Abstract
Purpose of Review:
Evidence suggests that the microbiome of the skin, gastrointestinal tract and airway contribute to health and disease. As we learn more about the role that the microbiota plays in allergic disease development, we can develop therapeutics to alter this pathway.
Recent Findings:
Epidemiologic studies reveal that an association exists between environmental exposures which alter the microbiota, and developing atopic dermatitis, food allergy and/or asthma. In fact, samples from the skin, gastrointestinal tract and respiratory tract, reveal distinct microbiotas compared to healthy controls, with microbial changes (dysbiosis) often preceding the development of allergic disease. Mechanistic studies have confirmed that microbes can either promote skin, gut and airway health by strengthening barrier integrity, or they can alter skin integrity and damage gut and airway epithelium.
Summary:
In this review, we will discuss recent studies that reveal the link between the microbiota and immune development, and we will discuss ways to influence these changes.
Keywords: Microbiome, Asthma, Food Allergy, Atopic Dermatitis, Immune Development
INTRODUCTION
The prevalence of allergic disease continues to increase in the developed world leading to an increase in the number of children diagnosed with respiratory allergies such as rhinitis and asthma, food allergy, and/or atopic dermatitis. The hygiene hypothesis suggests that individuals from larger households have lower rates of allergic rhinitis and asthma. The advent of sophisticated methods to detect bacteria has resulted in numerous studies investigating the association between bacteria and allergic disease. Associations between the microbiota and exposures (cesarean deliveries, formula feeding, prebiotic or probiotic use, high fat and low fiber diets, and antibiotic use during infancy), and the eventual development of allergic disease suggests that the human microbiota plays a central role in the regulation of this process. (1–5)
This review summarizes the current literature linking the microbiota and allergic diseases, and reviews experimental data suggesting potential mechanisms between the two. In addition, the authors will discuss clinical evidence that early interventions may alter the microbiome and may decrease development of allergic disease. Because findings differ for each of the three main allergic diseases, atopic dermatitis, food allergy, and asthma will be reviewed individually.
ATOPIC DERMATITIS
The skin microbiome is comprised of bacteria, fungi, viruses and archaeal communities, with bacteria (the microbiota) being the most widely studied. Healthy skin consists of a diverse community of microbes that has differing communities depending on the sampling site. Propionibacterium species predominate in sebaceous sites while Corynebacterium and Staphylococcus species are found in moist microenvironments.(6, 7) When changes in this “healthy microbiome” occur, allergic sensitization can ensue.
Infant Skin Microbiome
Early skin colonization of infants consists of four main genera: Staphylococcus, Streptococcus, Lactobacillus and Propionibacterium.(8) However, in infants with atopic dermatitis (AD), an increased prevalence of Staphylococcus aureus with a decrease in the commensal microbes, Propionibacterium, Streptococcus, Acinetobacter, Corynebacterium, and Prevotella, has been observed. (8–13) These changes in bacterial composition can impair the skin’s ability to prevent overgrowth of harmful bacteria. For example, coagulase negative commensal bacteria, including S. epidermidis, S. hominis, and S. lugdunensis, secrete antimicrobials that limit S. aureus overgrowth and biofilm formation. In individuals with AD the prevalence of these protective bacteria is decreased which can disrupt this protective process.(14) Despite evidence that an overgrowth of S. aureus precedes the development of atopic dermatitis, (15) colonization with other species of Staphylococcus is associated with lower risk of atopic dermatitis by one year of age, demonstrating that mechanistic pathways influencing AD development can be species specific. (16)
Skin Microbiota and Atopic Dermatitis
Regardless of the timing of presentation, numerous studies have shown that over 90% of patients with atopic dermatitis have Staphylococcus aureus colonization.(17) Furthermore, the proportion of S. aureus relative to other commensals increases during flares, with higher density associated with more severe AD. (12, 18, 19) Mechanistic studies investigating the association between the presence of S. aureus and AD severity have shown that S. aureus affects atopic dermatitis in several ways. S. aureus activates protease receptors to disrupt the epidermal barrier of patients with AD or mice with filaggrin loss of mutation functions.(20, 21). In addition, S. aureus releases endotoxins and enterotoxins which stimulate mast cells and cause inflammation and dysregulation of keratinocytes. S. aureus also upregulates production of type 2 cytokines such as TSLP, IL-4 and IL-13.(20) High IL-4 and IL-13 deplete keratinocyte-produced antimicrobial peptides (AMPs) needed to control pathogenic organisms, thereby allowing further destruction by pathogenic bacteria. Ultimately, TLR2-mediated sensing of S. aureus is impaired in Langerhans cells from AD skin causing a cycle of keratinocyte dysregulation and disruption of the skin microbiome.(22) In healthy skin, Staphylococcus epidermidis activates TLR2, which promotes tight junction protein expression and induces keratinocyte‐derived AMPs secretion; therefore when S. aureus is the predominating species colonizing the skin this protective process is less effective. (23, 24) In addition, coagulase negative bacteria including S. epidermidis, S. hominis, and S. lugdunensis secrete antimicrobials that limit S. aureus overgrowth and biofilm formation.(25) While most studies point to S. aureus preceding the overgrowth of atopic dermatitis, one recent study did not find a high prevalence of S. aureus in lesional skin of infants with AD, (16) suggesting that longitudinal studies are needed to determine if S. aureus or other microbes play a role in AD development.
Gut Microbiome and Atopic Dermatitis
A diminished diversity of the gut microbiome also shares a relationship with atopic dermatitis. For example, antibiotic use in the first two years of life is associated with an increased risk of atopic dermatitis, suggesting a link between changing the GI microbiota and skin immunity. (1, 8) Additionally, other evidence demonstrates a lack of Bacteroides diversity or a high prevalence of Clostridium difficile colonization by one year of age is associated with atopic dermatitis development by 2 years of age. (26–28) One explanation for this difference is that individuals with atopic dermatitis are missing mucin producing bacteria which provides food for the commensal bacteria of the gut. If this nutrition is lacking it is possible that pathogenic bacteria overgrowth occurs instead. (29) Furthermore, often times a lower abundance of Bifidobacterium is present in the intestine of these individuals, suggesting that immune mechanisms are activated differently if more than one allergic disease is present. (30) Interestingly, the gut microbiota of infants with atopic dermatitis changes if they have concomitant food allergy. The fecal microbiota of those with both atopic dermatitis and food allergy contained more Escherichia coli and Bifidobacterium pseudocatenulatum, and less Bifidobacterium breve, Bfidobacterium adolescentis, Faecalibacterium prausnitzii, and Akkermansia muciniphila than children who had atopic dermatitis without food allergy. (9) Understanding the mechanisms behind this difference will help improve methods of prevention and treatment.
