Abstract
A growing body of research is beginning to elucidate reasons people living with HIV (PLWHIV) might prefer oral daily antiretroviral treatment (ART) compared with emerging long-acting ART (LA-ART) or HIV remission strategies under investigation. Our objective is to provide qualitative insights into the reasons why PLWHIV might prefer one of these HIV control therapies over others. From May to August 2018, we implemented a semistructured cross-sectional survey of PLWHIV in the United States to better understand patient preferences around various HIV treatment and remission options. Using free text, respondents were asked to explain why they preferred one HIV control option over the other two. We analyzed responses to the open-ended survey questions on reasons for preferring oral daily ART versus LA-ART versus HIV remission strategies using conventional content analysis. The results showed that PLWHIV preferred oral daily ART because of its familiarity and known safety and efficacy profile, whereas those who preferred LA-ART would value the convenience it offers. Finally, HIV remission strategies would be preferred to avoid taking ART altogether. The qualitative results provide insights into reasons why PLWHIV in the United States might prefer oral daily ART versus novel therapies. More importantly, they provide information to better align HIV virological control strategies with end-user perspectives. To make informed choices around evolving HIV therapeutics, PLWHIV and HIV care providers would benefit from decision tools to better assess options and trade-offs. More research is needed on how best to effectively support PLWHIV and HIV care providers in shared decision-making.
Keywords: HIV control, antiretroviral treatment (ART), long-acting ART, HIV remission, HIV cure research, people living with HIV
Introduction
There are now >30 antiretroviral medications approved by the U.S. Food and Drug Administration,1 allowing people living with HIV (PLWHIV) to approach a near-normal life expectancy.2 Many of these medications are available as daily oral single-tablet regimens with high potency and low toxicity.3 Although antiretroviral treatment (ART) pill burden and side effect profiles have improved, maintaining lifelong ART adherence continues to be challenging as other barriers to adherence, such as stigma, remain unchanged.4 There are two major and concurrent lines of research focused on new HIV control options: (1) long-acting ART (LA-ART) formulations5,6 and (2) strategies for inducing durable ART-free HIV remission, with >250 active or completed clinical trials worldwide.7
On one hand, the advent of LA-ART represents a major paradigm shift in HIV therapy and seemingly obviates the need for daily ART adherence.6,8 Combination cabotegravir (CAB) and rilpivirine (RPV) regimens are currently in late-stage development as long-acting intramuscular injectable ART that may be coadministered monthly or bimonthly.5,6 On the other hand, remission strategies that would allow PLWHIV to maintain viral suppression for substantially extended periods after discontinuing oral ART are a global research focus.9 Such strategies for producing sustained ART-free virological remission are under early-phase investigations and include early ART, immune-based strategies, stem cell transplantations, cell and gene therapy approaches, and latency reversal agents.10
The importance of studying end-user perspectives in the development of new HIV control options is being increasingly recognized.9,11–13 A growing body of research is beginning to elucidate reasons PLWHIV might prefer oral daily ART to LA-ART or HIV remission strategies.9,14–18 For example, a recent conjoint analysis provided insights into the impact of specific attributes of LA-ART on acceptability among 56 PLWHIV recruited in Seattle, Washington, and Riverside, California.14 In this study, PLWHIV placed higher importance on the efficacy and dosing frequency of ART regimens.14 In an effort to better understand motivators and perceived benefits of various HIV control options, we implemented a cross-sectional survey of PLWHIV in the United States to better understand reasons patients might prefer various HIV treatment and remission options.9 Quantitative results were previously published.9 In this study, we report qualitative results from the semistructured survey asking PLWHIV to provide reasons why they might prefer oral daily ART over LA-ART or HIV remission strategies.
Methods
From May to August 2018, we implemented an online nationwide cross-sectional survey using Qualtrics (Provo, UT). Survey methodologies, inclusion/exclusion criteria, and recruitment methods are described elsewhere.9 All participants provided online informed consent before continuing to the survey. The study received approval from the University of North Carolina at Chapel Hill Non-Biomedical IRB (study #17-3084).
We asked participants to select a preferred hypothetical HIV control option from (1) standard oral daily ART, (2) LA-ART formulation (e.g., injectables or implantables) that would last for 1, 2, or 6 months, and (3) an HIV remission strategy that might keep HIV suppressed but about which less is known. Using free text, respondents were then asked to explain why they preferred this HIV control option over the other two.
