Abstract
Postmortem studies on the human brain reside at the core of investigations on neurologic and psychiatric disorders. Ground-breaking advances continue to be made on the pathologic basis of many of these disorders, at molecular, cellular, and neural connectivity levels. In parallel, there is increasing emphasis on improving methods to extract relevant demographic and clinical information about brain donors and, importantly, translate it into measures that can reliably and effectively be incorporated in the design and data analysis of postmortem human investigations. Here, we review the main source of information typically available to brain banks and provide examples on how this information can be processed. In particular, we discuss approaches to establish primary and secondary diagnoses, estimate exposure to therapeutic treatment and substance abuse, assess agonal status, and use time of death as a proxy in investigations on circadian rhythms. Although far from exhaustive, these considerations are intended as a contribution to ongoing efforts from tissue banks and investigators aimed at establishing robust, well-validated methods for collecting and standardizing information about brain donors, further strengthening the scientific rigor of human postmortem studies.
INTRODUCTION
Postmortem studies of the human brain are a fundamental component of investigations aimed at defining changes associated with brain disorders. Such studies uniquely access brain at the network, cellular, subcellular, and molecular levels, and represent the only approach capable of directly demonstrating pathology at these levels in the sechuman brain. In synergy with other key methodologic approaches, such as imaging studies, genetic investigations, experimental animals, and in vitro models, postmortem studies have led to ground-breaking advances toward our understanding of brain disorders. Important, and sometimes unsuspected, aspects of the pathophysiology of Alzheimer disease, Huntington disease, dementia, schizophrenia, and many other disorders have been discovered, in turn leading to increasingly sophisticated investigations and to novel therapeutic approaches.
These successes and other promising developments are accompanied by significant difficulties. Among the most challenging is obtaining and processing valid and adequate information about brain donors that can be used reliably by investigators. Required information is available from death certificates, medical records, interviews, or questionnaires provided by the family, and other sources so as to cover extended periods of time from the onset of a target disease until death. Indeed, postmortem investigations can uniquely provide an account of the full course of an individual’s illness, coalescing information provided by the family and clinicians about different stages of the disorder and changes over time. A basic challenge is to improve the scientific rigor of human postmortem studies by establishing robust validated methods for collecting and standardizing information about brain donors.
Here, we review information that can be gathered from documents such as death certificates, medical records, and questionnaires/interviews with donor families, which typically are collected by brain banks. We highlight the relevance of this information to investigations on brain disorders and suggest ways it can be used systematically. Given the limitations of space, we consider selected representative examples of important topics drawn primarilyfrom investigations on psychiatric disorders.
SOURCES OF INFORMATION
Death certificates
Death certificates are routinely requested by brain banks. They contain basic but useful information used to corroborate other documents. In the United States, information included in the death certificate varies from state to state. Particularly useful are demographic data and the cause of death.
Demographics
The following demographic information in death certificates is relevant to postmortem investigations: full name of the decedent, the date and place of birth, name at birth and any known aliases, sex, race, ancestry, ethnicity, age at death, location, date and time of death (the presumed time as well as the official pronounced time). Also informative are the decedent’s marital status, military service status, educational level, and occupation(s), which can be used as indicators of then level of functioning.
Cause of death
Death certificates typically state the manner of death (e.g., natural causes, accident, suicide, homicide), the primary cause of death, and circumstances that contributed to death. Significant clinical conditions, such as congestive heart failure, diabetes, neurologic or psychiatric disorders, injuries, and other complications contributing to the primary cause of death are often listed in sequence, stating their approximate timing prior to death. If an autopsy was performed, a medical examiner section is included, with information on the date and time of injuries that may have contributed to death, circumstances leading to them, site of occurrence (e.g., work, home), and whether emergency transportation was used. In addition, death certificates may state explicitly if tobacco or other substance use contributed to death, and whether a female decedent was pregnant at the time of death or within the year. This information can be particularly useful, providing insight into health and functioning status preceding the time of death.
Medical records
Medical records represent the primary source of information about brain donors. It is often possible to gather extensive records, which may include hospitalization admission and discharge summaries, outpatient medical visits, progress notes, treatment prescriptions, neurologic or psychiatric evaluations, and laboratory test results.
General hospital and emergency records
These records typically include notes on admission, progress, procedures, and discharge status. They detail clinical history, current neurologic and psychologic status, medicines prescribed, physical examination findings, clinical impressions, and plans. A summary of recent hospitalizations, including location, dates, procedures, status of treatment and recovery, age at the time of evaluation, medical conditions, and level of assistance required for activities of daily living, is included. If applicable, caretakers and assisted-living arrangements are also noted. Comments by family members may help determine whether unusual symptoms or behaviors arose, or if falls or episodes of loss of consciousness were associated with the hospitalization or emergency assessment. Over multiple hospitalizations, this section allows a history of specific medical conditions to be compiled.
The patient’s physical examination includes vital signs, weight, height, and body mass index, and examination of organ systems. Information on musculoskeletal status can be helpful in gauging physical disability, such as difficulties walking or standing, as well as the level of assistance needed. Neurologic notes may also comment on the patient’s alertness and responsiveness, and whether signs of depression or agitation were present. A psychiatric section, if included, can provide information about anxiety, agitation, depression, judgment, insight, delusions, euphoria, activity level, and suicidal ideation.
A medication report lists all medications taken at and during hospitalization. Modifications of the medication regimen may suggest poor treatment response or adverse effects. Such information can prove useful in postmortem investigations, as treatments for comorbid conditions may impact findings in diagnostic subjects or generate unexpected effects in controls.
Medical records also include considerations of the patient’s diagnosis, symptom types and severity, and expected progression. They also include recommendations for next steps in various aspects of treatment and assistance, and plans for aftercare, for pharmaceutical interventions, occupational, speech, or physical therapy, supervision, and/or assistant care, and suggest steps the family can take to support the patient.
Neurologic disorders
Intake notes state the patient’s current age, chief complaints, and primary diagnosis, and usually comment on other medical issues. The clinical history details how long the patient has been affected by a neurologic disorder, the nature of its onset and progression, and when medical assistance was first sought. The family history provides information on whether relatives are symptomatic, the patient’s parents are living, their cause of death if applicable, and any major health conditions they experienced. Information on neurodevelopment, academic performance, military service, legal difficulties, employment, housing, and substance abuse may be included. Daily living activities (walking, feeding, dressing, or personal hygiene) and hobbies, as well as functional impairment (speech or swallowing difficulties, choking, hypersalivation, or trouble speaking or being understood), are considered, as well as recent noteworthy events, such as syncope, delirium, or changes in consciousness. Mood or behavioral issues may be addressed; recent falls or injuries due to physical impairments, lack of insight, level of assistance provided, status of eyesight and hearing, sleep patterns and abnormalities, incontinence, and impotence are described. Neurologic notes include comments on the nature, duration, and current severity of particular neurologic signs and symptoms. The patient’s responses to medication trials will also be provided. Thus, such notes are an essential source of information on the severity of the illness and its progression.
Neurologic records typically include results of the physical examination, including assessment of pain status and examination of motor and sensory functions, speech impairment, facial expression, motor coordination, tremor, rigidity, agility, postural stability, gait, bradykinesia, and other details. A mental status section describes the patient’s appearance, mood, affect, sensations, thought process and content, behavior, judgment, and insight. Cognitive examinations focus on the patient’s level of attention, orientation, memory, speech and language. Mini Mental State Examination scores are often included, particularly for patients with dementia, and provide measures of cognitive impairment. Level of orientation to person, time and place, registration, recall, repetition, attention and calculation, language, and ability to follow simple commands are measured and reported.
A report on medicinal treatment is particularly important, as it can be used to assess exposure to therapeutic drugs, their doses, timing, and responses. As discussed below, this section is essential to postmortem investigations, as it allows estimates of exposure to pharmacologic treatment that can support assessments of whether the treatment may have caused, or masked, significant brain changes. Responsivity to specific pharmacologic treatments is also taken into account in the process of formulating a diagnosis postmortem. The medication section includes new medications added at that time, which is helpful in assessing medication history even when medical records through the patient’s lifetime are incomplete. In addition, progress and discharge notes usually outline therapeutic recommendations and describe the physician’s view of the expected progression of symptoms, and to what extent prognosis has been discussed with the patient and family. There may be mention of the level of the patient’s insight in the disorder and the prognosis, including life expectancy.
Psychiatric disorders
Admission notes describe the patient’s psychiatric symptoms and their progression, behavior prior to hospitalization, and specific circumstances leading to voluntary or involuntary hospitalization. Social, developmental, and substance abuse histories are often included in admission and discharge records. An initial care plan may list the patient’s assets, such as family support, abilities, and liabilities, such as lack of treatment adherence or insight into the disorder. Included also may be recommendations for treatment as well as short-term and long-term goals. The clinical and family history, physical, neurologic, and mental status examination findings, laboratory results, and other clinical details as for neurologic disorders also are included.
The mental status examination involves a subjective exploration of the patient’s experience. It includes a description of the patient’s appearance and behavior (including dysmorphic features and abnormal movments), speech, language, and thought, and mood state.. The presence of delusions and hallucinations is noted. Cognitive exam includes level of consciousness, orientation to date and place, and estimates of attention, memory, fund of knowledge, insight, and judgment.
