Abstract
Attempts to develop a protective human immunodeficiency virus (HIV) vaccine have had limited success, especially in terms of inducing protective antibodies capable of neutralizing different viral strains. As HIV transmission occurs mainly via mucosal surfaces, HIV replicates significantly in the gastrointestinal tract, and the oral route of vaccination is a very convenient one to implement worldwide, we explored three SIV vaccine modalities administered orally and composed of simian immunodeficiency virus (SIV) DNA priming with different boosting immunogens, with the goal of evaluating whether they could provide lasting humoral and cellular responses, including at mucosal surfaces that are sites of HIV entry. Twenty-four Cynomolgus macaques (CyM) were primed with replication-incompetent SIV DNA provirus and divided into three groups for the following booster vaccinations, all administered in the oral cavity: Group 1 with recombinant SIV gp140 and Escherichia coli heat-labile toxin adjuvant dmLT, Group 2 with recombinant SIV-Oral Poliovirus (SIV-OPV), and Group 3 with recombinant SIV-modified vaccinia ankara (SIV-MVA). Cell-mediated responses were measured using blood, lymph node, rectal and vaginal mononuclear cells. Significant levels of systemic and mucosal T-cell responses against Gag and Env were observed in all groups. Some SIV-specific plasma IgG, rectal and salivary IgA antibodies were generated, mainly in animals that received SIV DNA + SIV-MVA, but no vaginal IgA was detected. Susceptibility to infection after SIVmac251 challenge was similar in vaccinated and nonvaccinated animals, but acute infection viremia levels were lower in the group that received SIV DNA + SIV-MVA. Nonvaccinated CyM maintained central memory and total CD4+ T-cell levels in the normal range during the 5 months of postinfection follow-up as did the vaccinated animals, precluding evaluation of vaccine impact on disease progression. We conclude that the oral cavity vaccination tested in these regimens can stimulate cell-mediated immunity systemically and mucosally, but humoral response stimulation was limited with the doses and the vaccine platforms used.
Keywords: AIDS, SIV vaccine, DNA vaccine, mucosal immunity
Introduction
Natural transmission of HIV and SIV occurs predominantly via mucosal surfaces. Systemic dissemination occurs usually within a few days and, at that point, the intestinal mucosa is a site of major virus replication and CD4+ T-cell depletion in addition to lymphoid organs.1–5 To control both entry and systemic dissemination, an effective HIV vaccine may need to stimulate both arms of the adaptive immune system, eliciting cellular and humoral immunity systemically as well as at mucosal surfaces.
Among mucosal routes, the oral route of vaccination is particularly appealing because of its ease of administration, an issue particularly important in settings with limited health care resources. A few oral vaccines are in use: the attenuated oral poliovirus vaccine (OPV), the most widely used, the typhoid, the rotavirus, and three cholera vaccines, one based on a live-attenuated organism, and live vaccines have been shown to be on average more immunogenic than vaccines based on killed pathogens.6–11 These vaccines are administered in the oral cavity, but they are swallowed and induce immunity in both the oral cavity and the gastrointestinal (GI) tract. They are safe, provide a high level of protection, and provide evidence for oral vaccination as an important approach to disease prevention, especially when a pathogen enters via mucosal surfaces as is the case in the vast majority of HIV transmissions.
It is unclear to what extent oral vaccination could protect in the context of HIV/SIV infection.12–14 Data from mucosal immunization in humans indicate that responses are maximal at the site of antigen exposure and present to a lesser degree at other mucosal sites as well, supporting the notion of limited compartmentalization of the mucosal immune system.15–17 Immunization at one mucosal site can lead to an immune response at other mucosal effector sites, as immunologically competent cells with homing receptors specific for mucosal sites circulate among different sites, but there are differences in the magnitude observed at different sites. The highest antibody (Ab) titers are usually achieved at the mucosal site of antigen exposure and decrease at distant sites.17–21 Although HIV replicates at significant levels in the intestinal mucosa,1,22–24 it enters the body predominantly via the genital tract or the rectum, likely requiring more disseminated immunity than pathogens that infect exclusively orally.12–14
OPV is one of the most successful oral vaccines currently used in the world.25,26 It is very safe—in extremely rare cases, ∼1 in every 2.7 million first doses of the vaccine, OPV can cause paralysis26- and using a HIV-recombinant OPV could provide a cheap way to simultaneously immunize against HIV and poliovirus. While live-attenuated OPV has been replaced by the less efficacious but safer, inactivated poliovirus vaccine (IPV) in most countries, it is still in use in many countries. Previous testing of recombinant SIV-OPV, using a mixture of 20 recombinants expressing small SIV fragments, indicated that excellent antibody responses can be obtained with this approach, and partial protection from infection and disease was also achieved in Cynomolgus macaques (CyM).27–29 Furthermore, pre-existing immunity to OPV did not prevent development of immunity to a recombinant protein subsequently delivered by OPV.30 One of the shortcomings of the SIV-OPV was the apparent limited stimulation of cell-mediated immunity, at least in CyM, but technical limitations in analysis of T-cell responses at the time of these experiments may have affected the evaluation.
In previous preclinical trials, we achieved significant systemic and mucosal T-cell responses with a SIV or SHIV DNA-rMVA approach after oral and intestinal immunizations.31–35 However, IgG and IgA responses were sporadic, short lived, and usually undetectable by the day of challenge. Differences in systemic and vaginal anti-SIV responses were observed in animals vaccinated orally vs. intestinally, and these immunizations provided some protection from infection and some from disease progression. Our candidate vaccine, given via oral routes, resulted in two significant outcomes: intestinal immunization provided significant protection from infection but no protection from disease progression; oral cavity immunization provided significant protection from AIDS with >50% of the animals controlling viremia to undetectable levels after a peak of viremia.35
We reasoned that it could be interesting to investigate whether a strategy that simultaneously stimulates immunity in the oral cavity and in the intestine could provide protection at the level of both infection and disease progression. The OPV is suited for this goal, as when given orally it infects cells both in the oral cavity and in the intestine, therefore we opted to investigate an approach based on the combination of recombinant OPV and DNA immunization, where the latter is known to be effective to induce cell-mediated immunity. This platform was compared with two platforms, SIV DNA/MVA and SIV DNA/protein, that we previously utilized via mucosal routes.31–35 The nonhuman primate CyM species was selected because, at the time of this study, poliovirus, which provides the vector used in this vaccine approach, was thought not to be able to infect Rhesus macaques (RM) orally.36–38
We compared the immunogenicity and protection of oral SIV DNA vaccine regimens boosted with either the gp140 SIV Env protein, a SIV-recombinant attenuated oral poliovirus (SIV-OPV) expressing SIV Gag and SIV Env, based on an attenuated OPV vector,39 or SIV Gag, Pol, Env rMVA. We found that the most immunogenic approach was the SIV DNA/MVA, while the recombinant SIV DNA/OPV did not achieve the expected humoral responses we had anticipated, although it did stimulate significant cell-mediated immunity.
Materials and Methods
Vaccine formulations, vaccination arms, and SIVmac251 low-dose vaginal challenge
Twenty-four female CyM, used in this study, were housed at Biomere Biomedical Research Models, Worcester, MA, according to an approved protocol under the guidelines established by the Animal Welfare Act and the National Institute of Health Guide for the Care and Use of Laboratory Animals, and were divided into three groups. One animal in Group 3 died of unrelated causes during the vaccine phase leaving 7 animals in this group. Each animal received a total of three DNA doses on day 1, week 8, and week 16 that consisted of 1 mg of pVacc7 DNA (Fig. 1A). The DNA plasmid pVacc7 used for priming is a derivative of pVacc6 in which SIVmac239 env was replaced by SIVsmE543 env and includes a full SIVmac239 genome with multiple mutations in the NC basic domain, in the functional domains of RT, INT, and PR, and a stop codon at the beginning of the vpr gene. Gene expression is under the control of the CMV promoter, replacing the 5′ LTR, while the 3′ is replaced by a polyadenylation signal. The DNA sequence was confirmed by sequencing, and the profile of viral particles produced was evaluated by 293T transfection and subsequent Western blot using macaque SIV-positive sera.35 DNA was formulated in 1 mL of 20 mM DOTAP (1,2-dioleoyl-3-trimethylammonium-propane, cholesterol (1:1)(Encapsula Nano Sciences).
