Graphical abstract
Keywords: COVID-19 pneumonia, Down syndrome, Immune dysregulation, Immune activation
Abstract
We report two cases of Corona Virus Disease-19 (COVID-19) in patients with Down Syndrome (DS) and describe the identification, diagnosis, clinical course and management of the infection. Down Syndrome, which is caused by trisomy 21, is characterized by immune dysregulation, anatomical differences in the upper respiratory tract and higher rate of comorbidities. All these risk factors can contribute to more severe clinical presentations of COVID-19 in this population. It is essential to raise awareness of the clinical relevance of SARS-COV-2 infection in DS patients, as well as in other most vulnerable patients, in order to improve their management and treatment and to encourage vaccinating these individuals early, once a vaccination is available.
Introduction
In December 2019, the novel coronavirus SARS-CoV-2 was identified as the etiologic agent of the COronaVIrus Disease-19 (COVID-19) outbreak occurring in Wuhan, China (Zhu et al., 2020). The clinical spectrum of COVID-19 is wide, ranging from asymptomatic infection to severe disease and death. Pro-inflammatory factors play a central role in COVID-19 severity and mortality, inducing an excessive inflammatory and immune response, leading to acute respiratory distress (ARDS) and multi-organ failure (MOF) (Zhou et al., 2020).
Down Syndrome (DS) is the most common chromosomal abnormality in humans. It is caused by trisomy 21 (T21) and is characterized by an immune dysregulation that predisposes the individual to autoimmune disorders (Nisihara et al., 2007) and to anatomical differences in the upper respiratory tract (such as a smaller trachea enlarged adenoids/tonsils, glossoptosis, upper airway narrowing and tracheal bronchus airway malacia) that in turn predispose the individual to a high frequency of respiratory disease, which is the leading cause of mortality in persons with DS (Colvin and Yeager, 2017; Englund et al., 2013). In particular, lower respiratory tract infections (LRTIs) account for 43–78% of intensive care unit (ICU) admissions, and 50% of those admissions require ventilation support (Watts and Vyas, 2013).
Respiratory syncytial virus is the most common cause of LRTIs in DS, leading to severe bronchiolitis. Moreover, during the H1N1 pandemic, higher risks of hospitalization, endotracheal intubation (EI) and death have been reported in DS patients (Pérez-Padilla et al., 2010). Despite the increased rate of respiratory infections, no increased risk of SARS-CoV-2 infection in DS individuals has been described.
We report two COVID-19 cases in Caucasian patients with DS who, through a nasopharyngeal swab (NFS), tested positive for SARS-CoV-2 without other respiratory coinfections, with a chest computer tomography (CT) scan showing a bilateral interstitial pneumonia.
Case 1
A 59-year-old DS woman with previous history of congenital hydrocephalus, epilepsy and hypothyroidism, coming from a rehabilitation facility, was admitted on March 4th, 2020, to the Emergency Department (ED) because of acute dyspnoea. Physical examination showed Glasgow Coma Scale 12, body temperature (BT) 37.0 °C, blood pressure (BP) 80/50 mmHg, heart rate (HR) 90 bpm and peripheral oxygen saturation (SpO2) on ambient air (AA) 93% with a PaO2/FiO2 (P/F) ratio of 262 mmHg. Blood tests revealed severe lymphopenia (230 cells/μl), thrombocytopenia (60.000/μl), high C-reactive protein levels (CRP) (14.85 mg/dL, normal range <1) and mild hypokalaemia (2.81 mmol/L, normal range 3.5–5 mmol/L) (Table 1 ). D-dimer levels (370 ng/ml) were slightly above the normal value (<250 ng/ml). In the ED, the patient had multiple generalized seizure episodes, and P/F ratio fell to 162 mmHg. A cranial CT scan did not reveal CNS abnormalities, except for her tetraventricular hydrocephalus. Oxygen supplementation with O2 at 10 L/min, sub-cutaneous enoxaparin (4000 UI/daily) and intravenous (iv) sodium valproate (800 mg/daily), methylprednisolone (1 mg/Kg/daily), piperacillin-tazobactam (13.5 mg/daily) and noradrenaline (2 ml/h) were started.
Table 1.
