Abstract
Soon after reports of a novel coronavirus capable of causing severe pneumonia surfaced in late 2019, expeditious global spread of the Severe Acute Respiratory Distress Syndrome Coronavirus 2 (SARS-CoV-2) forced the World Health Organization to declare an international state of emergency. Although best known for causing symptoms of upper respiratory tract infection in mild cases and fulminant pneumonia in severe disease, Coronavirus Disease 2019 (COVID-19) has also been associated with gastrointestinal, neurologic, cardiac, and hematologic presentations. Despite concerns over poor specificity and undue radiation exposure, chest imaging nonetheless remains central to the initial diagnosis and monitoring of COVID-19 progression, as well as to the evaluation of complications. Classic features on chest CT include ground-glass and reticular opacities with or without superimposed consolidations, frequently presenting in a bilateral, peripheral, and posterior distribution. More recently, studies conducted with MRI have shown excellent concordance with chest CT in visualizing typical features of COVID-19 pneumonia. For patients in whom exposure to ionizing radiation should be avoided, particularly pregnant patients and children, pulmonary MRI may represent a suitable alternative to chest CT. Although PET imaging is not typically considered among first-line investigative modalities for the diagnosis of lower respiratory tract infections, numerous reports have noted incidental localization of radiotracer in parenchymal regions of COVID-19-associated pulmonary lesions. These findings are consistent with data from Middle East Respiratory Syndrome-CoV cohorts which suggested an ability for 18F-FDG PET to detect subclinical infection and lymphadenitis in subjects without overt clinical signs of infection. Though highly sensitive, use of PET/CT for primary detection of COVID-19 is constrained by poor specificity, as well as considerations of cost, radiation burden, and prolonged exposure times for imaging staff. Even still, decontamination of scanner bays is a time-consuming process, and proper ventilation of scanner suites may additionally require up to an hour of downtime to allow for sufficient air exchange. Yet, in patients who require nuclear medicine investigations for other clinical indications, PET imaging may yield the earliest detection of nascent infection in otherwise asymptomatic individuals. Especially for patients with concomitant malignancies and other states of immunocompromise, prompt recognition of infection and early initiation of supportive care is crucial to maximizing outcomes and improving survivability.
Abbreviations: SARS-CoV, Severe Acute Respiratory Syndrome Coronavirus; COVID-19, Coronavirus Disease 2019; CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; GGO, ground-glass opacity; RT-PCR, reverse transcription polymerase chain reaction; 18F-FDG, 18F-labelled fluorodeoxyglucose; SUVmax, maximum standardized uptake; MIP, maximum intensity projection; 68Ga-PSMA, 68Ga-labelled prostate-specific membrane antigen; 18F-choline, 18F-labelled choline
Introduction
Coronaviridae refers to a family of single-stranded positive-sense RNA viruses. Though most often associated with mild upper respiratory infections, two notable β-coronaviruses were well-known for causing severe disease with considerably high mortality rates in recent memory during the Severe Acute Respiratory Syndrome Coronavirus 1 (SARS-CoV-1) and Middle East Respiratory Syndrome (MERS) epidemics.1, 2, 3, 4, 5, 6 Even still, the novel SARS-CoV-2 β-coronavirus driving the current Coronavirus Disease 2019 (COVID-19) pandemic has spread with alarming rapidity across the globe, with transmission rates far in excess of those seen during prior β-coronavirus epidemics.7 Clinical manifestations of COVID-19 have typically been associated with respiratory complaints, though gastrointestinal, neurologic, cardiac, and hematologic presentations have also been observed.4 , 8, 9, 10, 11 Significant overlap in presentation with other respiratory tract infections, such as the influenza virus, makes diagnosis on the basis of clinical grounds challenging. This especially holds true during times of the year when the flu is epidemic.12 Symptomatologies ranging from congestion and rhinorrhea, to vomiting and diarrhea, and even to cerebrovascular accident and hemorrhagic encephalopathy have been reported.7, 8, 9, 10 , 13 Nevertheless, fever, cough, and shortness of breath remain the most common presenting symptoms, followed by myalgia or fatigue, sputum production, headache, and hemoptysis.1 , 6 , 10 , 12 , 14 Severe pathologic states have been associated with elevations in acute-phase reactants, including ferritin, D-dimers, and proinflammatory cytokines.7, 8, 9, 10 , 13 As specific treatments and vaccinations are as yet under active investigation, early recognition and prompt isolation protocols remain essential for curtailing viral spread and limiting morbidity and mortality.6 , 7 , 12 , 15, 16, 17
The utility of radiologic imaging in screening and diagnosis of suspected COVID-19 cases has been hotly debated since the start of the pandemic, with many professional societies and expert panels having previously issued recommendations against first-line chest imaging as a primary screening modality.15 , 18, 19, 20, 21, 22 Joint guidelines from the European Society of Radiology and the European Society of Thoracic Imaging as well as those issued by the Fleischner Society do recognize a limited role specifically for chest CT in resource-limited triage situations,23, 24, 25 though discussion of the merits of these approaches lies beyond the scope of this article. Nevertheless, chest imaging remains an integral component of the workup and staging of COVID-19, especially when assessing for complications or disease progression.5 , 15 , 18 , 23 , 26 , 27 As such, it remains essential for radiologists to be able to stratify cases of COVID-19 on the basis of radiologic imaging in order to identify those patients most at risk for imminent clinical decompensation.23 , 28 In this review, we aim to summarize the characteristic imaging findings associated COVID-19 pneumonia, with special focus on chest computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET).
