Abstract
Background:
Postsurgical secondary lymphedema is usually a progressive and lifelong condition lacking any curative treatment. The aim of this study was to develop new, simple surgical mouse models of chronic lymphedema, better simulating chronic nature of human postsurgical lymphedema.
Methods:
Two experimental mouse models of secondary lymphedema were created surgically without radiation by modifications of the previously described methods: the tail model and the hind limb model. Lymphedema formation was clinically assessed and quantitatively evaluated by measuring circumferences and limb volumes. Postmortem specimens were assessed histologically to examine the efficacy of the models.
Results:
In the tail models, although a substantial frequency of tail necrosis (30.0%) was noted and the increase in circumference was maintained for only limited times postoperatively depending on the particular tail model, the overall success rate was 65.0%. In the mouse hind limb model, the overall success rate was 88.9%, and the increased circumference and limb volume were maintained over the entire study period of 8 weeks. The overall success rate of the mouse hind limb model was significantly higher than that of the mouse tail model(s).
Conclusions:
We have successfully established modified mouse tail and hind limb lymphedema models via only surgical techniques without radiation, which have characteristics of chronic secondary lymphedema. The mouse hind limb model has a higher success rate than the mouse tail model and has advantages of having the healthy contralateral hind limbs as an internal control.
BACKGROUND
Impairment of lymphatic-transport capacity due to abnormal vessel development or damaged lymphatic vessels causes stagnation of water and associated proteins in the interstitium, which initiates an inflammatory reaction leading to fibrosis and impaired immune responses. Moreover, fatty degeneration of the connective tissue leads usually to a progressive and lifelong condition, lymphedema.1–5 The biology of lymphedema is complex and, as yet, poorly understood.1 Furthermore, current therapeutic option for treating lymphedema is limited to noncurative therapy such as conservative treatment, physical therapy, and surgical approaches. Thus, there is an obvious need for better molecular characterization of this disease process to elucidate the pathobiology of lymphatic insufficiency.6–9 To enhance our understanding of molecular biology and pathogenesis of this disease and to test emerging therapies such as gene therapy or cell therapy, establishing appropriate lymphedema models is crucial.
The aim of this study was to develop new, simple surgical mouse models of chronic lymphedema that better resemble the chronic conditions of human secondary lymphedema.
METHODS
Study Design and Animals
To create simple, reliable, and reproducible small animal models of chronic secondary lymphedema, we modified the method of Slavin et al.7 to create the mouse tail model and that of Olszewski et al.10 to generate the mouse hind limb model. All experiments were performed in accordance with the specifications of the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978), the Animal Welfare Act, and other Federal regulations, and the protocol was approved by our hospital’s Institutional Animal Care and Use Committee. We used 8-week-old BALB/C female mice for the tail model and 28-week-old C57BL/6 male mice for the hind limb model. Before and after the operations, all the mice were maintained in a temperature- and light-controlled environment with ad libitum access to normal chow and water. Analgesic (butorphanol 3.0 mg/kg) was administered subcutaneously every 4–6 hr postoperatively when necessary. The postoperative follow-up period was 8 weeks for all mice.
Surgical Techniques
Mice were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). After shaving and aseptic preparation with povidione/iodine solution, the mice were placed in the supine position. Prior to the operation, 0.1 mL of 0.1% Evans blue in saline was injected into the subcutaneous tissue in the midportion of the tail for the tail model and into the subcutaneous tissue in the dorsal surface of the paw for the hind limb model, in order to identify lymphatic vessels.
For the mouse tail model, under loupe magnification (3.5×), a circumferential skin incision was made through the dermis 5-mm distal from the tail base to sever the superficial dermal lymphatic vessels, with a 2-mm-wide skin bridge to improve the circulation of the tail and prevent tail necrosis. A 4-mm skin gap was created to prevent the development of collateral lymphatic flow. The dermal lymphatics under the skin bridge were destroyed bluntly, and some deep lymphatic vessels usually located in the lateral aspects of the tail adherent to the tail veins and stained with the Evans blue were ligated. After the operation, the circumferential incision site was protected with a rubber band to prevent restoration of the superficial lymphatics and infection. Three different kinds of operations were performed in the mouse tail model, according to the cutting and repair of the skin bridge: a no-cut operation without bridge cutting, a one-cut operation with cutting and repair of the distal bridge, and a two-cut model with proximal and distal cutting and repair (Supplemental Fig. 1).
