Graphical Abstract
Keywords: Covid-19, SARS-CoV-2, Anti-viral, Drug, Favipiravir, Remdesevir
Abstract
Coronavirus, known as the coronavirus pandemic, is continuing its spread across the world, with over 42 million confirmed cases in 189 countries and more than 1.15 million deaths. Although, scientists focus on the finding novel drugs and vaccine for SARS-CoV-2, there is no certain treatment for it. Antiviral drugs such as; oseltamivir, favipiravir, umifenovir, lopinavir, remdesivir, hydroxychloroquine, chloroquine, azithromycin, ascorbic acid, corticosteroids, are mostly used for patients. They prevent cytokine storm that is the main reason of deaths related to SARS-CoV-2. In addition, anti-inflammatory agents have critical roles to inhibit the lung injury and multisystem organ dysfunction. The combination with anti-viral drugs with other drugs displays high synergistic effects. In the present study, the drugs used for Covid-19 are analyzed and compare the efficiency for the Covid-19 patients from the different continents including USA, South Korea, Italy, Spain, Germany, Russia, Brazil, Turkey, and China. Nowadays, all countries tried to find vaccine and new drug candidates for SARS-CoV-2, but anti-viral drugs may be the best candidates for the treatment of Covid-19 before finding novel anti-Covid drug.
1. Introduction
COVID-19 is a new coronavirus disease identified in December 2019. Patients have clinical symptoms as dry cough, dyspnea, fever, and bilateral lungin-filtrateson imaging. Cases were all linked to Wuhan's Huanan Seafood Wholesale Market, trading in fish and a variety of live animal species including poultry, bats, marmots, and snakes. On 7th January 2020, The causative agent was identified from throat swab samples conducted by the Chinese Centre for Disease Control and Prevention (CCDC) and was named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Furthermore, it was called as COVID-19 by the World Health Organization (WHO) [1].
Infected patients have usually mild symptoms as dry cough, sore throat, and fever. Furthermore, the most of the cases have been resolved and according to the sexuality and age symptoms degree alters. For instance, 54.3% of those infected with SARS-CoV-2 are male with a median age of 56 years and patients requiring intensive care support were older and had multiple comorbidities including cardiovascular, cerebrovascular, endocrine, digestive, and respiratory disease. Those in intensive care were also more likely tore-port dyspnoea, dizziness, abdominal pain, and anorexia [1].
This respiratory disease is very severe and potentially fatal in some patients according to their age and chronic disease history. It has been reported that 15% of cases related to this disease are severe, and mortality rate varies between 1.5% and 10% [2]. As of May 25, 2020, over 5,43 million cases of COVID-19 were reported, with greater than 345,000 deaths. It was reported that 91.1% of cases were diagnosed with pneumonia [3]. The second most common diagnosis is acute respiratory distress syndrome (3.4%) [4]. Although the fatality rate of SARS-CoV-2 is lower than these of Middle East respiratory syndrome (MERS-CoV) and SARS-CoV, the higher contagiousness of SARS-CoV-2 is a significant risk for the healthcare system. Presently COVID-19 seems to spread from person to person as common cold or influenza viruses-ie, face to face contact with a sneeze or cough, or from contact with secretions of people who are infected.
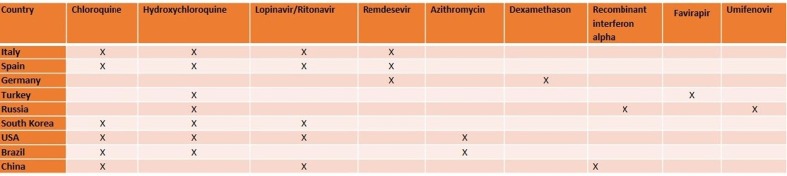
At present study, anti-viral drugs were analyzed to find the efficiency of the treatments of SARS-CoV-2 virus. It is known that countries used different drugs and drug combinations for patients. These therapy methods usually depend on the number of deaths and the number of patients used ventilation. Moreover, there is no certain treatments ways for COVID-19. In this study, different countries from all over the world were analyzed with their drugs which were used for COVID-19. We also displayed that total case numbers and deaths between January to September 2020. Interestingly, it was observed that second wave started before September 2020 all countries.
2. Statistics, Prevention, Diagnosis, and treatment
After COVID-19 appeared in Wuhan on December 1, 2019, it spread rapidly to other countries. Following the spread of COVID-19 to all continents, a pandemic was declared by WHO on March 11, 2020. The center of the pandemic that started in Asia shifted to Europe in mid-March and then to America. According to John Hopkins University, the total number of cases in the world was 54,518,771, the number of patients recovered was 38,259,383 while the total number of deaths was 1,319,267 until November 16, 2020. Besides, At November 16, 2020, while 15,316,990 of 15,415,926 active patients had COVID-19 with mild symptoms, 98,936 patients were in critical condition. Below the detailed information about the COVID-19 statistics of some countries in Europe, America, and Asia, the measures they have taken against this pandemic, and the treatment protocols is given.
2.1. Italy
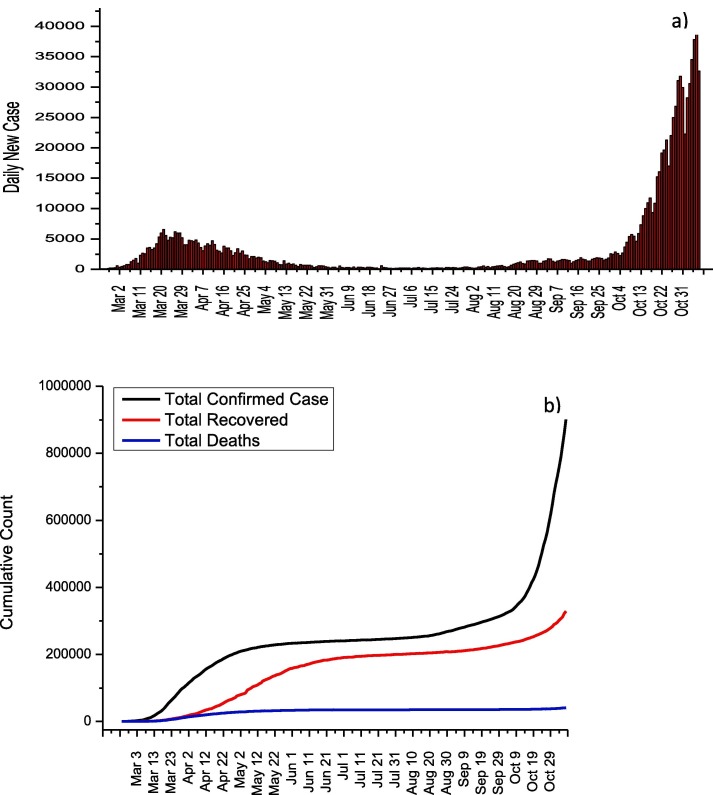
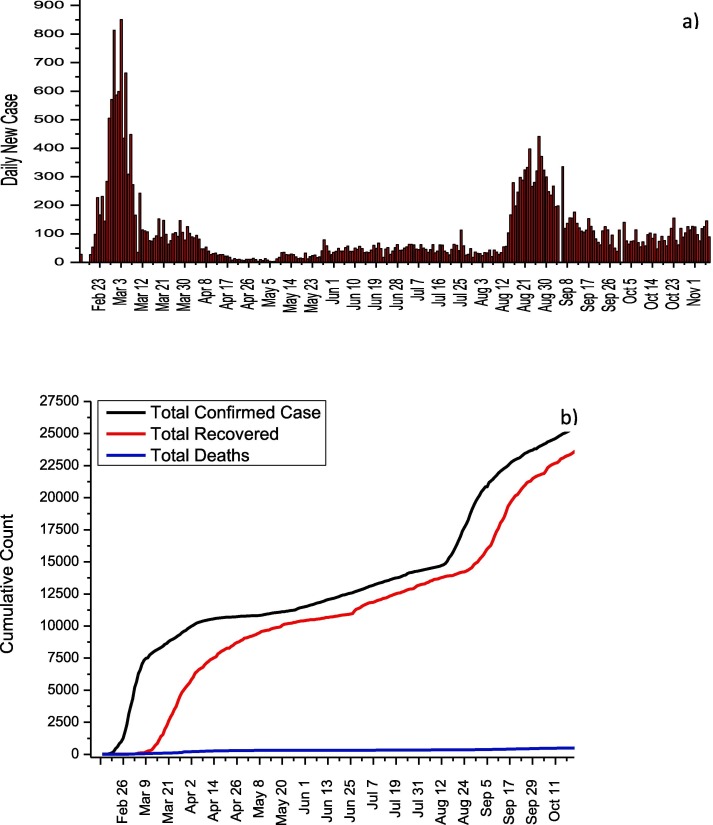
On 31 January, the first two cases in Italy were confirmed in Rome. The fact that serious measures began to be taken in 3 weeks after the first case appeared, caused the cases in Italy to spread rapidly. Then, various measures were put into effect by taking advantage of China's pandemic experience. It can be seen from Fig. 1 a that the number of daily cases reached their highest values, especially between 17 and 29 March and 3–7 November. On November 6, the highest number of cases was reached with 39,809 days of cases. Firstly, on February 23, entrance and exit restrictions were imposed to 11 towns in the north of Italy, and schools in this region were closed [5]. This restriction was expanded on 8 March by including the whole Lombardia region and 12 cities while quarantine measures applied to 16 million people were applied to the whole country on 9 March [6]. On March 11, the activities of businesses other than supermarkets, banks, and pharmacies were stopped, and these measures were further tightened [7]. Parks and many factories were closed on 19 and 21 March, respectively. It can be seen from Fig. 1b that, thanks to these measures, the rate of case increase has decreased and the total number of cases has reached a plateau. While a total of 902,409 cases and 41,063 deaths were reported in Italy until 7 November, the fatality rate was reported as 4.6%.The normalization effects in Italy are clearly seen from Fig. 1a and b. Especially after October 4, Italy is experiencing the second wave for COVID-19, and the increase in the number of daily cases continues rapidly. The treatment protocol published by the Italian Society of Infectious and Tropical Diseases for the treatment of COVID-19 is given in Table 1 [8].
