Abstract
Background:
Soft and hard artisanal cheeses are regularly consumed in Egypt. These products are usually processed from raw milk which may harbor many pathogenic and spoilage microorganisms.
Aim:
To evaluate the safety of some artisanal cheeses in Egypt, such as Ras, Domiati, and Mish, through chemical and microbiological examination.
Methods:
One hundred and fifty random samples of traditional Ras, Domiati, and Mish cheeses (50 each) were microbiologically and chemically analyzed. Counts of total bacteria, presumptive coliform, staphylococci, yeast, and mold were estimated. Furthermore, isolation of Escherichia coli and Staphylococcus aureus was performed, followed by PCR confirmation; isolates of E. coli were examined for the presence of virulence genes; on the other hand, the detection of the five classical enterotoxin genes of S. aureus was performed using multiplex PCR. Regarding chemical analysis, moisture, salt, and acidity content were measured. Correlations between chemical and microbial findings were investigated.
Results:
Mean counts of total bacteria, presumptive coliform, staphylococci, yeast, and mold were (2 × 108, 3 × 106 and 1 × 107 ), (3 × 105, 5 × 10 and 5 × 102), (1 × 106, 4 × 105and 1 × 105), (3 × 105, 1 × 105 and 5 × 105), and (7 × 103, 4 × 103 and 3 × 104) for Ras, Domiati and Mish cheeses, respectively. Serological identification of suspected E. coli revealed that E. coli O125 was isolated from Ras and Domiati samples, E. coli O18 was recovered from Ras samples, while E. coli O114 was isolated from Mish samples. PCR results revealed that all detected isolates of E. coli were positive for both iss (increased serum survival) and fimH (type 1 fimbriae) genes. Concerning isolated S. aureus, all examined products were harboring S. aureus enterotoxigenic strains, with seb and sed genes being the most common. The mean values of moisture, salt, and acidity were (30.03, 56.44, and 58.70), (3.30, 6.63, and 7.56) and (0.65, 0.68, and 0.50) for Ras, Domiati, and Mish cheeses, respectively.
Conclusion:
Enterotoxigenic S. aureus harboring seb gene and enteropathogenic E. coli (serogroups O18, O114, and O125) were frequently isolated from soft and hard artisanal cheeses in Egypt. Therefore, strict hygienic measures should be applied during their manufacture, handing, and distribution.
Keywords: Domiati, E. coli virulence genes, Mish, Ras, S. aureus enterotoxins
Introduction
Traditional Egyptian dairy products are an essential part of the daily diet in Egypt. Raw milk is the common factor in making all traditional products, which usually record high sensorial characteristics, as consumers mostly choose their dairy products based on its sensory properties, regardless if it is made from raw or pasteurized milk (Awad, 2006; El-Ghaish et al., 2010; Ewida and Hussein, 2019). Some traditional products have been traced back to the pharaoh era of 4,000 BC, and is then handed down from one generation to another. There are different types of traditional cheese in Egypt and among them are Ras, Domiati, and Mish (Benkerroum, 2012).
Ras cheese is a hard cheese commonly known in Egypt as “Romi”. It is one of the artisan type cheeses, which is produced often in small factories located in rural areas. It is mainly made from raw milk, either cow’s or a mixture of cow and buffalo milk, mostly without adding a starter culture, and fermentation and ripening occur only due to the native flora of raw milk, then it is kept to ripen for 3–8 months till a sharp flavor is acquired, similar to the Greek Kefalotyri cheese (Awad, 2006; El-Fadaly et al., 2015).
Domiati or Damietta cheese (Gebnah Domiata) is one of the most popular white, soft pickled cheeses. It is named after the Egyptian seaport city “Dumyât”. Typically, it is salty, unlike other pickled cheeses, as the salt is added directly to the raw milk at the proportion of 5%–14%, which varies according to season and ripening conditions (Robinson and Tamime, 1991).
Mish is one of the oldest indigenous milk products in Egypt that has a sharp harsh flavor with a relatively higher salt content and is mostly yellowish brown color. Karish cheese is considered as the basic raw material for such a product. Some other ingredients that may be included in Mish are sour milk, buttermilk, morta (a byproduct of ghee), different types of pepper (green, red, and paprika), table salt, and a portion of an old Mish which acts as the starter culture. All ingredients are brined under microaerophilic conditions mostly in earthenware containers for more than 1 year to obtain matured mish, which consists of Mish cheese and Mish slurry (pickling medium), and both are consumed (Tamime, 2006).
Raw milk used in the production of traditional dairy products and harbor a large number of native microflora that may participate in the development of a desirable aroma and an appropriate flavor, but it may also contain pathogenic microorganisms such as Escherichia coli, Staphylococcus aureus, and others that are considered as public health hazards, causing a serious food illness and may produce lethal toxins in the food (Awad et al., 2003; El-Ghaish et al., 2010). Moreover, undesirable spoilage organisms may also be found that cause defects and limits the shelf life of these products, rendering them unmarketable. Many other factors may cause an increase in the microbial load of traditional products, such as manufacture, handling, storage, and even marketing ways, which are still primitive and unhygienic (Osman et al., 2011).
The current study was conducted to assess the safety of some traditional cheeses in Egypt, such as Ras, Domiati, and Mish, through chemical and microbiological examination.
Materials and Methods
Samples collection
One hundred and fifty random samples of Ras, Domiati, and Mish cheeses (50 each) were collected from different Egyptian Governorates. The samples were identified, placed in an ice box, and examined immediately.
Chemical analysis
Moisture and acidity were analyzed according to the AOAC guidelines (Association of Official Analytical Chemists) (2000) and salt according to the APHA guidelines (American Public Health Association) (2004).