For the mouse hind limb model, under loupe magnification (3.5×), a 5-mm-wide circumferential incision including skin and all subcutaneous tissue was made in the upper thigh. The stained main lymph trunks remaining around the femoral vessels were identified and resected completely, and the skin edge was continuously sutured to the underlying muscle with an 8.0 Prolene suture, leaving a gap of 5 mm between the wound edges to block the superficial lymphatics and prevent the development of collateral lymphatic flow. After the operation, the circumferential incision sites were protected with rubber bands to prevent restoration of the superficial lymphatics and infection. One hind limb was operated on, and the contralateral healthy hind limb served as an internal control (Supplemental Fig. 2).
Quantitative and Histologic Evaluation of Lymphedema
Lymphedema formation was clinically assessed and quantitatively evaluated, preoperatively and twice weekly after the operation, by measuring the tail circumference at 3 locations, just distal and 1- and 3-cm distal from the skin incision, respectively, for the mouse tail model. For the mouse hind limb model, the hind limb circumference was measured at the midthigh and ankle, along with the limb volume. Volume measurements of the hind limb volume were made from the tip of the hind limb to the distal edge of the wound by the water displacement method. To adjust for the increased circumference and volume of the hind limb due to age-appropriate growth and individual differences between the mice, the circumference and volume of the contralateral healthy hind limb were also measured and compared with the operated hind limb. All the measurements of the tail and hind limbs were performed in triplicate, and the means were calculated. For the mouse tail model, the integrity of the lymphatic vasculature was evaluated by FITC (Fluorescein Isothiocyanate)-Dextran microlymphangiography preoperatively (control) and 4 and 8 weeks postoperatively, as described by Rutkowski et al.11 Clinical success was defined as sustained increased circumferences and limb volumes of at least 5% in the injured tail or hind limb by postoperative week 4.
For histologic evaluation, mice were killed by diethyl ether inhalation, preoperatively (control) and 2, 4, 8 weeks postoperatively. Tail and hind limb tissues were excised, flash frozen in liquid N2, and cryosectioned transversely into 12- and 60-μm sections. Samples were stained with hematoxylin and eosin by standard techniques.
Statistical Analysis
Data were expressed as means ± standard deviations. For numerical data, analysis of variance followed by two-tailed Student’s t-tests was used to assess differences between groups. A P value less than 0.05 was considered statistically significant.
RESULTS
The Mouse Tail Model
Sixty-seven mice underwent surgery to produce the mouse tail model: 60 for clinical evaluation and 7 for histologic evaluation. For clinical evaluation, 20 mice each were used to generate 3 types of mouse tail model (see Methods). Twenty-one mice (35.0%) were subsequently excluded: 3 (5.0%) due to death and 18 due to tail necrosis (30.0%). Tail necrosis occurred in all 3 mouse tail models: 5 (25.0%) in the no-cut, 5 (25.0%) in the one-cut, and 8 (40.0%) in the two-cut model. Thus, there was substantial tail necrosis but no statistically significant difference in this respect between the 3 mouse tail models. The 39 surviving mice without tail necrosis (65.0%) were clinically evaluated: 14 in the no-cut, 14 in the one-cut, and 11 in the two-cut model.
