Abstract
Background:
It is unknown whether an initial invasive strategy in patients with stable ischemic heart disease and at least moderate ischemia improves outcomes in patients with a history of heart failure (HF) or left ventricular dysfunction (LVD) when EF ≥35%, but <45%.
Methods:
Among 5179 participants randomized into the International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA), all of whom had LVEF ≥35%, we compared cardiovascular outcomes by treatment strategy in those with a history of HF or LV dysfunction (HF/LVD) at baseline versus those without HF/LVD. Median follow-up was 3.2 years.
Results:
There were 398 (7.7%) participants with HF/LVD at baseline of whom 177 had HF/LVEF>45%, 28 had HF/LVEF 35-45% and 193 had LVEF 35-45% but no prior history of HF. HF/LVD was associated with more comorbidities at baseline, particularly prior myocardial infarction (MI), stroke and hypertension. Compared to those without HF/LVD, those with HF/LVD were more likely to experience a primary outcome composite of cardiovascular death, nonfatal MI, or hospitalization for unstable angina, HF, or resuscitated cardiac arrest; four-year cumulative incidence rate (22.7% vs. 13.8%), cardiovascular death or MI (19.7% vs. 12.3%), and all-cause death or HF (15.0% vs. 6.9%). Those with HF/LVD randomized to the invasive versus conservative strategy had a lower rate of the primary outcome (17.2% vs. 29.3%, difference in 4-year event rate −12.1%; 95% CI: −22.6, −1.6%), whereas those without HF/LVD did not (13.0% vs. 14.6%, difference in 4-year event rate −1.6%; 95% CI: −3.8%, 0.7%; p-interaction = 0.055). A similar differential effect was seen for the primary outcome, all-cause mortality, and CV mortality when invasive versus conservative strategy associated outcomes were analyzed with LVEF as a continuous variable for those with and without prior HF.
Conclusion:
ISCHEMIA trial participants with stable ischemic heart disease and at least moderate ischemia with a history of HF or LVD were at increased risk for the primary outcome. In the small, high-risk subgroup with HF and LVEF 35-45%, an initial invasive approach was associated with a better event-free survival. This result should be considered hypothesis generating.
Keywords: ischemic heart disease, heart failure, left ventricular dysfunction, percutaneous coronary intervention, medical therapy
Ischemic heart disease is a common underlying cause of heart failure (HF) due to left ventricular dysfunction (LVD) arising from myocardial ischemia or infarction as well as common risk factors such as hypertension and diabetes.1 The prognosis of patients with HF and flow-limiting obstructive coronary artery disease is also poor.2 Prior studies failed to demonstrate a significant difference in all-cause mortality between medical therapy alone and surgical revascularization in patients with severe left ventricular LVD (LV ejection fraction [EF] ≤35%.3, 4 Long-term follow-up of STICH found surgical revascularization in addition to medical therapy in patients with HF and severe left ventricular LVD improved long-term survival as well as angina symptoms.5, 6 Nevertheless, the optimal management of patients with coronary disease and HF or LVD without markedly reduced LVEF remains uncertain.
Specifically, whether or not an invasive strategy with initial angiography and percutaneous coronary intervention (PCI)7 or coronary artery bypass graft (CABG) surgery if appropriate is superior to a conservative treatment with guideline-directed medical therapy (GDMT) alone for patients with stable ischemic heart disease and a history of HF or LVD is unknown.
The ISCHEMIA trial found no significant differences at 4 years in the occurrence of cardiovascular events among participants with at least moderate or severe ischemia randomized to a routine invasive therapy or a conservative approach with revascularization reserved for failure of medical treatment. 8 Importantly, patients with LVEF <35% or New York Heart Association (NYHA) functional class III or IV symptoms were excluded from ISCHEMIA. However, those with history of heart failure or mild-to-moderate LV dysfunction (EF 35%-45%) were included, providing a unique opportunity to explore the benefit of an invasive strategy in this subgroup. The objectives of our study were to: a) summarize baseline patient characteristics by history of HF or LVD; b) determine the association between history of HF or LVD and clinical outcomes; c) compare primary outcomes between initial invasive or conservative strategy according to whether history of HF or LVD was present at baseline; and d) explore hospitalization for HF during follow-up in the subgroups according to treatment.
Methods
The ISCHEMIA Trial
Deidentified participant data and data dictionary will be available starting June 30, 2022. Methods of data sharing to be determined based on National Institutes of Health data sharing policy and in discussion with the National Institutes of Health and the National Heart, Lung, and Blood Institute program officer. The design and results of the ISCHEMIA trial have been published.8, 9 In summary, ISCHEMIA was a randomized, controlled trial that included 5179 participants with at least moderate ischemia on noninvasive stress testing who were randomized 1:1 to a routine invasive strategy or conservative strategy with angiography reserved for failure of medical therapy. Participants were followed for a median of 3.2 years. Key exclusion criteria were >50% unprotected left main stenosis or non-obstructive coronary artery disease (CAD) on blinded coronary computed tomography angiography, estimated glomerular filtration rate (eGFR) <30 ml/min, recent MI (within 2 months prior enrollment), LVEF <35% by any imaging modality, NYHA Class III or IV, unacceptable angina on medical therapy at baseline, or PCI or CABG within 1 year prior to enrollment. Overall, cardiac catheterization was performed in 96% of the invasive group and 26% of the conservative group, and revascularization was performed in 80% of the invasive group and 21% of the conservative group (15% prior to sustaining a primary outcome). This subgroup analysis of patients with HF/LVD was prespecified and the statistical analysis plan was finalized before the main trial database was locked. All patients provided informed consent for participation in the ISCHEMIA trial. The protocol was approved by the institutional review board at New York University Grossman School of Medicine (the clinical coordinating center) and by the institutional review board and ethics committee at each participating site (see the Supplementary Appendix).
Definition of Heart Failure
History of HF was defined as having been diagnosed with HF prior to randomization regardless of LVEF. LVD was defined as LVEF ≥35% and <45% assessed by the ISCHEMIA core laboratory on the medical history/medical status form at randomization regardless of a history of HF. Participants were then categorized as HF with reduced ejection fraction (HFrEF) if LVEF <45% or HF with preserved ejection fraction (HFpEF) if LVEF ≥45%. These cut points for EF were chosen partly due to aspects of data collection. Specifically, when exact EF was unknown, the category “abnormal—moderate (LVEF 35%-44%)” on the case report form was used to classify patients as having LVD. 10–13 For the analyses using continuous EF, multiple imputation (average value across 100 imputed datasets) was used to assign an EF within the category for those without continuous EF value (Supplemental Methods, Imputation of LVEF). Continuous LVEF was available for 4633 of 5174. The remaining 541 had ejection fraction entered only as “Normal (EF>=55%),” “Abnormal - mild (45-54%),” or “Abnormal – moderate (35-44%).” Sensitivity analyses were performed using LVEF <50% to define LVD based on guidelines definition.14 For the sensitivity analyses using a 50% cutoff to define LV dysfunction, 36 patients without prior HF and EF between 45-54% were not included since specific LV function was unknown.