Medications are listed in admission and discharge notes, and provide information on medications provided before and during hospitalization, and their doses, usually with comments about their acceptance and helpful or adverse effects. The discharge summary often is a particularly rich source of information. It recapitulates the psychiatric symptoms experienced by the subject before and at admission, stressors that may have contributed to the illness, including health, housing, family, work, and legal issues, and circumstances leading to hospitalization. The patient’s mental status and behavior at admission and progress during hospitalization are noted. A summary of medication and treatment recommendations is included, as well as planning for post-discharge care and follow-up.
Progress notes
Progress notes are provided in medical records occasioned by clinical assessments, hospitalizations, and periods in assisted-living facilities, and concerning treatment recommendations. They provide accounts of the clinical status of the patient, including progression and severity of symptoms, modifications of treatment, assessment of treatment adherence, vital signs, levels of alertness, orientation, depression, and agitation as observed over time. When generated around the time of death, progress notes provide information on whether long-term treatment for neurologic or psychiatric disorders has been continued or substituted by palliative care. Pain relief and sedation introduced near the time of death provide important information about drug exposure within days of death. In addition, key information about specific agonal conditions, including coma, hypoxia, fever, seizures, dehydration, hypoglycemia, organ failure, head injury, and ingestion of neurotoxic substances at time of death, can be derived from these progress notes and used to calculate the agonal score, an important potential confound, related to the effects of end-of-life experiences, typically considered in human postmortem studies (see below) (Tomita et al., 2004).
Laboratory findings
The results of laboratory testing, such as blood and urine tests, imaging (e.g., magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography, X-rays), pathology (e.g., biopsies), and specialized testing (e.g., electroencephalograms, electrocardiograms, pulmonary function testing, and others), are routinely included in medical records. Blood tests carried out close to the time of death may include circulating concentrations of drugs as well as markers of inflammation, both of which can be particularly useful for postmortem investigations. Results from brain CT and MRI scans carried out to assess suspected brain trauma or following instances of delirium or sudden cognitive decline can be used to document atrophy, infarctions, hemorrhages, tumors, and bone fractures.
Questionnaires/interviews with prospective brain donors or family members
Many brain banks use clinical interviews and structured questionnaires, with prospective brain donors and close family members. Typically, these include questions about family medical history, major life events, premorbid and prodromal behavioral changes and symptoms, social history, effectiveness of various treatments, and hospitalizations. Such data gathering can be tailored for specific neuropsychiatric disorders. Here, we refer specifically to questionnaires filled in by close family members after brain donation, as it is the current approach used by this brain bank.
Questionnaires are often useful in developing a broad framework regarding the life of a donor and a timeline of symptomatology and treatment. Family medical history is especially valuable, as it includes major medical conditions, substance abuse, and suicides within the close and extended family, as well as causes of death of relatives, and can indicate whether there is a maternal or paternal lineage of neurologic or psychiatric symptoms or diagnoses. Information on early events in the donor’s life, such as birth complications, childhood illnesses, abuse, trauma, or neglect and potential toxin exposure, can represent vulnerability factors that are very relevant for postmortem studies. In addition, family members often can provide detailed descriptions of insidious early symptoms that may precede the clinical onset of psychiatric disorders or dementia. Changes in social behavior and school performance often are noted by family members well before clinical onset of a disorder, and may not be included in medical records. Similarly, family members may be aware of substance abuse that may have been concealed from clinicians. The donor’s educational level, marital and employment history, and living arrangements can provide additional measures of symptom severity and disability. Questionnaires often prove to be useful in assessing treatment adherence, an essential aspect of exposure to prescribed drugs. Finally, information in questionnaires can help in detailing circumstances surrounding traumatic brain injury, or leading to suicidal behavior.
Supplemental items
It is not unusual for family members of a donor to provide a brain bank with supplemental items, such as school grades, court order documents, police incident reports, and personal effects (diary entries, poems, drawings, or suicide notes). These items may be helpful in corroborating evidence gleaned from the medical records. For instance, school reports may provide evidence for cognitive changes during the prodromal period in some psychiatric disorders. Similarly, samples of writing or drawings may add evidence to estimates of cognitive functions and executive skills at distinct times in the life of the donor. Police incident reports may provide clues on substance abuse, the donor’s ability to follow society’s rules, and whether the donor was homeless.
USE OF INFORMATION SOURCES FOR POSTMORTEM INVESTIGATIONS
In aggregate, the sources of information described above offer needed data regarding individual brain donors, including clinical information on their primary clinical diagnosis, comorbidities, exposure to prescribed drugs and abused substances, functional status, and other variables. Such information is essential for the rigorous design and interpretation of human postmortem studies and to address neurobiologic aspects of major brain disorders in a credible manner. Basic demographic information, including age at death, sex, race, or ethnicity, is relatively easily recovered and does not require further comment. However, several critically important aspects of the antemortem history can be associated with major artifacts, are not as easily managed, and require further consideration. These include primary and comorbid diagnoses, clinical condition at the time of death, and both recent and previous exposure to prescribed treatments and substance abuse, as well as evidence of the presence of inflammation - all of which can impact neuropathologic findings.
Diagnosis
The nominal or presumptive diagnosis for each brain donor, as represented by the next of kin or legal representative at the time of brain donation, needs to be documented rigorously. Verification of diagnosis is of critical importance, as the intake diagnosis may not be accurate, typically for insufficiently detailed clinical information, or owing to pathologic findings that may not accord with initial clinical impressions. Of similar importance, the presumed absence of brain disorders in nominally healthy control donors needs to be corroborated. In order to formulate a final or distributive diagnosis, all available sources of information as described above are considered, in conjunction with a detailed neuropathology report typically issued for each brain donor.
Neurologic disorders
For neurologic disorders, review of available, typically extensive, medical records often provides a plausible presumptive diagnosis, particularly when the clinical manifestations and illness course have been clear and distinctive (e.g., Huntington disease). However, for many disorders, notably dementias and psychoses, diagnosis based on routine clinical information may not be sufficient. Discrepancies between antemortem clinical diagnoses and postmortem neuropathologic diagnoses are not unusual, and can be especially prevalent in some forms of dementia (Nelson et al., 2007; Weisman et al., 2007; Grandal Leiros et al., 2016). Therefore, a detailed postmortem neuropathologic analysis is critical to accurately confirm, or revise, a final diagnosis, on which subsequent research will be based.
Psychiatric disorders
In contrast to neurologic disorders, the diagnosis of psychiatric disorders is made exclusively on clinical grounds. For postmortem brain research, psychiatric diagnoses are based on the available sources of clinical information already summarized, and particularly on medical records and interview of relatives and completion of questionnaires by them. Prospective interviews of potential donors and interviews of family members, particularly when based on structured and validated clinical research procedures such as the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (DSM) of the American Psychiatric Association (American Psychiatric Association, 1994) are particularly useful in providing additional information on family and clinical history. Donor family members often are asked to fill out questionnaires specifically designed to ascertain the presence and absence of specific clinical features that can inform the diagnosis of specific psychiatric disorders. Although interviews and questionnaires usually are very useful, caution must be exercised, as the reliability and accuracy of information from family members can be limited, especially if a patient has not remained close to the relative informants. Systematic review of clinical records and information regarding the psychosocial functioning of the donor represent the most reliable approach to establishing a psychiatric diagnosis for postmortem brain donors.
Information extracted from medical records can be used to determine whether specific DSM diagnostic criteria are met. Lacking a defined neuropathology for most major psychiatric disorders, information from the neuropathology report cannot be used to corroborate psychiatric diagnoses, whereas such reports are particularly important in determining accurate diagnoses of neurologic conditions such as the type of dementia or other coarse brain pathology. Once all of the information is gathered, a consensus diagnostic conference involving at least two expert diagnosticians can be held to review the available information and to establish a consensus psychiatric diagnosis.
Systemic disorders
The determination of other systemic disorders, such as conditions of the heart, lung, liver, kidney, and other organ systems, can typically be derived from reviewing medical records, including laboratory and other test results. Such information is often crucial for studies investigating relationships between systemic and brain disorders.
Agonal factors
Agonal factors, i.e., medical conditions occurring during terminal illness just prior to death, such as coma, hypoxia, pyrexia, seizures, dehydration, hypoglycemia, multiple organ failure, head injury, and ingestion of neurotoxic substances at time of death, have been suggested to impact specific aspects of postmortem brain tissue, such as RNA integrity (Harrison et al., 1991a, b; Barton et al., 1993; Johnston et al., 1997; Hynd et al., 2003; Tomita et al., 2004). Interestingly, the impact of agonal factors on gene expression profile data was found to be more robust than other factors typically thought to have similar effects, such as postmortem time interval and freezer storage time (Tomita et al., 2004).
