Abstract
Introduction:
The frequency of glomerulonephritis (GN) is reported to be changing in the world over the past four decades. Few studies arise from the western region of Saudi Arabia.
Aims:
The aim of this study was to address the frequency of primary GN (1ry GN) and secondary GN (2ry GN) over a period of 26 years in the western region of Saudi Arabia and compare to previous data from other regions of the country.
Subjects and Methods:
The records of adult renal biopsies, 448 1ry GN and 263 2ry GN, are analyzed. Frequencies of GN subtypes are compared for period 1 (1988–19999) and period 2 (2000–2013).
Results:
Postinfectious GN (PIGN) and minimal change disease (MCD) show significant changes (P ≤ 0.05). PIGN increased to 6.5% in period 2 from 0% in period 1. MCD decreased to 5.9% in period 2 from 13.5% in period 1. Membranous GN is the most common 1ry GN for both periods with similar percentages (23.8% and 24.2%, respectively). Focal segmental glomerulosclerosis (FSGSC) is the second in period 2 (23%); immunoglobulin A nephropathy at 9.6% became the third, and MCD is the last place instead of the fourth in period 1. Lupus nephritis is the most common 2ry GN. Pooled data from Saudi studies show FSGSC the most common 1ry GN in both periods.
Conclusions:
The western region of Saudi Arabia presents with a different 1ry GN pattern than the rest of the country that is likely attributed to its unique geographical and environmental characteristics.
Keywords: Postinfectious glomerulonephritis, primary glomerulonephritis, Saudi Arabia, secondary glomerulonephritis, western region
INTRODUCTION
Epidemiologic studies of glomerular diseases provide important information about the evolution of glomerulonephritis (GN). Studies from different parts of the world reveal changing frequencies of glomerular diseases in response to variation in factors such as population demographics and environmental, genetic, and socioeconomic status. The rising frequency of membranous GN (MGN) has been related to environmental pollution as a result of industrialization.[1,2] The United States and Italy report a decline in MGN and an increased FSGSC.[3,4,5]
The central region of Saudi Arabia reports higher frequencies of FSGSC.[6,7,8,9,10,11,12,13,14,15,16,17] The western region of Saudi Arabia reported MGN as the most common 1ry GN.[18]
The purpose of this study is to address the frequency of 1ry and 2ry GN over a period of 26 years from the largest referral center in the western region. The study will also compare the changing GN pattern over the past three decades to the pooled data from Saudi Arabia studies.
SUBJECTS AND METHODS
Current study cases
The records of all adult (age above 18 years) adult native renal biopsies received in our center for the period of 1988–2013 were retrieved and retrospectively analyzed. Our hospital is the referral center for renal biopsy cases, with two renal pathologists and an electron microscopy (EM) unit. We cover cases from wide range of hospitals in the western region of Saudi Arabia.
The total number of adult renal biopsies received in the period of 1988–2013 is 809 cases. Eliminated cases from the study include insufficient tissues for diagnosis, nonglomerular diseases, normal glomeruli by all studies, and advanced global sclerosis. The cases which were analyzed in the study constitute 448 cases of 1ry GN and 263 cases of 2ry GN [Table 1].
Table 1.
Frequencies of renal diseases in 809 adult renal biopsies
| Diagnosis | n (%) |
|---|---|
| Primary GN | 448 (55.4) |
| Secondary GN | 263 (32.5) |
| Glomeruli no change | 11 (1.4) |
| Global glomerulosclerosis | 41 (5.1) |
| Others | 12 (1.5) |
| Insufficient tissue | 34 (4.2) |
| Total | 809 (100.0) |
All cases included in the study had been investigated by the three modalities: light microscopy, immunofluorescence (IF) labeling, and EM. Tissue handling and preparation was performed following the routine standard techniques in the EM unit and the hospital histopathology laboratory.
The selected cases of 1ry GN and 2ry GN categories were analyzed separately. Each GN category was further arranged into GN subtypes. Furthermore, the 2 GN categories are divided into two period groups based on the date the biopsy was performed. The two periods are as follows: period 1 representing the years 1988–19999 and period 2 representing the years 2000–2013.
Comparison of the frequency of 1ry GN and 2ry GN subtypes in the two periods was performed. Also correlation of GN subtypes frequencies with age groups and gender was performed.
Ethical Statement
The research is approved in the Pathology department board meeting - no. 128482/40 /d; Date 28 / 12 / 1440 Hijra (30/8/2019). Approved by the Dean of the College of Medicine; King Abdulaziz University - Approval no.128482/40 /d / 9; Date 11/ 01/ 1441 Hijra (11/09/2019). Consent forms are signed by patients before the renal biopsy which includes agreement to use tissue biopsies and patient's data for education and research purposes. The procedures follow the guidelines laid down in Declaration of Helsinki (year 2013).
Statistics
The Statistical Package for the Social Sciences (SPSS) version 22 (IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp.) was used to analyze the data. Descriptive analysis (frequencies and percentages) were used to obtain variable distribution. Chi-square (X2) was used to obtain the relationship between study variables at a significant level of 0.05; in addition, Bonferroni method was used to investigate the differences between each group within the variable.
