On April 2, 1986, a symposium comprised of multidisciplinary preclinical and clinical scientists and physicians was convened and the proceedings published.1 It addressed neuroendocrine disorders of the gastroenteropancreatic (GEP) systems and introduced preclinical and clinical applications of the somatostatin peptide analogue SMS 201–995 (octreotide [Sandostatin]). Three years later, in 1989, octreotide was the first Food and Drug Administration (FDA)-approved drug for the symptomatic control of carcinoid syndrome and the watery diarrhea syndrome of the pancreatic neuroendocrine tumor (NET), VIPoma.2 Now 30 years later, octreotide, and the recently FDA-approved agent lanreotide, continue as first-line therapies for all symptomatic NETs. Furthermore, the isotopie radiolabeling of octreotide specifically targets somatostatin receptors overexpressed on NETs and has heralded yet another FDA-approved diagnostic-therapeutic strategy termed theranostics, which allows an octreotide derivative to act as a radiodiagnostic molecular imaging agent and a radiotherapeutic product for the treatment of malignant endocrine tumors.3,4
To better understand the context of these developments, and the pivotal role native somatostatin and its long-acting analogues play in normal peptide regulation and neuropeptide excess associated with NETs, we delineate and define distinct eras in the history and discovery of gastrointestinal (GI) endocrinology. The major periods of gut endocrinology include: the physiology (“juice”) era, the clinical era, the peptide chemistry era, and the peptide diagnostic and therapeutic era.5
Building on the historical milestones recently addressed in reviews by O’Dorisio5 and Oberg,6 we address and expand on the two reviews where appropriate. Regarding 177Lu-DOTA(tyr)-octreotate and 90Y-DOTA-(tyr)-octreotide therapy in childhood and adult NETs, we highlight the collaboration between academia and industry in basic science and the clinical research that advanced LUTATHERA (Lu-177-dota-tate) to FDA approval as standard of care therapy for low-grade NETs.4 Examples of new radioisotopes and therapy compounds currently in development for diagnosis and therapy for high-grade NETs are also discussed.
Endocrinology began in 1902 with the physiology era, with the discovery of the “blood-borne chemical messenger” secretin by Bayliss and Starling.7 They demonstrated that HCI placed into canine duodenum resulted in the secretion of alkaline pancreatic juice. They called this substance “secretin” and later coined the term “hormone” derived from the Greek military word Opuaw, which means “I arouse/excite to activity.”5
In 1905, Edkins,8 a prominent physiologist, described a potent gastric acid secretagogue subsequently termed “gastrin.” It was not until 1961 that Gregory and Tracy9 identified the multigastrin forms confirming its biologic properties described by Edkins. Physiologists Ivy and Eldberg10 described their observation on hormone-mediated gallbladder contraction. They noted that the extract from dog proximal small intestine when injected intravenously into another dog was associated with gallbladder contraction. A second pair of physiologists, Harper and Raper,11 found that dog small intestine extract was associated with gallbladder contraction and pancreatic juice release and termed it “pancreozymin.” For a time, the abbreviation CCK/PZ was used. It was ultimately determined that pancreozymin was indeed cholecys-tokinin (CCK). This highlights the insensitivity of the bioassay techniques at the time, and the tremendous impact that radioimmunoassay (RIA) was to have on the neuroendocrine field.
A few years before the discovery of CCK, Banting and Best12 were able to stabilize sheep insulin and achieve glucose homeostasis in pancreatectomized dogs. Shortly after insulin stabilization, a patient with insulin-dependent diabetes mellitus was successfully treated with the extracted sheep insulin. This and other key insulin milestones were reviewed by the endocrinologist Forsham13 in 1982. In the United States, large-scale insulin production was achieved thanks to the strong collaboration between academia and industry (Eli Lilly) and NOVO-Nordisk in Europe. Diabetes mellitus represented a peptide-deficient state of the enteropancreatic axis as opposed to the excess peptide hormone production by functional NETs.
During the era of physiologic discovery, morphologists, anatomists, and pathologists were working to identify the GEP cells responsible for the secretion of these newly identified peptide hormone substances. Table 1 lists the key investigators who helped define the neuroendocrine cells of the GEP axis.5,6
Table 1.
Investigators who helped describe and identify the amine/peptide-secreting cells of the GEP system
| P. Langerhans | 1870s | Cell clusters on acinar cells | Langerhans and Moorison,14 1869 |
| E. Laguesse | 1890s | Islets of Langerhans | Langerhans and Moorison,14 1869 |
| R. P. Heidenhain | 1870 | EC EC-like: histamine secretion |
Heidenhain,151870 |
| A. Nikolas | 1891 | EC cells, Gl tract | Nikolas,16 1891 |
| N. Kulchitsky | 1897 | EC cells/crypts of Lieberkühn | Kulchitsky,17 1897 |
| M. Ciaccio | 1906 | EC cells in human Gl tract | Ciaccio,18 1906 |
| A. Gosset and P. Masson | 1914 | Diffuse endocrine system | Gosset and Masson,19 1914 |
| F. Feyter | 1938 | Helle zellen, clear cells; neuroendocrine | Feyter,20 1938 |
| F. Feyter | 1953 | Coined paracrine and neurocrine | Feyter,21 1953 |
Abbreviation: EC, enterochromaffin.
Data from O’Dorisio TM. Gut endocrinology: clinical and therapeutic impact. Am J Med. 1986;81(6B):1–7 and Öberg K. The Genesis of the Neuroendocrine Tumors Concept: From Oberndorfer to 2018. Endocrinol Metab Clin North Am. 2018;47(3):711–731.
By 1950 it was clear that, within the GEP system, there were many unexplained control systems and a larger number of different neuroendocrine cells and their secreting products and actions that were yet to be recognized. A prominent gastrophysiologist, Grossman,22 worked diligently to assign hormone and neuroenne action to the previously described substances, such as CCK and secretin. Until that time, the extracted peptide material was crude and only partially purified. This affected bioassay interpretation and assignment of the actual neuropeptides responsible for the observed actions. One example was the identification of incretin, a substance residing in the small intestine, released by a glucose load and affecting additional insulin secretion in a hormonal fashion. Moore23 is credited with the first physiologic observation of a small intestinal extract that lowered blood sugars in the presence of an increased glucose load into the duodenum. It is now accepted that incretin is caused, in part, by the two GI peptides glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide-1.24
The peptide chemistry era was ushered in with two remarkable discoveries in the 1950s and 1960s. The first was the development of column chromatography, which used cross-linked dextran gel for gel filtration of substances based on particle size and cross-linking and molecular weight.25 The resin columns used different size beads, which excluded extracts of different molecular weights and purification. The purification of gastrin from Zollinger-Ellison tumors by Gregory and Tracy in 1961 was an excellent example of the purification by column chromatography.5 Furthermore, Jorpes and Mutt from the Karolinska Institute in Stockholm, Sweden, purified several GI peptides extracted from porcine intestine using large-diameter columns.26,27 It has been estimated that the combined efforts of Gregory, Jorpes, and Mutt led to the purification of more than 14 GI neuropeptides that included GIP, vaso-active intestinal peptide (VIP), pancreastatin, and neuropeptide Y, and peptide tyrosine.
The second critical discovery was the development of RIA by Yalow and Berson in 1959.28,29 With highly purified GI peptides being rapidly produced, RIA used highly pure peptide standards in competitive protein binding curves with a short wavelength isotope, iodine 125- Kev 30, offering for the first time high peptide sensitivity and specificity measurements. Shortly after the initial description of RIA, McGuigan30 published the gastrin RIA. With the development of high-titer peptide antibodies for RIA, antibodies directed against peptide-secreting neuroendocrine cells using the immunohistochemical (IHC) methodology was simultaneously developed. Two active investigators in neuroendocrine clinical research in the mid-1970s were the endocrinologist S.R. Bloom and pathologist J.M. Polak. Together they identified by RIA and IHC many GI neuroendocrine secreting cells,31–33 and made a great contribution to GI neuroen-docrinology by the mid-1970s. The United States had also developed RIAs for the newly identified neuropeptides. The Mayo Clinic developed commercial neuropeptide RIAs under the supervision of V.L.W. Go. Also, The Ohio State University/University Reference Laboratory performed CLIA-certified GI neuropeptide RIAs under the supervision of S. Cataland and T.M. O’Dorisio. The most durable commercial neuropeptide RIA laboratory in the United States is the Inter Science Institute, which will soon celebrate 50 years of performing peptide RIAs.34
In 1977, R. Yalow received the Nobel prize in physiology and medicine “for the development of RIA of peptide hormones.” S. Berson had passed away and was not eligible posthumously. Dr Yalow shared the Nobel Prize that year with R. Guillemen and A. Schally “for their discoveries concerning the peptide hormone production of the brain.” Dr Guillemin and his colleagues had discovered somato-statin.35 Arimura and colleagues36,37 established an acetone extraction RIA to measure plasma somato-statin plasma in 1978. The impact that RIA had on GI peptide discovery is depicted in Fig. 1.
Fig. 1.