CT
Chest CT has frequently been used in the imaging of COVID-19 since the start of the pandemic, owing much to its affordability, availability, and detailed anatomic resolution.5 , 15 , 18 , 25 Bilateral lung involvement most often predominates, usually in a peripheral, subpleural, and posterior distribution.6 , 7 , 11 , 12 , 14 , 27, 28, 29, 30, 31, 32, 33, 34 Ground-glass opacities (GGOs) – defined as hazy opacities with preservation of the underlying vascular and bronchial architecture35 – have been most commonly observed, with occurrence rates ranging from 50% to 98% in the adult population2 , 12 , 14 , 28, 29, 30, 31, 32, 33 , 36 , 37 (Figs. 1 and 2 ). Reticular opacities – defined as thickening of pulmonary interstitial structures, including inter- and intralobular septa35 – have been reported as the next most common finding, with occurrence rates as high as 77%.7 , 14 , 29 , 30 , 36 The presence of consolidations – defined as dense regions of opacification with obfuscation of the underlying bronchovascular architecture35 – either alone or superimposed on concomitant GGOs (so-called “mixed lesions”) has reportedly been much lower, with estimates ranging between 24.2% and 64%2 , 6 , 14 , 27 , 28 , 30, 31, 32, 33 , 36, 37, 38 (Figs. 1 and 3 ). GGOs together with focal consolidation are thought to be indicators of organizing pneumonia, where lesion formation may be related to ongoing pulmonary edema with hyaline membrane formation.6 , 7 , 29 Crazy paving pattern – defined as GGOs with superimposed intralobular lines and interlobular septal thickening35 – is decidedly less common, with reported frequencies ranging from 5% to 36%.2 , 7 , 14 , 28, 29, 30 , 36 Ancillary findings, including adjacent pleural thickening, intralobular septal thickening, pulmonary vascular enlargement, subpleural lines, air bronchograms, and a reverse halo sign, have also been described2 , 3 , 7 , 12 , 14 , 27, 28, 29, 30, 31 , 36 (Fig. 2). While not typical features of disease, pleural effusion and mediastinal lymphadenopathy have been reported in a minority of cases and may herald poorer clinical outcomes.2 , 3 , 7 , 12 , 14 , 27, 28, 29, 30 , 36
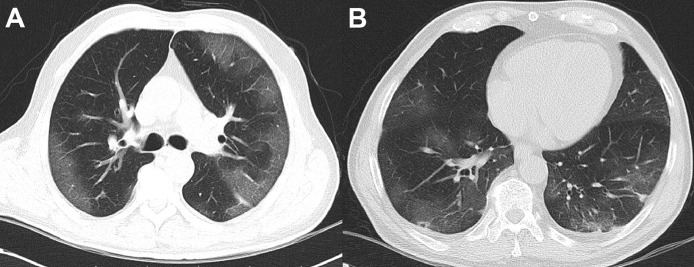
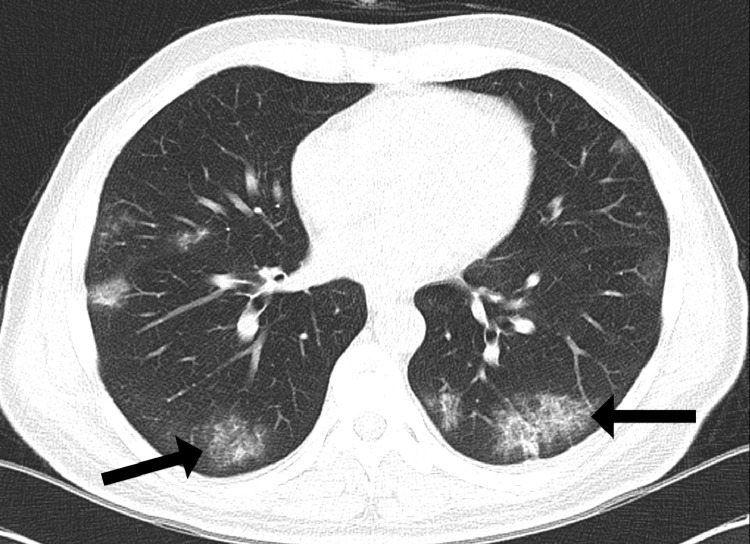
Figure 1.
A 64-year-old male presented with shortness of breath and fever. Noncontrast chest CT demonstrated bilateral peripheral ground glass opacities (GGOs) with tiny foci of superimposed consolidations.
Figure 2.
A 54-year-old male presented with cough and fever. Noncontrast chest CT demonstrated bilateral, peripheral ground-glass opacities (GGOs) superimposed on interstitial septal thickening (arrows).
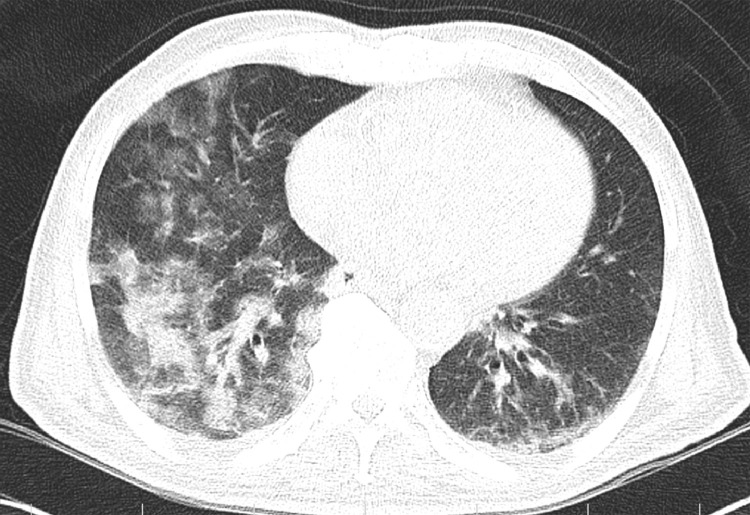
Figure 3.
An 86-year-old male presented with cough and fever. Noncontrast chest CT showed a consolidation-predominant pattern with bilateral lung involvement.
It is possible that the large discrepancies in reported occurrence rates in the published literature likely arise from factors of disease progression, patient characteristics, and overall severity of illness. For example, Li et al. found that the occurrence rates of linear opacities, consolidation, bronchial wall thickening, and crazy paving pattern were all much higher in critically ill patients.37 Likewise, Carotti et al. suggest that the development of consolidation may be related to disease progression coincident with alveolar accumulation of fibromyxoid exudates.7 As such, these lesions tend to be more prominent in middle to late disease and in patients over the age of 50 years.3 , 32 , 36 Similarly, reticular opacities were found to present much more commonly in younger populations, although these lesions were also seen to increase in frequency over the course of disease.3 , 7 Furthermore, while initial lesions may preferentially occupy the lung periphery, an increase in the number of lesions with centripetal spread has also been associated with disease progression.7 , 39, 40, 41 The severity of imaging manifestations tends to peak at approximately 10 days, with gradual resolution and decrease in number of lesions and lobes involved correlating with clinical improvement and recovery.7 , 28 , 36
Studies specifically focused in pediatric populations have suggested that children may experience milder, more focal disease in comparison to adults.42, 43, 44 For example, in a systematic review analysis of 39 published studies, Katal et al. found a predominance of typical GGOs in a unilateral, lower-lobe distribution.43 Normal chest CT in the setting of a positive reverse transcription polymerase chain reaction (RT-PCR) test was also found to be more common.42 , 43 , 45 In contrast, while pregnant patients may present initially with similar findings on chest CT as the general adult population, current literature as well as unpublished data from our group suggests that this population may in fact present more frequently with pleural effusions and be more prone to progression of GGOs to consolidative lesions.10 , 46 Nevertheless, practitioners are dutifully cautioned that imaging findings of COVID-19 pneumonia are highly nonspecific and share numerous overlapping features with both SARS-CoV-1 and MERS-CoV, as well as other viral pneumonias.2 , 7 , 14 , 26 , 28 , 30 , 31 , 36 , 47 In particular, herpes simplex virus, adenovirus, and cytomegalovirus pneumonias are all known to feature bilateral lung consolidation.26 In a comparative study of chest CT findings in COVID-19 pneumonia versus influenza virus pneumonia, Lin et al. found no difference in the presence of consolidation, GGOs, interlobular septal thickening, bronchial wall thickening, centrilobular nodules, mosaic attenuation, air bronchograms, crazy paving pattern, and laterality, though COVID-19 was found to present more frequently with the largest lesion closer to the pleura.48 As such, when faced with situations of diagnostic uncertainty, providers are encouraged to consider historical factors and clinical context as appropriate.