Fig. 1.
a) Daily new cases and b) total COVID-19 statistics for Italy.
Table 1.
COVID-19 treatment protocol proposed by the Italian Society of Infectious and Tropical Diseases.
| Treatment Protocols | ||
|---|---|---|
| Patient with mild symptoms but over 70 years or/and with different diseases or/and worsening conditions |
Lopinavir/Ritonavir + Chloroquine or Hydroxychloroquine | ➢ Lopinavir/Ritonavir: 200/50 mg twice daily➢ Chloroquine or Hydroxychloroquine: 500 mg or 200 mg twice daily |
| Darunavir /Ritonavir + Chloroquine or Hydroxychloroquine* or Darunavir/Cobicistat + Chloroquine or Hydroxychloroquine ** | ➢ Darunavir: 800 mg per day➢ Ritonavir: 100 mg per dayor➢ Darunavir: 800 mg per day➢ Cobicistat: 150 mg per day➢ Chloroquine: 500 mg twice per day for 20 days➢ Hydroxychloroquine: 200 mg twice a day | |
| Patient with severe symptoms | Remdesevir + Chloroquine or Hydroxychloroquine | ➢ Remdesevir: 200 mg for the first day, 100 mg daily for the following days➢ Chloroquine: 500 mg twice daily or Hydroxychloroquine: 200 mg twice per day |
If lopinavir is not available;
If Lopinavir/Ritonavir is not available
2.2. Spain
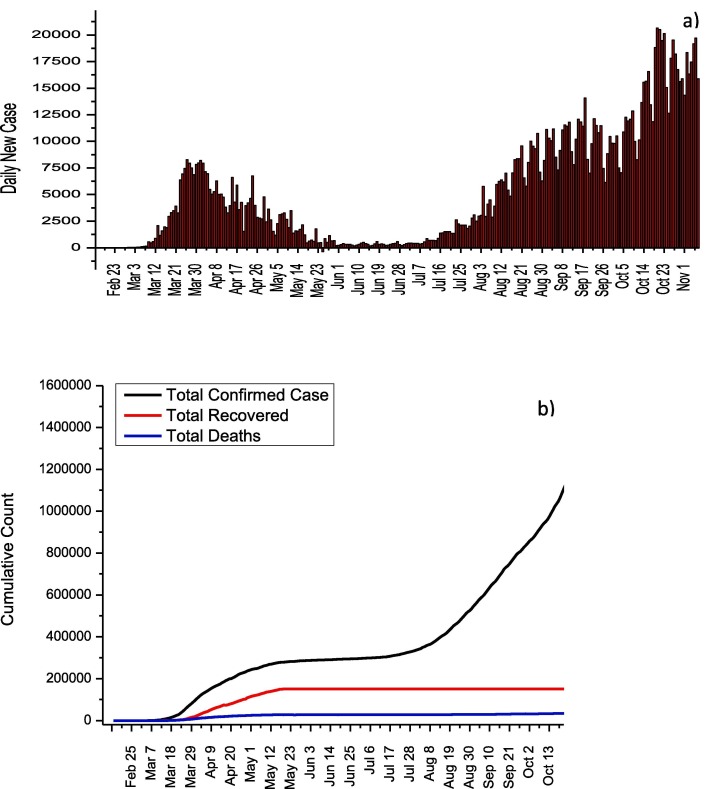
The first case in Spain was confirmed on January 31, and no new cases were reported almost during February [9]. While 1,399,169 cases were reported from the beginning of the pandemic process until 7 November, the deaths and recovery numbers were reported as 38,833 and 150,376. It is seen that the daily increase rate of COVID-19 cases in Spain gradually decreased after April (Fig. 2 a). This decrease can be explained by the declaration of a 15-day state of emergency by the Spanish government on March 13 and the implementation of quarantine conditions throughout the country [10]. The Spanish government has applied the Italian model in terms of social measures. Therefore, the daily number of cases in these two countries after the measures are parallel. In addition, the dramatic decrease in the number of daily cases in June is followed by the sharp increases in the number of daily cases as a result of the relaxation of quarantine conditions. This observed increase after 13 July can be clearly followed in Fig. 2b. In Spain, the maximum daily number of cases in first wave and second waves were determined as 8271 and 20640, respectively. The much higher number of infected cases in the second wave can be directly attributed to the elimination of nationwide measures.The usage information of drugs such as Remdesivir, Lopinavir/Ritonavir, Chloroquine/Hydroxychloroquine, Tocilizumab, Sarilumab, Ruxolitinib, Siltuximab, Baricitinib, Anakinra, Interferon Beta-1B, and Interferon Alpha-2B have been published by the Spanish Agency for Medicines and Health Products for COVID-19 (Table 2 ) [11]. However, the agency stated that this information is for informational purposes rather than a suggestion.
Fig. 2.
a) Daily new cases and b) total COVID-19 statistics for Spain.
Table 2.
COVID-19 treatment protocol recommended by the Spanish Agency for Medicines and Health Products.