Microbiological examination
Preparation of food homogenate and decimal dilutions, total bacterial count (TBC; CFU/g), presumptive coliform count (MPN/g), and isolation of E. coli were carried out according to the APHA guidelines (American Public Health Association) (2004).
Biochemical identification of E. coli (microscopic examination, oxidase, urea, IMVC, TSI, L-Lysin decarboxylase, carbohydrate fermentation tests, and confirmed tests for thermotolerant (fecal) coliforms) was carried out according to Silva et al.’s (2019) description.
Serological identification of E. coli was done using DENKA SEIKEN CO., LTD. kits. The molecular characterization, and virulence genes detection was performed using PCR, where DNA was extracted from the bacterial isolates using QIAamp® DNA Mini Kit (Catalogue no.51304) according to the instruction manual. PCR was done for characterization of 5 genes of E. coli using Emerald Amp GT PCR master mix, Takara (Catalogue No. RR310A), using 5 different pair of primers (as uniplex PCR) as mentioned in Table 1. The PCR products were electrophoresed in 1.5% agarose according to that described in Sambrook et al.’s (1989) study.
Table 1. Oligonucleotide primers’ sequences and PCR conditions.
| Target agent | Length of amplified product | Primer sequence (5’-3’) | Gene | Reference |
|---|---|---|---|---|
| S. aureus | 102 bp | GGTTATCAATGTGCGGGTGG | sea | Mehrotra et al., 2000x |
| CGGCACTTTTTTCTCTTCGG | ||||
| 278 bp | CCAATAATAGGAGAAAATAAAAG | sed | ||
| ATTGGTATTTTTTTTCGTTC | ||||
| 209 bp | AGGTTTTTTCACAGGTCATCC | see | ||
| CTTTTTTTTCTTCGGTCAATC | ||||
| 164 bp | GTATGGTGGTGTAACTGAGC | seb | ||
| CCAAATAGTGACGAGTTAGG | ||||
| 451 bp | AGATGAAGTAGTTGATGTGTATGG | sec | ||
| CACACTTTTAGAATCAACCG | ||||
| Primary denaturation: 94°C/5 minutes; secondary denaturation: 94°C/30 seconds; annealing: 57°C/40 seconds; extension: 72°C/45 seconds; No. of cycles: 35; final extension: 72°C/10 minutes. | ||||
| 1250 bp | AC GGAGTTACAAAGGACGAC | 23S rRNA | Straub et al., 1999 | |
| AGCTCAGCCTTAACGAGTAC | ||||
| Primary denaturation: 94°C/5 minutes; secondary denaturation: 94°C/30 seconds; annealing: 55°C/40 seconds; extension: 72°C/1.2 minutes.; No. of cycles: 35; final extension: 72°C/12 minutes. | ||||
| E. coli | 266 bp | ATGTTATTTTCTGCCGCTCTG | Iss | Yaguchi et al., 2007 |
| CTATTGTGAGCAATATACCC | ||||
| Primary denaturation: 94°C/5 minutes; secondary denaturation: 94°C/30 seconds; annealing: 54°C/30 seconds; extension: 72°C/30 seconds; No. of cycles: 35; final extension: 72°C/7 minutes. | ||||
| 614 bp | ACACTGGATGATCTCAGTGG | Stx1 | Dipineto et al., 2006 | |
| CTGAATCCCCCTCCATTATG | ||||
| Primary denaturation: 94°C/5 minutes; secondary denaturation: 94°C/30 seconds; annealing: 58°C/40 seconds; extension: 72°C/45 seconds; No. of cycles: 35; final extension: 72°C/10 minutes. | ||||
| 508 bp | TGCAGAACGGATAAGCCGTGG | fimH | Ghanbarpour and Salehi, 2010 | |
| GCAGTCACCTGCCCTCCGGTA | ||||
| Primary denaturation: 94°C/5 minutes; secondary denaturation: 94°C/30 seconds; annealing: 50°C/40 seconds; extension: 72°C/45 seconds; No. of cycles: 35; final extension: 72°C/10 minutes. | ||||
| 620 bp | GGT GGT GCA CTG GAG TGG | Tsh | Delicato et al., 2003 | |
| AGT CCA GCG TGA TAG TGG | ||||
| Primary denaturation: 94°C/5 minutes; secondary denaturation: 94°C/30 seconds; annealing: 54°C/40 seconds; extension: 72°C/45 seconds; No. of cycles: 35; final extension: 72°C/10 minutes. | ||||
| 720 bp | CGATTCTGGAAATGGCAAAAG | phoA | Hu et al., 2011 | |
| CGTGATCAGCGGTGACTATGAC | ||||
| Primary denaturation: 94°C/5 minutes; secondary denaturation: 94°C/30 seconds; annealing: 55°C/40 seconds; extension: 72°C/45 seconds; No. of cycles: 35; final extension: 72°C/10 minutes. | ||||
Staphylococci count (CFU/g) and S. aureus isolation were carried out according to the APHA guidelines (American Public Health Association) (2004).
Identification of suspected S. aureus strains (microscopic examination, catalase activity test, coagulase test, thermostable nuclease, and anaerobic utilization of mannitol) was carried out according to that described in Silva et al.’s (2019) study. Molecular characterization of S. aureus strains and enterotoxin genes detection was done as in E. coli. Six genes of S. aureus (5 enterotoxins done by multiplex PCR and 23S rRNA) and six pairs (5 as multiplex and 1 pair as uniplex PCR) were used (Table 1).
Yeast and mold counts (CFU/g) were conducted according to APHA’s guidelines (American Public Health Association) (2004).
Statistical analysis
The collected data were analyzed using Statistical Package for the Social Sciences Statistics 17 for Windows.
Ethical approval
In this study, no experimental live animals were used. All cheese samples were collected directly from the Egyptian local markets.