Data collection for 1ry glomerulonephritis in Saudi Arabia
Data regarding the frequencies of some major 1ry GN subtypes such as FSGSC, minimal change disease (MCD), immunoglobulin A nephropathy (IgAN), MGN, immunoglobulin M nephropathy (IgMN), membranoproliferative GN (MPGN), and postinfectious GN (PIGN) were extracted from original articles coming from Saudi Arabia over the past three decades. The purpose was to compare GN frequencies in the current study and GN frequencies from different parts of the country.
A total of 14 studies were retrieved, of which most of them were retrospective (n = 12) and two were prospective. Eight studies came from the central region, two from the western region, one from the eastern region, and three were multicenter studies from different regions of Saudi Arabia [Table 2].
Table 2.
Gender distribution of 2ry glomerulonephritis subtypes
| 2ry GN subtypes | Number | Ratio Male:female | |
|---|---|---|---|
| Male | Female | ||
| LN | 36 | 157 | 0.2:1 |
| DN | 12 | 15 | 0.8:1 |
| HTN | 6 | 3 | 2:1 |
| AS | 0 | 3 | 0:3 |
| CGN-AGBM | 1 | 0 | 1:0 |
| CGN-V | 5 | 2 | 2.5:1 |
| AM | 10 | 4 | 2.5:1 |
| CRYO | 2 | 1 | 2:1 |
| LCDD | 2 | 0 | 2:0 |
| MM | 3 | 0 | 3:0 |
| HSD | 1 | 0 | 1:0 |
| Total | 78 | 185 | 0.4:1 |
HTN: Hypertension nephropathy, DN: Diabetic nephropathy, AS: Alport syndrome, GN: Glomerulonephritis, CGN-AGBM: Crescentic GN-antiglomerular basement membrane, AM: Amyloid, CGN-V: Crescentic GN with vasculitis, CRYO: Cryoglobulinemia, LCDD: Light-chain deposition disease, HSD: Hemosiderosis, MM: Multiple myeloma, LN: Lupus nephritis
The data were then extracted from the publications for the total number of 1ry GN cases as well as the number of cases in each GN subtype and its percentage. The accumulated data from all studies were divided into two periods: period 1 (1983–2001) and period 2 (1998–2017), to align as much as possible with the present study two periods grouping.
The data are tabulated and pooled and recalculation of frequencies is made for some major 1ry GN subtypes on the pooled data, as shown in Table 3.
Table 3.
Pooled frequencies for some 1ry glomerulonephritis subtypes in Saudi Arabia
| 1ry GN subtype | Period, n (%) | |
|---|---|---|
| Period 1 (1983-2001) | Period 2 (1998-2017) | |
| FSGSC | 345 (29.0) | 322 (35.1) |
| MCD | 96 (8.1) | 90 (9.8) |
| IgAN | 108 (9.1) | 209 (22.8) |
| MGN | 127 (10.7) | 146 (15.9) |
| IgMN | 0 (0.0) | 4 (0.4) |
| MPGN | 183 (15.4) | 77 (8.4) |
| PIGN | 7 (0.6) | 13 (1.4) |
| Total | 1188 (100.0) | 918 (100.0) |
FSGSC: Focal segmental glomerulosclerosis, IgAN: Immunoglobulin A nephropathy, MPGN: Membranoproliferative GN, IgMN: Immunoglobulin M nephropathy, PIGN: Postinfectious glomerulonephritis, MCD: Minimal change disease, MGN: Membranous glomerulonephritis
Some publications (n = 4) are excluded from the pooled results for the following reasons:[9,10,18,19]
Incomplete IF and/or EM studies of the biopsy
Mixed-age groups pediatrics and adults
Data tabulated in percentages only
A study including some of the present study cases.
RESULTS
Primary glomerular disease frequencies
The total number for cases in period 2 is more than doubled (n = 322) compared with period 1 (n = 126).
The number of cases and percentages of each 1ry GN subtype is presented in Table 4. The changing ranks of 1ry GN subtypes in period 2 versus period 1 are demonstrated in Table 5.
Table 4.
