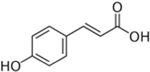
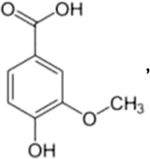
Abstract
Lignocellulosic biomass has been recognized as a promising feedstock for the fermentative production of biofuel. However, the pretreatment of lignocellulose generates a number of by-products, such as furfural, 5-hydroxylmethyl furfural (5-HMF), vanillin, vanillic acids and trans-p-coumaric acid (TPCA), which are known to inhibit microbial growth. This research explores the ability of Rhodococcus opacus PD630 to use lignocellulosic biomass for production of triacylglycerols (TAGs), a common lipid raw material for biodiesel production. This study reports that R. opacus PD630 can grow well in R2A broth in the presence of these model inhibitory compounds while accumulating TAGs. Furthermore, strain PD630 can use TPCA, vanillic acid, and vanillin as carbon sources, but can only use TPCA and vanillic acid for TAG accumulation. Strain PD630 can also grow rapidly on the hydrolysates of corn stover, sorghum, and grass to accumulate TAGs, suggesting that strain PD630 is well-suited for bacterial lipid production from lignocellulosic biomass.
Keywords: Lignocellulosic biomass, Pretreatment, Inhibitory compounds, Rhodococcus opacus PD630, Triacylglycerols (TAGs)
GRAPHICAL ABSTRACT

1. Introduction
Biodiesel is a promising liquid fuel alternative because it is renewable, nontoxic, and contributes much less greenhouse gas emissions to the environment. Biodiesel is made from biolipids, such as triacylglycerols (TAGs) with methanol through a transesterification reaction. Vegetable oils, animal oils/fats, and microbial lipids are available feed-stocks for TAGs. However, current biodiesel production is not cost-effective due to the high cost of the feed-stocks, which accounts for 70–75% of the total cost of biodiesel production (Chen et al., 2009b). Therefore, it is necessary to exploit a cheaper and more sustainable means for TAG production.
Lignocellulosic biomass, including forest and agricultural residues and commercial energy crops, represents the most abundant natural resource for the production of advanced biofuels. Fermentation of lignocellulosic biomass to biogas or ethanol has been well developed (Chandra et al., 2012; Mabee et al., 2011; Palmqvist and Hahn-Hagerdal, 2000; Schmitt et al., 2012; Spatari et al., 2010); however, few studies have explored the possibility of producing TAGs from lignocellulosic biomass for biodiesel production (Chen et al., 2009b; Kosa and Ragauskas, 2012; Zeng et al., 2013).
Lignocellulose consists of three types of polymers: 35–55% cellulose, 20–40% hemicellulose, and 10–25% lignin, all of which are strongly intermeshed and chemically bonded (Limayem and Ricke, 2012). Cellulose (consisting of D-glucose only), hemicellulose (consisting of pentoses (xylose and arabinose)) and hexoses (containing mannose, glucose, galactose, etc.) (Galbe and Zacchi, 2012) are bio-convertible. Pretreatment of lignocellulosic biomass is necessary for effective utilization of these carbon sources by biofuel-producing microorganisms (Chiaramonti et al., 2012; Galbe and Zacchi, 2012; Hendriks and Zeeman, 2009; Kwon et al., 2011; Park and Kim, 2012). However, current pretreatment strategies including thermal (hot water, steam) and chemical pretreatments (alkalis, acids or organic solvents) produce unwanted by-products (Chen et al., 2009b; Chiaramonti et al., 2012; Du et al., 2010; Kwon et al., 2011; Nlewem and Thrash Jr., 2010; Park and Kim, 2012). The profile of the pretreatment by-products may vary, depending on the pH and temperature used during the pretreatment. Two common groups of pretreatment by-products are furan and phenol derivatives. The most common furan derivatives are furfural from xylose and 5-hydroxymethylfurfural (5-HMF) from glucose (Almeida et al., 2009; Palmqvist and Hahn-Hagerdal, 2000). Phenol derivatives like vanillin, vanillic acids, and trans-p-coumaric acid (TPCA) are lignin degradation products (Du et al., 2010; Kosa and Ragauskas, 2012). These pretreatment by-products released to the downstream process can decrease the efficiency of biofuel production since they are known inhibitors for the growth of microorganisms (Almeida et al., 2009; Du et al., 2010; Kwon et al., 2011).
Bacteria with the ability to overcome the inhibition of these by-products and also use the downstream carbohydrates for producing TAGs would be candidates for bioconverting lignocellulosic biomass into valuable biodiesel. In recent years, several Rhodococcus species were demonstrated to have the ability to accumulate TAGs (Alvarez et al., 2000; Kosa and Ragauskas, 2012; Waltermann et al., 2000; Xiong et al., 2012). Rhodococcus strains, residing in soil and water environments, are known for their ability to degrade a wide range of compounds (Kosa and Ragauskas, 2012). Several studies have demonstrated that R. opacus strain PD630 is capable of accumulating TAGs up to 76% of cell dry weight under nitrogen-deficient conditions (Alvarez et al., 1996, 2000; Waltermann et al., 2000). Accordingly, R. opacus PD630 has become one of the most extensively studied TAG-producing microorganism among oleaginous bacterial strains (Alvarez et al., 1996, 2000; Hernandez and Alvarez, 2010; Kosa and Ragauskas, 2012; Waltermann et al., 2000), microalgae (Chen et al., 2009a), yeast (Chen et al., 2009b) and fungi (Zeng et al., 2013) species.
Lignin is known as the most recalcitrant component of lignocellulosic biomass, except through fungal biopulping (Kosa and Ragauskas, 2012). Recent studies have reported that several Rhodococcus strains are potential lignin degraders. For example, R. jostii RHA1 can breakdown lignocellulose to phenolic products (Ahmad et al., 2010) and R. opacus DSM 1069 and PD630 can convert lignin model compounds such as 4-hydroxybenzoic and vanillic acid to produce TAGs (Kosa and Ragauskas, 2012). Furthermore, the successful engineering of xylose (Xiong et al., 2012) and cellobiose (Hetzler and Steinbuchei, 2013; Schmitt et al., 2012) catabolic pathway in Rhodococcus strains indicates the possibility that lignocellulosic biomass can be completely utilized by Rhodococcus strains to produce TAGs.
