Abstract
Patient: Female, 47-year-old
Final Diagnosis: Intraperitoneal tuberculosis
Symptoms: Abdominal pain • decreased appetite
Medication: —
Clinical Procedure: —
Specialty: Infectious Diseases • Medicine, General and Internal
Objective:
Unusual clinical course
Background:
Extrapulmonary tuberculosis (TB) occurs in up to one-fifth of all cases of TB, with abdominal TB accounting for 5% of all cases. It is an uncommon diagnosis in the Western world, where it is primarily identified in immigrant and immunocompromised populations.
Case Report:
We review a case in which a 47-year-old Nepalese woman with a history of cognitive dysfunction secondary to epilepsy presented with decreased appetite and diffuse abdominal pain. She was hypoxic and febrile on initial exam, and imaging indicated lung consolidation, right-sided pleural effusion, and thickening and nodularity of the omentum with patchy wall thickening of the colon. After failing to improve on a standard antibiotic regimen for treatment of pneumonia and colitis, the differential was broadened to include TB. Interferon-γ release assay was subsequently found to be positive, and omental and peritoneal biopsies were obtained. The patient was started on an empiric course of rifampin, isoniazid, ethambutol, pyrazinamide, and pyridoxine. Laboratory testing revealed no immunochemical evidence of Mycobacterium species, however, Ziehl-Neelsen acid-fast stain was positive with rare acid-fast bacilli identified.
Conclusions:
Peritoneal TB carries significant morbidity and mortality if undiagnosed or untreated. Diagnosis is challenging in the absence of a single test that can confirm or exclude this condition. In combination with clinical suspicion, it is crucial to explore history regarding socio-epidemiology (travel, incarceration, occupation, homelessness, sick contacts) and immunological risk (drug use, chemotherapy) in patients with constitutional symptoms.
MeSH Keywords: Mycobacterium tuberculosis; Peritonitis, Tuberculous; Tuberculosis, Gastrointestinal
Background
Tuberculosis (TB) is an infectious disease caused in most cases by the bacterium Mycobacterium tuberculosis, which most commonly infects the lungs. This bacterium is primarily transmitted via droplets from infected individuals. TB is estimated to affect more than one-third of the world’s population [1]. According to the World Health Organization in 2018 approximately 10 million individuals were diagnosed with TB and 1.5 million died worldwide [2]. In the United States, TB was reported to have an incidence of 2.7 per 100,000 population, with approximately 8920 cases reported to the Centers for Disease Control and Prevention (CDC) [3].
Extrapulmonary TB occurs in up to one-fifth of all cases of TB, with intra-abdominal TB accounting for 5% of all cases [4–6]. Intra-abdominal TB encompasses TB infection of the gastrointestinal tract, lymph nodes, peritoneum, and/or intra-abdominal solid organs [7,8]. Infection with HIV is a major risk factor for intra-abdominal TB, as are liver cirrhosis, malignancy, immunosuppression with tumor necrosis factor inhibitors, and peritoneal dialysis [8]. In the Western world, the majority of cases of intestinal TB are seen in individuals who are immunocompromised (e.g., due to HIV infection) and among immigrants [9]. Here we present a case of an immigrant from Nepal who was hospitalized due to abdominal pain, generalized weakness, and failure to thrive. She was later found to have disseminated peritoneal TB.