| Treatment Protocols | |
|---|---|
| Remdesivir | ➢ 200 mg for the first day and 100 mg daily for 2–10 days |
| Lopinavir/Ritonavir | ➢ 200/50 mg twice per day |
| Chloroquine/Hydroxychloroquine | ➢ 400 mg per 12 h and 100 mg daily for 2–5 days |
| Tocilizumab | ➢ 600 mg for patients 75 kg➢ 400 mg for patients < 75 kg |
| Sarilumab | ➢ 200 or 400 mg per day |
| Ruxolitinib | ➢ 5 mg twice daily for 14 days |
| Siltuximab | ➢ 11 mg for 1 h➢ Other doses vary according to the CRP level |
| Baricitinib | ➢ 4 mg once daily for 7–14 days |
| Anakinra | ➢ 400 mg per day for a maximum of 15 days |
| Interferon Beta-1B and Interferon Alpha-2B | n.a. |
2.3. USA
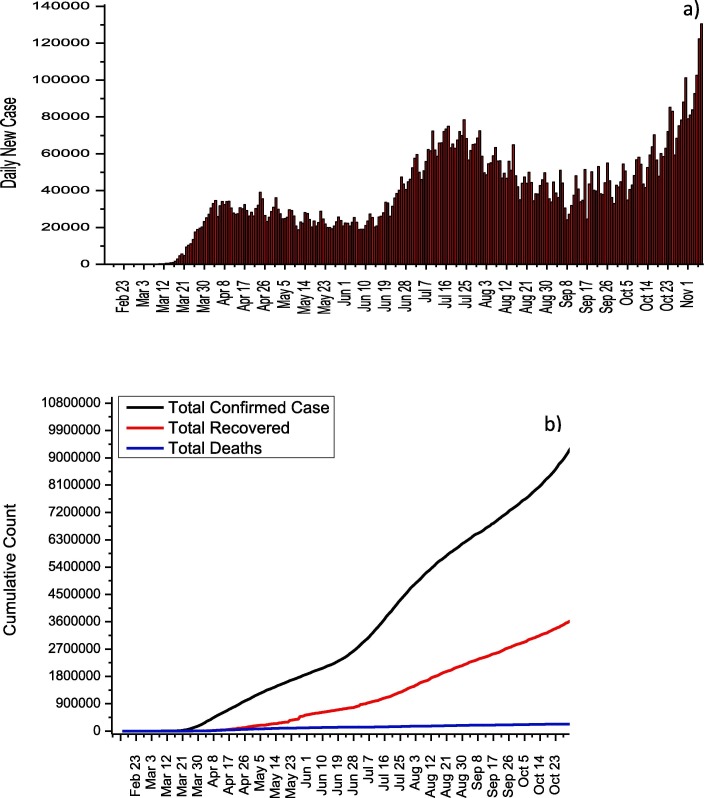
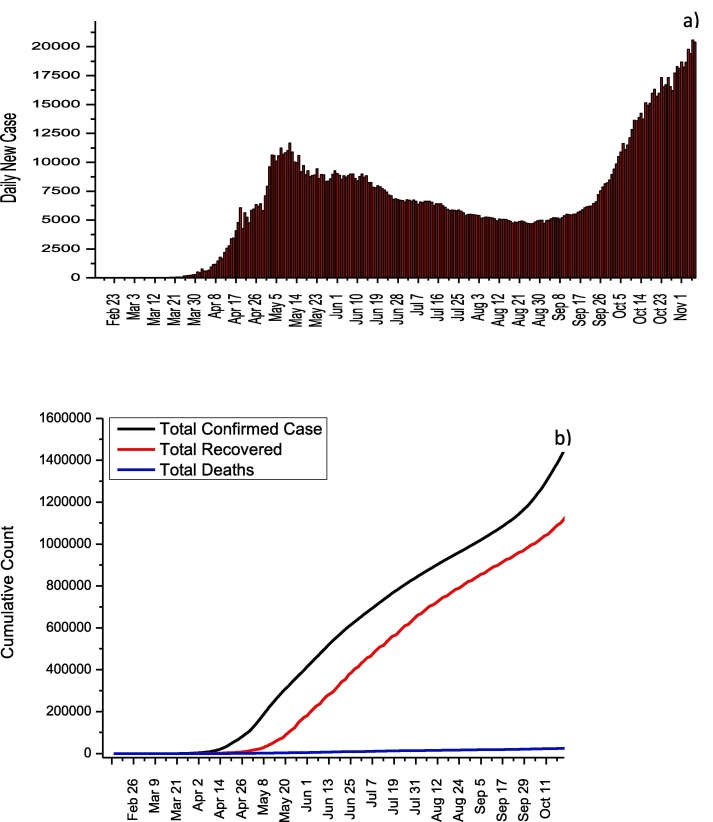
In U.S., 9,951,416 cases and 239,832 deaths from COVID-19 have been reported until 7 November. As seen in Fig. 3 a, no significant decreases in the number of cases were observed due to the lack of a stable quarantine throughout the country and the early initiation of normalization due to economic concerns. Thanks to the measures taken to maintain social distance across the country, the number of cases from April 5 to June 8 was reported around 35,000 while the highest daily number of cases was observed with 130,623 as of November 7. Although the increase in the number of daily cases accelerated after 8 June, after November 1, the increase in the number of daily cases is out of control (average daily number of cases is about 100,000).This increase is thought to be caused by the rapid normalization of America. Until November 7, the death rate from COVID-19 was reported as 2.41% while the recovery rate was reported as 38.7%. For the treatment of COVID-19 in the U.S. Azithromycin, Chloroquine, Hydroxychloroquine, Lopinavir/Ritonavir, and Remdesevir are recommended by the U.S. National Institutes of Health. The use of these antiviral drugs with their doses for adults is summarized in Table 3 [12].
Fig. 3.
a) Daily new cases and b) total COVID-19 statistics for USA.
Table 3.
COVID-19 treatment protocol recommended by the U.S. National Institutes of Health.
| Treatment Protocol | |
|---|---|
| Azithromycin* | ➢ Azithromycin: 500 mg for the first day, 250 mg for the next 2–5 days |
| Chloroquine** | ➢ Chloroquine: 1000 mg for the first day, 500 mg for the next 4–7 days |
| Hydroxychloroquine** | ➢ Hydroxychloroquine: 800 mg for the first day, 400 mg for the next 4–7 days |
| Lopinavir/Ritonavir | ➢ Lopinavir/Ritonavir: 400/100 mg twice per day for 10–14 days |
| Remdesevir*** | ➢ Remdesevir: 200 mg for the first day, 100 mg daily for the next 2–5 days |
The use of Azithromycin in combination with hydroxychloroquine was recommended.
Clinical trials are permitted for both hospitalized and non-hospitalized patients.
Remdesevir is recommended for use only in hospitalized COVID-19 patients due to its supply difficulties.
2.4. Brazil
The first case in Brasil was informed on 26 February 2020 [13]. The travel history and genetic findings of the first case show that the virus spread from northern Italy to Brazil [14]. Then, on February 3, an emergency was declared in Brazil [15]. On February 6, various measures such as restrictions on entry and exit from the country, quarantine measures, and exhumation were taken. Nevertheless, Imperial College (London, England), which compiles the transmission rates of COVID-19 by country, reported that Brazil has the highest spread rate [16]. Many researchers attribute the rapidly increasing number of cases in Brazil to President Bolsanaro's inadequate policies on COVID-19. As of November 7, a total of 5,640,952 cases were detected in Brazil. 414,531 of these cases are still infected and deaths from COVID-19 have been reported as 162,077 (Fig. 4 b).Besides, death and recovery rates were calculated as 2.87% and 89.8%. However, in Brazil, the number of cases has still not been controlled and the increase continues (Fig. 4a). A treatment protocol for COVID-19 has been published by the health ministry in Brazil. Accordingly, Chloroquine + Azitrophycin or Hydroxychloroquine + Azitrophycin antiviral drugs are used for patients with mild and moderate symptoms in adult patients. In severe patients, only hydroxychloroquine and azitrophycin are used together. A detailed treatment protocol for adult patients is given in Table 4 [17].
Fig. 4.
a) Daily new cases and b) total COVID-19 statistics for Brazil.
Table 4.
COVID-19 treatment protocol recommended by the Brazilian Ministry of Health.
| Treatment Protocols | ||
|---|---|---|
|
For Moderate Semptons |
Chloroquine + Azithromycin or Hydroxychloroquine + Azithromycin* |
➢ Chloroquine: 500 mg twice per day for the first day and 500 mg daily for the next 2–5 days➢ Azithromycin: 500 mg once a day for 5 days |
| ➢ Hydroxychloroquine: 400 mg twice daily for the first day and 400 mg daily for the next 2–5 days➢ Azithromycin: 500 mg per day for 5 days | ||
| For Severe Semptons | Hydroxychloroquine + Azithromycin* | ➢ Hydroxychloroquine: 400 mg twice per day for the first day and 400 mg daily for the next 2–5 days➢ Hydroxychloroquine: 500 mg once a day for 5 days |
2.5. Germany
The first COVID-19 case in Germany was detected by the German Ministry of Health on January 27, 2020 [18]. The pandemic process in Germany is managed by the Robert Koch Institute. As of 7 November, 644,048 cases, 11,306 deaths, and 411,997 recovered cases were reported. In Germany, similar to many other countries, it has taken important measures regarding social distance. The most striking of these is the prohibition of gathering more than two people as of March 22. In mid-March, borders with France, Switzerland, Austria, Denmark, and Luxembourg were closed, while flights to many countries were banned. In the state of Bavaria, a partial curfew was imposed. As a result of these measures, a rapid decrease in the number of daily cases was observed after the period of 25 March-4 April, when the number of daily cases was the highest (Fig. 5 a) for this period. The measures are taken to ensure social distance has a great contribution in controlling COVID-19 in Germany. After July, travel bans and contact restrictions were gradually lifted and the number of cases started to increase again (Fig. 5b). The second wave started on 3 August and has not reached the maximum point yet. The daily number of cases on November 7 is 23,399 and continues to increase.Germany is distinguished from other European countries with a particularly low mortality rate. Many researchers attribute this low mortality rate to the lower average age of the diagnosed population compared to other European countries [19]. Remdesevir is used as an antiviral in the treatment protocol recommended by the Robert Koch Institute (RCI). According to this algorithm, the use of 200 mg Remdesevir on the first day and 100 mg on the following days was deemed appropriate for adults and adolescents. It is stated that the use of Remdesevir should be between 5 and 10 days. On the other hand, RCI does not recommend the use of hydroxychloroquine and Lopinavir/Ritonavir. It has been stated that the use of these antiviral drugs for COVID-19 is the responsibility of doctors. The detailed treatment procedure recommended by RCI is given in Table 5 [20].