In this study, R. opacus PD630 was examined for its ability to grow in the presence of model inhibitory compounds of lignocellulosic hydrolysates and to use the model compounds as carbon sources and accumulate TAGs. To better understand the effects of these model inhibitory compounds on cell growth and TAG accumulation, the degradation mechanisms of each model inhibitory compound were studied. Furthermore, the chemical compositions of three hydrolysates from lignocellulosic biomass-corn stover, sorghum, and grass-were determined. The hydrolysates were produced by pretreating the lignocellulosic biomass with sodium hydroxide at low temperature and followed by enzymatic hydrolysis. Alkali pretreatment at low temperature was used in this study because the process has the advantages of effective delignification and retains the hemicellulose in the solids to increase yield (Park and Kim, 2012; Sills and Gossett, 2011). The results of growth tests incorporating these variables were discussed in terms of the suitability of strain PD630 for lipid production from lignocellulosic biomass.
2. Methods
2.1. Materials
Furfural, amido black, and acetic acid were purchased from Fisher Scientific (Pittsburgh, PA). 5-Hydroxymethylfurfural (5-HMF), vanillin, ethyl acetate and hexane were purchased from Acros Organics (Morris Plains, NJ). Vanillic acid, trans-p-coumaric acid (TPCA) and cellulase were purchased from Tokyo Chemical Industry Co., LTD (Japan). Glyceryl trioleate (TL), N, O-Bis (trimethylsilyl) trifluoro-acetamide (BSTFA) with trimethylchlorosilane (TMCS), pridine, xylose, glucose, arabinose, o-methoxylamine HCl, acetic anhydride were obtained from Sigma–Aldrich (St. Louis, MO). Glucosidase was purchased from MP Biomedicals (Solon, OH). [1,2- 13C2] D-glucose was purchased from Cambridge Isotope Laboratories, Inc. (MA, USA). Rhodococcus opacus PD630 (DSM 44193, hereafter referred as strain PD630) was purchased from DSMZ, Germany. The strain was streaked on Reasoner’s 2A (R2A) agar plates for short-term (2–3 weeks) preservation.
2.2. Effects of inhibitory compounds on cell growth and TAG production
To determine the maximum concentrations of inhibitory compounds that strain PD630 can tolerate, inhibition screening tests were conducted on R2A agar plates containing each of the model inhibitory compounds at different concentrations. For furfural and 5-HMF, the concentrations ranging from 0.05 to 5 g/L were used. For vanillin, vanillic acid and TPCA, the concentrations ranging from 0.05 to 2 g/L were used. The selection of concentration range for each inhibitory compound was based on reported values in different lignocellulosic hydrolysates (Almeida et al., 2009; Chen et al., 2009b). All plates were incubated at 30 °C for 5 days and checked daily for colony formation.
Results of the inhibitory screening tests were used to guide the experimental design of liquid growth tests to determine the inhibitory effects on bacterial cell growth and TAG accumulation. The experiments were conducted in 250-ml flasks containing 100 ml R2A broth amended with 0.2 g/L of one of the inhibitory compounds. The growth media were shaken for 1 h at 37 °C at 200 rpm to ensure complete dissolution of the inhibitory compounds and then filter-sterilized using a 0.22 μm Millex-GP syringe filter unit. A cell suspension pregrown from a single colony in 5 ml of R2A broth for 24–36 h at 150 rpm at 30 °C was used to inoculate the flasks. If not specified, a dilution ratio of 1:30 was applied to reach an initial optical density at 600 nm (OD600) of 0.1 in each flask. The flasks were then incubated at 30 °C at 150 rpm and monitored for cell growth (as OD600) over time. After 3 days of incubation, liquid samples were collected for TAG analysis.
2.3. Growth tests using inhibitory compounds as sole carbon sources
New sets of experiments were conducted to determine if any of these model inhibitory compounds can be used as a growth substrate for strain PD630. The growth tests were conducted as described above, except that a single or combination of inhibitory compounds in ammonium mineral salts (AMS) medium (Chu and Alvarez-Cohen, 1996): 18.57 mM NH4Cl, 0.98 mM K2SO4, 0.15 mM MgSO4 · 7H2O, 0.07 mM CaSO4 · 2H2O, 0.08 mM FeSO4 · 7H2O, 3.9 mM KH2PO4, 6.1 mM Na2HPO4, 0.001 mM KI, 0.002 mM ZnSO4 · 7H2O, 0.002 mM MnSO4 · H2O, 0.002 mM H3BO3, 0.004 mM CoMoO4 · H2O. Time-course liquid samples were collected and used to monitor cell growth (as OD600) to track inhibitory compounds and possible metabolites. When the cells reached the stationary growth phase, a second dose of the same inhibitory compound(s) (0.2 g/L) was added into the respective flasks to create a nitrogen-deficient condition – as a means to promote TAG accumulation. After receiving the second dose, the flasks were then incubated at 30 °C without shaking for 3 days before sampling for TAG analysis. For chemical analysis, liquid samples were filtered through 0.22 μm filter disks immediately after collection. The filtered samples were stored at −20 °C for later chemical analysis.
2.4. Growth tests using lignocellulosic hydrolysates
Experiments were also conducted to examine if strain PD630 could grow on different lignocellulosic biomass: grass, sorghum and corn stover. Grass was collected from local lawn, sorghum was provided by Dr. William Rooney, Texas A&M University (College Station, TX) and corn stover was provided by Dewberry Farm (Brookshire, TX). After drying at room temperature for at least 3 days, the biomass was pretreated with alkali and enzymatic hydrolysis to produce hydrolysate for the growth tests. Briefly, biomass (5 g) was pretreated in 1% (wt/vol) sodium hydroxide solution (100 ml) at 30 °C for 24 h (Sills and Gossett, 2011). The pretreated biomass was washed with deionized water at least three times to remove residual alkali and then dried at 50 °C for 3 h. Enzymatic hydrolysis was then conducted in 0.1 M sodium acetate buffer (100 ml, pH = 5.0) containing cellulase (35 mg/g biomass) (Hu et al., 2011) and glucosidase (64 U/g biomass) (Nlewem and Thrash Jr., 2010), and the mixture was incubated at 50 °C for 48 h. After centrifugation at 10,000 g for 10 min, supernatant was collected as the hydrolysate product, neutralized with sulfuric acid, and filter-sterilized for bacterial growth tests, as described above, and Gas Chromatography/Mass Spectrometry (GC/MS) analysis.
2.5. GC/MS
The model inhibitory compounds and degradation metabolites of these compounds were detected as trimethylsilyl (TMS) derivatives using GC/MS analysis (Raj et al., 2007). Briefly, filtered samples were extracted with ethyl acetate by 1:1 (vol/vol) ratio. The extracted samples (500 μl) were then evaporated to dryness under a stream of nitrogen gas, and reconstituted in 20 μl pyridine. To each solution, 40 μl of BSTFA with trimethylchlorosilane (TMCS) was added and the mixture was heated at 60 °C for 30 min. The derivatized mixture, as TMS derivatives, was then analyzed by GC/MS.