Information regarding agonal factors is particularly relevant to postmortem investigations focused on RNA expression, for tissue sample selection, and as a potential confounding variable. This information can be gathered from sources discussed above, such as medical records, donor family interviews, and questionnaires, as well as from toxicologic screening. For experimental design and statistical analysis purposes, it can be useful to conflate agonal factors and express them as an agonal score, as proposed by Tomita et al. (2004). A present/absent (1/0) score is assigned to the each of the following 10 factors: agonal duration, coma, hypoxia, pyrexia, seizures, dehydration, hypoglycemia, multiple organ failure, head injury, and ingestion of neurotoxic substances; the sum is the agonal score (ranging from 0 to 10). Agonal duration can be assigned a 0 for fast deaths, e.g., accidents or violent deaths, fast death due to natural causes (terminal phase less than 1 hour), or 1 for intermediate (terminal phase between 1 and 24 hours), and slow death (e.g., cancer or pulmonary diseases with a terminal phase longer than a day) (Tomita et al., 2004). Alternatively, agonal duration can be rated separately on a 1–4 rating scale, as proposed by Hardy et al. (1985), as: (1) violent and rapid death (e.g., accident, trauma, suicide: terminal phase <10 minutes); (2) fast death for natural causes (e.g., myocardial infarction terminal phase < 1 hour); (3) intermediate death (terminal phase: 1–24 hours); or (4) slow death (e.g., cancer: terminal phase > 1 day).
Exposure to pharmacologic treatment
Pharmacologic treatment for neurologic and psychiatric disorders typically is long-lasting, often for many years or decades. By virtue of their pharmacodynamic actions, medication treatments impact on molecular, cellular, and morphologic neural elements, and thus represent potential confounding factors in postmortem human brain studies. Notably, while pharmacologic treatments may induce differences between pathologic and control groups, they may be more likely to mask pathologic changes of interest. This is because pharmacologic treatments may tend to correct brain abnormalities intrinsic to a disorder rather than inducing additional ones. Evidence from postmortem studies supports this possibility. For instance, in a study testing the expression of the dopamine transporter protein in amygdala, we detected robust decreases in subjects with schizophrenia and a significant positive correlation between dopamine transporter expression and lifetime exposure to antipsychotics in subjects with schizophrenia (Markota et al., 2014). Thus, exposure to antipsychotics brought the numbers to these terminals toward normal values, consistent with the effects of these drugs to diminish dopaminergic neuro-transmission. Specifically, decreases of dopamine transporter in the context of otherwise normal densities of dopaminergic fibers would result in normal dopamine release but impaired reuptake, increasing availability of dopamine at the synapse and diffusion in the extrasynaptic space (Markota et al., 2014).
The need to account for exposure to pharmacologic treatment is well recognized in the field of human brain investigations, whether in vivo or postmortem. Several different approaches have been devised to address drug effects, including methods for estimating exposure to particular drugs, or families of drugs, inclusion in the study of a psychiatric control group that received similar treatment, and use of experimental animals to assess acute and chronic effects of specific drugs on the main outcome measures under study. Each of these approaches has advantages and weaknesses. Here, we focus on approaches aimed at estimating exposure to pharmacologic compounds on the basis of medical records. In the majority of cases, medical records contain sufficient, at times extensive, information on pharmacologic treatment, and often provide important information on the patient’s responsiveness to treatment and adherence to it (Roberts et al., 2009; Pantazopoulos et al., 2010).
Among several important aspects of rigorous assessments of medication exposure we highlight: (1) multidrug treatments; (2) shifts of pharmacologic treatment during the last few months before death, and/or older age; and (3) adherence to pharmacologic treatment.
Multidrug treatment
In the majority of patients with psychiatric disorders, different drugs and combinations of drugs will have been tried at different times during the course of the disorder. Pharmacologic approaches evolve according to the course of the disorder, response of the patient, availability of new medicines, and clinicians’ preferences, exposing a patient to a series of pharmacologic treatments. Importantly, some psychotropic drugs belong to large classes, such as antipsychotics and benzodiazepines, and each patient is often exposed to several members of each class during the course of the disorder. For instance, a patient with schizophrenia is often exposed to treatment with several different antipsychotics over the course of the illness, and patients with bipolar disorder may have been exposed to various drugs, including lithium, anticonvulsants, antipsychotics, antidepressants, and sedatives. Thus, there is a need for an approach that accounts for exposure to several drugs and can normalize doses for each member of a class to a standard comparator (Baldessarini, 2013). In addition to medicines intended to treat the primary brain disorder, others are often added later in life to address systemic comorbid conditions, such as diabetes or high blood pressure, or during the terminal phase as palliative care (e.g., opioids for pain). Many of these agents can affect the brain, and so need to be accounted for in interpreting findings from molecular and structural investigations. Expressing exposure to these medications may be challenging (see Table 14.1 for an example).
Table 14.1.
Example from a 72-year-old brain donor with bipolar disorder : medications at 1 year and 3 months before death
| 1 year before death |
| Valproic acid |
| Lorazepam |
| Zolpidem |
| 3 months before death |
| Lorazepam |
| Haloperidol |
| Ciprofloxacin |
| Piperacillin tazobactam |
| Metronidazole |
| Carbamazepine |
| Escitalopram oxalate |
| Loperamide |
| Metoprolol |
| Octreotide |
| Prednisone |
| Pantoprazole |
| Nitroglycerin |
| Enoxaparin sodium |
| Metoprolol tartrate |
| Hydromorphone Hydrochloride |
| Insulin |
| Bisoprolol |
| Glucagon |
| Furosemide |
| Esomeprazole magnesium |
| Ondansetron hydrochloride |
Timeframe of exposure to medications
As discussed above, an individual’s pharmacologic treatment may change several times across the course of the illness, creating a need for a time framework for considering medication exposure. In broad lines, it is important to consider whether a specific medication was administered chronically and whether there was exposure during the last period before death. Conceivably, both may impact the brain but, as we argue below, there are important reasons to consider them separately. With the exception of young brain donors with an acute cause of death, the last period before death is often characterized by a shift in medication regimen, which may include pharmacologic treatment consistent with palliative care and/or addressing intervening medical conditions. Particularly in elderly brain donors, two different aspects need consideration.
First, long-term treatment for specific brain disorders is often reduced or discontinued at some time before death, often following several years or even decades of exposure, and may be substituted with medicines that address emerging comorbidities, pain, depression, or agitation (Table 14.1). A striking example of this pattern is treatment with lithium for bipolar disorder. Lithium is used as a long-term treatment strategy, so that patients are often prescribed lithium over a period of many years, in quantities that may measure in kilograms (Fig. 14.1). Its major side-effects include nephrotoxicity, which may lead to discontinuation of treatment with lithium, especially in elderly patients whose renal function is compromised. Fig. 14.1 (bottom panel) shows data from a cohort of 27 subjects with bipolar disorder. While the majority of subjects had received between 1 and 8.6 kg of lithium over their lifetime, 18 out of 27 had not been treated with lithium during the last 6 months of life.
Fig. 14.1.
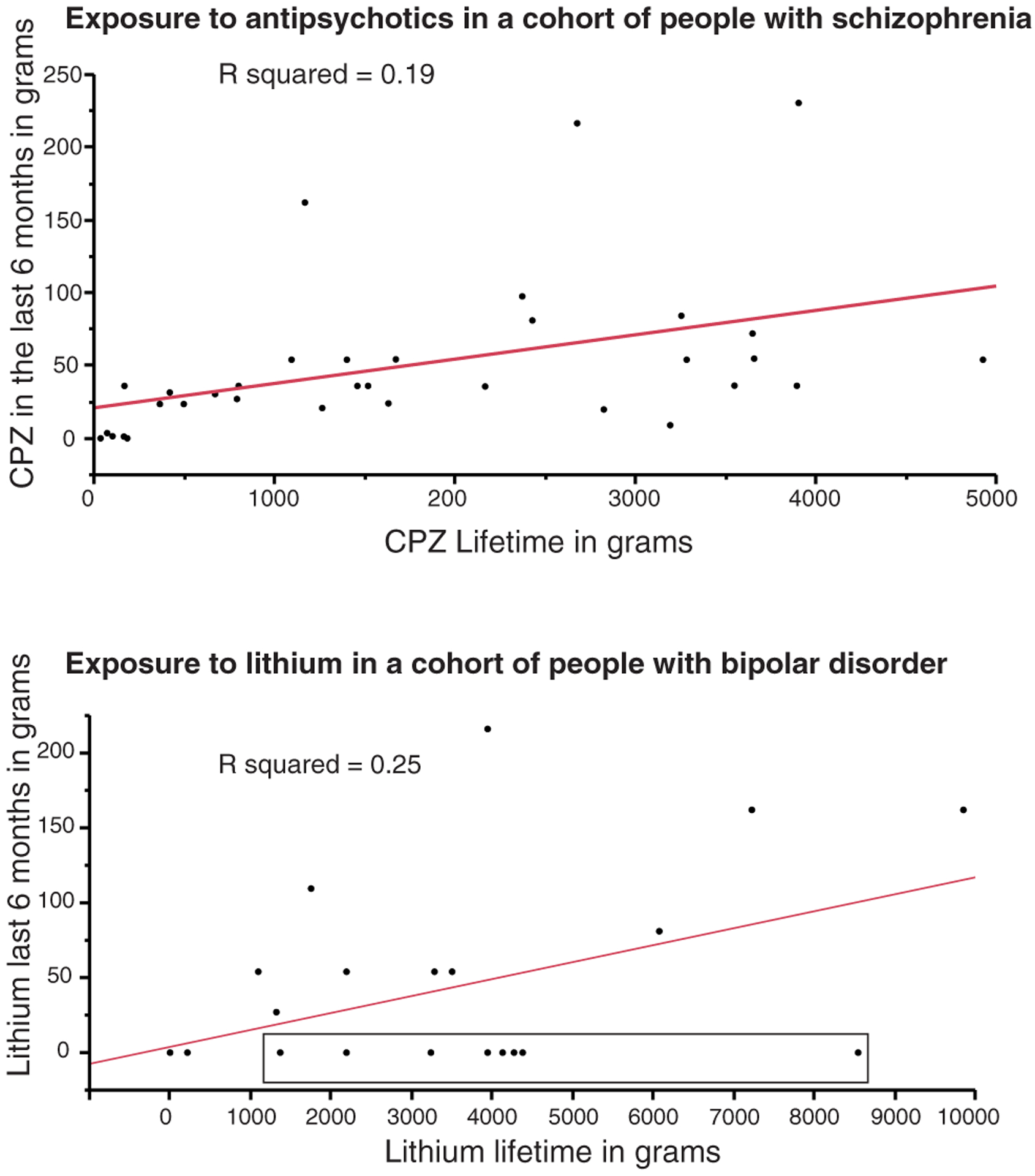
Correlation between lifetime and 6 months’ exposure to antipsychotics (top: chlorpromazine: CPZ) and (bottom) lithium in patients with schizophrenia and bipolar disorder, respectively. Lifetime and 6 months’ exposure before death was estimated according to the algorithm described in this chapter. The box in the graph at the bottom highlights subjects who received between 1 and 8.6 kg of lithium during their lifetime but were no longer exposed at 6 months before death.