MRI
An increasing number of case reports have confirmed visualization of incidental findings related to COVID-19 pneumonia in individuals who underwent MRI of the head and neck or chest for other clinical indications.49, 50, 51, 52, 53 Thus far, three studies have attempted to formally compare the ability of pulmonary MRI to resolve characteristic features of COVID-19 pneumonia against conventional chest CT. In a preliminary case series of eight patients, Torkian et al. demonstrated visualization of GGOs, consolidation, reticulation, and a reverse halo sign on multiple MRI sequences.34 T2-weighted turbo spin-echo turbo inversion recovery magnitude (T2W TSE-TIRM) was found to resolve lesions more brightly than the other sequences studied, likely highlighting areas of edema secondary to parenchymal inflammatory changes. Similarly, Ates et al. showed no significant differences in MRI detection of GGOs or consolidative lesions versus conventional CT.33 In their study, MRI had 91.7% sensitivity, 100% specificity, 100% positive predictive value, and 95.2% negative predictive value when using CT as the reference. In a prospective comparative analysis of ultrashort echo time MRI (UTE-MRI) vs conventional CT, Yang et al. further demonstrated high concordance in lesion detection.54 Their study reports substantial or excellent intermethod agreement for detecting typical findings of COVID-19 pneumonia, including pure GGOs, pure consolidation, and GGOs with consolidation; however, only fair to moderate lesion-based intermethod agreement was observed for the assessment of secondary signs, including pseudocavities, crazy paving pattern, and air bronchograms. Overall, these results are consistent with existing literature in related pulmonary infections suggesting that MRI adequately distinguishes between different stages of parenchymal infiltration in head-to-head comparisons with chest CT.55, 56, 57
Current guidelines from the American College of Radiology recommend limiting the use of MRI in confirmed or suspected SARS-CoV-2-positive patients.58 , 59 Concerns with regard to infectious transmission notwithstanding, pulmonary MRI has historically been limited by cardiac and respiratory motion artifact, low proton density of the parenchymal tissues, and susceptibility artifacts resulting from multiple soft-tissue-air interfaces.34 , 54, 55, 56, 57 , 60 Yet, these intrinsic properties of the lung parenchyma are in fact advantages when imaging pathology of the alveolar spaces, as GGOs and consolidation appear hyperintense relative to the surrounding tissues secondary to exudative fluid accumulation and increased proton density.33 , 34 , 55 , 56 , 60 Though not considered routine for evaluating suspected lower respiratory tract infections, pulmonary MRI may provide a viable alternative for imaging high-risk patient groups – such as pregnant patients and children – in whom exposure to ionizing radiation should be avoided.33 , 34 , 54 , 57 , 60
PET
18F-labelled fluorodeoxyglucose (18F-FDG) is a glucose analogue positron emitting radiotracer with a half-life of approximately 110 minutes. Although best known for its applicability in the functional imaging of malignancies, there exists a growing role for the use of 18F-FDG-PET in evaluation and characterization of infectious and inflammatory pulmonary conditions.27,39,61 Prior studies have established an ability for external imaging of intravenously injected radiotracers to noninvasively quantify the behavior of inflammatory cells in conditions such as chronic obstructive pulmonary disease (COPD) and asthma.62 Inflammatory reactions resulting in acute lung injury in the context of viral pneumonia are driven by chemokine recruitment of neutrophils, monocytes, and effector T-cells.39 , 63 Neutrophil activity in particular is highly reliant on anaerobic glycolysis and subsequent uptake of glucose from the surrounding microenvironment, manifesting as 18F-FDG-avid foci on PET imaging. When coupled with CT fusion underlays, PET/CT allows for detailed evaluation of both functional and anatomical processes, as well as non-invasive quantification of parenchymal and interstitial activity as a proxy for inflammatory cell behavior.27 , 61 , 62 , 64
Studies in MERS-CoV cohorts showed a marked increase in 18F-FDG-avid foci in infected patients with disease progression to pneumonia.65 In the case of subclinical infection without visualized pathologic changes by chest CT alone, Chefer et al. demonstrated increased 18F-FDG radiotracer uptake in axillary and mediastinal lymph nodes in MERS-infected rhesus macaques.63 18F-FDG nodal uptake in the setting of viral infection has previously been demonstrated, and can likely be attributed to increased leukocytic response in host lymphoid tissues.39 , 63 , 66 , 67 Given evidence that viral replication has been shown to occur in the absence of clinical manifestations of disease,68 18F-FDG PET/CT has been proposed as an alternative modality to detect early, subclinical infections, albeit with an associated radiation cost.39 , 63 Unfortunately, a paucity of data exists for the evaluation of SARS-CoV-1 infection by PET imaging, possibly due to a combination of factors related to more recent trends in the field regarding the use of functional imaging for investigations of inflammatory processes.27 , 61 , 62
Unsurprisingly, early case reports indicated that COVID-19 pneumonia presents similarly with 18F-FDG-avid parenchymal lesions.27 , 41 , 69 , 70 Qin et al. described a series of four patients who were admitted to hospital in Wuhan early in the pandemic and received 18F-FDG PET/CT during the course of acute illness. All patients demonstrated parenchymal 18F-FDG uptake in regions corresponding to GGOs and/or consolidative opacities, with maximum standardized uptake (SUVmax) values ranging from 4.6 to 12.2.71 Three out of the four patients in this case series also presented with radiotracer uptake in regional lymph nodes. Likewise, Zou and Zhu described an 18F-FDG-avid mass with a SUVmax of 4.9 in a middle-aged patient initially evaluated for suspicion of lung cancer.72 Radiotracer uptake was also noted in the right hilar and paratracheal lymph nodes, as well as in the bone marrow. A number of studies published in the ensuing weeks concordantly reported similar incidental pulmonary findings in otherwise asymptomatic or mildly symptomatic patients who received 18F-FDG PET/CT for oncologic indications, many of whom also demonstrated nodal involvement.40 , 73, 74, 75, 76, 77, 78, 79 These and other reports were recently quantified in a systematic review by Rafiee et al. which reported a mean SUVmax of 4.9 ± 2.3 in COVID-19-associated pulmonary lesions.11 Representative images of characteristic lesions are pictured in Figure 4, Figure 5, Figure 6 . In a case series of 5 patients, Scarlattei et al. even further observed radioactive localization of 68Ga-labelled prostate-specific membrane antigen (68Ga-PSMA) and 18F-labelled choline (18F-choline) to anatomic regions corresponding to subpleural GGOs in two patients receiving standard-of-care PET/CT for prostate cancer.77 68Ga-PSMA and 18F-choline are radiotracers frequently used to localize hypermetabolic lesions in the setting of prostate cancer,80 , 81 though the precise mechanisms driving pulmonary localization in the setting of acute infection may be an intriguing area for future investigation.