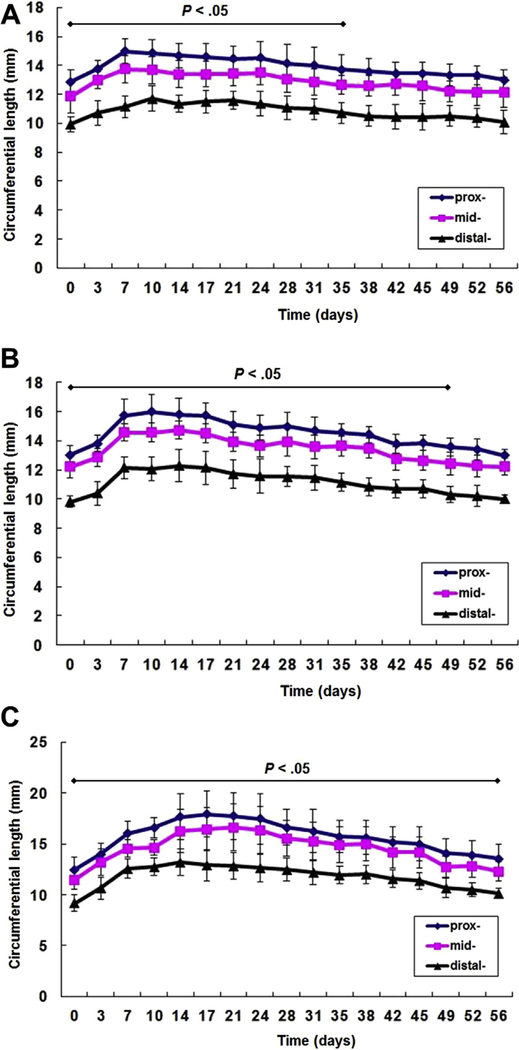
There was a statistically significant difference (P < 0.05) between postoperative tail circumference and preoperative circumference for the first postoperative 5 weeks in the no-cut model; in the one-cut model, a difference was maintained during the first postoperative 7 weeks, and in the two-cut model, it was maintained over the entire study period (postoperative 8 weeks) (Fig. 1).
Fig. 1.
Three mouse tail models: (A) no-cut model (n = 14), (B) one-cut model (n = 14), and (C) two-cut model (n = 11) (prox-: just distal from the skin incision, mid-: 1-cm distal from the skin incision, and distal-: 3-cm distal from the skin incision). Data are expressed as means ± standard deviations. A statistically significant difference (P < 0.05) was maintained during the first postoperative 35 days in the no-cut model, during the first postoperative 49 days in the one-cut model, and during the entire study period (postoperative 56 days) in the 2-cut model, compared with the preoperative tail circumference.
Fluorescence microlymphangiography was used to assess lymphatic drainage preoperatively and every 4 weeks postoperatively. In a healthy mouse tail, the injected FITC-Dextran was taken up by the lymphatics in the tail tip, and it was transported through the superficial network (Supplemental Fig. 3A). The intensity of fluorescence decreased as it traveled proximally and drained to the deeper vessels, and the superficial network was barely visible in most of the tail after 6 hr. However, in the operated mouse tail, FITC-Dextran was taken up by the lymphatics in the tail tip and remained in the lymphatic capillary network of the edematous tail even after 24 hr, suggesting delayed lymphatic drainage (Supplemental Fig. 3B, C).
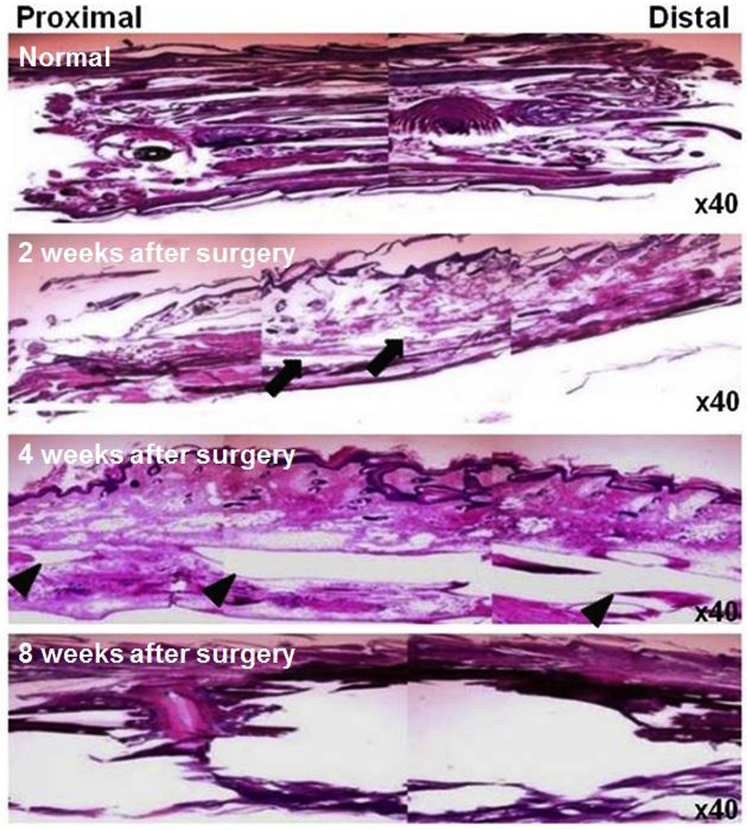
Light microscopic assessment of postmortem specimens from healthy mouse tails revealed dense collagen bundles arranged longitudinally. Narrow slit-like interstitial spaces were observed between the collagen bundles. Some hair follicles were also noted. In mice of the mouse tail model, marked interstitial edema in the superficial and deep connective tissue and dilated lymphatic vessels was noted in postoperative week 2. In postoperative week 4, some chronic changes characterized by diffuse fibrosis in both superficial and deep connective tissue, with compression of the skin began to appear, and the dilatation of lymphatic vessels became more prominent. By postoperative week 8, the connective tissue of the tail was completely replaced by dense fibrous tissue and calcified material (Fig. 2).