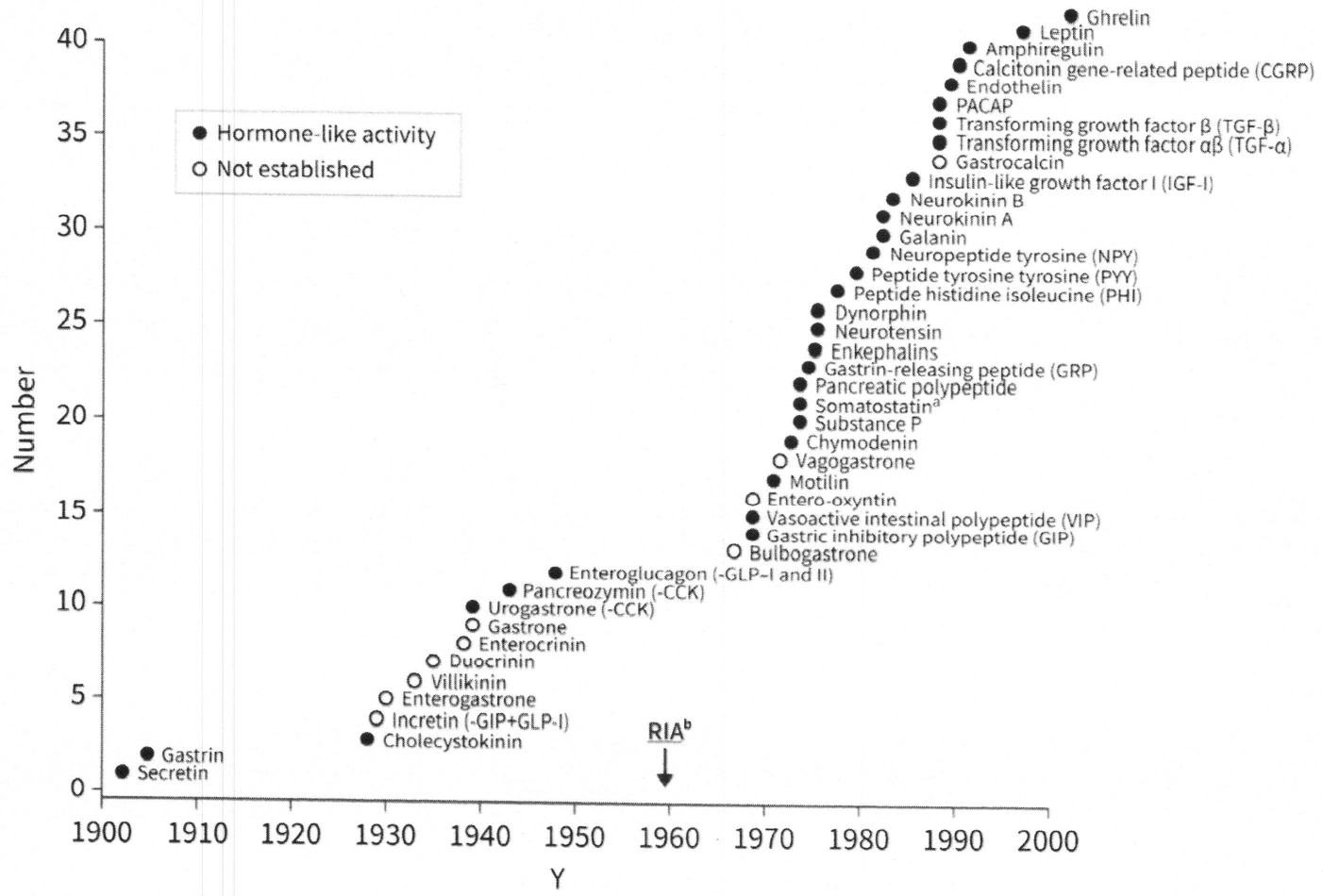
Peptides identified in the mammalian gastroenteropancreatic system. a Somatostatin has all regulatory actions: endocrine, paracrine, neuroendocrine, autocrine. b RIA (radioim-munoassay) first described in 1959.28,29
With the discovery of new peptides/hormones during this period between 1960 and 2000, it would be only a matter of time before the development and utility of some of these peptides became clinically relevant (the peptide therapy era). The clinical era, albeit, at a much slower pace, progressed in parallel with the discovery of GI hormones beginning in 1902. The pathologist S. Oberndorfer is credited with the description of small bowel tumors, which he termed “karzinoide,” meaning carcinoma-like. His publication in 190738 and description was the first to distinguish the slower growing tumors from true carcinomas. It was not until 1928 that he revised the initial description of benign tumors to more accurately reflect their malignant and metastatic potential.39 In his section on carcinoid tumors, Oberg6 cites a case report by Ransom40 from 1890 of a woman with probable malignant carcinoid tumors who flushed after eating. Oberg6 suggested this could be the first description of carcinoid syndrome. Lembeck,41 in 1953, confirmed that serotonin was present in an ileal carcinoid tumor, specifically within the enterochromaffin cells. The problem of being able to determine carcinoid tumors as functional serotonin-secreting tumors in the past has been caused by the difficulty in measuring the plasma serotonin, which has a short half-life of 35 seconds. This is been overcome with the addition of ascorbic acid to a whole-blood sample.
It has now been shown that ileal carcinoid tumors are the second-most frequent leading tumor of the GI tract. Moreover, they have a higher incidence than esophageal and gastric carcinomas combined.42 An insulin-secreting pancreatic NET was first described by Wilder and colleagues43 in 1927. It was a case that used a bioassay whereby they could demonstrate that the liver metastasis contained bioactive insulin. Whipple and Frantz44 published a large series of islet cell tumors associated with hypoglycemia. They also described what came to be known as Whipple triad: documented low blood sugar associated with symptoms of hypoglycemia, improved with glucose replacement.
In 1955, Zollinger and Ellison45 described two patients with abdominal pain, diarrhea (because of excess gastric acid secretion), and atypical bleeding ulcerations of the small bowel associated with NETs of the pancreas. Shortly after the initial description, the syndrome was named ulcerogenic syndrome and/or Zollinger-Ellison syndrome. One of the first two patients initially reported was a 17-year-old woman at the time of diagnosis. A few years following her subtotal gastrectomy, it was noted she was a member of a multiple endocrine neoplasia (MEN) syndrome type 1 (MEN-1) kindred, and one of the authors (T.M.O.) helped care for this patient for almost 20 years. Her weight had remained quite stable even in the presence of liver metastasis and subtotal gastrectomy. It was determined by RIA that her tumor secreted gastrin and somatostatin, and that the somatostatin acted, in part, as an antagonist to gastrin secretion on her liver tumors via a peptide-peptide interaction. She was never begun on octreotide, but her liver lesions remained stable until the time of her death at age 65. A similar case of a gastrinoma secreting somatostatin was discussed by O’Dorisio and colleagues in 1987.46
Gregory and coworkers47 identified gastrin from patients with Zollinger-Ellison syndrome, including one patient from the original Zollinger-Ellison publication. Using classic bioassay, they injected gastrinoma tumor extract intravenously into two dogs and showed profound gastric acid output. Their work validated Edkins8 physiologic observation in 1905 using canine stomach mucosa extracts.
In 1958, internist J.V. Verner and pathologist A.B. Morrison described two patients with fulminant secretory diarrhea, hypokalemia, achlorhydria (most often hypochlorhydria), and pancreatic NETs. The patient also had metabolic acidosis caused by bicarbonate loss in the diarrhea.48 Although initially termed “watery diarrhea, hypokalemia, achlorhydria,” it came to be called watery diarrhea syndrome. Because the neuromo-dulator VIP shares amino acid homology with secretin and GIP, both neuropeptides were thought to be the modulating peptide excess in the watery diarrhea syndrome. It was not until the purification of VIP by S. Said and V. Mutt and the development of the VIP RIA that the actual pathohumoral state of VIP and watery diarrhea syndrome was established.49,50
Although glucagonoma syndrome was first described in 1942, McGavran and co-workers51 reported a case of a patient with glucagonoma syndrome with elevated plasma glucagon levels, diabetes mellitus (type II), severe dermopathy, and a pancreatic NET in 1966. The immunoreactive-like glucagon RIA was performed by R.H. Unger and proved to be one of the best glucagon polyclonal antibodies (Unger 30K) for glucagon RIA in the United States. The association of the often-lethal thromboem-bolism in the glucagonoma syndrome was reported by Mallinson and coworkers in 1974.52
Somatostatinoma began to be described in the late 1970s. They are rare and are more often peripancreatic than pancreatic in location. These are considered asymptomatic but are associated with gallbladder disease (because of antagonism of CCK), diabetes mellitus (type II), and subclinical fat malabsorption (because of antagonism of pancreatic enzyme release).53
Although only 20% of pancreatic NETs are functional, these tumors of the GEP system predominantly secrete their excess peptides/amines in an endocrine manner. They may also secrete peptides or amines alien to their cell of origin in a paraendocrine (paracrine) fashion.46,54 Because of the ability to measure plasma neuropeptides by specific and sensitive RIA, and recognizing their physiologic and pathophysiologic actions, we can ascribe peptide/amine function to GEP NETs based on their syndrome/symptoms. Although it is currently accepted that GEP NETs derive from neuroendocrine cells/tissue, the amine precursor uptake decarboxylase hypothesis of Pearse55,56 remains appealing. His concept considered that the diffuse endocrine system is of neural crest origin. It was supported by the finding that neuron-specific enolase is present in all parts of the neuroendocrine cell system.57 Unfortunately, we have come to appreciate the inadequate specificity of neuron-specific enolase antibodies, which may have impacted on Pearse’s conclusion that the origin of neuroendocrine cells are derived from neural crest tissue. Later worked by Andrews and colleagues58 was an endodermal origin of neuroendocrine cells, and not necessarily neural crest origin. The amine precursor uptake decarboxylase concept, however, remains a convenient and practical framework to describe and study NETs.