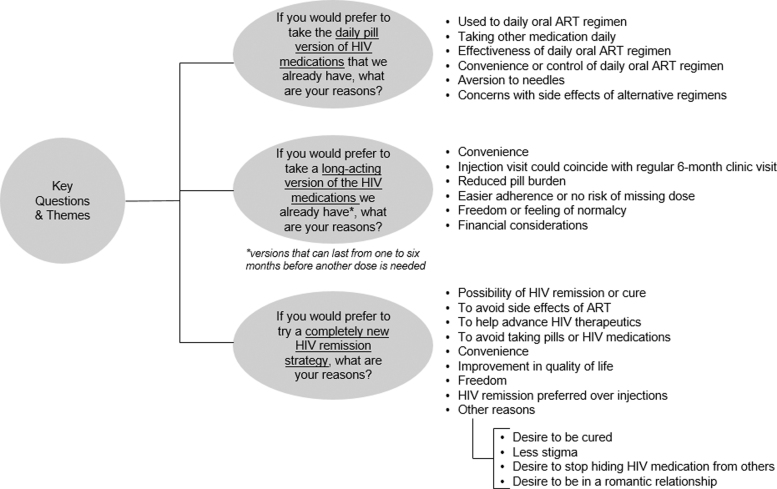
We analyzed responses to the open-ended survey questions on reasons for preferring oral daily ART versus LA-ART versus HIV remission strategies using conventional content analysis. We systematically organized text units into a structured format without using a pre-existing coding scheme. The lead author (K.D.) organized emergent themes, and for each HIV control option, responses were clustered into key themes. A research associate (K.E.P.) reviewed the responses and confirmed the themes that initially emerged (Fig. 1). Two members of the research team (K.D. and K.E.P.) organized quotations illustrating the main themes identified in open text fields (Supplementary Table S1).
FIG. 1.
Analytic coding tree—PLWHIV's preferences for oral daily ART versus LA-ART formulations versus HIV remission (United States, 2018). ART, antiretroviral treatment; LA-ART, long-acting antiretroviral treatment; PLWHIV, people living with HIV.
Results
There were 282 eligible respondents (mean age 47 years): 63% cisgender men, 35% cisgender women, and 1% transgender women (1% did not specify a gender).9 Participants were ethnically and racially diverse: 65% were white/Caucasian, 24% black/African American, 4% Asian, 4% multiracial, and 3% other.9
When presented with a choice between the three HIV control options, 9% of respondents preferred oral daily ART. Of 55% of respondents who would prefer LA-ART, dosing intervals substantially affected participant preferences, which were divided as follows: 6% reported that they would choose it if administered monthly, 7% if administered bimonthly, and 42% if administered every 6 months.9 In addition, 24% of respondents stated they would prefer an ART-free HIV remission strategy different from current regimens. Approximately 12% did not know which option they would choose.9
Of respondents who preferred to continue taking daily oral ART, their reasons were clustered into six key themes: (1) participants were used to their current oral daily ART, (2) they were also taking other non-HIV medications daily so taking one fewer daily pill would not make a difference, (3) they believed in the effectiveness of oral ART, (4) they preferred the convenience or control they had over oral ART, (5) they were averse to needles, and (6) they learned to manage side effects of oral daily ART and were concerned with potential side effects of alternative regimens (Fig. 1 and Supplementary Table S1).
Of respondents who preferred to take LA-ART, reasons were grouped into six key themes: (1) convenience of LA-ART formulation, (2) reduced pill burden, (3) easier adherence and no risk of inadvertently missing a dose, (4) freedom or feeling of normalcy, (5) financial considerations, and (6) preference for injection visits that would coincide with regular 6-monthly clinic visits (Fig. 1 and Supplementary Table S1).
Of those who preferred to try a completely new HIV remission strategy, reasons were categorized into nine key themes: (1) the possibility of achieving HIV remission or cure, (2) avoiding long-term ART side effects, (3) helping advance HIV therapeutics, (4) avoiding taking daily pills, (5) convenience, (6) improving their quality of life, (7) freedom from medication, (8) preference of HIV remission over injections, and (9) reduced stigma (e.g., desire to stop hiding HIV medications or to pursue romantic relationships without concern for taking pills) (Fig. 1 and Supplementary Table S1).
