Abstract
Purpose
To report updated analyses of the phase III CheckMate 214 trial with extended minimum follow-up assessing long-term outcomes with first-line nivolumab plus ipilimumab (NIVO+IPI) versus (vs) sunitinib (SUN) in patients with advanced renal cell carcinoma (aRCC).
Methods
Patients with aRCC with a clear cell component were stratified by International Metastatic Renal Cell Carcinoma Database Consortium risk and randomised to NIVO (3 mg/kg) plus IPI (1 mg/kg) every three weeks ×4 doses, followed by NIVO (3 mg/kg) every two weeks; or SUN (50 mg) once per day ×4 weeks (6-week cycle). Efficacy endpoints included overall survival (OS), progression-free survival (PFS) and objective response rate (ORR) per independent radiology review committee in patients with intermediate/poor-risk disease (I/P; primary), intent-to-treat patients (ITT; secondary) and in patients with favourable-risk disease (FAV; exploratory).
Results
Overall, 1096 patients were randomised (ITT: NIVO+IPI, n=550, SUN, n=546; I/P: NIVO+IPI, n=425, SUN, n=422; FAV: NIVO+IPI, n=125, SUN, n=124). After 4 years minimum follow-up, OS (HR; 95% CI) remained superior with NIVO+IPI vs SUN in ITT (0.69; 0.59 to 0.81) and I/P patients (0.65; 0.54 to 0.78). Four-year PFS probabilities were 31.0% vs 17.3% (ITT) and 32.7% vs 12.3% (I/P), with NIVO+IPI vs SUN. ORR remained higher with NIVO+IPI vs SUN in ITT (39.1% vs 32.4%) and I/P (41.9% vs 26.8%) patients. In FAV patients, the HRs (95% CI) for OS and PFS were 0.93 (0.62 to 1.40) and 1.84 (1.29 to 2.62); ORR was lower with NIVO+IPI vs SUN. However, more patients in all risk groups achieved complete responses with NIVO+IPI: ITT (10.7% vs 2.6%), I/P (10.4% vs 1.4%) and FAV (12.0% vs 6.5%). Probability (95% CI) of response ≥4 years was higher with NIVO+IPI vs SUN (ITT, 59% (0.51 to 0.66) vs 30% (0.21 to 0.39); I/P, 59% (0.50 to 0.67) vs 24% (0.14 to 0.36); and FAV, 60% (0.41 to 0.75) vs 38% (0.22 to 0.54)) regardless of risk category. Safety remained favourable with NIVO+IPI vs SUN.
Conclusion
After long-term follow-up, NIVO+IPI continues to demonstrate durable efficacy benefits vs SUN, with manageable safety.
Trial registration details
ClinicalTrials.gov identifier: NCT02231749.
Keywords: checkmate 214, nivolumab plus ipilimumab, advanced renal cell carcinoma, dual checkpoint inhibition, long-term follow-up
Key questions.
What is already known about this subject?
Nivolumab plus ipilimumab (NIVO+IPI) combination immunotherapy was the first to demonstrate superiority over sunitinib (SUN) in the first-line treatment of patients with advanced renal cell carcinoma (aRCC).
NIVO+IPI is currently approved for clinical use in patients with International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) intermediate/poor-risk disease based on results from the randomised phase III CheckMate 214 trial (NCT02231749).
What does this study add?
Here, we report durable efficacy benefits after long-term follow-up with NIVO+IPI over SUN in patients with aRCC in CheckMate 214.
With over 4 years of minimum follow-up, overall survival benefits with NIVO+IPI were maintained. Progression-free survival probabilities reached a plateau above 30% with NIVO+IPI in intent-to-treat and intermediate/poor-risk patient populations.
Objective response rates were consistent, with a complete response rate per independent radiology review committee over 10% as well as more ongoing responses and longer duration of response with NIVO+IPI regardless of IMDC risk group.
How might this impact on clinical practice?
These results continue to support the use of first-line NIVO+IPI to achieve meaningful and durable clinical outcomes in patients with aRCC.
Introduction
Many patients experience lasting benefits from immuno-oncology–based therapy due to improved efficacy together with favourable safety and quality-of-life outcomes. This new potential for prolonged survival underscores the importance of long-term updates to assess the durability of clinical benefits with first-line therapies for patients with advanced renal cell carcinoma (aRCC).1–3
Dual checkpoint inhibition with nivolumab plus ipilimumab (NIVO+IPI) is currently approved for the first-line treatment of patients with aRCC and International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) intermediate/poor (I/P)-risk disease, on the basis of results from the randomised phase III CheckMate 214 trial (NCT02231749). In the primary analysis of the CheckMate 214 trial (minimum follow-up, 17.5 months) in patients with I/P risk, overall survival (OS) was significantly superior (HR, 0.63; p<0.001) and confirmed objective response rate (ORR) was higher (42% versus (vs) 27%; p<0.001 per independent radiology review committee (IRRC)) with NIVO+IPI over sunitinib (SUN).4 Comparable OS benefits (HR 0.68; p<0.001) and improved ORR (39% vs 32%) were also observed with NIVO+IPI in intent-to-treat (ITT) patients.4 NIVO+IPI continued to demonstrate improved efficacy outcomes over SUN in both ITT and I/P-risk patients, including OS benefits and durable responses with additional follow-up.1 2 4 In IMDC favourable (FAV)-risk patients, ORR and progression-free survival (PFS) benefits were observed with SUN, yet the difference in OS outcomes between treatment arms was inconclusive.1 2 4
Here, we report extended 4-year minimum follow-up from the CheckMate 214 trial in ITT, I/P-risk and FAV-risk patients with aRCC. Survival outcomes, response per IRRC, durability of response and safety were assessed, providing the longest follow-up to date of an approved first-line checkpoint inhibitor-based therapy in aRCC.