Most enteropancreatic NETs are considered sporadic. However, inherited syndromes associated with pancreatic and thyroid NETs exist, and their genetics has been determined. These include MEN1 and MEN2 syndromes, von Hippel-Lindau disease, neurofibromatosis-1, and tuberous sclerosis. Although the inherited NET-associated syndromes are rare, we have clinically observed MEN1 and MEN2 more often in our NET clinics than von Hippel-Lindau disease or tuberous sclerosis. This may be caused, in part, by the functional nature of the pancreatic NETs in MEN1, and the diagnosis and management of pheochromocytoma in MEN2 syndromes. Norton and coworkers59 published a review of the genetics and clinical management of MEN1 and MEN2 in 2015. Table 2 represents the major NETs of the GEP system and their MEN associations.
Table 2.
Major neuroendocrine tumors of the GEP system and their associations with multiple endocrine neoplasias
| Gastrin | Zollinger-Ellison diarrhea, atypical Gl ulcers | Gastrinoma | 1 |
| Insulinoma | Hypoglycemia, symptomatic | Insulinoma | 1 |
| VIP | Watery diarrhea syndrome | VIPoma | 1 (rare) |
| Glucagon | Dermatosis, diabetes, thromboembolism | Glucagonoma | 1 (rare) |
| Somatostatin | Diabetes, fat malabsorption, cholelithiasis | Somatostatinoma | 1 (rare) 2 (very rare) |
| Pancreatic polypeptide | Asymptomatic | PP-oma | 1 |
| Calcitonin | Asymptomatic/diarrhea, flushing | Medullary thyroid carcinoma | 2 |
| Norepinephrine/epinephrine | Hypertension, tachycardia, pallor | Pheochromocytoma | 2 |
| Serotonin, substance P, neurotensin | Flushing, diarrhea, cool perspiration | Carcinoid | 1 (lung carcinoid) 2 (ileal carcinoid) |
MEN1, autosomal-dominant; gene resides on chromosome 11q13; 610 aa nuclear protein “MENIN” Tumor Suppressor. MEN2, autosomal-dominant, gene on chromosome 10q; a receptor tyrosine kinase protooncogene6,59.
Modified from O’Dorisio TM, Vinik Al. Pancreatic polypeptide in missed hormone-producing tumors of the gastrointestinal tract. In Cohen S, Soloway RD (Eds) Contemporary issues in gastroenterology. Edinburgh: Churchill-Livingston 1984;5:117–128; with permission.
Clinically affected MEN1 patients most often present initially with hypercalcemia and elevated parathyroid hormone or a history of parathyroid surgery and evidence of four gland parathyroid hyperplasia. The most common pituitary tumor, when present, is a prolactinoma, but can also be a growth hormone- or corticotropin-secreting tumor. The pancreatic NET, when it occurs, is most commonly an insulinoma or gastrinoma, but rare glucagonomas or VIPoma can also be seen in other members of MEN1 kindreds. The pancreatic NETs are most often multiple adenomas, one or two of which may grow while the other adenomas remain stable. Correcting the hypercalcemia when present before working up a functional PNET is recommended. Whereas carcinoid tumor can co-occur in MEN1 and MEN2 syndromes, the pulmonary carcinoid tumor seems more prevalent in MEN1 patients, in our experience.
Regarding MEN2 syndrome, the most frequent component of MEN2 almost always involves medullary thyroid carcinoma (MTC) from the neuroendocrine C-cells (parafollicular cells) of the thyroid gland. MTC is always present in MEN2 and constitutes the primary diagnosis. Parathyroid hyperplasia can also be present in MEN2A but is not as clinically frequent as seen in MEN1 patients. The third gland involved in MEN2 is the adrenal medulla, which gives rise to pheochromocytomas. Patients may present first with symptoms of pheochromocytoma and thereafter an MTC is discovered. Both MTC and pheochromocytoma are often bilateral. MEN2B is much less common (5%) than MEN2A, which accounts for 95% of MEN2 patients. MEN2B is clinically more aggressive, also consists of pheochromocytomas, but does not have parathyroid hyperplasia; it is also associated with a marfanoid body habitus and ganglioneuromas of the GI tract.59 Carcinoembryonic antigen with serial measurements of calcitonin is helpful in detecting early aggressivity and metastasis of MEN2-associated MTC.
The final portion of this review addresses selected aspects of the various peptide therapies that currently exist in the United States. The following schema represents a partial approach to the various therapeutic modalities (Fig. 2).
Fig. 2.
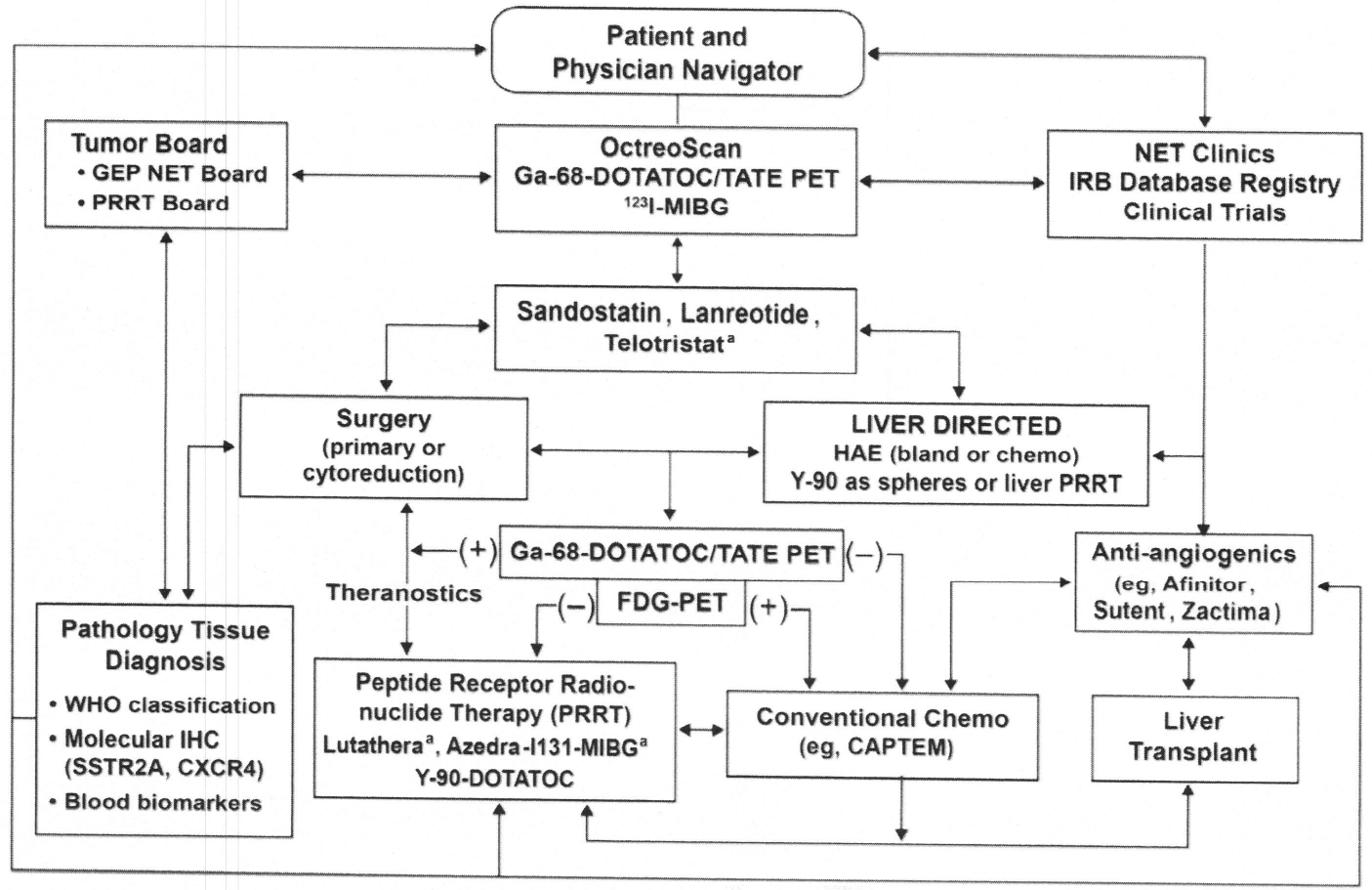
Carcinoid and neuroendocrine tumors: cancer management and treatment options of care. a Most recently FDA approved. FDG, fluorodeoxyglucose; WHO, World Health Organization. (Courtesy of Iowa NET Team, Iowa City, IA.)
As noted, the arrows are two-way, and designed to represent that electing one therapy does not eliminate its option at a different phase of treatment. It is important to demonstrate that any of the therapeutic options can be repeated. Most of the listed options are discussed by other authors in this issue. This review briefly address surgery/debulking and somatostatin and ianreotide therapy, and discusses the most recent FDA-approved peptide receptor radionuclide therapy (PRRT).