The frequencies of the 1ry glomerulonephritis subtypes
| GN categories | GN subtypes | Date category | Total | P | |
|---|---|---|---|---|---|
| Period 1 (1988-1999), n (%) | Period 2 (2000-2014), n (%) | ||||
| Primary GN | FSGSC | 27 (21.4)a | 74 (23.0)a | 101 | 0.016 |
| MCD | 17 (13.5)a | 19 (5.9)b | 36 | ||
| IgAN | 28 (22.2)a | 63 (19.6)a | 91 | ||
| MGN | 30 (23.8)a | 78 (24.2)a | 108 | ||
| IgMN | 8 (6.3)a | 27 (8.4)a | 35 | ||
| MPGN | 15 (11.9)a | 29 (9.0)a | 44 | ||
| MPGN III | 0 (0.0)a | 1 (0.3)a | 1 | ||
| DDD | 1 (0.8)a | 0 (0.0)a | 1 | ||
| PIGN | 0 (0.0)a | 21 (6.5)b | 21 | ||
| C1qN | 0 (0.0)a | 1 (0.3)a | 1 | ||
| IgGN | 0 (0.0)a | 2 (0.6)a | 2 | ||
| FGN | 0 (0.0)a | 7 (2.2)a | 7 | ||
| Total (n) | 126 | 322 | 448 | ||
a, b=Groups with same letter no significant difference. GN: Glomerulonephritis, DDD: Dense deposit disease, C1qN: Complement 1q nephropathy, IgGN: Immunoglobulin G nephropathy, FGN: Fibrillary GN, FSGSC: Focal segmental glomerulosclerosis, IgAN: Immunoglobulin A nephropathy, MPGN: Membranoproliferative GN, IgMN: Immunoglobulin M nephropathy, PIGN: Postinfectious glomerulonephritis, MCD: Minimal change disease, MGN: Membranous glomerulonephritis
Table 5.
Change in frequencies of 1ry glomerulonephritis subtypes in years 1988-1999 versus 2000-2013
| Period 1 (1988-1999) (%) | Rank (GN subtype) | Rank change | Rank (GN subtype) | Period 2 (2000-2014) (%) |
|---|---|---|---|---|
| 23.8 | MGN |  |
MGN | 24.2 |
| 22.2 | IgAN |  |
FSGSC | 23.0 |
| 21.4 | FSGSC | IgAN | 19.6 | |
| 13.5 | MCD |  |
MPGN | 9.0 |
| 11.9 | MPGN | IgMN | 8.4 | |
| 6.3 | IgMN | PIGN | 6.5 | |
| 0 | PIGN |  |
MCD | 5.9 |
MGN: Membranous glomerulonephritis, FSGSC: Focal segmental glomerulosclerosis, IgAN: Immunoglobulin A nephropathy, MPGN: Membranoproliferative GN, IgMN: Immunoglobulin M nephropathy, PIGN: Postinfectious glomerulonephritis, MCD: Minimal change disease, GN: Glomerulonephritis
The total number for cases in period 1 is 126 and for period 2 is 322 cases.
Of all the 1ry GN subtypes, there has been a significant change in the case percentages of PIGN and MCD with P ≤ 0.05.
PIGN cases in period 2 have increased to 6.5% from 0% in period 1. On the contrary, MCD cases have dropped from 13.5% in period 1 to 5.9% in period 2.
The most common 1ry GN for both periods remains MGN with similar case percentages (23.8% in period 1and 24.2% in period 2). FSGSC has become the second most common subtype in period 2 (at 23%) instead of IgAN, which now takes the third place (at 19.6%). With the significant drop in the percent of MCD cases, it now assumes the last place instead of the fourth in period 1.
Secondary glomerular disease frequencies
The total number of 2ry GN cases in period 2 has almost quadrupled (n = 207) compared with period 1 (n = 56).
The number of cases and percentages of 2ry is shown in Table 6. There is no significant difference in the percentages of case between the two periods with P = 0.492 except for the lupus nephritis (LN) cases. LN remains the most common 2ry GN in the two periods with a noticeable rise in percentage from 66% in period 1 to 75.4% in period 2. Diabetic nephropathy (DN) follows as the second most common 2ry GN, slightly decreasing from 14% in period 1 to 9.2% in period 2. The percent of hypertensive nephropathy (HTN) cases has not changed (3.6% and 3.4%, respectively). Cases of crescentic GN with vasculitis (CGN-V) have slightly risen from 1.9% to 5.4% of cases.
Table 6.
The frequencies of the 2ry glomerulonephritis subtypes
| GN categories | GN subtypes | Date category | Total | P | |
|---|---|---|---|---|---|
| Period 1 (1988-1999), n (%) | Period 2 (2000-2014), n (%) | ||||
| Secondary GN | LN | 37 (66.1)a | 156 (75.4)b | 193 | 0.492 |
| DN | 8 (14.3)a | 19 (9.2)a | 27 | ||
| HTN | 2 (3.6)a | 7 (3.4)a | 9 | ||
| AS | 1 (1.8)a | 2 (1.0)a | 3 | ||
| CGN-AGBM | 0 (0.0)a | 1 (0.5)a | 1 | ||
| CGN-V | 3 (5.4)a | 4 (1.9)a | 7 | ||
| AM | 3 (5.4)a | 11 (5.3)a | 14 | ||
| CRYO | 2 (3.6)a | 1 (0.5)a | 3 | ||
| LCDD | 0 (0.0)a | 2 (1.0)a | 2 | ||
| MM | 0 (0.0)a | 3 (1.4)a | 3 | ||
| H.SDROSIS | 0 (0.0)a | 1 (0.5)a | 1 | ||
| Total (n) | 56 | 207 | 263 | ||
a, b=Groups with same letter no significant difference. AS: Alport syndrome, GN: Glomerulonephritis, CGN-AGBM: Crescentic GN-antiglomerular basement membrane, CRYO: Cryoglobulinemia, LCDD: Light-chain deposit disease, H.SDROSIS: Hemosiderosis, MM: Multiple myeloma, AM: Amyloid, LN: Lupus nephritis, HTN: Hypertension nephropathy, DN: Diabetic nephropathy, CGN-V: Crescentic GN with vasculitis
Relationship between glomerulonephritis subtypes and age
Most of the 1ry GN subtypes occur at a younger age (between 19 and 29 years) except for FSGSC and MGN which occur at an older age (30–39 years). These are shown in Graph 1.
