Abstract
Object
Corona virus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which leads to acute respiratory infection symptoms. SARS-CoV-2 infection is not always limited to the respiratory tract, and renal infection and dysfunction have been shown to be specific risk factors for death. In addition, COVID-19 has a higher incidence, severity and mortality in men than women. This disparity is due to biological rather than comorbid or behavioral sex differences. Because the male reproductive system is unique, the function of sex hormones in COVID-19 infection may explain the differences between males and females. Understanding these factors will provide appropriate prevention measures and adequate triage strategies and guide the drug discovery process.
Methods
An electronic search was completed in PubMed, ARXIV, MEDRXIV and BIORXIV. The most relevant articles were systematically reviewed. In addition, single cell RNA sequencing analysis of tissue samples from human cell landscape was conducted.
Results
The influence of SARS-CoV-2 on the urogenital system, the possibility of urinary tract transmission and the functions of sex hormones were discussed in this review.
Conclusion
Corona viruses can invade the genitourinary system, causing urological symptoms. Identifying the potential genitourinary organ impairments and protecting them from damage are necessary. Since sex hormones have potential as specific drugs, the gonadal hormones substitution therapy should be considered in both sexes in the COVID-19 pandemic.
Keywords: Corona virus disease 2019, Genitourinary system, Sex-hormone, Angiotensin-converting enzyme 2, Transmembrane protease serine 2
1. Introduction
The recent outbreak of corona virus disease 2019 (COVID-19) is the third beta coronavirus pandemic that has occurred among humans, following severe acute respiratory syndrome (SARS) and Middle Eastern respiratory syndrome (MERS) [1]. By 12 June 2020, over 7.9 million people worldwide were infected, with a mortality rate of 5.9% among closed cases. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which leads to COVID-19 [[2], [3], [4]], is a large enveloped single-stranded RNA virus [5,6]. The following five steps summarize the process of viral invasion: Firstly, viruses bind to receptors of the host (attachment); Secondly, membrane fusion or endocytosis helps viruses enter host cells (penetration); Thirdly, viral release contents and viral RNA enter the nucleus inside the host cells for replication; Fourthly, viral proteins are produced by viral mRNA (biosynthesis). Lastly, novel viral particles are born (maturation) and released. The spike (S) envelope glycoproteins on coronavirus primarily determine host cell entry. S1 is produced through proteolytic cleavage of the S protein, which is responsible for receptor binding, while S2, the transmembrane C-terminal region of the S protein, accelerates membrane fusion. Hence, it is believed that the S protein engagement of the host cell receptor, together with the proteolytic cleavage of the protein, determines the host range and tissue preference of coronaviruses [7]. Recently, several studies demonstrated, either experimentally or bioinformatically, that angiotensin-converting enzyme 2 (ACE2) [8] and glucose-regulating protein 78 (GRP78) [9] were functional SARS-CoV-2 virus receptors of cells [6,[10], [11], [12], [13]], and transmembrane protease serine 2 (TMPRSS2) and FURIN protein were two proteases that process SARS-CoV-2 S protein to lead to efficient infection [11,12]. As the virus binds to cell receptors and enters the cell to complete intracellular replication, virus release occurs, inducing cytotoxicity, and the expression and distribution of the potential receptor determine the route of viral infection [14,15].
The virus seriously damages the respiratory system. Some patients experience acute respiratory infection symptoms and even develop acute respiratory failure, acute respiratory distress syndrome (ARDS) and other complications [3,4]. On the other hand, the occurrence of multiple organ dysfunction syndrome in patients suggests that the virus invades other organs as well [16]. Moreover, there is growing evidence that corona virus may also invade the genitourinary system, causing urology symptoms. A prospective cohort study of 701 COVID-19 patients revealed that elevated blood urea nitrogen (BUN), increased serum creatinine (Scr) and estimated glomerular filtration (eGFR) <60 mL/min/1.73 m2 occurred in 13.1%, 14.4% and 13.1% of COVID-19 patients, respectively. Additionally, the prevalence of acute kidney injury (AKI) was 5.1% [17]. From past experience, SARS-CoV infection was not always limited to the respiratory tract, and renal infection and dysfunction were specific risk factors for death. Routine routes of SARS-CoV-2 transmission consist of direct contact and respiratory droplets. SARS-CoV-2 is likely to propagate through high concentration aerosols in a relatively closed space [15]. Although SARS-CoV-2 was detected in blood, urine, anal swabs and oropharyngeal swabs from nine COVID-19 patients who were retested by quantitative real-time PCR (qRT-PCR) [18], whether 2019 new corona virus (2019-nCoV) can be transmitted through the urinary tract remains unknown. In addition, Wei et al. [19] discovered that COVID-19 had a higher incidence severity and mortality in men than in women. The extent to which this disparity is due to biological rather than comorbid or behavioral sex differences remains unknown. Because the male reproductive system is unique, the function of sex hormones in COVID-19 infection may explain the sex difference between males and females. Here, we conducted this review to understand the influence on the urogenital system, the possibility of urinary tract transmission and the functions of sex hormones. Understanding these factors will provide appropriate prevention measures and adequate triage strategies and guide the drug discovery process.
2. Renal
2.1. Epidemiological characteristics
Several studies have noted kidney impairment in patients with COVID-19. First, computed tomography (CT) scan of the kidney revealed decreased density, suggestive of edema and inflammation [17]. Pei et al. [20] found that 251 of 333 patients (75.4%) had anomalous AKI or urine dipstick tests, and 111 of the 162 (68.5%) patients experienced proteinuria remission. Li et al. [21] demonstrated that 63% of patients exhibited proteinuria; 19% experienced increased plasma creatinine levels; and 27% showed elevated urea nitrogen levels among a total of 59 kidney impairment patients with COVID-19 [21]. A total of 178 patients with COVID-19 from Wuhan Union Hospital were involved in another study. No patients (0%) showed elevated Scr, and five of 178 (2.8%) patients presented increased BUN. For 83 patients without any history of renal impairment who received conventional urine tests after hospitalization, 45 of 83 (54.2%) patients exhibited urinalysis abnormalities, such as hematuria, proteinuria and leukocyturia. The results demonstrated that urinalysis was better than blood chemistry tests in identifying the potential renal impairment of patients with COVID-19, and urinalysis could predict the disease severity [22]. In addition, a retrospective analysis of eGFR was conducted among 85 patients in Wuhan. Twenty-three of 85 (27.06%) patients exhibited acute renal failure (ARF). Elderly patients and people with comorbidities such as heart failure and hypertension developed ARF more easily [23].