SURGERY/CYTOREDUCTION
It is historically and presently accepted that the first best therapy in the management of NET is surgery, whenever possible. Less well-established is the overall survival (OS) improvement when the primary tumor is removed versus being unable to remove the primary tumor. This is caused, in part, by the reality that all surgical studies of this nature are retrospective and the assignment of patients to no surgery is inherently unethical. That said, there are publications that strongly suggest benefits in progression-free survival (PFS) and OS when the primary tumor and hepatic metastasis debulking is possible.60–63 At the University of Iowa, our endocrine surgeon (editor of the present monograph) is aggressive in removing primary PNETS and SBNETs, with liver tumor debulking (and liver sparing) when safe surgery and low patient risk are possible63
OCTREOTIDE/LANREOTIDE
Octreotide and Ianreotide remain as first-line management and therapy for functional and nonfunctional NETs. They are remarkable for their durability over decades and merit discussion. Octreotide (Sandostatin) and, somewhat later, Ianreotide (Somatu-line) were both developed as analogues (more properly, congeners) of the active ringed portion of native somatostatin-14.64–66 Their other natural form is somatostatin-28, representing an extension of somatostatin-14 away from its active eight amino acid ring.67 As noted from Fig. 1, somatostatin has been shown to have all four regulatory functions: (1) endocrine, (2) paracrine, (3) neurocrine, and (4) autocrine.67 Shortly after its initial discovery and publication in 1973 by Brazeau and colleagues,35 Sandoz (now Novartis) focused their medicinal chemists to synthesize a longer acting fragment of the eight amino acid ring of the native somatostatin to be used clinically. Almost 9 years later, octreotide (SMS 201–995, Sandostatin) was synthesized by Bauer and colleagues.64 Shown next is the amino acid structure of Sandostatin-native somatostatin-14 and the eight amino acid synthetic structures of octreotide and lanreotide both containing the D-isomers of the naturally occurring amino acids and the bioactive ringed portion. D-isomers prevent enzyme degradation, as compared with the naturally occurring L-form amino acids in somatostatin-14. Also documented in Fig. 3 showing the 1 to 14 somatostatin and octreotide and lanreotide is the purported amino acid, lysine, within the ringed portion considered to be the primary peptide binding site of somatostatin, octreotide, and lanreotide to the somatostatin receptor subtype 2 (sstr2A), the most prevalent receptor of the five somatostatin receptor subtypes on neuroendocrine cells and their NETs (see Fig. 3).67
Fig. 3.
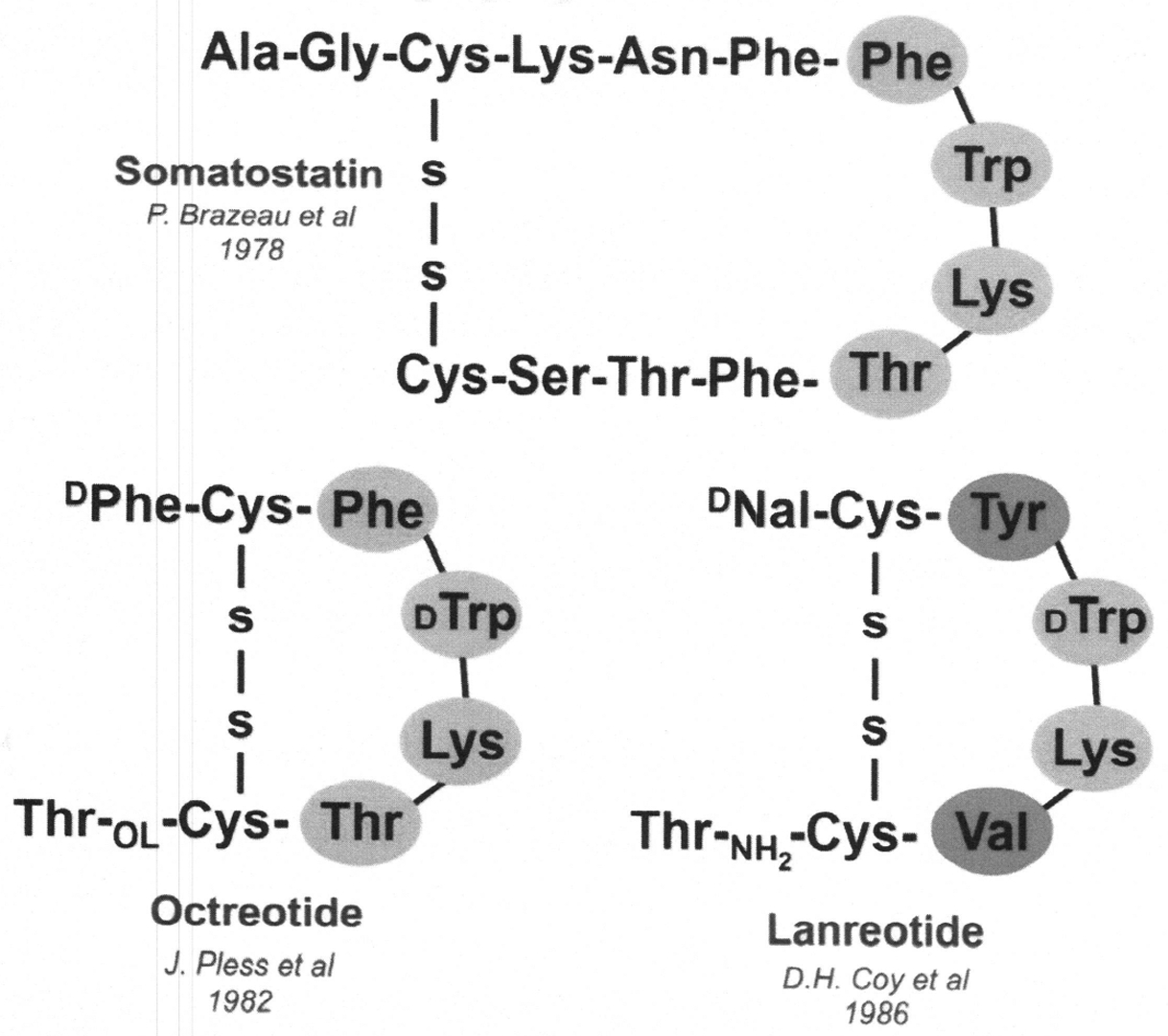
Somatostatin and its congeners.
It is important to appreciate that for 10 years following the discovery of native somatostatin, there was a large number of publications reporting somatostatin’s mechanisms of action and regulatory role in mammalian physiology68–70 and its regulatory role in NETs. Based on the information available on native somatostatin, and the wide-spread clinical use of octreotide, somewhat later the use of lanreotide was a reasonable and rational extension. Both analogues had a much longer plasma half-life than the naturally occurring somatostatin, and a much more potent and durable action on NETs.71–74 During the late 1980s, while octreotide and lanreotide were being used on a compassionate use basis to treat NETs and growth-hormone-secreting pituitary tumors causing acromegaly, there were efforts to characterize the various receptors on NETs. Reubi and coworkers75 mapped somatostatin receptor subtypes using autoradiographic techniques that included the use of 125lodine isotope labeled to the tyrosine substituted for phenylalanine in octreotide at position 3. Patel and Srikant76 demonstrated peptide analogue subtype selectivity using five clonal human somatostatin subtype receptors (hsstr1–5). During the same year, Bruns and colleagues77 discussed the molecular pharmacology of somatostatin-receptor subtypes. This was made possible for sstr2A by highly specific IHC with a commercially available polyclonal antibody, UMB-1 (Biotrend, Epitomics, Inc). Characterization of the UMB-1 antibody was published in 2012 by Korner and coworkers.78 As noted in our therapeutic schema, all patients coming to our NET clinics for the first time will have their tumor tissue stained (when available) for sstr2A. Qian and colleagues79 published a study examining the presence of somatostatin subtype receptors 1 to 5 and OS and PFS in patients with metastatic NETs. They concluded that patients with NETs that express sstr2A (but not sstrl, sstr3, sstr4, or sstr5) have a longer OS. They determined that tumors with sstr2A IHC that are treated with somatostatin analogues have a longer PFS.79
PEPTIDE RECEPTOR RADIONUCLIDE THERAPY AND THERANOSTICS
The remarkably persistent standard of care use of somatostatin analogues, especially octreotide, would almost naturally lead to PRRT of GEP NETs. We discuss the history of theranostics, from the FDA-approved diagnostic 68Ga-DOTATATE PET scan (NET-SPOT) and its therapeutic partner, 177Lu-DOTATATE PRRT, and allude to novel tumor targets that may lead to FDA approval in the near future.
Fig. 4 shows a timeline establishing the similar role that octreotide has had since its synthesis and clinical use in 1982 on PRRT.80 It depicts close interaction between discovery by academic centers and industry leading to clinical use of modified octreotide as a recently FDA-approved effective and rational therapy for NETS.81
Fig. 4.