The concentration of sugar (xylose, glucose and arabinose) in the hydrolysates of corn stover, sorghum, and grass was determined as methoxime per-acetate derivatives using GC/MS (Wahjudi et al., 2010). Aliquot of 1 μl of corn stover and sorghum hydrolystate and 10 μl of grass were evaporated to dryness and derivatized through a two-step derivatization prior to GC/MS analysis. The air-dried sample was first derivatized using 20 mg/mL O-methoxylamine HCl in 50 μl pyridine at 30 °C for 90 min. This was followed by adding 100 μl of acetic anhydride and the mixture was then heated at 45 °C for 60 min. To each sample, 1 μl of 0.4 μg/μl of [1,2 – 13C2] D-glucose was added as an internal standard prior to derivatization. Derivatized samples were air-dried and reconstituted in 50 μl of ethyl acetate before GC/MS analysis.
GC/MS analysis was performed on Ultra GC/DSQ (Thermo Electron, Waltham, MA) using electron impact ionization (EI). Rxi-5 ms was used as a gas chromatographic column with dimensions of 60 m length, 0.25 mm i.d., and 0.25 μm film thickness (Restek; Bellefonte, PA). Helium was used as a carrier gas at a constant flow of 1.5 ml/min. The injection volume was 1 μl and in splitless mode. The oven temperature was maintained at 50 °C for 5 min and raised to 320 °C at 20 °C/min. Mass spectrometer was operated in full scan mode.
For quantification, standard calibration curves were prepared by plotting peak area ratios of extracted ion chromatograms at m/z 131 for standards to the internal standard at m/z 133 vs. concentration (μg). Linear response 0.5–8.0 μg with linear regression of 0.9944 for glucose and 0.9968 were observed for both xylose and arabinose.
2.6. Lipid extraction and thin layer chromatography (TLC)
Forty-five milliliter of bacterial culture was pelleted at 5000 g for 15 min. The pellet was washed with deionized water once, and then resuspended in 1 ml of deionized water before transferring to a 20 ml glass vial containing 10 ml of chloroform/methanol mixture (2:1, vol:vol). After incubating at 37 °C, 200 rpm overnight, the extract mixture was centrifuged at 2500 g for 20 min to achieve a good phase separation. Then, 5 ml of the chloroform layer (the bottom layer) was transferred to a new glass vial. A gentle stream of air was used to evaporate the solvent in the vial to dryness and the dried lipids were reconstituted in 2 ml of hexane before TLC analysis. For samples collected from growth tests with inhibitory compounds, 200 ml of liquid culture was pelleted and 100 μl of hexane was used to dissolve the dried lipids. This allowed for a better resolution to be developed on the TLC plate.
Ten microliter (μl) of lipid samples (in hexane) and glyceryl trioleate (TL) standards (ranging from 0.4 to 200 μg) were applied to silica gel TLC plates (Product No. 4850–820, Whatman, Piscataway, NJ) and separated in a solvent system of hexane: diethyl ether: acetic acid (80:20:1, vol:vol) (Alvarez et al., 1996). After separation, the TLC plate was dried at 105 °C for 5 min, rinsed with 1 M sodium chloride solution for 15–20 min, and then stained in a 0.2% (wt/vol in 1 M sodium chloride) amido black solution for 15–20 min. After color was developed, the TLC plate was immersed in 1 M sodium chloride solution again to remove background dye before drying at 105 °C for 10 min.
The TAG content of each sample was determined by analyzing the image of the TLC plate using ImageJ software (National Institutes of Health, Bethesda, MD). A TAG standard curve was developed by correlating the loaded amount (μg) of TAG standards to “(area)/(mean gray value)” of the relevant spots developed on the TLC plate. The standard curve was then used to determine the TAG content of the each sample based on the corresponding spot on the TLC plate.
3. Results and discussion
3.1. Effects of inhibitory compounds on cell growth and TAG production
In this study, strain PD630 was used as a model oleaginous bacterium to examine its ability to grow in complex growth medium (R2A broth) in the presence of model inhibitory compounds: TPCA, vanillic acid, vanillin, furfural and 5-HMF. These five model inhibitory compounds were selected for this study as they are common pretreatment by-products of lignocellulosic biomass and they are known to inhibit microbial growth (Almeida et al., 2009; Du et al., 2010; Kosa and Ragauskas, 2012; Kwon et al., 2011; Nlewem and Thrash, 2010; Park and Kim, 2012).
Table 1 shows the results of the inhibition screening tests on R2A agar plates containing individual inhibitory compound over a range of concentrations. In the absence of inhibitory compounds, colonies appeared after two days of incubation. Tolerance to an inhibitory compound was judged if colonies appeared within 5 days of incubation. When high levels of some inhibitory compounds were present (5 g/L furfural, 2 g/L 5-HMF, and 1 g/L vanillin), colony formation was delayed from day 2 to day 3. Note that 5 g/L of furfural is the highest concentration reported in the lignocellulosic hydrolysates (Almeida et al., 2009; Chen et al., 2009b). Strain PD630 had a lower tolerance to phenol derived weak acids, especially vanillic acid (0.2 g/L). This concentration was used as the maximum concentration for each inhibitory compound in liquid-phase growth tests as described below.
Table 1.
Effects of model inhibitory compounds on colony formation of strain PD630.
| Model inhibitory compound | TPCA | Vanillic acid | Vanillin | Furfural | 5-HMF | |
 |
 |
 |
 |
|||
| Concentration (g/L) | 0.05 | + | + | + | + | + |
| 0.1 | + | + | + | + | + | |
| 0.2 | + | + | + | + | + | |
| 1.0 | + | – | + | + | + | |
| 2.0 | − | − | − | + | + | |
| 5.0 | N/A | N/A | N/A | + | − |
: Colony formation within 5 days;
: no colony formation within 5 days; “N/A”: not tested.