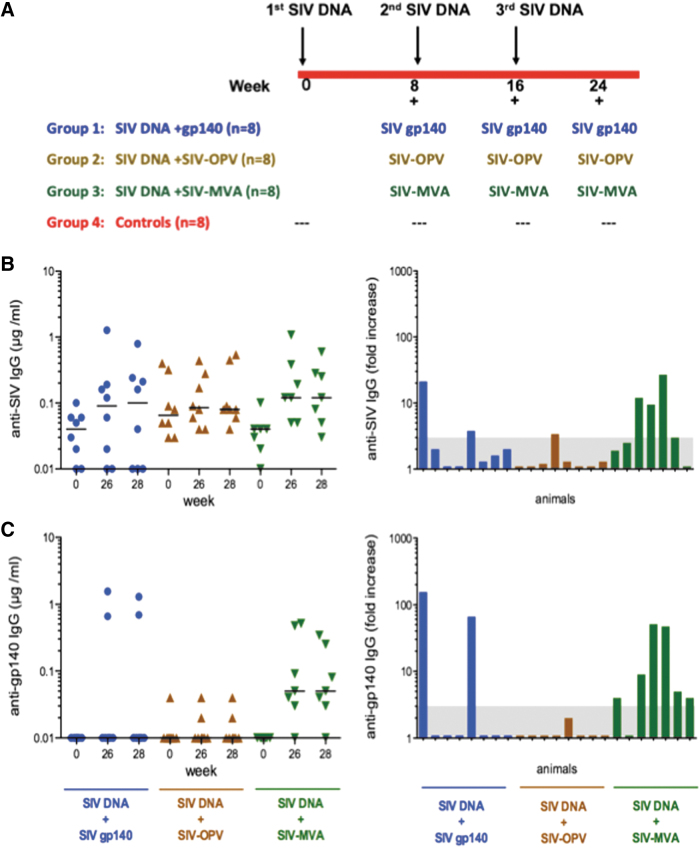
FIG. 1.
Study overview and SIV-specific systemic IgG responses in vaccinated animals. (A) Vaccination scheme and animal groups. Levels of antibodies to (B) SIV lysate and (C) SIV gp140 Env measured in plasma using ELISA. The left panels show the concentrations in individual animals before vaccination and at weeks 26 and 28 (corresponding to 2 and 4 weeks after the last immunization). Bars denote the median. Panels on the right show the fold increase over prevaccination level for each animal on week 26 when peak responses were typically observed. Postimmunization concentrations had to be threefold higher than the preimmune concentration to be considered significant.
On weeks 8, 16, and 24, Group 1 animals (n = 8) were immunized with 1 mg of SIV rgp140smE543 (a.a. 23–671; Genebank No. AAC56565; Immune Technology Corp, New York, NY) and 100 μg of E. coli double mutant heat-labile toxin (dmLT) adjuvant.40 Group 2 (n = 8) received 5 × 107 pfu SIV-OPV, and Group 3 (n = 8) received 5 × 108 pfu SIV gag,pol,env-expressing MVA (DR2 vector created by Dr. Bernie Moss, National Cancer Institute).41–44 SIV-OPV was constructed by inserting the sequence of the entire SIV gag (nucleotides 1309–2841 of SIVmac239) or SIV env (nucleotides 6860–9499)45 in the plasmid pSabin2-eGFP,39 replacing green fluorescent protein (gfp) between two poliovirus protease cleavage sites. SIV Gag and Env proteins are cleaved from the OPV polyprotein by the poliovirus protease and independently expressed intracellularly. The two corresponding viruses were mixed in equal amounts (2.5 × 107 each), and the mix was designated SIV-OPV. SIV-OPV and SIV-MVA recombinant vaccines were formulated in PBS in a final volume of 500 μL. Recombinant MVA and OPV doses were selected based on what is the optimal dose for each vector.
DNA and boosting vaccines were administered to the animals in the oral cavity, applied to the mucosa between the gum and the cheek while the animals were sedated. In the case of SIV-MVA, it was applied to the oral cavity mucosa by scarification, as normally done for poxvirus vaccination. Eight weeks after the last vaccination, vaccinated animals and controls were inoculated with a low dose (∼0.2 animal infectious dose50 (AID50) in a RM vaginal titration, corresponding to 100 TCID50) of the pathogenic SIVmac251 virus grown in RM PBMC (a gift from Dr. Nancy Miller and Dr. Ron Desrosiers, stock 2010 Day 8 SIVmac251), administered nontraumatically with a needleless tuberculin syringe as cell-free virus in the vagina.46 This virus stock is a highly diverse swarm of many different quasispecies of CCR5-tropic viruses.47 Challenge was repeated weekly until RT-PCR tests in two independent laboratories were positive for virus in plasma.
Specimens and sample processing
Blood and secretions were collected 2- and 4-weeks postvaccination, followed by monthly collection. Rectal, vaginal, and iliac lymph node tissues were biopsied on the day of the first vaccination and 2 weeks after each vaccination. Plasma and peripheral blood mononuclear cell (PBMCs) were isolated from ethylenediaminetetraacetic acid (EDTA) anti-coagulated whole blood according to the instructions of the manufacturer (Amersham Pharmacia, Uppsala, Sweden). Mononuclear cells (MNC) from lymph nodes and mucosal tissues were obtained from tissue biopsies. Briefly, after Telazol anesthesia, seven to eight biopsies/animal/time points were obtained from the rectum and vagina and lymph nodes using sterile forceps (for rectal and vaginal) and a small pinch biopsy device (Olympus endoscopic biopsy forceps). MNC from tissues were obtained with mechanical dissociation using GentleMACS dissociator (Miltenyi Biotech, Paris, France). Suspensions were passed through a 70 mm pore size cell-strainer and washed with 10% fetal bovine serum (FBS) in RPMI.48 PBMC and MNC are cryopreserved in FBS with 10% dimethyl sulfoxide (DMSO). Rectal, salivary and vaginal secretions were collected using Weck-Cel sponges (Beaver Visitec, Waltham, MA) premoistened with 50 μL of Dulbecco's phosphate-buffered saline as described previously.49
Evaluation of SIV-specific IgG in plasma samples and IgA in rectal and vaginal secretions
Antiviral and total IgA and IgG in secretions and IgG in plasma were measured by enzyme-linked immunosorbent assay (ELISA) as described.33,34,48,49 Briefly, for the SIV ELISAs, microtiter plates were coated overnight with 100 ng/well SIV rgp140smE543 (Immune Technology) or 100 μL per well of 1/400 aldrithiol-2-inactivated SIV particles (kindly provided by Dr. Jeff Lifson, Leidos Biomedical Research, Frederick, MD) that had been lysed with TritonX-100 detergent. Using monoclonal antibodies, the SIV lysate was found to contain gp41 but not gp120 at the 1/400 coating dilution used. For gp140 and SIV lysate assays, the standards were pooled serum or purified serum IgA from SIV-infected macaques, calibrated as previously described.32 Plates were developed using biotinylated goat antimonkey IgA or IgG (Rockland Immunochemicals, Gaithersburg, MD), neutravidin-labeled peroxidase and tetramethylbenzidene substrate (SouthenBiotech, Birmingham, AL). Before performing IgA assays, all samples were depleted of IgG using Protein G Sepharose as described because the goat antimonkey IgA was found to cross-react with monkey IgG.49 The concentration of anti-SIV IgA or IgG in each secretion was subsequently divided by the concentration of total IgA or IgG to obtain the specific activity (ng anti-SIV antibody per μg immunoglobulin). Specific activity or concentrations of SIV-specific IgG in plasma were considered significant if they were three-fold greater than those in pre-immune plasma.
IgG neutralization titers were measured as a function of Tat-induced luciferase reporter gene expression after single round of infection in either M7-Luc or TZM-bl cells.50 The viruses used in the neutralization assay were a TCLA stock of SIVmac251 (M7-Luc assay) and SIVmac239 (TZM-bl assay). Titers of SIV-specific neutralizing antibodies are the plasma dilution at which relative luminescence units were reduce 50% compared with virus control wells after subtraction of background.