Methods
Patients and treatment
The CheckMate 214 study design and statistical analyses details have been published previously.2 4 Briefly, adults with treatment-naïve aRCC with a clear cell component were randomised 1:1 to the NIVO+IPI or SUN arms and stratified by region and IMDC risk status (favourable, intermediate or poor).4–6 Combination NIVO 3 mg/kg and IPI 1 mg/kg was administered intravenously every 3 weeks for four doses, followed by NIVO 3 mg/kg monotherapy every 2 weeks. Patients were required to receive all four doses of NIVO+IPI before beginning NIVO monotherapy. SUN 50 mg was administered orally once daily for 4 weeks on and 2 weeks off in each 6-week cycle. Treatment continued until disease progression or unacceptable toxicity. A total of two dose reductions were permitted for SUN in 12.5 mg increments per day (for daily dose ≥25 mg); no dose reductions were allowed in the NIVO+IPI arm. The trial was stopped when NIVO+IPI demonstrated OS superiority over SUN in the primary efficacy population (database lock, 7 August 2017). A subsequent protocol amendment on 13 November 2017 permitted modifications to the NIVO+IPI arm, including the following: patients could discontinue after 2 years of study treatment even in the absence of disease progression or unacceptable toxicity; patients receiving NIVO maintenance therapy were permitted to switch to NIVO 240 mg every 2 weeks; and I/P-risk patients could cross over from SUN to NIVO+IPI.
Assessments
Primary endpoints included OS, PFS per IRRC and ORR per IRRC in the I/P-risk population. Secondary endpoints included OS, PFS and ORR in the ITT population, and the incidence rate of adverse events (AEs) among all treated patients. Exploratory endpoints included OS, PFS and ORR in FAV-risk patients. Response outcomes were confirmed and reported per IRRC using Response Evaluation Criteria in Solid Tumors (RECIST) V.1.1.7 Safety was assessed per the National Cancer Institute Common Terminology Criteria for Adverse Events, V.4.0.8 Treatment-related select AEs were prespecified, and defined as events that might be immune-mediated, differ from those caused by non-immunotherapeutic drugs, might require immunosuppression for management and whose early recognition might mitigate severe toxicity (including events in the skin, gastrointestinal, endocrine, hepatic, pulmonary or renal systems). A detailed characterisation of all responders, including durability of response, treatment-free interval and subsequent therapy outcomes, were conducted as post hoc analyses. Treatment-free interval was defined as the time between protocol therapy discontinuation until subsequent therapy initiation or last known date alive.1
Trial oversight
CheckMate 214 was approved by the institutional review board or ethics committee at each site and was conducted according to Good Clinical Practice guidelines, defined by the International Conference on Harmonisation. All patients provided written informed consent that was based on the Declaration of Helsinki principles. A data and safety monitoring committee reviewed efficacy and safety. The trial was designed by the authors in collaboration with the sponsors (Bristol Myers Squibb and ONO Pharmaceutical). Bristol Myers Squibb collected and analysed the data with the authors. A data confidentiality agreement was in place between Bristol Myers Squibb and the investigators. The authors vouch for the completeness and accuracy of the data and analyses and for the adherence of the trial to the protocol.
Statistical analysis
After the planned interim analysis met the prespecified boundary of statistical significance for OS, it was considered the final primary analysis per protocol.4 Descriptive p values were included in the present analyses to confirm consistency with the primary analysis as appropriate. Here, OS, PFS and duration of response were estimated using Kaplan-Meier methods.9 Stratified Cox proportional HRs and 95% CIs were calculated between treatment arms for OS and PFS (NIVO+IPI over SUN). ORR and the exact two-sided 95% CI were computed by Clopper-Pearson method; the two-sided p values were calculated per DerSimonian and Laird.10 11 Treatment-related AEs of interest were calculated in all treated patients. The incidence of corticosteroid use (≥40 mg prednisone daily or equivalent (PDE)) for management of treatment-related select AEs over time was analysed retrospectively for patients in the NIVO+IPI arm. The univariate Cox model was used to calculate the HR with 95% CI. All statistical analyses were done with SAS V.8.2 or East V.5.4.
Results
Patients
Among 1096 patients randomised, 550 patients in the ITT population were randomised to NIVO+IPI (425 I/P risk; 125 FAV risk) and 546 patients were randomised to SUN (422 I/P risk; 124 FAV risk; online supplemental figure 1). Efficacy analyses were conducted in ITT patients and are presented in all-risk, I/P-risk and FAV-risk groups. Overall, 547 patients in the NIVO+IPI arm and 535 patients in the SUN arm received treatment and were included in the safety analyses. Patients were enrolled from 16 October 2014 through 23 February 2016 (database lock, 25 February 2020). The minimum follow-up was 4 years (median follow-up=55 months), and 53 (10%) of 547 patients in the NIVO+IPI arm and 15 (3%) of 535 patients in the SUN arm continued therapy.
esmoopen-2020-001079supp001.pdf (583.9KB, pdf)
Key baseline characteristics were similar between treatment arms and across risk groups, as previously reported (online supplemental table 1).2 4 Among all treated patients in the NIVO+IPI arm, a median (range) of 14.0 doses (1 to 128) of NIVO and 4.0 doses (1 to 4) of IPI were received. The median duration of therapy (IQR) in all treated patients was 7.9 months (2.1 to 21.8) for patients treated with NIVO+IPI and 7.8 months (3.5 to 19.6) with SUN. Of all ITT patients, fewer treated with NIVO+IPI (53.5%; 294/550) received subsequent systemic therapy compared with patients treated with SUN (66.5%; 363/546; online supplemental table 2). In the NIVO+IPI arm, the most common subsequent systemic therapies included SUN (23.5%; 129/550), pazopanib (19.3%; 106/550) and axitinib (18.0%; 99/550). In the SUN arm, the most common subsequent systemic therapies included NIVO (40.1%; 219/546), axitinib (24.9%; 136/546) and cabozantinib (16.3%; 89/546).