To date, the rate of AKI among COVID-19 patients is highly variable. AKI has been found to occur in up to 27% of patients [23]. Another study showed that 29% of 52 severe COVID-19 pneumonia patients had complications associated with AKI [24]. In addition, Huang et al. [4] noted that AKI occurred in three of 41 (7%) patients. After improving the scale of patient sampling, a study from Cheng's group [17] noted that 36 of 701 (5.1%) patients were defined as having AKI, and AKI stage III occurred among 2% (14/701) of patients and was correlated with an elevated in-hospital mortality risk. A comprehensive study involving 6395 COVID-19 patients showed that the incidence of AKI was 4.3%. The severity of pneumonia was a risk factor for AKI; compared with the nonsevere group, the comorbidity of chronic kidney disease (CKD) (odds ratio [OR]: 3.28) and complications of AKI (OR: 11.02) were significantly elevated in the severe group [25]. Furthermore, Scr, the ratio of abnormal Scr, BUN and the ratio of abnormal BUN were markedly elevated in the severe group compared with their levels in the nonsevere group [25]. Pei et al. [20] found that severe AKI caused high mortality in COVID-19 patients, with a rate of 11.2% (28/251) in the AKI group versus 1.2% (1/82) in the control group. In Zhou et al.’s [26] study, which included 191 COVID-19 patients, among 33 confirmed COVID-19 patients who further developed AKI, 32 patients did not survive. The data are provided in Table 1.
Table 1.
Progress review of the effects on the kidney in patients with COVID-19.
| Author [reference number] | Accessed date | Major element | Patient or material | Main conclusion |
|---|---|---|---|---|
| Su, et al. [28] | 2020.4.20 | Kidney lesions in fatal COVID-19 | 26 COVID-19 patients | The virus particles were identified in the cytoplasm of renal proximal tubular epithelium as well as in the podocytes and less so in distal tubules. |
| Qi, et al. [85] | 2020.4.18 | The major system that is vulnerable to COVID-19 infection | 31 organs from nine major human systems | The respiratory system, digestive system and reproductive system are at the top-risk level to COVID-19 infection. |
| Zhu, et al [86] | 2020.3.15 | Successful recovery of COVID-19 pneumonia in a renal transplant recipient | 1 COVID-19 patient | When treating pneumonia due to opportunistic virus infection following kidney transplantation, a reduction or even temporary discontinuation of immunosuppressants is a common strategy and allows recipients the opportunity to reacquire anti-infection immunity within a short period, which is conducive to eliminating the virus. |
| Xu, et al. [87] | 2020.3.6 | Clinical manifestations and pathological features of COVID-19 | 34 COVID-19-related articles | The most common clinical manifestation of COVID-19 was fever; the most common patterns on chest imaging findings were ground-glass opacity; the pathology showed that the manifestations of COVID-19 were similar to SARS. |
| Diao, et al. [23] | 2020.4.10 | Kidney injury in COVID-19 | 85 COVID-19 patients | Viruses can not only directly infect human kidney tubules to induce acute tubular damage but also initiate CD68+ macrophage together with complement C5b-9 deposition to mediate tubular pathogenesis. |
| Liu, et al. [25] | 2020.5.2 | The relationship between COVID-19 and kidney disease | 6395 COVID-19 patients | The chronic kidney disease and acute kidney injury are susceptible to occur in patients with severe COVID-19. |
| Lin, et al. [56] | 2020.2.18 | ACE2 gene expressions in all cell types in healthy kidneys and bladders | Normal kidney samples from three healthy donors | The levels of ACE2 are detectable both in kidney and bladder. Kidney proximal tubule cells have higher expression percentages than bladder epithelial cells. |
| Hatem, et al. [88] | 2020.4.27 | Survival rate in acute kidney injury superimposed COVID-19 patients | 2290 abstracts | Severe AKI in patients with COVID-19 is an ominous clinical predictor and is associated with high mortality. |
| Zhou, et al. [22] | 2020.4.6 | Urinalysis detects the early renal-impairment in patients with COVID-19 | 178 COVID-19 patients | Urinalysis is better in unveiling potential kidney impairment of COVID-19 patients than blood chemistry test and urinalysis could be used to reflect and predict the disease severity. |
| Pei, et al. [20] | 2020.4.12 | Kidney Involvement in COVID-19 | 333 COVID-19 patients | Renal abnormalities occurred in the majority of patients with COVID-19 pneumonia and were associated with higher mortality. |
ACE2, angiotensin-converting enzyme 2; COVID-19, corona virus disease 2019; SARS, severe acute respiratory syndrome; AKI, acute kidney injury.
2.2. Potential mechanism of injury
The possible mechanisms of renal involvement in these cases may be didactically split into four fields: Direct cytotoxicity, cytokine damage, organ crosstalk and systemic effects.
2.2.1. Direct cytotoxicity
We suspect that the direct cytotoxicity of SARS-CoV-2 causes tubular damage. From a pathological report of COVID-19 patients, hematoxylin-eosin (H&E) staining revealed that renal tissues from autopsies showed severe acute tubular necrosis, luminal brush border sloughing lymphocyte infiltration and vacuole degeneration [22]. Immunohistochemistry (IHC) reported that the nucleoprotein (NP) antigen of SARS-CoV-2 accumulated in renal tubules. To estimate whether highly expressed ACE2 could induce SARS-CoV replication in tubular cells, electron microscopy (EM) was applied to reveal that virus-like particles are visible in the kidney [23]. This result was consistent with the findings of a previous study. Chu et al. [27] successfully proved the presence of SARS-CoV viral particles using EM in kidney specimens from autopsies of SARS patients with AKI. In another autopsy of 26 COVID-19 patients, nine of 26 had clinical features of renal injury, including increased Scr or new-onset proteinuria. Through light microscopy, diffuse injury of the proximal tubule, together with pigmented casts and occasional hemosiderin granules, was observed. There were obvious erythrocyte aggregates blocking the capillary lumen without fibrinoid material or platelets. Evidence of interstitial inflammation, vasculitis and hemorrhage was also absent. EM observation revealed coronavirus particle clusters with characteristic spikes in the podocytes and tubular epithelium. Furthermore, the upregulation of ACE2 was found in COVID-19 patients, and immunostaining with nucleoprotein antibody of SARS-CoV-2 was positive in tubule cells [28].
2.2.2. Cytokine damage
Cytokine release syndrome (CRS), also called “cytokine storm”, can occur in different conditions, such as hemophagocytic syndrome, sepsis and chimeric antigen receptor T cell therapy (CARTT) [29]. The incidence of CRS in COVID-19 patients has been reported since the first record of this disease [30]. In CRS patients, AKI might occur as an outcome of intrarenal inflammation, volume depletion, increased vascular permeability and cardiomyopathy, which can result in type 1 cardiorenal syndrome. However, cytokine generation tends to be induced by extracorporeal membrane oxygenation (ECMO), continuous kidney replacement therapy (CKRT) and invasive mechanical ventilation. Proinflammatory IL-6 is considered the most principle causative cell cytokine in CRS. Among COVID-19 patients, the concentration of IL-6 in plasma is increased in ARDS patients who accept advanced support therapies [31]. In addition, Diao et al. [23] analyzed renal tissues from six patients with postmortem examinations. IHC showed that a large number of CD68+ macrophages were recruited by SARS-CoV-2 virus to infiltrate into the tubulointerstitium, revealing that tubular damage might be induced by proinflammatory cytokines derived from macrophages [23]. They also found that SARS-CoV-2 virus could initiate complement C5b-9 deposition and assembly on tubules. Both experimental and clinical models revealed that the abnormal serum-derived complement composition in the tubular lumen resulted in complement C5b-9 assembly (via the alternative pathway) at the topmost brush-like border of TECs (tubular epithelial cells), which could be an essential factor in the pathogenesis of tubulointerstitial damage [31,32].