Discussion
This brief report provides insights into some of the factors that could influence the preferences of PLWHIV in the United States toward different HIV therapeutic strategies, including preferences for either oral daily ART versus emerging LA-ART, or remission options under clinical development. The results showed that PLWHIV who preferred oral daily ART noted its familiarity and known safety and efficacy profile, whereas those who preferred LA-ART particularly valued the convenience it offers. Finally, participants who preferred an HIV remission strategy, wanted to avoid taking ART altogether. The results provide information to better align HIV virological control strategies with end-user perspectives.9,13
Reasons why PLWHIV might prefer to stay on oral daily ART regimens reflect the tremendous scientific achievements for the past three decades to make therapies more potent, safe, convenient, and tolerable.3,19 Nevertheless, challenges remain, such as drug–drug interactions, real and perceived long-term toxicity, earlier onset of comorbidities, and suboptimal adherence.6 Moreover, for some populations, notably adolescents and young adults, ART adherence remains a significant barrier.20
LA-ART may improve adherence and limit HIV stigma associated with daily pill taking.6 As shown in a recent study among 374 PLWHIV in the United States, the most common benefit of LA-ART would be eliminating the need to remember to take daily pills.15 Clinical research has demonstrated high acceptability of LA-ART, with 97% of CAB+RPV trial participants who switched to LA-ART preferring monthly injections over previous oral daily ART.21 Reasons why PLWHIV might prefer LA-ART in our study corroborate results from studies conducted among participants in the Phase IIb and Phase III LATTE-1 LA-ART trials, including improved convenience, freedom, greater peace of mind and confidentiality, and the psychosocial and emotional benefits associated with not being constantly reminded of one's HIV status.16,22 Intermittent dosing of LA-ART may also reduce the anxiety of being completely off ART.22 It must be acknowledged, however, that LATTE trial participants may have had a high a priori interest in LA-ART, which may explain their high affinity for this strategy.
Potential drawbacks of LA-ART, however, include possible side effects (such as injection site reactions), frequent dosing, and the need to adhere to clinic/injection visits.17 Furthermore, PLWHIV with other chronic conditions may not see major reductions in their daily pill burden, which would be the case if such individuals were taking additional ART medications or medications for other diseases or conditions.17 In our survey, acceptance for LA-ART seemed higher if injections could coincide with clinic visits every 6 months. These results differ from a cross-sectional survey conducted among 303 youth living with HIV aged 13–24 years in the United States that found greater acceptance for injections taken every 3 months, paralleling contraceptive (i.e., Depo-Provera or medroxyprogesterone) injections.23 Our results also contradict results from a cross-sectional survey conducted among adults living with HIV in North and South Carolina who showed greatest interest in single weekly pills and least interest in regimens taken biannually.24 These data indicate that more research is needed to contextualize the diverse findings and support scaling up efforts of LA-ART. As well, given the significant differences that women express in their preferences for various hormonal contraceptive methods, similar research focused on women should keep this in mind and perhaps incorporate contraceptive preferences as covariables.
PLWHIV provided reasons why sustained ART-free remission strategies may be desirable in the future. Importantly, the continued salience of stigma in people's lives was clear.22,25,26 Our findings are concordant with previous research on preferable attributes of HIV remission or cure strategies–such as the desire to completely eliminate HIV,9 to stop taking ART,9,25 or to have improved romantic relationships.25 Results also indicate the need to appreciate psychosocial and emotional aspects of novel HIV control options.27 PLWHIV indicated that they strongly valued improvements in quality of life, yet there can be a fundamental tension between desire for an improved quality of life and need to interrupt HIV treatment to show efficacy of HIV remission strategies associated with the possibility of transmitting HIV to a sexual partner if virological suppression is lost.28–30 For the full potential of HIV control regimens to be realized, PLWHIV will also want freedom from the possibility of transmitting HIV to sexual partners.31 If PLWHIV adopt LA-ART, it will be more difficult for them to subsequently engage in HIV remission research requiring ART interruptions to show efficacy of interventions.