Similarly, large doses of antipsychotics, typically prescribed over decades, may be reduced, or even discontinued, later in life, either because of lessening of symptom severity or due to diminished tolerability (Auslander and Jeste, 2004) (Fig. 14.1, top panel). These observations support the idea that estimates of medication exposure limited to the last few months before death may only weakly reflect preceding exposure over decades (Fig. 14.1). We propose that long-term treatment, often spanning the majority of a patient’s adult life, is highly likely to induce important changes in the brain, which may persist even after the treatment is diminished or discontinued. Thus, it is critical to account for chronic exposure as a key potential confounding variable in human studies.
Second, even when medicines for a primary brain disorder are continued until death, others are often added later in life to address intervening medical conditions, such as diabetes, high blood pressure, pain, inflammatory or autoimmune diseases, or symptoms of depression or anxiety. Among these, benzodiazepines and analgesics, particularly opioids, are commonly encountered and relevant. Notably, prescription drugs, including benzodiazepines and opioids, are commonly abused by elderly people (Koechl et al., 2012). These additional medications are often disregarded in analyses of postmortem data, but are likely to have significant effects on the brain, and should be considered carefully.
The preceding considerations underscore the importance of estimating medication exposure within an extended timeframe, distinguishing between long-term and recent exposure and accounting for both. We suggest three distinct time windows: (1) medicines present at the time of death, supported by toxicologic testing; (2) medicines prescribed during the last 3 months of life, as estimated from medical records, expressed in total grams over 3 months, and encompassing all medications prescribed, whether for a primary brain disorder or for comorbid conditions; and (3) medicines prescribed long-term, as estimated from medical records, expressed in total grams, adjusted for treatment adherence, and including only medicines used to treat a primary brain disorder (Fig. 14.2).
Fig. 14.2.
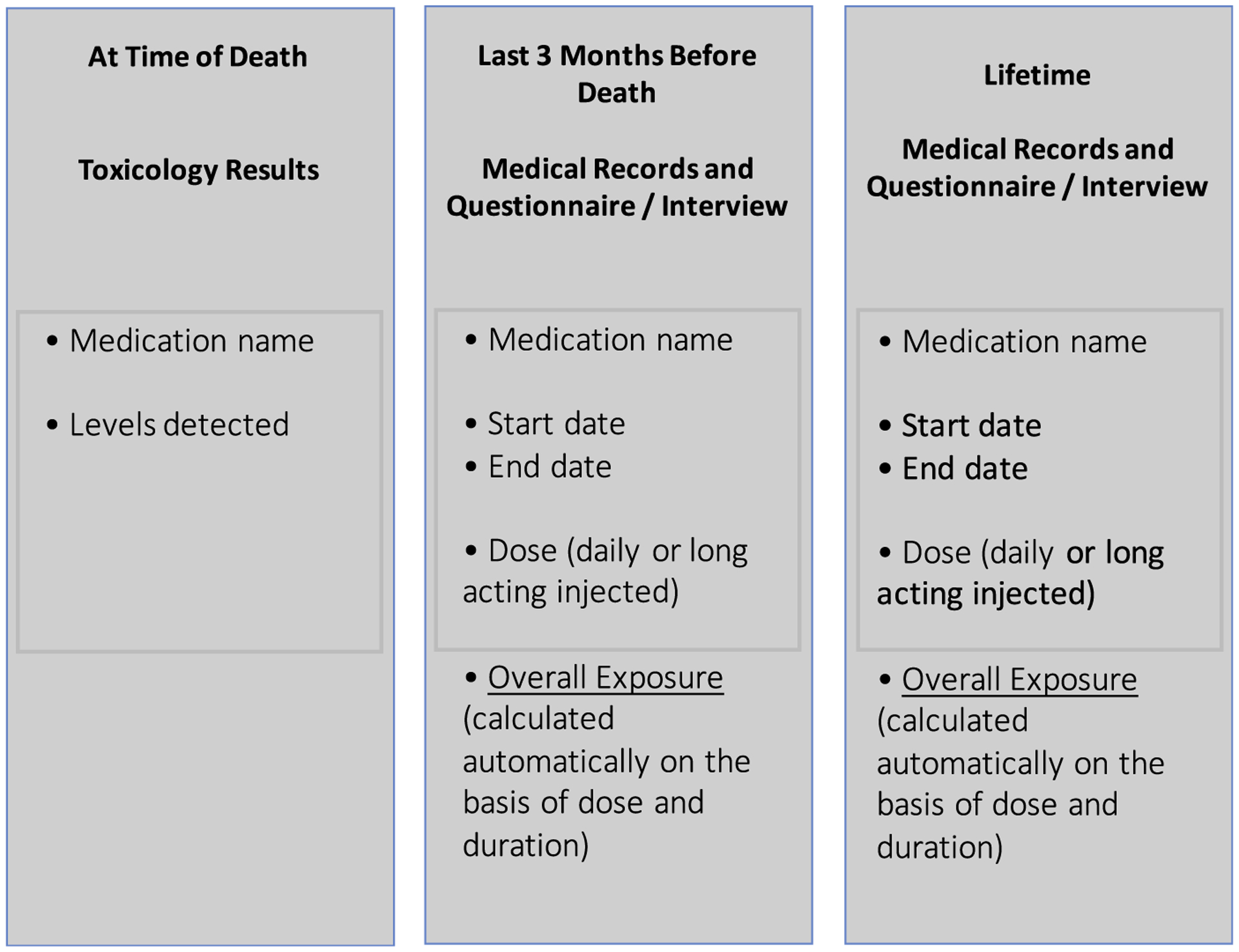
Suggested time framework for estimates of medication exposure in postmortem studies.
Treatment adherence
Adherence is the extent to which patients are able to follow recommendations for prescribed treatments (Vrijens et al., 2012; Hugtenburg et al., 2013). Nonadherence to treatment represents a particularly insidious clinical problem and in efforts to estimate exposure to medicines accurately. Although reports on nonadherence vary, it has been estimated that 20–50% of psychiatric patients are noncompliant or only partially compliant; among those with psychotic disorders, incomplete adherence can be as prevalent as 70–80% (Breen and Thornhill, 1998). For instance, in the course of the National Institute of Mental Health-sponsored academic Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study, 74% of patients discontinued or required a change of treatment before 18 months (Lieberman et al., 2005). Therefore, assessments of drug exposure calculated on the basis of medical records may overestimate the actual drug intake if not corrected for nonadherence.
Many factors can contribute to treatment nonadherence, including voluntary and involuntary reasons (Farooq and Naeem, 2014). Adverse effects, such as weight gain, perceived cognitive impairment, tremor, and excessive sedation, or either lack of symptoms or limited benefits are prevalent bases for nonadherence (Farooq and Naeem, 2014; Mago et al., 2014; Frank et al., 2015; Leclerc et al., 2015). However, factors such as cost of the medications, education of the patient and family with regard to the disorder and its pharmacologic treatment, and the patient’s living arrangements or homelessness also affect adherence (Zivin et al., 2009; Foster et al., 2012; Sajatovic et al., 2013; Farooq and Naeem, 2014; Levin et al., 2014). Albeit far from exact, levels of treatment-adherence can be derived from antemortem clinical and pharmacy records and family questionnaires, which may address nonadherence directly and list some of the risk factors cited above. In particular, clinicians often remark on treatment adherence in the intake and discharge summaries, and mention factors such as frequent changes of medications based on adverse effects, living arrangements, and use of long-acting formulations, intended to improve treatment adherence. All this information can be used to adjust estimates of drug exposure (Berretta et al., 2007; Pantazopoulos et al., 2007, 2010; Markota et al., 2014).
Estimating medication exposure
We propose an approach that allows estimation of exposures to relevant drugs, or families of drugs normalized to a standard comparator, within specific time windows, and adjustment of the estimates for information on treatment adherence. Information on exposure within approximately 24 hours of death can be obtained directly from toxicologic assays of some agents. Information on exposure during any time window, such as the last 3 months of life or across a lifetime, can be estimated by multiplying the estimated daily dose (EDD) by the estimated number of days of treatment (END) and dividing by 1000 to convert milligrams to grams. For instance, exposure to 300 mg lithium twice daily for 3 months would result in a total exposure of 54 grams. Exposure for 3 years would total 657 grams.