2.2.3. Organ crosstalk
As mentioned above, some results indicated the close association between alveolar and tubular damage in ARDS (the lung–kidney axis) [33]. The overproduction of cytokines is related to bidirectional damage to the lung–kidney. Injured kidney TEC increased IL-6, and in clinical and experimental studies, upregulation of IL-6 serum concentration in AKI was related to pulmonary hemorrhage and higher alveolar-capillary permeability. Notably, an excessively high level of anti-inflammatory mediator consistency might predispose the patient to a condition of relative immunosuppression. In addition, ARDS can cause kidney medullary hypoxia, which is an additional insult to tubular cells [34]. Heart–kidney crosstalk might also lead to the occurrence of AKI in COVID-19 patients. For example, acute viral myocarditis and CRS cardiomyopathy can both result in kidney vein hypotension, congestion and kidney hypoperfusion, with a decline in glomerular filtration rate.
2.2.4. Systemic effects
Fluid expansion can result in a positive balance of fluid in shock sufferers. This expansion has an unfavorable influence on ARDS by increasing leakage of alveolar capillaries. In some AKI patients, fluid expansion worsened kidney vein congestion, resulting in kidney compartment syndrome. We conjecture that there is a similar occurrence of clinical signs in COVID-19 patients; Su et al. [28] analyzed the kidney histopathology of 26 patients postmortem and found a common morphologic change. Erythrocytes stagnated in the capillaries of the peritubal and glomerular lumen without platelets, fibrin thrombi, red blood cell fragments or fibrinoid necrosis. This result demonstrated the possibility of renal venous congestion. Moreover, a negative fluid balance status may cause renal damage as well. Interestingly, less aggregation of red blood cells was observed in the capillaries of peritubal in patients with predominant glomerular loop occlusion, which is usually correlated with a relatively long hypotension duration [28]. In addition, rhabdomyolysis, hyperkalemia and metabolic acidosis may occur in COVID-19 patients, which are nearly always correlated with hemodynamic instability as well.
2.3. ScRNA analysis
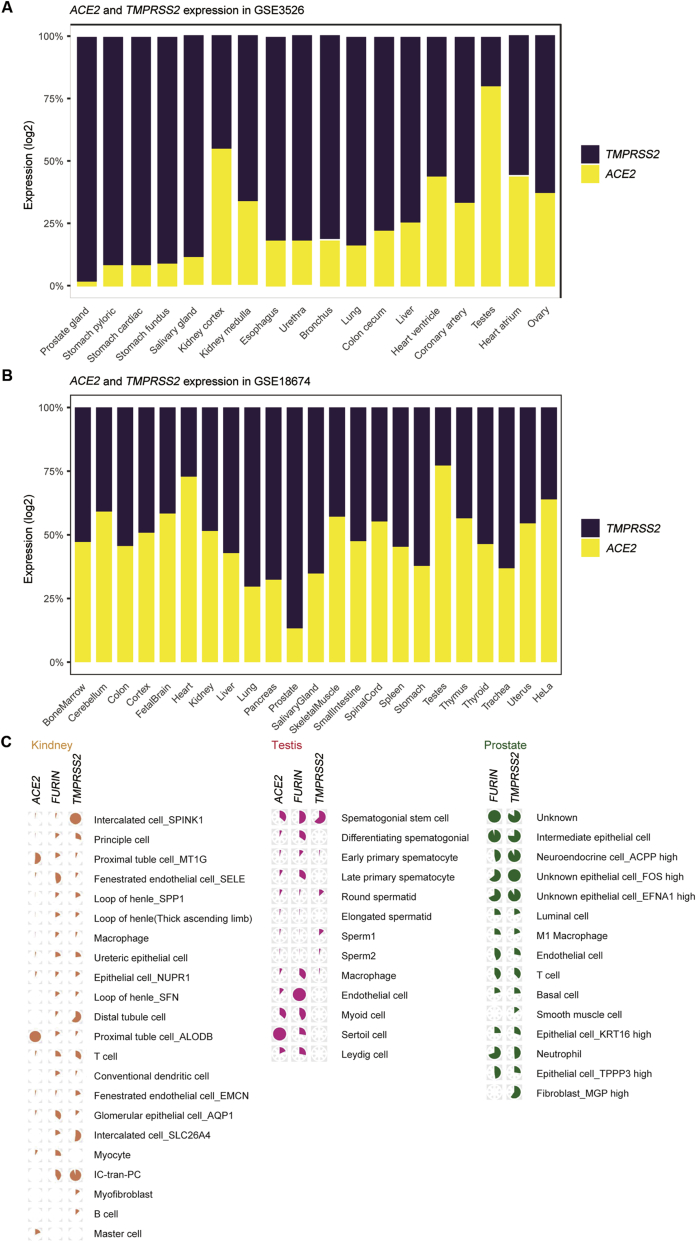
As shown in Fig. 1, we found that the expression of ACE2 was highly specific. In addition to the significant expression in proximal tubule cells (MT1G and ALDOB) and relatively marked expression in mast cells and myocytes, the expression of ACE2 in other types of cells was very low. This phenomenon positively correlated with the observations for renal histopathology. Proximal straight tubule cells were potential host cells targeted by SARS-CoV-2 by scRNA-seq analysis. Regarding TMPRSS2, its expression was more extensive, and almost all types of cells in kidney tissue exhibited increased or decreased expression. The mRNA expression of TMPRSS2 was higher in intercalated cells (SPINK1 and SLC26A4) and IC-tran-PC cells, followed by distal tubule cells. The expression of FURIN was similar, and extensive enrichment was observed in multiple cells, particularly in fenestrated endothelial cells and IC-tran-PC cells. Interestingly, neither FURIN nor TMPRSS2 was observed in ACE2-overexpressing mast cells.
Figure 1.
Single-cell transcriptomic profiling of tissue samples from human cell landscape. (A) The mRNA expression patterns of ACE2 and TMPRSS2 in GSE3526; (B) The mRNA expression patterns of ACE2 and TMPRSS2 in GSE18674; (C) Cell-specific expression patterns of ACE2, FURIN and TMPRSS2 in genitourinary system. ACE2, angiotensin-converting enzyme 2; TMPRSS2, transmembrane protease serine 2.
Zhou et al. [35] analyzed scRNA data in the adrenal gland. For the ACE2 median expression group, while FURIN was readily detected in all the tissue cells, TMPRSS2 expression could be readily detected in adrenal gland cells. In the presence of viremia, the most vulnerable target might be the adrenal gland.