Immunophenotyping and intracellular cytokine staining
105 MNC and 106 PBMCs were incubated for 14 h with medium and 1 μg/mL pools of 15-mer SIV Gag or SIV Env peptides. Cells incubated with 10 ng/mL PMA (4-α-phorbol 12-myristate 13-acetate; Sigma) and 1 μg/mL ionomycin (Sigma) or without any stimulation (unstimulated) provide respectively positive and negative controls. Cultures contained Brefeldin A (BD GolgiPlug Cat. # 555029; BD Biosciences) and 1 mg/mL of anti-CD49d and anti-CD28. PBMCs and MNC were washed, stained for surface markers in the dark, followed by fixation, permeabilization. Characterization of CD4+ and CD8+ T cells in PBMC was conducted according to previously published procedures.51 After the permeabilization, cells were intracellularly stained for cytokine expression with anticytokine antibodies for 1 h in the dark according to previously described procedures.52
The following antibodies were used in this study: anti-CD3-pacific blue/PerCp-Cy5.5 (clone SP34–2), anti-CD4-Amcyan (clone L200), anti-CD8-APC-Cy7 (clone RPA-T8), anti-TNFα-PE (clone MAb11), anti-IFNγ-Alexa Fluor-700 (clone B27), IL-2-APC (clone MQ1–17H12), anti-CD95-FITC (clone DX2), and anti-CD28-Pe-Cy5 (clone CD28.2). Gates for all antibodies were estimated in Fluorescence Minus One (FMO) staining samples. A viability dye (VIVID, LIVE/DEAD kit; Invitrogen) was added to the antibody cocktail to exclude dead cells. The acquisition of fluorescence data was done on LSRII flow cytometry using FACSDIVA software. The data were analyzed using FlowJO version 10.5.2 software (TreeStar, Ashland, OR). Data for peptide-stimulated populations are reported as percentage, determined after subtracting the percentage of positive cells detected in unstimulated cells for each sample. Evaluation of single, double, or triple-positive cells was carried out using FlowJo Boolean gate.
Viral load quantitation
Plasma SIV RNA levels were measured by real-time RT-PCR assay in Dr. Lifson's facility as described.53 The Lifson assay has a threshold sensitivity of 30 copy equivalents per milliliter. Interassay variation is <25% (coefficient of variation). Mean viral loads were calculated by transforming the number in its logarithmic value and averaging the logarithmic values of all the animals of the group at one specific time point.
Euthanasia
Animals were euthanized because of closure of the study, or earlier if they developed signs and symptoms consistent with the definition of AIDS. AIDS was defined as being SIV+ (detectable viremia) and experiencing one of the following criteria: 1—weight loss >15% in 2 weeks or >30% in 2 months; 2—documented opportunistic infection; 3—persistent anorexia >3 days without explicable cause; 4—severe, intractable diarrhea, 5—progressive neurologic signs, 6—significant cardiac and/or pulmonary signs, 7—loss of CD4+ T cells <200 or 10%.
Statistical analysis
Calculations and statistical analyses were performed using the GraphPad Prism version 7 software. Two-tailed Fisher's exact test was used to compare the frequency of IgA responses between groups. Between-group comparisons were carried out by two-tailed, t-test or Mann–Whitney test, and among four groups one-way ANOVA and Mann–Whitney test were used. Results of statistical analyses were considered significant if they produced p values ≤.05. Display of multicomponent distributions was performed with SPICE v5.2 (freely available from http://exon.niaid.nih.gov/spice/).54
Results
Vaccine data
Oral vaccination can stimulate systemic and mucosal anti-SIV responses
We reasoned that the combination of DNA, an excellent stimulator of T-cell responses, and SIV-OPV could provide a very interesting vaccine platform to explore and compare to what we accomplished using SIV DNA+ SIV-MVA via the oral route and with a vaccine composed of SIV DNA+ SIVgp140, which constitutes a more heavily explored approach to SIV and HIV immunization.
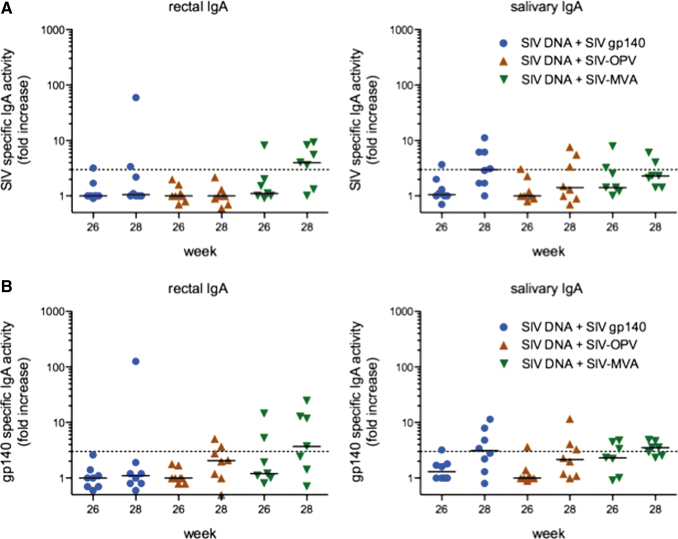
During the vaccination phase of the study, the systemic and mucosal antibody responses were measured in plasma and secretions. The oral vaccination elicited systemic IgG responses to SIV antigens in only two of eight Group 1 and one of eight Group 2 animals on weeks 26 and 28, corresponding to 2 and 4 weeks after the last vaccination (Fig. 1B, C). Plasma IgG antibodies against SIV lysate or gp140 Env were more frequently observed in Group 3 animals immunized with SIV DNA/rMVA (Fig. 1B, C). These IgG responses were negative in neutralization assays. A similar trend was observed for rectal IgA responses (Fig. 2A, B). The oral cavity vaccination stimulated rectal IgA responses to SIV antigens in only two Group 1 and Group 2 animals, whereas five of the seven animals in Group 3 had detectable anti-SIV or -gp140 Env IgA in rectal secretions. The same number of Group 3 animals also developed salivary IgA antibodies to SIV antigens, and these responses were detected in 50% of Group 1 animals that were orally boosted with SIV gp140 and dmLT adjuvant. However, only three of the eight Group 2 animals boosted with SIV-OPV had SIV-specific salivary IgA antibodies (Fig. 2A, B), and none of the animals in any group were found to have SIV-specific IgA in vaginal secretions (not shown). Thus, the SIV-OPV boost failed to stimulate the significant antibody responses we expected from an OPV-based approach.
FIG. 2.
Rectal and salivary IgA responses in vaccinated animals. Shown are the postimmunization fold increases in specific activity (ng of specific antibody per μg total IgA) to (A) SIV lysate and (B) SIV gp140 Env measured in rectal and salivary secretions. Fold increases were calculated by dividing the week 26 or 28 specific activity by that measured on week 0. To be considered significant, the specific activity had to exceed the dashed line (representing the mean + 3SD of negative controls) in the graphs. Bars represent the median for each group. SIV, simian immunodeficiency virus.
These results indicate that the oral cavity immunization is a suitable route to induce systemic, salivary, and rectal humoral responses using diverse approaches, but appropriate doses and formulations need to be systematically evaluated to identify those that more consistently generate antibodies.
Vaccine-induced systemic and mucosal SIV-specific CD4+ and CD8+ T-cell responses
As anti-SIV cell-mediated responses, in particular CD8+ T-cell responses, are critical in viremia control and long-term protection from disease progression, we evaluated the levels and breath of vaccine-induced cell-mediated immunity. Virus-specific T-cell responses were measured by intracellular staining and flow cytometric analysis at multiple time points, and are reported as percentage of antigen-specific CD4+ and CD8+ T-cells producing interferon gamma (IFNγ), interleukin-2 (IL-2), and tumor necrosis factor alpha (TNFα) in MNC after stimulation with SIV Gag or Env peptide. The analysis of the systemic SIV-specific immune responses, measured during immunization in PBMC and reported as the sum of anti-Gag and anti-Env percentages, revealed that all three vaccine modalities could stimulate significant anti-SIV cell-mediated responses in PBMCs and that, although consistently slightly higher in Group 3, these differences were not statistically significant (Fig. 3A).
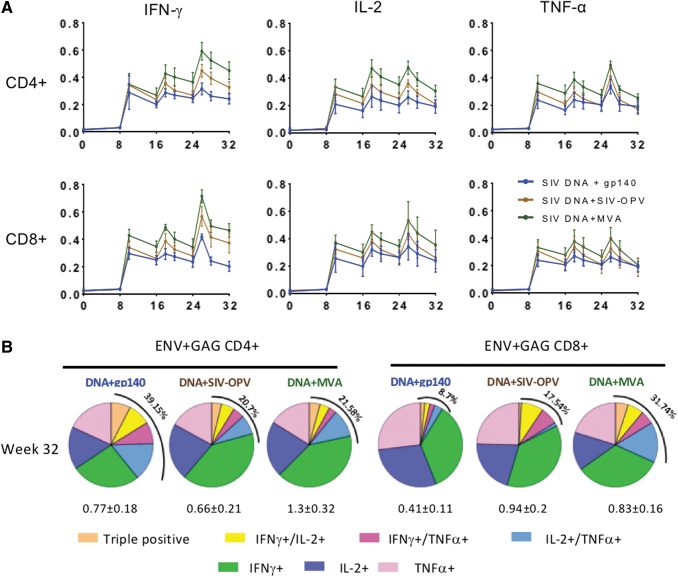
FIG. 3.