Treatment
Recent treatments for atopic dermatitis address the microbiota of the skin, with S. aureus as the main target. Emollients and anti-inflammatory medications are initially used to improve the epidermal barrier and prevent S. aureus from predominating. Antimicrobials are then used to directly combat S. Aureus. (8) New treatment strategies aim to add helpful bacteria to the skin rather than eliminate unwanted microorganisms. For example, the addition of topical Roseomonas mucosa and Vitreoscilla filiformis bacterial lysate has been shown to improve inflammation and severity of eczema. (31) Additionally, autologous microbiome transplant of S. hominis and S. epidermidis has also been efficacious in controlling S. aureus overgrowth. (32)
Improving the gut microbiome has been another target in the treatment of atopic dermatitis. Supplementation with probiotics and prebiotics is one intervention being assessed. Some success has been seen with probiotics. Prenatal and post-natal administration of the probiotics Bifidobacterium breve M-16V and Bifidobacterium longum BB536 reduced the risk of developing atopic dermatitis during the first 18 months of life. (33, 34) Additionally, prenatal and post‐natal treatment with Lactobacillus combined with Bifidobacterium reduced the risk of developing atopic dermatitis.(35) However, other studies have found conflicting results for probiotic use. For example, a recent randomized controlled trial administered Lactobacillus rhamnosus to children with a parental history of asthma for the first 6 mo of life. (36) Compared to those who received placebo, supplementation did not prevent the development of eczema or asthma by 2 years of age, suggesting that Bifidobacterium may play a larger role in allergic sensitization than Lactobacillus.
FOOD ALLERGY
Method of Delivery
The gut microbiome is mostly comprised of E. Coli and enterococcus species immediately after birth.(37) These microbes provide an oxygen rich environment for Bifidobacterium, Lactobacillus, Bacteroides, and Clostridium to proliferate. In infants born by cesarean section, Clostridium predominates over Bifidobacterium species, while the inverse is observed with vaginal deliveries.(38) Furthermore, infants born by cesarean section are colonized by maternal skin and hospital derived microbes, (39) suggesting that method of delivery may play a role in the early development of the microbiota and potentially the development of food allergy.
The Early Microbiome and Breastfeeding
Breastfed infants have less overall gut diversity within the first few weeks of life and are mostly colonized by Bifidobacterium. (40) An increased prevalence of Clostridium species compared to Bifidobacterium species at 3 weeks of age is associated with developing a food allergy in the first year of life. (40, 41) Furthermore, decreased Bifidobacterium and Lactobacillus species at 1–2 months of age increases the risk of developing allergies by 5 years of age. (42, 43) One possible explanation for this link between bacteria and decreased food allergy is that Bifidobacterium releases SCFAs (butyrate and propionate) and lowers stool pH, thereby creating an unfavorable environment for pathogenic bacteria. In mouse studies, non-digestible oligosaccharides and SCFAs decrease IgE mediated basophil degranulation and reduce the development of food allergies, further supporting a link between high levels of Bifidobacterium and food allergy. (44) Interestingly, formula is lacking Bifidobacterium further suggesting that early exposure to specific bacteria through the diet is key to food allergy prevention.(45)
Maternal Diet
Maternal diet may influence the infant microbiota and development of food allergy. The presence of Prevotella in maternal stool is associated with a decreased risk of their infant developing food allergy. (46) Prevotella is less prevalent in the Western world and is a microbe know for fermenting fiber and producing SCFAs. Maternal diets high in fat and fiber are associated with lower risk of food allergy development in offspring and this risk was further decreased if the mother’s stool contained Prevotella copri. Furthermore, P. copri is more prevalent in women from larger households and in women who did not receive antibiotics during pregnancy, two known protective associations in the development of allergic diseases. (46) Additionally, maternal peanut ingestion during pregnancy and lactation paired with early infant introduction to peanut allergen was shown to be associated with a decreased risk of sensitization to peanut allergen compared to early infant diet introduction alone.(47) However, another study found increased sensitization in offspring of mothers who consumed peanuts during pregnancy. (48) Insufficient data exists at this time to recommend a modification of the maternal diet during pregnancy to reduce the risk of food allergy.
Infant Diet
When considering introduction of solid foods, the benefits of SCFAs have been demonstrated. In a subset study of 301 children in the Protection Against Allergy Study in Rural Environments, the consumption of yogurt, fish, vegetables, and fruits within the first year of life was found to be associated with increased butyrate in stool samples by one year of age. In those children with butyrate and propionate levels over the 95th percentile, decreased sensitization to food allergens was discovered between the ages of 3 to 6 years of age.(4) Furthermore, another cohort study out of the United Kingdom found that diets rich in fruits, vegetables, and home prepared foods (as opposed to commercial infant foods) were associated with lower rates of food allergy by age 2 years of age. (49) These data could be secondary to the production of SCFAs by commensal bacteria, but more evidence is needed. Animal studies are currently underway and will help define mechanisms between bacteria and food allergy. (50)
Early food introduction to a diverse range of foods has been linked to lower incidence of food allergy.(49, 51, 52) The most substantial study supporting this is The Learning Early About Peanut Allergy trial, which revealed that introducing peanut allergen into an infant’s diet at 4–6 months of age reduced the incidence of peanut allergy. (51, 53) Interestingly, infants with Staphylococcus skin colonization were more likely to develop allergy suggesting that skin colonization may also contribute to allergic sensitization. In addition to early introduction of peanut, cheese consumption is associated with a reduced risk for food allergy, potentially due to its microbial composition and/or its relatively high content of SCFAs.(4) Further research is needed to delineate the relationship between specific foods and microbial development.
Gastrointestinal Microbiota and Food Allergy
The microbiota of the GI tract changes over the first three years of life, with the neonatal and infant microbiota influenced by method of delivery, breastfeeding, and solid food introduction as discussed above. (54, 55) Microbiota differences have been observed in patients with established food allergy, and they differ based on the food allergen studied. For example, studies report a higher prevalence of Lachnospiraceae, Streptococcaceae, and Leuconostocaceae in children with egg allergy (56), and an increase in Lachnospiraceae and Ruminocaceae in those with milk allergy.(57) Interestingly, further differences exist between those who have resolution of their allergy. An observational study investigating the microbiota of 226 infants between 3–16 months of age with milk allergy found that the presence of Clostridia and Firmicutes in the gut was associated with resolution of milk allergy by 8 years of age.(58) Further studies are needed to determine if specific species of these bacteria could be used to treat food allergy.