Case Report
The patient was a 47-year-old Nepalese woman with a history of uncontrolled epilepsy leading to severe cognitive dysfunction. At baseline the patient was minimally verbal, unable to perform her activities of daily living, and unable to understand or communicate in English. Her last hospitalization had been 3 months prior at which time she was treated for seizures related to medication noncompliance due to financial difficulties. The patient presented to the Emergency Department with a 1-week history of decreased appetite and generalized abdominal pain. On initial evaluation, she was hypoxic and required oxygen at 4 L/min via nasal cannula. Her blood pressure was 142/85 mmHg; heart rate, 135 beats/minute; and temperature, 38.7°C. The results from her laboratory tests revealed a white blood cell count of 16 700/μL (normal 3900–9500/μL), hematocrit of 35.2% (normal 29.4–47%), and platelet count of 622 000/μL (normal 140 000–366 000/μL]. Liver and kidney function tests were within normal limits; however, albumin was noted to be low at 2.1 g/dL (normal 3.5–5.7 g/dL). Chest X-ray revealed bilateral pleural effusions with pulmonary edema and atelectasis (Figure 1). Computed tomography (CT) of the chest, abdomen, and pelvis with contrast revealed consolidation of the bilateral lower lobes of the lungs (right greater than left) and moderate-sized right-sided loculated pleural effusion (Figure 2). Moderate ascites was seen in the abdomen, with thickening and nodularity of the omentum and patchy wall thickening of the colon (Figure 3). Pulmonary embolism and bowel obstruction were ruled out. The patient was treated empirically with a course of intravenous vancomycin, cefepime, metronidazole, and azithromycin to cover bacterial pneumonia and colitis.
Figure 1.
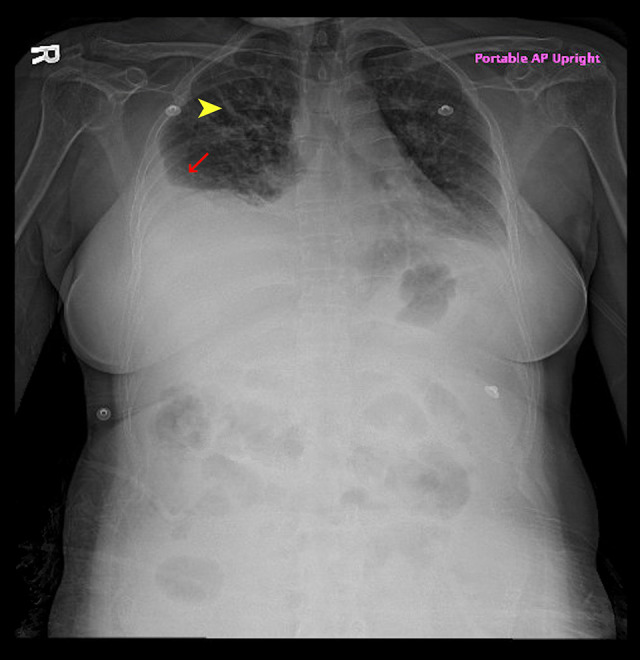
X-ray of the chest revealing pulmonary edema (yellow arrow) and pleural effusion (red arrow).
Figure 2.
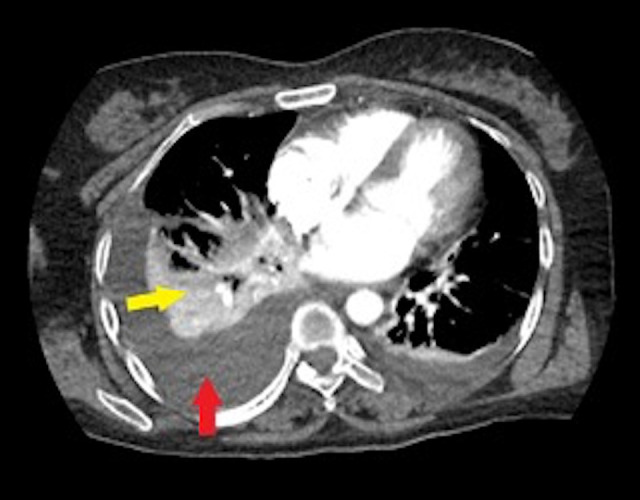
Computed tomography of the chest indicating pleural effusion (red arrow) and consolidation (yellow arrow).