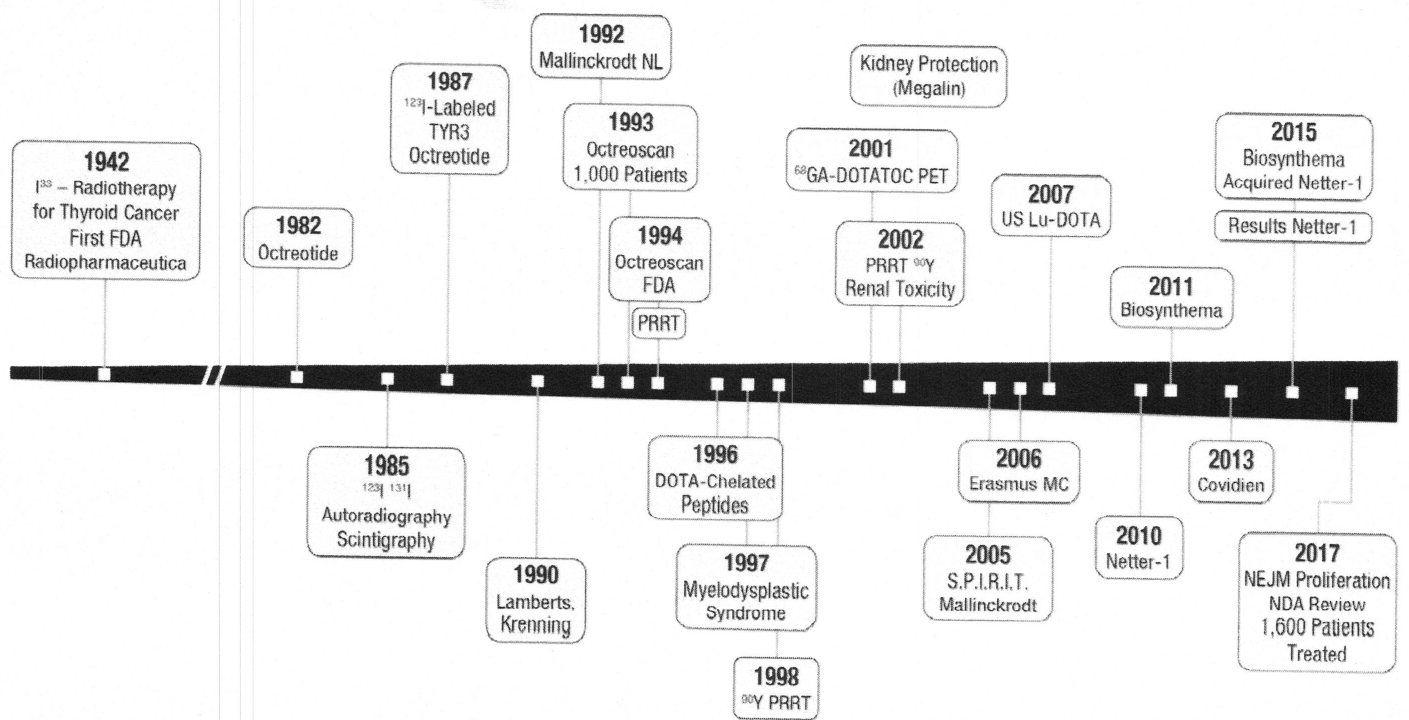
A timeline of octreotide culminating in development of PRRT and theranostics.
An excellent review of PRRT development was recently published by Levine and Krenning.82 Noted in Fig. 4, targeted radiotherapy began with the work of S. Hertz using 131Iodine treatment of severe hyperthyroidism.83 Although his work had been done well before the 1946 JAMA publication,83 World War II interrupted Dr Hertz’ investigative efforts. During the 1940s, Dr Hertz recognized that thyroid targeting with 131Iodine made it possible to successfully treat patients with thyroid cancer. The Society of Nuclear Medicine and Molecular Imaging established a yearly Sol Hertz Award in 2016, recognizing “individuals who have made outstanding contributions to radionuclide therapy.”
The first use of the term “peptide receptor” concept probably derives from the work by the Erasmus University group, headed by Krenning84 and Maecke and colleagues.85 There are early publications by Krenning and colleagues84 that not only address the peptide receptor acronym, but also demonstrate proof of concept using 111In-octreotide as an imaging and therapeutic agent given to a single patient. Mueller-Brand with Maecke and their colleagues reported on the “powerful new tool,” DOTA-TOC (a chelator attached to [tyr3]-octreotide), for sstr2A in metastatic NET subjects. This was the first use of β emitting energy, 90Yttrium, coupled with the DOTA chelator.86
Krenning and his group at Erasmus pursued diagnostic imaging with 123Iodine-labeled Tyr3-octreotide achieving successful scintigraphic imaging.87 By 1990, they had successfully developed 111Indium-labeled DTPA (chelator) attached to pentetreotide (OctreoScan) and reported their 1000-patient experience in 1993.88 The Octreoscan was ultimately used for diagnostic entry criteria in the registered NETTER-1 trial for PRRT4 coupled with therapy using 177Lu-DOTATATE. Since 2001, the improved diagnostic scan for detection of somatostatin receptor positivity was developed using the 68Ga-DOTA-somatostatin analogue, thereby increasing the sensitivity and specificity by using PET instead of single-photon emission computed tomography (SPECT). In 2007, Baum and colleagues published their work on the highly sensitive and specific 68Ga-DOTA-(tyr3)-octreotide (TOC) and DOTA-(tyr3-octreotide, substituted threonine for threoninolol-octreotide [TATE]) PET imaging.89 It was, however, the use of the diagnostic OctreoScan with 177Lu-DOTATATE that led to the first FDA-approved, European Medicines Agency-approved radiopharmaceutical for PRR.81
Noted in Fig. 5, the term “theranostics” was coined by Rosch and Baum in 2011.90 The theranostics concept is the use of the identical linker molecule (peptide in this case) with the chelator “cage” for 68Ga for diagnosis, and the 177Lu/90Y for therapy. A good description of PRRT and of theranostics is depicted in Fig. 5.85
Fig. 5.
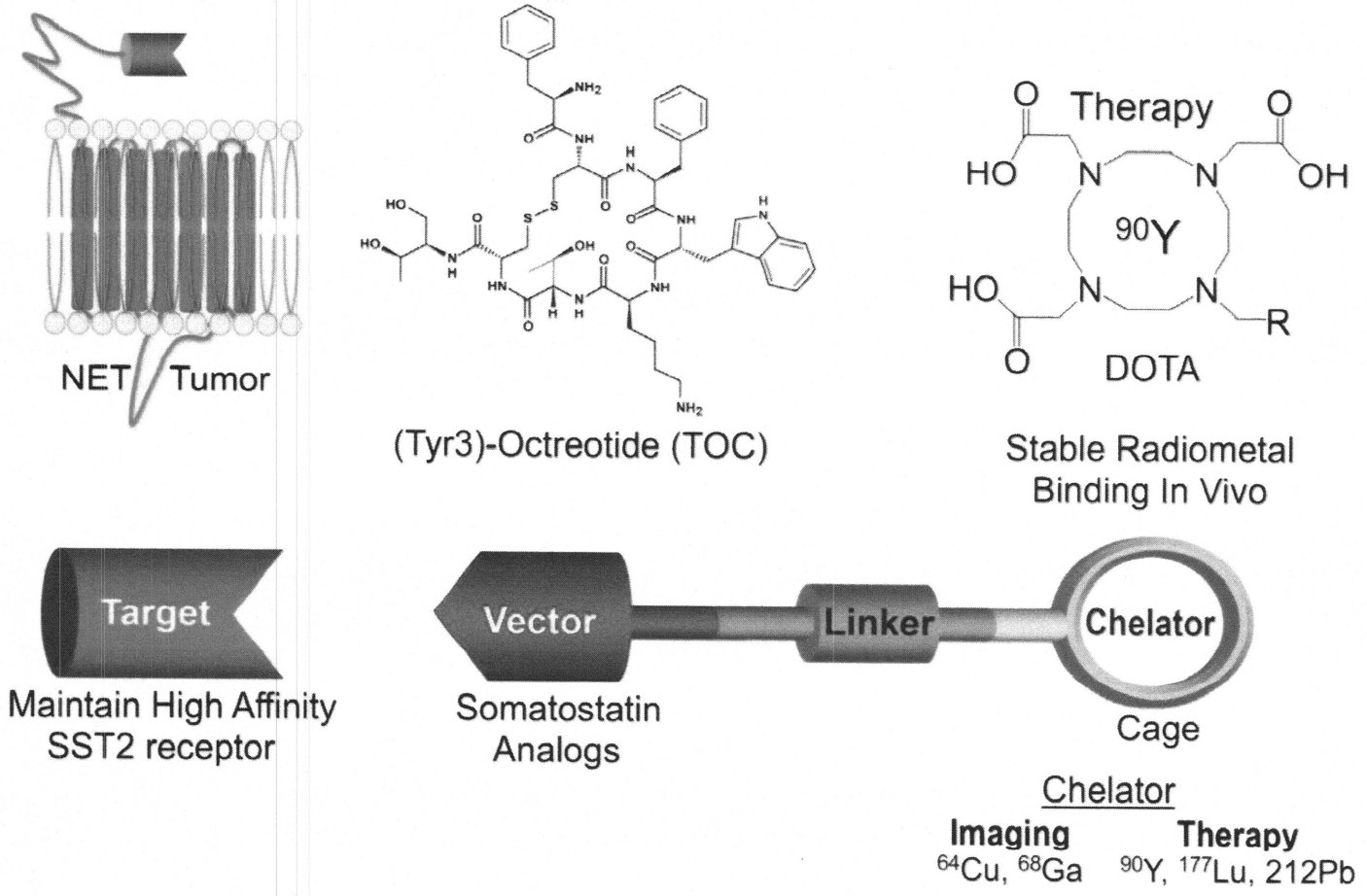
Targeted molecular imaging and therapy (PRRT) and theranostics concept. Theranostics is the use of diagnostic radionuclides (68Cu, 68Ga) and therapeutic radionuclides (90Y, 177Lu, 212Pb) labeled to the same somatostatin congener (TOC or TATE).90 (Courtesy of H. Maecke, University Hospital, Basel, CH.)