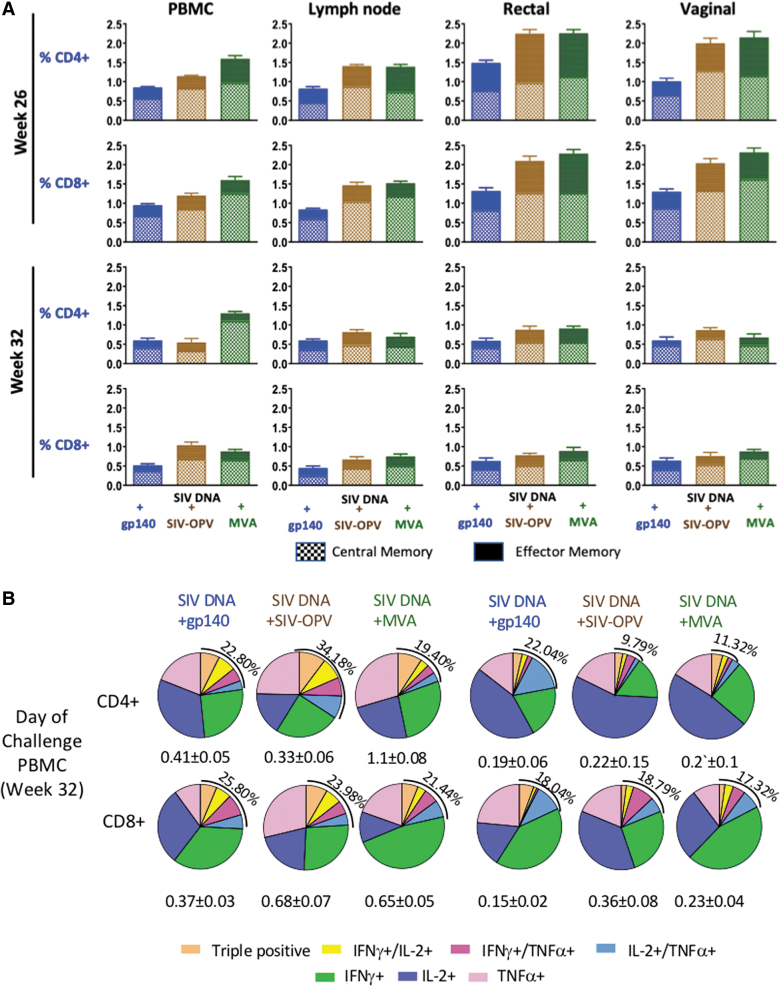
Circulating cell-mediated total percentages of SIV Gag and SIV Env-specific T-cell responses during immunization phase. (A) Geometric means of each vaccinated group is shown during the immunization phase as percentage of CD4+ T-cells and CD8+ T-cells producing IFNγ, TNFα, and IL-2, measured in PBMC by ICS upon stimulation with Gag or Env peptide pools. The graphs show the total SIV-specific T-cell responses (Gag plus Env) for vaccinated groups: SIV-DNA+SIV-gp140 vaccinated animals are represented in blue (Group 1), SIV-DNA+SIV-OPV in brown (Group 2), SIV-DNA+SIV-MVA in green (Group 3). Statistical significance between groups was tested with one-way ANOVA assay (p < .05). (B) Qualitative analysis of systemic SIV Gag and Env-specific cell-mediated responses for each vaccinated group 2 weeks on the first day of challenge (week 32), 8 weeks after last immunization. Pie graphs representing the diversity of CD4+ (left panels) and CD8+ T-cell responses (right panels) with different colors representing proportionally to percentages of the production of IFNγ, TNFα, and IL-2 alone and the simultaneous production of ≥2 cytokines combined are shown. The total mean percentage of SIV-specific multifunctional responses for each group is shown on the upper left side of each pie. ICS, intracellular cytokine staining; PBMC, peripheral blood mononuclear cell. IFNγ, interferon gamma; IL-2, interleukin 2; TNFα, tumor necrosis factor alpha.
We also evaluated long-term magnitude and functionality of these anti-SIV responses, measuring the fraction of SIV-specific CD4+ and CD8+ T cells producing TNFα, IFNγ, and IL-2 as single, double, or triple-positive cells, on week 32, 2 months after the last immunization, when the immune response has usually contracted to the memory level (Fig. 3B). On the day of challenge, Group 3 maintained levels of anti-SIV CD4+ T-cell immune responses ∼2-fold higher than the other groups; the same was true for anti-SIV CD8+ T-cell responses when both Groups 2 and 3 were compared with Group 1. We found that these responses were mainly monofunctional and predominantly made of cells producing IFNγ in all groups (Fig. 3B). However, variable percentages of multifunctional responses, varying between 8.7% and 38.15% of the total when both CD4+ and CD8+ T-cells were considered, were present in the different groups.
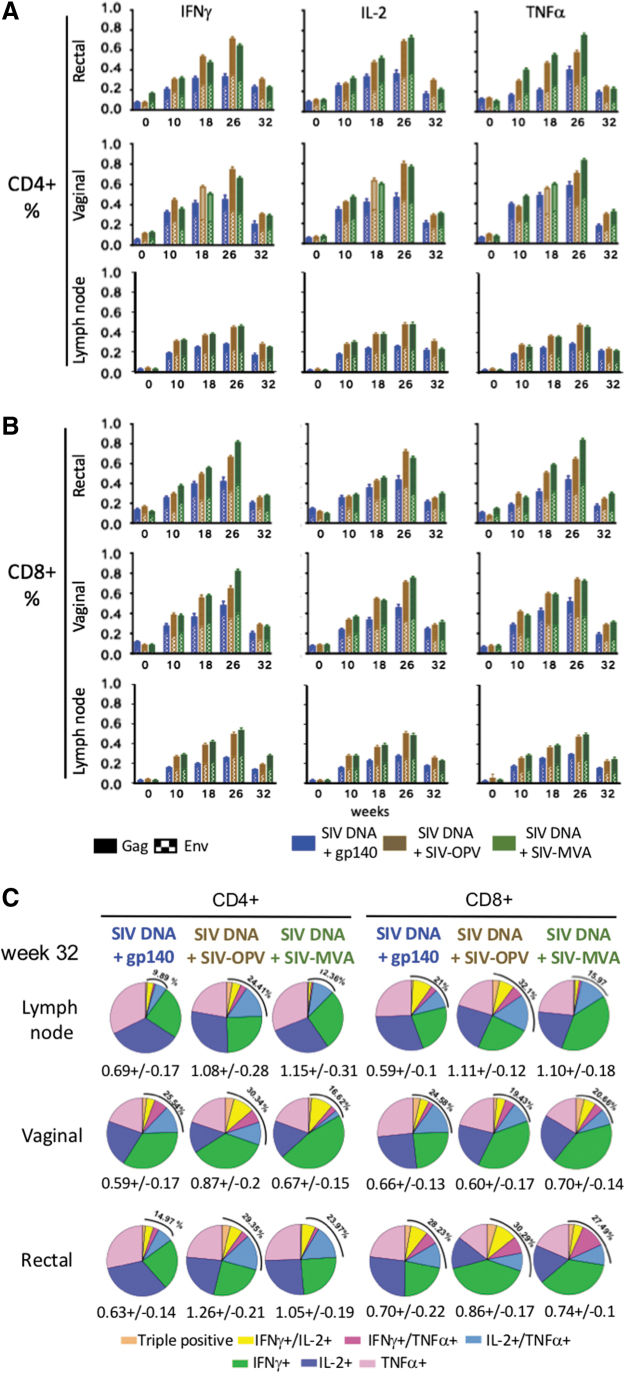
We evaluated the ability of the vaccine candidate to induce SIV-specific T-cell responses in tissues during the time course of the immunization regimen by investigating the percentages of SIV-specific mucosal MNC present in inguinal lymph nodes, rectal and vaginal biopsies, collected during immunization. Samples were collected at five time points, on day of first immunization, 2 weeks after second, third and fourth immunization, when the responses were likely to reach peak, and 8 weeks after the last immunization, when the effector component of the response is usually substantially reduced. Oral vaccine administration stimulated CD4+ and CD8+ T-cell responses at all examined sites with variable magnitude that increased over the course of the immunizations. When SIV-specific responses were analyzed in rectal, vaginal, and lymph node MNC on week 26, 2 weeks after the last immunization, significant levels of Gag and Env specific for CD4+ and CD8+ T-cell responses were observed in all vaccinated groups in all tissues (Fig. 4A). The differences in SIV-specific immune responses did not reach significance when compared between the groups, although rectal and vaginal responses were consistently higher than those in lymph node MNC. On week 32, 8 weeks after last immunization, the responses had contracted to a frequency varying from ∼0.2% to 0.4% for the individual cytokines with T cells producing IL-2, or IFNγ or TNFα similarly represented in SIV-specific rectal and vaginal CD4+ and CD8+ T cells and values varying between 0.6% and 1.2% (Fig. 4A, B).