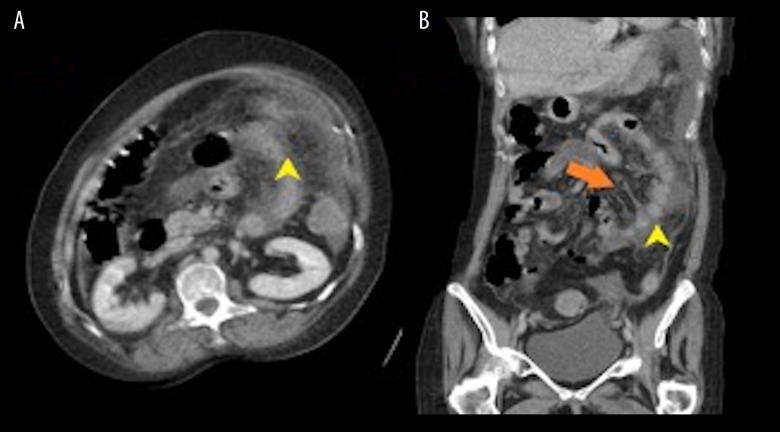
Figure 3.
(A, B) Computed tomography of the abdomen with contrast. Notice the mild patchy thickening of the colon (yellow arrow) and slight soft-tissue thickening and nodularity of the omentum (orange arrow).
Due to minimal oral intake during initial hospitalization and an 8.6-kg weight loss since the patient’s hospitalization 3 months earlier, a nasogastric tube was placed to ensure adequate nutrition. Further evaluation of the pleural effusion with thoracentesis yielded 600 mL of cloudy, yellow pleural fluid. Fluid analysis yielded the following results: protein, 3 g/dL; glucose, 132 mg/dL; lactic dehydrogenase, 149 U/L; nucleated cells, 1180/mm3; and red blood cells, 600/mm3. No acid-fast bacilli (AFB) were identified. Adenosine deaminase (ADA) was not obtained. Pleural fluid and blood cultures were negative, and lower respiratory culture grew normal oral flora. The pleural effusion quickly reaccumulated, prompting placement of a chest tube. The patient continued to be intermittently febrile; however, cultures were negative, and antibiotics were discontinued after a 10-day course. Owing to continued fevers, the patient was screened for HIV, and the test was negative.
Imaging was reviewed once again, and because the patient had immigrated to the United States 6 years earlier from a country with a high prevalence of TB, concern for TB emerged. The patient had no known personal history of or exposure to TB, and her vaccination status was unknown. An interferon-γ release assay was subsequently completed and found to be positive. Three separate sputum samples were collected and were negative for AFB. The patient underwent omental and peritoneal biopsy, as well as a lumbar puncture to rule out meningitis due to TB. Intraoperatively, diffuse studding of the peritoneum was observed (Figure 4). Omental and peritoneal biopsies revealed caseating granulomas; however, stains for AFB and fungi were negative. Ascitic fluid was additionally negative for AFB, but it was not sent for further fluid analysis. Lumbar puncture showed nucleated cells at 0/mm3; fluid red blood cells, 2435/mm3; total protein, 77 mg/dL; and glucose, 69 mg/dL. Again, culture was negative for AFB. Samples were sent to the CDC for further analysis. Polymerase chain reaction (PCR) testing of the omental biopsy showed no immunohistochemical evidence of mycobacterial species. In addition, Mycobacterium genus 16S rRNA PCR assay was negative, as was M. tuberculosis complex IS6110 PCR assay for mycobacteria including atypical AFB. Ziehl-Neelsen acid-fast stain, however, was positive with rare AFB.
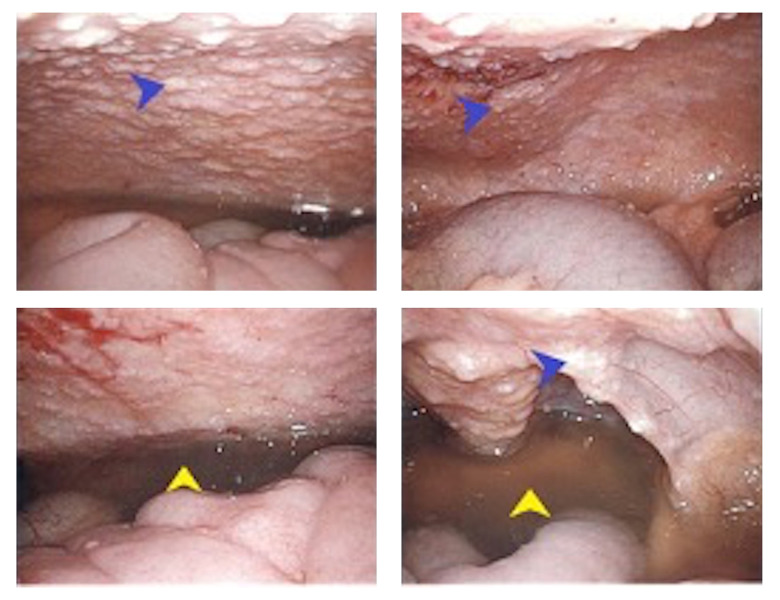
Figure 4.