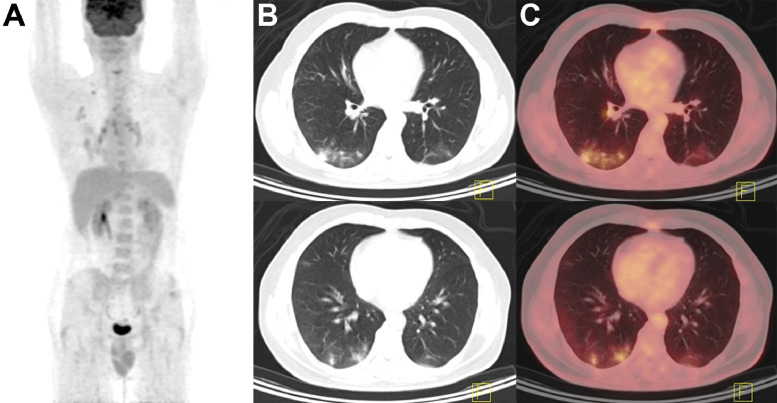
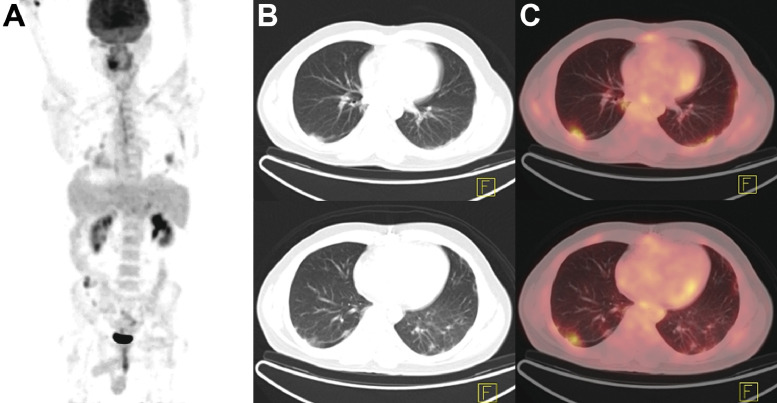
Figure 4.
Staging PET/CT in a patient with biopsy-proven low-grade non-Hodgkin's lymphoma. (A), Maximum intensity projection (MIP) showed precarinal, subcarinal, right infraclavicular, right lower paratracheal, and right hilar lymphadenopathy, with a SUVmax of 6.2. (B–C), Incidental note was made of poorly-defined hypermetabolic airspace consolidations superimposed on ground-glass opacities in dependent segments of the bilateral lungs (B, low-dose CT; C, PET/CT fusion). Serologic testing confirmed the presence of SARS-CoV-2 infection. MIP, maximum intensity projection
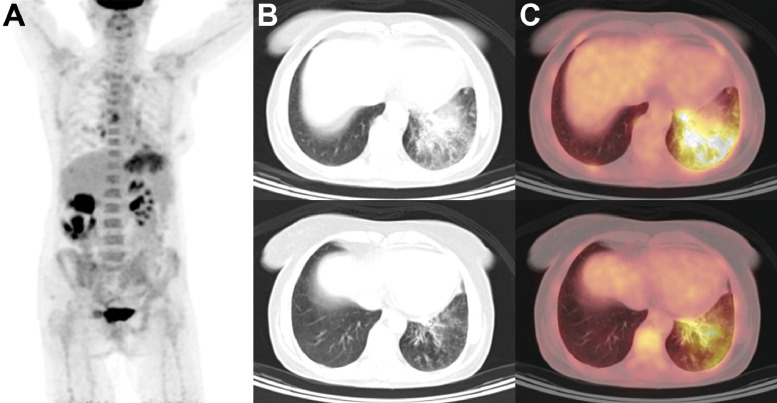
Figure 5.
Restaging PET/CT in a patient with history of well-differentiated adenocarcinoma of the sigmoid colon. (A), Multiple hypoechoic hepatic and periportal lymph nodes noted on recent ultrasound demonstrated no focal hypermetabolism on maximum intensity projection (MIP) to suggest metastatic disease; (B), however, multifocal, patchy consolidations in a peripheral, subpleural distribution were identified in the bilateral lungs on low-dose CT. (C), Corresponding hypermetabolic parenchymal foci were identified on PET/CT fusion, concerning for an infectious or inflammatory process. The patient tested positive for SARS-CoV-2 infection by RT-PCR. MIP, maximum intensity projection
Figure 6.
Restaging PET/CT to evaluate for treatment response in a patient with known diffuse large B-cell lymphoma and recent chemotherapy. (A), Maximum intensity projection (MIP) demonstrated hypermetabolic mediastinal lymphadenopathy, with a SUVmax of 7.5. (B–C), Ill-defined ground-glass opacities with superimposed consolidations were also noted in the left lung base, along with corresponding 18F-FDG uptake on PET overlay (B, low-dose CT; (C), PET/CT fusion). A diagnosis of COVID-19 pneumonia was confirmed by RT-PCR testing. MIP, maximum intensity projection.