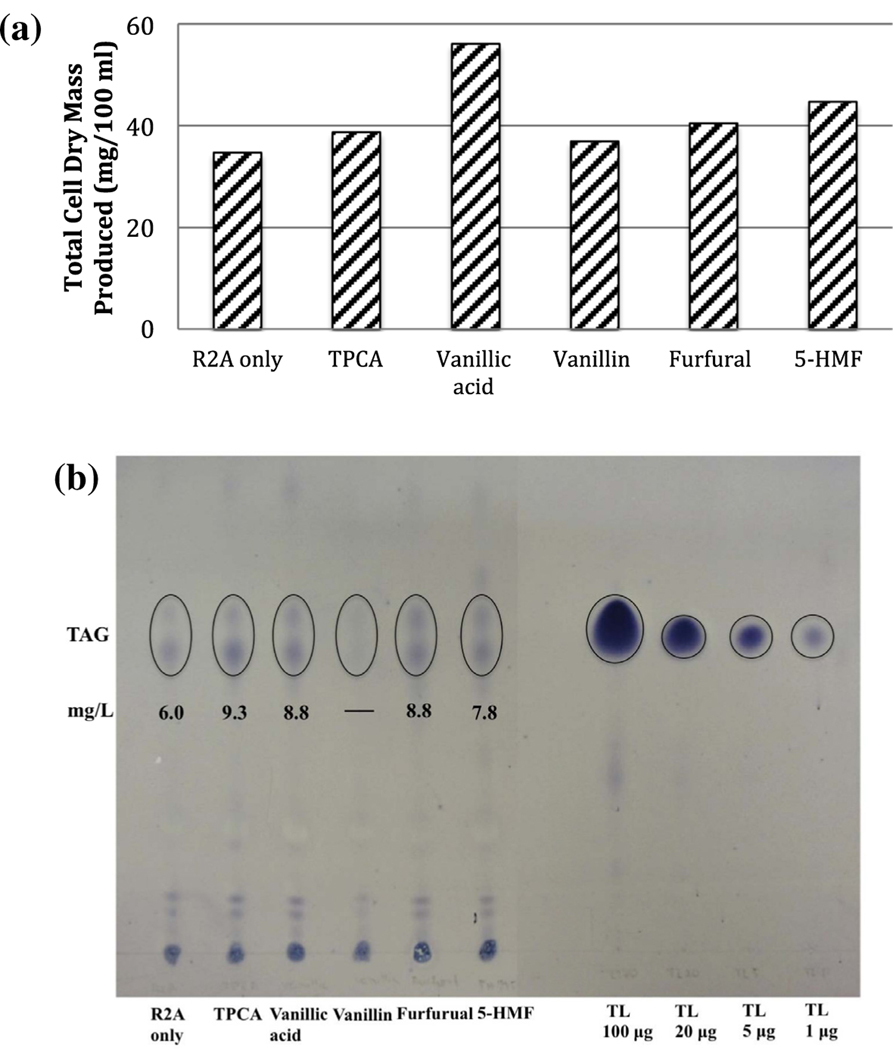
An unexpected result was obtained in the 3-day aerobic growth experiments using R2A broth medium with 0.2 g/L of individual inhibitory compound. The total dry cell mass of strain PD630 was higher when grown on R2A broth with each inhibitory compound than that on R2A broth alone (34.7 mg) (Fig. 1a). The highest biomass (56 mg) was observed when strain PD630 was grown in R2A with vanillic acid. These results suggested that strain PD630 might be able to use some of the inhibitory compounds as additional carbon source for biomass production. This hypothesis was later tested and confirmed by our follow-up growth tests with inhibitory compounds as sole carbon source (see results in Section 3.2).
Fig. 1.
Strain PD630 grown with R2A broth containing inhibitory compounds. Cells were grown under aerobic condition with R2A broth and 0.2 g/L of each inhibitory compound. (a) Total cell dry mass produced in 100 ml culture; (b) TLC results showing TAG contents of strain PD630 grown in R2A containing inhibitory compounds (left), as calculated using a glycerol trioleate TAG standard (TL, right).
Different inhibitory compounds showed different effects on TAG production by strain PD630. When an additional dose of inhibitory compound was added during the stationary growth phase, the presence of vanillin (0.2 g/L) inhibited TAG accumulation in strain PD630 (TAG content was below detection limit as determined by TLC, see Fig. 1b). To our surprise, compared to the TAG content in strain PD630 when grown with R2A broth only, higher quantity of TAGs was accumulated in strain PD630 when grown with R2A broth in the presence of the other four inhibitory compounds: vanillic acid, TPCA, furfural, and 5-HMF (Fig. 1b). One explanation for these observations is that the addition of these inhibitory compounds may interfere with the cell growth and thus indirectly forced the bacteria to switch gene regulation to the TAG accumulation pathway (Alvarez et al., 2000; Hernandez and Alvarez, 2010; Hernandez et al., 2012). Alternatively, strain PD630 may use these inhibitory compounds as carbon sources. The later hypothesis is supported by our results of growth tests using these inhibitory compounds as sole carbon sources.
3.2. Model inhibitory compounds as carbon sources for strain PD630
The ability of strain PD630 to grow on model inhibitory compounds was examined in AMS medium containing individual model inhibitory compound (0.2 g/L) or their combinations (Table 2). No significant cell growth was observed for 14 days when furfural or 5-HMF was provided as the only carbon source, suggesting that strain PD630 could not use furfural or 5-HMF for growth. On the other hand, strain PD630 was able to grow on vanillin, vanillic acid and TPCA at different rates, reaching maximum OD600 = 0.3–0.5, from an initial OD600 = 0.1. As shown in Table 2, it took 5 days for strain PD630 to grow with vanillin, but only 1 day or 2 days when grown with TPCA and vanillic acid, respectively.
Table 2.
Growth of strain PD630 with model inhibitory compounds.
| Model inhibitory compound | Time for complete degradationa (days) | Time to reach maximum OD600b (days) | Maximum OD600 observed | Metabolite detected | |
|---|---|---|---|---|---|
| Vanillin | <6 | 5 | 0.3 | Vanillyl alcohol 3,4-Dihydroxybenzyl alcohol | |
| Vanillic acid | >2 | 2 | 0.4 | 2-Methoxyhydroquinone 3,4 Dihydroxybenzoic acid | |
| TPCA | 1 | 1 | 0.4 | 4-Hydroxybenzoic acid 3,4-Dihydroxybenzoic acid | |
| Mix-1 | Vanillin Vanillic acid |
>7 7 |
7 | 0.4 | Vanillyl alcohol 2-Methoxyhydroquinone |
| Mix2–2 | Vanillic acid TPCA |
1 1 |
1 | 0.9 | 4-Hydroxybenzoic acid |
| Mix3 | Vanillin Vanillic acid TPCA |
>3 1 1 |
2 | 0.9 | Vanillyl alcohol 4-Hydroxybenzoic acid 3,4- Dihydroxybenzyl alcohol 2-Methoxyhydroquinone |
| Mix5 | Furfural 5-HMF Vanillin Vanillic acid |
<1 3 7 7 |
2 | 0.6 | Furan-2-carboxylic acid 5-Hydroxyfuran-2-carboxylic acid Vanillyl alcohol 2-Methoxyhydroquinone 4Hydroxybenzoic acid 3,4-Dihydroxybenzene alcohol |
| TPCA | 2 | ||||
Days it took for individual compound to reach to undetectable concentration or to trace amount as determined by GC/MS, depending on the days that sample were collected for growth test.
Day when the cells reached maximum OD600.