Results
Chemical analysis
The chemical analysis of the examined samples, including moisture, salt, and acidity, is illustrated in Table 2; for Ras cheese samples, the mean values were 30.03, 3.30, and 0.65, respectively. The mean values of Domiati cheese samples were 56.44, 6.63, and 0.68, respectively, and were 58.70, 7.56, and 0.50, respectively, for Mish samples.
Table 2. Chemical analysis of examined samples (n = 50 each).
| Samples | Moisture % | Salt % | Acidity % | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Mean ± SE | Min. | Max. | Mean ± SE | Min. | Max. | Mean ± SE | |
| Ras cheese | 22.35 | 39.96 | 30.03 ± 0.59 | 2.00 | 4.40 | 3.30 ± 0.10 | 0.36 | 0.97 | 0.65 ± 0.02 |
| Domiati cheese | 48.92 | 62.92 | 56.44 ± 0.50 | 3.51 | 8.78 | 6.63 ± 0.20 | 0.36 | 2.16 | 0.68 ± 0.04 |
| Mish | 43.67 | 69.38 | 58.70 ± 0.97 | 3.51 | 9.33 | 7.56 ± 0.29 | 0.14 | 1.44 | 0.50 ± 0.05 |
Microbiological examination
As reported in Table 3, the TBCs of Ras, Domiati, and Mish samples were 2 × 108, 3 × 106, and 1 × 107, respectively, while coliforms were determined in 44%, 48%, and 92% of that examined in Ras, Domiati, and Mish samples, with a mean values of 3 × 105, 5 × 10, and 5 × 102, respectively. The examined samples were positive for E. coli in 8% of Ras cheese samples and in 4% of both Domiati and Mish samples, respectively, as shown in Table 5. The serological identification of the suspected isolated E. coli using slide agglutination test (Table 5) revealed that E. coli O125 was isolated from Ras and Domiati cheese samples; E. coli O18 was isolated from Ras samples, while E. coli O114 was detected in Mish samples. The molecular characterization of E. coli showed that all E. coli strains isolated from the present study were positive for phoA gene (Fig. 1). All isolates of E. coli lacked both stx1 and tsh genes; on the other hand, all detected isolates were positive for both iss and fimH genes (Figs. 2–5). Staphylococci were found in 92%, 88%, and 100% of Ras, Domiati, and Mish samples with mean values of 1 × 106, 4 × 105, and 1 × 105, respectively (Table 3). The incidences of S. aureus in the examined Ras, Domiati, and Mish samples based on biochemical identification were 26%, 36%, and 18%, as presented in Table 6. The number of molecularly identified S. aureus isolates by using 23S rRNA for the examined Ras, Domiati, and Mish samples was 2, 13, and 6, respectively (Fig. 6). The detection of the five classical enterotoxin genes of S. aureus revealed that the two strains identified from Ras cheese were enterotoxigenic strains having seb and sed genes; in Domiati samples, 10 strains were enterotoxigenic strains harboring seb, sed, and see genes, while the 6 strains from Mish samples were enterotoxigenic strains harboring seb gene (Fig. 7). The mean values of yeast / mold counts of the examined Ras, Domiati and Mish samples were 3 × 105/7 × 103, 1 × 105/ 4 × 103, and 5 × 105/3 × 104 as reported in Table 3.
Table 3. Microbiological evaluation of examined samples (CFU/g) (n = 50 each).
| Ras cheese | Domiati cheese | Mish | ||
|---|---|---|---|---|
| Total bacterial count | No. (%) of positive samples | 50 (100%) | 50 (100%) | 50 (100%) |
| Min. | 5 × 105 | 7 × 104 | 7 × 103 | |
| Max. | 1 × 109 | 1 × 107 | 1 × 108 | |
| Mean ± SE | 2 × 108 ± 0.5 × 108 | 3 × 106 ± 0.4 × 106 | 1 × 107 ± 0.2 × 107 | |
| Coliforms count (MPN/g) | No. (%) of positive samples | 22 (44%) | 24 (48%) | 46 (92%) |
| Min. | 2 × 104 | 4 × 10 | 2 × 10 | |
| Max. | 2 × 106 | 2 × 102 | 2 × 103 | |
| Mean ± SE | 3 × 105 ± 1 × 105 | 5 × 10 ± 0.1 × 10 | 5 × 102 ± 0.8 × 102 | |
| Staphylococcus count | No. (%) of positive samples | 46 (92%) | 44 (88%) | 50 (100%) |
| Min. | 1 × 104 | 1 × 104 | 7 × 102 | |
| Max. | 6 × 106 | 2 × 106 | 9 × 105 | |
| Mean ± SE | 1 × 106 ± 0.2 × 106 | 4 × 105 ± 0.7×105 | 1 × 105 ± 0.2 × 105 | |
| Yeast count | No. (%) of positive samples | 45 (90%) | 50 (100%) | 46 (92%) |
| Min. | 1 × 104 | 3 × 103 | 4 × 103 | |
| Max. | 1 × 106 | 8 × 105 | 1 × 106 | |
| Mean ± SE | 3 × 105 ± 0.6 × 105 | 1 × 105 ± 0.2 × 105 | 5 × 105 ± 0.6 × 105 | |
| Mold count | No. (%) of positive samples | 25 (50%) | 22 (44%) | 36 (72%) |
| Min. | 2 × 103 | 1 × 103 | 3 × 102 | |
| Max. | 2 × 104 | 2 × 104 | 1 × 105 | |
| Mean ± SE | 7 × 103 ± 0.9 × 103 | 4 × 103 ± 0.9 × 103 | 3 × 104 ± 0.7 × 104 | |
Table 5. Biochemical, serological, molecular identification, and incidence of virulence genes of E. coli isolated from examined samples using PCR.