For imaging of COVID-19, preliminary reports suggest that 18F-FDG PET/CT is a highly sensitive modality despite suffering from poor specificity.27 , 69 , 77 In otherwise asymptomatic patients, incidental detection of subclinical disease burden may play an important role in curbing asymptomatic viral spread.41 , 82 As Alberts et al. astutely point out, the low-dose CT performed in conjunction with PET imaging is nonetheless a diagnostic CT and may show early parenchymal changes warranting confirmatory laboratory testing.82 Furthermore, timely recognition of SARS-CoV-2 infection in highly vulnerable populations may additionally aid clinicians in identifying patients most at risk for fulminant decompensation. For example, Polverari et al. reported the presence of multiple 18F-FDG-avid foci corresponding to GGOs visualized on accompanying low-dose CT (right lower lobe SUVmax = 5.9; left lower lobe SUVmax = 7.9) in the lungs of a patient who underwent 18F-FDG PET/CT for suspected recurrence of non–small cell lung cancer.76 The suspicious left-upper lobe nodule was notably not visualized as a hypermetabolic focus. It was determined that the PET-avid foci were likely related to an acute inflammatory process, and the patient subsequently tested positive for SARS-CoV-2 infection by RT-PCR. The patient rapidly progressed over the ensuing days, requiring intensive care unit hospitalization 3 days following 18F-FDG PET/CT acquisition. Physicians practicing in endemic regions are thus cautioned to maintain a high index of suspicion for incidental findings of early infection, where prompt recognition and anticipatory care may improve outcomes in otherwise highly susceptible populations.11
18F-FDG PET/CT may also yield additional information regarding ongoing inflammatory changes. Data from Qin et al. suggest that lesions demonstrating greater 18F-FDG uptake may correlate with higher erythrocyte sedimentation rates and take a longer time to heal.39,41,71 Lutje et al. further propose that 18F-FDG PET/CT may also be useful in assessing for changes in other organ systems, including the heart, kidneys, and gastrointestinal tract.41 However, while Zou and Zhu did note foci of bone marrow uptake in their patient,72 Qin et al., found no evidence of disseminated disease and instead posit that SARS-CoV-2 may exhibit some degree of tissue tropism.71 18F-FDG PET/CT also has the potential to evaluate for the presence of concomitant infections in situations of diagnostic uncertainty. For instance, Kamani et al. described a case of COVID-19 diagnosed in a patient with chronic osteitis of the left first metatarsal and positive bacterial hemocultures who had 18F-FDG PET/CT performed for investigation of suspected concomitant prosthetic valve infection.83 Following laboratory confirmation of SARS-CoV-2 infection, the patient was subsequently found to have hypermetabolic foci in the bilateral lungs, with a SUVmax of 7.6 in regions corresponding to GGOs with partial consolidation. The expected hypermetabolic focus in the left foot was also observed. Evidence of lymphadenitis was detected in the subcarinal, paratracheal, and hilar nodes bilaterally, with a SUVmax of 6.1. No evidence of prosthetic valve infection was detected. Thus, we observe that 18F-FDG PET/CT may serve a complementary role in narrowing the differential diagnosis in patients presenting with multiple possible infectious sources.
Nevertheless, multiple authors staunchly recommend against the use of PET/CT as a primary diagnostic modality for investigating cases of suspected COVID-19 in the emergency setting.11 , 27 , 41 , 69 , 84 , 85 As noted by Rafiee et al. PET is an expensive imaging modality associated with prolonged acquisition times and increased radiation burden in comparison to conventional chest X-ray and chest CT.11 Joob and Wiwanitkit similarly note that the technical complexity of PET imaging prolongs exposure times for nuclear medicine staff, placing them at greater risk for nosocomial transmission.84 Thus, imaging of persons-under-investigation is contingent upon adequate supplies of personal protective equipment for patients and staff,86 , 87 which, especially in the early days of the pandemic, were severely limited by catastrophic interruptions to supply chains worldwide.88 In addition, cleaning protocols can take as long as one full hour when factoring in the need to both sanitize the bay and scanner and allow for thorough ventilation and air-exchange in cases of confirmed or suspected SARS-CoV-2 infection.59 , 86 , 87 , 89 Taken together, these risks cannot justify acquisition of PET imaging solely for the purposes of diagnostic investigations for COVID-19. While Lee and Blazak do suggest a role for assessing persistent morphologic changes from a functional standpoint,85 such applications warrant further study prior to implementation in standard-of-care clinical workflows.
Conclusion
Chest imaging continues to remain essential to the monitoring and staging of COVID-19 pneumonia. By now, characteristic features on chest CT have been well described; typically, these can include findings of GGOs and reticular opacities with or without superimposed consolidations, often residing in the peripheral and posterior lung fields bilaterally. Yet, as these findings are nonspecific and overlap considerably with other causes of viral pneumonia, consideration of alternative diagnoses is warranted in patients with negative confirmatory assays. Though pulmonary MRI is not typically considered among first-line modalities for the investigation of suspected lower respiratory tract infections, initial studies have reported high concordance with chest CT for detecting features of COVID-19 pneumonia in head-to-head comparisons. For patient groups in whom excessive or repeated exposure to ionizing radiation should be avoided, pulmonary MRI may yet provide a viable alternative. Early reports from SARS-CoV-2 positive patients who received 18F-FDG PET/CT for other indications confirm the presence of hypermetabolic foci in anatomic distributions corresponding to COVID-19-associated pulmonary lesions. While preliminary data would suggest that PET/CT is a highly sensitive modality for the detection of COVID-19 pneumonia, concerns over cost, undue radiation burden, and prolonged exposure times for nuclear medicine staff limit its applicability as a primary first-line imaging modality. Nonetheless, radiologists and nuclear medicine physicians practicing in endemic areas must remain vigilant in scrutinizing all scans for evidence of otherwise asymptomatic infection. Early detection of SARS-CoV-2 infection in patients who receive nuclear medicine imaging for unrelated clinical indications, such as the evaluation and staging of malignancy, is essential for providing prompt anticipatory care to vulnerable populations at high risk for rapid clinical decompensation.