Graph 1.

Relationship between 1ry GN subtypes and age
LN is diagnosed mostly in the younger age group of 19–29 years. The remaining 2ry GNs occur at an older age; DN, HTN, and CGN-V are diagnosed mostly at 50–59 years, multiple myeloma at >60 years, and amyloid at 40–49 years. These are shown in Graph 2.
Graph 2.

Relationship between 2ry GN subtypes and age
Gender distribution of glomerulonephritis subtypes
1ry GN is more common in males with total male: female ratio of 1.4:1 with the exception of dense deposit disease and fibrillary GN that appear more common in females [Table 7].
Table 7.
Gender distribution of 1ry glomerulonephritis subtypes
| 1ry GN subtypes | Number | Ratio Male:Female | |
|---|---|---|---|
| Male | Female | ||
| FSGSC | 61 | 40 | 1.5:1 |
| MCD | 23 | 13 | 1.8:1 |
| IgAN | 51 | 40 | 1.3:1 |
| MGN | 65 | 43 | 1.5:1 |
| IgMN | 18 | 17 | 1.1:1 |
| MPGN | 24 | 20 | 1.2:1 |
| MPGN III | 1 | 0 | 1:0 |
| DDD | 0 | 1 | 0:1 |
| PIGN | 11 | 10 | 1.1:1 |
| C1qN | 1 | 0 | 1:0 |
| IgGN | 2 | 0 | 2:0 |
| FGN | 1 | 6 | 0.2:1 |
| Total | 258 | 190 | 1.4:1 |
GN: Glomerulonephritis, DDD: Dense deposit disease, C1qN: Complement 1q nephropathy, IgGN: Immunoglobulin G nephropathy, FGN: Fibrillary GN, FSGSC: Focal segmental glomerulosclerosis, IgAN: Immunoglobulin A nephropathy, MPGN: Membranoproliferative GN, IgMN: Immunoglobulin M nephropathy, PIGN: Postinfectious glomerulonephritis, MCD: Minimal change disease, MGN: Membranous glomerulonephritis
The two most common 2ry GN subtypes (LN and DN) are female predominant, with a male: female ratio of 0.2:1 and 0.8:1, respectively. The remaining 2ry GN subtypes are male predominant, as shown in Table 2.
Total primary glomerular disease cases pooled from the previous Saudi Arabia studies
The details of the Saudi Arabia published data for 1ry GN subtypes are shown in Table 8, and Table 3 presents the pooled data of 1ry GN subtypes in Saudi Arabia for two time periods: period 1 (1983–2001) and period 2 (1998–2017).
Table 8.
Frequencies of some 1ry glomerulonephritis subtypes in Saudi Arabia
| Periods | Study; author | GN subtype, n (%) | Total cases number (1ry GN) | Remarks | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FSGSC | MCD | IgAN | MGN | IgMN | MPGN | PIGN | ||||
| 1998-2017 | AlFaadhel et al., 2019[6] | 213 (39.8) | 37 (6.9) | 156 (29.2) | 91 (17) | 38 (7.1) | 535 | Retrosp; Multic; W-C- | ||
| 2010-2012 | Al-Homrany et al., 2019[7] | 48 (14.2) | 19 (5.6) | 35 (10.3) | 39 (11.5) | 4 (1.2) | 30 (8.8) | 9 (2.7) | 209 | Prosp; Multic; E-W-C- |
| 2007-2016 | AlMatham et al., 2017[8] | 36 (35.3) | 27 (26.5) | 8 (7.8) | 13 (12.7) | 6 (5.9) | 4 (3.9) | 102 | Retrosp; Single c; C- | |
| 2005-2009 | Nawaz et al., 2013[9] | ×(27.6) | ×(17.7) | ×(11.5) | ×(9.9) | 192 | Retrosp; Single c; Ped. Adults; C- * | |||
| 2005-2010 | Saleemi et al., 2012[10] | 30 (23.8) | 17 (13.5) | 7 (5.6) | 14 (11.1) | 15 (11.9) | 4 (3.2) | 123 | Retrosp; Single c; no EM; C- * | |
| 1988-2005 | Jalalah 2009[18] | 63 (21.3) | 16 (5.4) | 52 (17.6) | 76 (25.7) | 23 (7.8) | 34 (11.5) | 6 (2) | 296 | Retrosp; Single c; W- * |
| 1998-2005 | Alkhunaizi[11] | 25 (35) | 7 (10) | 10 (14) | 3 (4) | 3 (4) | 72 | Retrosp; Single c; E- | ||
| 1990-1999 | Bernieh et al., 2000[19] | 13 (15.3) | 25 (29.4) | 13 (15.3) | 3 (3.5) | 7 (8.2) | 5 (5.9) | 70 | Retrosp; Single c; LM only; W- * | |
| 1999 | Mousa et al., 2000[12] | 15 (31) | 7 (14.3) | 4 (8.1) | 1 (2) | 34 | Prosp; Single c; C- | |||
| 1994-1999 | Mitwalli et al., 2000[13] | 44 (22) | 11 (5.5) | 13 (16.5) | 5 (2.5) | 20 (10) | 127 | Retrosp; Single c; C- | ||
| Before 2000 | Huraib et al., 2000[14] | 125 (21.3) | 68 (11.6) | 38 (6.5) | 62 (10.6) | 122 (20.7) | 612 | Retrosp; Multic; E-W-C- | ||
| 1990-2001 | Al Wakeel et al., 2004 [15] | 56 (47.6) | 13 (10.8) | 21 (17.5) | 20 (16.7) | 4 (3.3) | 4 (3.3) | 120 | Retrosp; Single c; C- | |