The ability of strain PD630 to grow with mixtures of these inhibitory compounds was also investigated (Table 2). When a mixture of vanillin, vanillic acid, and TPCA (hereafter referred as Mix3) was used, strain PD630 started to grow in the first day, and reached a maximum OD600 = 0.9 on day 2. The results were consistent with the observation that strain PD630 could grow on these three compounds with a different order of preference (TPCA > vanillic acid > vanillin) (Table 2), when individual compound was used as carbon source. A delayed growth pattern was observed when all five model inhibitory compounds (hereafter referred as Mix5) were present, starting to grow and then reaching to a maximum OD600 = 0.6 on day 2.
The longer time to initiate the growth and the lower maximum optical density might be due to potential inhibition effects of the two compounds, furfural and 5-HMF. The degradation mechanism of the inhibitory compounds in the mixture is more complex since it may involve cross-inhibition. It was also possible that furfural and 5-HMF are cometabolic substrates that consume reducing energy in strain PD630, leading to less biomass production (see Section 3.3 for details).
Experiments were also designed to examine whether vanillin, vanillic acid or TPCA can be used for TAG accumulation. During the inhibition study, higher TAG contents were observed when strain PD630 grown with R2A broth containing TPCA or vanillic acid (Fig. 1a and b), suggesting that vanillic acid and TPCA served as an additional carbon source for TAG accumulation. To further examine this possibility, a second dose of inhibitory compound was added after the cells reach the stationary growth phase to create a nitrogen deficient condition to maximize the TAG accumulation (Alvarez et al., 1996, 2000). It was expected that the addition of the carbon source would suddenly change the carbon to nitrogen ratio to a high level.
The TLC results showed that TPCA and vanillic acid, but not vanillin, can promote TAG accumulation in strain PD630 (Fig. 2). Both TPCA-grown and vanillic acid-grown cells produced higher TAG contents (1.4 mg/L of TAGs) than that of vanillin-grown cells (0.05 mg/L). The results are consistent with the findings from the growth tests using R2A broth with inhibitory compounds under aerobic conditions; the presence of 0.2 g/L vanillin inhibited TAG accumulation (Fig. 1b). The possible reason for the low TAG accumulation in vanillin-grown PD630 is discussed in Section 3.3. Nevertheless, TPCA and vanillic acid are found to be alternative carbon sources for TAG accumulation in strain PD630.
Fig. 2.
TLC results showing TAG contents of strain PD630 following growth on ammonium mineral salts medium (AMS) using 0.2 g/L of TPCA, vanillic acid or vanillin as the sole carbon source (left), as calculated using a TL standard (right).
3.3. Analysis of the degradation of model inhibitory compounds
To better understand the effects of model inhibitory compounds on the growth of strain PD630 and TAG accumulation, samples taken from cell growth study were used for analysis of the degradation of model inhibitory compounds and their metabolites. Samples were extracted with ethyl acetate and subjected to GC/MS analysis as described. The substrates and their metabolites were detected as trimethylsilyl (TMS) derivatives. The results obtained from GC/MS data along with growth experiments are summarized in Table 2.
3.3.1. Degradation of individual model inhibitory compounds
TPCA and vanillic acid are phenol derivatives, carboxylic acid model compounds tested for the degradation study. As seen in Table 2, TPCA is the most rapidly degraded inhibitory compound tested; it was metabolized within a day. Trace amount of 4-hydroxybenzoic acid and 3,4-dihydroxybenzoic acid were detected as TPCA metabolites. The degradation pathway of TPCA is similar to the degradation pathway of ferulic acid in strain Nocardia sp. DSM 1069 (Eggeling and Sahm, 1980), where the side chain (propenoic acid) was shortened to yield 3,4 dihydroxybenzoic acid and followed by demethylation and successive hydroxylation to form 4-hydroxybenzoic acid (Scheme 1 in supplementary material). Vanillic acid, on the other hand, was degraded relatively slower than TPCA. Similar to TPCA, vanillic acid seems to go through demethylation followed by hydroxylation to yield 3,4 dihydroxybenzoic acid. In addition to this metabolite, decarboxylated form of vanillic acid, 2-methoxyhydroquinone, was also detected. This oxidative process of vanillic acid is commonly observed in fungi (Krings et al., 2001; Shimizu et al., 2005). Metabolites of vanillic acid and TPCA are very short-lived, subjected to aromatic ring cleavage and further degradation thereafter.
As discussed in Section 3.2, TPCA-grown PD630 yielded the highest content of TAGs (1.4 mg/l) of all model compounds tested. The high yield of TAGs coincides with the fast metabolism of TPCA. Even though the TAG accumulation in strain PD630 using vanillic acid is comparable to TPCA, the cell yield was not as high (Fig. 1b). Recently, Kosa et al. observed slower degradation of vanillic acid in comparison to 4-hydroxycarboxyic acid (Kosa and Ragauskas, 2012). In addition, lower yield of TAGs and lower cell numbers were also noted for vanillin. Although no additional test was performed, compounds with longer side chains (propionic acid) such as TPCA might be of preference for strain PD630 to use as a sole source of carbon, in comparison to shorter side chain compounds like vanillic acid.
Vanillin is a non-carboxylic acid aromatic model compound tested for degradation study. A reduced form of vanillin, vanillyl alcohol, was detected as a major metabolite; vanillyl alcohol can be demethylated to form 3,4-dihydroxybenzyl alcohol which was also detected in trace amounts. Similarly, conversion of vanillin to vanillyl alcohol and vanillic acid using other strains was previously reported (Krings et al., 2001; Shimizu et al., 2005). This process was also reported to be a reversible process (Krings et al., 2001). However, no formation of vanillic acid as a metabolite of vanillin was observed in strain PD630. Despite the complete metabolism of vanillin, inhibition of TAG formation was observed (Fig. 2) and cell growth was not maximized until day 5 (Table 2). To further understand the degradation kinetics of vanillin and its TAG inhibition nature, three different mixes of model compounds namely, Mix2–1 (vanillin and vanillic acid), Mix2–2 (vanillic acid and TPCA), and Mix3 (vanillic acid, vanillin and TPCA) were used as carbon sources.
3.3.2. Degradation of mixed model inhibitory compounds
When Mix2–1 was used as the source of carbon for strain PD630, the degradation of vanillic acid was delayed until day 7. Vanillin degradation was not detected even though vanillyl alcohol formation was observed gradually to day 7. It is also true that cells did not grow for over 7 days. When Mix2–2 was used, vanillic acid and TPCA were degraded and cells grew to their maximum density within a day. As seen in Table 2, the degradation rates of these model inhibitors compounds (vanillin, vanillic acid, Mix2–1 and Mix2–2) were consistent with the cell growth rates of strain PD630. The delay in degradation of vanillic acid in Mix2–1 could be due to the conversion of vanillin to vanillic acid in similar manner to the previous report (Krings et al., 2001; Shimizu et al., 2005). In addition, the presence of vanillyl alcohol in equilibration with vanillin could cause the delay of growth and the inhibition of TAG accumulation when vanillin was present in the mixture.