Efficacy
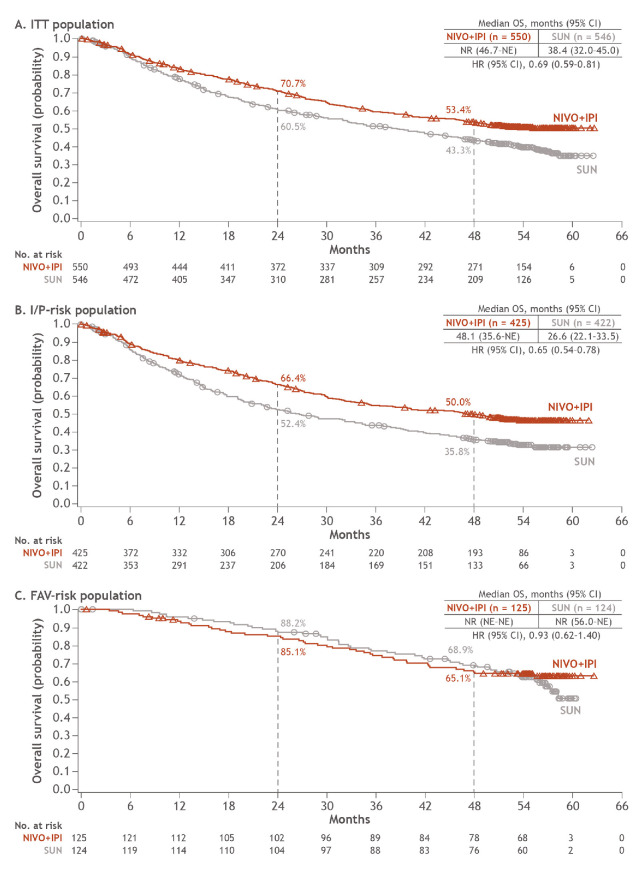
Superior OS with NIVO+IPI vs SUN was sustained in the ITT population (HR 0.69; 95% CI 0.59 to 0.81) as well as in patients with I/P risk (HR 0.65; 95% CI 0.54 to 0.78). Median OS with NIVO+IPI was not reached vs 38.4 months with SUN in the ITT population and 48.1 vs 26.6 months in patients with I/P risk. OS probabilities at 4 years were 53.4% with NIVO+IPI vs 43.3% with SUN in ITT patients and 50.0% vs 35.8% in patients with I/P risk. In patients with FAV risk, the difference in OS was not statistically significant (HR 0.93; 95% CI 0.62 to 1.4) and median OS was not reached in either arm (figure 1). OS probabilities at 4 years were similar between arms at 65.1% vs 68.9% with NIVO+IPI vs SUN.
Figure 1.
OS in ITT, I/P-risk and FAV-risk patients. FAV, favourable; I/P, intermediate/poor; ITT, intent-to-treat; NE, not estimable; NIVO+IPI, nivolumab plus ipilimumab; NR, not reached; OS, overall survival; SUN, sunitinib.
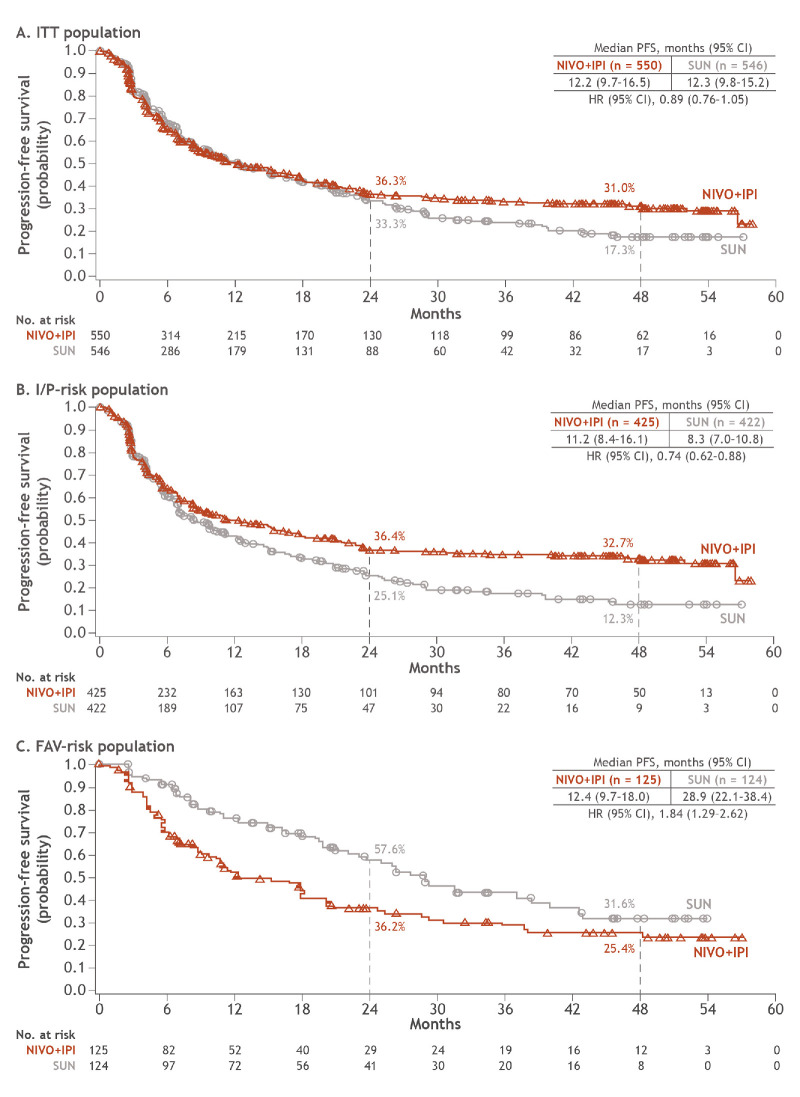
PFS outcomes favoured NIVO+IPI vs SUN and remained consistent with previous reports in ITT (HR 0.89; 95% CI 0.76 to 1.05) and I/P-risk patients (HR 0.74; 95% CI 0.62 to 0.88); PFS probabilities remained above 30% with NIVO+IPI in both patient groupings (figure 2). Median (95% CI) PFS with NIVO+IPI vs SUN was 12.2 months (9.7 to 16.5) vs 12.3 months (9.8 to 15.2) in ITT patients and 11.2 months (8.4 to 16.1) vs 8.3 months (7.0 to 10.8) in patients with I/P risk. The 4-year PFS probabilities were 31.0% vs 17.3% and 32.7% vs 12.3%, with NIVO+IPI vs SUN, in ITT and I/P risk patients, respectively. In patients with FAV risk, PFS outcomes favoured SUN (HR 1.84; 95% CI 1.29 to 2.62), with 4-year PFS probabilities of 25.4% with NIVO+IPI vs 31.6% with SUN. However, the difference in PFS probabilities across arms has decreased over time.