Exposure to compounds belonging to large classes of drugs, such as antipsychotics, antidepressants, and anticonvulsants, can be estimated by using a standard comparator (Baldessarini and Tarazi, 1995; Baldessarini, 2013). For instance, exposure to an antipsychotic can be calculated by normalizing to chlorpromazine doses (CPZeq):
These calculations can be modified to account for poor adherence of approximately 33%:
Finally, to account for dosage changes, or for switches to a different compound from the same family, two or more time periods can be added:
Estimating lifetime drug exposure, as just described or as proposed by others (Ray et al., 2014), may be particularly valuable in assessing effects of long-term treatments. Similar approaches have also been used to assess treatment response versus treatment resistance (Roberts et al., 2009). We emphasize that the approach proposed here produces estimated, rather than directly measured, values and the accuracy of such estimates depends greatly on the availability of adequate medical records. These estimates need to be used with great caution, and validated by supplementary approaches whenever possible. Nevertheless, they represent a critical and necessary factor for rigorous analyses of human postmortem studies.
Exposure to drugs of abuse
Estimating substance use is often difficult, whether in clinical settings or for in vivo or postmortem human studies, posing a challenge to clinicians and investigators. Substance use disorders represent a prevalent co-occurring condition in psychiatric patients. Reported prevalence of such substance abuse ranges from 15% to 50% of patients, varying with diagnosis and duration of illness, age, type of substance, geographic location, and culture (Lasser et al., 2000; Conway et al., 2006; Barnett et al., 2007; Katz et al., 2008; National Collaborating Centre, 2011; Wisdom et al., 2011; Chand et al., 2014; Saban et al., 2014). Alcohol, cannabis, and nicotine are especially commonly abused substances in psychiatric patients, whereas estimates of rates of use of cocaine, amphetamines, and opioids are more variable (Green et al., 2004; Barnett et al., 2007; National Collaborating Centre, 2011; Thoma and Daum, 2013). Typically, brain donations received through medical examiners have a higher incidence of substance abuse than those obtained through community referrals by family members or clinical staff members.
As noted above for exposure to therapeutic drugs, it is important to consider substance abuse within a timeframe, as use of these substances varies over the course of the illness. Cannabis and class A drugs (including hallucinogens, opioids, and stimulants) may be mostly used during the prodromal period and around the time of clinical onset of the disorder, while nicotine and alcohol misuse may be much more long-lasting (Lasser et al., 2000; Barnett et al., 2007; National Collaborating Centre, 2011; Wisdom et al., 2011). For instance, estimates of substance abuse at the time of the first psychotic episode have identified cannabis abuse in 28–50% of patients, alcohol abuse at 21–43%, and class A drugs approximately 55% (Green et al., 2004; Barnett et al., 2007), although lower rates have also been reported (Chand et al., 2014). However, approximately 50% of patients with substance abuse became abstinent or markedly decreased drug consumption following a first psychotic episode, as shown by a meta-analysis including studies published between 1990 and 2009 (Wisdom et al., 2011). Use of alcohol and nicotine, instead, often persists. A study on smoking rates showed that people with a psychiatric illness are nearly twice as likely to smoke, particularly with anxiety disorders, major depression, bipolar disorder, and personality disorders (Lasser et al., 2000).
Information about substance abuse during the overall course of major psychiatric illnesses, and particularly at older ages, is very limited. However, hints can be gleaned from estimates of substance abuse in elderly persons without a psychiatric diagnosis, in whom abuse is particularly likely with prescribed medicines, including benzodiazepines and opioids, as well as alcohol (National Collaborating Centre, 2011; Koechl et al., 2012). In general, the preceding findings suggest that, in people with psychiatric disorders, frequent early exposure to a variety of substances, including cannabis, cocaine, and amphetamines, in younger years and early in the course of their illness may be followed by alcohol and nicotine misuse later on, and in elderly patients, by abuse of prescribed medicines with psychotropic effects. It is reasonable to postulate that exposure to such substances at various stages of life may contribute to some abnormalities detected in brain tissue of subjects with psychiatric disorders. Arguably, a brief period of drug exposure during late adolescence or early adulthood may be of less concern than protracted misuse of alcohol or nicotine during adult life, or of prescription drugs later in life. Abuse of benzodiazepines (as well as barbiturates, neuroactive steroids, anesthetics, and alcohol) later in life may be especially relevant to aspects of the pathophysiology of psychiatric disorders involving changes in GABAergic systems (Benes and Berretta, 2001; Costa et al., 2004; Lewis et al., 2005; Coghlan et al., 2012).
Estimates of drug exposure in brain donors at different stages of life require a combination of approaches designed to assess recent and past use (for detailed review, see Lehrmann et al., 2008). While it may not be possible to obtain reliable estimates using the methods described above for prescribed drugs, information from toxicologic screening, family interviews, questionnaires, and medical records can be used to assess the presence and extent of substance abuse. Toxicologic assays of amphetamine, barbiturates, benzodiazepines, cannabinoids, cocaine, opiates, and phencyclidine can be especially helpful (see Chapter 11). Assays of hair may be particularly advantageous, as they typically reveal use within recent months, much longer than assays of transient concentrations in urine or blood (Lehrmann et al., 2008). Structured next-of-kin interviews and questionnaires also are often useful in assessing substance abuse throughout life, and particularly regarding exposure to alcohol and nicotine. In addition, medical records may contain comments about substance abuse at different times. Although information from family interviews, questionnaires, and medical records cannot easily be transformed into quantitative values, ranking systems (e.g., heavy, moderate, occasional, or nonuser) or simply use versus nonuse can be devised in order to include substance abuse as a potential confounding variable in statistical models (Pantazopoulos et al., 2010; Cobb et al., 2013; Kunii et al., 2014; Ray et al., 2014). As noted above for estimates of medication exposure, the approaches proposed here are often used by investigators with other methods, such as including subjects with similar substance abuse but a dissimilar neuropsychiatric disorder, or by factoring in data derived from animal models.
Inflammatory and immune responses
For the past decade there has been increasing evidence of immune system abnormalities in chronic psychiatric illnesses, including schizophrenia. Manifestations of autoimmunity and inflammation in schizophrenia include elevated concentrations of C-reactive protein (CRP) or cytokines, and abnormalities of blood lymphocytes. Other lines of evidence for immune system dysfunction include increased prevalence of prenatal maternal infections (Fond et al., 2015) and increased prevalence of autoimmune disease in patients with schizophrenia as well as their first-degree relatives (Miller et al., 2011). Immune system-related genes, including for cytokines, cytokine pathways, and the major histocompatibility complex, all have been associated with schizophrenia (Girgis et al., 2014; Reus et al., 2015; Sekar et al., 2016; Trepanier et al., 2016).
This area of research is complex, and many potential mechanisms whereby immune system dysfunction might contribute to the pathophysiology of schizophrenia have been proposed; they involve a complex cascade of microglial activation, upregulated cytokine signaling, edema, apoptosis, and gliosis. Miller and colleagues (2011) summarize three theories regarding an immune basis for schizophrenia: (1) chronically activated macrophages and T lymphocytes are fundamental mediators of schizophrenia; (2) a shift from cytotoxic immune function and toward antibody-dependent immune response is primary; and (3) the hypothesis that activated central nervous system microglia release proinflammatory cytokines and free radicals that cause abnormal neurogenesis, neuronal degradation, and white-matter abnormalities.
It has been unclear to what extent the elevation in CRP in schizophrenia is due to specific factors associated directly with this disorder or due to its comorbidities. CRP, an acute-phase reactant, is activated by proinflammatory cytokines and produced by the liver; it is a marker of systemic inflammation (Lichtenstern et al., 2012). Many comorbidities of schizophrenia are associated with elevated CRP. These include high basal metabolic index, metabolic syndrome, and tobacco smoking (Joseph et al., 2015). Many studies did not control for potential confounding factors, but in recent studies that have controlled for such features, the most dominant association was with the diagnosis of schizophrenia itself (Miller et al., 2013). Considering the high rate of metabolic comorbidity, schizophrenia may represent a multisystemic inflammatory disease affecting the brain and other organs. A better understanding of the pathophysiology of schizophrenia is necessary for new drug design. Interventions directed at lowering the level of CRP and other inflammatory markers with acetylsalicylic acid and other nonsteroidal anti-inflammatory drugs appear promising (Fond et al., 2015). Together, these considerations suggest that it is important for postmortem studies to take into consideration variables potentially reflecting immune activation during the life of the individual, such as comorbid diseases, particularly autoimmune disorders and metabolic syndrome, or near the time of death, such as presence of infection, and laboratory data such as CRP.
Use of medical records for the neuropathology report
The purpose of the neuropathologic examination is to provide an essential comprehensive description of pathologic changes in the postmortem brain, which is a basis for establishing the presence of neurologic disorders, including comorbid conditions, and confirming absence of brain pathology in healthy controls. Such information is critical in supporting valid comparisons of diseased to control brain tissue specimens. A description of the complex process underlying neuropathologic examination is beyond the scope of this chapter. However, it is relevant to mention here that, in addition to macroscopic and microscopic brain examinations, the neuropathologic report is based on information derived from a broad range of sources, including those discussed above.