We must acknowledge study limitations. Survey questions were hypothetical, and the sample may have been skewed toward PLWHIV most interested in advancing HIV therapeutics. In addition, the survey questions were presented without regard to, or mention of, external or structural factors. Thus, the nuanced reasoning for preferring HIV control methodologies could have been limited by the form of the questions posed. Results could be enhanced with in-depth interviews with PLWHIV to delve deeper into reasons for preferring various HIV control options. Additional limitations are discussed in previously published research.9
Conclusions
A nuanced understanding of patient preferences will contribute to a patient-centered drug development process that fully engages the communities for which it is intended and not one guided solely by advances in the scientific understanding of virology, immunology, and biochemistry.32 Acceptability research should become a critical adjunct to ongoing biomedical research efforts aimed at improving HIV control options.17,22,33–35 To make informed choices around evolving HIV therapeutics, PLWHIV and HIV care providers would benefit from decision tools to better assess options and trade-offs. More research is needed on how best to effectively support PLWHIV and HIV care providers in shared decision-making.
Supplementary Material
Acknowledgments
We are extremely grateful to all the survey participants. We also thank all community members who diligently reviewed the survey instrument, particularly Dawn Averitt, Krista Martel, and Maria Mejia.
Author Disclosure Statement
K.D., D.M.C., K.E.P., J.T.K., P.S., and J.A.S. have no conflict of interest to declare. D.E. participates in unpaid activities that are not part of this study for Gilead Sciences, Inc. T.P. has received research funding from Gilead Sciences, Inc.
Funding Information
This study was supported by grant funding from Gilead Sciences, Inc. Gilead Sciences, Inc. has had no input into the development or content of these materials. K.D. received support from R21MH118120, amfAR Institute for HIV Cure Research (amfAR 109301), UM1AI126620 (BEAT-HIV Collaboratory) co-funded by NIAID, NIMH, NINDS, and NIDA and AI131385 (P01 Smith—Revealing Reservoirs during Rebound (R3)—Last Gift). P.S. and K.D. received support from R21MH122280.
Supplementary Material
References
- 1. U.S. DHHS: FDA-Approved HIV Medicines. AIDSinfo (2020). Available at https://aidsinfo.nih.gov/understanding-hiv-aids/fact-sheets/21/58/fda-approved-hiv-medicines, accessed April28, 2020
- 2. Marcus JL, Chao CR, Leyden WA, et al. : Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. JAIDS 2016;73:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nachega J, Patienti J, Uthman O: Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: A meta-analysis of randomized controlled trials. Clin Infect Dis 2014;58:1297–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Buell KG, Chung C, Chaudhry Z, Puri A, Nawab K, Ravindran RP: Lifelong antiretroviral therapy or HIV cure: The benefits for the individual patient. AIDS Care 2016;28:242–246 [DOI] [PubMed] [Google Scholar]
- 5. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. : Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017;390:1499–1510 [DOI] [PubMed] [Google Scholar]
- 6. Amico RD, Margolis DA: Long-acting injectable therapy: An emerging paradigm for the treatment of HIV infection. Curr Opin HIV AIDS 2020;15:13–18 [DOI] [PubMed] [Google Scholar]
- 7. TAG: Research Toward a Cure Trials. (2019). Available at www.treatmentactiongroup.org/cure/trials accessed July17, 2020
- 8. Czarnogorski M: Using implementation science to better integrate novel long-acting injectable therapy into routine HIV care. J Acquir Immune Defic Syndr 2019;82:286–288 [DOI] [PubMed] [Google Scholar]
- 9. Dubé K, Eskaf S, Evans D, et al. : The dose response: Perceptions of people living with HIV in the United States on alternatives to oral daily antiretroviral therapy. AIDS Res Hum Retroviruses 2020;36:324–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deeks SG, Lewin SR, Ross AL, et al. : International AIDS society global scientific strategy: Towards an HIV cure 2016. Nat Med 2016;22:839–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. FDA: The Voice of the Patient. 2014. Available at: https://www.fda.gov/media/88257/download
- 12. FDA: Backgrounder for FDA's HIV Patient-Focused Drug Development and HIV Cure Research Public Meeting. (2013). Available at www.fda.gov/downloads/ForIndustry/UserFees/PrescriptionDrugUserFee/UCM354549.pdf accessed July17, 2020
- 13. Dubé K, Barr L, Palm D, Brown B, Taylor J: Putting participants at the centre of HIV cure research. Lancet HIV 2019;3018:18–19 [DOI] [PubMed] [Google Scholar]