Figure 2.
PFS per IRRC in ITT, I/P-risk and FAV-risk patients. FAV, favourable; I/P, intermediate/poor; IRRC, independent radiology review committee; ITT, intent-to-treat; NIVO+IPI, nivolumab plus ipilimumab; PFS, progression-free survival; SUN, sunitinib.
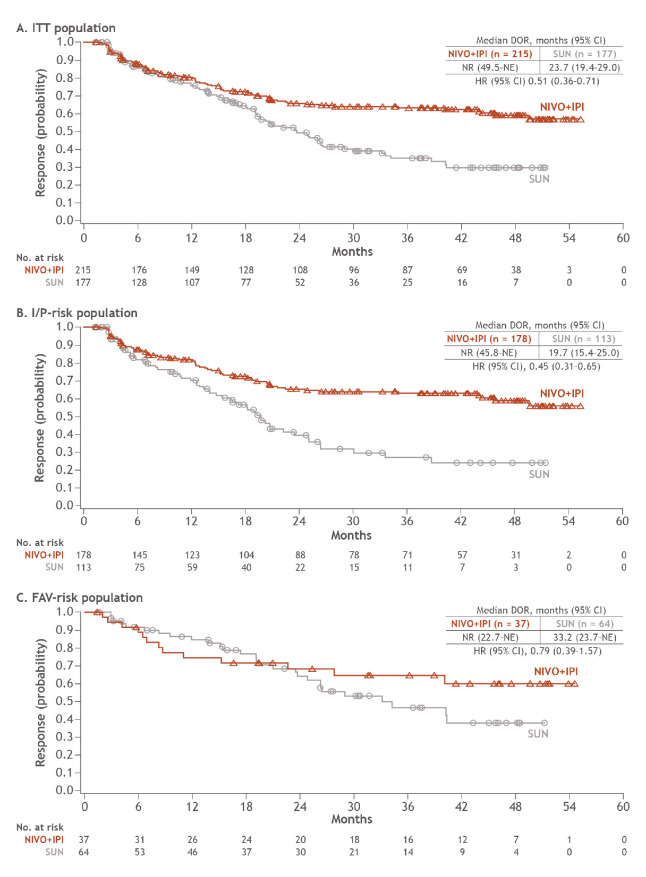
ORR (95% CI) per IRRC for patients in the ITT group was 39.1% (35.0 to 43.3) with NIVO+IPI vs 32.4% (28.5 to 36.5) with SUN and 41.9% (37.1 to 46.7) vs 26.8% (22.6 to 31.3) in patients with I/P risk (table 1). In patients with FAV risk, ORR was lower with NIVO+IPI vs SUN (29.6% vs 51.6%); however, the complete response rate was greater with NIVO+IPI over SUN regardless of baseline risk assessment: ITT (10.7% vs 2.6%), I/P risk (10.4% vs 1.4%) and FAV risk (12.0% vs 6.5%; table 1). Median (range) time to response with NIVO+IPI was 2.8 months (0.9 to 35.0) vs 4.0 months (0.6 to 23.6) with SUN among ITT patients. Across IMDC risk groups, median duration of response with NIVO+IPI has not yet been reached in any group vs 23.7, 19.7 and 33.2 months with SUN (ITT, I/P-risk and FAV-risk groups, respectively; figure 3). Additionally, the probability of response (95% CI) lasting at least 4 years was higher with NIVO+IPI vs SUN (ITT, 59% (0.51 to 0.66) vs 30% (0.21 to 0.39); I/P risk, 59% (0.50 to 0.67) vs 24% (0.14 to 0.36); and FAV risk, 60% (0.41 to 0.75) vs 38% (0.22 to 0.54)). More patients had ongoing responses with NIVO+IPI vs SUN regardless of risk group (table 1).
Table 1.