Fig. 2.
Representative light microscopic findings on postmortem specimens from healthy and operated mouse tails. Note the characteristic histologic findings of chronic lymphedema in the mouse tail model. Normal mouse tail shows compact fibrous tissue (top panel). Stromal edema is seen (arrows) in tail tissue at 2 weeks after surgery (second panel) and markedly dilated lymphatics are observed (arrowheads) at 4 weeks after surgery (third panel). Tissue is totally disrupted at 8 weeks after surgery (bottom panel). All figures are hematoxylin and eosin stained (×40 objective lens).
The Mouse Hind Limb Model
Thirty-five mice underwent surgery for the mouse hind limb model: 27 for clinical evaluation and 8 for histologic evaluation. Three of the 27 mice for clinical evaluation (11.1%) died during the study period and were excluded from the study. In the surviving 24 mice (88.9%), the operated hind limb gradually became edematous.
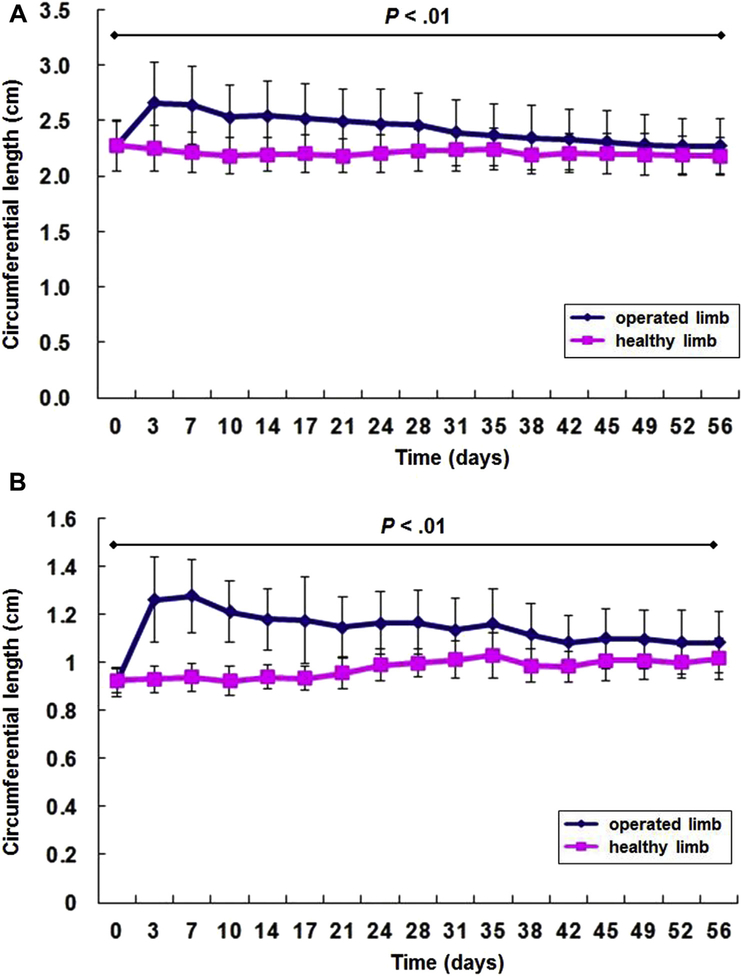
Postoperative hind limb circumference measured at the midthigh, and the ankle was significantly greater than in the contralateral healthy hind limb during the entire study period (postoperative 8 weeks) (P < 0.01) (Fig. 3). Postoperative hind limb volumes measured by the water displacement test were also significantly different during the entire study period (P < 0.01) (Fig. 4).