| 1989-1994 | Mitwalli et al., 1996[16] | 60 (32.3) | 2 (1.1) | 20 (10.8) | 20 (10.8) | 14 (7.5) | 147 | Retrosp; Single c; C- | ||
| 1983-1988 | Akhtar et al., 1990[17] | 45 (30.4) | 2 (1.3) | 9 (6) | 16 (10.8) | 22 (14.8) | 3 (2) | 148 | Retrosp; Single c; C- | |
*Removed from further analysis. Retrosp: Retrospective, Prosp: Prospective, Multic: Multicenter, Single C: Single center, W: Western region, E: Eastern region, C: Central region, Ped: Pediatrics, GN: Glomerulonephritis
FSGSC is the most common 1ry GN in Saudi Arabia for both periods 1 and 2. It accounts for 29% and 35% of the cases, respectively. IgAN cases have notably increased from 9.1% to 22.8% in period 2. MCD, MGN, IgMN, and PIGN showed a slight increase in the percentage of cases in period 2. On the contrary, MPGN cases have declined from 15.4% to 8.4%.
DISCUSSION
The current study reports results of adult renal biopsy-confirmed glomerular disease cases from our center database over the past three decades. Our institution is the largest tertiary care center for patients who are residing in the western region of Saudi Arabia. This study identified a significant shift in the frequencies of some GN subtypes over the past three decades (1988–2013).
The most prominent change is a marked increase in the frequency of PIGN from 0% of the 1ry GN cases in period 1 (1988–1999) to 6.5% in period 2 (2000–2013). No such marked elevation in PIGN frequency is identified in studies from Saudi Arabia.
The most prominent change in 1ry GN cases is the marked increase in PIGN from 0% in period 1 (1988–1999) to 6.5% in period 2 (2000–2013). No such marked elevation was identified in Saudi Arabia studies. Europe and the United States have reported increased PIGN cases in adults and the elderly.[20,21,22] In the past four decades, the change grew from 4% to 6% to 34% of the cases.[22]
The true incidence of PIGN in the world is difficult to estimate. It is underreported due to its transient nature that is shadowed by the clinical presentation.[23]
Scientists believed for the longest time that only streptococci caused PIGN. Now, we realized that the disease is caused by organisms other than Group A streptococci including other types of bacteria, mycobacteria, fungi, parasites, and viruses. Hence, the name changed to PIGN instead of poststreptococcal GN.[23]
The change in demographics has attributed to the increasing PIGN cases in Europe and the United States. Life expectancy improved, and the infectious agents, such as methicillin-resistant Staphylococcus aureus and Gram-negative bacteria, have evolved.[21,22]
Unlike other regions in Saudi Arabia, the western region, where population in this study resides, is known to receive mass gatherings of pilgrimages from around the world all year round to visit the holy mosques for Hajj and Umrah; this would be accompanied by higher risks of infections spread among the public.[24] Hence, this may have contributed to the elevation of PIGN in Western Saudi Arabia.
Another significant observation in the current study is the decreasing MCD cases from 13.5% in period 1 to 5.9% in period 2. Saudi Arabia studies varied in MCD reporting. Cases ranged from 1.1% to 29.4% [Table 8]. Similarly, worldwide studies showed MCD cases ranging from 0 to 39.5% of the cases over the past three decades.[25,26]
Malaysia reported MCD as the most common 1ry GN.[27,28] Korea also reported the same between 1970 and 1990, but that was followed by a decline in the later years of 1990–2011.[26,29,30] This variation could be attributed to the different renal biopsy policies or to environmental factors, such as allergens or pollution, that may influence the pathogenesis of MCD.
In the current study, MGN is the most common 1ry GN in both periods. In contrast to other studies from Saudi Arabia, they reported FSGSC as the most common 1ry GN [Graph 3].[6,7,8,9,10,11,12,13,14,15,16,17]
Graph 3.