ORR and BOR per IRRC at 4 years minimum follow-up in ITT, I/P-risk and FAV-risk patients
| Intent-to-treat | Intermediate/poor risk | Favourable risk | ||||
| NIVO+IPI (n=550) |
SUN (n=546) |
NIVO+IPI (n=425) |
SUN (n=422) |
NIVO+IPI (n=125) |
SUN (n=124) |
|
| Confirmed ORR (95% CI), % p value |
39.1 (35 to 43) | 32.4 (29 to 37) | 41.9 (37 to 47) | 26.8 (23 to 31) | 29.6 (22 to 38) | 51.6 (43 to 61) |
| 0.0134 | <0.0001 | 0.0005 | ||||
| Best overall response, n (%) | ||||||
| Complete response | 59 (10.7) | 14 (2.6) | 44 (10.4) | 6 (1.4) | 15 (12.0) | 8 (6.5) |
| Partial response | 156 (28.4) | 163 (29.9) | 134 (31.5) | 107 (25.4) | 22 (17.6) | 56 (45.2) |
| Stable disease | 198 (36.0) | 230 (42.1) | 131 (30.8) | 187 (44.3) | 67 (53.6) | 43 (34.7) |
| Progressive disease | 97 (17.6) | 77 (14.1) | 82 (19.3) | 71 (16.8) | 15 (12.0) | 6 (4.8) |
| Unable to determine | 38 (6.9) | 57 (10.4) | 32 (7.5) | 48 (11.4) | 6 (4.8) | 9 (7.3) |
| Not reported | 2 (0.4) | 5 (0.9) | 2 (0.5) | 3 (0.7) | 0 | 2 (1.6) |
| Ongoing response, n (%) |
n=215 140 (65.1) |
n=177 92 (52.0) |
n=178 116 (65.2) |
n=113 56 (49.6) |
n=37 24 (64.9) |
n=64 36 (56.3) |
BOR, best overall response; FAV, favourable-risk disease; I/P, intermediate/poor-risk disease; IRRC, independent radiology review committee; ITT, intent-to-treat; NIVO+IPI, nivolumab plus ipilimumab; ORR, objective response rate; SUN, sunitinib.
Figure 3.
DOR per IRRC in ITT, I/P-risk and FAV-risk patients. DOR, duration of response; FAV, favourable; I/P, intermediate/poor; IRRC, independent radiology review committee; ITT, intent-to-treat; NE, not estimable; NIVO+IPI, nivolumab plus ipilimumab; NR, not reached; SUN, sunitinib.
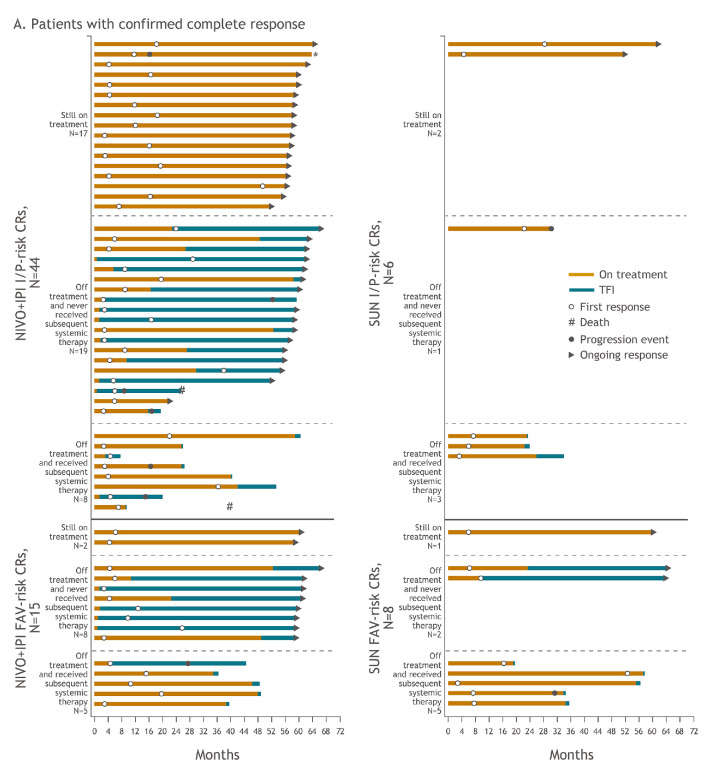
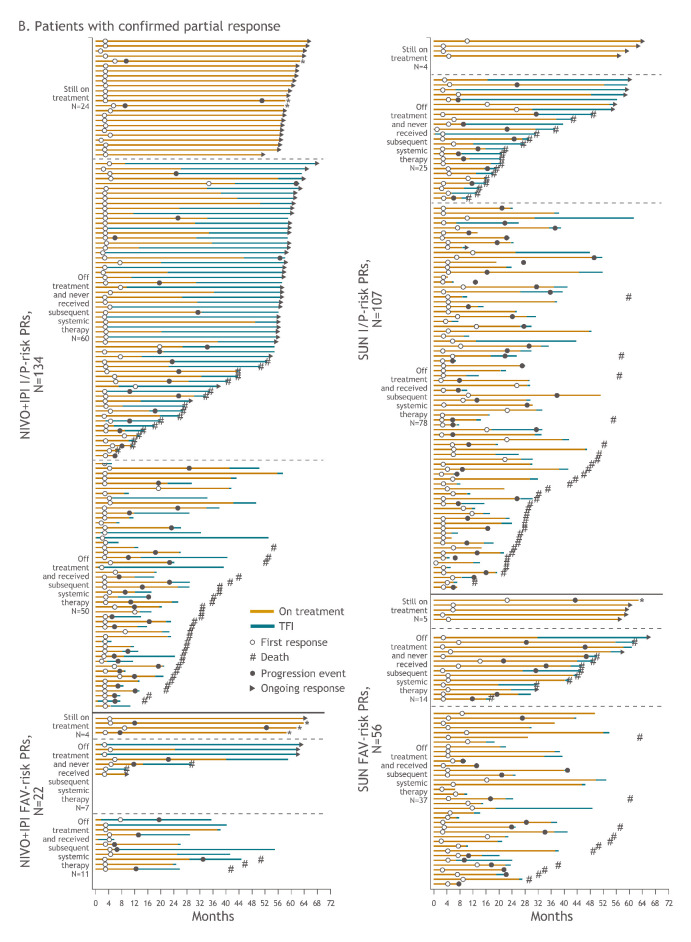
Additional analyses were conducted to assess long-term outcomes in complete and partial responders, including treatment discontinuation, subsequent systemic therapy and treatment-free interval outcomes. Treatment-free interval was assessed in patients with I/P and FAV risk in both arms (figure 4a, figure 4b). Of all 59 patients who achieved complete responses with NIVO+IPI, 19 (32.2%) were still on therapy, 27 (45.8%) discontinued therapy and did not require subsequent systemic therapy and 13 (22.0%) received subsequent systemic therapy after discontinuation. With SUN, of the 14 patients who achieved complete responses, 3 (21.4%) were still on therapy, 3 (21.4%) discontinued therapy and did not require subsequent systemic therapy and 8 (57.1%) received subsequent systemic therapy after discontinuation. In the 156 patients who achieved partial responses with NIVO+IPI, 28 (17.9%) were still on therapy, 67 (42.9%) discontinued therapy and did not require subsequent systemic therapy and 61 (39.1%) received subsequent systemic therapy after discontinuation. Among the 163 patients with partial responses treated with SUN, 9 (5.5%) were still on therapy, 39 (23.9%) discontinued therapy and did not require subsequent systemic therapy and 115 (70.6%) received subsequent systemic therapy after discontinuation.