Fig. 3.
The circumferences of the hind limbs of the operated and the contralateral healthy control limbs (n = 24) are significantly different (P < 0.01) during the entire study period (postoperative 56 days): (A) midthigh circumferences and (B) ankle circumferences. Data are expressed as means ± standard deviations.
Fig. 4.
The limb volumes of the operated and contralateral healthy control limbs (n = 24) are significantly different (P < 0.01) during the entire study period (postoperative 56 days). Data are expressed as means ± standard deviations.
Light microscopic assessment of postmortem specimens from healthy mouse hind limbs revealed well-defined epidermis, dermis, subcutaneous fat, and skeletal muscle. Neither interstitial edema nor dilatation of lymphatic vessels was noted. In the mouse hind limb model, typical histologic findings of chronic lymphedema were noted at postoperative 8 weeks: a highly thickened epidermis, a very cellular dermis, and an overall increase in the thickness of the tissues in the lymphedematous hind limb. In 1 mouse, we made an interesting, incidental histologic observation, namely, of a high-grade spindle cell sarcoma in a tissue sample at postoperative 8 weeks. The tumor cells had diffusely infiltrated the dermis and deep fascial layer. Although the tumor mainly consisted of an area of clearly undifferentiated cells, some parts of the tumor contained poorly formed vascular structures, and these findings were compatible with a lymphangiosarcoma (Supplemental Fig. 4).
The overall success rates were 65.0% for the mouse tail model and 88.9% for the mouse hind limb model based on quantitative evaluation. The success rates of the 2 models were significantly different (P = 0.02). While in the mouse tail model, the increased circumference was maintained for a limited postoperative period depending on the type of tail model, the increased circumference and hind limb volume were sustained for the entire study period of 8 weeks in the mouse hind limb model.
DISCUSSION
Lymphedema is a progressive and lifelong pathologic condition in which microcirculatory imbalances of tissue fluid equilibrium are created by loss of lymphatic-transport capacity.6–13 Unfortunately, no curative treatment exists for this potentially debilitating condition, and current treatments for chronic lymphedema, which include conservative treatments such as lifelong physiotherapy, surgical removal of edematous tissue (reducing operation), or lymphovenous microsurgical bypass, can only slow its progression.14,15 For these reasons, there has been substantial interest in new therapeutic development such as gene-mediated or cell-mediated lymphangiogenesis, and these new approaches may ultimately reverse the clinical course of chronic lymphedema.16–19 However, such approaches will require suitable preclinical animal models of chronic lymphedema to precisely test their therapeutic responses and their safety.
Previously, several animal models of chronic secondary lymphedema have been developed for studying pathophysiology and therapy for lymphedema. While studies used a large animal model for induction of chronic lymphedema, mice are still preferable for research for many reasons: (1) mice are easy to handle and breed, and there is a wide range of inbred, transgenic, and knockout strains available; (2) they have the advantage of cost-effectiveness; and (3) there is a great range of research tools available for mice studies, such as antibodies and databases.4 The mouse tail lymphedema model has provided a straightforward and reproducible system for lymphatic researchers and has been used widely to advance our understanding of surgically induced lymphedema, although it is to some extent different from the human disease from an anatomical and physiological standpoint.5,7,11 Recently, to overcome the limitations of tail models, lymphedema has been induced in the mouse hind limb by preoperative or postoperative radiation and lymphatic vessel ablation, which recapitulate the human disease more precisely compared with the mouse tail model. The mouse hind limb model would be more useful to enhance our understanding of molecular biology and pathogenesis of this disease and to test emerging therapies such as gene-mediated or cell-mediated lymphangiogenesis due to its longer duration of lymphedema and its similarity to human counterparts such as arm or leg lymphedema.4,5 However, surgery with preoperative or postoperative radiation is too complicated to be widely applied in conventional laboratory settings. Thus, in this study, we sought to create lymphedema models in mice only by surgery without radiation, which are sustainable, easy to create, allows repeated physiologic measurement.