On the top right-hand panel is the DOTA-chelator with its “cage” containing the therapeutic radiometal, 90Y. The top middle panel is the DOTA chelator attached to (tyr3)-octreotide (TOC) and considered to be the vector binding to the tumor’s sstr2A. The top left panel is the tumor membrane with the sstr2A dangling externally above the membrane. For a single receptor (sstr2A) binding to a single ligand (tyr3)-octreotide, four properties are necessary85: (1) high sstr2A expression on the NET, (2) the native peptide (somatostatin) sequence being known, (3) high affinity/specificity/avidity for the target (sstr2A), and (4) the analogues (vector, ligand) are synthetically feasible (50 amino acid residues or less).
The next major breakthrough in theranostics is likely to be the introduction of new radioisotopes and new targeting agents in addition to DOTATOC and DOTATATE. 177Lutetium and 90Yttrium are primarily β emitters with path lengths greater than the average diameter of a single cell, thus generating “cross-fire” effects of cytotoxicity to adjacent cells and normal tissues, a Particle energy emitters, such as 225Ac (astatine) and 212Pb (lead) coupled to a high-affinity somatostatin analogue, are likely to deliver a higher energy dose to a single cell with little or no cross-fire when targeted to tumors expressing high levels of sstr2A. Recent in vitro experiments in cell lines expressing sstr2 have shown a six-fold increase in radiation dose/cell from 213Bi-DOTATOC compared with 177Lu-DOTATATE.91 Importantly, these results substantiate emerging evidence that α emitters may be more effective for PRRT because of preferred dose deposition within the cell nucleus (Table 3).92
Table 3.
Radionuclides currently in use for theranostics
| 225Ac (α) | <0.1 | 5.59 |
| 212Pb (α) | <0.1 | 6.0–8.8a |
| 177Lu (β) | 1.7 | 0.134 |
| 90Yt (β) | 0.05–12 | 0.933 |
212Pb decays to 212Bi (36%) and 212Po (64%) with respective α energies.
The contribution of internalization to targeting efficiency was then investigated within the geometry of cells by modifying the uptake of α or β emitting radionucleotides from complete membrane bound (0% internalized) to fully internalized (100%). The up-take values arising with 100% internalization were approximately 1.5-fold higher for the entire cell, and 2- to 3-fold higher for the nucleus when compared with binding to the cell membrane, α Emitters deposit significantly higher doses for the tumor metastasis of various sizes relative to β emitters representing 60 to 140 times higher doses for 212Pb and 213Bi, and 190 to 620 times higher doses for 225Ac when compared with the β emitter, 177Lu (Azure). Importantly, these results substantiate emerging evidence that a emitters may be more effective for PRRT because of preferred dose deposition within the cell nucleus.
Most NETs are low grade 1 and 2 with Ki-67 less than 20%. Grade 3 NETS and neuroendocrine carcinomas (NECs) have less response to either 177Lu- or 90Y-DOTA-TOC or DOTATATE. These high-grade tumors have a Ki-67 of 55% to 90% and often lack the sstr2A expression, leading investigators to try to identify theranostics targets for grade 3 NETS and NECs. CXCR4 is a chemokine receptor that like sstr2A is a G-protein-coupled receptor expressed on the cell surface and internalized on binding of a high-affinity ligand.93 We and others have identified CXCR4 expression on NEC, multiple myeloma, and leukemia.94–98 The ligand for CXCR4 is a small peptide SDF-1, also known as CXCLI2. Pentixafor is a CXCR4 antagonist that can be complexed to the DOTA chelator. GA68-Pentixifor and 177Lu-Pentixifor are, thus, a theranostic pair. 177Lu can be used for SPECT imaging and diagnosis: however, 68Ga-Pentixifor is improved as a PET imaging agent with a sensitivity of 3 to 5 mm as compared with 10 to 15 m sensitivity for SPECT. 177Lu-Pentixather has shown promise as a radiother-apeutic drug in early studies for patients with multiple myeloma and leukemia,94 but CXCR4 is expressed on hematopoietic progenitors and stromal cells, leading to concern for bone marrow toxicity. A recent preclinical study has demonstrated that bone marrow from mice treated with 177Lu-Pentixather is able to serve as a graft in lethally irradiated animals, providing support for the use of 177Lu-Pentixather in aggressive malignancies with dosimetry of bone marrow dose to prevent toxicity.99 Patients with multiple myeloma or leukemia treated with 177Lu-Pentixather routinely receive autologous hematopoietic stem cell rescue.
177LU-PSMA-617 has primarily been tested in Germany under compassionate use circumstances and relapsed metastatic castrate-resistant prostate cancer and has only recently been introduced into US clinical trials. A retrospective analysis of 145 patients treated in 12 European centers demonstrated greater than 50% decrease in prostate-specific antigen levels in most patients.100 Hematologic toxicity was low even in these heavily pretreated patients; dry mouth related to high uptake of the 177Lu-PMSA-617 in salivary glands was the primary toxicity along with fatigue and nausea.101 These preliminary results support the conduct of controlled phase 2 trials that are currently ongoing in Europe and the United States.
Although most theranostic compounds are peptide agonists targeting G-protein-coupled receptors, several antagonist molecules are in preclinical and early clinical use. One example is a peptide ligand, 68Ga-DOTA-bombesin (neoBOMB), targeting the gastrin-releasing peptide receptor known to be expressed in prostate cancer cells.102 This gastrin-releasing peptide receptor antagonist has shown promising results in animal models and its first PET imaging in humans has been recently reported. Similarly, an antagonist at the somatostatin receptor has been introduced as a theranostic pair in NETs. 68Ga/177Lu-DOTA-JR-11 seems to bind to many more sites/cells than either DOTATOC or DOTATATE in low-grade NETs. It is in early phase trials with little information on safety or efficacy available at this time.
KEY POINTS.
Although most theranostic compounds are peptide agonists targeting G-protein-coupled receptors, several antagonist molecules are in preclinical and early clinical use.
One example is a peptide ligand, 68Ga-DOTA-bombesin (neoBOMB) targeting the gastrin releasing peptide (GRP) receptor known to be expressed in prostate cancer cells.
This GRPR antagonist has shown promising results in animal models and its first PET imaging in humans has been recently reported.
Similarly, an antagonist at the somatostatin receptor has been introduced as a theranostic pair in neuroendocrine tumors.
ACKNOWLEDGMENTS
A special acknowledgment for Ms Sue Kieffer for her careful editorial input and bibliography research and accuracy.