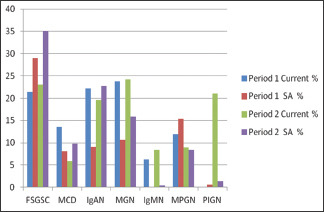
Comparison between the frequencies of 1ry GN subtypes in the current study and pooled data in Saudi Arabia
Studies from other parts of the world reported variable results. The United States and Brazil reported similar results to Saudi with FSGSC being the most common 1ry GN,[3,31] whereas IgAN is recorded as the most common 1ry GN in Australia, France, Italy, Japan, Singapore, and China.[1,2,4,32,33,34,35] This is also found in a systematic review of the world literature which concluded that IgAN is the most common 1ry GN followed by MGN then FSGSC.[36] Few studies from Pakistan and Japan reported similar results to the current study where MGN is the most common 1ry GN.[37,38] In the current study the frequency of FSGSC has slightly increased in period 2 to be 23% where it was 21.4% in period 2, and this is not a significant rise as noticed in other studies where FSGSC is particularly reported to increase.
The variations in 1ry GN worldwide can be associated with several factors: geographic location, socioeconomic status, ethnicity, and environmental factors such as infections and pollution. With the improvement in the socioeconomic status, we observe a decrease in IgAN, MGN, and PIGN which might be related to decreasing infectious antigens.[2] MGN may increase due to the rising environmental pollution from industrialization such as heavy metals and organic solvents.[39]
For 2ry GN subtypes, there is no difference between period 1 and period 2 case percentages. However, the total number of biopsies has increased in period 2. LN remains the most common 2ry GN in the two periods. On the contrary, DN frequency decreased in period 2 to become 9.2% from a frequency of 14.3% in period 1. Similarly, LN is reported as the most common 2ry GN in other studies from Saudi Arabia.[6,7,9,10,11,12,13,14,16,17]
Nowadays, we witness a dramatic increase in the number of renal biopsies for 1ry GN and 2ry GN. One can explain this with increasing population size and improving health care in the country. The primary care facilities have grown in number, and the referrals to tertiary care centers have become more efficient. This reflects on the increased life expectancy of the population to 75 years.[24]
CONCLUSIONS
The western region of Saudi Arabia presents with a different 1ry GN pattern than the rest of the country; somewhat that is likely attributed to its unique geographical and environmental characteristics. Fewer reports are published from this area which necessitates the development of a Saudi Renal Biopsy Registry as suggested by many other studies.[7,14] Al-Homrany published a plan for establishing a national registry which would unify policies and protocols for renal biopsy to ensure a better understanding of GN pathogenesis and proper patient management.[40] The number of renal biopsies is increasing in all centers across the country and the number of nephrologists and renal pathologists is increasing as well, and an important achievement would be the establishment of the Saudi Renal Biopsy Registry.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hou JH, Zhu HX, Zhou ML, Le WB, Zeng CH, Liang SS, et al. Changes in the spectrum of kidney diseases: An analysis of 40,759 biopsy-proven cases from 2003 to 2014 in China. Kidney Dis (Basel) 2018;4:10–9. doi: 10.1159/000484717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woo KT, Chan CM, Chin YM, Choong HL, Tan HK, Foo M, et al. Global evolutionary trend of the prevalence of primary glomerulonephritis over the past three decades. Nephron Clin Pract. 2010;116:c337–46. doi: 10.1159/000319594. [DOI] [PubMed] [Google Scholar]
- 3.O'Shaughnessy MM, Hogan SL, Poulton CJ, Falk RJ, Singh HK, Nickeleit V, et al. Temporal and demographic trends in glomerular disease epidemiology in the Southeastern United States, 1986-2015. Clin J Am Soc Nephrol. 2017;12:614–23. doi: 10.2215/CJN.10871016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaza G, Bernich P, Lupo A 'Triveneto' Register of Renal Biopsies (TVRRB) Renal biopsy in chronic kidney disease: Lessons from a large Italian registry. Am J Nephrol. 2013;37:255–63. doi: 10.1159/000348566. [DOI] [PubMed] [Google Scholar]