Studies investigating the mechanisms between food allergy and the microbiota have found that symbiotic bacteria have been shown to assist in intestinal integrity and immune system development/regulation. For example, commensal bacteria induce intestinal T-cells to differentiate into T-regulatory cells (59, 60) and the SCFAs, butyrate and propionate, drive T-regulatory differentiation (61, 62) and decrease pro-inflammatory mediator production from dendritic cells.(61) In addition, high fiber diets have been shown to protect mice from developing peanut sensitization due to the increase in fiber fermenting anaerobic bacteria producing SCFA which increase T-regulatory cells and dendritic cell tolerogenesis.(63)
As discussed above, numerous studies have demonstrated an association between the microbiome and the development of food allergy. Further mechanistic studies are needed to help us better understand how these bacteria lead to food allergy in some, but not all, infants.
Treatment
Despite our lack of understanding of these underlying mechanisms, studies investigating various treatment modalities have been performed. Treatments that intervene with the microbiome include prebiotics, probiotics, synbiotics, and fecal microbiota transplantation. The benefit of adding prebiotics to mimic human milk in formula-fed infants to decrease food allergy has not been demonstrated. In regard to probiotics, Lactobacillus rhamnosus supplementation in children with milk allergy has been shown to reduce the development of other allergic diseases and hasten resolution of milk allergy.(64) Use of this same supplement alongside oral desensitization to peanut resulted in a majority of participants achieving tolerance, however the study lacked a probiotic only and oral immunotherapy only groups for comparison.(65) Recent reviews of probiotics in food allergy concluded that insufficient data exists to recommend probiotic supplementation at this time. (66) Based on these data, the World Allergy Organization has suggested that probiotics can be used in certain high-risk populations making it clear that its recommendations are based on low-quality evidence.(67) Further research is needed in this area. Synbiotics and fecal microbiota transfers trials are still in their infancy and have yet to produce reliable results. Currently the use of partially hydrolyzed infant formula with added synbiotics compared to regular infant formula is being investigated. Fecal microbiota transfer studies in murine models have revealed that colonization of milk sensitized, germ-free mice with bacteria from healthy infant stool decreased the systemic allergic response compared to uncolonized mice.(68) In humans, fecal microbiota transfers have been studied in irritable bowel disease and Clostridium difficile infection (69, 70) however data for peanut allergy treatment with fecal microbiota transfers are still undergoing several phase 1 clinical trials (NCT02960074).
ASTHMA
The early work of Bisgaard et al. showed that bacterial colonization of the hypopharynx at one month of age was associated with early wheeze and the subsequent development of asthma.(71) Furthermore, they found that early colonization with Streptococcus pneumoniae, Moraxella catarrhalis, and Haemophilus influenzae was associated with increased levels of total IgE and blood eosinophil counts (71). The same group demonstrated that bacterial infections of the airway were associated with acute episodes of wheeze in children, independent of viral infection, (72) suggesting bacteria could be an early predictive marker or a causal factor in the development of wheeze and childhood asthma.
Environmental Impact
It has now been well established that a child’s environment can influence health outcomes. Multiple studies have found that children raised in the setting of rural farm communities have a decreased likelihood of developing asthma (73–79). Furthermore, some studies have looked into specific exposures, finding that pig farming, farm milk consumption (80), and time spent working in animal sheds and barns are protective (78). Meanwhile sheep farming, hare/rabbit farming, and hay feed exposure increased the risk of developing asthma (78). Endotoxin levels found in the dust near these farming environments have been the focus of studies examining the differences between these various farm-related exposures. In comparing the rural farm environment of Indiana Amish versus South Dakota Hutterite children, it was found that endotoxin levels were 6.8 times greater in dust from the homes of the Amish community. (73) Interestingly, the prevalence of asthma in the Amish cohort of children was 4 times lower than that of the Hutterite. The exposure to greater microbial diversity, found in various farming communities, seems to exhibit protection from the development of asthma. It has been suggested by many that the early-in-life exposure to higher levels of environment-specific endotoxins plays a significant role in innate immunity activation and its ability to suppress inflammatory responses (73, 78, 79). While the knowledge that certain farm exposures protect against asthma development is helpful, it is not a practical solution to raise all children on farms. A recent study investigated the mechanisms behind this exposure finding that mice exposed to dust from Amish farms have less airway hyper-reactivity and eosinophilia. Furthermore, knocking out steps in innate immunity pathways eliminated these protective effects, demonstrating that high endotoxin exposure is needed to develop innate immune responses. (75)
Method of Delivery
With an increase in the number of caesarian sections being performed in industrialized countries coinciding with an increase in asthma prevalence, method of delivery has become an area of interest in regard to the neonatal microbiome. Multiple studies have found an association between delivery via caesarian section and childhood asthma, with a recent meta-analysis finding that caesarian section increased the risk of childhood asthma by 20% (81). The microbiota within the nares, skin, and oral cavity are different between caesarian and vaginally delivered neonates.(82) Furthermore, neonates born via caesarian section showed greater nasopharyngeal microbiota instability longitudinally as well as decreased abundance of Corynebacterium and Dolosigranulum (83), bacteria associated with an absence of wheeze or asthma. Not only is the nasopharyngeal microbiota affected but the gut microbiota also appears to play a role in early airway immune responses. Independent of intrapartum antibiotic administration, vaginally delivered infants have a higher abundance of Bifidobacterium and E. coli with a lower abundance of Staphylococcus and Klebsiella. The same study also found that the composition of the GI microbiota at one week of life was associated with the number of respiratory infections reported in infants over the first year of life, suggesting that both the respiratory and gut microbiota play a role in asthma development. (84) However, other studies have found that while mode of delivery was associated with different patterns of microbiota in the neonate, when the microbiota was sampled again at 6 weeks of age, the differences no longer remained (82). Further investigations are needed to determine if this early difference in the microbiome at birth is associated with long term outcomes of asthma.
Breastfeeding
Many studies have shown a beneficial association between breastfeeding and respiratory health (85–89). Infants exclusively breastfeed for the first six weeks of life have a more stable microbiota profile comprised mainly of Dolosigranulum and Corynebacterium. Breastfeeding for a longer period of time (3 months) was also associated with prolonged elevated abundance of Dolosigranulum and Corynebacterium, thereby providing greater microbiota stability to the developing airway (90). In addition, breastfeeding is associated with fewer parent-reported respiratory infections (91, 92), suggesting that this prolonged microbiota stability is stimulating immune system development.