Study Outcomes
The primary outcome was the composite of cardiovascular death, MI, resuscitated cardiac arrest, or hospitalization for unstable angina or HF. Other outcomes were all-cause death, cardiovascular death, MI, hospitalization for UA, hospitalization for HF.9 Outcomes of special interest were the composite of all-cause death or HF hospitalization, Rose dyspnea questionnaire score ≥215 assessed at baseline and at every study visit, and HF symptoms defined as NYHA II or more among those without NYHA II or more at baseline.
Statistical analysis
Continuous variables are summarized with median (25th, 75th percentile) and categorical variables with number (percentage). Summary statistics are provided for those with and without prior HF or LVD and are shown separately for the HFpEF and HFrEF in the subset with HF or LVD. The history of HF or LVD groups (yes versus no) are compared with Wilcoxon rank sum, chi-square, and Fisher’s exact tests as appropriate. No testing was done to compare HFrEF and HFpEF.
Cox proportional hazards regression models were fit for each outcome where prior HF or LVD was the independent variable of interest. Models were run without adjustment and were repeated adjusting for the same adjustment covariates as those in the primary manuscript with the exception of LVEF (age, sex, eGFR, and diabetes). The same transformations and inclusion of cubic restricted splines for covariates needed in the primary analysis were used. Results are presented as hazard ratio (95% CI) and p-value for the unadjusted and adjusted analyses as well as the cumulative incidence rate (95% CI) and number of events.
The analyses in the second objective were repeated where the independent variable of interest was HF type. HF type had three levels to preserve the sample size (no prior HF and LVEF ≥ 45, LVEF <45 with or without prior HF, and prior HF with LVEF ≥ 45). Even so, statistical power is limited, and the HF type groups are small given that the trial excluded patients with LVEF <35%. Restricted cubic splines were used to test whether there was a non-linear association between continuous LVEF and each outcome in Cox proportional hazards regression models. No outcomes had non-linear relationships with EF so a hazard ratio and 95% CI corresponding to a 10 unit increase in LVEF was given for each outcome. The results are presented unadjusted and adjusted as described above. Sensitivity analyses were also performed using EF <50% to define LVD.
For assessing the presence of a treatment interaction with HF/LVD, we focused on 4-year cumulative event rates and assessed whether differences in 4-year event rates for invasive minus conservative were consistent for those with and without HF/LVD (Supplemental Methods, Calculating 95% confidence intervals for the differences in the CIF estimates).A forest plot was used to show those differences for those with and without prior HF or LVD for each outcome along with interaction p-values (Supplemental Methods, Calculation of interaction p-values for the test of differences in CIF differences). Predicted primary outcome rates at 4 years across continuous EF values were calculated for each prior HF and treatment group using Cox proportional hazards models where LVEF was included as a restricted cubic spline. These predicted rates were plotted along with the half width 95% confidence interval for the event rate difference between treatment groups. At any point on the x axis where neither line touches the shaded area indicates a confidence interval that does not include zero at that point.
A Cox proportional hazards regression model was fit for the all-cause mortality outcome. The model includes prior HF, age, sex, eGFR, diabetes, and baseline LVEF as adjustment variables and hospitalization for HF as a time dependent covariate. Hospitalization for HF was defined as a time dependent variable that takes the value of zero for participants who never had a hospitalization for HF event. For those having hospitalization for heart failure, it takes the value of zero before the hospitalization for HF and one in the time after hospitalization for HF.
Results
Study Participants
A total of 5174 out of 5179 participants randomized into the main ISCHEMIA trial were included in this analysis. Five participants were excluded: 4 had an LVEF <35% and in 1 the LVEF was missing. HF/LVD was present in 398/5174 (7.7%) participants of whom 221 (55.5%) had LVEF <45% and 205 (51.5%) had a prior clinical diagnosis of HF; 28 patients (0.6%) had prior heart failure and LVEF <45%. Baseline characteristics according to HF classification are presented in Table 1 (Supplemental Table I). Overall, participants with HF/LVD were slightly older, with more prior MI, revascularization, and comorbidities than participants without HF/LVD. At baseline, 50% had NYHA class II symptoms while only 17% without HF/LVD had class II symptoms. Participants with HF/LVD had similar use of statins, but more ACE/ARB, diuretics, beta blockers, and anticoagulation at baseline than those without HF/LVD. Similar results were seen when LVD was defined as LVEF<50% (Supplemental Table II).
Table 1.
Baseline Patient Characteristics According to Prior Heart Failure or Left Ventricular Dysfunction (LVD)
| History of HF/LVD |
|||||
|---|---|---|---|---|---|