| Types of samples | Samples containing E. coli strains identified biochemically | Strains identified serologically | Strains identified by phoA gene | Strains harbor Stx1 gene | Strains harbor Tsh gene | Strains harbor Iss gene | Strains harbor fimH gene | |||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | serotype | |||||||
| No. | type | No. | No. | No. | No. | No. | ||||
| Ras cheese | 4 | 8 | 4 | 3 | O125 | 4 | Nil | Nil | 4 | 4 |
| 1 | O18 | |||||||||
| Domiati cheese | 2 | 4 | 2 | 2 | O125 | 2 | 2 | 2 | ||
| Mish | 2 | 4 | 2 | 2 | O114 | 2 | 2 | 2 | ||
Fig. 1. Agarose gel electrophoresis of the PCR product of phoA gene for DNA extracted from analyzed E. coil isolates. Lane L: ladder; Lane P: positive control. Lanes 1–8: showing positive E. coil strains at 720 bp.
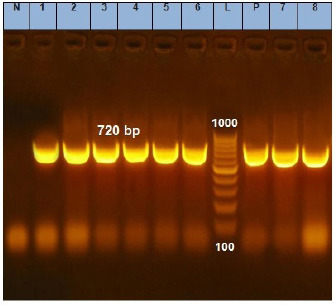
Fig. 2. Agarose gel electrophoresis of E. coil stx1 virulence gene. Lane L: ladder; Lane P: positive control. Lanes 1–8: showing negative strains at 614 bp.
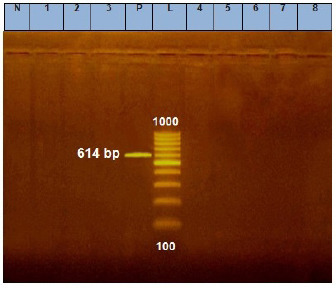
Fig. 5. Agarose gel electrophoresis of E. coil fimH virulence gene. Lane L: ladder; Lane P: positive control. Lanes 1–8: showing positive strains at 508 bp.
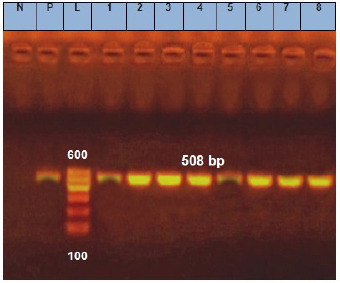
Table 6. Biochemical, molecular identification, and incidence of enterotoxin genes of S. aureus isolated from examined samples using multiplex PCR.
| Types of samples | Samples harbor coagulase positive S. aureus | Strains identified by 23S rRNA | Samples harbor enterotoxigenic strains | Type of SEs | |||||
|---|---|---|---|---|---|---|---|---|---|
| sea | seb | sec | sed | see | |||||
| No. | % | No. | No. | No. | No. | No. | No. | No. | |
| Ras cheese | 13 | 26 | 2 | 2 | Nil | 2 | Nil | 1 | Nil |
| Domiati cheese | 18 | 36 | 13 | 10 | 10 | 3 | 1 | ||
| Mish | 9 | 18 | 6 | 6 | 6 | Nil | Nil | ||
Fig. 6. Agarose gel electrophoresis of the PCR product of 23S rRNA gene for DNA extracted from analyzed S. aureus isolates. Lane L: ladder; Lane P: positive control. Lanes 1–21: showing positive S. aureus strains at 1,250 bp.
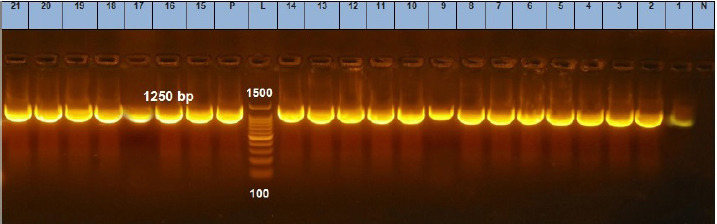
Fig. 7. Agarose gel electrophoresis showing multiplex PCR amplification products for S. aureus enterotoxin genes. Lanes L, ladder lane P: positive control. Staphylococcal enterotoxin A (sea) positive isolates at 102 bp. Staphylococcal enterotoxin D (sed) positive isolates at 278 bp. Staphylococcal enterotoxin E (see) positive isolates at 209 bp. Staphylococcal enterotoxin B (seb) positive isolates at 164 bp. Staphylococcal enterotoxin C (sec) positive isolates at 451 bp.
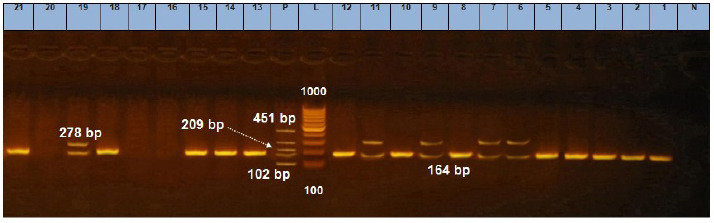
Fig. 3. Agarose gel electrophoresis of E. coil tsh virulence gene. Lane L: ladder; Lane P: positive control. Lanes 1–8: showing negative strains at 620 bp.
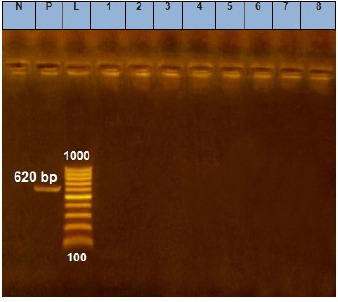
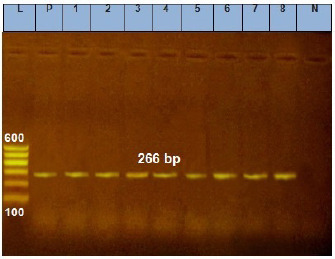
Fig. 4. Agarose gel electrophoresis of E. coil iss virulence gene. Lane L: ladder; Lane P: positive control. Lanes 1–8: showing positive strains at 266 bp.