FIG. 4.
Tissue SIV-specific cell-mediated T-cell responses during immunization. SIV Gag+Env-specific (A) CD4+ and (B) CD8+ T-cell responses were detected in MNC isolated from vaginal and rectal and lymph node biopsies during the immunization protocol, and the group average is represented as a bar graph for each group. Each bar represents the sum of Gag- and Env-specific T-cells producing IFNγ, IL-2, and TNFα evaluated as individual cytokines after stimulation with Gag or Env peptide pools. (C) Functionality of lymph node, vaginal and rectal SIV-specific T-cell responses on the first day of challenge (week 32). Pie graphs illustrate the diversity of Gag+Env-specific CD4+ T-cells and CD8+ T-cells producing IFNγ, TNFα, and IL-2 and colors are proportionate to the percentages of single, double, or triple positives for each group. The SIV-specific T-cell mean percentage of the total CD4+ or CD8+ T cells is shown for each group beneath each pie. The total mean percentage of SIV-specific multifunctional responses for each group is shown on the upper left side of each pie. MNC, mononuclear cell.
When multifunctionality of antigen-specific responses was investigated with Boolean analysis on the same time point samples, a large fraction of the response, ranging from 70% to 90% of the total, was characterized by monofunctional cells producing each of the three cytokines tested. However, polyfunctional responses were detectable in all tissues with frequency varying from ∼10% to 32% of the total (Fig. 4C).
Analysis of SIV-specific CD4+ and CD8+ T-cell responses memory and effector subsets
We compared the central memory (CM) and effector memory (EM) subset fractions of the cell-mediated response in blood and tissue MNC at peak after the last immunization (week 26) and 2 months after the last immunization (week 32). On week 26, CM or EM varied within a twofold range among the three immunization regimens, both being consistently lower in Group 1 that received boosting immunizations not including a SIV-Gag antigen but only SIV Env (Fig. 5A). However, differences between groups did not reach statistical significance, probably because of the small size of the groups and of the variability in the magnitude of the responses among animals within the same groups. On week 32, the SIV responses were also of comparable magnitude in all three groups, although reduced compared with week 26 peak responses (Fig. 5A). When the distribution between the CM and EM CD4+ and CD8+ T cells was evaluated for each animal within each vaccination group, the average group CM/EM ratio of SIV-specific cells present in tissue compartments on week 32 was higher compared with week 26 for most comparisons (p < .05), supporting a larger contraction of the EM than the CM immune response since its peak and the persistence of significant CM antigen-specific responses.
FIG. 5.
Central memory (CD95+/CD28+) and effector memory (CD95+/CD28−) SIV-specific cell-mediated T-cell responses during the immunization protocol. (A) SIV Gag+Env-specific CD4+ and CD8+ T-cell responses were detected in PBMC and in MNC isolated from lymph node, vaginal and rectal biopsies 2 weeks after last immunization (week 26) and on first day of challenge (week 32), and the group average is represented as a bar graph for each group. Each bar represents the sum of anti-CM and EM Gag + Env-specific T-cells producing IFNγ, IL-2, and TNFα evaluated as individual cytokines. CD3+ T cells were gated within total PMBC, CD4+ and CD8+ T cells were gated within the CD3+ population, and percentages of cytokine+ cells were estimated in these populations for each cytokine and added to provide the total. (B) Functionality of PBMC SIV-specific T-cell responses on first day of challenge (week 32). Pie graphs illustrate the diversity of Gagmen-specific CD4+ T-cells and CD8+ T-cells producing IFNγ, TNFα, and IL-2 as percentages of single, double, or triple positives for each group. The SIV-specific T-cell mean percentage of the total CD4+ or CD8+ T cells is shown for each group beneath each pie.
When the fraction of antigen-specific responses producing the same cytokine alone or in combination was compared with each of the other two cytokines in CM and EM of SIV-specific CD4+ and CD8+ T cells, IL-2 was the predominantly expressed cytokine in CD4+/EM+ and CD8+/EM T cells of Group 2 (Fig. 5B, p < .05) and the multifunctional CM CD4+ T-cell SIV Gag+Env-specific responses were a significantly larger percentage than that of the EM CD4+ fraction (p < .05). The other two groups had similar levels of CM and EM antigen-specific multifunctional responses, supporting a more prolonged survival of EM responses in these groups and in CD8+ CM and EM responses the largest fraction of antigen-specific cells secreted IFNγ (p < .05).
When all the above data are considered together, we concluded that all vaccine modalities given in the oral cavity were similarly effective in stimulating cell-mediated T-cell responses at multiple sites, including sites of HIV exposure, but much less effective at stimulating humoral responses. The vaccination utilizing the SIV DNA-MVA modality produced more consistent humoral results, although some low-level responses were observed in a couple of animals of the other two groups as well. These data indicate that the oral cavity can be explored as a site to stimulate broadly distributed responses but higher doses of vaccine, more immunizations, or different formulations might be necessary.
Resistance to challenge and disease progression
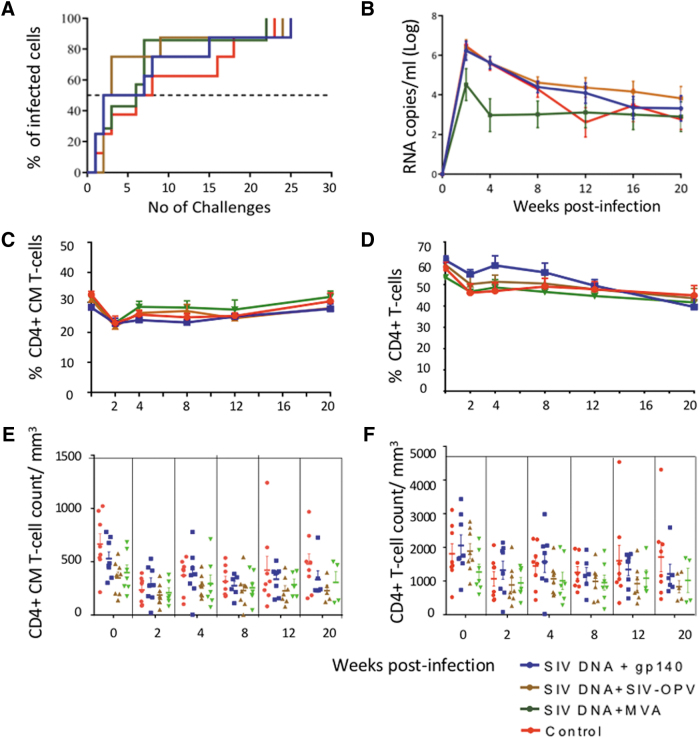
To evaluate levels of protection provided by the vaccination, animals were vaginally challenged with repeated low doses of SIVmac251 (Fig. 6). No significant differences were observed when the median number of challenges was compared among groups, indicating that the levels of immune responses present in the vaginal tissues were not sufficient to provide protection (Fig. 6A). When viral loads were evaluated, early control of viremia from peak levels to week 8 was observed in Group 3 compared with other groups. However, after that, all three groups equally controlled viremia (Fig. 6B). The level of viremia control observed in the control group was higher than that previously observed in infected RM32,33,35 and viremia was at least half log lower than what is normally observed for CyM and ∼2 logs of what was observed in another study where SIV-OPV was investigated in CyM.29,55,56
FIG. 6.
Low-dose vaginal SIVmac251 challenge outcome. (A) The Kaplan–Meier graph represents the number of nontraumatic challenges received for each vaccinated group until a positive infection was detected in plasma. The nontraumatic challenges were stopped after 26 challenges when all the animals had become positive. The dotted line intersects the point in each curve corresponding to 50% of the animals being infected. (B) Average viral loads in the groups. (C) PBMC CM (CD95+/CD28+) CD4+ T-cell percentages postinfection. (D) PBMC total CD4+ T-cell percentages postinfection. (E, F) Absolute number of PBMCs CM (E) and total78 CD4+ T-cells during the course of the infection.