Peritoneal nodularity (blue arrows) and ascites (yellow arrows) noted intraoperatively.
The patient was started on a 2-month course of rifampin, isoniazid, ethambutol, pyrazinamide, and pyridoxine. A percutaneous endoscopic gastrostomy tube was placed to ensure adequate nutrition. At discharge, the patient was afebrile and did not require any supplemental oxygen, and improvement in abdominal pain was reported. After the initial 2 months of therapy, the patient was transitioned to rifampin and isoniazid to complete an additional 4 months of therapy with the Department of Health. Unfortunately, the final clinical course is unknown because the patient was lost to hospital follow-up.
Discussion
Peritoneal TB can be a challenging disease to diagnose at presentation owing to its insidious onset, nonspecific symptoms, and the limitations of diagnostic testing [10]. It can mimic various other conditions such as Crohn disease, malignancy, or atypical infections (such as Yersinia or amoebiasis), cirrhosis with ascites, or spontaneous bacterial peritonitis [5].
Symptoms may be present for weeks to months before diagnosis. The subsequent delay in diagnosis can lead to a delay in treatment, resulting in high morbidity and mortality [11]. Therefore, peritoneal TB should remain in the differential diagnosis in all patients from high-risk populations who present with nonspecific symptoms such as weight loss, fever, nausea, vomiting, ascites, and abdominal pain [8,12].
Infection may occur via reactivation of latent TB or via ingestion of mycobacteria (with ingestion of infected milk or sputum) [7]. In the setting of active pulmonary or miliary TB, infection can additionally occur via hematogenous spread or contiguous spread from adjacent organs. Lymphatic spread has also been described from infected lymph nodes [7]. In the case described above wherein there is presumed pulmonary TB with evidence of abdominal TB, either ingestion of mycobacteria or hematogenous spread may have occurred. Abdominal TB has been described in 3 different clinical forms: wet type (with ascites), dry type (with abdominal swelling), or fibrotic type (with abdominal masses comprising omental and mesenteric thickening) [13]. A combination of these 3 types can occur and presentation may vary, as was seen in our patient who had mixed-type presentation with the presence of ascites and omental and mesenteric thickening [13].
In cases in which intra-abdominal TB is suspected, initial work-up consists of abdominal imaging with either ultrasound or CT. Imaging may help to rule out potential alternative cases and can reveal involvement of the peritoneum, omentum, and abdominal organs as well as the presence of ascites and/or lymphadenopathy [13,14]. It is important to note that findings in intra-abdominal TB are nonspecific and may be present in other bacterial intra-abdominal infections. This was the case in our patient wherein she was treated for colitis and pneumonia with broad-spectrum antibiotics.
If ascites is present, the next step in the workup involves paracentesis. Accumulation of ascites due to peritoneal TB classically results in straw-colored fluid with lymphocytic predominance and white blood cell count of 150–4000 cells/mm3; however, neutrophilic predominance has been reported in patients undergoing peritoneal dialysis [15]. The sensitivity of ascitic fluid on AFB smear and culture for mycobacteria is low overall, but when positive, it is quite specific [16]. Activity of ADA is also considered useful in diagnosis of abdominal TB, and multiple studies have determined that the optimal cutoff for ADA level for diagnosis is >30 IU/L with 100% sensitivity and 98% specificity in noncirrhotic patients [17,18]. Whereas, in cirrhotic patients, a lower ADA cutoff of 27 IU/L was found to have 100% sensitivity and 93.3% specificity [17]. Additionally, measurement of interferon-γ in ascitic fluid has been shown to have high specificity (98%) with a sensitivity of 93% when 3.2 U/mL is used as a cutoff [17].