Fig. 5.
a) Daily new cases and b) total COVID-19 statistics for Germany.
Table 5.
COVID-19 treatment protocol recommended by the RKI.
| Treatment Protocols | |
|---|---|
| Remdesevir* | ➢ 200 mg for the first day and 100 mg daily for 2–5 days |
| Dexamethason | ➢ 6 mg daily for maximum of 10 days |
It is used in the presence of pneumonia due to COVID-19, and the treatment is continued for maximum of 10 days
2.6. South Korea
The first COVID-19 case in South Korea appeared on January 20, and the maximum number of cases was observed on March 3 [21]. The rapid increase in the number of cases in the early days has been associated with a ritual held in a church in Daegu [22]. The total number of infected, dead, and recovered until 7 November 2020 has been reported as 27,284,517, and 24,910, respectively. South Korea's mortality rate from COVID-19 was determined as 1.89%. But thanks to the early social isolation measures, the epidemic was brought under control in a short period. Also, with the disciplined implementation of lock-down measures, test policy, and social isolation measures, the daily number of cases was reduced to 22 on April 17, 2020 (Fig. 6 ) [23]. The number of cases followed this decreasing trend until May 26, 2020. However, as a result of the normalization policy followed subsequently, a slight increase was observed in the number of daily cases. The daily cases, which increased again in August and September, was brought under control towards the end of September. For the moderate and severe cases, Central Clinical Task Force has suggested Lopinavir 400 mg/Ritonavir 100 mg twice a day or Hydroxychloroquine 400 mg/Chloroquine 500 mg orally per day [24]. In addition, Remdesevir was added to this treatment protocol, which lasted 7–10 days [25]. The details of this treatment protocol is summarized in Table 6 .
Fig. 6.
a) Daily new cases and b) total COVID-19 statistics for South Korea.
Table 6.
COVID-19 treatment protocol recommended by South Korean Central Clinical Task Force [24]
| Treatment Protocol | |
|---|---|
| Lopinavir/Ritonavir or Chloroquine or Hydroxychloroquine | ➢ Lopinavir: 400 mg twice per day➢ Ritonavir: 100 mg twice per dayor➢ 500 mg per dayor➢ 400 mg per day |
2.7. Russia
On January 31, the first two COVID-19 cases in Russia were confirmed by Russian Ministry of Health [26]. Although some researchers claim that there is a delay in COVID-19 measures, many measures have been taken in Russia to reduce the number of COVID-19 cases. The most striking of these is the decision to close the workplaces between 30 March and 11 May [27]. While all international flights in the country were banned on March 27, curfews were declared in many states, especially Moscow Municipality, on March 29 [28]. With the reopening of workplaces as of May, the normalization process started. Almost all workplaces became open on June 8 [29]. In the following period, Russia has reached the highest number of daily cases on May 11 with a daily number of 11,656 cases. Even though a gradual decrease in the number of daily cases has been achieved with the social isolation measures taken, the fact that the duration of these measures was not long enough resulted in the increase in the number of cases after 8 August. Before the number of daily cases in the first wave was reduced to below 6000, the second wave started around 8 September and the increase in the number of daily cases accelerated. Until 7 November, 1,753,836 COVID-19 cases have been detected in Russia (Fig. 7 ). The total number of deaths and total recovered cases and mortality rates to date have been reported as 30,251, 1,342,814 and 1.72, respectively. The COVID-19 treatment procedure recommended by the Russian Ministry of Health is summarized in Table 7 . Accordingly, Recombinant interferon-alpha + Hydroxychloroquine and Umifenovir + Hydroxychloroquine are primarily used in the treatment of COVID-19 (Table 7) [30]. In cases where hydroxychloroquine is not available, the use of Recombinant interferon-alpha + Mefloquine and Umifenovir + Mefloquine drugs has been recommended. In addition, it is recommended to use Recombinant interferon-alpha + Umifenovir drugs in case the side effects of Hydroxychloroquine and Mefloquine are detected.
Fig. 7.
a) Daily new cases and b) total COVID-19 statistics for Russia.
Table 7.
COVID-19 treatment protocol recommended by the Russian Ministry of Health.
| Treatment Protocols | |
|---|---|
| Recombinant interferon alpha + Hydroxychloroquine | ➢ Recombinant interferon alpha: 15000–18000 IU per day➢ Hydroxychloroquine: 600 mg for the first day, 400 mg for the second day, then 200 mg per day for 5 days |
| Umifenovir + Hydroxychloroquine | ➢ Umifenovir: 200 mg per 6 h➢ Hydroxychloroquine: 600 mg for the first day, 400 mg for the second day, then 200 mg per day for 5 days |