CM CD4+ T-cell counts, which usually decline earlier after infection than total CD4+T-cell counts in RM, recovered after the declined observed 2 week postinfection to levels comparable with those preinfection and remained stable during the postinfection follow-up in all groups (Fig. 6C, group average, Fig. 6E, individual absolute counts/mm3). Total CD4+ T-cell counts declined ∼10% 2 weeks after infection in all groups and remained at that level for the remaining of the follow-up (Fig. 6D, group percentage averages, Fig. 6F, individual absolute counts/mm3, p = ns when time 0 values were compared with those of week 20 postinfection), indicating that the levels of viremia were compatible with preservation of these cells for the length of the follow-up in vaccinated group and controls. Although a milder course of SIV infection in CyM versus RM has been reported, preservation of CD4+ T cells occurred in these animals at levels that were not statistically different than when noninfected, unlike what was observed by others in SIV-infected CyM, where significant decrease to <50% of preinfection value in both CM and total CD4+ T cells has been observed [Fig. 2 in55]. The unusual resistance of these control animals to SIV-mediated CD4+ T-cell decline reduced the ability to reveal any level protection from the vaccination, if any had been induced.
Discussion
As of today, only one vaccine modality tested in clinical trials and administered intramuscularly (i.m.) achieved partial protection (31.2% efficacy), the RV-144 ALVAC-HIV (v CP1521) plus AIDSVAX,57 not only supporting the feasibility of achieving protection but also requiring further improvement. However, the recent trial HVTN702 in South Africa, based on the same modality of vaccination, was terminated because of lack of efficacy.58 This trial utilized MF59 instead of Alum, and the difference in outcome was predicted in a preclinical SIVmac251 trial in RM that compared side by side the two adjuvants, supporting the different contributions of each vaccine adjuvant to the vaccine outcome and the need to test each variable independently.59 Achievement of appropriate antibody responses, in particular neutralizing antibody, is considered the holy grail of the successful HIV vaccine [60–62 and references therein].
In previous studies in RM, we have shown that mucosal immunization stimulating cell-mediated immune responses can achieve protection from disease progression but did not protect from acquisition of infection, although in some cases infection was delayed.32–34,63 Intranasal vaccination was more efficient in eliciting cellular and humoral virus-specific responses at mucosal sites than the same regime administered systemically (i.m.) and provided better protection from disease progression after rectal or vaginal challenge. SIV-specific CD4+ and CD8+ IFNγ producing T cells present at the time of challenge correlated with the subsequent control of the viremia and longer survival of these animals. However, it did not stimulate significant humoral responses in the circulation or vaginal mucosa. In a recent study, where HIV-MVA followed by recombinant trimeric HIV gp120 with dmLT was administered with a pressurized, needle-free injection devise both buccally and sublingually in RM, Jones et al. found that this immunization strategy provided an effective route to induce immunity and partial protection against rectal SHIV challenge.64
The study reported here was initiated with the goal of using a vector based on OPV, a virus known to be excellent at stimulating long-lived antibody responses and in particular mucosal IgA, to improve the stimulation of humoral immunity and the oral cavity route as easily accessible and practical in resource-limited settings. The importance of mucosal IgA responses is highlighted in humans by the detection of HIV-specific IgA in semen or vaginal secretions of some cohorts of HIV-1 resistant, heavily exposed but seronegative sex workers, which has been interpreted as indication that local IgA, induced by viral exposure, can protect during subsequent exposures.65–68 Secretory IgA antibodies have been shown to inhibit host entry and dissemination of pathogens into systemic compartment, and this mechanism, combined with others, such as antibody-dependent cellular cytotoxicity (ADCC) by FcαR+ tissue macrophages, could work for HIV as well.69–71
An additional modality capable of inducing significant antibodies is the use of a purified protein as priming and/or boosting tool of antipathogen responses. Comparative studies in humans and NHP that utilized protein immunogens efficiently internalized at all mucosal surfaces established that local immunization stimulates mucosal IgA responses often limited to the vaccination site or of less magnitude at more distant sites (reviewed in72). These differential antibody responses may be less apparent with replicating vaccines. For example, a replication-competent adenovirus type 5 vector with SIV genes was recently reported to induce SIV-specific mucosal IgA responses in multiple mucosal tissues regardless of whether it was administered to female macaques by the sublingual, rectal, vaginal, or nasal + intratracheal mucosal routes.73 The protein dose used here did not provide satisfactory results and may have been too low, as uptake of topically applied proteins in the oral cavity is inefficient. Antigens formulated in conjunction with adjuvants, as we did in this immunization, avoid inducing tolerance and failure to stimulate an immune response is dose- or antigen dependent.74–76 Achieving immune responses with oral protein immunization may require testing multiple doses, formulations, and methods of application to find the dose high enough to induce the optimal response.
The approaches used here and compared with the DNA/MVA platform previously used did not consistently stimulate the desired humoral responses but were able to stimulate cell-mediated responses. As the last boost did not include the SIV DNA component and managed to increase the levels of cell-mediated responses, it provided the evidence that these different boosting modalities were immunogenic on their own. In the case of the gp140 boost, increasing the amount of protein, and perhaps adjuvant, could work in a more significant and consistent way. In the case of the SIV-OPV vectors, a few issues could have affected the outcome. The vaccination was done with sedated animals that were not swallowing, possibly limiting the spreading of it to the entire gastrointestinal tract. Alternatively, the recombinant SIV-OPV stability in vivo may have been more limited than what was observed in vitro, with reduced in vivo viral replication, and the possibility that a higher dose could have provided better results. The OPV recombinant previously used carried much smaller inserts than those employed here, and that feature may have favored better in vivo replication.27–29
Despite these shortcomings, this study shows that the oral cavity can be considered a useful route of vaccination, as all platforms achieved significant levels of cell-mediated immunity and sporadic levels of humoral immunity, leaving open the possibility of improvement for the latter if more doses, formulations, and schedules are evaluated. Additional studies should aim at improving vaccine platforms used via this route, as it is highly amenable to its employment in resource-limited environments. An unexpected result at the time of challenge was the viremia control observed in the naive animals, being better than that normally observed in CyM. This species was selected because previous results indicated that RM could not be infected orally with OPV. No significant decline of CM CD4+ T cells or total CD4+ T cells was observed during the 5 months after infection. This occurrence prevented the evaluation of the vaccination on the preservation of the immune system. In addition to species-specific issues, it is possible that the virus stock used in this study may not have had the same virulence as other related similar stocks. As RMs have been recently shown to be infectable orally77 and postinfection data in these species have been more consistent in our hands, future experiments in this species with additional recombinant SIV- and SHIV-OPV will be pursued.
Acknowledgments
We thank Dr. David Montefiori (Duke University, Durham, NC) for carrying out the neutralization assay on plasma samples, Dr. Jeff Lifson (Leidos Biomedical Research, Inc., Frederick National Laboratory, Frederick, MD) for evaluating plasma SIV viral loads, Robert L. Wilson for analyses of antibody responses, and Olga Nichols for production of the SIV-MVA in the LSU Health Sciences Vector Core Facility.
Authors' Contribution
Omkar Chaudhary obtained PBMC and MNC from blood and tissues, carried out analysis of immune response in samples, prepared the first partial draft of the article and graphs for the figures reporting the cell-mediated immune responses, contributed to some statistical analysis, and revised final draft of the article.
Lingyun Wang obtained PBMC and MNC from blood and tissues, carried out qualitative evaluation of SIV plasma viral loads weekly after challenge, prepared graphs for some figures, contributed to some statistical analysis, and revised final draft of the article.
Deepanwita Bose obtained PBMC and MNC from blood and tissues, carried out analysis of immune responses in a subset of samples and evaluated CD4+ T cell counts, prepared graphs for some figures, contributed to some statistical analysis, and revised final draft of the article.
Vivek Narayan constructed and tested SIV-OPV recombinants, and revised final draft of the article.
Ming Te Yeh provided the pSabin2-eGFP vector, advised all technical aspects of oral poliovirus vaccine work and titrated the SIV-OPV virus stocks, revised final draft of the article.
Angela Carville supervised vaccinations, sampling and animal care at the Biomere facility, revised final draft of the article.
John D. Clements provided the double mutant heat-labile toxin (dmLT) adjuvant, critically contributed to the interpretation of the technical and intellectual content and to the editing of the article.
Raul Andino provided the OPV vector, supervised Dr. Yeh's work, and critically revised final version of the article.