| No History of HF/LVD (N=4,776) | Overall (N=398) | LVEF 35%-45% (n=221) * | LVEF>45% (n=177) | p** | |
| Demographics | |||||
| Age, years | 0.004 | ||||
| n | 4776 | 398 | 221 | 177 | |
| Median (Q1, Q3) | 65 (58, 71) | 66 (59, 72) | 64 (57, 71) | 67 (61, 74) | |
| Female sex | 1085/4776 (22.7%) | 83/398 (20.9%) | 28/221 (12.7%) | 55/177 (31.1%) | 0.393 |
| Race | <.001 | ||||
| White | 3083/4728 (65.2%) | 315/396 (79.5%) | 153/220 (69.5%) | 162/176 (92.0%) | |
| Asian | 1431/4728 (30.3%) | 54/396 (13.6%) | 46/220 (20.9%) | 8/176 (4.5%) | |
| Black or African American | 178/4728 (3.8%) | 26/396 (6.6%) | 20/220 (9.1%) | 6/176 (3.4%) | |
| Other | 36/4728 (0.8%) | 1/396 (0.3%) | 1/220 (0.5%) | 0 | |
| NYHA Class | <.001 | ||||
| None | 3049/4776 (63.8%) | 112/398 (28.1%) | 94/221 (42.5%) | 18/177 (10.2%) | |
| I | 910/4776 (19.1%) | 86/398 (21.6%) | 45/221 (20.4%) | 41/177 (23.2%) | |
| II | 817/4776 (17.1%) | 200/398 (50.3%) | 82/221 (37.1%) | 118/177 (66.7%) | |
| Medical history | |||||
| Angina | 4276/4776 (89.5%) | 362/398 (91.0%) | 194/221 (87.8%) | 168/177 (94.9%) | 0.370 |
| MI | 844/4762 (17.7%) | 146/395 (37.0%) | 77/219 (35.2%) | 69/176 (39.2%) | <.001 |
| PCI | 912/4772 (19.1%) | 137/398 (34.4%) | 65/221 (29.4%) | 72/177 (40.7%) | <.001 |
| CABG | 172/4776 (3.6%) | 31/398 (7.8%) | 13/221 (5.9%) | 18/177 (10.2%) | <.001 |
| PVD | 177/4765 (3.7%) | 26/398 (6.5%) | 10/221 (4.5%) | 16/177 (9.0%) | 0.005 |
| Stroke | 129/4775 (2.7%) | 22/398 (5.5%) | 13/221 (5.9%) | 9/177 (5.1%) | 0.001 |
| Hypertension | 3464/4761 (72.8%) | 322/395 (81.5%) | 167/218 (76.6%) | 155/177 (87.6%) | <.001 |
| Diabetes | 1978/4776 (41.4%) | 184/398 (46.2%) | 106/221 (48.0%) | 78/177 (44.1%) | 0.061 |
| Chronic lung disease | 261/4765 (5.5%) | 37/396 (9.3%) | 14/220 (6.4%) | 23/176 (13.1%) | 0.002 |
| Hospitalization for heart failure | 0 | 56/398 (14.1%) | 12/221 (5.4%) | 44/177 (24.9%) | <.001 |
| Medical Therapy at Randomization | |||||
| Aspirin | 4305/4772 (90.2%) | 338/398 (84.9%) | 189/221 (85.5%) | 149/177 (84.2%) | <.001 |
| Statin | 4525/4771 (94.8%) | 374/398 (94.0%) | 209/221 (94.6%) | 165/177 (93.2%) | 0.452 |
| High intensity statin | 1766/4771 (37.0%) | 144/398 (36.2%) | 86/221 (38.9%) | 58/177 (32.8%) | 0.740 |
| ACE/ARB | 3085/4772 (64.6%) | 328/398 (82.4%) | 170/221 (76.9%) | 158/177 (89.3%) | <.001 |
| Beta blockers | 3805/4772 (79.7%) | 351/398 (88.2%) | 190/221 (86.0%) | 161/177 (91.0%) | <.001 |
| Aldosterone antagonist | 79/4772 (1.7%) | 36/398 (9.0%) | 18/221 (8.1%) | 18/177 (10.2%) | <.001 |
| Diuretic | 935/4772 (19.6%) | 146/398 (36.7%) | 65/221 (29.4%) | 81/177 (45.8%) | <.001 |
| Antiarrhythmics | 58/4740 (1.2%) | 10/392 (2.6%) | 1/218 (0.5%) | 9/174 (5.2%) | 0.027 |
| Anticoagulants | 155/4729 (3.3%) | 48/397 (12.1%) | 25/220 (11.4%) | 23/177 (13.0%) | <.001 |
| Dual antiplatelet | 1149/4772 (24.1%) | 78/398 (19.6%) | 48/221 (21.7%) | 30/177 (16.9%) | 0.044 |
| QOL | |||||
| SAQ Angina Frequency Score | 0.902 | ||||
| n | 4262 | 380 | 204 | 176 | |
| Median (Q1, Q3) | 90 (70, 100) | 90 (70, 100) | 90 (70, 100) | 85 (65, 100) | |
| Stress imaging and ETT detail | |||||
| Degree of ischemia† | 0.015 | ||||
| None | 225/4764 (4.7%) | 29/398 (7.3%) | 14/221 (6.3%) | 15/177 (8.5%) | |
| Mild | 327/4764 (6.9%) | 25/398 (6.3%) | 13/221 (5.9%) | 12/177 (6.8%) | |
| Moderate | 1550/4764 (32.5%) | 150/398 (37.7%) | 74/221 (33.5%) | 76/177 (42.9%) | |
| Severe | 2601/4764 (54.6%) | 193/398 (48.5%) | 119/221 (53.8%) | 74/177 (41.8%) | |
| Uninterpretable | 61/4764 (1.3%) | 1/398 (0.3%) | 1/221 (0.5%) | 0 | |
| Anterior ischemia | 1183/3525 (33.6%) | 106/357 (29.7%) | 70/201 (34.8%) | 36/156 (23.1%) | 0.139 |
| Duke Prognostic Score | 0.055 | ||||
| n | 2791 | 195 | 111 | 84 | |
| Median (Q1, Q3) | 5 (5, 6) | 5 (5, 6) | 5 (5, 6) | 5 (5, 6) | |
| Number of 50% Diseased Vessels by CCTA† | 0.361 | ||||
| 0 | 4/2789 (0.1%) | 0 | 0 | 0 | |
| 1 | 659/2789 (23.6%) | 38/195 (19.5%) | 22/111 (19.8%) | 16/84 (19.0%) | |
| 2 | 875/2789 (31.4%) | 62/195 (31.8%) | 33/111 (29.7%) | 29/84 (34.5%) | |
| 3 | 1251/2789 (44.9%) | 95/195 (48.7%) | 56/111 (50.5%) | 39/84 (46.4%) | |
| LM ≥ 50% Stenosis by CCTA | 37/3586 (1.0%) | 3/255 (1.2%) | 2/150 (1.3%) | 1/105 (1.0%) | 0.747 |
| Proximal LAD ≥ 50% Stenosis by CCTA | 1649/3487 (47.3%) | 98/249 (39.4%) | 57/147 (38.8%) | 41/102 (40.2%) | 0.015 |
| Number of 70% Diseased Vessels by CCTA | 0.165 | ||||
| 0 | 288/2383 (12.1%) | 13/175 (7.4%) | 7/99 (7.1%) | 6/76 (7.9%) | |
| 1 | 960/2383 (40.3%) | 66/175 (37.7%) | 42/99 (42.4%) | 24/76 (31.6%) | |
| 2 | 673/2383 (28.2%) | 58/175 (33.1%) | 28/99 (28.3%) | 30/76 (39.5%) | |
| 3 | 462/2383 (19.4%) | 38/175 (21.7%) | 22/99 (22.2%) | 16/76 (21.1%) | |
| Proximal LAD ≥ 70% Stenosis by CCTA | 762/3478 (21.9%) | 43/249 (17.3%) | 28/147 (19.0%) | 15/102 (14.7%) | 0.086 |
ACE/ARB indicates angiotensin-converting enzyme/ angiotensin II receptor blocker; CABG, coronary artery bypass graft; CCTA, coronary computed tomography angiography; ETT, exercise tolerance testing; HF/LVD, Heart Failure/ Left ventricular dysfunction;; LAD, left anterior descending; LM, left main ; NYHA, New York Heart Association; MI, myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease ;Q1, first quartile; Q3, third quartile; QOL, Quality of Life; SAQ, Seattle Angina Questionnaire;
Only 28 patients had LVEF between 35%-45% and also a prior history of heart failure.