Standard components of a neuropathologic examination include the following: (1) intake summary, including age, sex, principal clinical diagnosis, duration of disease, family history of the disorder, cause of death, and whether brain death may have occurred; (2) reports by a trained brain bank dissectionist describing the condition and abnormalities observed in the brain at the time of its arrival (meningeal abnormalities, gross lesions, atherosclerotic plaques, brain softening, brain weight, weight of the half-brain to be fixed in formalin, extra tissue such as pituitary, dura); (3) report of the pathologist or diener who removed the brain from the skull after death (clinical diagnosis, brain weight); (4) autopsy report for the rest of the body if this was done; (5) family questionnaire filled in by next of kin (with principal clinical diagnosis, possible alternative diagnoses, other clinical conditions, family history of similar conditions, diseases, and causes of death for near relatives, medications, and related information); (6) clinical records from physicians, including imaging and laboratory reports.
These records provide information concerning the last clinical diagnosis (e.g., dementia with Lewy bodies) and alternative diagnoses considered (e.g., Alzheimer disease, corticobasal degeneration). They can be used to suggest additional tissue blocks to take for examination beyond the standard set, e.g., extra substantia nigra in Parkinson disease (and in control brains). These extra sections provide additional diagnostic information and tissue blocks for future research.
Once neuropathologic diagnoses are obtained after microscopic examination of stained tissue sections, a comment section is written that explains these pathologic diagnoses and addresses concerns raised by the family and clinicians who had seen the patient. Examples include principal disorders that lack identifying histo-pathologic characteristics (e.g., bipolar disorder), or when the neuropathologic diagnosis differs from the clinical diagnosis (e.g., Alzheimer disease versus a clinical diagnosis of frontotemporal dementia). The neuropathologic report as well as the clinical information listed above are then used to arrive at a working consensus diagnostic category to be used to guide future research use of tissue from a particular brain specimen.
Time of death as a proxy for circadian rhythm
Mounting evidence from animal and cell culture studies shows that the expression of several molecules involved in circadian rhythm regulation varies in a circadian manner, in turn driving rhythmic expression of down-stream molecular cascades (Reppert and Weaver, 2002; Hastings and Herzog, 2004; Guilding and Piggins, 2007; see Chapter 15). These molecular rhythms have been observed in the suprachiasmatic nucleus of the hypothalamus, termed the “master clock” (Reppert and Weaver, 2002; Hastings and Herzog, 2004; Torres-Farfan et al., 2006; Ansari et al., 2009; Ji et al., 2010; Wreschnig et al., 2014), as well as in many brain regions, including the amygdala and parts of the thalamus (Chaudhury and Colwell, 2002; Lamont et al., 2005; Granados-Fuentes et al., 2006; Guilding and Piggins, 2007; Verwey et al., 2007; Fuller et al., 2008; Carneiro and Araujo, 2009; Mistlberger, 2009; Verwey and Amir, 2009; Wang et al., 2009, 2011; Albrecht et al., 2013). These findings have raised the question of whether similar rhythmic molecular expression occurs in the human brain. If so, circadian rhythm expression changes may contribute to variation among subjects within a cohort, rendering it necessary to consider time of day when quantifying protein or gene expression in human postmortem studies. Recent studies have successfully addressed this question by using time of death, easily obtained from the death certificate, as a proxy for circadian time to analyze rhythmically expressed molecules in human postmortem brain studies to assess circadian-like expression changes and assess differences in rhythms between diagnosis groups (Zhou et al., 2001; Hofman, 2003; van Wamelen et al., 2013; Li, 2014; Bunney et al., 2015; Chen et al., 2016; Pantazopoulos et al., 2017).
There are several limitations and potential confounding factors to account for when examining circadian rhythm relationships in postmortem brain tissue. First, time of death represents a single measure per subject at a specific time point, rather than repeated measures across time. Therefore, relationships of time of death with expression of specific molecules needs to be validated by comparisons to molecule- and region-specific rhythmic expression in animal models (Chaudhury and Colwell, 2002; Lamont et al., 2005; Granados-Fuentes et al., 2006; Guilding and Piggins, 2007; Verwey et al., 2007; Fuller et al., 2008; Carneiro and Araujo, 2009; Mistlberger, 2009; Verwey and Amir, 2009, Wang et al., 2009; Pantazopoulos et al., 2011; Albrecht, 2013; Albrecht et al., 2013). Measurements in cerebrospinal fluid samples taken at different time points from the same human subjects have been used in some studies to evaluate circadian changes (Rubinow, 1986). A few studies have also used geographic location and date of death of each subject, in order to adjust time-of-death values to the local timing of sunrise (Li, 2014; Bunney et al., 2015; Chen et al., 2016). However, this adjustment was not used in several human postmortem studies which successfully measured rhythmic molecular expression in various brain regions and validated it by comparing it with established rhythms in rodents (Zhou et al., 2001; Hofman, 2003; van Wamelen et al., 2013; Pantazopoulos et al., 2017). For instance, in a recent study, analyses based on time of death showed “rhythmic-like’ changes of somatostatin expression in normal human amygdala (Pantazopoulos et al., 2017). In people with bipolar disorder, this rhythmic expression was found to be altered in the same region (Pantazopoulos et al., 2017). These findings are consistent with those reported in rodent amygdala, where similar rhythmic somatostatin expression was observed, and with findings in cerebrospinal fluid of live human subjects with mood disorders (Rubinow, 1986; Albrecht et al., 2013).
“Text mining clinical information”
A promising approach for obtaining clinical information pertaining to symptoms and treatment is the analysis of electronic health records. For instance, McCoy et al. (2015) based their approach on the research domain criteria, a proposed dimensional model of psychopathology, to design an elegant method of extracting clinical dimensions from electronic medical records using information retrieval and natural language. Although perhaps still in early stages, the application of these approaches to extracting information relative to brain donors may be potentially ground-breaking for postmortem investigations. They may allow to report, and perhaps quantify, on specific clinical domains for each brain donor, thus allowing postmortem studies to progress into a modern, dimensional approach to investigations on psychiatric disorders.
In addition, mining of clinical records can leverage knowledge of prognosis indicators to assess the severity of the disorder. Here, we use schizophrenia as an example of the parameters commonly used to formulate the prognosis of this disorder, that can be extracted from medical records and used to estimate severity. The clinical outcome of schizophrenia is relatively poor. Disease trajectories display a high level of heterogeneity, from nearly normal routine functioning to the need for custodial care, with most patients experiencing periodic fluctuations in the severity of symptoms, even with sustained treatment. Poor clinical outcome is variously defined as greater number of hospitalizations, more severe symptoms, lack of remission, general dysfunction, and poor occupational functioning. Predictors of course and outcome include: premorbid functioning, cognitive functioning (Green, 1996), negative symptoms, substance abuse, insidious (as opposed to acute) onset of illness, various sociodemographic characteristics, certain clinical features at the time of first episode, treatment response, and duration of untreated illness (McGlashan, 2008) (Table 14.2).
Table 14.2.
Most common indicators of poor prognosis and good prognosis in patients with schizophrenia
| Poor prognostic indicators | Good prognostic indicators |
|---|---|
| Negative symptoms | Negative symptoms not prominent |
| Poor premorbid functioning | Good premorbid functioning |
| Cognitive impairment | No cognitive impairment |
| Insidious onset | Acute onset |
| Male sex | Female sex |
| Single | Married |
| Longer duration of untreated psychosis | Shorter duration of untreated psychosis |
| Substance abuse | No substance abuse |
| Early onset (age <18 years) | Later onset (age >45 years) |
No single feature is strongly associated with long-term prognosis. Among the few clinical features with robust predictive power even early in the course of schizophrenia are negative symptoms of social withdrawal, passivity, and cognitive impairment (Ventura et al., 2015). The predictive capacity of other variables, such as gradual versus acute onset, attenuates with time. In first-episode cases, male sex, being unmarried, premorbid social withdrawal, and insidious onset predict poor outcome over the next 2–5 years (Leung and Chue, 2000; Juola et al., 2013). Cognitive functioning in early stages of schizophrenia predicts global functioning over several years of follow-up, and an earlier age of onset may bode poorly for future outcome.
Structural brain imaging changes are subtle and present in many different patterns, and have not provided compelling diagnostic or predictive value (Fusar-Poli and Meyer-Lindenberg, 2016). In recent years, machine learning methods have been employed to extract MRI volumetric data with the goal of discriminating between normal controls and psychotic disorder subjects and contributing to predictions of subsequent illness course (Mourao-Miranda et al., 2012). This technique is not employed routinely.
Indicators of prognosis that are commonly documented in clinical records include: age of onset, sex, education, whether employed or receiving disability payments, and marital status. The Positive and Negative Syndrome Scale, a good measurement of positive and negative symptoms, is not routinely scored and recorded, but data-mining algorithms using natural language processing (see above) have been developed to extract this information from electronic medical records (Patel et al., 2015). Documentation of cognitive impairment, though a core feature of psychotic disorders, often is cursory. Structural brain imaging is frequently performed as part of initial evaluations of new psychotic illnesses. There is substantial overlap between normal controls and schizophrenia cases, and routine readings usually result in a “normal” interpretation (Fusar-Poli and Meyer-Lindenberg, 2016). A variety of laboratory studies are routinely performed as part of initial assessment to rule out treatable causes of psychotic symptoms secondary to a nonpsychiatric medical disorder and to establish a baseline of function/body mass index in anticipation of future morbidity, iatrogenic and otherwise. CRP is not among them. These studies often have very limited prognostic value.