In patients without ascites, tissue biopsy should be considered because it aids in histological and microbiological diagnosis. A variety of techniques, including endoscopic, laparoscopic, and percutaneous ultrasound or CT-guided interventions, are available for biopsy. Which intervention is used to obtain biopsies depends on the organ is involved and the location and accessibility of the involved lesion on imaging. Peritoneoscopy with peritoneal biopsy is the criterion standard for diagnosis of peritoneal TB because it additionally allows for direct visualization of peritoneal involvement, which may not be readily apparent on imaging [19]. Peritoneal biopsy can reveal thickened peritoneum with or without yellow-white tubercles as well as dense adhesions [19,20]. Caseating granulomas are often identified on histology in intra-abdominal TB; however, stains for AFB are positive in only 50–63% of cases, while culture is positive in 70% of cases [11,20].
Positive tuberculin skin test and interferon-γ release assay results may be observed in patients with abdominal TB, but they are of limited value because they cannot differentiate between active and latent TB and may be positive in those who previously received vaccination against TB [16]. TB PCR testing of the tissue is very specific, but it lacks sensitivity and is positive in only 25–70% of cases [18–20]. Newer testing, such as the GeneXpert MTB/RIF assay for detecting Mycobacterium tuberculosis complex and resistance to rifampin, is promising. It has high sensitivity and specificity and can detect culture-negative M. tuberculosis infections and it provides more rapid results in hospitals where it is available [21]. Unfortunately, this testing was not available for our patient at the time of diagnosis.
Aside from testing for HIV, further investigations to rule out any underlying immunocompromising condition were deferred while the patient was inpatient. She had no known history of recurrent infections in the past or any pattern to suggest that she was immunocompromised. Presence of disseminated TB is very uncommon in the immunocompetent population, and the consideration for atypical infections such as TB is much lower in the differential diagnoses in patients such as ours. Unfortunately, at this time no single test has superior sensitivity or specificity for diagnosing abdominal TB, and a negative test result does not completely rule out the disease. In patients with nonspecific symptoms and suggestive radiological findings, clinical improvement with antitubercular therapy can also be considered diagnostic [11].
Conclusions
Abdominal TB is an uncommon cause of disease in the developed world. It carries significant risk for morbidity and mortality if left undiagnosed or untreated, but diagnosis is challenging in the absence of a single test that can confirm or exclude this condition. In patients with evidence of pulmonary TB, extrapulmonary/disseminated spread must be considered. In cases in which pulmonary disease is not as obvious or is absent, clinical suspicion must remain high. It is crucial to explore history regarding socio-epidemiology (travel, incarceration, occupation, homelessness, sick contacts) and immunological risk (drug use, chemotherapy) in patients with constitutional symptoms.
Acknowledgments
We would like to acknowledge Helen Houpt, MSLS, AHIP, and Elizabeth Morgan, MLS, for their help with our literature search.
Footnotes
Conflicts of interest
None.