P-values comparing any history of HF/LVD versus none (first 2 columns)
Uninterpretable degree of ischemia set to missing for chi-square test; Zero vessels with 50% stenosis is combined with one vessel for chi-square test.
HF/LVD and clinical outcomes
Participants with HF/LVD had a higher rate of the primary composite outcome of cardiovascular death, nonfatal MI, or hospitalization for unstable angina, HF, or resuscitated cardiac arrest than participants without HF/LVD (adjusted HR 1.43; 95%CI: 1.12 - 1.82). Similar results were seen for cardiovascular death or MI, and hospitalization for HF (Table 2) and also when LVD was defined as LVEF<50% (Supplemental Table III). These results were primarily noted in the subgroup with reduced LVEF (Supplemental Table IV). The associations between baseline LVEF as a continuous variable and trial outcomes are shown in Supplemental Table V.
Table 2.
Association between history of HF/LVD at baseline and outcomes
| History of HF/LVD | No History of HF/LVD | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable Label | N events | CIF | N events | CIF | Unadjusted HR (95% CI) | Unadjusted p | Adjusted* HR (95% CI) | Adjusted* p |
| Primary outcome | 78 | 22.68 (17.79, 27.95) | 592 | 13.78 (12.67, 14.93) | 1.68 (1.32, 2.12) | <0.001 | 1.43 (1.12, 1.82) | 0.004 |
| CV death/MI | 67 | 19.74 (15.11, 24.82) | 523 | 12.26 (11.20, 13.37) | 1.61 (1.25, 2.08) | <0.001 | 1.37 (1.06, 1.78) | 0.016 |
| All-cause Death | 40 | 11.60 (8.05, 15.85) | 249 | 6.05 (5.25, 6.92) | 2.00 (1.43, 2.79) | <0.001 | 1.58 (1.13, 2.22) | 0.008 |
| CV Death | 33 | 9.51 (6.35, 13.44) | 170 | 4.15 (3.50, 4.88) | 2.42 (1.67, 3.52) | <0.001 | 1.89 (1.30, 2.77) | <0.001 |
| Primary Definition of MI | 44 | 13.25 (9.48, 17.65) | 399 | 9.26 (8.35, 10.23) | 1.38 (1.01, 1.89) | 0.041 | 1.22 (0.89, 1.67) | 0.223 |
| Unstable Angina | 3 | 0.78 (0.22, 2.14) | 45 | 1.05 (0.77, 1.42) | 0.84 (0.26, 2.70) | 0.771 | 0.83 (0.26, 2.69) | 0.758 |
| Hospitalization for Heart Failure | 17 | 4.46 (2.50, 7.25) | 59 | 1.30 (0.96, 1.73) | 3.61 (2.10, 6.20) | <0.001 | 2.73 (1.57, 4.75) | <0.001 |
| All-cause death or HF Hosp | 52 | 14.95 (10.91, 19.59) | 287 | 6.88 (6.04, 7.80) | 2.29 (1.70, 3.08) | <0.001 | 1.80 (1.33, 2.43) | <0.001 |
| HF death | 4 | 1.22 (0.31, 3.44) | 9 | 0.18 (0.07, 0.40) | 5.61 (1.72, 18.26) | 0.004 | 4.19 (1.25, 14.08) | 0.021 |
| NYHA Class II or greater† | 62 | 34.43 (27.10, 41.87) | 984 | 27.77 (26.18, 29.38) | 1.31 (1.01, 1.70) | 0.038 | 1.32 (1.02, 1.70) | 0.038 |
| Rose Dyspnea Scale 2 or more‡ | 91 | 40.63 (33.56, 47.58) | 1026 | 33.77 (31.91, 35.64) | 1.28 (1.03, 1.58) | 0.026 | 1.23 (0.99, 1.53) | 0.064 |
CV indicates cardiovascular; CIF, Cumulative incidence function; HF/LVD, heart failure/ left ventricular dysfunction; HR, hazard ratio; MI, myocardial infarction; NYHA, New York Heart Association
Adjusted for age (restricted cubic spline), sex, eGFR (restricted cubic spline), and diabetes.
NYHA Class II or greater outcome assessed at baseline and in each study visit is restricted to 4158 participants without NYHA Class II or greater at baseline.
Rose Dyspnea Scale 2 or more outcome assessed at baseline and in each study visit is restricted to 3566 participants without Rose Dyspnea Scale 2 or more at baseline.
Clinical Outcomes by HF/LVD with an Invasive or Conservative Strategy
In participants without HF/LVD, there was no observed difference between the invasive and conservative strategies for the primary outcome (difference in 4-year event rate −1.6%; 95% CI: −3.8%, 0.7%) (Figure 1). However, for the subgroup with HF/LVD, those assigned to the invasive strategy had lower rates of the primary outcome when compared with those assigned to the conservative strategy (difference in 4-year event rate −12.1%; 95%CI: −22.6, −1.6%; p-interaction: 0.055) (Figure 2, Supplemental Table VI). Similar results were seen for cardiovascular death or MI. Similar patterns were seen when LVD was defined as LVEF<50% (Supplemental Figures I and II). When adjusted proportional hazards regression models were used to test for a differential treatment effect in those with and without HF/LVD, findings were consistent with those testing for a difference in CIF differences (Supplemental Figure III). The probabilities of the primary outcome, all-cause mortality and CV mortality according to baseline LVEF analyzed as a continuous variable are presented in Figure 3 for those with and without a history of HF. In patients without history of heart failure, there were no observed differences in the primary outcome, all-cause mortality and CV mortality between treatment groups across the spectrum of LVEF (Figure 3 A, C, E). However, patients with a history of heart failure and LVD treated with the initial invasive strategy had lower observed rates of the primary outcome, all-cause mortality and CV mortality than patients treated initially with the conservative strategy (Figure 3 B, D, F). For the primary outcome in patients with a history of HF (Fig 3B) the lack of overlap with the between group confidence interval for a segment of the EF curve compared to complete overlap in fig 3A, is consistent with the overall test of interaction (p=.055) noted above. In participants without HF/LVD, the invasive strategy was associated with higher rates of hospitalization for HF (Figure 1), which was not seen in patients with HF/LVD assigned to the invasive strategy. Of the 51 patients randomized to the invasive group who were hospitalized for HF, 38 (75%) had revascularization prior to hospitalization for HF, 25 (66%) with PCI and 13 (34%) with CABG.
Figure 1. Association between randomized treatment and outcomes for patients with and without HF/LVD at baseline.