Figure 4A.
Figure 4B.
Treatment-free interval and response outcomes in patients with confirmed complete response (A) and confirmed partial response (B) in I/P-risk and FAV-risk patients. Of all-risk patients with confirmed complete response, 13 versus 8 received subsequent systemic therapy with NIVO+IPI versus SUN. Of all-risk patients with confirmed partial response, 61 versus 115 received subsequent systemic therapy with NIVO+IPI versus SUN. These patients may have stopped therapy due to investigator-assessed progression or other protocol-specified reason such as toxicity (data not shown). The decision to start subsequent systemic therapy in either arm was made by the investigator based on expert opinion and treatment guidelines, and these data were not formally collected. *Denotes patients who were treated beyond confirmed blinded independent central review–assessed progression. CR, complete response; FAV, favourable; I/P, intermediate/poor; NIVO+IPI, nivolumab plus ipilimumab; PR, partial response; SUN, sunitinib.
Safety
Consistent with previous reports,2 4 similar overall rates of treatment-related AEs of any grade occurred in the NIVO+IPI and SUN arms with extended follow-up (514/547 (94.0%) vs 521/535 (97.4%) patients), and there were fewer grade 3 to 4 treatment-related AEs with NIVO+IPI vs SUN (47.9% vs 64.1%; online supplemental table 3). Treatment-related AEs leading to discontinuation within 30 days of the last dose occurred in 124 (22.7%) patients in the NIVO+IPI arm and in 70 (13.1%) patients in the SUN arm. No additional treatment-related deaths were reported since the primary analysis. The overall incidence of treatment-related select AEs with NIVO+IPI was similar to previous reports (online supplemental table 3).2 4 In total, 159 (29.1%) of 547 patients treated with NIVO+IPI received corticosteroids (≥40 mg PDE) to manage any-grade treatment-related select AEs, 106 (19.4%) patients received ≥40 mg PDE continuously for ≥2 weeks and 54 (9.9%) received ≥40 mg PDE continuously for ≥30 days.
Discussion
With an extended minimum follow-up of 4 years, the longest in a phase III trial of a checkpoint inhibitor-based combination regimen for first-line treatment of patients with aRCC, NIVO+IPI continues to demonstrate superior long-term survival benefits in both ITT patients and in patients with I/P risk. The HR for OS with NIVO+IPI vs SUN has remained stable over time in ITT and I/P-risk patients, ranging from 0.68 and 0.63 at 17.5 months follow-up to 0.69 and 0.65 at 48 months follow-up, respectively. Moreover, after 4 years, ≥50% of patients in the ITT and I/P-risk populations were alive, with median OS not reached in ITT patients. ORR remained consistent and significantly in favour of NIVO+IPI over SUN. In addition, more responses were complete, more responses were ongoing and duration of response was longer with NIVO+IPI, with median duration of response not reached at 4 years minimum follow-up with the combination immunotherapy regimen in any IMDC risk group. Interestingly, of patients achieving partial or complete responses, a greater proportion of patients treated with NIVO+IPI discontinued therapy without progression and did not receive subsequent systemic therapy. Furthermore, no new safety signals were observed with longer follow-up. The overall incidence of treatment-related events, those leading to discontinuation and select (potentially immune-mediated) AEs (including the proportion of patients who required prednisone or equivalent to manage these events) was similar to previous reports.1 2 4
In patients with FAV risk, an OS benefit for either treatment arm remains inconclusive with 4 years minimum follow-up. Median PFS was longer and ORR was higher with SUN, yet the complete response rate was double with NIVO+IPI with more ongoing responses, compared with SUN. Although no significant benefit with NIVO+IPI has emerged in this exploratory cohort, the difference in OS between arms continues to evolve with long-term follow-up. The HR for OS with NIVO+IPI vs SUN has decreased over time from 1.45 at 17.5 months minimum follow-up to 0.93 at 4 years minimum follow-up.4 Additionally, a recent separate analysis suggests that while OS has appeared similar among FAV-risk patients between treatment arms, the way these patients experienced OS was notably different: SUN patients spent more time on protocol treatment with toxicity, whereas NIVO+IPI patients spent more time off treatment without toxicity.12
IMDC risk assessment was specifically derived from patients treated with vascular endothelial growth factor -targeted therapies, but it is now routinely applied to all patients with aRCC including those treated with immuno-oncology–based therapy.5 13 Identifying patients who will survive longer or develop a long-term response with immuno-oncology–based regimens via additional markers of response is a clear unmet need.14 15 The ongoing demonstration of survival and response benefits with NIVO+IPI vs SUN through 4 years of follow-up in both the primary I/P-risk population and the secondary ITT population suggests that a range of patients with aRCC across the spectrum of IMDC risk may derive long-term clinical benefit with NIVO+IPI.