References:
- 1.World Health Organization (WHO) Global tuberculosis report 2019. Geneva: WHO; 2019. https://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 2.Dheda K, Barry CE, 3rd, Maartens G. Tuberculosis. Lancet. 2016;387(10024):1211–26. doi: 10.1016/S0140-6736(15)00151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control Prevention (CDC) Tuberculosis – data and statistics. Atlanta (GA): Centers for Disease Control and Prevention; 2019. https://www.cdc.gov/tb/statistics/default.htm. [Google Scholar]
- 4.Yang Z, Kong Y, Wilson F, et al. Identification of risk factors for extrapulmonary tuberculosis. Clin Infect Dis. 2004;38(2):199–205. doi: 10.1086/380644. [DOI] [PubMed] [Google Scholar]
- 5.Mehta JB, Dutt A, Harvill L, Mathews KM. Epidemiology of extrapulmonary tuberculosis. A comparative analysis with pre-AIDS era. Chest. 1991;99(5):1134–38. doi: 10.1378/chest.99.5.1134. [DOI] [PubMed] [Google Scholar]
- 6.Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004;120(4):316–53. [PubMed] [Google Scholar]
- 7.Debi U, Ravisankar V, Prasad KK, et al. Abdominal tuberculosis of the gastrointestinal tract: Revisited. World J Gastroenterol. 2014;20(40):14831–40. doi: 10.3748/wjg.v20.i40.14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans RP, Mourad MM, Dvorkin L, Bramhall SR. Hepatic and intra-abdominal tuberculosis: 2016 update. Curr Infect Dis Rep. 2016;18(12):45. doi: 10.1007/s11908-016-0546-5. [DOI] [PubMed] [Google Scholar]
- 9.Jehangir W, Khan R, Gil C, et al. Abdominal tuberculosis: An immigrant’s disease in the United States. N Am J Med Sci. 2015;7(6):247–52. doi: 10.4103/1947-2714.157484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uygur-Bayramicli O, Dabak G, Dabak R. A clinical dilemma: Abdominal tuberculosis. World J Gastroenterol. 2003;9(5):1098–101. doi: 10.3748/wjg.v9.i5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasheed S, Zinicola R, Watson D, et al. Intra-abdominal and gastrointestinal tuberculosis. Colorectal Dis. 2007;9(9):773–83. doi: 10.1111/j.1463-1318.2007.01337.x. [DOI] [PubMed] [Google Scholar]
- 12.Hadara Y, Kunimura T, Sasaki Y, et al. Clinicial. Peritoneal tuberculosis: challenging to diagnose, but curable. Clinical Case Reports: Open Access. 2020;3(1):140. [Google Scholar]
- 13.Abu-Zidan FM, Sheek-Hussein M. Diagnosis of abdominal tuberculosis: Lessons learned over 30 years: Pectoral assay. World J Emerg Surg. 2019;14(1):33. doi: 10.1186/s13017-019-0252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demirkazik FB, Akhan O, Ozmen MN, Akata D. US and CT findings in the diagnosis of tuberculous peritonitis. Acta Radiol. 1996;37(4):517–20. doi: 10.1177/02841851960373P217. [DOI] [PubMed] [Google Scholar]
- 15.Quantrill SJ, Woodhead MA, Bell CE, et al. Peritoneal tuberculosis in patients receiving continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 2001;16(5):1024–27. doi: 10.1093/ndt/16.5.1024. [DOI] [PubMed] [Google Scholar]
- 16.Marshall JB. Tuberculosis of the gastrointestinal tract and peritoneum. Am J Gastroenterol. 1993;88(7):989–99. [PubMed] [Google Scholar]
- 17.Liao YJ, Wu CY, Lee SW, et al. Adenosine deaminase activity in tuberculous peritonitis among patients with underlying liver cirrhosis. World J Gastroenterol. 2012;18(37):5260–65. doi: 10.3748/wjg.v18.i37.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanai FM, Bzeizi KI. Systematic review: Tuberculous peritonitis – presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther. 2005;22(8):685–700. doi: 10.1111/j.1365-2036.2005.02645.x. [DOI] [PubMed] [Google Scholar]
- 19.Koff A, Azar MM. Diagnosing peritoneal tuberculosis. BMJ Case Rep. 2020;13(2):e233131. doi: 10.1136/bcr-2019-233131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhargava DK, Shriniwas, Chopra P, et al. Peritoneal tuberculosis: Laparoscopic patterns and its diagnostic accuracy. Am J Gastroenterol. 1992;87(1):109–12. [PubMed] [Google Scholar]
- 21.Patil N, Saba H, Marco A, et al. Initial experience with GeneXpert MTB/RIF assay in the Arkansas Tuberculosis control program. Australas Med J. 2014;7(5):203–7. doi: 10.4066/AMJ.2014.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]