Laboratory parameters of the two patients with Down Syndrome.
| Days after disease onset | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 |
4 |
7 |
8 |
11 |
12 |
|||||||
| Case 1 | Case 2 | Case 1 | Case 2 | Case 1 | Case 2 | Case 1 | Case 2 | Case 1 | Case 2 | Case 1 | Case 2 | |
| WBC (cells/μL) | 6540 | 3710 | 7410 | 3660 | 8777 | 8450 | 24100 | 7990 | 8820 | |||
| Lymphocyte (cells/μL; %) | 230; 3,5 | 482; 13 | 500; 6,8 | 1070; 29,2 | 600; 6,8 | 610; 7,2 | 810; 3,4 | 680; 8,5 | 770; 8.7 | |||
| CPR (mg/dL) | 14,85 | 4.6 | 2,11 | 7.82 | 0,37 | 5.1 | ||||||
| AST (U/L) | 25 | 30 | 14 | 71 | 13 | 17 | 24 | 31 | ||||
| ALT (U/L) | 6 | 26 | 11 | 128 | 9 | 12 | 54 | 52 | ||||
| Total bilirubin (mg/dL) | 0.22 | 0,77 | 0.67 | 1,51 | 1,08 | 0.51 | 0.45 | |||||
| GGT (U/L) | 20 | 31 | 45 | 28 | 25 | 41 | 45 | |||||
| D-dimer (ng/mL) | 370 | 1266 | 531 | 993 | ||||||||
WBC whole blood count, CPR c-protein reactive, AST aspartate aminotransferase, ALT Alanine transaminase.
From days 1 to 5, she was in a serious clinical but stable condition, with a stable 200 mmHg P/F ratio. At day 3, lopinavir/ritonavir therapy (400 mg/100 mg/daily) was started. At day 6, the patient’s condition became critical, with a P/F ratio persistently below 200 mmHg. From day 6 to 9, O2 supplementation was increased to 12 L/min, without improvement. Blood tests revealed severe hypokalaemia (1.7 mmol/L) and hypocalcaemia (6.2 mg/dl; normal range: 8.6–10.4), mild hypernatremia and an increased lactate dehydrogenase (LDH) level (314 U/L; normal range: 120–246). The patient died on March 17, 2020.
Case 2
A 42-year-old DS woman with hypothyroidism was admitted on March 24th 2020 to the ED because of fever in the 6 previous days. She lived in a home setting where her brother had been diagnosed with COVID-19 pneumonia. Physical examination showed BT 37 °C, BP 130/80 mmHg, HR 100 bpm and SpO2 95% on AA, with a P/F ratio of 538 mmHg. Blood tests showed lymphopenia (500 cells/μl), thrombocytopenia (99,000/μl) and increased CRP level: 4.63 mg/dl (Table 1). Hydroxychloroquine (400 mg/daily), lopinavir/ritonavir (400/100 gr/daily) and IV ceftriaxone (2 grams/daily) were started. From days 2 to 5, increased LDH (293 U/L), CRP (7.82), ferritin (1.633 ng/ml) and D-dimer (1.266 ng/ml) levels, thrombocytopenia (79,000/μl), lymphopenia (600/μl) and aspartate transaminase (AST) 71 UI/l were recorded, and a gradual reduction of P/F ratio to 140 mmHg was observed. IV methylprednisolone (1 mg/Kg/daily) and increased oxygen supplementation until continuous positive airway pressure (CPAP) supplementation were prescribed. On day 6, considering a P/F ratio of 100 mmHg during CPAP, the patient received IV 400 mg sarilumab therapy, underwent endotracheal intubation and was transferred to an ICU. She was mechanically ventilated for 5 days with a progressive improvement, and on day 29, she was discharged, with two consecutive NFS resulted negative for SARS-CoV-2.
Discussion and conclusions
We described two patients with DS affected by COVID-19: one of them had an unfavourable clinical outcome. DS presents with a complex clinical spectrum of variable features affecting most organ systems. Various genetic mechanisms may be contributors to the phenotype of DS, and more than 80 clinical features with variation in number and in severity are reported in DS (Epstein et al., 1991; Patterson, 2007). Our two patients had different features of DS, with case 1 presenting a more complexed clinical DS with congenital hydrocephalus and epilepsy.
The two individuals were of different ages. The first patient was close to the maximum lifespan for DS, and an older adult is commonly considered to be an independent risk factor for an unfavourable outcome in patients with COVID-19 (Zhou et al., 2020).