- 14. Simoni JM, Tapia K, Lee SJ, et al. : A conjoint analysis of the acceptability of targeted long-acting injectable antiretroviral therapy among persons living with HIV in the U.S. AIDS Behav 2020;24:1226–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dandachi D, Dang BN, Lucari B, Swindells S, Giordano TP: Acceptability and preferences for long-acting antiretroviral formulations among people with HIV infection. AIDS Care 2020;0:1–9 [DOI] [PubMed] [Google Scholar]
- 16. Mantsios A, Murray M, Karver TS, et al. : Efficacy and freedom: Patient experiences with the transition from daily oral to long-acting injectable antiretroviral therapy to treat HIV in the context of phase 3 trials. AIDS Behav 2020;DOI: 10.1007/s10461-020-02918-x. [DOI] [PubMed] [Google Scholar]
- 17. Simoni JM, Beima-Sofie K, Mohamed ZH, et al. : Long-acting injectable antiretroviral treatment a qualitative study among US providers, adults living with HIV, and parents of youth living with HIV. AIDS Patient Care STDS 2019;33:104–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saberi P, Eskaf S, Sauceda JA, Dubé K: Perceptions of HIV virologic control strategies among younger and older age groups of people living with HIV in the United States: A cross-sectional survey. AIDS Res Hum Retroviruses 2020;36:606–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ: The challenge of finding a cure for HIV infection. Science 2009;323:1304–1307 [DOI] [PubMed] [Google Scholar]
- 20. Griffith D, Agwu A: Caring for youth living with HIV across the continuum: Turning gaps into opportunities. AIDS Care 2017;29:1205–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murray M, Antela A, Mills A, et al. : Patient views on long-acting HIV treatment: Cabotegravir+rilpivirine as maintenance therapy (ATLAS 48 weeks results). Int AIDS Soc 2019;MOAB0103 [Google Scholar]
- 22. Kerrigan D, Mantsios A, Gorgolas M, et al. : Experiences with long acting injectable ART: A qualitative study among PLHIV participating in a phase II study of cabotegravir+rilpivirine (LATTE-2) in the United States and Spain. PLoS One 2018;13:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weld ED, Rana MS, Dallas RH, et al. : Interest of youth living with HIV in long-acting antiretrovirals. JAIDS 2019;80:190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Derrick CB, Ostermann J, Weissman SB, et al. : Who wants to switch? Gauging patient interest in novel antiretroviral therapies. Open Forum Infect Dis 2018;5:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dubé K, Hosey L, Starr K, et al. : Participant perspectives in an HIV cure-related trial conducted exclusively in women in the United States: Results from AIDS clinical trials group (ACTG) 5366. AIDS Res Hum Retroviruses 2020;36:268–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dubé K, Dee L, Evans D, et al. : Perceptions of equipoise, risk—Benefit ratios, and ‘Otherwise Healthy Volunteers’ in the context of early-phase HIV cure research in the United States: A qualitative inquiry. J Empir Res Hum Res Ethics 2018;13:3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dubé K, Taylor J, Sylla L, et al. : ‘Well, It's the Risk of the Unknown … Right?’: A qualitative study of perceived risks and benefits of HIV cure research in the United States. PLoS One 2017;12:e0170112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dubé K, Evans D, Dee L, et al. : “We Need to Deploy Them Very Thoughtfully and Carefully”: Perceptions of analytical treatment interruptions in HIV cure research in the United States. AIDS Res Hum Retroviruses 2018;34:67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Power J, Westle A, Dowsett GW, et al. : Perceptions of HIV cure research among people living with HIV in Australia. PLoS One 2018;13:e0202647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Power J, Dowsett GW, Westle A, et al. : The significance and expectations of HIV cure research among people living with HIV in Australia. PLoS One 2020;15:e0229733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sylla L, Evans D, Taylor J, et al. : If we build it, will they come? Perceptions of HIV cure-related research by people living with HIV in four U.S. cities—A qualitative focus group study. AIDS Res Hum Retroviruses 2018;34:56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. amfAR: Long-acting HIV treatment and prevention are coming: Preparing for potential game changers. 2018. Available at: https://www.amfar.org/uploadedFiles/_amfarorg/Articles/On_The_Hill/2018/overview.pdf
- 33. Dubé K, Sylla L, Dee L, et al. Research on HIV cure: Mapping the ethics landscape. PLoS Med 2017;14:e1002470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grossman CI, Ross AL, Auerbach JD, et al. : Towards multidisciplinary HIV-cure research: Integrating social science with biomedical research. Trends Microbiol 2016;24:5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dubé K, Auerbach JD, Stirratt MJ, Gaist P: Applying the behavioural and social sciences research (BSSR) functional framework to HIV cure research. J Int AIDS Soc 2019;22:e25404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.