2.4. Therapy
The implementation of potential interventions such as continues renal replacement therapy (CRRT) is strongly suggested to protect renal function in patients with COVID-19, especially for ARF cases, and may be an important approach to prevent fatality. Hemoperfusion should be applied for ≥2 h for at least 3 consecutive days. During the procedure, anticoagulation along with citrate or heparin should be used to ensure blood flow >120 mL/min, preventing circuit clotting. The cartridge adsorptive capacity tends to be exhausted after 4 h, and then the therapy is completed. ECMO supports both the lungs and heart and is suggested to be applied in conjunction with CRRT. We suggest connecting the CRRT circuit straight to the ECMO apparatus. Several different methods can be applied for removing cytokines: First, the neutro-macroporous sorbent is used for direct hemoperfusion, as cytokine removal is mainly carried out using a neutro-macroporous sorbent; second, plasma is separated from whole blood and further adsorption on a resin; third, CKRT with adsorptive properties via hollow fiber filters is chosen; lastly, CKRT of high dose is concluded with high cut-off (HCO) or medium cut-off (MCO) membranes. CKRT filters with particular membranes, including acrylonitrile, sodium methallyl sulfonate plus polyethyleneimine and polymethylmethacrylate, also adsorb cytokines. These filters need to be replaced every 24 h due to adsorptive site saturation [[36], [37], [38]].
3. Testis
3.1. Epidemiological characteristics
Based on recent bioinformatics evidence [38,39], the testis may be infected by SARS-CoV-2, causing urgent doubt about whether the virus can be transmitted sexually. This is important for the formulation of the principles for treatment and guidelines for sexual behavior among males with COVID-19. As shown in a randomized controlled trial, the serum sex-related hormone ratio of testosterone (T) to luteinizing hormone (LH) and the ratio of follicle-stimulating hormone (FSH) to LH, which to some degree represent reproductive ability, exhibited an obvious difference between infected individuals and healthy controls [40]. However, several studies with limited cases analyzed that performed semen analysis exhibited a negative viral result by reverse transcription-polymerase chain reaction (RT-PCR) [[41], [42], [43]]. Notably, a total of 38 male patients aged 15–50 years provided semen samples for the detection of new coronavirus nucleic acids [42]. Li et al. [44] found that the RT-PCR tests of six samples were positive. Among the samples, two were from patients in the recovery period, and four were from patients in the acute infection period [44]. Warnings have already been given by the Society for Assisted Reproductive Technology (SART) and the American Society for Reproductive Medicine (ASRM) [45]. The advice issued was that ART patients, gamete donors, gestational carriers and expectant parents who meet the diagnostic criteria for SARS-CoV-2 infection need to avoid becoming pregnant or taking part in any programs for fertility. The Center for Diseases Control and Prevention (CDC) (Washington DC, USA) has also stated that it is unclear whether nonrespiratory bodily fluids from an infected patient, including urine, vomit, semen or breast milk, can contain viable, infectious viruses [46]. In Italy, all gamete donors were required to be interviewed by authorities for recent travel to high-risk areas and/or the presence of respiratory symptoms. Cessation of donation during a 2-week time period from the end of symptoms was implemented if donors had respiratory symptoms or returned from a high-risk area [47].
3.2. ScRNA analysis
In the testis, the expression levels of ACE2, FURIN and TMPRSS2 were all high in spermatogonial cells. Sertoli cells and endothelial cells had the highest ACE2 and FURIN mRNA expression, respectively.
The expression of ACE2 was abundant in the testis. We observed that ACE2 is expressed in all Sertoli cells, a large portion of spermatogonial stem cells and myoid cells, and a small amount of other types of cells. FURIN was more highly expressed in early spermatogenesis cells (spermatogonial stem cells, differentiating spermatogonia, early and late primary spermatocytes), various stromal cells (Sertoli cells, myoid cells and Leydig cells) and endothelial cells, but almost no expression was observed in sperm cells after round spermatids. TMPRSS2 was only highly expressed in spermatogonial stem cells and round spermatids, but was lowly expressed in other cells (Fig. 1).
3.3. Hypogonadism in the progression of COVID-19 infection
As shown in a recent study, viral infection itself can worsen the function of Leydig cells and consequent hypogonadism [40]. Several studies have revealed that hypogonadism is correlated to chronic obstructive pulmonary disease (COPD), with a prevalence ranging from 22% to 69% in men [48]. In this situation, low levels of testosterone led to a decline in overall strength capacity and respiratory muscle activity [38]. Normal circulating levels of testosterone exhibited a protective effect on a few respiratory outcomes (i.e., forced expiratory volume in one second [FEV1] and forced vital capacity [FVC]) [38]. A single-center study reported that peak oxygen consumption was improved in men who received testosterone replacement therapy [49].
As proinflammatory cytokines show a vital function in the process of SARS-CoV-2 infection, the activity of cytokines and related receptors can be reduced during treatment. In this situation, testosterone could reduce inflammation. In fact, some results revealed that hypogonadism was correlated with upregulated proinflammatory cytokines and that testosterone treatment downregulated TNF-α, IL-1β and IL-6 [50]. In addition, the relationship between an accumulation of proinflammatory factors and a decline in testosterone was frequently observed in elderly people [51] and in patients with stable coronary heart disease [52]. According to the above evidence, the hypothesis arose that testosterone played a role in the cascade of events causing progression of SARS-CoV-2 infection due to the cytokine storm. Upregulation of inflammatory cytokines accompanied by the decline in androgens and estrogens in aging men may establish a nonpositive association between testosterone levels and mortality associated with COVID-19 [53].
4. Prostate
4.1. Epidemiological characteristics
A single-cell RNA sequencing dataset found that ACE2 and TMPRSS2 are coexpressed in prostate epithelial cells. Based on this situation, we proposed the hypothesis that the prostate may be a target organ of SARS-CoV-2. However, few studies have focused on the effect of SARS-CoV-2 on the prostate, and there is a lack of pathological autopsy reports on the prostate. Only two complete autopsies of SARS-CoV-2-positive individuals in Oklahoma revealed a grossly normal prostate [50]. In addition, Quan et al. [54] collected the expressed prostatic secretion (EPS) of 23 confirmed mild and common patients, which tested nonpositive for SARS-CoV-2 RNA. In this research, EPS was tested only in mild and common patients. Thus, large samples and long-term follow-up are needed.
4.2. ScRNA analysis
Using a publicly available single-cell RNA sequencing dataset, Song et al. [55] analyzed 24 519 normal prostate epithelial cells for the expression of ACE2 and TMPRSS2. In this dataset, 0.32% of all epithelial cells (78 of 24 519), 0.47% of all stromal cells (10 of 2113), 0.06% of endothelial cells (1 of 1586) and 0.22% of leukocytes (1 of 459) expressed ACE2. TMPRSS2 was expressed in 18.65% of all epithelial cells (4573 of 24 519), 41.74% of all stromal cells (882 of 2113), 16.71% of endothelial cells (265 of 1586) and 52.07% of leukocytes (239 of 459). They found TMPRSS2 and ACE2 coexpressing cells in epithelial cells, with a higher proportion in club and hillock cells. Double-positive cells could potentially serve as reservoirs for SARS-CoV-2 infiltration and damage to the prostate; however, they constitute approximately 0.07% of all prostate epithelial cells. They found that 0.61% of prostate club cells coexpress ACE2 and TMPRSS2 and that club cells have a markedly lower proportion of double-positive cells. The implications of SARS-CoV-2 prostate infiltration via club cells are unclear and warrant further investigation (Fig. 1).