In Mix3, no additional metabolite was detected other than the metabolites of individual model compounds. Unlike Mix2–1, degradation of vanillic acid and growth rate of strain PD630 were not deterred by the presence of vanillin in Mix3. This result is consistent with Mix2–2. The presence of the readily degradable TPCA is responsible for the fast cell growth. In addition, the degradation of vanillyl alcohol was observed on day 2 unlike those observed in the two other mixes (Mix2–1 and Mix2–2) (Fig. 3a). This clearly indicates the presence of vanillyl alcohol to be a contributing factor for delayed cell growth and inhibition of TAG accumulation. This is also true for Mix5 despite the presence of additional inhibitors (furfural and 5-HMF).
Fig. 3.
Degradation of Mix3 (a) and Mix5 (b) with the cell growth. Left y-axis represents the normalized concentration of each compound and the right y-axis represents the growth of the PD630 as optical density (OD600). For simplicity of this figure, intensities of each peak were normalized relative to the most abundant peak of each compound.
In Mix5, the degradation of TPCA was delayed by only one day in comparison to that in Mix3. Despite the presence of two additional model compounds and their metabolites, for simplicity and as a direct comparison to Mix3, only degradation of vanillin, vanillyl alcohol, vanillic acid and TPCA was observed (Fig. 3b). As seen from Fig. 3b, vanillin was degraded to about 80% of its original concentration. However, vanillic acid was degraded to about 10% of its original concentration and started to increase again to its 90% concentration in 7 days. Since vanillin was not degraded after 36 h, it is impossible for vanillin to be converted to vanillic acid during the first 36 h. The upsurge of vanillic acid coincided with the metabolism of vanillyl alcohol, suggesting that oxidation of vanillyl alcohol to vanillic acid could be possible. However, this could not be confirmed from Mix2–1, as both vanillin and vanillic acid were not degraded in 7 days. Again as noticed above, the degradation of vanillyl alcohol coincides with cell growth.
Furfural and 5-HMF were observed to be completely degraded in the presence of the other three model compounds, even though no growth was observed when they were used individually. Furfural was degraded to furan-2-carboxylic acid as proposed by previously (Koopman et al., 2010), while 5-HMF was metabolized to 5-(hydroxymethyl)furan-2-carboxylic acid and an unidentified compound. 5-HMF might have been partially converted into furan-2-carboxylic acid after subsequent oxidation and decarboxylation (Scheme 1 in supplementary material). The complete degradation of furfural and 5-HMF in the mixture rather than individually indicates cometabolism may exist in the degradation of Mix5. As the number of model compounds increase, the degradation mechanisms become further complicated. However, the ultimate target of these metabolic analyses was to determine if PD630 could grow on the real lignocellulosic hydrolysates, which is shown in Section 3.4.
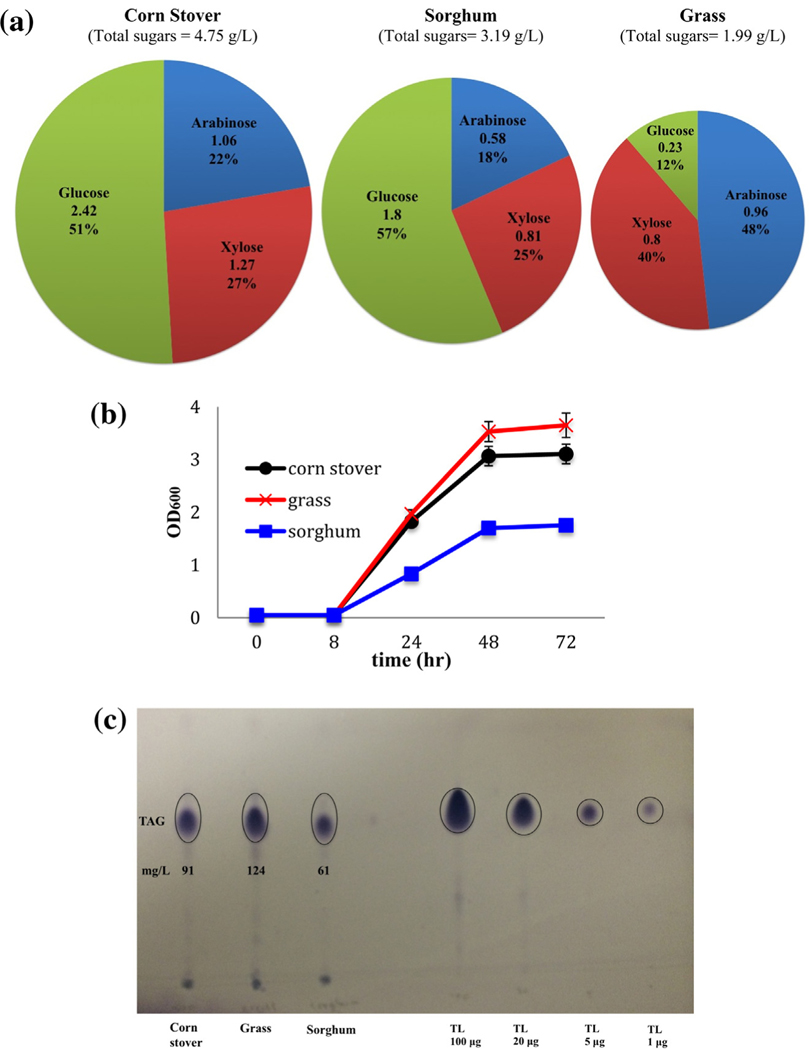
3.4. Growth of strain PD630 with lignocellulosic hydrolysates
Altogether, strain PD630 can tolerate model lignocellulosic inhibitory compounds at low levels, and use some of the compounds as carbon sources to accumulate TAGs. Experiments were conducted to further investigate its ability to grow on three different lignocellulosic hydrolysates produced from sorghum, corn stover, and grass. In this study, alkali pretreatment (sodium hydroxide solution) at low temperature was used to reduce the production of inhibitory compounds (Chiaramonti et al., 2012; Nlewem and Thrash, 2010; Park and Kim, 2012). A number of acids and aldehydes were detected in the three different hydrolysates (Table 3). Among these chemicals, 4-hydroxy benzaldehyde and 4-hydroxy benzoic acid have been reported as lignocellulose pretreatment by-products in hydrolysates of corn stover, hybrid poplar and pine (Du et al., 2010). Possibly due to detection limits and different pretreatment methods, 4-hydroxybenzoic acid was not detected in the corn stover hydrolysate. Also among the five model inhibitory compounds, only TPCA was detected in all the three hydrolysates, and vanillic acid was detected in corn stover and grass hydrolysates (Table 3). Five different types of sugars were identified in these hydrolysates; these include glucose, xylose, galactose, mannose and arabinose (Fig. 4a). Compared to the other two sugars, glucose, xylose and arabinose are the dominant sugars in all three hydrolysates. Corn stover hydrolysate contains much higher glucose content than those of grass and sorghum. As shown in Fig. 4b and c, strain PD630 can grow well on all three hydrolysates, with a lower biomass and TAG production when cultured with sorghum hydrolysate. Despite grass hydrolysate having the least glucose content among the three hydrolysates, the highest biomass and TAG production was achieved when grass hydrolysate was used. The different biomass and TAG production with these three hydrolysates might be explained by the complex composition in the hydrolysates. Excluding the detected sugars, about 20 different compounds were identified in the grass hydrolysates, indicating a more complex composition of grass hydrolysate than the other two (11 for corn stover and 9 for sorghum) (Table 3). The complex composition in the grass hydrolysate may provide additional carbon sources for strain PD630 to use. Overall, based on the growth tests with lignocellulosic hydrolysates, missing or negligible amount of vanillin, furfural or 5-HMF did not affect the growth or TAG accumulation of strain PD630.