Extended follow-up reported in this study provides further evidence for the clinically meaningful, durable benefits of first-line NIVO+IPI in patients with aRCC and continues to support NIVO+IPI as a first-line treatment option for patients with aRCC.
Acknowledgments
The patients and families who made this study possible. The clinical study teams who participated in the study. The study was supported by Bristol Myers Squibb. All authors contributed to and approved the presentation; writing and editorial assistance was provided by Rachel Lieberman, PhD, of Parexel, funded by Bristol Myers Squibb. Dako, an Agilent Technologies, Inc company, for collaborative development of the PD-L1 IHC 28-8 pharmDx assay (Santa Clara, California). Bristol Myers Squibb (Princeton, New Jersey) and ONO Pharmaceutical Company Ltd (Osaka, Japan).
Footnotes
Presented at: These data were presented in part at the European Society for Medical Oncology Virtual Congress 2020, 19 to 21 September. Poster 711P.
Contributors: All authors listed have provided substantial contributions to the conception or design of the work, or the acquisition, analysis or interpretation of data, drafting the work or revising it critically for important intellectual content, final approval of the version published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Please see Acknowledgements section of the manuscript for further contributions.
Funding: This work was supported by Bristol Myers Squibb (Princeton, New Jersey) and ONO Pharmaceutical Company Ltd (Osaka, Japan). Authors received no financial support or compensation for publication of this manuscript. The University of Texas MD Anderson Cancer Center is supported by the National Institutes of Health (grant P30 CA016672). Patients treated at Memorial Sloan Kettering Cancer Center were supported in part by Memorial Sloan Kettering Cancer Center Support Grant (Core Grant, number P30 CA008748).
Competing interests: LA reports consulting fees from Pfizer, Novartis, Bristol Myers Squibb (BMS), Ipsen, Roche, MSD, AstraZeneca, Merck, Amgen, Astellas and Exelixis; and other fees from Corvus Pharmaceuticals and Peloton Therapeutics. NMT reports research funding from BMS, Calithera Biosciences, Nektar Therapeutics, Exelixis, Pfizer, Novartis, Arrowhead Pharmaceuticals, Mirati Therapeutics, Takeda, Epizyme and Eisai Medical Research; consulting and advisory fees from BMS, Calithera Biosciences, Nektar Therapeutics, Exelixis, Pfizer, Novartis, Eisai Medical Research, Ipsen, Lilly Oncology, Neoleukin Therapeutics, Surface Oncology, ONO Pharmaceutical and Oncorena; and travel accommodations and expenses from BMS, Calithera Biosciences, Nektar Therapeutics, Exelixis, Pfizer, Novartis, Eisai Medical Research, Ipsen, Lilly Oncology, Neoleukin Therapeutics, Surface Oncology, ONO Pharmaceutical and Oncorena. MB reports advisory and speaker fees from Roche, MSD, BMS, AstraZeneca and Sanofi. DM reports research funding from BMS, Merck, Genentech/Roche, Novartis, Peloton Therapeutics, Alkermes and Prometheus Laboratories; consulting or advisory fees from BMS, Merck, Genentech/Roche, Pfizer, Exelixis, Novartis, X4 Pharma, Array BioPharma, Peloton Therapeutics, EMD Serono, Jounce Therapeutics, Alkermes and Lilly; and other fees from Beth Israel Deaconess Medical Center. ERP reports research funding (institutional) from BMS, AstraZeneca, Merck, Peloton Therapeutics, Pfizer and Astellas; consulting fees from AstraZeneca, BMS, Genentech, Merck, Pfizer, Clovis, Exelixis, Incyte, Seattle Genetics, Janssen, Flatiron Health, Infinity Pharma and McKesson; fees for development of educational presentations from BMS and Merck; and US Patent Application No. 14/588,503. PB reports consulting or advisory fees from BMS, Pfizer, MSD Oncology, Novartis, Ipsen, Roche and Janssen Cilag; travel accommodations and expenses from Amgen; and honoraria from Astellas Pharma. CP reports consulting or advisory fees from BMS, MSD, Pfizer, Ipsen Eusa, Eisai and General Electric; speaker fees from BMS, MSD, Pfizer, Ipsen, Eusa, Eisai, General Electric, Janssen and AstraZeneca; research funding from Pfizer; expert testimony fees from Pfizer and Eusa; and travel accommodations and expenses from Roche. TP reports consulting fees from AstraZeneca, BMS, Exelixis, Incyte, Ipsen, Merck/MSD, Pfizer, Seattle Genetics, Merck Serono, Astellas, Johnson & Johnson, Eisai and Roche; honoraria from AstraZeneca, BMS, Exelixis, Incyte, Ipsen, Merck/MSD, Pfizer, Seattle Genetics, Merck Serono, Astellas, Johnson & Johnson, Eisai and Roche; research funding (institutional) from AstraZeneca, Roche, BMS, Exelixis, Ipsen, Merck/MSD, Novartis, Pfizer, Seattle Genetics, Merck Serono, Astellas, Johnson & Johnson and Eisai; and travel accommodations and expenses from Roche, Pfizer, MSD, AstraZeneca and Ipsen. FD reports research funding (institutional) from MSD, Pfizer and Ipsen. SG reports research funding from Bayer, BMS, Pfizer, Novartis, Corvus, Pfizer, Acceleron, Merck, Agensys, Seattle Genetics, Calithera, Immunomedics, Corvus and Eisai; and consulting or advisory fees from