In recent years, actuarial methods have been developed to combine baseline characteristics with functional measurements to estimate prognosis. These approaches use probabilistic models based on Bayes’ theorem and decision analysis techniques (Schubert et al., 2015). This methodology is finding increasing applications in psychiatry, particularly where highly complex data and multimodal data sets are analyzed together.
CONCLUSIONS
The discussion and examples provided in this chapter are intended to contribute to growing efforts toward rigorous and extensive use of source information on brain donors in the context of research on brain disorders. Extensive information on brain donors is often available, and all efforts should be made to obtain it consistently. We suggest that the main challenge is to devise robust approaches to extracting data from available sources and translating into measures that can reliably and effectively be incorporated in the design and data analysis of postmortem human investigations.
ACKNOWLEDGMENTS
The authors are grateful to NIH, for funding the Harvard Brain Tissue Resource Center through the NIH-NeuroBiobank HHSN-271-2013-00030C - The National Institute of Mental Health (NIMH), National Institute of Neurological Diseases and Stroke (NINDS) and Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). Their sincere gratitude also goes to brain donors and their families, without whom research on brain disorders would not be possible.
References
- Albrecht U (2013). Circadian clocks and mood-related behaviors. Handb Exp Pharmacol: 227–239. [DOI] [PubMed] [Google Scholar]
- Albrecht A, Thiere M, Bergado-Acosta JR et al. (2013). Circadian modulation of anxiety: a role for somatostatin in the amygdala. PLoS One 8: e84668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (1994). Diagnostic and statistical manual of mental disorders, fourth edition American Psychiatric Association, Arlington, VA. [Google Scholar]
- Ansari N, Agathagelidis M, Lee C et al. (2009). Differential maturation of circadian rhythms in clock gene proteins in the suprachiasmatic nucleus and the pars tuberalis during mouse ontogeny. Eur J Neurosci 29: 477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auslander LA, Jeste DV (2004). Sustained remission of schizophrenia among community-dwelling older outpatients. Am J Psychiatry 161: 1490–1493. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ (2013). Chemotherapy in psychiatry: pharmacologic basis of treatments for major mental illness, Springer, New York. [Google Scholar]
- Baldessarini RJ, Tarazi FI (1995). Pharmacotherapy of psychosis and mania In: Brunton LL, Lazo JS, Parker KL (Eds.), Goodman and Gilman’s the pharmacological basis of therapeutics, 11th edn McGraw-Hill, New York. [Google Scholar]
- Barnett JH, Werners U, Secher SM et al. (2007). Substance use in a population-based clinic sample of people with first-episode psychosis. Br J Psychiatry 190: 515–520. [DOI] [PubMed] [Google Scholar]
- Barton AJ, Pearson RC, Najlerahim A et al. (1993). Pre- and post-mortem influences on brain RNA. J Neurochem 61: 1–11. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S (2001). GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 25: 1–27. [DOI] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Lange N (2007). Neuron numbers and volume of the amygdala in subjects diagnosed with bipolar disorder or schizophrenia. Biol Psychiatry 62: 884–893. [DOI] [PubMed] [Google Scholar]
- Breen R, Thornhill JT (1998). Noncompliance with medication for psychiatric disorders. CNS Drugs 9: 457–471. [Google Scholar]
- Bunney BG, Li JZ, Walsh DM et al. (2015). Circadian dysregulation of clock genes: clues to rapid treatments in major depressive disorder. Mol Psychiatry 20: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro BT, Araujo JF (2009). The food-entrainable oscillator: a network of interconnected brain structures entrained by humoral signals? Chronobiol Int 26: 1273–1289. [DOI] [PubMed] [Google Scholar]
- Chand P, Thirthalli J, Murthy P (2014). Substance use disorders among treatment naive first-episode psychosis patients. Compr Psychiatry 55: 165–169. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Colwell CS (2002). Circadian modulation of learning and memory in fear-conditioned mice. Behav Brain Res 133: 95–108. [DOI] [PubMed] [Google Scholar]
- Chen CY, Logan RW, Ma T et al. (2016). Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc Natl Acad Sci U S A 113: 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb JA, Simpson J, Mahajan GJ et al. (2013). Hippocampal volume and total cell numbers in major depressive disorder. J Psychiatr Res 47: 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan S, Horder J, Inkster B et al. (2012). GABA system dysfunction in autism and related disorders: from synapse to symptoms. Neurosci Biobehav Rev 36: 2044–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS et al. (2006). Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry 67: 247–257. [DOI] [PubMed] [Google Scholar]
- Costa E, Davis JM, Dong E et al. (2004). A GABAergic cortical deficit dominates schizophrenia pathophysiology. Crit Rev Neurobiol 16: 1–23. [DOI] [PubMed] [Google Scholar]
- Farooq S, Naeem F (2014). Tackling nonadherence in psychiatric disorders: current opinion. Neuropsychiatr Dis Treat 10: 1069–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond G, D’albis MA, Jamain S, et al. 2015. The promise of biological markers for treatment response in first-episode psychosis: a systematic review. Schizophr Bull, 41, 559–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster A, Gable J, Buckley J (2012). Homelessness in schizophrenia. Psychiatr Clin North Am 35: 717–734. [DOI] [PubMed] [Google Scholar]
- Frank E, Ozon C, Nair V et al. (2015). Examining why patients with attention-deficit/hyperactivity disorder lack adherence to medication over the long term: a review and analysis. J Clin Psychiatry 76: e1459–e1468. [DOI] [PubMed] [Google Scholar]
- Fuller PM, Lu J, Saper CB (2008). Differential rescue of light- and food-entrainable circadian rhythms. Science 320: 1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Meyer-Lindenberg A (2016). Forty years of structural imaging in psychosis: promises and truth. Acta Psychiatr Scand 134: 207–224. [DOI] [PubMed] [Google Scholar]
- Girgis RR, Kumar SS, Brown AS (2014). The cytokine model of schizophrenia: emerging therapeutic strategies. Biol Psychiatry 75: 292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Fuentes D, Tseng A, Herzog ED (2006). A circadian clock in the olfactory bulb controls olfactory responsivity. J Neurosci 26: 12219–12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandal Leiros B, Perez Mendez LI, Zelaya Huerta MV et al. (2016). Prevalence and concordance between the clinical and the post-mortem diagnosis of dementia in a psychogeriatric clinic. Neurologia. 10.1016/j.nrl.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Green MF (1996). What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 153: 321–330. [DOI] [PubMed] [Google Scholar]
- Green AI, Tohen MF, Hamer RM et al. (2004). First episode schizophrenia-related psychosis and substance use disorders: acute response to olanzapine and haloperidol. Schizophr Res 66: 125–135. [DOI] [PubMed] [Google Scholar]
- Guilding C, Piggins HD (2007). Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain? Eur J Neurosci 25: 3195–3216. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Wester P, Winblad B et al. (1985). The patients dying after long terminal phase have acidotic brains; implications for biochemical measurements on autopsy tissue. J Neural Transm 61: 253–264. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Barton AJ, Najlerahim A et al. (1991a). Regional and neuronal reductions of polyadenylated messenger RNA in Alzheimer’s disease. Psychol Med 21: 855–866. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Procter AW, Barton AJ et al. (1991b). Terminal coma affects messenger RNA detection in post mortem humantemporalcortex.BrainResMolBrainRes 9:161–164. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Herzog ED (2004). Clock genes, oscillators, and cellular networks in the suprachiasmatic nuclei. J Biol Rhythms 19: 400–413. [DOI] [PubMed] [Google Scholar]
- Hofman MA (2003). Circadian oscillations of neuropeptide expression in the human biological clock. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 189: 823–831. [DOI] [PubMed] [Google Scholar]
- Hugtenburg JG, Timmers L, Elders PJ et al. (2013). Definitions, variants, and causes of nonadherence with medication: a challenge for tailored interventions. Patient Prefer Adherence 7: 675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynd MR, Lewohl JM, Scott HL et al. (2003). Biochemical and molecular studies using human autopsy brain tissue. J Neurochem 85: 543–562. [DOI] [PubMed] [Google Scholar]
- Ji LD, Xu J, Wu DD et al. (2010). Association of disease-predisposition polymorphisms of the melatonin receptors and sunshine duration in the global human populations. J Pineal Res 48: 133–141. [DOI] [PubMed] [Google Scholar]
- Johnston NL, Cervenak J, Shore AD et al. (1997). Multivariate analysis of RNA levels from postmortem human brains as measured by three different methods of RT-PCR. Stanley Neuropathology Consortium.J NeurosciMethods 77: 83–92. [DOI] [PubMed] [Google Scholar]
- Joseph J, Depp C, Martin AS et al. (2015). Associations of high sensitivity C-reactive protein levels in schizophrenia and comparison groups. Schizophr Res 168: 456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juola P, Miettunen J, Veijola J et al. (2013). Predictors of short- and long-term clinical outcome in schizophrenic psychosis - the Northern Finland 1966 Birth Cohort study. Eur Psychiatry 28: 263–268. [DOI] [PubMed] [Google Scholar]
- Katz G, Durst R, Shufman E et al. (2008). Substance abuse in hospitalized psychiatric patients. Isr Med Assoc J 10: 672–675. [PubMed] [Google Scholar]
- Koechl B, Unger A, Fischer G (2012). Age-related aspects of addiction. Gerontology 58: 540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunii Y, Hyde TM, Ye T et al. (2014). Revisiting DARPP-32 in postmortem human brain: changes in schizophrenia and bipolar disorder and genetic associations with t-DARPP-32 expression. Mol Psychiatry 19: 192–199. [DOI] [PubMed] [Google Scholar]