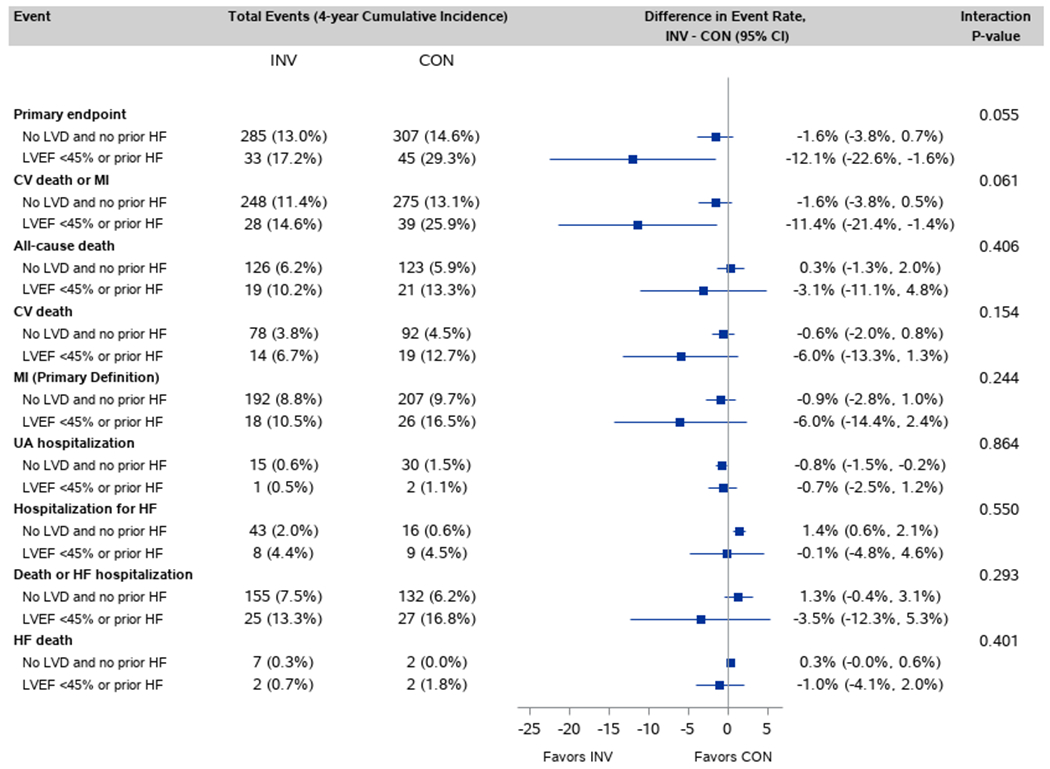
Of 2586 INV participants, 214 had prior HF/LVD. Of 2588 CON participants, 184 had prior HF/LVD
Figure 2.
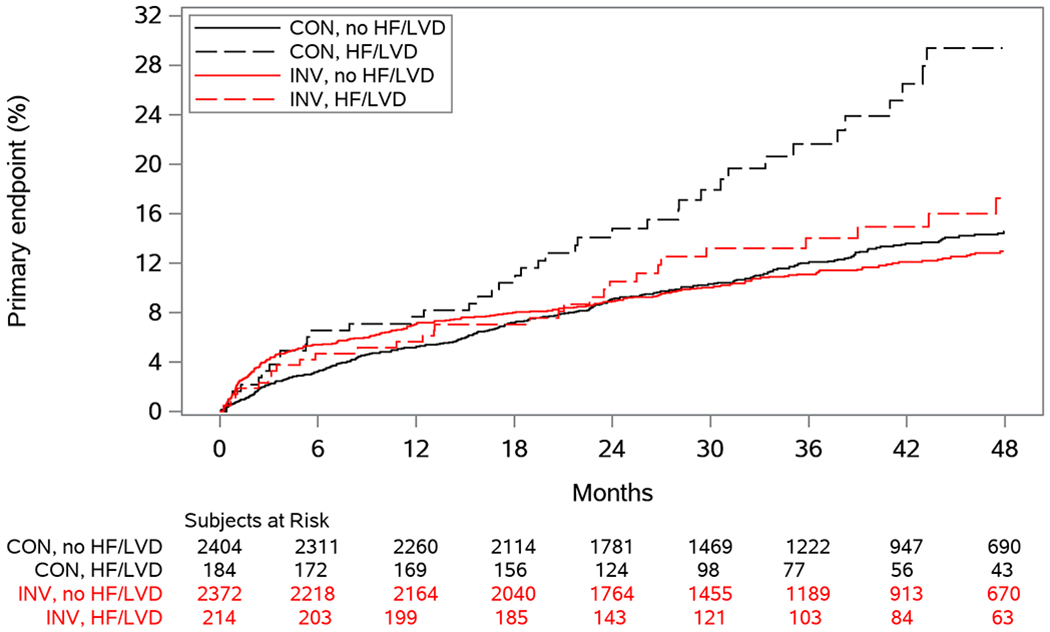
Cumulative incidence curves for the primary endpoint according to randomized treatment and history of HF/LVD
Figure 3. Predicted probability of primary outcome, all-cause mortality, and CV mortality according to baseline LVEF.
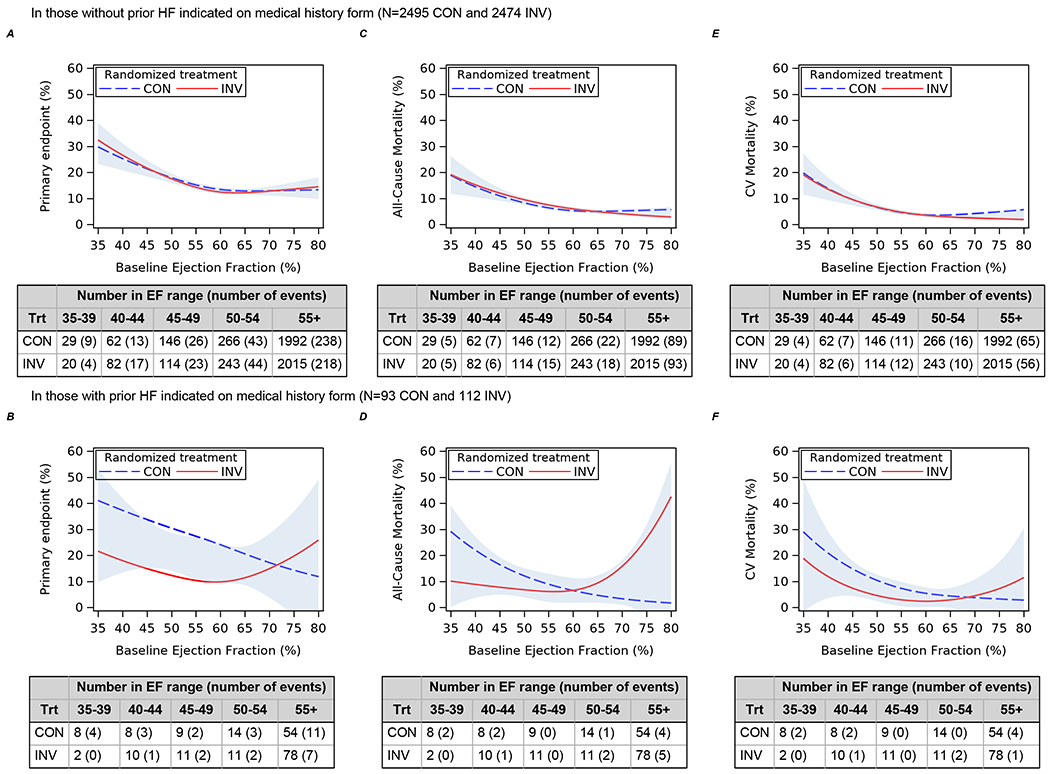
A, C, E) In those without prior HF indicated on medical history form. B, D, F) In those with prior HF indicated on medical history form. The blue shading indicates the half-width confidence intervals for the difference in event rates for invasive and conservative strategies.