Disclosures
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Footnotes
Conflicts of Interest: None.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng Y, Wang L, Ben S. Meta-analysis of chest CT features of patients with COVID-19 pneumonia. J Med Virol. 2020 doi: 10.1002/jmv.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song F, Shi N, Shan F, et al. Emerging 2019 novel Coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295:210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katal S, Balakrishnan S, Gholamrezanezhad A. Neuroimaging and neurologic findings in COVID-19 and other coronavirus infections: A systematic review in 116 patients. J Neuroradiol. 2020 doi: 10.1016/j.neurad.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kooraki S, Hosseiny M, Myers L, et al. Coronavirus (COVID-19) outbreak: What the Department of Radiology should nnow. J Am Coll Radiol. 2020;17(4):447–451. doi: 10.1016/j.jacr.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernheim A, Mei X, Huang M, et al. Chest CT findings in Coronavirus disease-19 (COVID-19): Relationship to duration of infection. Radiology. 2020;295 doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carotti M, Salaffi F, Sarzi-Puttini P, et al. Chest CT features of coronavirus disease 2019 (COVID-19) pneumonia: Key points for radiologists. Radiol Med. 2020;125:636–646. doi: 10.1007/s11547-020-01237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fields BKK, Demirjian NL, Balakrishnan S, et al. Coronavirus disease 2019 (COVID-19): An update on neurologic sequelae. Neurodiem. 2020 https://www.neurodiem.com/news/coronavirus-disease-2019-covid-19-an-update-on-neurologic-sequelae-5kjyB0CXWwiwdicqPIu8Fn Published April 22, 2020. Accessed October 27, 2020. [Google Scholar]
- 9.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with Coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behzad S, Aghaghazvini L, Radmard AR, et al. Extrapulmonary manifestations of COVID-19: Radiologic and clinical overview. Clin Imaging. 2020;66:35–41. doi: 10.1016/j.clinimag.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rafiee F, Keshavarz P, Katal S, et al., Coronavirus Disease 2019 (COVID-19) in molecular imaging: A systematic review of incidental detection of SARS-CoV-2 pneumonia on PET studies. Semin Nucl Med. (in press). doi: 10.1053/j.semnuclmed.2020.10.002 [DOI] [PMC free article] [PubMed]
- 12.Zhao W, Zhong Z, Xie X, et al. Relation between chest CT findings and clinical conditions of Coronavirus disease (COVID-19) pneumonia: A multicenter study. AJR Am J Roentgenol. 2020;214:1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 13.Okabayashi T, Kariwa H, Yokota S, et al. Cytokine regulation in SARS coronavirus infection compared to other respiratory virus infections. J Med Virol. 2006;78:417–424. doi: 10.1002/jmv.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J, Wu X, Zeng W, et al. Chest CT findings in patients with Coronavirus disease 2019 and its relationship with clinical features. Invest Radiol. 2020;55:257–261. doi: 10.1097/RLI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fields BKK, Demirjian NL, Gholamrezanezhad A. Coronavirus Disease 2019 (COVID-19) diagnostic technologies: A country-based retrospective analysis of screening and containment procedures during the first wave of the pandemic. Clin Imaging. 2020;67:219–225. doi: 10.1016/j.clinimag.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salehi S, Abedi A, Balakrishnan S, et al. Coronavirus disease 2019 (COVID-19) imaging reporting and data system (COVID-RADS) and common lexicon: A proposal based on the imaging data of 37 studies. Eur Radiol. 2020;30:4930–4942. doi: 10.1007/s00330-020-06863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan J, St. Pierre JM, Pickering TA, et al. Coronavirus disease 2019 (COVID-19): A modeling study of factors driving variation in case fatality rate by country. Int J Environ Res Public Health. 2020;17:8189. doi: 10.3390/ijerph17218189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demirjian NL, Fields BKK, Song C, et al. Impacts of the Coronavirus disease 2019 (COVID-19) pandemic on healthcare workers: A nationwide survey of United States radiologists. Clin Imaging. 2020;68:218–225. doi: 10.1016/j.clinimag.2020.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mossa-Basha M, Medverd J, Linnau K, et al. Policies and uidelines for COVID-19 preparedness: Experiences from the University of Washington. Radiology. 2020 doi: 10.1148/radiol.2020201326. [DOI] [PubMed] [Google Scholar]
- 20.Mossa-Basha M, Meltzer CC, Kim DC, Tuite MJ, Kolli KP, Tan BS. Radiology Department Preparedness for COVID-19: Radiology scientific expert panel. Radiology. 2020 doi: 10.1148/radiol.2020200988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ACR recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection. American College of Radiology website.https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. Published March 11, 2020. Updated March 22, 2020. Accessed October 28, 2020.
- 22.Simpson S, Kay FU, Abbara S, et al. Radiological Society of North America Expert Consensus Statement on reporting Chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA - Secondary Publication. J Thorac Imaging. 2020;35:219–227. doi: 10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Revel MP, Parkar AP, Prosch H, et al. European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). COVID-19 patients and the radiology department - advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI) Eur Radiol. 2020 doi: 10.1007/s00330-020-06865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin GD, Ryerson CJ, Haramati LB, et al. The role of chest imaging in patient management during the COVID-19 pandemic: A multinational consensus statement from the Fleischner Society. Radiology. 2020 doi: 10.1148/radiol.2020201365. Published April 7, 2020. Accessed April 8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demirjian NL, Fields BKK, Gholamrezanezhad A. Role of chest CT in resource-driven healthcare systems. AJR Am J Roentgenol. 2020;215:W36. doi: 10.2214/AJR.20.23498. [DOI] [PubMed] [Google Scholar]