Airway Microbiome and Asthma
Whether a causal relationship exists between the airway microbiota and asthma remains unknown. However, multiple studies have attempted to map out the upper airway microbiota in order to investigate a possible relationship between dysbiosis and respiratory illness, wheeze, and asthma. Prospective cohort studies have found that six dominant genera make up the upper airway microbiota, ranging from early childhood through late adolescence. Throughout this time period the dominant genera in the upper airway microbiota are Moraxella, Streptococcus, Corynebacterium, Alloiococcus, Haemophilus, and Staphylococcus (93–95). Alterations in the development of the upper airway microbiota are associated with an increased risk of upper respiratory tract infections (URIs) during the first few years of life. (95, 96) Increases in the abundance of Streptococcus and Haemophilus (97) is associated with RSV bronchiolitis, while RV bronchiolitis is associated with an increased abundance of Haemophilus and Moraxella. (98) (91, 93, 99, 100). In contrast, a high abundance of Corynebacterium confers protective effects leading to stabilization of airway microbiota and milder disease, (90, 91, 93, 100, 101) suggesting that bacteria play an active role in the immune responses to infection. In a study of the upper and lower airway microbiome and transcriptome, it was suggested that in the nasal airway of non-asthmatics, Corynebacterium negatively interacted with genes that promoted inflammation and therefore conferred protection (100). The relationship between Moraxella and respiratory illness remains ill-defined with studies showing conflicting data. While some are associating its abundance with microbial stability and lack of respiratory illness(91), others are finding that a microbial profile dominated by Moraxella correlates with increased instances of respiratory illness and asthma exacerbations(71, 94). Murine models do support a detrimental role, as colonization with Moraxella led to strong inflammatory responses and elevated neutrophilic infiltrates (102, 103). In another study, isolated strains of Moraxella from nasal secretions of asthmatics were utilized for inoculation of airway epithelial cell cultures. It was found that Moraxella isolates increased epithelial damage as well as gene expression of pro-inflammatory cytokines(94). These studies point to the importance of understanding the evolution of early life airway microbiota and identifying a ‘critical window’ in which intervention could alter the trajectory of respiratory health. Further supporting these findings, neonatal mice exhibited a two-week window following birth in which microbial exposure and diversity correlated with stabilization of the lung microbiota. Dysregulation of the microbiota in the time window led to sustained susceptibility to allergic airway inflammation into adulthood (104). Additional studies are still needed to confirm whether distinct microbiota profiles trigger harmful responses in the upper airway.
Treatment
While numerous studies are underway, few interventions have been published. Administration of Lactobacillus reuteri to mice attenuated recruitment of airway eosinophils and prevented allergen-induced airway hyperresponsiveness.(105) In infants, one recent study examined third trimester supplementation with fish oil and high dose Vitamin D. (106) At one month of age, the infant airway microbiota contained fewer bacteria associated with asthma development compared to placebo matched controls. Interestingly, the vaginal microbiota was not altered in this study, suggesting that prenatal supplementation does not harm the maternal microbiota. Randomized control trials with long-term follow-up are needed to determine if these early interventions prevent allergic diseases during childhood.
CONCLUSIONS
A growing body of evidence links the skin, gut, and respiratory microbiota with allergic diseases. Commensal bacteria is associated with “healthy” immune development, while a higher abundance of pathogenic bacteria is associated with weakened mucosal protection and the upregulation of inflammatory cytokines (Figure 1). However, studies describing the timing behind these microbial changes and immune development are lacking, and the ideal moment to intervene before detrimental immune changes occur is needed. Once this “window of opportunity” is determined, better methods to prevent allergic disease can be achieved.
Figure 1:
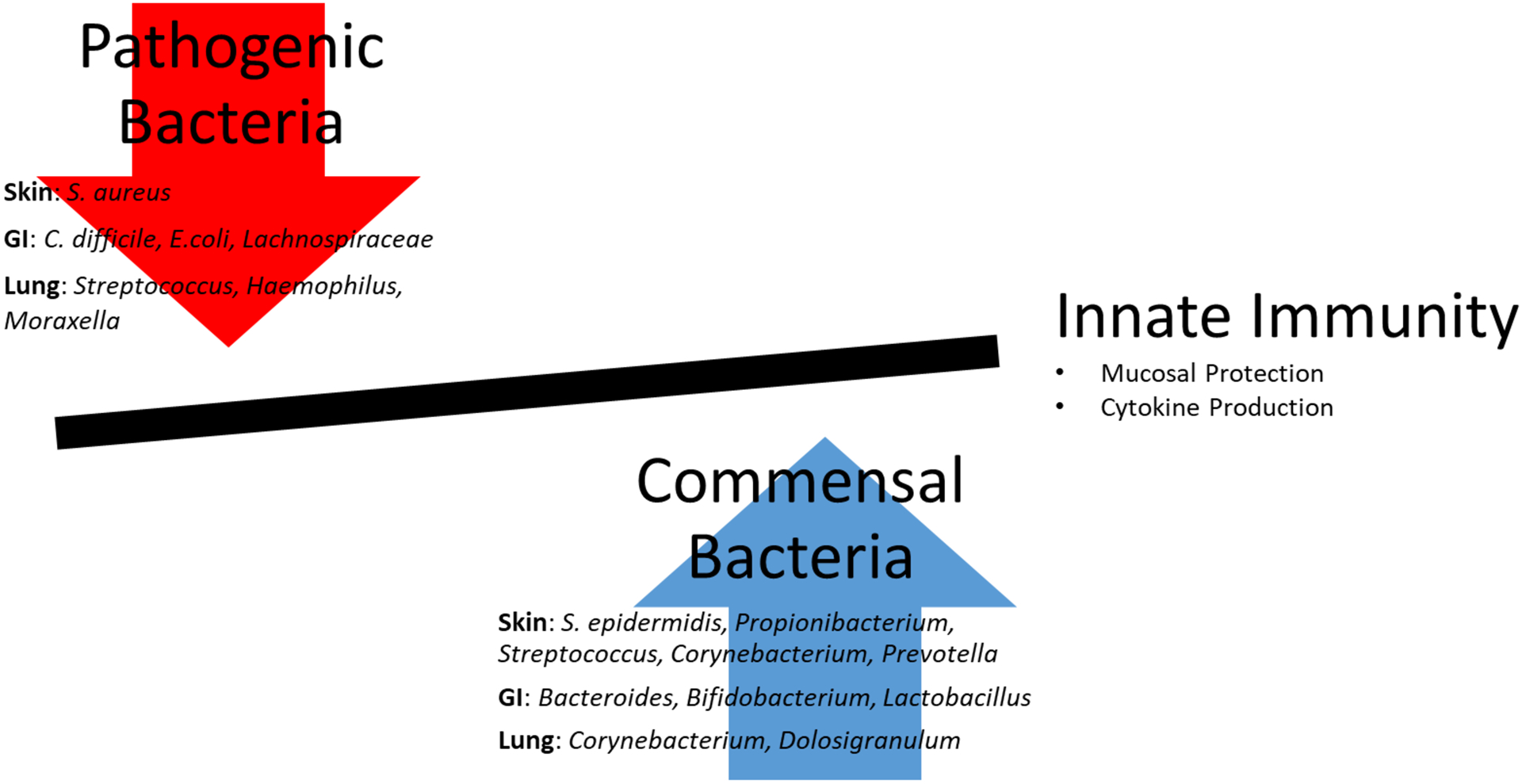
An increase in pathogenic bacteria is associated with a decrease in innate immune responses including a decrease in mucosal protection and an upregulation of inflammatory cytokines leading to an increase in allergic sensitization. In contrast, an increase in commensal bacteria is associated with activation of innate immunity and prevention of allergic sensitization.