Pamela A. Kozlowski provided the SIV-MVA, supervised evaluation of antibody responses, composed the corresponding figures, critically contributed to the interpretation of the technical and intellectual content and to the editing of the article.
Anna Aldovini designed the experiments, secured funding, coordinated, supervised, and troubleshooted all aspects of the execution, interpreted the data, wrote the article, and edited the figures.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by grant NIH-R01DE026325 to AA.
References
- 1. Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M: Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 2005;434:1093–1097 [DOI] [PubMed] [Google Scholar]
- 2. Veazey RS, DeMaria M, Chalifoux LV, et al. : Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 1998;280:427–431 [DOI] [PubMed] [Google Scholar]
- 3. Barratt-Boyes SM, Soloff AC, Gao W, et al. : Broad cellular immunity with robust memory responses to simian immunodeficiency virus following serial vaccination with adenovirus 5- and 35-based vectors. J Gen Virol 2006;87(Pt 1):139–149 [DOI] [PubMed] [Google Scholar]
- 4. Gordon SN, Cervasi B, Odorizzi P, et al. : Disruption of intestinal CD4+ T cell homeostasis is a key marker of systemic CD4+ T cell activation in HIV-infected individuals. J Immunol 2010;185:5169–5179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belyakov IM, Berzofsky JA: Immunobiology of mucosal HIV infection and the basis for development of a new generation of mucosal AIDS vaccines. Immunity 2004;20:247–253 [DOI] [PubMed] [Google Scholar]
- 6. Cryz SJ Jr., Que JU, Levine MM, Wiedermann G, Kollaritsch H: Safety and immunogenicity of a live oral bivalent typhoid fever (Salmonella typhi Ty21a)-cholera (Vibrio cholerae CVD 103-HgR) vaccine in healthy adults. Infect Immun 1995;63:1336–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guzman CA, Borsutzky S, Griot-Wenk M, et al. : Vaccines against typhoid fever. Vaccine 2006;24:3804–3811 [DOI] [PubMed] [Google Scholar]
- 8. O'Ryan M: Rotarix (RIX4414): An oral human rotavirus vaccine. Expert Rev Vaccines 2007;6:11–19 [DOI] [PubMed] [Google Scholar]
- 9. Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. : Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006;354:11–22 [DOI] [PubMed] [Google Scholar]
- 10. Ryan ET, Calderwood SB, Qadri F: Live attenuated oral cholera vaccines. Expert Rev Vaccines 2006;5:483–494 [DOI] [PubMed] [Google Scholar]
- 11. Taylor DN, Tacket CO, Losonsky G, et al. : Evaluation of a bivalent (CVD 103-HgR/CVD 111) live oral cholera vaccine in adult volunteers from the United States and Peru. Infect Immun 1997;65:3852–3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aldovini A: Mucosal Vaccination for Prevention of HIV Infection and AIDS. Curr HIV Res 2016;14:247–259 [DOI] [PubMed] [Google Scholar]
- 13. Kozlowski PA, Aldovini A: Mucosal Vaccine Approaches for Prevention of HIV and SIV Transmission. Curr Immunol Rev 2019;15:102–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu H, Wang X, Veazey RS: Mucosal immunology of HIV infection. Immunol Rev 2013;254:10–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kantele A, Hakkinen M, Moldoveanu Z, et al. : Differences in immune responses induced by oral and rectal immunizations with Salmonella typhi Ty21a: Evidence for compartmentalization within the common mucosal immune system in humans. Infect Immun 1998;66:5630–5635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kozlowski PA, Cu-Uvin S, Neutra MR, Flanigan TP: Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect Immun 1997;65:1387–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kozlowski PA, Williams SB, Lynch RM, et al. : Differential induction of mucosal and systemic antibody responses in women after nasal, rectal, or vaginal immunization: Influence of the menstrual cycle. J Immunol 2002;169:566–574 [DOI] [PubMed] [Google Scholar]
- 18. Johansson EL, Wassen L, Holmgren J, Jertborn M, Rudin A: Nasal and vaginal vaccinations have differential effects on antibody responses in vaginal and cervical secretions in humans. Infect Immun 2001;69:7481–7486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rudin A, Johansson EL, Bergquist C, Holmgren J: Differential kinetics and distribution of antibodies in serum and nasal and vaginal secretions after nasal and oral vaccination of humans. Infect Immun 1998;66:3390–3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rudin A, Riise GC, Holmgren J: Antibody responses in the lower respiratory tract and male urogenital tract in humans after nasal and oral vaccination with cholera toxin B subunit. Infect Immun 1999;67:2884–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Russell MW, Moldoveanu Z, White PL, Sibert GJ, Mestecky J, Michalek SM: Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the Cholera toxin B subunit. Infect Immun 1996;64:1272–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Asiedu CK, Goodwin KJ, Balgansuren G, et al. : Elevated T Regulatory Cells in Long-Term Stable Transplant Tolerance in Rhesus Macaques Induced by Anti-CD3 Immunotoxin and Deoxyspergualin. J Immunol 2005;175:8060–8068 [DOI] [PubMed] [Google Scholar]
- 23. Brenchley JM, Schacker TW, Ruff LE, et al. : CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med 2004;200:749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mehandru S, Poles MA, Tenner-Racz K, et al. : Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med 2004;200:761–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kew OM, Sutter RW, de Gourville EM, Dowdle WR, Pallansch MA: Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annu Rev Microbiol 2005;59:587–635 [DOI] [PubMed] [Google Scholar]
- 26. Global Polio Eradication Initiative, World Health Organization, 1211 Geneva, Switzerland. Available at www.polioeradication.org/Polioandprevention/Thevaccines/Oralpoliovaccine(OPV).aspx accessed May2020
- 27. Crotty S, Andino R: Poliovirus vaccine strains as mucosal vaccine vectors and their potential use to develop an AIDS vaccine. Adv Drug Deliv Rev 2004;56:835–852 [DOI] [PubMed] [Google Scholar]
- 28. Crotty S, Lohman BL, Lu FX, Tang S, Miller CJ, Andino R: Mucosal immunization of cynomolgus macaques with two serotypes of live poliovirus vectors expressing simian immunodeficiency virus antigens: Stimulation of humoral, mucosal, and cellular immunity. J Virol 1999;73:9485–9495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crotty S, Miller CJ, Lohman BL, et al. : Protection against simian immunodeficiency virus vaginal challenge by using Sabin poliovirus vectors. J Virol 2001;75:7435–7452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mandl S, Hix L, Andino R: Preexisting immunity to poliovirus does not impair the efficacy of recombinant poliovirus vaccine vectors. J Virol 2001;75:622–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Manrique M, Kozlowski PA, Cobo-Molinos A, et al. : Immunogenicity of a vaccine regimen composed of simian immunodeficiency virus DNA, rMVA, and viral particles administered to female rhesus macaques via four different mucosal routes. Journal of Virology 2013;87:4738–4750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Manrique M, Kozlowski PA, Cobo-Molinos A, et al. : Long-term control of simian immunodeficiency virus mac251 viremia to undetectable levels in half of infected female rhesus macaques nasally vaccinated with simian immunodeficiency virus DNA/recombinant modified vaccinia virus Ankara. J Immunol 2011;186:3581–3593 [DOI] [PubMed] [Google Scholar]
- 33. Manrique M, Kozlowski PA, Wang SW, et al. : Nasal DNA-MVA SIV vaccination provides more significant protection from progression to AIDS than a similar intramuscular vaccination. Mucosal Immunol 2009;2:536–550 [DOI] [PubMed] [Google Scholar]
- 34. Manrique M, Micewicz E, Kozlowski PA, et al. : DNA-MVA vaccine protection after X4 SHIV challenge in macaques correlates with day-of-challenge antiviral CD4+ cell-mediated immunity levels and postchallenge preservation of CD4+ T cell memory. AIDS Res Hum Retroviruses 2008;24:505–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Manrique M, Kozlowski PA, Cobo-Molinos A, et al. : Resistance to infection, early and persistent suppression of simian immunodeficiency virus SIVmac251 viremia, and significant reduction of tissue viral burden after mucosal vaccination in female rhesus macaques. J Virol 2014;88:212–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iwasaki A, Welker R, Mueller S, Linehan M, Nomoto A, Wimmer E: Immunofluorescence analysis of poliovirus receptor expression in Peyer's patches of humans, primates, and CD155 transgenic mice: Implications for poliovirus infection. J Infect Dis 2002;186:585–592 [DOI] [PubMed] [Google Scholar]