- Lamont EW, Robinson B, Stewart J et al. (2005). The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc Natl Acad Sci U S A 102: 4180–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S et al. (2000). Smoking and mental illness: a population-based prevalence study. JAMA 284: 2606–2610. [DOI] [PubMed] [Google Scholar]
- Leclerc E, Noto C, Bressan RA et al. (2015). Determinants of adherence to treatment in first-episode psychosis: a comprehensive review. Rev Bras Psiquiatr 37: 168–176. [DOI] [PubMed] [Google Scholar]
- Lehrmann E, Afanador ZR, Deep-Soboslay A et al. (2008). Postmortem diagnosis and toxicological validation of illicit substance use. Addict Biol 13: 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A, Chue P (2000). Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand Suppl 401: 3–38. [DOI] [PubMed] [Google Scholar]
- Levin JB, Seifi N, Cassidy KA et al. (2014). Comparing medication attitudes and reasons for medication nonadherence among three disparate groups of individuals with serious mental illness. J Nerv Ment Dis 202: 769–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW (2005). Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6: 312–324. [DOI] [PubMed] [Google Scholar]
- Li JZ (2014). Circadian rhythms and mood: opportunities for multi-level analyses in genomics and neuroscience: circadian rhythm dysregulation in mood disorders provides clues to the brain’s organizing principles, and a touchstone for genomics and neuroscience. Bioessays 36: 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstern C, Brenner T, Bardenheuer HJ et al. (2012). Predictors of survival in sepsis: what is the best inflammatory marker to measure? Curr Opin Infect Dis 25: 328–336. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, Mcevoy JP et al. (2005). Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353: 1209–1223. [DOI] [PubMed] [Google Scholar]
- Mago R, Borra D, Mahajan R (2014). Role of adverse effects in medication nonadherence in bipolar disorder. Harv Rev Psychiatry 22: 363–366. [DOI] [PubMed] [Google Scholar]
- Markota M, Sin J, Pantazopoulos H et al. (2014). Reduced dopamine transporter expression in the amygdala of subjects diagnosed with schizophrenia. Schizophr Bull 40: 984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccoy TH, Castro VM, Rosenfield HR et al. (2015). A clinical perspective on the relevance of research domain criteria in electronic health records. Am J Psychiatry 172: 316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcglashan TH (2008). Premorbid adjustment, onset types, and prognostic scaling: still informative? Schizophr Bull 34: 801–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W et al. (2011). Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 70: 663–6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Gassama B, Sebastian D et al. (2013). Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 73: 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE (2009). Food-anticipatory circadian rhythms: concepts and methods. Eur J Neurosci 30: 1718–1729. [DOI] [PubMed] [Google Scholar]
- Mourao-Miranda J, Reinders AA, Rocha-Rego V et al. (2012). Individualized prediction of illness course at the first psychotic episode: a support vector machine MRI study. Psychol Med 42: 1037–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centre National Collaborating (2011). Psychosis with coexisting substance misuse: assessment and management in adults and young people, Leicester UK, The British Psychological Society and The Royal College of Psychiatrists. [PubMed] [Google Scholar]
- Nelson PT, Jicha GA, Schmitt FA et al. (2007). Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol 66: 1136–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazopoulos H, Lange N, Baldessarini RJ et al. (2007). Parvalbumin neurons in the entorhinal cortex of subjects diagnosed with bipolar disorder or schizophrenia. Biol Psychiatry 61: 640–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazopoulos H, Woo T-UW, Lim MP et al. (2010). Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch Gen Psychiatry 67: 155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazopoulos H, Dolatshad H, Davis FC (2011). A fearinducing odor alters PER2 and c-Fos expression in brain regions involved in fear memory. PLoS One 6: e20658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazopoulos H, Wiseman JT, Markota M et al. (2017). Decreased numbers of somatostatin-expressing neurons in the amygdala of subjects with bipolar disorder or schizophrenia: relationship to circadian rhythms. Biol Psychiatry 81: 536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Jayatilleke N, Broadbent M et al. (2015). Negative symptoms in schizophrenia: a study in a large clinical sample of patients using a novel automated method. BMJ Open 5: e007619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray MT, Shannon Weickert C, Webster MJ (2014). Decreased BDNF and TrkB mRNA expression in multiple cortical areas of patients with schizophrenia and mood disorders. Transl Psychiatry 4: e389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR (2002). Coordination of circadian timing in mammals. Nature 418: 935–941. [DOI] [PubMed] [Google Scholar]
- Reus GZ, Fries GR, Stertz L et al. (2015). The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 300: 141–154. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Roche JK, Conley RR et al. (2009). Dopaminergic synapses in the caudate of subjects with schizophrenia: relationship to treatment response. Synapse 63: 520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow DR (1986). Cerebrospinal fluid somatostatin and psychiatric illness. Biol Psychiatry 21: 341–365. [DOI] [PubMed] [Google Scholar]
- Saban A, Flisher A, Laubscher R et al. (2014). The association between psychopathology and substance use: adolescent and young adult substance users in inpatient treatment in Cape Town, South Africa. Pan Afr Med J 17 (Suppl 1): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajatovic M, Levin J, Ramirez LF et al. (2013). Prospective trial of customized adherence enhancement plus long-acting injectable antipsychotic medication in homeless or recently homeless individuals with schizophrenia or schizoaffective disorder. J Clin Psychiatry 74: 1249–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, De Rivera H et al. (2016). Schizophrenia risk from complex variation of complement component 4. Nature 530: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert KO, Clark SR, Baune BT (2015). The use of clinical and biological characteristics to predict outcome following. First Episode Psychosis 49 (1): 24–35. [DOI] [PubMed] [Google Scholar]
- Thoma P, Daum I (2013). Comorbid substance use disorder in schizophrenia: a selective overview of neurobiological and cognitive underpinnings. Psychiatry Clin Neurosci 67: 367–383. [DOI] [PubMed] [Google Scholar]
- Tomita H, Vawter MP, Walsh DM et al. (2004). Effect of agonal and postmortem factors on gene expression profile: quality control in microarray analyses of postmortem human brain. Biol Psychiatry 55: 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Farfan C, Seron-Ferre M, Dinet V et al. (2006). Immunocytochemical demonstration of day/night changes of clock gene protein levels in the murine adrenal gland: differences between melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. J Pineal Res 40: 64–70. [DOI] [PubMed] [Google Scholar]
- Trepanier MO, Hopperton KE, Mizrahi R et al. (2016). Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry 21: 1009–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wamelen DJ, Aziz NA, Anink JJ et al. (2013). Suprachiasmatic nucleus neuropeptide expression in patients with Huntington’s disease. Sleep 36: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Subotnik KL, Gitlin MJ et al. (2015). Negative symptoms and functioning during the first year after a recent onset of schizophrenia and 8 years later. Schizophr Res 161: 407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwey M, Amir S (2009). Food-entrainable circadian oscillators in the brain. Eur J Neurosci 30: 1650–1657. [DOI] [PubMed] [Google Scholar]
- Verwey M, Khoja Z, Stewart J et al. (2007). Differential regulation of the expression of Period2 protein in the limbic forebrain and dorsomedial hypothalamus by daily limited access to highly palatable food in food-deprived and free-fed rats. Neuroscience 147: 277–285. [DOI] [PubMed] [Google Scholar]
- Vrijens B, De Geest S, Hughes DA et al. (2012). A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 73: 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xu L, Wang E (2009). Robustness and coherence of a three-protein circadian oscillator: landscape and flux perspectives. Biophys J 97: 3038–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AY, Lohmann KM, Yang CK et al. (2011). Bipolar disorder type 1 and schizophrenia are accompanied by decreased density of parvalbumin- and somatostatin-positive interneurons in the parahippocampal region. Acta Neuropathol 122: 615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman D, Cho M, Taylor C et al. (2007). dementia with Lewy bodies, Braak stage determines phenotype, not Lewy body distribution. Neurology 69: 356–359. [DOI] [PubMed] [Google Scholar]
- Wisdom JP, Manuel JI, Drake RE (2011). Substance use disorder among people with first-episode psychosis: a systematic review of course and treatment. Psychiatr Serv 62: 1007–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreschnig D, Dolatshad H, Davis FC (2014). Embryonic development of circadian oscillations in the mouse hypothalamus. J Biol Rhythms 29: 299–310. [DOI] [PubMed] [Google Scholar]
- Zhou JN, Riemersma RF, Unmehopa UA et al. (2001). Alterations in arginine vasopressin neurons in the suprachiasmatic nucleus in depression. Arch Gen Psychiatry 58: 655–662. [DOI] [PubMed] [Google Scholar]
- Zivin K, Madden JM, Graves AJ et al. (2009). Cost-related medication nonadherence among beneficiaries with depression following Medicare Part D. Am J Geriatr Psychiatry 17: 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]