Discussion
Chemical analysis
The chemical parameters, including moisture, salt, and acidity, are shown in Table 2; for Ras cheese samples, nearly similar results were obtained by Ahmed (2016) and El-Refaay et al. (2016) concerning salt and acidity, while higher results were recorded by Awad et al. (2003), Osman et al. (2011), and Dahmash et al. (2019) for salt and moisture. For Domiati cheese samples, nearly similar results for salt were obtained by Aly et al. (2007) and El-Baradei et al. (2007), higher results recorded by El-kholy et al. (2014), El-Zahar (2014), and El-Refaay et al. (2016) for salt and acidity. The results for the Mish samples were in agreement with El-Zahar (2014) and El-Refaay et al. (2016). The apparent variation among the examined parameters in different examined samples could be attributed to variances in composition, properties of raw milk, and biodiversity of the organisms in each product, as well as other ingredients employed in the manufacture of traditional raw products, especially with lack of Egyptian standards for such products (Aly et al., 2007).
Microbiological examination
Total bacterial, presumptive coliform, yeast and mold counts were considered the most significant indices for the microbial quality (APHA (American Public Health Association), 2004). Nearly similar results of TBC for Ras cheese samples were obtained by Awad et al. (2003), El-Zahar (2014), and El-Leboudy et al. (2014), while lower results were found by Osman et al. (2011) and Abd El-Halem et al. (2019). The results of TBC for Domiati cheese samples were in agreement with those obtained by Aly et al. (2007), Sayed et al. (2011), and Ibrahim et al. (2015a), and higher results were obtained by El Sayed et al. (2011), while lower findings were obtained by El-kholy et al. (2014) and Ibrahim et al. (2015b). Nearly similar TBC results for Mish samples were reported by Zaki and Shokry (1988) and El-Zahar (2014). TBC is not directly related to the presence of pathogens or toxins, but it may be a convenient indicator of poor sanitation, defects in process control systems, and/or contaminated raw ingredients (APHA (American Public Health Association), 2004; Silva et al., 2019). There was a negative correlation between TBC, salt, and acidity in the all examined samples, but was not significant as not all but most microorganisms were suppressed by salt and acidity content (Aly et al., 2007). Nevertheless, there was a positive significant correlation between TBC and moisture in Domiati and Mish cheese samples (Table 4). The relatively high moisture allows the growth of different types of desirable and undesirable microorganisms that may be introduced through raw milk used or during the processing steps (Aly et al., 2007).
Table 4. Statistical correlation between chemical and microbiological analyses.
| Moisture % | Salt % | Acidity % | ||||
|---|---|---|---|---|---|---|
| Pearson correlation | Sig. | Pearson correlation | Sig. | Pearson correlation | Sig. | |
| Ras cheese | ||||||
| TBC | 0.215 | 0.134 | −0.151 | 0.295 | −0.171 | 0.235 |
| Coliforms count | 0.430a | 0.002a | −0.394a | 0.005a | −0.048 | 0.739 |
| Staph. count | 0.182 | 0.207 | −0.197 | 0.170 | −0.088 | 0.542 |
| Yeast count | 0.067 | 0.644 | −0.070 | 0.630 | −0.169 | 0.240 |
| Mold count | 0.169 | 0.241 | −0.094 | 0.518 | −0.078 | 0.589 |
| Domiati cheese | ||||||
| TBC | 0.326b | 0.021b | −0.170 | 0.237 | −0.237 | 0.097 |
| Coliforms count | 0.229 | 0.110 | −0.806a | 0.000a | −0.103 | 0.475 |
| Staph. count | 0.388a | 0.005a | −0.267 | 0.061 | −0.391a | 0.005a |
| Yeast count | 0.115 | 0.427 | −0.306b | 0.031b | −0.245 | 0.086 |
| Mold count | 0.062 | 0.668 | −0.142 | 0.327 | −0.256 | 0.073 |
| Mish | ||||||
| TBC | 0.423b | 0.022b | −0.169 | 0.381 | −0.047 | 0.807 |
| Coliforms count | 0.039 | 0.788 | −0.590a | 0.000a | −0.165 | 0.215 |
| Staph. count | 0.131 | 0.364 | −0.315b | 0.026b | −0.516a | 0.000a |
| Yeast count | 0.556a | 0.000 | −0.221 | 0.123 | −0.481a | 0.000a |
| Mold count | 0.054 | 0.707 | −0.144 | 0.317 | −0.259 | 0.070 |
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
The presence of coliforms in cheese is objectionable as it reflects unsanitary conditions during production, handling, storage, and even distribution (Sayed et al., 2011). Its existence may constitute a biological hazard since coliforms are implicated in many reported cases of food poisoning. Moreover, coliforms possess an economic significance as products contaminated with coliforms will be of inferior quality and unmarketable (Martin et al., 2016). Nearly similar results of coliforms for Ras cheese were obtained by El-Zahar (2014), lower results were obtained by Osman et al. (2011), Awad et al. (2003), and Abd El-Halem et al. (2019), while higher results recorded by Abd elsalam (2014) and Ahmed (2016). For Domiati cheese samples, higher findings were introduced by Ibrahim et al. (2015b) and Abd Allah et al. (2017), while lower results were recorded by Sayed et al. (2011). For Mish samples, higher results were recorded by El-Zahar (2014). There was a positive significant correlation between coliform count and moisture in Ras cheese samples (Table 4). The comparatively low incidence of coliforms in Ras cheese samples may be related to low moisture content and consequently low water activity that will influence microbial growth (Trmčić et al., 2016). Salt content had a significant negative correlation with coliforms in all examined samples as added salt not only enhances flavor but it also acts as a preservative; the anti-microbial activity of salt is owed to its ability to decrease water activity in food by damaging the microbial cell membrane (Ayyash and Shah, 2011). Concerning E. coli incidence rate, higher results for Ras cheese samples were obtained by Ombarak et al. (2016) and Abd El-Halem et al. (2019). Nearly similar results in Domiati cheese samples were recorded by Sayed et al. (2011), while El-kholy et al. (2014) and Ibrahim et al. (2015b) obtained higher results. For Mish samples, higher results obtained by