Table 3.
Compounds identified as TMS derivatives in ethyl acetate extracts from each lignocellulosic hydrolysate.
| Corn stover | Grass | Sorghum | Identified compounds |
|---|---|---|---|
| − | + | − | 3-Phenylpropanoic acid |
| + | − | + | 2,2-Dimethyl-1,3-dioxolane-4,5-dicarboxylic acid dimethyl ester |
| + | + | − | Nonanoic acid |
| + | + | − | 4-Hydroxy benzaldehyde |
| − | + | − | 5-Methylpyrimidine-2,4-diol |
| − | + | − | Decanoic acid |
| + | + | + | 3-Methoxy benzaldehyde |
| − | + | − | 4-Hydroxyphenethyl alcohol |
| − | + | − | 2-Hydroxy-3-phenylpropanoic acid |
| − | + | + | 4-Hydroxybenzoic acid |
| − | + | + | Dodecanoic acid |
| + | + | + | D-xylose |
| + | + | − | Vanillic acid |
| + | + | + | cis-p-Coumaric acid |
| − | + | − | Tetradecanoic acid |
| − | + | − | Sebacic acid |
| − | + | − | 3,5-Dimethyl-4-hydroxybenzoic acid |
| + | + | + | 3,4-Dimethoxycinnamic acid |
| + | + | + | trans-p-Coumaric acid (TPCA) |
| + | + | + | Hexadecanoic acid |
| + | + | + | 4-Methoxy-3-hydroxycinnamic acid |
| + | + | − | Octadecanoic acid |
: Present;
: absent.
Fig. 4.
Strain PD630 can grow on lignocellulosic hydrolysates while accumulating TAGs. (a) Distribution of three dominant sugars (glucose, xylose and arabinose in g/L and %). (b) Strain PD630 can grow on three different lignocellulosic hydrolysates produced from corn stover, grass, and sorghum. (c) TLC results showing TAG contents of strain PD630 grown with three different hydrolysates (left), as calculated using a TL standard (right).
4. Conclusions
This study demonstrated that strain PD630 can tolerate low concentration of five model inhibitory compounds. Furthermore, strain PD630 can use vanillic acid and TPCA for cell growth and TAG accumulation. Though vanillin can be used as carbon source, TAGs are unable to be accumulated. While strain PD630 cannot use furfural and 5-HMF as carbon sources, the strain can degrade them cometabolically. Importantly, strain PD630 is able to grow rapidly on the hydrolysates of grass, corn stover and sorghum while accumulating TAGs. These results indicate that it is possible to culture the oleaginous strain PD630 on lignocellulosic biomass for biodiesel production.
Supplementary Material
HIGHLIGHTS.
Strain PD630 can tolerate low concentration of five model inhibitory compounds.
Strain PD630 can use vanillic acid and TPCA for cell growth and TAG accumulation.
Vanillin can be used as a carbon source, but TAGs are not accumulated.
Strain PD630 can grow on three lignocellulosic hydrolysates and accumulate TAGs.
Acknowledgements
The authors thank the National Science Foundation, United States (Award #1134488) for supporting this research. Thanks also go to Dr. William Rooney and Dewberry Farm for providing sorghum and corn stover used in this study.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.biortech.2014.02.133.