| Recombinant interferon alpha + Mefloquine* | ➢ Recombinant interferon alpha: 15000–18000 ME per day➢ Mefloquine: 500 mg for the first and second days, then 250 mg for 5 days * |
| Umifenovir + Mefloquine* | ➢ Umifenovir: 200 mg per 6 h➢ Mefloquine: 500 mg for the first and second days, then 250 mg for 5 days |
| Recombinant interferon alpha + Umifenovir** | ➢ Recombinant interferon alpha: 15000–18000 ME per day➢ Umifenovir: 200 mg per 6 h for 5 days |
If hydroxychloroquine is not available
If the use of hydroxychloroquine and mefloquine has side effects
2.8. Turkey
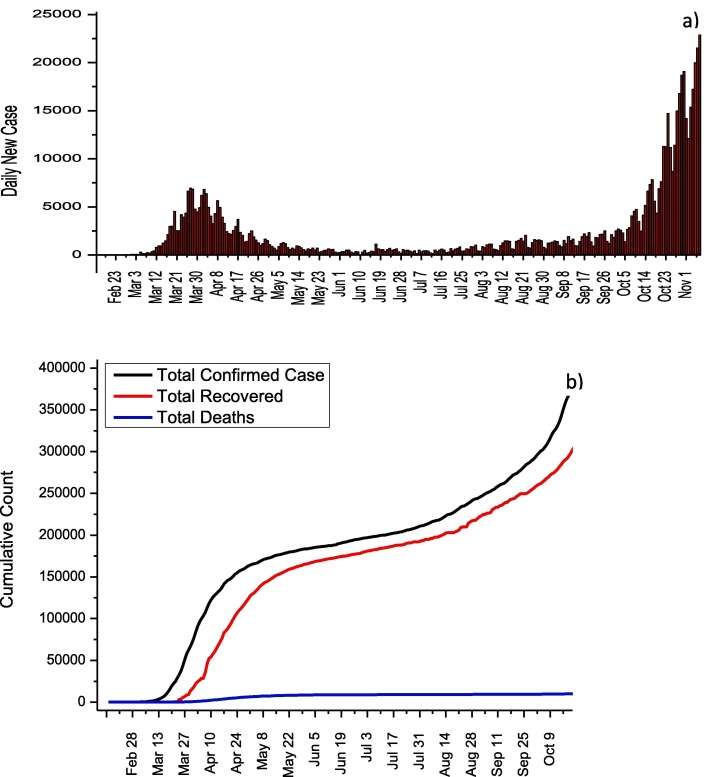
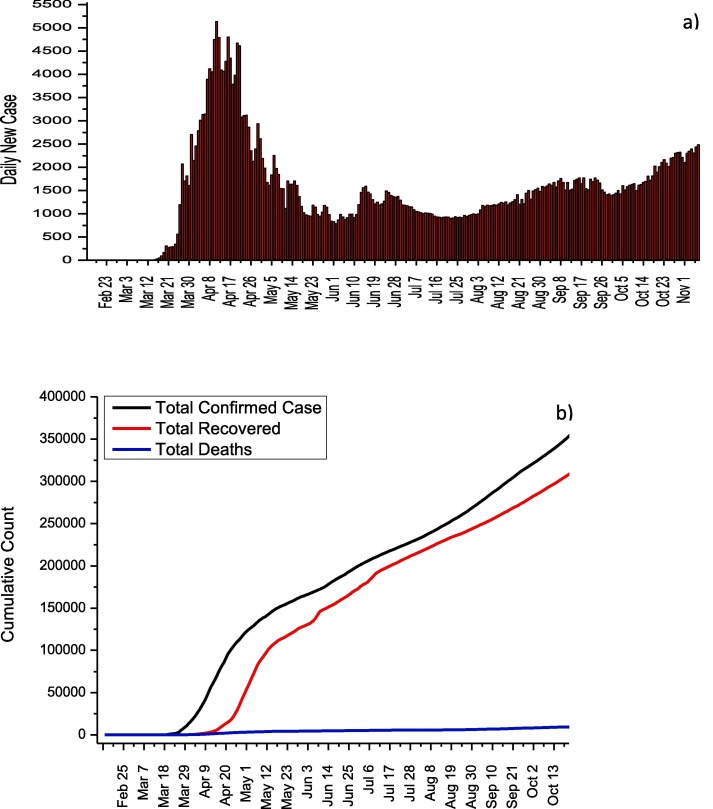
In Turkey, the first cases of COVID-19 were detected at March 11, 2020. But before this date, all flights with China, South Korea, and Italy were stopped, while land borders with Iran and Iraq were closed. Thanks to these measures, the first cases in Turkey have been identified almost one month later compared to other European and Asian countries. COVID-19 experiences of other countries were taken into account, and schools were closed on March 12 after the first confirmed case. Besides, bars, theaters, cinemas, gyms, and cafes were closed on March 16. On March 21, flight bans were extended for additional 46 countries while travel restrictions were imposed on all metropolitan cities on April 4. Curfew was ordered by Ministry of Interior for April 10–13, April 18–19, April 23–26, May 9–10, May 16–19 and May 23–26. The highest number of cases was reached on April 11 with 5,138 daily case, and this number dropped to 839 on 31 May in virtue of social isolation measures. As of May 6, it was announced by the Ministry of Health that normalization would begin, and these measures were gradually removed. As observed in other countries, the removal of the measures in Turkey has led to an increase in the number of day cases (Fig. 8 a). The effect of normalization on the total number of cases is clearly seen in Fig. 8b. As of 7 November, 391,719 total number of cases, 336,221 total recovered and 10,803 total deaths were reported while the mortality rate was calculated as 2.76. The Ministry of Health explains the relatively low mortality rate with the early initiation of antiviral medication. Table 8 summarizes the adult patient treatment protocol recommended by the Ministry of Health. Hydroxychloroquine and favirapir are used for adult patients who show mild, moderate or severe symptoms (Table 8) [31]. Lopinavir/Ritonavir is recommended for pregnant women.
Fig. 8.
a) Daily new cases and b) total COVID-19 statistics for Turkey.
Table 8.
COVID-19 treatment protocol recommended by the Turkish Ministry of Health.
| Treatment Protocols | ||
|---|---|---|
| For Mild/Moderate /Severe Semptons |
Hydroxychloroquine and/or Favirapir |
➢ 2x200 mg for 5 days |
| ➢ 2 × 1600 mg loading and 2 × 600 mg maintenance for 5 days | ||
| Treatment in pregnant women with definite diagnosis of COVID-19 | Lopinavir/Ritonavir | ➢ 200/50 mg every 12 h for 10–14 days |
2.9. China
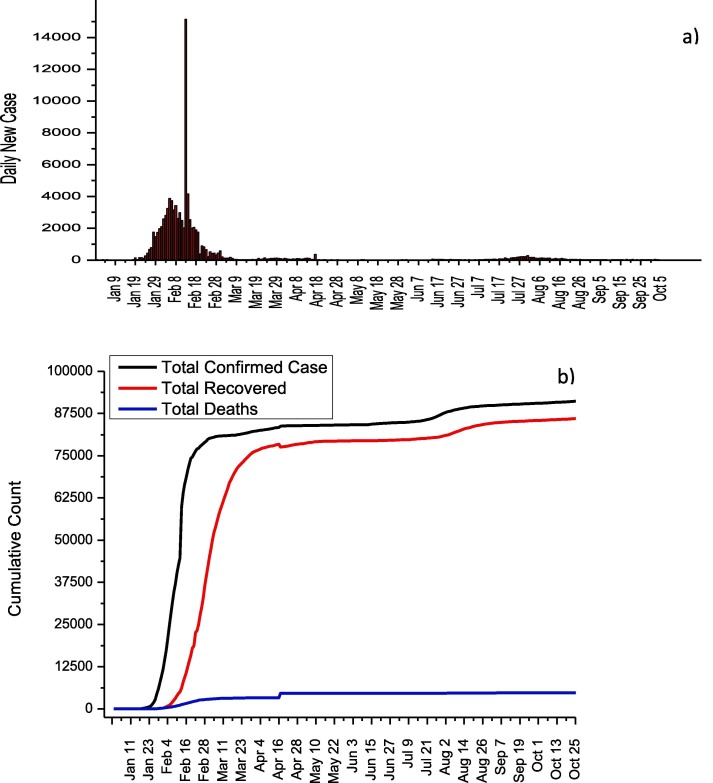
The first COVID-19 case emerged in Wuhan, China on December 1, 2019, and the first death in China was reported on January 10. The highest number of daily cases in China was reported as 15,141 on February 13. It has been announced that the total number of deaths, total recovery and total cases until November 7 are 4,741, 86,344 and 91,622, respectively (Fig. 9 ). The genome sequencing of SARS-CoV-2 was determined on January 10, and rapid tests were developed by the China CDC. On January 16, the national health commission announced that it has published the first diagnosis and treatment protocol for COVID-19. On January 23, travels to Wuhan were stopped and a disciplined quarantine was imposed. In addition, the activities of public transportation vehicles were stopped to reduce human mobility in Wuhan city. This quarantine has been expanded to include 15 cities in the Hubei region. Early application of diagnostic tests and isolation of even patients with mild symptoms in facilities such as stadiums are the most important factors for controlling the spread of COVID-19. The closure of schools and factories also played an vital role in reducing the number of cases. As a result of these measures, the number of daily cases was reduced to 90 on March 30. No second wave has been seen for COVID-19 in China and it is one of the most successful countries in terms of controlling the disease. Several antiviral drugs namely Chloroquine, Abidol, Interferon, Lopinavir/Ritonavir, and Ribavirin are recommended by China National Health Commission and their usage details are given in Table 9 [32].
Fig. 9.
a) Daily new cases and b) total COVID-19 statistics for China.
Table 9.