Acknowledgments
The following grants supported this research:
National Institute for Allergy, Infections Disease (NIAID) K23 Career development Award 1 K23 AI135094-01
American Academy of Allergy, Asthma and Immunology (AAAAI) Foundation Faculty Development Award
National Institute of Health Loan Repayment Program L40 AI096442
Footnotes
Author Disclaimers: All authors have nothing to disclose.
References
- 1.Shu SA, Yuen AWT, Woo E, Chu KH, Kwan HS, Yang GX, et al. Microbiota and Food Allergy. Clin Rev Allergy Immunol. 2019;57(1):83–97. [DOI] [PubMed] [Google Scholar]
- 2.Marrs T, Sim K. Demystifying Dysbiosis: Can the Gut Microbiome Promote Oral Tolerance Over IgE-mediated Food Allergy? Current Pediatric Review. 2018;14(3):156–63. [DOI] [PubMed] [Google Scholar]
- 3.Cukrowska B. Microbial and Nutritional Programming-The Importance of the Microbiome and Early Exposure to Potential Food Allergens in the Development of Allergies. Nutrients. 2018;10(10):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunyavanich B. Food allergy and the microbiome: Current understandings and future directions. Journal of Allergy & Clinical Immunology. 2019;144(6):1468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmiechen Zoe C. KAW, Frischmeyer-Guerrerio Pamela A.. Recent developments in understanding the mechanisms of food allergy. Wolters Kluwer Health. 2019;21(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh J, Byrd AL, Deming C, Conlan S, Program NCS, Kong HH, et al. Biogeography and individuality shape function in the human skin metagenome. Nature. 2014;514(7520):59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Findley K, Oh J, Yang J, Conlan S, Deming C, Meyer JA, et al. Topographic diversity of fungal and bacterial communities in human skin. Nature. 2013;498(7454):367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lunjani N, Satitsuksanoa P, Lukasik Z, Sokolowska M, Eiwegger T, O’Mahony L. Recent developments and highlights in mechanisms of allergic diseases: Microbiome. Allergy. 2018;73(12):2314–27. [DOI] [PubMed] [Google Scholar]
- 9.Fieten KB, Totte JEE, Levin E, Reyman M, Meijer Y, Knulst A, et al. Fecal Microbiome and Food Allergy in Pediatric Atopic Dermatitis: A Cross-Sectional Pilot Study. International Archives of Allergy & Immunology. 2018;175(1–2):77–84. [DOI] [PubMed] [Google Scholar]
- 10.Jensen-Jarolim E, Pali-Scholl I, Roth-Walter F. Outstanding animal studies in allergy II. From atopic barrier and microbiome to allergen-specific immunotherapy. Current Opinion in Allergy & Clinical Immunology. 2017;17(3):180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramsey MM, Freire MO, Gabrilska RA, Rumbaugh KP, Lemon KP. Staphylococcus aureus Shifts toward Commensalism in Response to Corynebacterium Species. Front Microbiol. 2016;7:1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22(5):850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fyhrquist N, Ruokolainen L, Suomalainen A, Lehtimaki S, Veckman V, Vendelin J, et al. Acinetobacter species in the skin microbiota protect against allergic sensitization and inflammation. J Allergy Clin Immunol. 2014;134(6):1301–9 e11. [DOI] [PubMed] [Google Scholar]
- 14.Sugimoto S, Iwamoto T, Takada K, Okuda K, Tajima A, Iwase T, et al. Staphylococcus epidermidis Esp degrades specific proteins associated with Staphylococcus aureus biofilm formation and host-pathogen interaction. J Bacteriol. 2013;195(8):1645–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meylan P, Lang C, Mermoud S, Johannsen A, Norrenberg S, Hohl D, et al. Skin Colonization by Staphylococcus aureus Precedes the Clinical Diagnosis of Atopic Dermatitis in Infancy. J Invest Dermatol. 2017;137(12):2497–504. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy EA, Connolly J, Hourihane JO, Fallon PG, McLean WHI, Murray D, et al. Skin microbiome before development of atopic dermatitis: Early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J Allergy Clin Immunol. 2017;139(1):166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Munoz-Planillo R, Hasegawa M, et al. Staphylococcus delta-toxin induces allergic skin disease by activating mast cells. Nature. 2013;503(7476):397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **18.Tauber M, Balica S, Hsu CY, Jean-Decoster C, Lauze C, Redoules D, et al. Staphylococcus aureus density on lesional and nonlesional skin is strongly associated with disease severity in atopic dermatitis. J Allergy Clin Immunol. 2016;137(4):1272–4 e3. [DOI] [PubMed] [Google Scholar]; COMMENT: Staphylococcus aureus abundance increases during skin flares. Increased abundance of S. aureus is assocaited with increased severity of atopc dermatitis.