5. Bladder
Lin et al. [56] found low expression levels of ACE2 in all bladder epithelial cell types based on the public bladder dataset of twelve cell types. The concentration of ACE2 showed a decreasing trend from the outer layer of the bladder epithelium (umbrella cells) to the inner layer (basal cells), with the intermediate cells exhibiting moderate concentrations. Other cell types, such as immune and endothelial cells, were mostly negative for ACE2. The percentage of ACE2-positive cells in umbrella cells of the bladder (1.3%) was obviously lower than the expression in the renal epithelium. The current reports of COVID-19 patients have not shown positive detection of the virus in urine samples. However, a previous analysis of SARS patients indicated that the SARS virus could survive in urine at detectable levels [57]. The detection of SARS virus in the urine implied the possibility of viral release from infected bladder epithelial cells and transmission via the urinary tract.
BCG, either in the form of instillations or chemotherapy, is applied in the optimization of disease control in nonmuscle invasive bladder cancer (NMIBC) [58]. We currently understand the mechanism of action of BCG intravesical instillations, and it seems to be attributed to a local immune response, which is characterized by increased expression of cytokines in the bladder wall and urine and by an influx of mononuclear, dendritic, and granulocyte cells. The populations in countries vaccinated with BCG appeared to have lower morbidity and mortality rates [59]. Whether intravesical injection of BCG has any protective effect against COVID-19 needs to be explored [60].
6. Sex hormone
One of the most widely available types of epidemiologic data related to COVID-19 is sex-related mortality. A few researchers have regarded male sex as a poor prognostic factor based on evidence supporting a higher predominance of male deaths caused by COVID-19 deaths in some countries [61]. Men represented 73% of deaths in China [62], 70% in Italy [19] and 59% in South Korea [63]. One recent review, which collected data from 77 932 patients from all available epidemiological studies, revealed a correlation between male sex and higher mortality [19]. Indeed, Channappanavar et al. [64] revealed that female mice are less susceptible to SARS-CoV infection than age-matched male mice. The mortality rate increased as a result of estrogen receptor antagonist treatment or ovariectomy in female mice. Thus, this result suggested that the estrogen receptor signaling pathway had a protective function in mice infected with SARS-CoV [64]. In this situation, it is of benefit to know the pathologic mechanisms by analyzing a possible hormonal dependency of the activity and expression of ACE2 and TMPRSS2 in various organs according to epidemiologic evidence.
6.1. Estrogen and ACE2
Epidemiological and experimental data strongly indicated that ERs activated by estrogen successfully drove immune protection against respiratory virus infections, including both influenza and corona viruses [64]. Stronger immune responses in females are attributed to the stronger regulatory pathway of estrogen–ER–aromatase–estrogen playing a significant role in reproductive functions [65]. Estrogen activates ERs in accumulated neutrophils, macrophages and monocytes, increasing the production of proinflammatory cytokines and chemokines, such as IL-12, TNFα and CCL2, inducing the production of type I and III interferon (IFN), which are important for decreasing the virus titer and are induced by ER activation in lymphocytes [66,67]. In addition, proinflammatory cytokines and chemokines upregulate aromatase expression and induce the conversion from androgens to estrogens. Thus, we consider using estrogen treatment to induce the estrogen–ER–aromatase–estrogen circuit, promoting the decrease in virus titer, the restoration of destroyed tissue and the alleviation of inflammation. Currently, among available estrogen preparations, only Premarin can induce beneficial effects in humans without adverse reactions, as it exhibits similar DNA repair and genome stabilizer functions as endogenous estrogen [68].
As the ACE2 gene is located on the X chromosome and estrogen increases the expression of ACE2, ACE2 expression is higher in females than in males. ACE2 is expressed both in adult Leydig cells of humans and mice in a testosterone-independent manner. In these cells, ACE2 has been regarded as a functional gene in steroidogenesis [69]. The expression of ACE2 increases as LH increases in ovarian granulosa cells and can be stimulated with human chorionic gonadotropic (hCG) to improve the ACE2-angiotensin (1–7)-Mas system [70]. In addition to the expression of ACE2 in gonadal tissue, some studies have reported that the activity and expression of ACE2 were affected by sex hormones in the mouse myocardium, renal tissue and adipose tissue [[71], [72], [73], [74]]. Ovariectomy in female mice resulted in a decline in the activity of ACE2 in mouse adipose tissue that led to hypertension. However, 17β-estradiol administration could restore this phenomenon [72]. In the myocardium, ACE2 expression, together with cardiac hypertrophy, was significantly increased in spontaneously hypertensive male rats compared to its expression in female rats. Then, after orchiectomy, significant declines in the expression of ACE2 and cardiac hypertrophy were observed, with subsequent improvements in cardiac performance. In female mice, ovariectomy resulted in an upregulation of the expression of ACE2 and cardiac hypertrophy, and worse activity of the heart pump was observed [71]. Since ACE2 expression in the myocardium seems to be regulated by androgens [71], the function of androgen receptor (AR) gene polymorphisms in the pathogenesis of hypertension and cardiovascular adverse events in male patients with COVID-19 cannot be excluded.
Physiologically, ACE2 is part of the renin-angiotensin system (RAS) and functions as a key regulator of systemic blood pressure through the cleavage of angiotensin 1 (Ang 1) to generate the inactive Ang 1–9 peptide, and it directly metabolizes Ang 2 to generate Ang 1–7, limiting its effects on fibrosis and vasoconstriction. In addition to being a functional receptor for SARS-CoV-2, ACE2 has been implicated in lung disease, diabetes and cardiovascular pathologies [72]. Although ACE2 expression is associated with susceptibility to SARS-CoV-2 infection, the mechanism between ACE2 and SARS-CoV-2 remains unclear. In fact, ACE2 was suggested to play a protective role, as its overexpression attenuates lung inflammation [73]. We speculate that it also plays the same role in the genitourinary system. In a cohort of 12 COVID-19 patients, levels of circulating Ang 2 were significantly elevated compared to the levels in healthy controls (linearly associated with viral load), suggesting a direct link between downregulation of tissue ACE2 with systemic RAS imbalance and facilitating the development of multiorgan damage in COVID-19 patients [74,75]. Although ACE2 facilitates viral entry at the epithelial surface, the ACE2/Ang 1–7 axis can be carefully manipulated to curtail SARS-CoV-2 infection while affording maximal protective effects against lung and renal damage in COVID-19 patients, which represents a potential target for therapeutic intervention [76,77]. Currently, in two phase II clinical trials, administration of ACE2 was proven to reduce systemic inflammation and shift the RAS peptide balance away from Ang 2 toward Ang 1–7 [78,79]. In addition, potential therapeutic strategies may include preventing the binding of SARS-CoV-2 and blocking the receptor-binding domain (RBD) of the viral S-protein by human ACE2. In addition to this RBD-blocking strategy, other possible treatment options may include localized use of ACE2 antibody, small molecule inhibitors, ACE2-derived peptides or single chain antibody fragment against ACE2.