COVID-19 treatment protocol recommended by the China National Health Commission.
| Treatment Protocols | ||
|---|---|---|
| General Treatment |
Interferon alpha | ➢ 5 million U 2 times per day |
| Lopinavir/Ritonavir | ➢ 200/50 mg twice a day for maximum 10 days | |
| Ribavirin + Lopinavir/Ritonavir Or Ribavirin + Interferon | ➢ 500 mg, 2 or 3 times for maximum 10 days | |
| Chloroquine phosphate | ➢ 500 mg twice a day during 7 days for weigh over 50 kg | |
| Abidol | ➢ 200 mg for three times a day during maximum 10 days | |
*Usage of 3 or more antiviral drugs are not recommended at the same time
3. Drugs: Structures and biological properties
3.1. Hydroxychloroquine/chloroquine
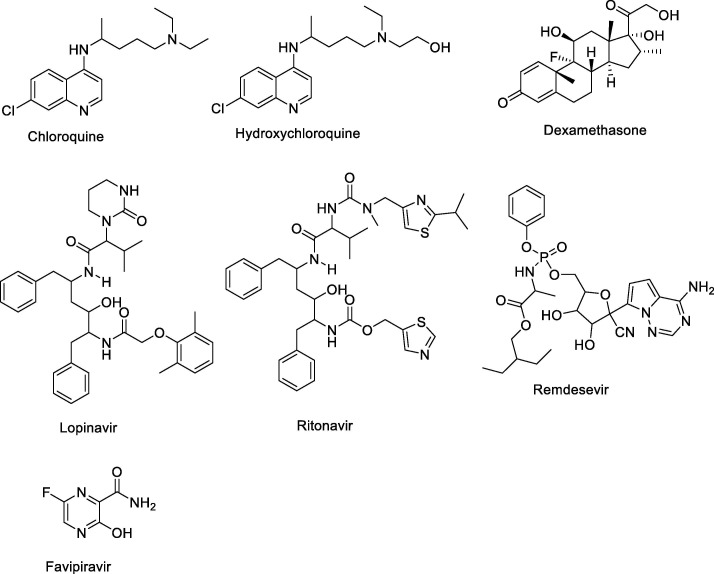
Chloroquine (alkylated 4-aminoquinolines) was synthesized in 1934, then it was used for the treatments of malaria in 1945 (Fig. 10 ) [33]. It is a weak bases due to nitrogene atoms on the structure. Before chloroquine, the natural quinine, isolated from cinchona tree bark, was known as an antimalarial agent. Then, hydroxychloroquine (HCQ) sulfate was synthesized and found as a new antimalarial drug with less toxicity and less blood-retinal barrier permeability in 1946. HCQ has one more hydroxyl groups on the structures than CQ, so it created novel and more affectivity with more polarity and lower lipophilic effect. In addition, intracellular pH levels in immune cells were increased by HCQ to prevent downstream immune cell interaction, antigen processing, and cytokine responses. It causes pH interferes with viral entry into endosomes and blocks viral-endosome fusion. After starting pandemia, HCQ and CQ were tested for the treatments of corona virus. It was found that hydroxychloroquine displayed high activity (EC50 = 0.72 μM) than chloroquine (EC50 = 5.47 μM) against SARS-CoV-2 [34]. Moreover, there were many in vitro and in vivo studies about HCQ and CQ for COVID-19 all over the worlds. Studies showed that HCQ could be critical roles in combination with other drugs, but it needs to be improved for clinical trials. HCQ and CQ display mild adverse effects included rash and headache.
Fig. 10.
The used drugs against COVID-19.
3.2. Lopinavir/Ritonavir
Ritonavir, known as antiretroviral, have been used as a protease inhibitors since 2000 [35], [36] (Fig. 10). The effects of protease inhibitors were described by the binding of lipoprotein particles or the blocking of their binding to the LDL receptor, which disrupts the mechanisms responsible for intracellular synthesis, storage, and release of cholesterol. It has been used for the treatment of HIV-1 infection and improved its potantial medicine for COVID-19 [37] (Fig. 11 ). The drug concentration may have critical roles for the treatments of diseases, so lopinavir is used with ritonavir. This combination creates more activity against COVID-19. On the other hand, there is a gastrointestinal effects in patients.
Fig. 11.
Chemical structures of anti-viral drugs used for the treatments of SARS-CoV-2.
3.3. Remdesevir
Remdesivir, developed by Gilead Sciences, is a member of antiviral drugs with broad-spectrum (Fig. 10). It was used for RNA virus infections treatments by acting adenosine nucleotide analogue [38]). Although, remdesevir has adverse effects including the elevation of hepatic enzymes, renal impairments, and hypotension, FDA was approved for the treatments of COVID-19 last days. Recent studies improved that remdesiver displayed high anti-viral activity at lower values of the EC50 (1.13 μM). Last clinical trials offer that remdesevir could be used for COVID-19 patients with low oxygen saturation and critical patients on ventilation [39]. The mortality rate significantly decreased from 11 to 7.1% when remdesevir was used. Antiviral activity against several coronaviruses including SARS-CoV and MERS-CoV, remdesivir was also reported via in vitro and in vivo studies. On the other hand, Remdesevir was reported some adverse effect such as; hepatic enzymes, diarrhea, rash, renal impairment, hypotension, organ-dysfunction syndrome, septic shock, acute kidney injury, and hypotension.
3.4. Dexamethasone (Corticosteroids)
Recently, Oxford University announced that Dexamethasone, corticosteroid, is the most effective drug for the treatments of COVID-19 [40] (Fig. 10). Two thousand patients saves life against the new type of corona virus in England. Dexamethasone prevents the cytokine storm in patients, which is the result of an excessive immune systems [41]. When the steroid drug dexamethasone was used against COVID-19, it reduced deaths by one-third in ventilator-dependent patients and by one-fifth in patients with less oxygen saturation. COVID-19 cause the severe systemic inflammatory response such as lung injury, ARDS, and multisystem organ dysfunction. Therefore, anti-inflammatory effects of corticosteroid therapy could be eliminating these viral complications. On the other hand, dexamethasone was used more than 14 days; it did not reduce the mortality [42]. Some case reports displayed that high dose of corticosteroids could be multi-organ dysfunction and a potentially increased risk of deaths. As a result, the therapy of dexamethasone is not clear for the treatments of SARS-CoV-2, but it may be applied with combination with other anti-viral drugs.
3.5. Favipiravir
Favipiravir (6-fluoro-3-hydroxy-2-pyrazinecarboxamide) was synthesized by Toyama Chemical Co., Ltd. in 2002 and approved in 2014 [43] (Fig. 10). It is an anti-viral drug that inhibits the RNA-dependent RNA polymerase of influenza virus. Favipiravir displays a broad range of influenza viruses, including A(H1N1), A(H5N1) and the recently emerged A(H7N9) avian virus [44]. After starting COVID-19, many countries used favipiravir against corona virus treatment. Some clinical applications show that high dose of favipiravir (1800–2400 mg of first day) could be the best way for the decreasing corona effects, followed by maintenance doses ranging from 300 mg to 1800 mg next days [45], [46]. Moreover, favipiravir have high activity first five days after getting corona virus (Fig. 11). Interestingly, it was first approved anti-covid drug in China. Nowadays, many countries improved its affectivity to prevent COVID-19.The side-effect of favipiravir is acceptable with asymptomatic hyperuricemia and reversible elevation in transaminases.
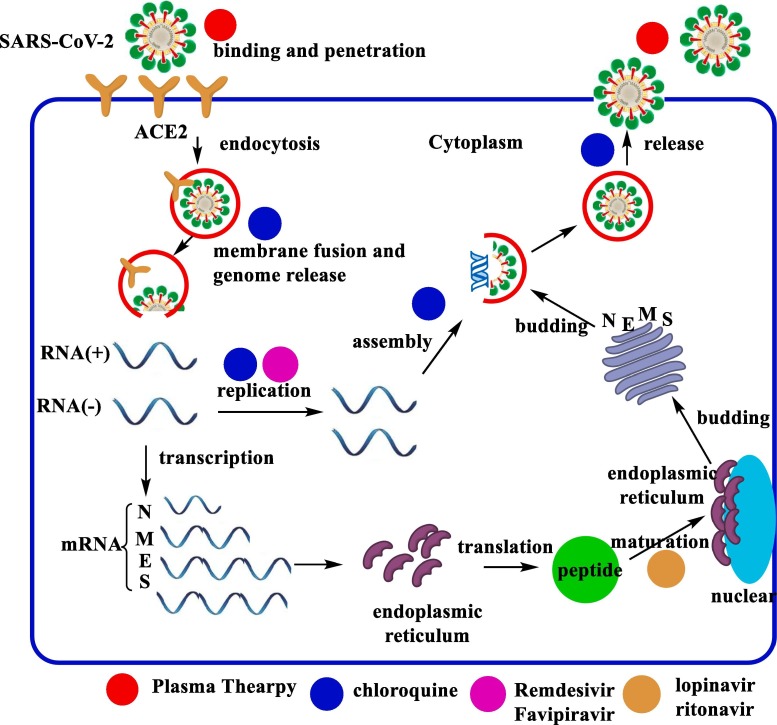
3.6. The mechanism of anti-Covid drugs
The proposed mechanism of actions of anti-COVID-19 drugs was shown in Fig. 12 . The treatments are depended on the life cycle of SARS-CoV-2 in host cell [47]. The detail mechanism could be summarized as;
Fig. 12.