Association between hospitalization for HF during follow-up and all-cause death
Participants who were hospitalized for HF during the study also had a higher rate of death than those who did not. There were 263 deaths (1.6 deaths per 100 patient years of follow-up) in the time without hospitalization for HF and 26 deaths (24.8 deaths per 100 patient years of follow-up) after hospitalization for HF. By multivariable analysis, hospitalization for HF was an independent predictor of mortality (adjusted HR 7.11. 95% CI 4.57-11.06, p<0.0001).
Discussion
Among patients with stable ischemic heart disease and at least moderate ischemia enrolled in the ISCHEMIA trial, participants with HF/LVD had higher rates of cardiovascular events than those without HF/LVD. In addition, there was a lower rate of the primary outcome as well as CV death or MI for participants with HF/LVD randomized to the initial invasive strategy, but no difference between strategies in patients without HF/LVD. This difference in outcomes was driven by a large effect of the invasive strategy on the subgroup of patients with heart failure and LVEF 35-45%. There was no evidence of a similar benefit in patients with LVEF 35-45% in the absence of symptoms or in patients with HFpEF (Figure 3). When outcomes for invasive versus conservative strategy were compared using LVEF as a continuous variable, this similar differential effect was noted for the primary outcome, all-cause mortality, and CV mortality for those with prior HF vs those without prior HF. Finally, regardless of baseline HF/LVD, participants who were hospitalized for HF during the study had a higher subsequent risk of death than those who did not. Our study confirms the higher risk of patients with ischemic heart disease and HF/LVD and provides important new insights into the management approach for such patients with mid-range LVEF 35% to 44%. Our results were generally consistent when LVD was defined as LVEF <50%.
In the past decade, several trials have addressed whether an invasive strategy or a conservative strategy with initial optimal medical therapy was the best approach for the management of patients with stable ischemic heart disease. 16–18 Optimal medical therapy with lifestyle and pharmacologic interventions reduces the risk of myocardial infarctions and resulting damage to the myocardium. Medical therapy also treats hypertension and other risk factors thereby reducing their contribution to left ventricular hypertrophy and dilation. In the Surgical Treatment for Ischemic Heart Failure (STICH) trial, surgical revascularization was compared with medical therapy alone in 1212 randomized patients with ischemic cardiomyopathy and LVEF <35%.5 After a median follow-up of 56 months, there was no significant difference in mortality between treatment groups.5, 19 Similar results were seen in the HEART study4, although the study was small and underpowered. However, patients undergoing surgical revascularization had significantly lower rates of cardiovascular death and the combined outcome of all-cause death or hospitalization for cardiovascular causes compared with the medical therapy group after 10 years of follow-up, especially if age less than 60 .5 These results support surgical therapy in patients with LVEF<35%. On the other hand, non-randomized studies have shown that PCI with newer generation drug-eluting stents might be an alternative to CABG in patients with multi-vessel disease and LVEF ≤35%.20 Less is known about percutaneous intervention in this setting or in those with LVEF >35% but <50%. The COURAGE trial randomized 2287 patients with stable coronary disease, whom only 5% had prior HF, to PCI plus medical therapy or medical therapy alone and found similar rates of all-cause death and non-fatal myocardial infarction between groups.17,21 BARI-2D found no differences between groups of patients with coronary artery disease and type 2 diabetes assigned to either prompt revascularization (PCI and CABG strata) or medical therapy alone in the rates of all-cause death and cardiovascular events.18 However, in high-risk patients, including those with LVEF<50% and more extensive coronary disease, the rates of death, MI, and stroke at 5 years were significantly lower among those undergoing CABG when compared with the group receiving medical therapy alone.22
There are a number of pathophysiologic reasons why those with a mild-moderate LVD may benefit from revascularization. First, performing revascularization improves blood flow to ischemic myocardium, either reducing dysfunction or recruiting segments previously hibernating.23 Revascularization is a key strategy to improve cardiac reserve in HF. Coronary artery disease (CAD) is common in patients with HFpEF and is associated with increased mortality and greater deterioration in ventricular function.24–27 Although revascularization can lead to myocardial injury as evidenced by cardiac magnetic resonance,28 it may be associated with preservation of cardiac function and improved outcomes in patients with CAD.26 Our results suggest that when myocardial ischemia is associated with known functional impairment of the myocardium, patients are more likely to derive benefit from revascularization. CAD is the only therapeutic target in HFpEF. We observed no benefit and a possible higher rate of death in participants with a prior history of HF with normal EF assigned to an initial invasive strategy, although the numbers were quite small. Thus, treating ischemia more aggressively in patients with LVD might provide incremental benefit from recruitment of hibernating myocardium and/or symptom relief more pronounced than in patients without HF.
Interestingly, we observed higher rates of subsequent HF diagnoses and hospitalizations among those without HF/LVD at baseline randomized to an invasive strategy. This finding was unexpected and deserves further investigation.
Our findings should be interpreted in light of several limitations. First, there were a small number of patients with clinical events in the HF/LVD, including only 28 patients with prior history of HF and LVD, and therefore, our results should be interpreted as hypothesis-generating. LVEF has inherent measurement variation, so although all measurements were performed in core laboratories, LVEF recorded at trial inclusion may have varied around the somewhat arbitrary cut point of >35%. HF is the result of factors other than ischemia even in a high-risk population such as this one, so the strength of association between an initial invasive approach and HF outcomes may be lessened by other HF etiologies over time. We instructed sites to record NYHA class only if HF was present, but instructions may not have been followed perfectly. We may also underestimate the benefit of revascularization due to incomplete revascularization in the invasive arm as well as use of revascularization for refractory symptoms in the conservative arm. Unfortunately, the type of diuretic was not collected in our study and therefore the differentiation between thiazides (usually used for hypertension) and loop diuretic (generally used for HF) could not be made. Finally, revascularization in the invasive strategy was a mixture of CABG and PCI, which differ fundamentally in their acute risks and benefits as well as in their long-term protection from future events.29 Further follow-up should provide additional information regarding the robustness of our findings and whether there are longer-term benefits of the invasive approach in patients with prior HF/LVD.