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Neuroendocrine disorders of the gastroenteropancreatic system. Clinical applications of the somatostatin analogue SMS 201–995 (Octreotide, Sandostatin) April 2–4, 1986, San Diego, California: Am J Med 1986;81 (6B): 1–101. O’Dorisio TM, eds. [PubMed] [Google Scholar]
- 2.Approved drug products with therapeutic equivalence evaluations, 39th edition US Department of Health and Human Services Food and Drug Administration; 2019:vol. 3;326. [Google Scholar]
- 3.Bushnell DL Jr, O’Dorisio TM, O’Dorisio MS, et al. 90Y-edotreotide for metastatic carcinoid refractory to octreotide. J Clin Oncol 2010;28(10): 1652–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strosberg J EI-Haddad G, Wolin E, et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med 2017;376(2); 125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Dorisio TM. Gut endocrinology: clinical and therapeutic impact. Am J Med 1986;81(6B):1–7. [DOI] [PubMed] [Google Scholar]
- 6.Öberg K The genesis of the neuroendocrine tumors concept: from Oberndorfer to 2018. Endocrinol Metab Clin North Am 2018;47(3):711–31. [DOI] [PubMed] [Google Scholar]
- 7.Bayliss WM, Starling EH. Preliminary communication on the causality of the so called “preferential reflex secretion” of the pancreas. Lancet 1902;1:813–4. [Google Scholar]
- 8.Edkins JS. On the chemical mechanism of gastric secretion. Lancet 1905; 11: 156–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregory RA, Tracy HJ. The preparation and properties of gastrin. J Physiol 1961;156:523–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivy AC, Eidberg E. A hormone mechanism for gallbladder contraction and evaluation. Am J Physiol 1928;86:599–613. [Google Scholar]
- 11.Harper AA, Raper HS. Pancreozymin, a stimulant of the secretion of pancreatic enzymes in extracts of the small intestine. J Physiol 1943; 102(1): 115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banting FG, Best CH. The internal secretion of the pancreas. J Lab Clin Med 1922;251–66. [PubMed] [Google Scholar]
- 13.Forsham PH. Milestones in the 60-year history of insulin (1922–1982). Diabetes Care 1982;5(Suppl 2): 1–3. [DOI] [PubMed] [Google Scholar]
- 14.Langerhans P, Moorison H. Contributions to the microscopic anatomy of the pancreas. Baltimore (MD): John Hopkins Press; 1937. [Google Scholar]
- 15.Heidenhain RP. Untersuchungen über den Bau der Labdrusen. Arch Mikr Anat 1870;6:368. [Google Scholar]
- 16.Nikolas A Recherches sur I’epithelium de I-intestin grele. Intern Monatssch Anat Physiol 1891; 1. [Google Scholar]
- 17.Kulchitsky N Zur Frage über den Bau des Darmkanals. Arch Mikr Anat 1897; 49:7–35. [Google Scholar]
- 18.Ciaccio M Sur une nouvelle espece cellulaire dans les glandes de Lieberkuhn. C R Seances Soc Biol Fil 1906;60:76–7. [Google Scholar]
- 19.Gosset A, Masson P. Tumeurs endocrines de I’appendice. Presse Med 1914;25: 237–9. [Google Scholar]
- 20.Feyter F Ueber diffuse endokrine epithealiale organe. Zentralbl Innere Med 1938;545:31–41. [Google Scholar]
- 21.Feyter F Ueber die peripherin endkrinem (Parakrinim) drusen des menschen. Vienna (Austria): W, Maudrich Verlag; 1953. [Google Scholar]
- 22.Ml Grossman. General concepts In: Bloom SR, Polak JM, editors. Gut hormones. Edinburgh (Scotland): Churchill-Livingstone; 1981. p. 17–22. [Google Scholar]
- 23.Moore B On the treatment of diabetus mellitus by acid extract of duodenal mucous membrane. Biochem J 1906;1(1):28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rehfeld JF. The origin and understanding of the incretin concept. Front Endocrinol (Lausanne) 2018;9:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porath J, Flodin P. A new method for the detection of amino-acids, peptides, proteins and other buffering substances on paper. Nature 1951:168(4266): 202–3. [DOI] [PubMed] [Google Scholar]
- 26.Jorpes JE, Mutt V. The preparation of secretin. Biochem J 1952;52(2):328–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jorpes JE, et al. Further purification of cholecystokinin and pancreozymin. Acta Chem Scand 1964;18:2408–12. [Google Scholar]
- 28.Yalow RS, Berson SA. Assay of plasma insulin in human subjects by immunological methods. Nature 1959; 184(Suppl 21): 1648–9. [DOI] [PubMed] [Google Scholar]
- 29.Yalow RS, Berson SA. Immunoassay of endogenous plasma insulin in man. J Clin Invest 1960;39:1157–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGuigan JE. Antibodies to the carboxyl-terminal tetrapeptide of gastrin. Gastroenterology 1967;53(5):697–705. [PubMed] [Google Scholar]
- 31.Bloom SR, Bryant MG, Polak JM. Proceedings: distribution of gut hormones. Gut 1975;16:821. [PMC free article] [PubMed] [Google Scholar]
- 32.Solica E, Polak JM, Larsson L-l, et al. Update on Lohsane classification of endocrine cells In: Bloom SR, Polak JM, editors. Gut hormone. 2nd edition Edinburgh (Scotland): Churchill Livingstone; 1981. p. 96–100. [Google Scholar]
- 33.Bloom SR, Polak JM. Glucagonomas, VIPomas and somatostatinomas. Clin Endocrinol Metab 1980;9(2):285–97. [DOI] [PubMed] [Google Scholar]
- 34.Al Vinik, O’Dorisio TM, Woltering E, et al. Neuroendocrine tumors: a comprehensive guide to diagnosis and management. 5th edition InterScience Institute; 2012. [Google Scholar]
- 35.Brazeau P, Vale W, Burgus R, et al. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 1973; 179(4068):77–9. [DOI] [PubMed] [Google Scholar]
- 36.Arimura A, Coy DH, Chihara M, et al. Somatostatin In: Bloom SR, Polak JM, editors. Gut hormone. Edinburgh (Scotland): Churchill Livingstone; 1976. p. 437–41. [Google Scholar]
- 37.Rehfeld JF. The changing concept of gut endocrinology In: Wabitsch M, Posovszy C, editors. Developmental biology of gastrointestinal hormones, vol. 32 Basel (Switzerland): Karger; 2017. p. 8–19. Endocr Dev. [DOI] [PubMed] [Google Scholar]
- 38.Oberndorfer S Karzenoide tumoren des dünndarms. Frankf Z Pathol 1907; 1 : 426–32. [Google Scholar]
- 39.Oberndorfer S Karzinoide handuch der speziellen, handbuch der speziellen pathologischen anatomie und histologie. Berlin: Springer; 1928. p. 814–47. [Google Scholar]
- 40.Ransom W A case of primary carcinoma of the ileum. Lancet 1890;2:1020–3. [Google Scholar]
- 41.Lembeck F 5-Hydroxytryptamine in carcinoid tumor. Nature 1953;172:910–1. [Google Scholar]
- 42.Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3(10): 1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilder RM, Allan FN, Power WH, et al. Carcinoma of the islands of the pancreas: hyperinsulinism and hypoglycemia. JAMA 1927;89:348–55. [Google Scholar]
- 44.Whipple AO, Frantz VK. Adenoma of islet cells with hyperinsulinism: a review. Ann Surg 1935; 101 (6): 1299–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zollinger RM, Ellison EH. Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas. Ann Surg 1955;142(4):709–23. [PMC free article] [PubMed] [Google Scholar]
- 46.O’Dorisio TM, Mekhjian HS, Ellison EC, et al. Role of peptide radioimmunoassay in understanding peptide-peptide interactions and clinical expression of gastro-enteropancreatic endocrine tumors. Am J Med 1987;82(Suppl 5B):60–7. [DOI] [PubMed] [Google Scholar]
- 47.Gregory RA, Grossman Ml, Tracy HJ, et al. Nature of the gastric secretagogue in Zollinger-Ellison tumours. Lancet 1967;2(7515):543–4. [DOI] [PubMed] [Google Scholar]
- 48.Verner JV, Morrison AB. Islet cell tumor and a syndrome of refractory watery diarrhea and hypokalemia. Am J Med 1958;25(3):374–80. [DOI] [PubMed] [Google Scholar]
- 49.Said SI, Faloona GR. Elevated plasma and tissue levels of vasoactive intestinal polypeptide in the watery-diarrhea syndrome due to pancreatic, bronchogenic and other tumors. N Engl J Med 1975;293(4): 155–60. [DOI] [PubMed] [Google Scholar]
- 50.Gaginella TS, O’Dorisio TM. Vasoactive intestinal polypeptide neuromodulation of intestinal secretion In; Binder HJ, editor. Mechanism of intestinal secretion. New York: Alan R Liss; 1979. p. 231–47. [Google Scholar]
- 51.McGavran MH, Unger RH, Recant L, et al. A glucagon-secreting alpha-cell carcinoma of the pancreas. N Engl J Med 1966;274(25): 1408–13. [DOI] [PubMed] [Google Scholar]
- 52.Mallinson CN, Cox B, Bloom SR. Proceedings: plasma levels of amino acids and glucagon in patients with pancreatic glucagonomas. Gut 1974;15(4):340. [PubMed] [Google Scholar]
- 53.Ganda OP, Weir GC, Soeldner JS, et al. “Somatostatinoma”: a somatostatin-containing tumor of the endocrine pancreas. N Engl J Med 1977;296(17):963–7. [DOI] [PubMed] [Google Scholar]
- 54.Vinik I, Strodel WE, O’Dorisio TM. Endocrine tumors of the gastroenteropancreatic (GEP) axis In: Santern RJ, Manni A, editors. Endocrine related tumors. Boston: Martinus Nijhoff; 1984. p. 305–45. [Google Scholar]
- 55.Pearse AG. Common cytochemical properties of cells producing polypeptide hormones, with particular reference to calcitonin and the thyroid C cells. Vet Ree 1966:79(21):587–90. [DOI] [PubMed] [Google Scholar]
- 56.Pearse AG. The APUD concept and hormone production. Clin Endocrinol Metab 1980;9(2):211–22. [DOI] [PubMed] [Google Scholar]