Dawoud et al. (2018). All isolated E. coli serotypes (O125, O18, and O114) belonging to enteropathogenic E. coli mainly infect infants younger than 2 years. Acute watery diarrhea, vomiting, and low-grade fever are the most detected symptoms. E. coli O18 also results in extra intestinal infections as neonatal meningitis, septicemia, pyelonephritis, and pneumonia (Stenutz et al., 2006; Dedeić-Ljubović et al., 2009; Ewida and Hussein, 2019). The molecular characterization of E. coli was carried out using phoA gene (alkaline phosphatase) as a target gene for detecting E. coli (Thong et al., 2011) and all E. coli strains were positive for (phoA) gene. E. coli bacteria possesses multiple virulence genes from which four genes were detected (stx1, tsh, iss, and fimH); all detected isolates were positive for both (iss) and (fimH) genes. The virulence gene iss encodes a protein associated with increased serum survival of E. coli in humans and is considered as a protective factor for the pathogen against phagocytosis. E. coli acquires this gene to have the ability to cause extra intestinal infections, such as neonatal meningitis and sepsis (Sarowska et al., 2019). The adhesion criteria of E. coli are related to fimH virulence gene which is important for both normal ecosystem and pathogenicity of E. coli. Although 95% of E. coli isolates show expression for type 1 fimbriae, the gene is not essential for the presence of E. coli in the colon. However, it helps E. coli to transitory colonize in the oropharyngeal portal which is significant for normal fecal/oral transmission of E. coli among hosts, as well as it plays an important role in the incidence of urinary tract infections caused by E. coli (Sokurenko et al., 1998; Sarowska et al., 2019).
Staphylococci are widespread microorganisms that are present everywhere. Staphylococci outbreaks are usually caused by food handlers with bad personal hygiene (EL-Kholy et al., 2014). Our results (Table 4) showed that there was a significant positive correlation between moisture and staphylococci count and a significant negative correlation between it and acidity in Domiati cheese samples. While staphylococci count significantly decreases with increase in salt and acidity in Mish samples, the growth of staphylococci relies mainly on many parameters, such as temperature, pH, salt, water activity, and oxidation reduction potential; their growth can be decreased by the integration of two or more parameters (Bellio et al., 2019). Staphylococcus aureus is incriminated in 9,000 deaths every year; it is regarded as the third most serious pathogen causing foodborne illnesses worldwide (Ahmed et al., 2019). The presence of S. aureus in dairy products is regarded as a serious public health hazard as the organism may lose its viability during manufacturing processes but its preformed heat stable enterotoxin will still present and induce vomiting and diarrhea within 1–6 hours. However, food poisoning is not only the main health problem concerning S. aureus, but it also causes toxic shock syndrome, pneumonia, post-operative wound infection, and skin infections (FDA (Food and Drug Administration), 2012; EL Malt, 2013; Bellio et al., 2019). Large numbers of extracellular proteins and toxins are produced by S. aureus. The most important toxins are Staphylococcal enterotoxins (SEs), which are SEA, SEB, SEC, SED, and SEE, that cause 95% of staphylococcal food poisoning cases, while only 5% of outbreaks occurred by newly described SEs (SEG-SEI, SEIJ, SEIQ, SER-SET, and SEIU-SEIV) and TSST-1. SEs are soluble in water, stable against most proteolytic enzymes (pepsin and trypsin), especially SEB, and heat-resistant even after boiling for 30 minutes (Bhunia, 2008; Ahmed et al., 2019). The detection of the five classical enterotoxin genes of S. aureus (Fig. 7) revealed that Ras cheese strains harbor the seb and sed genes, while Domiati cheese strains were harboring seb, sed, and see genes. Moreover, Mish samples S. aureus strains harbor the seb gene. In Egypt, Abolghait et al. (2020) recently reported similar results that methicillin-resistant S. aureus isolated from chicken meat and giblets often produces staphylococcal enterotoxin B (SEB) in non-refrigerated raw chicken livers. Production of enterotoxins by S. aureus is influenced by a combination of several factors, such as moisture, water activity, salt, acidity, and temperature as well as microbial competition (FDA (Food and Drug Administration), 2012; Bellio et al., 2019).
The low pH and the high nutritional profile of most cheeses are favorable for the growth of yeasts and molds. Their presence in cheese not only influences the sensory characteristics and decreases the grading of cheese but it also possesses a potential hazard. Some of them have the ability to produce several toxins, such as aflatoxins, and others have the ability to cause human and animal pathogenicity (Ledenbach and Marshall, 2009; ELbagory et al., 2014; El-Fadaly et al., 2015). Nearly similar results of yeast and mold counts for Ras samples were obtained by El-Zahar (2014) and El-Fadaly et al. (2015), and lower counts were recorded by ELbagory et al. (2014). Nearly similar results in Domiati cheese were obtained by Sayed et al. (2011), higher results were obtained by Aly et al. (2007), and lower results were recorded by Ibrahim et al. (2015b). The results of Mish samples are well in line with those reported by El-Zahar (2014) and El-Refaay et al. (2016). In Mish samples, a significant positive correlation was found between yeast and moisture, as well as a significant negative correlation with acidity. The higher moisture content of the product, the more susceptibility for spoilage, off flavors, and over ripening, which result in more breakdown of acids, sugars, proteins, and lipids (Morales et al., 2003; Aly et al., 2007). Corresponding to the relatively high counts of yeasts and molds in the examined samples, using raw milk, poor cleaning practices, and inadequate ripening and storage conditions are the main causes (D’Amico, 2014).