- 37. Sabin AB: Pathogenesis of poliomyelitis; reappraisal in the light of new data. Science 1956;123:1151–1157 [DOI] [PubMed] [Google Scholar]
- 38. Takahashi Y, Misumi S, Muneoka A, et al. : Nonhuman primate intestinal villous M-like cells: An effective poliovirus entry site. Biochem Biophys Res Commun 2008;368:501–507 [DOI] [PubMed] [Google Scholar]
- 39. Yeh MT, Bujaki E, Dolan PT, et al. : Engineering the live-attenuated polio vaccine to prevent reversion to virulence. Cell Host Microbe 2020;27:736–751 e738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Clements JD, Norton EB: The Mucosal Vaccine Adjuvant LT(R192G/L211A) or dmLT. mSphere 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moss B, Carroll MW, Wyatt LS, et al. : Host range restricted, non-replicating vaccinia virus vectors as vaccine candidates. Adv Exp Med Biol 1996;397:7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sutter G, Moss B: Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci U S A 1992;89:10847–10851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Curtis AD, 2nd, Jensen K, Van Rompay KKA, Amara RR, Kozlowski PA, De Paris K: A simultaneous oral and intramuscular prime/sublingual boost with a DNA/Modified Vaccinia Ankara viral vector-based vaccine induces simian immunodeficiency virus-specific systemic and mucosal immune responses in juvenile rhesus macaques. J Med Primatol 2018;47:288–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Curtis AD, 2nd, Walter KA, Nabi R, et al. : Oral Coadministration of an Intramuscular DNA/Modified Vaccinia Ankara Vaccine for Simian Immunodeficiency Virus Is Associated with Better Control of Infection in Orally Exposed Infant Macaques. AIDS Res Hum Retroviruses 2019;35:310–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kestler H, Kodama T, Ringler D, et al. : Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science 1990;248:1109–1112 [DOI] [PubMed] [Google Scholar]
- 46. Regoes RR: The role of exposure history on HIV acquisition: Insights from repeated low-dose challenge studies. PLoS Comput Biol 2012;8:e1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Keele BF, Li H, Learn GH, et al. : Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med 2009;206:1117–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guadalupe M, Reay E, Sankaran S, et al. : Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol 2003;77:11708–11717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kozlowski PA, Lynch RM, Patterson RR, Cu-Uvin S, Flanigan TP, Neutra MR: Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J Acquir Immune Defic Syndr 2000;24:297–309 [DOI] [PubMed] [Google Scholar]
- 50. Montefiori DC: Evaluating neutralizing antibodies against HIV, SIV and SHIV in luciferase reporter gene assays. In: Current Protocols in Immunology (Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, Coico R, eds.) Vol 1 Hoboken, NJ, John Wiley & Sons, 2004, pp. 12.11.11–12.11.15 [DOI] [PubMed] [Google Scholar]
- 51. Mansfield KG, Veazey RS, Hancock A, et al. : Induction of disseminated Mycobacterium avium in simian AIDS is dependent upon simian immunodeficiency virus strain and defective granuloma formation. Am J Pathol 2001;159:693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Manrique M, Micewicz E, Kozlowski PA, et al. : DNA-MVA vaccine protection after X4 SHIV challenge in macaques correlates with day-of-challenge antiviral CD4+ cell-mediated immunity levels and postchallenge preservation of CD4+ T cell memory. AIDS Res Hum Retroviruses 2008;24:505–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lifson JD, Rossio JL, Piatak M Jr., et al.: Role of CD8(+) lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J Virol 2001;75:10187–10199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Roederer M, Nozzi JL, Nason MC: SPICE: Exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 2011;79:167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chebloune Y, Moussa M, Arrode-Bruses G, et al. : A single lentivector DNA based immunization contains a late heterologous SIVmac251 mucosal challenge infection. Vaccine 2020;38:3729–3739 [DOI] [PubMed] [Google Scholar]
- 56. Karlsson I, Malleret B, Brochard P, et al. : Dynamics of T-cell responses and memory T cells during primary simian immunodeficiency virus infection in cynomolgus macaques. J Virol 2007;81:13456–13468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. : Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009;361:2209–2220 [DOI] [PubMed] [Google Scholar]
- 58. Cohen J: Combo of two HIV vaccines fails its big test. Science 2020;367:611–612 [DOI] [PubMed] [Google Scholar]
- 59. Vaccari M, Gordon SN, Fourati S, et al. : Adjuvant-dependent innate and adaptive immune signatures of risk of SIVmac251 acquisition. Nat Med 2016;22:762–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cheng HD, Grimm SK, Gilman MS, et al. : Fine epitope signature of antibody neutralization breadth at the HIV-1 envelope CD4-binding site. JCI Insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Karuna ST, Corey L: Broadly Neutralizing Antibodies for HIV Prevention. Annu Rev Med 2020;71:329–346 [DOI] [PubMed] [Google Scholar]
- 62. Sok D, Burton DR: Recent progress in broadly neutralizing antibodies to HIV. Nat Immunol 2018;19:1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang SW, Kozlowski PA, Schmelz G, et al. : Effective induction of simian immunodeficiency virus-specific systemic and mucosal immune responses in primates by vaccination with proviral DNA producing intact but noninfectious virions. J Virol 2000;74:10514–10522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jones AT, Shen X, Walter KL, et al. : HIV-1 vaccination by needle-free oral injection induces strong mucosal immunity and protects against SHIV challenge. Nat Commun 2019;10:798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Beyrer C, Artenstein AW, Rugpao S, et al. : Epidemiologic and biologic characterization of a cohort of human immunodeficiency virus type 1 highly exposed, persistently seronegative female sex workers in northern Thailand. Chiang Mai HEPS Working Group. J Infect Dis 1999;179:59–67 [DOI] [PubMed] [Google Scholar]
- 66. Clerici M, Barassi C, Devito C, et al. : Serum IgA of HIV-exposed uninfected individuals inhibit HIV through recognition of a region within the alpha-helix of gp41. Aids 2002;16:1731–1741 [DOI] [PubMed] [Google Scholar]
- 67. Clerici M, Salvi A, Trabattoni D, et al. : A role for mucosal immunity in resistance to HIV infection. Immunol Lett 1999;66:21–25 [DOI] [PubMed] [Google Scholar]
- 68. Lo Caputo S, Trabattoni D, Vichi F, et al. : Mucosal and systemic HIV-1-specific immunity in HIV-1-exposed but uninfected heterosexual men. Aids 2003;17:531–539 [DOI] [PubMed] [Google Scholar]
- 69. Corthesy B: Multi-faceted functions of secretory IgA at mucosal surfaces. Front Immunol 2013;4:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Duchemin M, Khamassi M, Xu L, Tudor D, Bomsel M: IgA Targeting Human Immunodeficiency Virus-1 Envelope gp41 Triggers Antibody-Dependent Cellular Cytotoxicity Cross-Clade and Cooperates with gp41-Specific IgG to Increase Cell Lysis. Front Immunol 2018;9:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ruprecht RM, Marasini B, Thippeshappa R: Mucosal Antibodies: Defending Epithelial Barriers against HIV-1 Invasion. Vaccines (Basel) 2019;7:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Neutra MR, Kozlowski PA: Mucosal vaccines: The promise and the challenge. Nat Rev Immunol 2006;6:148–158 [DOI] [PubMed] [Google Scholar]
- 73. Xiao P, Patterson LJ, Kuate S, et al. : Replicating adenovirus-simian immunodeficiency virus (SIV) recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal SIV(mac251) challenge. J Virol 2012;86:4644–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Coffman RL, Sher A, Seder RA: Vaccine adjuvants: Putting innate immunity to work. Immunity 2010;33:492–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Coquet JM, Rausch L, Borst J: The importance of co-stimulation in the orchestration of T helper cell differentiation. Immunol Cell Biol 2015;93:780–788 [DOI] [PubMed] [Google Scholar]
- 76. Mestecky J, Russell MW, Elson CO: Perspectives on mucosal vaccines: Is mucosal tolerance a barrier? J Immunol 2007;179:5633–5638 [DOI] [PubMed] [Google Scholar]
- 77. Shen L, Chen CY, Huang D, et al. : Pathogenic events in a nonhuman primate model of oral poliovirus infection leading to paralytic poliomyelitis. J Virol 2017;91:e02310-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tracey I, Lane J, Chang I, Navia B, Lackner A, Gonzalez RG: 1H magnetic resonance spectroscopy reveals neuronal injury in a simian immunodeficiency virus macaque model. J Acquir Immune Defic Syndr Hum Retrovirol 1997;15:21–27 [DOI] [PubMed] [Google Scholar]