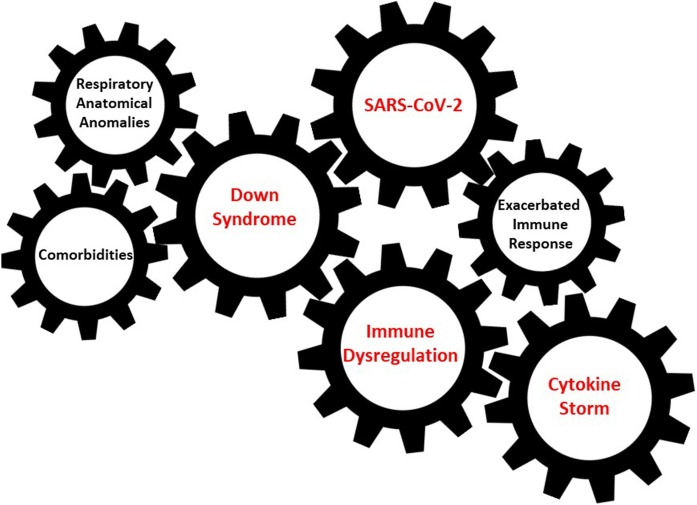
Both individuals presented lymphopenia at admission. The first patient had a stable course of disease for 10 days, requiring low-flow oxygen supplemental therapy and without major inflammatory index alterations, until precipitated. The second patient showed a progressive increase in both inflammation indexes and need of oxygen supplementation until CPAP support and mechanical ventilation by EI was required. As for SARS-CoV-1 and Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infections, severe respiratory deterioration in COVID-19 is usually related to an exacerbated immune response to the virus, leading to the cytokine release syndrome (Picchianti Diamanti et al., 2020, Channappanavar and Perlman, 2017). Patients with severe COVID-19 had lymphopenia along with high levels of circulating pro-inflammatory cytokines compared to patients with mild COVID-19 illness. SARS-CoV-2 cytokine production appears to be related to Type I and III interferons (IFNs) and is followed by infiltration and activation of different immune cells, followed by hyperproduction of cytokines, exacerbated immune activation and progressive decline of respiratory function (Tisoncik et al., 2012). Studies have shown in DS the occurrence of an interferonopathic signature with a dysregulation of IFN-inducible cytokines causing a chronic inflammation state (Sullivan et al., 2016, Sullivan et al., 2017), along with a widespread hypersensitivity to IFN stimulation across the human immune system (Araya et al., 2019). Recently, in an animal model carrying triplication of these IFN receptors, activation of Toll-like receptors and consequent induction of the IFN antiviral response led to a cytokine storm with a worsening of liver pathology and rapid death (Tuttle et al., 2020) (Fig. 1 ).
Fig. 1.
Interaction between SARS-CoV-2 and immune dysregulation and other potential risk factors present in individuals with Down Syndrome.
The first patient did not receive immunomodulating therapy as it was not yet available at the time of admission, while the second one was administered the anti-IL-6 agent sarilumab, and in the following days, she had a progressive improvement of respiratory function until recovery. Retrospective studies indicated that an elevated level of IL-6, an inflammatory cytokine, was associated with a risk of mortality in COVID-19, suggesting that it may act as a critical mediator for respiratory failure, shock and MOF (Ruan et al., 2020). Immunomodulatory agents targeting directly the key cytokines may also help to alleviate hyperinflammation symptoms in severe cases (Cantini et al., 2020a, Cantini et al., 2020b, Cantini et al., 2020c). Nevertheless, a phase 2/3 double blind randomized clinical trial, evaluating the clinical efficacy of sarilumab relative to the control arm in adult patients hospitalized with COVID-19 receiving mechanical ventilation at baseline, was terminated prematurely because the trial did not meet its primary and key secondary endpoints (clinicaltrial.gov).
In the light of such considerations, the different course of the two patients can be ascribed to: i) different expression and features of the DS, ii) different age and life expectancy of the two cases, iii) different host-virus interaction leading to a different immunological response that in the second patient resulted in a cytokine storm and iv) different outbreak period in which the infection was contracted. Case 1 was admitted at the beginning of the epidemic, when there was a limited knowledge of the clinical management of COVID-19. Case 2 was admitted about 20 days later when pathogenic mechanisms, drug protocols and indications for invasive respiratory support were more clearly defined.
In conclusion, individuals with DS, due to the high rate of comorbidities, anatomical differences in the upper respiratory tract and immune dysregulation, manifest several risk factors for respiratory infections and unfavourable outcome. Recognition of the clinical relevance of DS in the management of COVID-19 is crucial, and individuals with DS are among the priority candidates for early immunosuppression therapy, for available antiviral therapies and, finally, for SARS-CoV-2 vaccination, once available.