6.2. Androgen and TMPRSS2
As an important accidental discovery in medicine, many of the insights associated with TMPRSS2 come from cancer studies. TMPRSS2 is a widely studied androgen-regulated gene in prostate cancer. TMPRSS2 is mainly expressed on the luminal side of the prostate epithelium, and compared to noncancerous tissue, the expression of TMPRSS2 is higher in prostate tumor tissue [80]. Prostate tumor cell lines significantly drive the expression of TMPRSS2 in response to androgens [81], contributing to prostate cancer pathogenesis by aberrantly upregulating the expression of oncogenes. Notably, the TMPRSS2 gene is related to the most common gene fusion events: The most common somatic gene rearrangement involving TMPRSS2 with ETS family members of carcinogenic transcription factors is ERG [80]. While androgen usually does not directly regulate ERG, the AR regulatory elements of TMPRSS2 are juxtaposed with the ERG gene via gene fusion. Consequently, the gene ERG is regulated by AR signaling and is highly expressed in prostate tumors that harbor the TMPRSS2-ERG fusion protein [81]. Interestingly, TMPRSS2-ERG fusion is less frequent in prostate cancers in both Asian and black populations than in populations of men of European ancestry. The relevance of this situation to the current COVID-19 pandemic is not yet clear.
An open problem is to what extent susceptibility could inhibit the androgen signaling pathway and potentially reduce SARS-CoV-2 infection. A subsequent question is to what degree AR can regulate the expression of TMPRSS2 protein in the lung or other tissues associated with viral entry. The current results demonstrated that AR is expressed in the respiratory tract epithelium in humans and mice, particularly in bronchial epithelial cells and type 2 pneumocytes. The transcript of TMPRSS2 was upregulated over two-fold by administrating androgen to a lung cancer cell line, with AR protein androgen-dependent loading onto the enhancer of TMPRSS2 [81]. However, it is still unknown whether AR signaling antagonists can eliminate the expression of TMPRSS2 in a lung adenocarcinoma cell line or in normal human respiratory epithelium.
If observational studies further confirm that AR signaling inhibition is a feasible strategy, then some therapeutics, including darolutamide, apalutamide, enzalutamide or chemical gonadal ablation, which significantly decrease the activity of AR signaling, should be repurposed for treatment of COVID-19 patients. Related trials can be rapidly performed, according to the known safety profile of these therapies in both males and females, as well as their immediate availability. The protease inhibitors nafamostat, aerosolized aprotinin and camostat have been proven to attenuate TMPRSS2 protease activity, in addition to the theoretical potential for AR-targeted therapies to regulate the expression of TMPRSS2. Since April 6, 2020, at least one phase 1–2 camostat clinical trial for COVID-19 (ClinicalTrials.gov, NCT04321096) has been initiated. In addition, discoveries in cancer studies indicated that bromhexine acted as an effective and specific protease inhibitor for TMPRSS2. The prevalence of prostate tumor metastasis was decreased by systemic administration of bromhexine without proof of systemic toxicity [82]. It is notable for clinical trials of TMPRSS2 inhibitors that in clinical trials of TMPRSS2 inhibitors, the potential physiological mechanisms of TMPRSS2 in normal tissues and cells are not yet fully known. TMPRSS2 induces proteolytic cascades in the regular prostate and leads to prostate-specific antigen activation, which is a protease related to ejaculate production [84]. Thus, the influence on male reproductive function remains to be debated. Notably, TMPRSS2 seems unimportant in other organs, as of its effects can be redundant due to the effects of other proteases. A compelling finding was recorded in a cancer study conducted with a TMPRSS2 knockout mouse model, where TMPRSS2 seemed completely dispensable for normal growth as well as organ function [83].
7. Conclusion
In conclusion, the function of gonadal hormones and potential substitution therapy should be considered in both sexes in the COVID-19 pandemic. Patients with hypogonadism could temporarily stop taking testosterone (or LH/hCG) or be given a lower dose. Conversely, estrogen substitution therapy should be assessed in postmenopausal or hypogonadal females. Therefore, it is useful to monitor levels of serum testosterone closely in such patients, and this work should continue even after the end of the acute phase of the disease.
Author contribution
Study design: Shancheng Ren, Chao Qin.
Data acquisition: Kuangzheng Liu, Xinglin Chen.
Data analysis: Yuqing Wu, Xiaohan Ren.
Drafting of manuscript: Kuangzheng Liu.
Critical revision of the manuscript: Shancheng Ren, Chao Qin.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Second Military Medical University.
Contributor Information
Shancheng Ren, Email: renshancheng@gmail.com.
Chao Qin, Email: nmuqinchao@163.com.
References
- 1.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A. Severe acute respiratory syndrome-related coronavirus: the species and its viruses—A statement of the Coronavirus Study Group. bioRxiv. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li W., Sui J., Huang I.C., Kuhn J.H., Radoshitzky S.R., Marasco W.A. The S proteins of human coronavirus NL63 and severe acute respiratory syndrome coronavirus bind overlapping regions of ACE2. Virology. 2007;367:367–374. doi: 10.1016/j.virol.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Millet J.K., Whittaker G.R. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–134. doi: 10.1016/j.virusres.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibrahim I.M., Abdelmalek D.H., Elshahat M.E., Elfiky A.A. COVID-19 spike-host cell receptor GRP78 binding site prediction. J Infect. 2020;80:554–562. doi: 10.1016/j.jinf.2020.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conaldi P.G., Biancone L., Bottelli A., De Martino A., Camussi G., Toniolo A. Distinct pathogenic effects of group B coxsackieviruses on human glomerular and tubular kidney cells. J Virol. 1997;71:9180–9187. doi: 10.1128/jvi.71.12.9180-9187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nowakowski T.J., Pollen A.A., Di Lullo E., Sandoval-Espinosa C., Bershteyn M., Kriegstein A.R. Expression analysis highlights AXL as a candidate Zika virus entry receptor in neural stem cells. Cell Stem Cell. 2016;18:591–596. doi: 10.1016/j.stem.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu J., Gong E., Zhang B., Zheng J., Gao Z., Zhong Y. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang P., Liu J., Xu W., Luo Q., Deng K., Lin B. Novel coronavirus can be detected in urine, blood, anal swabs and oropharyngeal swabs samples. MedRxiv. 2019:2020. doi: 10.1101/2020.02.21.20026179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei X., Xiao Y., Wang J., Chen R., Zhang W., Yang Y. Sex differences in severity and mortality among patients with COVID-19: evidence from pooled literature analysis and insights from integrated bioinformatic analysis. arXiv. 2020;2003:13547. https://arxiv.org/abs/2003.13547 [Google Scholar]