- 26.Lim ZY, Khoo HW, Hui TCH, et al. Variable computed tomography appearances of COVID-19. Singapore Med J. 2020;61:387–391. doi: 10.11622/smedj.2020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katal S, Amini H, Gholamrezanezhad A. PET in the diagnostic management of infectious/inflammatory pulmonary pathologies: A revisit in the era of COVID-19. Nucl Med Commun. 2020 doi: 10.1097/MNM.0000000000001299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ojha V, Mani A, Pandey NN, et al. CT in coronavirus disease 2019 (COVID-19): A systematic review of chest CT findings in 4410 adult patients. Eur Radiol. 2020;30:6129–6138. doi: 10.1007/s00330-020-06975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye Z, Zhang Y, Wang Y, et al. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): A pictorial review. Eur Radiol. 2020;30:4381–4389. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/s1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 Novel Coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosseiny M, Kooraki S, Gholamrezanezhad A, et al. Radiology perspective of Coronavirus Disease 2019 (COVID-19): Lessons from severe acute respiratory syndrome and middle East Respiratory syndrome. AJR Am J Roentgenol. 2020;214:1078–1082. doi: 10.2214/AJR.20.22969. [DOI] [PubMed] [Google Scholar]
- 33.Ates OF, Taydas O, Dheir H. Thorax magnetic resonance imaging findings in patients with Coronavirus Disease (COVID-19) Acad Radiol. 2020;27:1373–1378. doi: 10.1016/j.acra.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torkian P, Rajebi H, Zamani T, et al. Magnetic resonance imaging features of coronavirus disease 2019 (COVID-19) pneumonia: The first preliminary case series. Clin Imaging. 2020;69:261–265. doi: 10.1016/j.clinimag.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner society: Glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 36.Salehi S, Abedi A, Balakrishnan S, et al. Coronavirus disease 2019 (COVID-19): A systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215:87–93. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 37.Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020;55:327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inui S, Fujikawa A, Jitsu M, et al. Chest CT findings in cases from the cruise ship “Diamond Princess” with Coronavirus Disease 2019 (COVID-19) Radiology. 2020;2 doi: 10.1148/ryct.2020200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng Y, Lei L, Chen Y, et al. The potential added value of FDG PET/CT for COVID-19 pneumonia. Eur J Nucl Med Mol Imaging. 2020;47:1634–1635. doi: 10.1007/s00259-020-04767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Albano D, Bertagna F, Bertoli M, et al. Incidental findings suggestive of COVID-19 in asymptomatic patients undergoing nuclear medicine procedures in a high-prevalence region. J Nucl Med. 2020;61:632–636. doi: 10.2967/jnumed.120.246256. [DOI] [PubMed] [Google Scholar]
- 41.Lutje S, Marinova M, Kutting D, et al. Nuclear medicine in SARS-CoV-2 pandemia: 18F-FDG-PET/CT to visualize COVID-19. Nuklearmedizin. 2020;59:276–280. doi: 10.1055/a-1152-2341. [DOI] [PubMed] [Google Scholar]
- 42.Hoang A, Chorath K, Moreira A, et al. COVID-19 in 7780 pediatric patients: A systematic review. E Clin Med. 2020;24 doi: 10.1016/j.eclinm.2020.100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katal S, Johnston SK, Johnston JH, et al. Imaging findings of SARS-CoV-2 infection in pediatrics: A systematic review of coronavirus disease 2019 (COVID-19) in 850 patients. Acad Radiol. 2020 doi: 10.1016/j.acra.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia W, Shao J, Guo Y, et al. Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol. 2020;55:1169–1174. doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kooraki S, Hosseiny M, Gholamrezanezhad A. Radiologic findings of Coronavirus Disease (COVID-19): Clinical correlation is recommended. AJR Am J Roentgenol. 2020;215:W7. doi: 10.2214/AJR.20.23211. [DOI] [PubMed] [Google Scholar]
- 46.Liu H, Liu F, Li J, et al. Clinical and CT imaging features of the COVID-19 pneumonia: Focus on pregnant women and children. J Infect. 2020;80:e7–e13. doi: 10.1016/j.jinf.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koo HJ, Lim S, Choe J, et al. Radiographic and CT features of viral pneumonia. Radiographics. 2018;38:719–739. doi: 10.1148/rg.2018170048. [DOI] [PubMed] [Google Scholar]
- 48.Lin L, Fu G, Chen S, et al., CT manifestations of Coronavirus Disease (COVID-19) pneumonia and influenza virus pneumonia: A comparative study. AJR Am J Roentgenol 2020:1-9. doi: 10.2214/AJR.20.23304 [DOI] [PubMed]
- 49.Angelini V, Villanacci A, Belotti A, et al. Incidental whole-body MRI evidence of COVID-19 in an asymptomatic patient in a high prevalence region. Egypt J Radiol Nucl Med. 2020;51 doi: 10.1186/s43055-020-00288-x. [DOI] [Google Scholar]
- 50.Kaiser Ururahy Nunes Fonseca E, Chate RC, Neto RS, et al. Findings of COVID-19 on magnetic resonance imaging. Radiology. 2020;2 doi: 10.1148/ryct.2020200193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Langenbach MC, Hokamp NG, Persigehl T, et al. MRI appearance of COVID-19 infection. Diagn Interv Radiol. 2020;26:377–378. doi: 10.5152/dir.2020.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith P, Bilello M, Mohan S. Neuro-thoracic radiologists "Corner": Incidental pulmonary findings on a neck MRI leading to the diagnosis of COVID-19. AJNR Am J Neuroradiol. 2020;41:E78–E79. doi: 10.3174/ajnr.A6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jain R, Young M, Dogra S, et al. Surprise diagnosis of COVID-19 following neuroimaging evaluation for unrelated reasons during the pandemic in hot spots. AJNR Am J Neuroradiol. 2020;41:1177–1178. doi: 10.3174/ajnr.A6608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang S, Zhang Y, Shen J, et al. Clinical potential of UTE-MRI for assessing COVID-19: Patient- and lesion-based comparative analysis. J Magn Reson Imaging. 2020;52:397–406. doi: 10.1002/jmri.27208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leutner CC, Gieseke J, Lutterbey G, et al. MR imaging of pneumonia in immunocompromised patients: Comparison with helical CT. AJR Am J Roentgenol. 2000;175:391–397. doi: 10.2214/ajr.175.2.1750391. [DOI] [PubMed] [Google Scholar]
- 56.Ohno Y, Koyama H, Yoshikawa T, et al. Pulmonary high-resolution ultrashort TE MR imaging: Comparison with thin-section standard- and low-dose computed tomography for the assessment of pulmonary parenchyma diseases. J Magn Reson Imaging. 2016;43:512–532. doi: 10.1002/jmri.25008. [DOI] [PubMed] [Google Scholar]
- 57.Syrjala H, Broas M, Ohtonen P, et al. Chest magnetic resonance imaging for pneumonia diagnosis in outpatients with lower respiratory tract infection. Eur Respir J. 2017;49 doi: 10.1183/13993003.01303-2016. [DOI] [PubMed] [Google Scholar]
- 58.ACR Guidance on COVID-19 and MR Use. American college of radiology website.https://www.acr.org/Clinical-Resources/Radiology-Safety/MR-Safety/COVID-19-and-MR-Use. Accessed October 31, 2020.