- 19.Kemter AM, Nagler CR. Influences on allergic mechanisms through gut, lung, and skin microbiome exposures. J Clin Invest. 2019;130:1483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakatsuji T, Chen TH, Two AM, Chun KA, Narala S, Geha RS, et al. Staphylococcus aureus Exploits Epidermal Barrier Defects in Atopic Dermatitis to Trigger Cytokine Expression. J Invest Dermatol. 2016;136(11):2192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirasawa Y, Takai T, Nakamura T, Mitsuishi K, Gunawan H, Suto H, et al. Staphylococcus aureus extracellular protease causes epidermal barrier dysfunction. J Invest Dermatol. 2010;130(2):614–7. [DOI] [PubMed] [Google Scholar]
- 22.Iwamoto K, Numm TJ, Koch S, Herrmann N, Leib N, Bieber T. Langerhans and inflammatory dendritic epidermal cells in atopic dermatitis are tolerized toward TLR2 activation. Allergy. 2018;73(11):2205–13. [DOI] [PubMed] [Google Scholar]
- 23.Seite S, Bieber T. Barrier function and microbiotic dysbiosis in atopic dermatitis. Clin Cosmet Investig Dermatol. 2015;8:479–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geoghegan JA, Dufrene YF. Mechanomicrobiology: How Mechanical Forces Activate Staphylococcus aureus Adhesion. Trends Microbiol. 2018;26(8):645–8. [DOI] [PubMed] [Google Scholar]
- 25.Di Domenico EG, Cavallo I, Capitanio B, Ascenzioni F, Pimpinelli F, Morrone A, et al. Staphylococcus aureus and the Cutaneous Microbiota Biofilms in the Pathogenesis of Atopic Dermatitis. Microorganisms. 2019;7(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas CL, Fernandez-Penas P. The microbiome and atopic eczema: More than skin deep. Australas J Dermatol. 2017;58(1):18–24. [DOI] [PubMed] [Google Scholar]
- 27.Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut. 2007;56(5):661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol. 2012;129(2):434–40, 40 e1–2. [DOI] [PubMed] [Google Scholar]
- 29.Lee MJ, Kang MJ, Lee SY, Lee E, Kim K, Won S, et al. Perturbations of gut microbiome genes in infants with atopic dermatitis according to feeding type. J Allergy Clin Immunol. 2018;141(4):1310–9. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe S, Narisawa Y, Arase S, Okamatsu H, Ikenaga T, Tajiri Y, et al. Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. J Allergy Clin Immunol. 2003;111(3):587–91. [DOI] [PubMed] [Google Scholar]
- 31.Myles IA EN, Anderson ED, et al. First‐in‐human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakatsuji T CT, Narala S, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Translation Medicine. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tadao Enomoto MS, Keiji Nishimori, Shinichiro Shimazu, Akira Yoshida, Kazuko Yamada, Fukumi Furukawa, Takemasa Nakagawa, Naotake Yanagisawa, Noriyuki Iwabuchi, Toshitaka Odamaki, Fumiaki Abe, Jiro Nakayama, Jin-zhong Xiao. Effects of Bifidobacterial Supplementation to Pregnant Women and Infants in the Prevention of Allergy Development in Infants and on Fecal Microbiota. Allergology International. 2014;63(4):575–85. [DOI] [PubMed] [Google Scholar]
- 34.Chernikova D, Yuan I, Shaker M. Prevention of allergy with diverse and healthy microbiota: an update. Current Opinion in Pediatrics. 2019;31(3):418–25. [DOI] [PubMed] [Google Scholar]
- 35.Dotterud CK OS, Johnsen R, Oien T. Probiotics in pregnant women to prevent allergic disease: a randomized, double-blind trial. Br J Dermato. 2010;163(3):616–23. [DOI] [PubMed] [Google Scholar]
- 36.Cabana MD MM, Caughey AB, et al. Pediatrics . Early Probiotic Supplementation for Eczema and Asthma Prevention: A Randomized Controlled Trial. . Pediatrics. 2017;140(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell host & microbe. 2015;17(6):852. [DOI] [PubMed] [Google Scholar]
- 38.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118(2):511–21. [DOI] [PubMed] [Google Scholar]
- 39.Gronlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr. 1999;28(1):19–25. [DOI] [PubMed] [Google Scholar]
- 40.Kalliomaki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. 2001;107(1):129–34. [DOI] [PubMed] [Google Scholar]
- 41.Bunyavanich S. Food allergy: could the gut microbiota hold the key? Nat Rev Gastroenterol Hepatol. 2019;16(4):201–2. [DOI] [PubMed] [Google Scholar]
- 42.Bisgaard HL N; Bonnelykke K; Chawes BL; Skov T; Paudan-Müller G; Stokholm J; Smith B; Krogfelt KA. Reduced Diversity of the Intestinal Microbiota During Infancy Is Associated With Increased Risk of Allergic Disease at School Age. J Allergy Clin Immunol. 2011;128. [DOI] [PubMed] [Google Scholar]
- 43.Sjogren YM, Jenmalm MC, Bottcher MF, Bjorksten B, Sverremark-Ekstrom E. Altered early infant gut microbiota in children developing allergy up to 5 years of age. Clinical & Experimental Allergy. 2009;39(4):518–26. [DOI] [PubMed] [Google Scholar]
- 44.Hayen SM, den Hartog Jager CF, Knulst AC, Knol EF, Garssen J, Willemsen LEM, et al. Non-Digestible Oligosaccharides Can Suppress Basophil Degranulation in Whole Blood of Peanut-Allergic Patients. Front Immunol. 2018;9:1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roger LC, Costabile A, Holland DT, Hoyles L, McCartney AL. Examination of faecal Bifidobacterium populations in breast- and formula-fed infants during the first 18 months of life. Microbiology. 2010;156(Pt 11):3329–41. [DOI] [PubMed] [Google Scholar]
- **46.Peter J Vuillermin MOH, Fiona Collier,Allen Katrina J.,Tang Mimi L. K.,Harrison Leonard C., Carlin John B.,Saffery Richard,Ranganathan Sarath,Sly Peter D., Gray Lawrence,Molloy John,Pezic Angela, Conlon Michael,Topping David,Nelson Karen, Mackay Charles R.,Macia Laurence,Koplin Jennifer,Dawson Samantha L., Moreno-Betancur Margarita,Ponsonby Anne-Louise,the J. Craig Venter Institute, and the BIS Investigator Group. Maternal carriage of Prevotella during pregnancy associates with protection against food allergy in the offspring. Nature Communications. 2020;11(1452). [DOI] [PMC free article] [PubMed] [Google Scholar]; COMMENT:Nested case-cohort study revealing maternal carriage of Prevotella copri in stool during pregnancy was strongly associated with the absence of food allergy development in offspring.
- 47.Tracy J ABB Pitt, Chan-Yeung Moira, Chan Edmond S., Watson Wade T. A., Chooniedass Rishma, Azad Meghan B.. Reduced risk of peanut sensitization following exposure through breast-feeding and early peanut introduction. Journal of Allergy & Clinical Immunology. 2017. [DOI] [PubMed] [Google Scholar]
- 48.Sicherer S Maternal consumption of peanut during pregnancy is associated with peanut sensitizaiton in atopic infants. Journal of Allergy & Clinical Immunology. 2010;126(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Venter PhD Carina KMP, Holloway PhD John W., Silveira PhD Lori J., Fleischer MD David M., Dean PhD Taraneh,Arshad MD S. Hasan. Different Measures of Diet Diversity During Infancy and the Association with Childhood Food Allergy in a UK Birth Cohort Study Journal of Allergy & Clinical Immunology. 2020. [DOI] [PubMed] [Google Scholar]
- 50.Musso Paola EC, Bernardini Roberto. Human Microbiome and Allergic Diseases in Children: Pathogenetic Role and Therapeutic Options. Current Pediatric Reviews. 2019;15. [DOI] [PubMed] [Google Scholar]
- **51.Du Toit George GR, Sayre Peter H., Bahnson Henry T.,Radulovic Suzana,Santos Alexandra F.,Brough Helen A., Phippard Deborah,Basting Monica, Feeney Mary,Turcanu Victor, Sever Michelle L., Lorenzo Margarita Gomez,Plaut Marshall,Lack Gideon. Randomized Trial of Peanut Consumption in Infants at Risk for Peanut Allergy. The New England Journal of Medicine. 2015;372(9). [DOI] [PMC free article] [PubMed] [Google Scholar]; COMMENT: Early Introduction of peanut significantly decreased the frequency of developing peanut allergy in a high-risk infant cohort.