- 20.Pei G., Zhang Z., Peng J., Liu L., Zhang C., Yu C. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol. 2020;31:1157–1165. doi: 10.1681/ASN.2020030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z., Wu M., Yao J., Guo J., Liao X., Song S. Caution on kidney dysfunctions of COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.02.08.20021212. [DOI] [Google Scholar]
- 22.Zhou H., Zhang Z., Fan H., Liu J., Li M., Dong Y. Urinalysis, but not blood biochemistry, detects the early renal-impairment in patients with COVID-19. medRxiv. 2020 doi: 10.1101/2020.04.03.20051722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diao B., Wang C., Wang R., Feng Z., Tan Y., Wang H. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv. 2020 doi: 10.1101/2020.03.04.20031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y., Zhang Z., Pan X., Xing G., Zhang Y., Liu Z.S. The chronic kidney disease and acute kidney injury involvement in COVID-19 pandemic: a systematic review and meta-analysis. medRxiv. 2020;2020 doi: 10.1101/2020.04.28.20083113. 2004.2028.20083113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu K.H., Tsang W.K., Tang C.S., Lam M.F., Lai F.M., To K.F. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67:698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su H., Yang M., Wan C., Yi L., Tang F., Zhu H. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98:219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neelapu S.S., Tummala S., Kebriaei P., Wierda W., Gutierrez C., Locke F.L. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.David S., Biancone L., Caserta C., Bussolati B., Cambi V., Camussi G. Alternative pathway complement activation induces proinflammatory activity in human proximal tubular epithelial cells. Nephrol Dial Transplant. 1997;12:51–56. doi: 10.1093/ndt/12.1.51. [DOI] [PubMed] [Google Scholar]
- 32.Cybulsky A.V., Takano T., Papillon J., Khadir A., Liu J., Peng H. Complement C5b-9 membrane attack complex increases expression of endoplasmic reticulum stress proteins in glomerular epithelial cells. J Biol Chem. 2002;277:41342–41351. doi: 10.1074/jbc.M204694200. [DOI] [PubMed] [Google Scholar]
- 33.Panitchote A., Mehkri O., Hastings A., Hanane T., Demirjian S., Torbic H. Factors associated with acute kidney injury in acute respiratory distress syndrome. Ann Intensive Care. 2019;9:74. doi: 10.1186/s13613-019-0552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Husain-Syed F., Slutsky A.S., Ronco C. Lung–kidney cross-talk in the critically Ill patient. Am J Respir Crit Care Med. 2016;194:402–414. doi: 10.1164/rccm.201602-0420CP. [DOI] [PubMed] [Google Scholar]
- 35.Zhou L., Niu Z., Jiang X., Zhang Z., Zheng Y., Wang Z. Systemic analysis of tissue cells potentially vulnerable to SARS-CoV-2 infection by the protein-proofed single-cell RNA profiling of ACE2, TMPRSS2 and Furin proteases. iScience. 2020;28:101744. doi: 10.1016/j.isci.2020.101744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dastan F., Saffaei A., Mortazavi S.M., Jamaati H., Adnani N., Roudi S.S. Continues renal replacement therapy (CRRT) with disposable hemoperfusion cartridge: a promising option for severe COVID-19. J Glob Antimicrob Resist. 2020;21:340–341. doi: 10.1016/j.jgar.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams E., Mousa A.Y. Achieving a popliteal venous access for RRT in critically ill COVID-19 patient in prone position. J Vasc Surg Cases Innov Tech. 2020;6:266–268. doi: 10.1016/j.jvscit.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui P., Chen Z., Wang T., Dai J., Zhang J., Ding T. Clinical features and sexual transmission potential of SARS-CoV-2 infected female patients: a descriptive study in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.02.26.20028225. [DOI] [Google Scholar]
- 40.Ma L., Xie W., Li D., Shi L., Mao Y., Xiong Y. Effect of SARS-CoV-2 infection upon male gonadal function: a single center-based study. medRxiv. 2020 doi: 10.1101/2020.03.21.20037267. [DOI] [Google Scholar]
- 41.Song C., Wang Y., Li W., Hu B., Chen G., Xia P. Detection of 2019 novel coronavirus in semen and testicular biopsy specimen of COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.03.31.20042333. [DOI] [Google Scholar]
- 42.Paoli D., Pallotti F., Colangelo S., Basilico F., Mazzuti L., Turriziani O. Study of SARS-CoV-2 in semen and urine samples of a volunteer with positive naso-pharyngeal swab. J Endocrinol Invest. 2020;43:1819–1822. doi: 10.1007/s40618-020-01261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song C., Wang Y., Li W., Hu B., Chen G., Xia P. Absence of 2019 novel coronavirus in semen and testes of COVID-19 patients. Biol Reprod. 2020;3:4–6. doi: 10.1093/biolre/ioaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li D., Jin M., Bao P., Zhao W., Zhang S. Clinical characteristics and results of semen tests among men with Coronavirus Disease 2019. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.ASRM/SART SART and ASRM issue advice for infertility patients concerning the novel coronavirus (COVID-19) ASRM Bulletin. 2020;22 https://www.sart.org/news-and-publications/news-and-research/press-releases-and-bulletins/sart-and-asrm-issue-advice-for-infertility-patients-concerning-the-novel-coronavirus-covid-19 [Google Scholar]
- 46.Centers for Disease Control and Prevention Clinical questions about COVID-19: questions and answers. https://www.cdc.gov/coronavirus/2019-ncov/hcp/faq.html
- 47.La Marca A., Niederberger C., Pellicer A., Nelson S.M. COVID-19: lessons from the Italian reproductive medical experience. Fertil Steril. 2020;113:920–922. doi: 10.1016/j.fertnstert.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Balasubramanian V., Naing S. Hypogonadism in chronic obstructive pulmonary disease: incidence and effects. Curr Opin Pulm Med. 2012;18:112–117. doi: 10.1097/MCP.0b013e32834feb37. [DOI] [PubMed] [Google Scholar]
- 49.Caminiti G., Volterrani M., Iellamo F., Marazzi G., Massaro R., Miceli M. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure a double-blind, placebo-controlled, randomized study. J Am Coll Cardiol. 2009;54:919–927. doi: 10.1016/j.jacc.2009.04.078. [DOI] [PubMed] [Google Scholar]
- 50.Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maggio M., Basaria S., Ceda G.P., Ble A., Ling S.M., Bandinelli S. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest. 2005;28:116–119. [PubMed] [Google Scholar]
- 52.Nettleship J.E., Pugh P.J., Channer K.S., Jones T., Jones R.D. Inverse relationship between serum levels of interleukin-1 beta and testosterone in men with stable coronary artery disease. Horm Metab Res. 2007;39:366–371. doi: 10.1055/s-2007-976543. [DOI] [PubMed] [Google Scholar]
- 53.Chen J.W., Jiang Q., Xia X., Liu K., Yu Z., Tao W. Individual variation of the SARS-CoV2 receptor ACE2 gene expression and regulation. Aging Cell. 2020;7 doi: 10.1111/acel.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quan W., Zheng Q., Tian J., Chen J., Liu Z., Chen X. No SARS-CoV-2 in expressed prostatic secretion of patients with coronavirus disease 2019: a descriptive multicentre study in China. medRxiv. 2020 doi: 10.1101/2020.03.26.20044198. [DOI] [Google Scholar]
