inhibition of miR‐155 promotes recovery after experimental mouse stroke. J Neurosci. 2015;35(36):12446‐12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Liu DZ, Jickling GC, Ander BP, et al. Elevating microRNA‐122 in blood improves outcomes after temporary middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2016;36(8):1374‐1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Xu LJ, Ouyang YB, Xiong X, Stary CM, Giffard RG. Post‐stroke treatment with miR‐181 antagomir reduces injury and improves long‐term behavioral recovery in mice after focal cerebral ischemia. Exp Neurol. 2015;264:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mirzaei H, Momeni F, Saadatpour L, et al. MicroRNA: Relevance to stroke diagnosis, prognosis, and therapy. J Cell Physiol. 2018;233(2):856‐865. [DOI] [PubMed] [Google Scholar]
- 125. Lai CP, Breakefield XO. Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front Physiol. 2012;3(1):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Alvarez‐Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341‐345. [DOI] [PubMed] [Google Scholar]
- 127. Katakowski M, Buller B, Zheng X, et al. Exosomes from marrow stromal cells expressing miR‐146b inhibit glioma growth. Cancer Lett. 2013;335(1):201‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9(6):871‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Shimbo K, Miyaki S, Ishitobi H, et al. Exosome‐formed synthetic microRNA‐143 is transferred to osteosarcoma cells and inhibits their migration. Biochem Biophys Res Commun. 2014;445(2):381‐387. [DOI] [PubMed] [Google Scholar]
- 130. Zhang ZG, Chopp M. Promoting brain remodeling to aid in stroke recovery. Trends Mol Med. 2015;21(9):543‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Xin H, Li YI, Liu Z, et al. MiR‐133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome‐enriched extracellular particles. Stem Cells. 2013;31(12):2737‐2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Xu B, Zhang YU, Du X‐F, et al. Neurons secrete miR‐132‐containing exosomes to regulate brain vascular integrity. Cell Res. 2017;27(7):882‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Latronico MV, Condorelli G. Therapeutic use of microRNAs in myocardial diseases. Curr Heart Fail Rep. 2011;8(3):193‐197. [DOI] [PubMed] [Google Scholar]
- 134. Port JD, Sucharov C. Role of microRNAs in cardiovascular disease: therapeutic challenges and potentials. J Cardiovasc Pharmacol. 2010;56(5):444‐453. [DOI] [PubMed] [Google Scholar]
- 135. Cipollini V, Troili F, Giubilei F. Emerging biomarkers in vascular cognitive impairment and dementia: from pathophysiological pathways to clinical application. Int J Mol Sci. 2019;20(11):2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Yin KJ, Olsen K, Hamblin M, Zhang J, Schwendeman SP, Chen YE. Vascular endothelial cell‐specific microRNA‐15a inhibits angiogenesis in hindlimb ischemia. J Biol Chem. 2012;287(32):27055‐27064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. López‐Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Dastur DK. Cerebral blood flow and metabolism in normal human aging, pathological aging, and senile dementia. J Cereb Blood Flow Metab. 1985;5(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 139. Boulias K, Horvitz HR. The C. elegans microRNA mir‐71 acts in neurons to promote germline‐mediated longevity through regulation of DAF‐16/FOXO. Cell Metab. 2012;15(4):439‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Toledano H, D'Alterio C, Czech B, Levine E, Jones DL. The let‐7‐Imp axis regulates ageing of the Drosophila testis stem‐cell niche. Nature. 2012;485(7400):605‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ugalde AP, Español Y, López‐Otín C. Micromanaging aging with miRNAs: new messages from the nuclear envelope. Nucleus. 2011;2(6):549‐555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this review article as no new data were created or analyzed in this review manuscript.