- 52.Roduit Caroline RF, Depner Martin, Schaub Bianca, Loss Georg, Genuneit Jon, Pfefferle Petra, Hyvärinen Anne, Karvonen Anne M., Riedler Josef, et al. Increased food diversity in the first year of life is inversely associated with allergic diseases. Journal of Allergy & Clinical Immunology. 2014;133(4):1056–64. [DOI] [PubMed] [Google Scholar]
- 53.du Toit G, Sayre PH, Roberts G, Lawson K, Sever ML, Bahnson HT, Fisher HR, Feeney M, Radulovic S, Basting M, Plaut M, Lack G Allergen specificity of early peanut consumption and effect on development of allergic disease in the Learning Early About Peanut Allergy study cohort. Journal of Allergy & Clinical Immunology. 2018;141(4):1343–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yassour M, Vatanen T, Siljander H, Hamalainen AM, Harkonen T, Ryhanen SJ, et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci Transl Med. 2016;8(343):343ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fazlollahi M, Chun Y, Grishin A, Wood RA, Burks AW, Dawson P, et al. Early-life gut microbiome and egg allergy. Allergy. 2018;73(7):1515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berni Canani R, Sangwan N, Stefka AT, Nocerino R, Paparo L, Aitoro R, et al. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2016;10(3):742–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bunyavanich S, Shen N, Grishin A, Wood R, Burks W, Dawson P, et al. Early-life gut microbiome composition and milk allergy resolution. Journal of Allergy & Clinical Immunology. 2016;138(4):1122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478(7368):250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34(5):794–806. [DOI] [PubMed] [Google Scholar]
- 61.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–50. [DOI] [PubMed] [Google Scholar]
- 63.Tan J, McKenzie C, Vuillermin PJ, Goverse G, Vinuesa CG, Mebius RE, et al. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016;15(12):2809–24. [DOI] [PubMed] [Google Scholar]
- 64.Berni Canani R, Di Costanzo M, Bedogni G, Amoroso A, Cosenza L, Di Scala C, et al. Extensively hydrolyzed casein formula containing Lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow’s milk allergy: 3-year randomized controlled trial. J Allergy Clin Immunol. 2017;139(6):1906–13 e4. [DOI] [PubMed] [Google Scholar]
- 65.Tang ML, Ponsonby AL, Orsini F, Tey D, Robinson M, Su EL, et al. Administration of a probiotic with peanut oral immunotherapy: A randomized trial. J Allergy Clin Immunol. 2015;135(3):737–44 e8. [DOI] [PubMed] [Google Scholar]
- 66.Cuello-Garcia CA, Brozek JL, Fiocchi A, Pawankar R, Yepes-Nunez JJ, Terracciano L, et al. Probiotics for the prevention of allergy: A systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2015;136(4):952–61. [DOI] [PubMed] [Google Scholar]
- 67.Fiocchi A, Pawankar R, Cuello-Garcia C, Ahn K, Al-Hammadi S, Agarwal A, et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Probiotics. World Allergy Organ J. 2015;8(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao W, Ho HE, Bunyavanich S. The gut microbiome in food allergy. Ann Allergy Asthma Immunol. 2019;122(3):276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. The New England journal of medicine. 2013;368(5):407–15. [DOI] [PubMed] [Google Scholar]
- 70.Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312(17):1772–8. [DOI] [PubMed] [Google Scholar]
- 71.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, et al. Childhood asthma after bacterial colonization of the airway in neonates. The New England journal of medicine. 2007;357(15):1487–95. [DOI] [PubMed] [Google Scholar]
- 72.Bisgaard H, Hermansen MN, Bonnelykke K, Stokholm J, Baty F, Skytt NL, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. Bmj. 2010;341:c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. The New England journal of medicine. 2016;375(5):411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ, et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 2015;349(6252):1106–10. [DOI] [PubMed] [Google Scholar]
- 75.Kirjavainen PV, Karvonen AM, Adams RI, Taubel M, Roponen M, Tuoresmaki P, et al. Farm-like indoor microbiota in non-farm homes protects children from asthma development. Nat Med. 2019;25(7):1089–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holbreich M, Genuneit J, Weber J, Braun-Fahrlander C, Waser M, von Mutius E. Amish children living in northern Indiana have a very low prevalence of allergic sensitization. J Allergy Clin Immunol. 2012;129(6):1671–3. [DOI] [PubMed] [Google Scholar]
- 77.Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrlander C, et al. Exposure to environmental microorganisms and childhood asthma. The New England journal of medicine. 2011;364(8):701–9. [DOI] [PubMed] [Google Scholar]
- 78.Ege MJ, Frei R, Bieli C, Schram-Bijkerk D, Waser M, Benz MR, et al. Not all farming environments protect against the development of asthma and wheeze in children. J Allergy Clin Immunol. 2007;119(5):1140–7. [DOI] [PubMed] [Google Scholar]
- 79.Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. The New England journal of medicine. 2002;347(12):869–77. [DOI] [PubMed] [Google Scholar]
- 80.Waser M, Michels KB, Bieli C, Floistrup H, Pershagen G, von Mutius E, et al. Inverse association of farm milk consumption with asthma and allergy in rural and suburban populations across Europe. Clin Exp Allergy. 2007;37(5):661–70. [DOI] [PubMed] [Google Scholar]
- 81.Darabi B, Rahmati S, HafeziAhmadi MR, Badfar G, Azami M. The association between caesarean section and childhood asthma: an updated systematic review and meta-analysis. Allergy Asthma Clin Immunol. 2019;15:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, Aagaard KM. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. 2017;23(3):314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bosch A, Levin E, van Houten MA, Hasrat R, Kalkman G, Biesbroek G, et al. Development of Upper Respiratory Tract Microbiota in Infancy is Affected by Mode of Delivery. EBioMedicine. 2016;9:336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reyman M, van Houten MA, van Baarle D, Bosch A, Man WH, Chu M, et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat Commun. 2019;10(1):4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duijts L, Jaddoe VW, Hofman A, Moll HA. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. 2010;126(1):e18–25. [DOI] [PubMed] [Google Scholar]