- 55.Song H., Seddighzadeh B., Cooperberg M.R., Huang F. Expression of ACE2, the SARS-CoV-2 receptor, and TMPRSS2 in prostate epithelial cells. bioRxiv. 2020 doi: 10.1101/2020.04.24.056259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin W., Hu L., Zhang Y., Ooi J.D., Meng T., Jin P. Single-cell analysis of ACE2 expression in human kidneys and bladders reveals a potential route of 2019-nCoV infection. bioRxiv. 2020 doi: 10.1101/2020.02.08.939892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yam W.C., Chan K.H., Poon L.L., Guan Y., Yuen K.Y., Seto W.H. Evaluation of reverse transcription-PCR assays for rapid diagnosis of severe acute respiratory syndrome associated with a novel coronavirus. J Clin Microbiol. 2003;41:4521–4524. doi: 10.1128/JCM.41.10.4521-4524.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen Z., Shen T., Wientjes M.G., O'Donnell M.A., Au J.L. Intravesical treatments of bladder cancer: review. Pharm Res (N Y) 2008;25:1500–1510. doi: 10.1007/s11095-008-9566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ouanes Y., Bibi M., Baradai N., Boukhris M., Chaker K., Kacem A. Does BCG protect against SARS-CoV-2 infection?: elements of proof. medRxiv. 2020 doi: 10.1101/2020.05.01.20087437. [DOI] [Google Scholar]
- 60.Moorlag S., Arts R.J.W., van Crevel R., Netea M.G. Non-specific effects of BCG vaccine on viral infections. Clin Microbiol Infect. 2019;25:1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 61.Mo P., Xing Y., Xiao Y., Deng L., Zhao Q., Wang H. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020:ciaa270. doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Br Med J. 2020;368:m129. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Korean Society of Infectious Diseases Report on the epidemiological features of coronavirus disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Kor Med Sci. 2020;35:e112. doi: 10.3346/jkms.2020.35.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Channappanavar R., Fett C., Mack M., Ten Eyck P.P., Meyerholz D.K., Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198:4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suba Z. DNA stabilization by the upregulation of estrogen signaling in BRCA gene mutation carriers. Drug Des Dev Ther. 2015;9:2663–2675. doi: 10.2147/DDDT.S84437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Newton A.H., Cardani A., Braciale T.J. The host immune response in respiratory virus infection: balancing virus clearance and immunopathology. Semin Immunopathol. 2016;38:471–482. doi: 10.1007/s00281-016-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kadel S., Kovats S. Sex hormones regulate innate immune cells and promote sex differences in respiratory virus infection. Front Immunol. 2018;9:1653. doi: 10.3389/fimmu.2018.01653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suba Z. Activating mutations of ESR1, BRCA1 and CYP19 aromatase genes confer tumor response in breast cancers treated with antiestrogens. Recent Pat Anti-Cancer Drug Discov. 2017;12:136–147. doi: 10.2174/1574892812666170227110842. [DOI] [PubMed] [Google Scholar]
- 69.Douglas G.C., O'Bryan M.K., Hedger M.P., Lee D.K., Yarski M.A., Smith A.I. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145:4703–4711. doi: 10.1210/en.2004-0443. [DOI] [PubMed] [Google Scholar]
- 70.Honorato-Sampaio K., Pereira V.M., Santos R.A., Reis A.M. Evidence that angiotensin-(1–7) is an intermediate of gonadotrophin-induced oocyte maturation in the rat preovulatory follicle. Exp Physiol. 2012;97:642–650. doi: 10.1113/expphysiol.2011.061960. [DOI] [PubMed] [Google Scholar]
- 71.Dalpiaz P.L., Lamas A.Z., Caliman I.F., Ribeiro R.F., Jr., Abreu G.R., Moyses M.R. Sex hormones promote opposite effects on ACE and ACE2 activity, hypertrophy and cardiac contractility in spontaneously hypertensive rats. PloS One. 2015;10 doi: 10.1371/journal.pone.0127515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gupte M., Thatcher S.E., Boustany-Kari C.M., Shoemaker R., Yiannikouris F., Zhang X. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler Thromb Vasc Biol. 2012;32:1392–1399. doi: 10.1161/ATVBAHA.112.248559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gupte M., Boustany-Kari C.M., Bharadwaj K., Police S., Thatcher S., Gong M.C. ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2008;295:R781–R788. doi: 10.1152/ajpregu.00183.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ji H., Menini S., Zheng W., Pesce C., Wu X., Sandberg K. Role of angiotensin-converting enzyme 2 and angiotensin (1–7) in 17 beta-oestradiol regulation of renal pathology in renal wrap hypertension in rats. Exp Physiol. 2008;93:648–657. doi: 10.1113/expphysiol.2007.041392. [DOI] [PubMed] [Google Scholar]
- 75.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hemnes A.R., Rathinasabapathy A., Austin E.A., Brittain E.L., Carrier E.J., Chen X. A potential therapeutic role for angiotensin-converting enzyme 2 in human pulmonary arterial hypertension. Eur Respir J. 2018;51:1702638. doi: 10.1183/13993003.02638-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khan A., Benthin C., Zeno B., Albertson T.E., Boyd J., Christie J.D. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21:234. doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tomlins S.A., Rhodes D.R., Perner S., Dhanasekaran S.M., Mehra R., Sun X.W. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 81.Lucas J.M., Heinlein C., Kim T., Hernandez S.A., Malik M.S., True L.D. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4:1310–1325. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stopsack K.H., Mucci L.A., Antonarakis E.S., Nelson P.S., Kantoff P.W. TMPRSS2 and COVID-19: serendipity or opportunity for intervention? Canc Discov. 2020;10:779–782. doi: 10.1158/2159-8290.CD-20-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bennett C.L., Price D.K., Kim S., Liu D., Jovanovic B.D., Nathan D. Racial variation in CAG repeat lengths within the androgen receptor gene among prostate cancer patients of lower socioeconomic status. J Clin Oncol. 2002;20:3599–3604. doi: 10.1200/JCO.2002.11.085. [DOI] [PubMed] [Google Scholar]
- 84.Tahmineh M., Fatemeh H., Neda G., Babak E., Atousa Y., Ghomareza H. COVID-19 and multiorgan failure: a narrative review on potential mechanisms. J Mol Histol. 2020;51:613–628. doi: 10.1007/s10735-020-09915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qi J., Zhou Y., Hua J., Zhang L., Bian J., Liu B. The scRNA-seq expression profiling of the receptor ACE2 and the cellular protease TMPRSS2 reveals human organs susceptible to COVID-19 infection. bioRxiv. 2020 doi: 10.1101/2020.04.16.045690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu L., Shu H., Li H., Tao Q., Zhou J., Chen G. Slow recovery from critical coronavirus disease 2019 pneumonia in an immunosuppressed renal transplant recipient with early acute cardiorenal syndrome. Cardiorenal Med. 2020;28:1–6. doi: 10.1159/000510916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu L., Mao Y., Chen G. Risk factors for 2019 novel coronavirus disease (COVID-19) patients progressing to critical illness: a systematic review and meta-analysis. Aging. 2020;12:12410–12421. doi: 10.18632/aging.103383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hatem A., Ahmed D., Mahmoud M.M., Sohail A.S., Lenar Y., Jyoti B. Survival rate in acute kidney injury superimposed COVID-19 patients: a systematic review and meta-analysis. Ren Fail. 2020;42:393–397. doi: 10.1080/0886022X.2020.1756323. [DOI] [PMC free article] [PubMed] [Google Scholar]