According to the existing methods,7,10 we created a mouse tail model using 8-week-old BALB/C female mice and a mouse hind limb model using 28-week-old C57BL/6 male mice. However, in our pilot study, these models gave unsatisfactory long-term results. Several authors have reported that the mouse tail model of lymphedema is reproducible and reliable and has many characteristics of chronic secondary lymphedema.2,7,11,12 However, in our pilot study, this model gave satisfactory results only up to 10 postoperative days. Furthermore, after 10 days, there was a substantial rate of tail necrosis probably due to dry-up followed by thrombosis of the extremely small tail vessels and deterioration of the circulation (Supplemental Fig. 5). To reduce the incidence of tail necrosis and induce longer duration of lymphedema, we modified the model with a skin bridge, 2-mm in width, to improve circulation in the tail and the circumferential incision site was protected with a rubber band to prevent restoration of the superficial lymphatics. However, in spite of acceptable results in clinical, microlymphangiographic, and histologic studies, a high rate of tail necrosis still occurred. We thus decided to develop another model, the mouse hind limb model. Our initial results of the mouse hind limb model were satisfactory in the early postoperative period. However, during the follow-up, there was rapid healing of the area of skin and subcutaneous excised tissue in some cases, followed by the development of collateral lymphatic flow and a return to normal limb size (Supplemental Fig. 6). We therefore modified the mouse hind limb model as follows: (1) after lymphatic vessel ablation, the skin wound edges were continuously sutured to the underlying muscle leaving a gap of 5 mm between the 2 sides of wound edges to block the rapid regrowth of superficial lymphatics and prevent the development of collateral lymphatic flow; and (2) after the operation, the circumferential incision sites were protected with rubber bands to prevent restoration of the superficial lymphatics and infection. Our modified hind limb model gave satisfactory results in clinical and histologic studies over the entire study period of 8 weeks.
Intriguingly, in histologic examination of our mouse hind limb model, we encountered interesting incidental findings in a tissue sample at the postoperative week 8. Light microscopic assessment of the postmortem specimen revealed a high-grade spindle cell sarcoma compatible with lymphangiosarcoma as well as typical findings of chronic lymphedema. In humans, lymphangiosarcoma is the only malignant disease of the lymphatics, and the etiology is largely unknown.20–22 Chronic lymphedema is a widely recognized risk factor for lymphangiosarcoma.21 Typically, lymphangiosarcoma occurs in patients who have been treated for breast cancer with mastectomy and/or radiotherapy and who have developed a significant degree of chronic edema for many years.20–22 Other chronic lymphedema resulting from a congenital, idiopathic, traumatic, or infectious cause also predisposes the patients to lymphangiosarcoma.21 Although the true prevalence of the condition is not known, Mulvenna et al.23 found only 6 cases of lymphangiosarcoma in more than 5,000 patients with lymphedema over 30 years. Consequently, this finding points to the reliability of our mouse hind limb model as a chronic secondary lymphedema model.
Several limitations should be noted. Although clinical and histologic studies showed that our models are reliable and consistent for investigating chronic secondary lymphedema, we were unable to perform fluorescence microlymphangiography studies in the hind limb model due to the large size of limbs that do not allow microscopic examination to better characterize the lymphatic networks. In the mouse tail models, despite our modification, there was a substantial occurrence of tail necrosis in all 3 types of models, and we could not adjust for the increase in circumference in the controls due to age-appropriate growth.
In conclusion, despite the aforementioned potential limitations, our modified mouse tail and hind limb lymphedema models via only surgical techniques without radiation are simple, reproducible, and reliable, with many of the characteristics of chronic secondary lymphedema; however, in the mouse tail model, there was a substantial frequency of tail necrosis. On the other hand, the mouse hind limb model had a higher success rate and adequate internal controls in healthy contralateral hind limbs.