The mechanism of actions of anti-Covid drugs and life cycle of SARS-CoV-2 in host cell.
3.6.1. Binding and penetration;
The human angiotensin-converting enzyme 2 (ACE2) is the main cell receptor for COVID-19, so SARS-CoV-2 entry into host cells by using ACE2 receptor on the cell membrane [48]. Recently researchers suggested that the S genetic code of the receptor binding spike protein plays critical roles to entry into the host cell. On the other hand, general functions including protein assembly, envelope generation, encasing the RNA, budding, and pathogenesis are controlled by the nucleocapsid (N), the small envelope (E) and membrane (M) proteins. When the antibodies against SARS-CoV-2, obtained by plasma cells, could be neutralize the virus to decrease its pathogenicity (Fig. 12) [49].
3.6.2. Genome release;
Thegenome of SARS-CoV-2 can be released after the process of membrane fusion. Hydroxychloroquine or chloroquine increase the pH of the intranuclear body, lysosome, and Golgi bod, genome replication, and assembly of mature viral particles [50]. The pH interferes with SARS-CoV-2 entry into endosomes and blocks viral-endosome fusion (Fig. 12).
3.6.3. Genome replication:
The positive (+)-sense genomic RNA forms the synthesisof negative (−)-sense RNA, followed by the template to give the RNA chain of progeny virus. Remdesivir and Favipiravir are improved that they can be integrated into the RNA chain of progeny SARS-CoV-2 as the substrate of the RNA-dependent RNA polymerase (RdRp). Remdesivir and Favipiravir inhibit the replication of viral genomes (Fig. 12) [51], [52], [53].
3.6.4. Protein biosynthesis;
The negative (−)-sense RNA is used as atemplate with mRNAs transcribed to direct the protein biosynthesis of SARS-CoV-2 in the cytoplasm. The enzyme 3-chymotrypsin-like protease (3CLpro) has a crucial role in processing this RNA.The 3CLpro is inactivated by Lopinavir/ritonavir (Fig. 12) [54].
3.6.5. Assembly;
The genomic RNA andvirion proteins are assembled to give the infective form of SARS-CoV-2 (Fig. 12).
3.6.6. Release;
Final step of the life cycle is releasing SARS-CoV-2 from the host cell via exocytosis (Fig. 12).
4. Conclusion
COVID-19 causes the 42 million case and 1.15 million deaths all over the world. The number of cases and deaths in many countries, especially Germany, France, and Italy, reached the highest levels of after the months. The highest number of cases was the USA with 7.5 million cases, is followed by India with 6.7 million cases and Brazil with 5 million cases. Interestingly, the number of new cases in Russia increases each day compared to the other countries.
Recent clinical reports displayed that the specific treatment methods for SARS-CoV-2 is not known. However, some antiviral drugs and agents, such as oseltamivir, favipiravir, umifenovir, lopinavir, remdesivir, hydroxychloroquine, chloroquine, azithromycin, ascorbic acid, corticosteroids, have been used for the treatment of SARS-CoV-2. Some of them reduce cytokine storm that is the main reason of mortality related to SARS-CoV-2. Moreover, they used as anti-inflammatory agents which prevent the lung injury and multisystem organ dysfunction. Many of these drugs are used in combination with anti-viral drugs to create synergistic effects, such as hydroxychloroquine and favipiravir; Lopinavir/Ritonavir and hydroxychloroquine etc. Many of them are more effective when used early stage of the SARS-CoV-2 infection. For example, favipiravir have the highest activity against SARS-CoV-2 in the first 5 days.
Today, there is no certain treatments ways for COVID-19. At the present study, we analyzed different countries from all over the world and compare the drugs for the treatments of COVID-19. Total case numbers and deaths from January 2020 to November 2020 were also shown. It was observed that second wave started, so new drugs will be investigated for long term therapy. As a result, all countries tried to find new vaccine against SARS-CoV-2, and they also tried to find new drug candidates to prevent from virus. Anti-viral drugs may be best candidates for the treatments of COVID-19 before finding novel anti-Covid drugs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The author (A. Kivrak) would like to acknowledge networking contribution by the COST Action CA17104 “New diagnostic and therapeutic tools against multidrug resistant tumours”.
References
- 1.Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A., Iosifidis C., Agha R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19) International Journal of Surgery. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedersen S.F., Ho Y.-C. SARS-CoV-2: a storm is raging. J. Clin. Investig. 2020;130(5) doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ronco C., Reis T. Kidney involvement in COVID-19 and rationale for extracorporeal therapies. Nat. Rev. Nephrol. 2020:1–3. doi: 10.1038/s41581-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y., Sun W., Li J., Chen L., Wang Y., Zhang L., et al. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. MedRxiv. 2020 [Google Scholar]
- 5.Rotondi V, Andriano L, Dowd JB, Mills MC. Early evidence that social distancing and public health interventions flatten the COVID-19 curve in Italy. 2020.
- 6.Bernucci C., Brembilla C., Veiceschi P. Effects of the COVID-19 Outbreak in Northern Italy: Perspectives from the Bergamo Neurosurgery Department. World Neurosurgery. 2020;137 doi: 10.1016/j.wneu.2020.03.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Italian GovernmentPresidency of the Council of Ministers. Coronavirus, le misure adottate dal Governo. 2020.
- 8.Italian Society of Infectious and Tropical Diseases, Handbook for the care of people with disease-COVI 19, Edition 2.0, March 13, 2020, https://www.simit.org/news/11-vademecum-per-la-cura-delle-persone-con-malattia-da-covid-19.
- 9.Sisó-Almirall A., Kostov B., Mas-Heredia M., Vilanova-Rotllan S., Sequeira-Aymar E., Sans-Corrales M., et al. Prognostic factors in Spanish COVID-19 patients: A case series from Barcelona. PLoS ONE. 2020;15(8) doi: 10.1371/journal.pone.0237960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ministerio de Sanidad, Comparecencia Sobre Las Actuaciones Desarrolladas En Relación Con El Coronavirus (COVID-19), 2020, https://www.mscbs.gob.es/gabinete/notasPrensa.do?metodo=detalle&id=4825.
- 11.Spanish Agency for Medicines and Health Products, Tratamientos disponibles sujetos a condiciones especiales de acceso para el manejo de la infección respiratoria por SARS-CoV-2, 2020, https://www.aemps.gob.es/la-aemps/ultima-informacion-de-la-aemps-acerca-del-covid%E2%80%9119/tratamientos-disponibles-para-el-manejo-de-la-infeccion-respiratoria-por-sars-cov-2/?lang=en.
- 12.U.S. National Institutes of Health, Characteristics of Potential Antiviral Agents Under Evaluation for Treatment of COVID-19, 2020, https://www.covid19treatmentguidelines.nih.gov/tables/table-2b/.
- 13.Marson F.A.L., Ortega M.M. COVID-19 in Brazil. Pulmonology. 2020;26(4):241–244. doi: 10.1016/j.pulmoe.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Morales A.J., Gallego V., Escalera-Antezana J.P., Méndez C.A., Zambrano L.I., Franco-Paredes C., et al. COVID-19 in Latin America: The implications of the first confirmed case in Brazil. Travel Med. Infect. Dis. 2020;35 doi: 10.1016/j.tmaid.2020.101613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Croda JHR, Garcia LP. Resposta imediata da Vigilância em Saúde à epidemia da COVID-19. SciELO Public Health; 2020. [DOI] [PubMed]
- 16.Garcia LP, Duarte E. Intervenções não farmacológicas para o enfrentamento à epidemia da COVID-19 no Brasil. SciELO Public Health; 2020. [DOI] [PubMed]
- 17.Ministério Da Saúde, Orientações Do Ministério Da Saúde Para Manuseio Medicamentoso Precoce De Pacientes Com Diagnóstico Da COVID-19, 2020, https://saude.gov.br/images/pdf/2020/August/12/COVID-11ago2020-17h16.pdf.