Bayer, BMS, Exelixis, Corvus, Genentech, Sanofi/Genzyme, Pfizer, Seattle Genetics, Eisai, Merck and EMD Serono. CKK reports advisory board fees from Janssen, Astellas, Pfizer, Ipsen, Eisai, Roche, Merck and BMS; and lecture fees from Pfizer, Ipsen, Eisai and BMS. HG reports advisory board fees from Pfizer, Astellas, Ipsen, Roche and BMS. M-OG reports research funding from Novartis, BMS and Intuitive Surgical; advisory fees from Novartis, BMS, Pfizer, Bayer HealthCare, Astellas, Intuitive Surgical, AstraZeneca, MSD, Janssen Cilag, ONO Pharmaceutical, Ipsen Pharma and Merck Serono; and lecture fees from Novartis, BMS, Pfizer, Astellas, Hexal, Apogepha, AstraZeneca, MSD, ONO Pharmaceutical, Ipsen Pharma, Medac and Merck Serono. YT reports research funding from ONO Pharmaceutical, Pfizer and Takeda; consulting or advisory fees from ONO Pharmaceutical; and honoraria from ONO Pharmaceutical, BMS and Pfizer. DC reports research funding (institutional) from Janssen Oncology; consulting or advisory fees from Janssen Oncology, Roche/Genentech, Astellas Pharma, AstraZeneca, Novartis, Ipsen, BMS, MSD Oncology, Bayer, Lilly, Sanofi, Pierre Fabre and Boehringer Ingelheim; and travel accommodations and expenses from Pfizer, Roche, BMS and AstraZeneca Spain. BIR reports research funding from Pfizer, Merck, GNE/Roche, Aveo, AstraZeneca and BMS; consulting fees from BMS, Pfizer, GNE/Roche, Aveo, Novartis, Synthorx, Peloton, Compugen, Merck, Arravive, Surface Oncology and 3D Medicines; and stock ownership in PTC therapeutics. TKC reports clinical trial grants from BMS, Exelixis, Pfizer, Merck, AstraZeneca, Lilly, Eisai, Novartis, GSK and EMD Serono; consulting or advisory fees from BMS, Exelixis, Pfizer, Merck, AstraZeneca, Lilly, Eisai, Novartis, GSK and EMD Serono; manuscript preparation fees from BMS, Exelixis, Pfizer, Merck, AstraZeneca, Lilly, Eisai, Novartis, GSK and EMD Serono; travel accommodations and expenses from BMS, Exelixis, Pfizer, Merck, AstraZeneca, Lilly, Eisai, Novartis, GSK and EMD Serono; and patent related to biomarkers of immune-oncology and cfmethDNA pending. SSS is an employee of and has stock ownership in BMS. MBM is an employee of and has stock ownership in BMS. RJM reports research funding (institutional) from Pfizer, Novartis, Eisai, Genentech, Roche and BMS; and consulting or advisory fees from Pfizer, Novartis, Eisai, Exelixis, Merck, Genentech, Incyte, Lilly, Roche and BMS.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. Bristol Myers Squibb’s policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Motzer RJ, Escudier B, McDermott DF, et al. Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J Immunother Cancer 2020;8:e000891. 10.1136/jitc-2020-000891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol 2019;20:1370–85. 10.1016/S1470-2045(19)30413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufman HL, Atkins MB, Subedi P, et al. The promise of Immuno-oncology: implications for defining the value of cancer treatment. J Immunother Cancer 2019;7:129. 10.1186/s40425-019-0594-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heng DYC, Xie W, Regan MM, et al. External validation and comparison with other models of the International metastatic renal-cell carcinoma database Consortium prognostic model: a population-based study. Lancet Oncol 2013;14:141–8. 10.1016/S1470-2045(12)70559-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heng DYC, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009;27:5794–9. 10.1200/JCO.2008.21.4809 [DOI] [PubMed] [Google Scholar]
- 7.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute Common terminology criteria for adverse events (CTCAE), version 4.0. Available: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40 [Accessed 10 August 2020].
- 9.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81. 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 10.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934;26:404–13. 10.1093/biomet/26.4.404 [DOI] [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-Analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 12.Regan MM, Jegede OA, Mantia C, et al. Treatment-free survival, with and without toxicity, after immuno-oncology versus targeted therapy for advanced renal cell carcinoma: 42-month results of CheckMate 214. European Society of Medical Oncology Virtual Congress, 2020: 713P. [Google Scholar]
- 13.Labriola MK, Batich KA, Zhu J, et al. Immunotherapy is changing first-line treatment of metastatic renal-cell carcinoma. Clin Genitourin Cancer 2019;17:e513–21. 10.1016/j.clgc.2019.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raimondi A, Randon G, Sepe P, et al. The evaluation of response to immunotherapy in metastatic renal cell carcinoma: open challenges in the clinical practice. Int J Mol Sci 2019;20:4263. 10.3390/ijms20174263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Vida A, Strijbos M, Hutson T. Predictive and prognostic biomarkers of targeted agents and modern immunotherapy in renal cell carcinoma. ESMO Open 2016;1:e000013. 10.1136/esmoopen-2015-000013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-001079supp001.pdf (583.9KB, pdf)