Supplementary Material
Acknowledgments
This work was supported in part by grants from NIH (HL127759, DP3DK094346, and DP3DK 108245 to Y-.S.Y.), the Bio & Medical Technology Development Program of the NRF funded by the Korean government (MSIP) (No 2015M3A9C6031514), and the Korea Health Technology R&D Project through the KHIDI, funded by the Ministry of Health & Welfare, Republic of Korea (No HI15C2782).
Footnotes
Conflict of interests: The authors have no conflict of interests in relation to this article.
SUPPLEMENTARY DATA
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.avsg.2017.01.023.
REFERENCES
- 1.Tabibiazar R, Cheung L, Han J, et al. Inflammatory manifestations of experimental lymphatic insufficiency. PLoS Med 2006;3:e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin WS, Rockson SG. Animal models for the molecular and mechanistic study of lymphatic biology and disease. Ann N Y Acad Sci 2008;1131:50–74. [DOI] [PubMed] [Google Scholar]
- 3.Yang CY, Nguyen DH, Wu CW, et al. Developing a lower limb lymphedema animal model with combined lymphadenectomy and low-dose radiation. Plast Reconst Surg Glob Open 2014;2:e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oashi K, Furukawa H, Oyama A, et al. A new model of acquired lymphedema in the mouse hind limb: a preliminary report. Ann Plast Surg 2012;69:565–8. [DOI] [PubMed] [Google Scholar]
- 5.Bramos A, Perrault D, Yang S, et al. Prevention of postsurgical lymphedema by 9-cis retinoic acid. Ann Surg 2016;264:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rockson SG. Lymphedema. Am J Med 2001;110:288–95. [DOI] [PubMed] [Google Scholar]
- 7.Slavin SA, Van den Abbeele AD, Losken A, et al. Return of lymphatic function after flap transfer for acute lymphedema. Ann Surg 1999;229:421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mortimer PS. Therapy approaches for lymphedema. Angiology 1997;48:87–91. [DOI] [PubMed] [Google Scholar]
- 9.Campisi C, Boccardo F, Alitta P, et al. Derivative lymphatic microsurgery: indications, techniques, and results. Microsurgery 1995;16:463–8. [DOI] [PubMed] [Google Scholar]
- 10.Olszewski W, Machowski Z, Sokolowski J, et al. Experimental lymphedema in dogs. J Cardiovasc Surg 1968;9:178–83. [PubMed] [Google Scholar]
- 11.Rutkowski JM, Moya M, Johannes J, et al. Secondary lymphedema in the mouse tail: Lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc Res 2006;72:161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin WS, Szuba A, Rockson SG. Animal models for the study of lymphatic insufficiency. Lymphat Res Biol 2003;1:159–69. [DOI] [PubMed] [Google Scholar]
- 13.Szuba A, Rockson SG. Lymphedema: classification, diagnosis and therapy. Vasc Med 1998;3:145–56. [DOI] [PubMed] [Google Scholar]
- 14.Foldi E The treatment of lymphedema. Cancer 1998;83:2833–4. [DOI] [PubMed] [Google Scholar]
- 15.Witte MH, Bernas MJ, Martin CP, et al. Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc Res Tech 2001;55:122–45. [DOI] [PubMed] [Google Scholar]
- 16.Rivard A, Isner JM. Angiogenesis and vasculogenesis in treatment of cardiovascular disease. Mol Med 1998;4:429–40. [PMC free article] [PubMed] [Google Scholar]
- 17.Baumgartner I, Pieczek A, Manor O, et al. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation 1998;97:1114–23. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med 1999;5:1359–64. [DOI] [PubMed] [Google Scholar]
- 19.Karkkainen MJ, Saaristo A, Jussila L, et al. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci U S A 2001;98:12677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gajraj H, Barker SG, Burnand KG, et al. Lymphangiosarcoma complicating chronic primary lymphoedema. Br J Surg 1987;74:1180. [DOI] [PubMed] [Google Scholar]
- 21.Sun S, Guan JL. Modeling lymphangiosarcoma in mice. Cell Cycle 2016;15:1801–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer 2003;98:1716–26. [DOI] [PubMed] [Google Scholar]
- 23.Mulvenna PM, Gillham L, Regnard CF. Lymphangiosarcoma—experience in a lymphedema clinic. Palliat Med 1995;9:55–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.