- 5.Dragovic D, Rosenstock JL, Wahl SJ, Panagopoulos G, DeVita MV, Michelis MF. Increasing incidence of focal segmental glomerulosclerosis and an examination of demographic patterns. Clin Nephrol. 2005;63:1–7. doi: 10.5414/cnp63001. [DOI] [PubMed] [Google Scholar]
- 6.AlFaadhel T, Alsuwaida A, Alsaad K, Almezaini L, Ahmed N, AlHamad MY, et al. Prevalence and 20-year epidemiological trends of glomerular diseases in the adult Saudi population: A multicenter study. Ann Saudi Med. 2019;39:155–61. doi: 10.5144/0256-4947.2019.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Homrany M, Alghamdi S, Al-Hwiesh A, Mousa D, Alwakeel J, Mitwalli A, et al. Pattern of renal diseases and the need for establishment of renal biopsy registry in Saudi Arabia. Saudi J Kidney Dis Transpl. 2019;30:628–33. doi: 10.4103/1319-2442.261335. [DOI] [PubMed] [Google Scholar]
- 8.AlMatham KI, AlFayez AF, AlHarthi RA, AlMutairi FS, Alrasheedi FS, Mustafa A, et al. Glomerulonephritis disease pattern at Saudi tertiary care center. Saudi Med J. 2017;38:1113–7. doi: 10.15537/smj.2017.11.21119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nawaz Z, Mushtaq F, Mousa D, Rehman E, Sulaiman M, Aslam N, et al. Pattern of glomerular disease in the Saudi population: A single-center, five-year retrospective study. Saudi J Kidney Dis Transpl. 2013;24:1265–70. doi: 10.4103/1319-2442.121288. [DOI] [PubMed] [Google Scholar]
- 10.Saleemi AN, Nabi Z, Ali S, Salloom A, El Nasri A, Albaqumi M. Spectrum of biopsy-proven glomerular disease in Al Qassim region: A single centre experience. Saudi J Kidney Dis Transpl. 2012;23:1084–7. doi: 10.4103/1319-2442.100963. [DOI] [PubMed] [Google Scholar]
- 11.Alkhunaizi AM. Pattern of renal pathology among renal biopsy specimens in Eastern Saudi Arabia. Saudi Med J. 2007;28:1676–81. [PubMed] [Google Scholar]
- 12.Mousa DH, Al-Hawas FA, Al-Sulaiman MH, Al-Khader AA. A prospective study of renal biopsies performed over one-year at the Riyadh armed forces hospital. Saudi J Kidney Dis Transpl. 2000;11:449–54. [PubMed] [Google Scholar]
- 13.Mitwalli AH, Al Wakeel J, Abu-Aisha H, Alam A, Al Sohaibani M, Tarif N, et al. Prevalence of glomerular diseases: King Khalid university hospital, Saudi Arabia. Saudi J Kidney Dis Transpl. 2000;11:442–8. [PubMed] [Google Scholar]
- 14.Huraib S, Al Khader A, Shaheen FA, Abu Aisha H, Souqiyyeh MZ, Al Mohana F, et al. The spectrum of glomerulonephritis in Saudi Arabia: The results of the Saudi registry. Saudi J Kidney Dis Transpl. 2000;11:434–41. [PubMed] [Google Scholar]
- 15.Al Wakeel JS, Mitwalli AH, Tarif N, Alam AA, Hammad D, Abu-Aisha H, et al. Spectrum and outcome of primary glomerulonephritis. Saudi J Kidney Dis Transpl. 2004;15:440–6. [PubMed] [Google Scholar]
- 16.Mitwalli AH, Al Wakeel JS, Al Mohaya SS, Malik HG, Abu-Aisha H, Hassan OS, et al. Pattern of glomerular disease in Saudi Arabia. Am J Kidney Dis. 1996;27:797–802. doi: 10.1016/s0272-6386(96)90516-8. [DOI] [PubMed] [Google Scholar]
- 17.Akhtar M, Qunibi W, Taher S, Ginn E, Furayh O, Sanjad S, et al. Spectrum of renal disease in Saudi Arabia? Ann Saudi Med. 1990;10:37–44. Doi: 10.5144/0256-4947.1990.37. [Google Scholar]
- 18.Jalalah SM. Patterns of primary glomerular diseases among adults in the western region of Saudi Arabia. Saudi J Kidney Dis Transpl. 2009;20:295–9. [PubMed] [Google Scholar]
- 19.Bernieh B, Sirwal IA, Abbadi MA, Ashfaquddin M, Mohammad AO. The spectrum of glomerulonephritis in adults in Madinah Munawarah region. Saudi J Kidney Dis Transpl. 2000;11:455–60. [PubMed] [Google Scholar]
- 20.Stratta P, Segoloni GP, Canavese C, Sandri L, Mazzucco G, Roccatello D, et al. Incidence of biopsy-proven primary glomerulonephritis in an Italian province. Am J Kidney Dis. 1996;27:631–9. doi: 10.1016/s0272-6386(96)90096-7. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Iturbe B, Musser JM. The current state of poststreptococcal glomerulonephritis. J Am Soc Nephrol. 2008;19:1855–64. doi: 10.1681/ASN.2008010092. [DOI] [PubMed] [Google Scholar]