- 86.Lopez-Alarcon M, Villalpando S, Fajardo A. Breast-feeding lowers the frequency and duration of acute respiratory infection and diarrhea in infants under six months of age. J Nutr. 1997;127(3):436–43. [DOI] [PubMed] [Google Scholar]
- 87.Paricio Talayero JM, Lizan-Garcia M, Otero Puime A, Benlloch Muncharaz MJ, Beseler Soto B, Sanchez-Palomares M, et al. Full breastfeeding and hospitalization as a result of infections in the first year of life. Pediatrics. 2006;118(1):e92–9. [DOI] [PubMed] [Google Scholar]
- 88.Tarrant M, Kwok MK, Lam TH, Leung GM, Schooling CM. Breast-feeding and childhood hospitalizations for infections. Epidemiology. 2010;21(6):847–54. [DOI] [PubMed] [Google Scholar]
- 89.Azad MB, Vehling L, Lu Z, Dai D, Subbarao P, Becker AB, et al. Breastfeeding, maternal asthma and wheezing in the first year of life: a longitudinal birth cohort study. Eur Respir J. 2017;49(5). [DOI] [PubMed] [Google Scholar]
- 90.Bosch A, de Steenhuijsen Piters WAA, van Houten MA, Chu M, Biesbroek G, Kool J, et al. Maturation of the Infant Respiratory Microbiota, Environmental Drivers, and Health Consequences. A Prospective Cohort Study. American journal of respiratory and critical care medicine. 2017;196(12):1582–90. [DOI] [PubMed] [Google Scholar]
- 91.Biesbroek G, Tsivtsivadze E, Sanders EA, Montijn R, Veenhoven RH, Keijser BJ, et al. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. American journal of respiratory and critical care medicine. 2014;190(11):1283–92. [DOI] [PubMed] [Google Scholar]
- 92.Biesbroek G, Bosch AA, Wang X, Keijser BJ, Veenhoven RH, Sanders EA, et al. The impact of breastfeeding on nasopharyngeal microbial communities in infants. American journal of respiratory and critical care medicine. 2014;190(3):298–308. [DOI] [PubMed] [Google Scholar]
- 93.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell host & microbe. 2015;17(5):704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McCauley K, Durack J, Valladares R, Fadrosh DW, Lin DL, Calatroni A, et al. Distinct nasal airway bacterial microbiotas differentially relate to exacerbation in pediatric patients with asthma. J Allergy Clin Immunol. 2019;144(5):1187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **95.Teo SM, Tang HHF, Mok D, Judd LM, Watts SC, Pham K, et al. Airway Microbiota Dynamics Uncover a Critical Window for Interplay of Pathogenic Bacteria and Allergy in Childhood Respiratory Disease. Cell host & microbe. 2018;24(3):341–52.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]; COMMENT: Longitudinal study of the nasopharyngeal microbiota from early infancy to 5 years of age. Shows asymptomatic colonization of the nasopharynx in early-sensitized children by Streptococcus, Haemophilus, and Moraxella increased the risk of chronic wheeze at 5 years of age.
- 96.Hyde ER, Petrosino JF, Piedra PA, Camargo CA Jr., Espinola JA, Mansbach JM. Nasopharyngeal Proteobacteria are associated with viral etiology and acute wheezing in children with severe bronchiolitis. J Allergy Clin Immunol. 2014;133(4):1220–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rosas-Salazar C, Shilts MH, Tovchigrechko A, Schobel S, Chappell JD, Larkin EK, et al. Differences in the Nasopharyngeal Microbiome During Acute Respiratory Tract Infection With Human Rhinovirus and Respiratory Syncytial Virus in Infancy. J Infect Dis. 2016;214(12):1924–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Caliskan M, Bochkov YA, Kreiner-Moller E, Bonnelykke K, Stein MM, Du G, et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. The New England journal of medicine. 2013;368(15):1398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rosas-Salazar C, Shilts MH, Tovchigrechko A, Schobel S, Chappell JD, Larkin EK, et al. Nasopharyngeal Lactobacillus is associated with a reduced risk of childhood wheezing illnesses following acute respiratory syncytial virus infection in infancy. J Allergy Clin Immunol. 2018;142(5):1447–56.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **100.Chun Y, Do A, Grishina G, Grishin A, Fang G, Rose S, et al. Integrative study of the upper and lower airway microbiome and transcriptome in asthma. JCI Insight. 2020;5(5). [DOI] [PMC free article] [PubMed] [Google Scholar]; COMMENT: Study linking the microbiome and transcriptome in asthmatic versus healthy children. Corynebacterium in the nasal microbiome shows negative associations with many genes related to inflammation in healthy children. This protective effect was attenuated in children with severe persistent asthma.
- 101.Kloepfer KM, Sarsani VK, Poroyko V, Lee WM, Pappas TE, Kang T, et al. Community-acquired rhinovirus infection is associated with changes in the airway microbiome. J Allergy Clin Immunol. 2017;140(1):312–5 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hassan F, Ren D, Zhang W, Merkel TJ, Gu XX. Moraxella catarrhalis activates murine macrophages through multiple toll like receptors and has reduced clearance in lungs from TLR4 mutant mice. PLoS One. 2012;7(5):e37610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alnahas S, Hagner S, Raifer H, Kilic A, Gasteiger G, Mutters R, et al. IL-17 and TNF-alpha Are Key Mediators of Moraxella catarrhalis Triggered Exacerbation of Allergic Airway Inflammation. Front Immunol. 2017;8:1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gollwitzer ES, Saglani S, Trompette A, Yadava K, Sherburn R, McCoy KD, et al. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat Med. 2014;20(6):642–7. [DOI] [PubMed] [Google Scholar]
- 105.Forsythe P, Inman MD, Bienenstock J. Oral treatment with live Lactobacillus reuteri inhibits the allergic airway response in mice. American journal of respiratory and critical care medicine. 2007;175(6):561–9. [DOI] [PubMed] [Google Scholar]
- 106.Hjelmso MH, Shah SA, Thorsen J, Rasmussen M, Vestergaard G, Mortensen MS, et al. Prenatal dietary supplements influence the infant airway microbiota in a randomized factorial clinical trial. Nat Commun. 2020;11(1):426. [DOI] [PMC free article] [PubMed] [Google Scholar]