Funding
This work was supported by Line one-Ricerca Corrente ‘Infezioni Emergenti e Riemergenti’ and by Progetto COVID 2020 12371675 both funded by the Italian Ministry of Health.
Ethical approval
This study was approved by the Spallanzani Institute Ethical Board, and patients’ written informed consent for publication was collected.
Conflict of interest
All authors have no conflict of interest to declare.
Acknowledgments
COVID-19 INMI Study Group: Maria Alessandra Abbonizio, Chiara Agrati, Fabrizio Albarello, Gioia Amadei, Alessandra Amendola, Mario Antonini, Raffaella Barbaro, Barbara Bartolini, Martina Benigni, Nazario Bevilacqua, Licia Bordi, Veronica Bordoni, Marta Branca, Paolo Campioni, Maria Rosaria Capobianchi, Cinzia Caporale, Ilaria Caravella, Fabrizio Carletti, Concetta Castilletti, Roberta Chiappini, Carmine Ciaralli, Francesca Colavita, Angela Corpolongo, Massimo Cristofaro, Salvatore Curiale, Alessandra D’Abramo, Cristina Dantimi, Alessia De Angelis, Giada De Angelis, Rachele Di Lorenzo, Federica Di Stefano, Federica Ferraro, Lorena Fiorentini, Andrea Frustaci, Paola Gallì, Gabriele Garotto, Maria Letizia Giancola, Filippo Giansante, Emanuela Giombini, Maria Cristina Greci, Giuseppe Ippolito, Eleonora Lalle, Simone Lanini, Daniele Lapa, Luciana Lepore, Andrea Lucia, Franco Lufrani, Manuela Macchione, Alessandra Marani, Luisa Marchioni, Andrea Mariano, Maria Cristina Marini, Micaela Maritti, Giulia Matusali, Silvia Meschi, Francesco Messina Chiara Montaldo, Silvia Murachelli, Emanuele Nicastri, Roberto Noto, Claudia Palazzolo, Emanuele Pallini, Virgilio Passeri, Federico Pelliccioni, Antonella Petrecchia, Ada Petrone, Nicola Petrosillo, Elisa Pianura, Maria Pisciotta, Silvia Pittalis, Costanza Proietti, Vincenzo Puro, Gabriele Rinonapoli, Martina Rueca, Alessandra Sacchi, Francesco Sanasi, Carmen Santagata, Silvana Scarcia, Vincenzo Schininà, Paola Scognamiglio, Laura Scorzolini, Giulia Stazi, Francesco Vaia, Francesco Vairo, Maria Beatrice Valli.
Contributor Information
on behalf of INMI COVID-19 study groups:
Abbonizio, Chiara Agrati, Fabrizio Albarello, Gioia Amadei, Alessandra Amendola, Mario Antonini, Raffaella Barbaro, Barbara Bartolini, Martina Benigni, Nazario Bevilacqua, Licia Bordi, Veronica Bordoni, Marta Branca, Paolo Campioni, Maria Rosaria Capobianchi, Cinzia Caporale, Ilaria Caravella, Fabrizio Carletti, Concetta Castilletti, Roberta Chiappini, Carmine Ciaralli, Francesca Colavita, Angela Corpolongo, Massimo Cristofaro, Salvatore Curiale, Alessandra D’Abramo, Cristina Dantimi, Alessia De Angelis, Giada De Angelis, Rachele Di Lorenzo, Federica Di Stefano, Federica Ferraro, Lorena Fiorentini, Andrea Frustaci, Paola Gallì, Gabriele Garotto, Maria Letizia Giancola, Filippo Giansante, Emanuela Giombini, Maria Cristina Greci, Giuseppe Ippolito, Eleonora Lalle, Simone Lanini, Daniele Lapa, Luciana Lepore, Andrea Lucia, Franco Lufrani, Manuela Macchione, Alessandra Marani, Luisa Marchioni, Andrea Mariano, Maria Cristina Marini, Micaela Maritti, Giulia Matusali, Silvia Meschi, Francesco Messina Chiara Montaldo, Silvia Murachelli, Emanuele Nicastri, Roberto Noto, Claudia Palazzolo, Emanuele Pallini, Virgilio Passeri, Federico Pelliccioni, Antonella Petrecchia, Ada Petrone, Nicola Petrosillo, Elisa Pianura, Maria Pisciotta, Silvia Pittalis, Costanza Proietti, Vincenzo Puro, Gabriele Rinonapoli, Martina Rueca, Alessandra Sacchi, Francesco Sanasi, Carmen Santagata, Silvana Scarcia, Vincenzo Schininà, Paola Scognamiglio, Laura Scorzolini, Giulia Stazi, Francesco Vaia, Francesco Vairo, and Maria Beatrice Valli
References