- 22.Nasr SH, Markowitz GS, Stokes MB, Said SM, Valeri AM, D'Agati VD. Acute postinfectious glomerulonephritis in the modern era: Experience with 86 adults and review of the literature. Medicine (Baltimore) 2008;87:21–32. doi: 10.1097/md.0b013e318161b0fc. [DOI] [PubMed] [Google Scholar]
- 23.Kanjanabuch T, Kittikowit W, Eiam-Ong S. An update on acute postinfectious glomerulonephritis worldwide. Nat Rev Nephrol. 2009;5:259–69. doi: 10.1038/nrneph.2009.44. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Regional Office for the Eastern Mediterranean Saudi Arabia Health Profile World Health Organization. 2015 [Google Scholar]
- 25.Parichatikanond P, Chawanasuntorapoj R, Shayakul C, Choensuchon B, Vasuvattakul S, Vareesangthip K, et al. An analysis of 3,555 cases of renal biopsy in Thailand. J Med Assoc Thai. 2006;89(Suppl 2):S106–11. [PubMed] [Google Scholar]
- 26.Choi IJ, Jeong HJ, Han DS, Lee JS, Lee HY, Kim PK. An analysis of 2361 cases of renal biopsy in Korea. Yonsei Med J. 1991;32:9–15. doi: 10.3349/ymj.1991.32.1.9. [DOI] [PubMed] [Google Scholar]
- 27.Khoo JJ. Renal biopsies in Johor: A 7-year study. Malays J Pathol. 2001;23:101–4. [PubMed] [Google Scholar]
- 28.Bavanandan S, Kun LS. 5th Report of the The Malaysian Registry of Renal Biopsy Ch 2 Primary Glomerulonephritis. 2012 [Google Scholar]
- 29.Choi IJ, Jeong HJ, Han DS, Lee JS, Choi KH, Kang SW, et al. An analysis of 4,514 cases of renal biopsy in Korea. Yonsei Med J. 2001;42:247–54. doi: 10.3349/ymj.2001.42.2.247. [DOI] [PubMed] [Google Scholar]
- 30.Chang JH, Kim DK, Kim HW, Park SY, Yoo TH, Kim BS, et al. Changing prevalence of glomerular diseases in Korean adults: A review of 20 years of experience. Nephrol Dial Transplant. 2009;24:2406–10. doi: 10.1093/ndt/gfp091. [DOI] [PubMed] [Google Scholar]
- 31.Polito MG, de Moura LA, Kirsztajn GM. An overview on frequency of renal biopsy diagnosis in Brazil: Clinical and pathological patterns based on 9,617 native kidney biopsies. Nephrol Dial Transplant. 2010;25:490–6. doi: 10.1093/ndt/gfp355. [DOI] [PubMed] [Google Scholar]
- 32.Jegatheesan D, Nath K, Reyaldeen R, Sivasuthan G, John GT, Francis L, et al. Epidemiology of biopsy-proven glomerulonephritis in Queensland adults. Nephrology (Carlton) 2016;21:28–34. doi: 10.1111/nep.12559. [DOI] [PubMed] [Google Scholar]
- 33.Simon P, Ramee MP, Boulahrouz R, Stanescu C, Charasse C, Ang KS, et al. Epidemiologic data of primary glomerular diseases in western France. Kidney Int. 2004;66:905–8. doi: 10.1111/j.1523-1755.2004.00834.x. [DOI] [PubMed] [Google Scholar]
- 34.Sugiyama H, Yokoyama H, Sato H, Saito T, Kohda Y, Nishi S, et al. Japan Renal Biopsy Registry: The first nationwide, web-based, and prospective registry system of renal biopsies in Japan. Clin Exp Nephrol. 2011;15:493–503. doi: 10.1007/s10157-011-0430-4. [DOI] [PubMed] [Google Scholar]
- 35.Zhou FD, Zhao MH, Zou WZ, Liu G, Wang H. The changing spectrum of primary glomerular diseases within 15 years: A survey of 3331 patients in a single Chinese centre. Nephrol Dial Transplant. 2009;24:870–6. doi: 10.1093/ndt/gfn554. [DOI] [PubMed] [Google Scholar]
- 36.McGrogan A, Franssen CF, de Vries CS. The incidence of primary glomerulonephritis worldwide: A systematic review of the literature. Nephrol Dial Transplant. 2011;26:414–30. doi: 10.1093/ndt/gfq665. [DOI] [PubMed] [Google Scholar]
- 37.Hashmi AA, Hussain Z, Edhi MM, Mumtaz S, Faridi N, Khan M. Insight to changing morphologic patterns of glomerulopathy in adult Pakistani patients: An institutional perspective. BMC Res Notes. 2016;9:73. doi: 10.1186/s13104-016-1876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokoyama H, Sugiyama H, Narita I, Saito T, Yamagata K, Nishio S, et al. Outcomes of primary nephrotic syndrome in elderly Japanese: Retrospective analysis of the Japan Renal Biopsy Registry (J-RBR) Clin Exp Nephrol. 2015;19:496–505. doi: 10.1007/s10157-014-1022-x. [DOI] [PubMed] [Google Scholar]
- 39.Xu X, Wang G, Chen N, Lu T, Nie S, Xu G, et al. Long-Term Exposure to Air Pollution and Increased Risk of Membranous Nephropathy in China. J Am Soc Nephrol. 2016;27:3739–46. doi: 10.1681/ASN.2016010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Homrany M. Need for renal biopsy registry in Saudi Arabia. Saudi J Kidney Dis Transpl. 2008;19:346–9. [PubMed] [Google Scholar]