Conclusion
Enterotoxigenic S. aureus harboring seb gene and enteropathogenic E. coli (serogroups O18, O114, and O125) were frequently isolated from soft and hard artisanal cheeses in Egypt. Therefore, strict hygienic measures should be applied during their manufacture, handing, and distribution. Also, more awareness training programs for producers of dairy products should be conducted about how to produce safe products through the implementation of the recent food safety management systems as good manufacturing practices as well as hazard analysis and critical control points in production sites. Also, consumers should be aware of the correct handling and storage methods in order to maintain product safety.
Authors’ contribution
Ashraf Ahmed Moawad and Eman Fathi Abdel-Latif designed and supervised the study as well as reviewed and approved the final version of the submitted manuscript. Ola Wagih Hegab performed the experiments, analyzed, interpreted the data, and drafted the manuscript.
Conflict of interest
All authors declare that they have no conflict of interest.
References
- Abd Allah M.A.F., Ibrahim E.M.A., El Barbary H.A., Mohammed M.O. M. S. Thesis, Fac. Vet. Med. Banha, Egypt: Benha Univ; 2017. Quality assessment of soft cheese related to some pathogenic bacteria. [Google Scholar]
- Abd El-Halem S.G.A., El Derea H.B., Tahoun M.K., Abd El Hamed I.A. M. S. Thesis, High Inst. Pub. Health. Alexandria, Egypt: Alexandria Univ; 2019. Foodborne pathogens in milk and dairy products: prevalence and antibiotic resistance. [Google Scholar]
- Abd elsalam A.B. Ph. D. Thesis, Fac. Vet. Med. Giza, Egypt: Cairo Univ; 2014. Prevalence of biological hazards in milk and some dairy products in Egyptian markets. [Google Scholar]
- Abolghait S.K., Fathi A.G., Youssef F.M., Algammal A.M. Methicillin-resistant Staphylococcus aureus (MRSA) isolated from chicken meat and giblets often produces staphylococcal enterotoxin B (SEB) in non-refrigerated raw chicken livers. Int. J. Food Microbiol. 2020;328:108669. doi: 10.1016/j.ijfoodmicro.2020.108669. [DOI] [PubMed] [Google Scholar]
- Ahmed A.A., Maharik N.M.S., Valero A., Kamal S. M. Incidence of enterotoxigenic Staphylococcus aureus in milk and Egyptian artisanal dairy products. Food Control. 2019;104:20–27. [Google Scholar]
- Ahmed L.I. Ph. D. Thesis, Fac. Vet. Med. Giza, Egypt: Cairo Univ; 2016. Deteriorating microorganisms in milk and some dairy products. [Google Scholar]
- Aly S.A., Morgan S.D., Moawad A.A., Metwally B. N. Effect of moisture, salt content and pH on the microbiological quality of traditional Egyptian Domiati cheese. Assiut Vet. Med. J. 2007;53:68–81. [Google Scholar]
- AOAC (Association of Official Analytical Chemists) Official methods of analysis of AOAC international. 17th. Rockville, MD: AOAC Int; 2000. [Google Scholar]
- APHA (American Public Health Association) Standard methods for examination of dairy products. 17th. Washington DC: 2004. [Google Scholar]
- Awad S. Texture and flavour development in Ras cheese made from raw and pasteurised milk. Food Chem. 2006;97:394–400. [Google Scholar]
- Awad S., El Attar A., Ayad E.H.E., El Soda M. Characteristic of Egyptian market Ras cheese; 1- sensory evaluation, rheological, physico-chemical properties and microbiological analysis. Egyp. J. Dairy Sci. 2003;31:289–303. [Google Scholar]
- Ayyash M.M., Shah N.P. The effect of substitution of NaCl with KCl on chemical composition and functional properties of low-moisture Mozzarella cheese. J. Dairy Sci. 2011;94:3761–3768. doi: 10.3168/jds.2010-4103. [DOI] [PubMed] [Google Scholar]
- Bellio A., Chiesa F., Gallina S., Bianchi D.M., Macori G., Bossi D., Nia Y., Mutel I., Messio S., Hennekinne J-A., Decastelli L. Insight into the distribution of staphylococci and their enterotoxins in cheeses under natural conditions. Front. Microbiol. 2019;9:1–8. doi: 10.3389/fmicb.2018.03233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkerroum N. Traditional fermented foods of North African countries: technology and food safety challenges with regard to microbiological risks. Comp. Rev. Food Sci. Food Saf. 2012;12:54–89. [Google Scholar]
- Bhunia A.K. New York, NY: Springer Science+Business Media; 2008. Foodborne microbial pathogens mechanisms and pathogenesis. [Google Scholar]
- D’Amico D.J. Microbiological quality safety issues in cheese making. In Cheese and microbes. In: Donnelly C.W., editor. Washington, DC: American Society Microbiol Press; 2014. pp. 251–309. [Google Scholar]
- Dahmash M.S., Nazem A.M., Khalil R.H. M. S. Thesis, Fac. Vet. Med. Alexandria, Egypt: Alexandria Univ; 2019. Histamine producing bacteria and some biogenic amines in Ras cheese. [Google Scholar]
- Dawoud M.E.A., Mawgoud Y.A., Hussein A., Rezq M.A.A. Detection of mastitis pathogens by multiplex PCR in raw milk and some dairy products from Menoufia Governorate, Egypt. Curr. Sci. Int. 2018;7:535–540. [Google Scholar]