- Araya P., Waugh K.A., Sullivan K.D., Núñez N.G., Roselli E., Smith K.P., et al. Trisomy 21 dysregulates T cell lineages toward an autoimmunity-prone state associated with interferon hyperactivity. PNAS USA. 2019;116:24231–24241. doi: 10.1073/pnas.1908129116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantini F., Niccoli L., Matarrese D., Nicastri E., Stobbione P., Goletti D. Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J Infect. 2020;81(2):318–356. doi: 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantini F., Niccoli L., Nannini C., Matarrese D., Natale M.E.D., Lotti P., et al. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J Infect. 2020;81(4):647–679. doi: 10.1016/j.jinf.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantini F., Goletti D., Petrone L., Najai Fard S., Niccoli L., Foti R. Immune therapy, or antiviral therapy, or both for COVID-19: a systematic review. Drugs. 2020;80(18):1929–1946. doi: 10.1007/s40265-020-01421-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin K.L., Yeager M.E. What people with Down Syndrome can teach us about cardiopulmonary disease. Eur Respir Rev. 2017;26:143. doi: 10.1183/16000617.0098-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund A., Jonsson B., Zander C.S., Gustafsson J., Annerén G. Changes in mortality and causes of death in the Swedish Down syndrome population. Am J Med Genet A. 2013;161A:642–649. doi: 10.1002/ajmg.a.35706. [DOI] [PubMed] [Google Scholar]
- Epstein C.J., Korenberg J.R., Anneren G., Antonarakis S.E., Ayme S., Courchesne E., et al. Protocols to establish genotype-phenotype correlations in Down syndrome. Am J Hum Genet. 1991;49:207–235. [PMC free article] [PubMed] [Google Scholar]
- Nisihara R.M., Skare T.L., Silva M.B., Messias-Reason I.T., Oliveira N.P., Fiedler P.T., et al. High positivity of anti-CCP antibodies in patients with Down syndrome. Clin Rheumatol. 2007;26:2031–2035. doi: 10.1007/s10067-007-0606-1. [DOI] [PubMed] [Google Scholar]
- Patterson D. Genetic mechanisms involved in the phenotype of Down syndrome. Ment Retard Dev Disabil Res Rev. 2007;13(3):199–206. doi: 10.1002/mrdd.20162. PMID: 17910086. [DOI] [PubMed] [Google Scholar]
- Pérez-Padilla R., Fernández R., García-Sancho C., Franco-Marina F., Aburto O., Lopez-Gatell H., Bojórquez I. Pandemic (H1N1) 2009 virus and Down syndrome patients. Emerg Infect Dis. 2010;16:1312–1314. doi: 10.3201/eid1608.091931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picchianti Diamanti A., Rosado M.M., Pioli C., Sesti G., Laganà B. Cytokine Release Syndrome in COVID-19 Patients, A New Scenario for an Old Concern: The Fragile Balance between Infections and Autoimmunity. Intern J Mol Sci. 2020;21(9):3330. doi: 10.3390/ijms21093330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;3 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K.D., Lewis H.C., Hill A.A., Pandey A., Jackson L.P., Cabral J.M., et al. Trisomy 21 consistently activates the interferon response. Elife. 2016;5 doi: 10.7554/eLife.16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K.D., Evans D., Pandey A., Hraha T.H., Smith K.P., Markham N., et al. Trisomy 21 causes changes in the circulating proteome indicative of chronic autoinflammation. Sci Rep. 2017;7(1):1–11. doi: 10.1038/s41598-017-13858-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle K.D., Minter R., Waugh K.A., Araya P., Ludwing M., Sempeck C., et al. JAK1 inhibition blocks lethal sterile immune responses: implications for COVID-19 therapy. bioRxiv. 2020 doi: 10.1101/2020.04.07.024455. [DOI] [Google Scholar]
- Watts R., Vyas H. An overview of respiratory problems in children with Down’s syndrome. Arch Dis Child. 2013;98(10):812–817. doi: 10.1136/archdischild-2013-304611. [DOI] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]