References
- Ahmad M, Taylor CR, Pink D, Burton K, Eastwood D, Bending GD, Bugg TDH, 2010. Development of novel assays for lignin degradation: comparative analysis of bacterial and fungal lignin degraders. Mol. BioSyst 6, 815–821. [DOI] [PubMed] [Google Scholar]
- Almeida JRM, Bertilsson M, Gorwa-Grauslund MF, Gorsich S, Liden G, 2009. Metabolic effects of furaldehydes and impacts on biotechnological processes. Appl. Microbiol. Biotechnol 82, 625–638. [DOI] [PubMed] [Google Scholar]
- Alvarez HM, Mayer F, Fabritius D, Steinbuchel A, 1996. Formation of intracytoplasmic lipid inclusions by Rhodococcus opacus strain PD630. Arch. Microbiol 165, 377–386. [DOI] [PubMed] [Google Scholar]
- Alvarez HM, Kalscheuer R, Steinbuchel A, 2000. Accumulation and mobilization of storage lipids by Rhodococcus opacus PD630 and Rhodococcus ruber NCIMB 40126. Appl. Microbiol. Biotechnol 54, 218–223. [DOI] [PubMed] [Google Scholar]
- Chandra R, Takeuchi H, Hasegawa T, 2012. Methane production from lignocellulosic agricultural crop wastes: a review in context to second generation of biofuel production. Renew. Sustain. Energy Rev 16, 1462–1476. [Google Scholar]
- Chen W, Zhang C, Song L, Sommerfeld M, Hu Q, 2009a. A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J. Microbiol. Methods 77, 41–47. [DOI] [PubMed] [Google Scholar]
- Chen X, Li Z, Zhang X, Hu F, Ryu DDY, Bao J, 2009b. Screening of oleaginous yeast strains tolerant to lignocellulose degradation compounds. Appl. Biochem. Biotechnol 159, 591–604. [DOI] [PubMed] [Google Scholar]
- Chiaramonti D, Prussi M, Ferrero S, Oriani L, Ottonello P, Torre P, Cherchi F, 2012. Review of pretreatment processes for lignocellulosic ethanol production, and development of an innovative method. Biomass Bioenergy 46, 25–35. [Google Scholar]
- Chu KH, Alvarez-Cohen L, 1996. Trichloroethylene degradation by methane-oxidizing cultures grown with various nitrogen sources. Water Environ. Res 68, 76–82. [Google Scholar]
- Du B, Sharma LN, Becker C, Chen S, Mowery RA, van Walsum P, Chambliss CK, 2010. Effect of varying feedstock-pretreatment chemistry combinations on the formation and accumulation of potentially inhibitory degradation products in biomass hydrolysates. Biotechnol. Bioeng 107, 430–440. [DOI] [PubMed] [Google Scholar]
- Eggeling L, Sahm H, 1980. Degradation of coniferyl alcohol and other lignin-related aromatic compounds by Nocardia sp. DSM 1069. Arch. Microbiol 126, 141–148. [Google Scholar]
- Galbe M, Zacchi G, 2012. Pretreatment: the key to efficient utilization of lignocellulosic materials. Biomass Bioenergy 46, 70–78. [Google Scholar]
- Hendriks ATWM, Zeeman G, 2009. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol 100, 10–18. [DOI] [PubMed] [Google Scholar]
- Hernandez MA, Alvarez HM, 2010. Glycogen formation by Rhodococcus species and the effect of inhibition of lipid biosynthesis on glycogen accumulation in Rhodococcus opacus PD630. FEMS Microbiol. Lett 312, 93–99. [DOI] [PubMed] [Google Scholar]
- Hernandez MA, Arabolaza A, Rodriguez E, Gramajo H, Alvarez HM, 2012. The atf2 gene is involved in triacylglycerol biosynthesis and accumulation in the oleaginous Rhodococcus opacus PD630. Appl. Microbiol. Biotechnol 97, 2119–2130. [DOI] [PubMed] [Google Scholar]
- Hetzler S, Steinbuchei S, 2013. Establishment of cellobiose utilization for lipid production in Rhodococcus opacus PD630. Appl. Environ. Microbiol 79, 3122–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Arantes V, Saddler JN, 2011. The enhancement of enzymatic hydrolysis of lignocellulosic substrates by the addition of accessory enzymes such as xylanase: is it an additive or synergistic effect? Biotechnol. Biofuels 4, 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman F, Wierckx N, de Winde JH, Ruijssenaars HJ, 2010. Identification and characterization of the furfural and 5-(hydroxymethyl)furfural degradation pathways of Cupriavidus basilensis HMF14. PNAS 107, 4919–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosa M, Ragauskas AJ, 2012. Bioconversion of lignin model compounds with oleaginous Rhodococci. Appl. Microbiol. Biotechnol 93, 891–900. [DOI] [PubMed] [Google Scholar]
- Krings U, Pilawa S, Theobald C, Berger RG, 2001. Phenyl propenoic side chain degradation of ferulic acid by Pycnoporus cinnabarinus -elucidation of metabolic pathways using [5–2H]-ferulic acid. J. Biotechnol 85, 305–314. [DOI] [PubMed] [Google Scholar]
- Kwon Y, Ma A, Li Q, Wang F, Zhuang G, Liu C, 2011. Effect of lignocellulosic inhibitory compounds on growth and ethanol fermentation of newly-isolated thermotolerant Issatchenkia orientalis. Bioresour. Technol 102, 8099–8104. [DOI] [PubMed] [Google Scholar]
- Limayem A, Ricke SC, 2012. Lignocellulosic biomass for bioethanol production: current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci 38, 449–467. [Google Scholar]
- Mabee WE, McFarlane PN, Saddler JN, 2011. Biomass availability for lignocellulosic ethanol production. Biomass Bioenergy 35, 4519–4529. [Google Scholar]
- Nlewem KC, Thrash ME Jr., 2010. Comparison of different pretreatment methods based on residual lignin effect on the enzymatic hydrolysis of switchgrass. Bioresour. Technol 101, 5426–5430. [DOI] [PubMed] [Google Scholar]
- Palmqvist E, Hahn-Hagerdal B, 2000. Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour. Technol 74, 25–33. [Google Scholar]
- Park YC, Kim JS, 2012. Comparison of various alkaline pretreatment methods of lignocellulosic biomass. Energy 47, 31–35. [Google Scholar]
- Raj A, Reddy MMK, Chandra R, 2007. Identification of low molecular weight aromatic compounds by gas chromatography–mass spectrometry (GC–MS) from kraft lignin degradation by three Bacillus sp. Int. Biodeterior. Biodegradation 59, 292–296. [Google Scholar]
- Schmitt E, Bura R, Gustafson R, Cooper J, Vajzovic A, 2012. Converting lignocellulosic solid waste into ethanol for the State of Washington: an investigation of treatment technologies and environmental impacts. Bioresour. Technol 104, 400–409. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Yuda N, Nakamura T, Tanaka H, Wariishi H, 2005. Metabolic regulation at the tricarboxylic acid and glyoxylate cycles of the lignin-degrading basidiomycete Phanerochaete chrysosporium against exogenous addition of vanillin. Proteomics 5, 3919–3931. [DOI] [PubMed] [Google Scholar]
- Sills DL, Gossett JM, 2011. Assessment of commercial hemicellulases for saccharification of alkaline pretreated perennial biomass. Bioresour. Technol 102, 1389–1398. [DOI] [PubMed] [Google Scholar]
- Spatari S, Bagley DM, Maclean HL, 2010. Life cycle evaluation of emerging lignocellulosic ethanol conversion technologies. Bioresour. Technol 101, 654–667. [DOI] [PubMed] [Google Scholar]
- Wahjudi PN, Patterson ME, Lim S, Yee JK, Mao CS, Lee W-NP, 2010. Measurement of glucose and fructose in clinical samples using gas chromatography/mass spectrometry. Clin. Biochem 43, 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltermann M, Luftmann H, Baumeister D, Kalscheuer R, Steinbuchel A, 2000. Rhodococcus opacus strain PD630 as a new source of high-value single-cell oil? Isolation and characterization of triacylglycerols and other storage lipids. Microbiology 146, 1143–1149. [DOI] [PubMed] [Google Scholar]
- Xiong X, Wang X, Chen S, 2012. Engineering of a Xylose metabolic pathway in Rhodococcus Strains. Appl. Environ. Microbiol 78, 5483–5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Zheng Y, Yu Z, Yu L, Gao D, Chen S, 2013. Lignocellulosic biomass as a carbohydrate source for lipid production by Mortierella isabellina. Bioresour. Technol 128, 385–391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.