- 18.Böhmer M.M., Buchholz U., Corman V.M., Hoch M., Katz K., Marosevic D.V., et al. Investigation of a COVID-19 outbreak in Germany resulting from a single travel-associated primary case: a case series. Lancet. Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stafford N. Covid-19: Why Germany’s case fatality rate seems so low. BMJ. 2020;369 doi: 10.1136/bmj.m1395. [DOI] [PubMed] [Google Scholar]
- 20.Koch-Institut Robert. Ständiger Arbeitskreis der Kompetenz- und Behandlungszentren für Krankheiten durch hochpathogene Erreger, Hinweise zu Erkennung. Diagnostik und Therapie von Patienten mit COVID-19. 2020 [Google Scholar]
- 21.Shim E., Tariq A., Choi W., Lee Y., Chowell G. Transmission potential and severity of COVID-19 in South Korea. International Journal of Infectious Diseases. 2020 doi: 10.1016/j.ijid.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shim E., Tariq A., Choi W., Lee Y., Chowell G. Transmission potential and severity of COVID-19 in South Korea. International Journal of Infectious Diseases. 2020;93:339–344. doi: 10.1016/j.ijid.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim C. As Covid-19 cases rise, South Korea raises virus threat level to its maximum. Vox Vox2020 2020.
- 24.Singh A.K., Singh A., Shaikh A., Singh R., Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: A systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab Syndr. 2020;14(3):241–246. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Republic of Korea, Ministry of Food and Drug Safety, Korea Centers for Disease Control and Prevention, Special import approved for COVID-19 treatment, 2020, https://www.mfds.go.kr/eng/brd/m_64/view.do?seq=30&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1.
- 26.Reshetnikov V, Mitrokhin O, Shepetovskaya N, Belova E, Jakovljevic M. Organizational measures aiming to combat COVID-19 in the Russian Federation: the first experience. Expert Review of Pharmacoeconomics & Outcomes Research 2020:null-null. [DOI] [PubMed]
- 27.Anna Shashina M.N. COVID-19: Guidance for Employers in Russia. Bird & Bird. 2020 [Google Scholar]
- 28.Åslund A. Responses to the COVID-19 crisis in Russia, Ukraine, and Belarus. Eurasian Geogr. Econ. 2020:1–14. [Google Scholar]
- 29.Krasilnikov S. Coronavirus in Russia: The Latest News. The Moscow Times2020.
- 30.Ministry of Health of Russia, Bpeмeнныe Meтoдичecкиe Peкoмeндaции, 2020, https://static-0.rosminzdrav.ru/system/attachments/attaches/000/050/033/original/RESP_REC_V2.pdf.
- 31.T.C. Sağlık Bakanlığı Halk Sağlığı Genel Müdürlüğü, COVID-19 (SARS-CoV-2 Enfeksiyonu) Erişkin Hasta Tedavisi, 2020, https://covid19.saglik.gov.tr/Eklenti/38355/0/covid-19rehberieriskinhastatedavisipdf.pdf.
- 32.Chinese National Health Commission, Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th edition). 2020.
- 33.Ridley R.G. Medical need, scientific opportunity and the drive for antimalarial drugs. Nature. 2002;415(6872):686–693. doi: 10.1038/415686a. [DOI] [PubMed] [Google Scholar]
- 34.Gautret P., Lagier J.C., Parola P., Hoang V., Meddeb L., Mailhe M., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56(1) doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Thompson M.A., Aberg J.A., Cahn P., Montaner J.S.G., Rizzardini G., Telenti A., et al. Antiretroviral Treatment of Adult HIV Infection 2010 Recommendations of the International AIDS Society-USA Panel. Jama-Journal of the American Medical Association. 2010;304(3):321–333. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 36.Riddler S.A., Haubrich R., DiRienzo A.G., Peeples L., Powderly W.G., Klingman K.L., et al. Class-sparing regimens for initial treatment of HIV-1 infection. N. Engl. J. Med. 2008;358(20):2095–2106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao B., Wang Y., Wen D., Liu W., Wang J.L., Fan G., et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis J.S., Ferreira D., Denholm J.T., Tong S.Y.C. Clinical trials for the prevention and treatment ofCOVID-19: current state of play. Med. J. Aust. 2020;213(2):86–93. doi: 10.5694/mja2.50673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Javorac D., Grahovac L., Manic L., Stojilkovic N., Andelkovic M., Bulat Z., et al. An overview of the safety assessment of medicines currently used in the COVID-19 disease treatment. Food Chem. Toxicol. 2020;144 doi: 10.1016/j.fct.2020.111639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen MP, George M, Gilroy D, Sofat R. Beyond dexamethasone, emerging immuno-thrombotic therapies for COVID-19. British Journal of Clinical Pharmacology. [DOI] [PubMed]
- 41.Doyle L.W., Bowman E., Callanan C., Carse E., Casalasz D., Charlton M.P., et al. Postnatal corticosteroids and sensorineural outcome at 5 years of age. J. Paediatr. Child Health. 2000;36(3):256–261. doi: 10.1046/j.1440-1754.2000.00493.x. [DOI] [PubMed] [Google Scholar]
- 42.Voto C., Berkner P., Brenner C. Overview of the Pathogenesis and Treatment of SARS-CoV-2 for Clinicians. A Comprehensive Literature Review. Cureus. 2020;12(9) doi: 10.7759/cureus.10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ajeet, Aggarwal B, Verma SK. Favipiravir May Acts as COVID-19 Main Protease PDB ID 6LU7 Inhibitor: Docking Analysis. Biointerface Research in Applied Chemistry 2020; 10 (6):6821-6828.
- 44.Arouche T.D., Reis A.F., Martins A.Y., Costa J.F.S., Carvalho R.N., Neto A. Interactions Between Remdesivir, Ribavirin, Favipiravir, Galidesivir, Hydroxychloroquine and Chloroquine with Fragment Molecular of the COVID-19 Main Protease with Inhibitor N3 Complex (PDB ID:6LU7) Using Molecular Docking. J. Nanosci. Nanotechnol. 2020;20(12):7311–7323. doi: 10.1166/jnn.2020.18955. [DOI] [PubMed] [Google Scholar]
- 45.Dauby N, Van Praet S, Vanhomwegen C, Veliziotis I, Konopnicki D, Roman A. Tolerability of favipiravir therapy in critically ill patients with COVID-19: A report of four cases. Journal of Medical Virology. [DOI] [PubMed]
- 46.Nittari G., Pallotta G., Amenta F., Tayebati S.K. Current pharmacological treatments for SARS-COV-2: A narrative review. Eur. J. Pharmacol. 2020 doi: 10.1016/j.ejphar.2020.173328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El Kantar S., Nehmeh B., Saad P., Mitri G., Estephan C., Mroueh M., et al. Derivatization and combination therapy of current COVID-19 therapeutic agents: a review of mechanistic pathways, adverse effects, and binding sites. Drug Discovery Today. 2020;25(10):1822–1838. doi: 10.1016/j.drudis.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang L., Chen Y., Xiao J., Luo W., Li F., Wang Y., et al. Progress in the Research and Development of Anti-COVID-19 Drugs. Frontiers. Public Health. 2020 doi: 10.3389/fpubh.2020.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng M., Song L. Novel antibody epitopes dominate the antigenicity of spike glycoprotein in SARS-CoV-2 compared to SARS-CoV. Cell. Mol. Immunol. 2020;17(5):536–538. doi: 10.1038/s41423-020-0385-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rolain J.M., Colson P., Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int. J. Antimicrob. Agents. 2007;30(4):297–308. doi: 10.1016/j.ijantimicag.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016 doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang M.L., Cao R.Y., Zhang L.K., Yang X.L., Liu J., Xu M.Y., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldhill D.H., te Velthuis A.J.W., Fletcher R.A., Langat P., Zambon M., Lackenby A., et al. The mechanism of resistance to favipiravir in influenza. PNAS. 2018;115(45):11613–11618. doi: 10.1073/pnas.1811345115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nukoolkarn V., Lee V.S., Malaisree M., Aruksakulwong O., Hannongbua S. Molecular dynamic simulations analysis of ritronavir and lopinavir as SARS-CoV 3CL(pro) inhibitors. J. Theor. Biol. 2008;254(4):861–867. doi: 10.1016/j.jtbi.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]