Conclusions
In ISCHEMIA, patients with stable ischemic heart disease and HF/LVD had worse outcomes than patients without HF/LVD. Patients with HF/LVD assigned to an initial invasive treatment strategy had better clinical outcomes when compared with a conservative strategy, whereas no such difference was present in those without HF/LVD. This result was driven by a large effect in 28 patients with HF and an LVEF 35-45% with little evidence of an effect for those with HFpEF or an LVEF 35-45% who did not have symptoms. Our findings require confirmation in larger data-sets.
Supplementary Material
Clinical Perspective.
What is new:
This is a contemporary study comparing an initial invasive versus conservative strategy in patients with stable ischemic heart disease and history of heart failure and/or left ventricular dysfunction (HF/LVD).
We found a lower rate of the primary outcome and secondary outcome (CV death or MI) for participants with HF/LVD randomized to the invasive strategy.
These results were primarily driven by left ventricular dysfunction (EF<45%) in the HF/LVD group.
In participants without HF/LVD we found no difference between the strategies in the primary outcome and secondary outcome (CV death or MI).
What are the clinical implications?
Patients with stable ischemic heart disease and at least moderate ischemia with a history of HF or LVD are at increased risk for cardiovascular events and deserve diligent efforts to optimize evidence-based medical therapy.
For patients with myocardial ischemia, HF and LVEF 35-45%, an early invasive strategy might improve event-free survival.
However, this may not be true for patients with heart failure and preserved LVEF or patients with a reduced EF who do not have clinical HF.
Further evidence is required to confirm these findings.
Acknowledgments
Funding Sources: NIH grants U01HL105907, U01HL105462, U01HL105561, U01HL105565
This project was supported in part by Clinical Translational Science Award Nos. 11UL1 TR001445 and UL1 TR002243 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences, the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the Department of Health and Human Services.
Disclosure statements
Dr. Lopes reports grants from National Heart, Lung and Blood Institute, during the conduct of the study; other from Bayer, other from Boehringer Ingleheim, grants and other from Bristol-Myers Squibb, other from Daiichi Sankyo, grants and other from Glaxo Smith Kline, grants and other from Medtronic, other from Merck, grants and other from Pfizer, other from Portola, grants and other from Sanofi, outside the submitted work
Dr. Alexander reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study;
Susanna Stevens reports grants from National Heart, Lung and Blood Institute during the conduct of the study
Dr. Reynolds reports grants from National Heart, Lung and Blood Institute during the conduct of the study; non-financial support from Abbott Vascular, non-financial support from Siemens, non-financial support from BioTelemetry, outside the submitted work;
Dr. Stone reports grants and personal fees from National Heart, Lung, and Blood Institute , during the conduct of the study; personal fees from Terumo, personal fees from Amaranth, personal fees from Shockwave, personal fees and other from Valfix, personal fees from TherOx, personal fees from Reva, personal fees from Vascular Dynamics, personal fees from Robocath, personal fees from HeartFlow, personal fees from Gore, personal fees from Ablative Solutions, personal fees from Matrizyme, personal fees from Miracor, personal fees from Neovasc, personal fees from V-wave, personal fees from Abiomed, personal fees from Claret, personal fees from Sirtex, personal fees and other from Ancora, personal fees and other from Qool Therapeutics, other from Cagent, other from Applied Therapeutics, other from Biostar family of funds, other from MedFocus family of funds, personal fees and other from SpectraWave, personal fees from MAIA Pharmaceuticals, personal fees and other from Orchestra Biomed, other from Aria, personal fees from Vectorious, other from Cardiac Success, outside the submitted work; .
Dr. Pina reports grants from National Heart, Lung and Blood Institute, during the conduct of the study
Dr. Rockhold reports grants from NIH Grant, during the conduct of the study; grants and personal fees from Janssen, personal fees from Merck HeathCare KGaA, personal fees from Merck Research Labs, personal fees from Novo Nordisk, personal fees from KLSMC, personal fees from Aldeyra, personal fees from Rhythm , grants and personal fees from AstraZeneca, personal fees from Complexa, grants and personal fees from Eidos, other from Athira, other from Spencer Healthcare, other from GlaxoSmithKline, personal fees from Phathom, outside the submitted work
Dr. Elghamaz reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study;
Dr. Lopez-Sendon reports grants from National Heart, Lung, and Blood Institute, during the conduct of the study; grants from Bayer, grants and personal fees from Pfizer, personal fees from Menarini, grants and personal fees from Sanofi, grants from Merk, grants and personal fees from Boeringher Infleheim, grants from Amgen, outside the submitted work
Dr. Farsky reports grants from National Heart, Lung and Blood Institute, during the conduct of the study
Dr. Chernyavskiy reports grants from National Heart, Lung and Blood Institute, during the conduct of the study
Dr. Diaz reports grants from National Heart, Lung and Blood Institute, during the conduct of the study
Dr. Phaneuf reports grants from National Heart, Lung and Blood Institute, during the conduct of the study
Dr. de Belder reports grants from National Heart, Lung and Blood Institute, during the conduct of the study
Dr. Ma reports grants from National Heart, Lung and Blood Institute, during the conduct of the study
Dr. Guzman reports grants from National Heart, Lung and Blood Institute, during the conduct of the study
Dr. Khouri reports grants from National Heart, Lung and Blood Institute, during the conduct of the study
Dr. Sionis reports grants from National Heart, Lung and Blood Institute, during the conduct of the study
Dr. Hausenloy reports grants from National Heart, Lung and Blood Institute, during the conduct of the study
Dr. Doerr reports grants from National Heart, Lung and Blood Institute, during the conduct of the study
Dr. Selvanayagam reports grants from National Heart, Lung and Blood Institute, during the conduct of the study;
Dr. Maggioni reports grants from National Heart, Lung and Blood Institute, during the conduct of the study; personal fees from Bayer, personal fees from Fresenius, personal fees from Novartis, outside the submitted work
Dr. Hochman is PI for the ISCHEMIA trial for which, in addition to support by National Heart, Lung, and Blood Institute grant, devices and medications were provided by Abbott Vascular; Medtronic, Inc.; St. Jude Medical, Inc.; Volcano Corporation; Arbor Pharmaceuticals, LLC; AstraZeneca Pharmaceuticals, LP; Merck Sharp & Dohme Corp.; Omron Healthcare, Inc.; and financial donations from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP.
Dr. Maron reports grants from National Heart, Lung, and Blood Institute during the conduct of the study.