- 57.Schmechel D, Marangos PJ, Brightman M. Neurone-specific enolase is a molecular marker for peripheral and central neuroendocrine cells. Nature 1978: 276(5690):834–6. [DOI] [PubMed] [Google Scholar]
- 58.Andrew A, Kramer B, Rawdon BB. The origin of gut and pancreatic neuroendocrine (APUD) cells: the last word? J Pathol 1998;186:117–8. [DOI] [PubMed] [Google Scholar]
- 59.Norton JA, Krampitz G, Jensen RT. Multiple endocrine neoplasia: genetics and clinical management. Surg Oncol Clin N Am 2015;24(4):795–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Givi B, Pommier SJ, Thompson AK, et al. Operative resection of primary carcinoid neoplasms in patients with liver metastases yields significantly better survival. Surgery 2006;140(6):891–7. [DOI] [PubMed] [Google Scholar]
- 61.Hill JS, McPhee JT, McDade TP, et al. Pancreatic neuroendocrine tumors: the impact of surgical resection on survival. Cancer 2009;115(4):741–51. [DOI] [PubMed] [Google Scholar]
- 62.Woltering EA, Voros BA, Beyer DT, et al. Aggressive surgical approach to the management of neuroendocrine tumors: a report of 1,000 surgical cytoreductions by a single institution. J Am Coll Surg 2017;224(4):434–47. [DOI] [PubMed] [Google Scholar]
- 63.Scott AT, Breheny PJ, Keck KJ, et al. Effective cytoreduction can be achieved in patients with numerous neuroendocrine tumor liver metastases (NETLMs). Surgery 2019;165:166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bauer W, Briner U, Doepfner W, et al. SMS 201–995: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci 1982; 31(11):1133–40. [DOI] [PubMed] [Google Scholar]
- 65.Liebow C, Reilly C, Serrano M, et al. Somatostatin analogues inhibit growth of pancreatic cancer by stimulating tyrosine phosphatase. Proc Natl Acad Sci USA 1989;86(6):2003–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rossowski WJ, Coy DH. Specific inhibition of rat pancreatic insulin or glucagon release by receptor-selective somatostatin analogs. Biochem Biophys Res Commun 1994;205(1):341–6. [DOI] [PubMed] [Google Scholar]
- 67.O’Dorisio TM, Redfern S. Somatostatin and somatostatin-like peptides: clinical research and clinical applications In: Mazzaferro EL, et al. , editors. Advances in endocrinology and metabolism, Vol. 1 St Louis (MO): Mobley; 1980. p. 175–230. [Google Scholar]
- 68.Somatostatin Reichlin S.. N Engl J Med 1983;309(24):1495–501. [DOI] [PubMed] [Google Scholar]
- 69.Somatostatin Reichlin S. (second of two parts). N Engl J Med 1983;309(25): 1556–63. [DOI] [PubMed] [Google Scholar]
- 70.Yamada RT, Chide T. Somatostatin In: Malkhlouf GM, Schults SG, editors. Handbook of physiology: section 6: the gastrointestinal system volume ii: neural and endocrine biology. Bethesda (MD): Oxford University Press; 1989. p. 431–53. [Google Scholar]
- 71.Lamberts SW, van der Lely AJ, de Herder WW, et al. Octreotide. N Engl J Med 1996;334(4):246–54. [DOI] [PubMed] [Google Scholar]
- 72.Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009;27(28):4656–63. [DOI] [PubMed] [Google Scholar]
- 73.Caplin ME, Pavel M, Cwikia JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014;371(3):224–33. [DOI] [PubMed] [Google Scholar]
- 74.Battershill PE, Clissold SP. Octreotide. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in conditions associated with excessive peptide secretion. Drugs 1989;38(5):658–702. [DOI] [PubMed] [Google Scholar]
- 75.Reubi JC, Maurer R, von Werder K, et al. Somatostatin receptors in human endocrine tumors. Cancer Res 1987;47(2):551–8. [PubMed] [Google Scholar]
- 76.Patel YC, Srikant CB. Subtype selectivity of peptide analogs for all five cloned human somatostatin receptors (hsstr 1–5). Endocrinology 1994;135(6):2814–7. [DOI] [PubMed] [Google Scholar]
- 77.Bruns C, Weckbecker G, Raulf F, et al. Molecular pharmacology of somatostatin-receptor subtypes. Ann N Y Acad Sci 1994;733:138–46. [DOI] [PubMed] [Google Scholar]
- 78.Körner M, Waser B, Schonbrunn A, et al. Somatostatin receptor subtype 2A immunohistochemistry using a new monoclonal antibody selects tumors suitable for in vivo somatostatin receptor targeting. Am J Surg Pathol 2012;36(2): 242–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qian ZR, Li T, Ter-Minassian M, et al. Association between somatostatin receptor expression and clinical outcomes in neuroendocrine tumors. Pancreas 2016; 45(10): 1386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harris AG. The clinical development of the somatostatin analogue octreotide. Rotterdam (Netherlands): PhD Thesis, Erasmus University, Department of Internal Medicine; 2003. [Google Scholar]
- 81.Hennrich U, Kopka K. Lutathera®: The First FDA- and EMA-approved radio-pharmaceutical for peptide receptor radionuclide therapy. Pharmaceuticals (Basel) 2019; 12(3) [pii:E114]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Levine R, Krenning EP. Clinical history of the theranostic radionuclide approach to neuroendocrine tumors and other types of cancer: historical review based on an interview of Eric P. Krenning by Rachel Levine. J Nucl Med 2017;58(Suppl 2): 3S–9S. [DOI] [PubMed] [Google Scholar]
- 83.Hertz S, Roberts A. Radioactive iodine in the study of thyroid physiology; the use of radioactive iodine therapy in hyperthyroidism. J Am Med Assoc 1946; 131:81–6. [DOI] [PubMed] [Google Scholar]
- 84.Krenning EP, Kooij PP, Bakker WH, et al. Radiotherapy with a radiolabeled so-matostatin analogue, [111ln-DTPA-D-Phe1]-octreotide. A case history. Ann N Y Acad Sci 1994;733:496–506. [DOI] [PubMed] [Google Scholar]
- 85.Heppeler A, Froidevaux S, Eberle AN, et al. Receptor targeting for tumor localisation and therapy with radiopeptides. Curr Med Chem 2000;7(9):971–94. [DOI] [PubMed] [Google Scholar]
- 86.Otte A, Jermann E, Behe M, et al. DOTATOC: a powerful new tool for receptor-mediated radionuclide therapy. Eur J Nucl Med 1997;24(7):792–5. [DOI] [PubMed] [Google Scholar]
- 87.Lamberts SW, Bakker WH, Reubi JC, et al. Somatostatin-receptor imaging in the localization of endocrine tumors. N Engl J Med 1990;323(18): 1246–9. [DOI] [PubMed] [Google Scholar]
- 88.Krenning EP, Kwekkeboom DJ, Bakker WH, et al. Somatostatin receptor scintigraphy with [1111n-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med 1993;20(8):716–31. [DOI] [PubMed] [Google Scholar]
- 89.Antunes P, Ginj M, Zhang H, et al. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging 2007;34(7):982–93. [DOI] [PubMed] [Google Scholar]
- 90.Rösch F, Baum RP. Generator-based PET radiopharmaceuticals for molecular imaging of tumours: on the way to THERANOSTICS. Dalton Trans 2011; 40(23):6104–11. [DOI] [PubMed] [Google Scholar]
- 91.Chan HS, de Blois E, Morgenstern A, et al. In vitro comparison of 213Bi- and 177Lu-radiation for peptide receptor radionuclide therapy. PLoS One 2017: 12(7):e0181473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Azure MT, Archer RD, Sastry KS, et al. Biological effect of lead 212 localized in the nucleus of mammalian cells: role of recoil energy in the radiotoxicity of internal alpha particle emitters. Radiat Res 1994;140(2):273–83. [PMC free article] [PubMed] [Google Scholar]
- 93.Scarlett KA, White EZ, Coke CJ, et al. Agonist-induced CXCR4 and CB2 heter-odimerization inhibits Galpha13/RhoA-mediated migration. Mol Cancer Res 2018;12:1541–7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Philipp-Abbrederis K, Herrmann K, Knop S, et al. In vivo molecular imaging of chemokine receptor CXCR4 expression in patients with advanced multiple myeloma. EMBO Mol Med 2015;7(4):477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Asfour I, Afify H, Elkourashy S, et al. CXCR4 (CD184) expression on stem cell harvest and CD34(+) cells post-transplant. Hematol Oncol Stem Cell Ther 2017;10(2):63–9. [DOI] [PubMed] [Google Scholar]
- 96.Lapa C, Herrmann K, Schirbel A, et al. CXCR4-directed endoradiotherapy induces high response rates in extramedullary relapsed multiple myeloma. Theranostics 2017;7(6): 1589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Muhlethaler-Mottet A, Liberman J, Ascencao K, et al. The CXCR4/CXCR7/CXCL12 axis is involved in a secondary but complex control of neuroblastoma metastatic cell homing. PLoS One 2015;10(5):e0125616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Werner RA, Weich A, Higuchi T, et al. Imaging of chemokine receptor 4 expression in neuroendocrine tumors: a triple tracer comparative approach. Theranostics 2017;7(6): 1489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Habringer S, Lapa C, Herhaus P, et al. Dual targeting of acute leukemia and supporting niche by CXCR4-directed theranostics. Theranostics 2018;8(2): 369–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rahbar K, Ahmadzadehfar H, Kratochwil C, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med 2017;58(1):85–90. [DOI] [PubMed] [Google Scholar]
- 101.Heck MM, Retz M, Tauber R, et al. PSMA-targeted radioligand therapy in prostate cancer. Urologe A 2017;56(1):32–9 [in German]. [DOI] [PubMed] [Google Scholar]
- 102.Nock BA, Kaloudi A, Lymperis E, et al. Theranostic perspectives in prostate cancer with the gastrin-releasing peptide receptor antagonist Neo Bombi: preclin-ical and first clinical results. J Nucl Med 2017;58(1):75–80. [DOI] [PubMed] [Google Scholar]