- 59.Kooraki S, Hosseiny M, Raman SS, et al. Coronavirus Disease 2019 (COVID-19) precautions: What the MRI suite should know. J Am Coll Radiol. 2020;17:830. doi: 10.1016/j.jacr.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caro-Dominguez P, Shelmerdine SC, Toso S, et al. Collaborators of the European Society of Paediatric Radiology Cardiothoracic Task F. Thoracic imaging of coronavirus disease 2019 (COVID-19) in children: a series of 91 cases. Pediatr Radiol. 2020;50:1354–1368. doi: 10.1007/s00247-020-04747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Capitanio S, Nordin AJ, Noraini AR, et al. PET/CT in nononcological lung diseases: Current applications and future perspectives. Eur Respir Rev. 2016;25:247–258. doi: 10.1183/16000617.0051-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones HA, Marino PS, Shakur BH, et al. In vivo assessment of lung inflammatory cell activity in patients with COPD and asthma. Eur Respir J. 2003;21:567–573. doi: 10.1183/09031936.03.00048502. [DOI] [PubMed] [Google Scholar]
- 63.Chefer S, Thomasson D, Seidel J, et al. Modeling [(18)F]-FDG lymphoid tissue kinetics to characterize nonhuman primate immune response to Middle East respiratory syndrome-coronavirus aerosol challenge. EJNMMI Res. 2015;5:65. doi: 10.1186/s13550-015-0143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu YM, Nortmann CA. Pixel-feature hybrid fusion for PET/CT images. J Digit Imaging. 2011;24:50–57. doi: 10.1007/s10278-009-9259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Das KM, Lee EY, Langer RD, et al. Middle east respiratory syndrome Coronavirus: What does a radiologist need to know? AJR Am J Roentgenol. 2016;206:1193–1201. doi: 10.2214/AJR.15.15363. [DOI] [PubMed] [Google Scholar]
- 66.Scharko AM, Perlman SB, Hinds PWn, et al. Whole body positron emission tomography imaging of simian immunodeficiency virus-infected rhesus macaques. Proc Natl Acad Sci U S A. 1996;93:6425–6430. doi: 10.1073/pnas.93.13.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brust D, Polis M, Davey R, et al. Fluorodeoxyglucose imaging in healthy subjects with HIV infection: impact of disease stage and therapy on pattern of nodal activation. AIDS. 2006;20:495–503. doi: 10.1097/01.aids.0000210603.40267.29. [DOI] [PubMed] [Google Scholar]
- 68.Haagmans BL, van den Brand JM, Provacia LB, et al. Asymptomatic Middle East respiratory syndrome coronavirus infection in rabbits. J Virol. 2015;89:6131–6135. doi: 10.1128/JVI.00661-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maurea S, Mainolfi CG, Bombace C, et al. FDG-PET/CT imaging during the Covid-19 emergency: a southern Italian perspective. Eur J Nucl Med Mol Imaging. 2020;47:2691–2697. doi: 10.1007/s00259-020-04931-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tulchinsky M, Fotos JS, Slonimsky E. Incidental CT Findings Suspicious for COVID-19-Associated pneumonia on nuclear medicine examinations: Recognition and management plan. Clin Nucl Med. 2020;45:531–533. doi: 10.1097/RLU.0000000000003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qin C, Liu F, Yen TC, et al. (18)F-FDG PET/CT findings of COVID-19: A series of four highly suspected cases. Eur J Nucl Med Mol Imaging. 2020;47:1281–1286. doi: 10.1007/s00259-020-04734-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zou S, Zhu X. FDG PET/CT of COVID-19. Radiology. 2020;296:E118. doi: 10.1148/radiol.2020200770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Amini H, Divband G, Montahaei Z, et al. A case of COVID-19 lung infection first detected by [18F]FDG PET-CT. Eur J Nucl Med Mol Imaging. 2020;47:1771–1772. doi: 10.1007/s00259-020-04821-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Czernin J, Fanti S, Meyer PT, et al. Nuclear medicine operations in the Times of COVID-19: Strategies, precautions, and experiences. J Nucl Med. 2020;61:626–629. doi: 10.2967/jnumed.120.245738. [DOI] [PubMed] [Google Scholar]
- 75.Mattoli MV, Taralli S, Pennese E, et al. Atypical presentation of COVID-19 incidentally detected at 18F-FDG PET/CT in an asymptomatic oncological patient. Clin Nucl Med. 2020;45:e383–e385. doi: 10.1097/RLU.0000000000003175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Polverari G, Arena V, Ceci F, et al. (18)F-Fluorodeoxyglucose uptake in patient with asymptomatic severe acute respiratory syndrome Coronavirus 2 (Coronavirus Disease 2019) referred to positron emission tomography/computed tomography for NSCLC restaging. J Thorac Oncol. 2020;15:1078–1080. doi: 10.1016/j.jtho.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scarlattei M, Baldari G, Silva M, et al. Unknown SARS-CoV-2 pneumonia detected by PET/CT in patients with cancer. Tumori. 2020;106:325–332. doi: 10.1177/0300891620935983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Setti L, Kirienko M, Dalto SC, et al. FDG-PET/CT findings highly suspicious for COVID-19 in an Italian case series of asymptomatic patients. Eur J Nucl Med Mol Imaging. 2020;47:1649–1656. doi: 10.1007/s00259-020-04819-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kirienko M, Padovano B, Serafini G, et al. CT, [(18)F]FDG-PET/CT and clinical findings before and during early Covid-19 onset in a patient affected by vascular tumour. Eur J Nucl Med Mol Imaging. 2020;47:1769–1770. doi: 10.1007/s00259-020-04822-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mazzola R, Francolini G, Triggiani L, et al. Metastasis-directed therapy (SBRT) guided by PET-CT (18)F-CHOLINE versus PET-CT (68)Ga-PSMA in castration-sensitive oligorecurrent prostate cancer: A comparative analysis of effectiveness. Clin Genitourin Cancer. 2020 doi: 10.1016/j.clgc.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 81.Zhou J, Gou Z, Wu R, et al. Comparison of PSMA-PET/CT, choline-PET/CT, NaF-PET/CT, MRI, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: A systematic review and meta-analysis. Skeletal Radiol. 2019;48:1915–1924. doi: 10.1007/s00256-019-03230-z. [DOI] [PubMed] [Google Scholar]
- 82.Alberts I, Vollnberg B, Sachpekidis C, et al. Incidental SARS-CoV-2-related findings in asymptomatic patients in [(18)F]-FDG-PET/CT-potential insights. Eur J Nucl Med Mol Imaging. 2020;47:2068–2069. doi: 10.1007/s00259-020-04869-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kamani CH, Jreige M, Pappon M, et al. Added value of (18)F-FDG PET/CT in a SARS-CoV-2-infected complex case with persistent fever. Eur J Nucl Med Mol Imaging. 2020;47:2036–2037. doi: 10.1007/s00259-020-04860-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Joob B, Wiwanitkit V. 18F-FDG PET/CT and COVID-19. Eur J Nucl Med Mol Imaging. 2020;47:1348. doi: 10.1007/s00259-020-04762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee JC, Blazak JK. The use of positron emission Ttmography in Coronavirus Disease 2019 cases. J Thorac Oncol. 2020;15:e131. doi: 10.1016/j.jtho.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Azam SA, Myers L, Fields BKK, et al. Coronavirus disease 2019 (COVID-19) pandemic: Review of guidelines for resuming non-urgent imaging and procedures in radiology during Phase II. Clin Imaging. 2020;67:30–36. doi: 10.1016/j.clinimag.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Charters PFP, Little D, Rodrigues JCL, et al. (18)FDG-PET/CT findings in COVID-19: A single centre retrospective radiological review. BJR Case Rep. 2020;6 doi: 10.1259/bjrcr.20200091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fields BKK, Demirjian NL, DeBoer C, et al: 3D printing novel PPE for response to COVID-19 related shortages. In: Proc SPIE 11583, 16th International Symposium on Medical Information Processing and Analysis, 115830O; 2020. 10.1117/12.2579555 [DOI]
- 89.Nakajima K, Kato H, Yamashiro T, et al. COVID-19 pneumonia: Infection control protocol inside computed tomography suites. Jpn J Radiol. 2020 doi: 10.1007/s11604-020-00948-y. [DOI] [PMC free article] [PubMed] [Google Scholar]