Abbreviations and Acronyms
- CABG
coronary artery bypass graft
- CAD
coronary artery disease
- CI
confidence interval
- CV
Cardiovascular
- EF
ejection fraction
- HF
heart Failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with preserved ejection fraction
- HR
hazard ratio
- GDMT
guideline-directed medical therapy
- LVD
left ventricular dysfunction
- LVEF
left ventricular ejection fraction
- MI
myocardial infarction
- NYHA
New York Heart Association
- PCI
percutaneous coronary intervention
- UA
unstable angina
Footnotes
REFERENCES
- 1.Bui AL, Horwich TB and Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D, et al. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–72. [DOI] [PubMed] [Google Scholar]
- 3.Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cleland JGF, Calvert M, Freemantle N, Arrow Y, Ball SG, Bonser RS, Chattopadhyay S, Norell MS, Pennell DJ and Senior R. The Heart Failure Revascularisation Trial (HEART). 2011;13:227–233. [DOI] [PubMed] [Google Scholar]
- 5.Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, Michler RE, Bonow RO, Doenst T, Petrie MC, et al. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N Engl J Med. 2016;374:1511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jolicoeur EM, Dunning A, Castelvecchio S, Dabrowski R, Waclawiw MA, Petrie MC, Stewart R, Jhund PS, Desvigne-Nickens P, Panza JA, et al. Importance of angina in patients with coronary disease, heart failure, and left ventricular systolic dysfunction: insights from STICH. J Am Coll Cardiol. 2015;66:2092–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perera D, Clayton T, Petrie MC, Greenwood JP, O’Kane PD, Evans R, Sculpher M, McDonagh T, Gershlick A, de Belder M, et al. Percutaneous Revascularization for Ischemic Ventricular Dysfunction: Rationale and Design of the REVIVED-BCIS2 Trial: Percutaneous Coronary Intervention for Ischemic Cardiomyopathy. JACC Heart Fail. 2018;6:517–526. [DOI] [PubMed] [Google Scholar]
- 8.Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, Chaitman BR, Senior R, Lopez-Sendon J, Alexander KP, et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med. 2020;382:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ischemia Trial Research Group, Maron DJ, Hochman JS, O’Brien SM, Reynolds HR, Boden WE, Stone GW, Bangalore S, Spertus JA, Mark DB, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: Rationale and design. Am Heart J. 2018;201:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2019;381:1609–1620. [DOI] [PubMed] [Google Scholar]
- 11.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–92. [DOI] [PubMed] [Google Scholar]
- 12.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67. [DOI] [PubMed] [Google Scholar]
- 13.Kelly JP, Mentz RJ, Mebazaa A, Voors AA, Butler J, Roessig L, Fiuzat M, Zannad F, Pitt B, O’Connor CM, et al. Patient selection in heart failure with preserved ejection fraction clinical trials. J Am Coll Cardiol. 2015;65:1668–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 15.Rose GA and Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ. 1968;56:1–188. [PubMed] [Google Scholar]
- 16.Bangalore S, Maron DJ, Stone GW and Hochman JS. Routine Revascularization versus Initial Medical Therapy for Stable Ischemic Heart Disease: A Systematic Review and Meta-Analysis of Randomized Trials. Circulation. 2020;In Press DOI 10.1161/CIRCULATIONAHA.120.048194. [DOI] [PubMed] [Google Scholar]
- 17.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–16. [DOI] [PubMed] [Google Scholar]
- 18.Group BDS, Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panza JA, Ellis AM, Al-Khalidi HR, Holly TA, Berman DS, Oh JK, Pohost GM, Sopko G, Chrzanowski L, Mark DB, et al. Myocardial Viability and Long-Term Outcomes in Ischemic Cardiomyopathy. N Engl J Med. 2019;381:739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bangalore S, Guo Y, Samadashvili Z, Blecker S and Hannan EL. Revascularization in Patients With Multivessel Coronary Artery Disease and Severe Left Ventricular Systolic Dysfunction: Everolimus-Eluting Stents Versus Coronary Artery Bypass Graft Surgery. Circulation. 2016;133:2132–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancini GB, Bates ER, Maron DJ, Hartigan P, Dada M, Gosselin G, Kostuk W, Sedlis SP, Shaw LJ, Berman DS, et al. Quantitative results of baseline angiography and percutaneous coronary intervention in the COURAGE trial. Circ Cardiovasc Qual Outcomes. 2009;2:320–7. [DOI] [PubMed] [Google Scholar]
- 22.Brooks MM, Chaitman BR, Nesto RW, Hardison RM, Feit F, Gersh BJ, Krone RJ, Sako EY, Rogers WJ, Garber AJ, et al. Clinical and angiographic risk stratification and differential impact on treatment outcomes in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Circulation. 2012;126:2115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerber BL, Wijns W, Vanoverschelde JL, Heyndrickx GR, De Bruyne B, Bartunek J and Melin JA. Myocardial perfusion and oxygen consumption in reperfused noninfarcted dysfunctional myocardium after unstable angina: direct evidence for myocardial stunning in humans. J Am Coll Cardiol. 1999;34:1939–46. [DOI] [PubMed] [Google Scholar]
- 24.Pfeffer MA, Shah AM and Borlaug BA. Heart Failure With Preserved Ejection Fraction In Perspective. Circ Res. 2019;124:1598–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trevisan L, Cautela J, Resseguier N, Laine M, Arques S, Pinto J, Orabona M, Barraud J, Peyrol M, Paganelli F, et al. Prevalence and characteristics of coronary artery disease in heart failure with preserved and mid-range ejection fractions: A systematic angiography approach. Arch Cardiovasc Dis. 2018;111:109–118. [DOI] [PubMed] [Google Scholar]
- 26.Hwang SJ, Melenovsky V and Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63:2817–27. [DOI] [PubMed] [Google Scholar]
- 27.Choudhury L, Gheorghiade M and Bonow RO. Coronary artery disease in patients with heart failure and preserved systolic function. Am J Cardiol. 2002;89:719–22. [DOI] [PubMed] [Google Scholar]
- 28.Lim CC, Cuculi F, van Gaal WJ, Testa L, Arnold JR, Karamitsos T, Francis JM, Digby JE, Antoniades C, Kharbanda RK, et al. Early diagnosis of perioperative myocardial infarction after coronary bypass grafting: a study using biomarkers and cardiac magnetic resonance imaging. Ann Thorac Surg. 2011;92:2046–53. [DOI] [PubMed] [Google Scholar]
- 29.Alexander JH and Smith PK. Coronary-Artery Bypass Grafting. N Engl J Med. 2016;374:1954–64. [DOI] [PubMed] [Google Scholar]
- 30.Aalen O Nonparametric Estimation of Partial Transition Probabilities in Multiple Decrement Models. Annals of Statistics. 1978;6:534–545. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