- Dedeić-Ljubović A., Hukić M., Bekić D., Zvizdić A. Frequency and distribution of diarrhoeagenic Escherichia coli strains isolated from pediatric patients with diarrhoea in Bosnia and Herzegovina. Bosnian J. Basic Med. Sci. 2009;9:148–155. doi: 10.17305/bjbms.2009.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delicato E.R., de Brito B.G., Gaziri L.C.J., Vidotto M.C. Virulence-associated genes in Escherichia coli isolates from poultry with colibacillosis. Vet. Microbiol. 2003;94:97–103. doi: 10.1016/s0378-1135(03)00076-2. [DOI] [PubMed] [Google Scholar]
- Dipineto L., Santaniello A., Fontanella M., Lagos K., Fioretti A., Menna L.F. Presence of Shiga toxin-producing Escherichia coli O157:H7 in living layer hens. Lett. Appl. Microbiol. 2006;43:293–295. doi: 10.1111/j.1472-765X.2006.01954.x. [DOI] [PubMed] [Google Scholar]
- EL Malt L.M. Occurrence of enterotoxigenic staphylococcus aureus in some cheese varieties in Aswan city – Upper Egypt. Assiut Vet. Med. J. 2013;59:62–70. [Google Scholar]
- El Sayed M.A., Hosny I.M., El Kholy W.I., El Dairouty R.K., Mohamed S.H.S. Microbiological evaluation of Egyptian white soft cheeses style. J. Am. Sci. 2011;7:517–526. [Google Scholar]
- ELbagory A.M., Eid A.M., Hammad A.M., Dawood S.A. Prevalence of fungi in locally produced cheese and molecular characterization of isolated toxigenic molds. Benha Vet. Med. J. 2014;27:9–20. [Google Scholar]
- El-Baradei G., Delacroix-Buchet A., Ogier J.C. Biodiversity of bacterial ecosystems in traditional Egyptian Domiati cheese. Appl. Environ. Microbil. 2007;73:1248–1255. doi: 10.1128/AEM.01667-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Fadaly H.M., El-Kadi S.M., Hamad M.N., Habib A.A. Isolation and identification of Egyptian Ras cheese (Romy) contaminating fungi during ripening period. J. Microbiol. Res. 2015;5:1–10. [Google Scholar]
- El-Ghaish S., Dalgalarrondo M., Choiset Y., Sitohy M., Ivanova I., Haertlé T., Chobert J. Screening of strains of lactococci isolated from Egyptian dairy products for their proteolytic activity. Food Chem. 2010;120:758–764. [Google Scholar]
- El-kholy A.M., El-shinawy S.H., Meshref A.M.S., Korany A. M. Microbiological quality of domiati cheese and the influence of probiotics on the behavior of Staphylococcus aureus Escherichia coli O157:H7 in Domiati cheese. J. Food Saf. 2014;34:396–406. [Google Scholar]
- El-Leboudy A.A., Amer A.A., Youssef M.R. Assessment of sanitary measures of ras cheese in manufacturing dairy plant in alexandria governorate. Alexandria J. Vet. Sci. 2014;40:77–84. [Google Scholar]
- El-Refaay D.G.A., Salama E.M., Saad A.H., Ibrahim J.I. M. S. Thesis, Fac. Vet. Med. Ismailia, Egypt: Suez Canal Univ; 2016. Mycological studies on some types of cheese. [Google Scholar]
- El-Zahar K.M. Biogenic amines and microbiological profile of Egyptian cheeses. African J. Food Sci. 2014;2:18–26. [Google Scholar]
- Ewida R.M., Hussein A.A.A. Occurrence of virulent and antibiotic-resistant enteropathogenic and Shiga toxin- producing Escherichia coli in some milk products sold in Assiut city, Egypt. J. Adv. Vet. Res. 2019;8:38–42. [Google Scholar]
- Lampel K.A., Al-Khaldi S., Cahill S.M., editors. FDA (Food and Drug Administration) Bad bug book foodborne pathogenic microorganisms and natural toxins. 2nd. 2012. pp. 87–91.
- Ghanbarpour R., Salehi M. Determination of adhesion encoding genes in Escherichia coli isolates from omphalitis of chicks. Am. J. Anim. Vet. Sci. 2010;5:91–96. [Google Scholar]
- Hu Q., Tu J., Han X., Zhu Y., Ding C., Yu S. Development of multiplex PCR assay for rapid detection of Riemerella anatipestifer, Escherichia coli, and Salmonella enterica simultaneously from ducks. J. Microbiol. Methods. 2011;87:64–69. doi: 10.1016/j.mimet.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Ibrahim G.A., Sharaf O.M., and Abd El-Khalek A.B. Microbiological quality of commercial raw milk, Domiati cheese and Kareish cheese. Middle East J. Appl. Sci. 2015a;5:171–176. [Google Scholar]
- Ibrahim J.I., Salama E., Saad A., Helmy A.A. Microbial quality of some dairy products in Ismailia city. In 2nd Conference of Food Safety. Fac. Vet. Med., Suez Canal Univ. 2015b;1:14–21. [Google Scholar]
- Ledenbach L.H., Marshall R.T. Microbiological spoilage of dairy products. In: Sperber W.H., Doyle M.P, editors. In Compendium of the microbiological spoilage of foods beverages. Berlin, Germany: Springer; 2009. pp. 41–67. [Google Scholar]
- Martin N.H., Trmčić A., Hsieh T., Boor K.J., Wiedmann M. The evolving role of coliforms as indicators of unhygienic processing conditions in dairy foods. Front. Microbiol. 2016;7:1–8. doi: 10.3389/fmicb.2016.01549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrotra M., Wang G., Johnson W.M. Multiplex PCR for detection of genes for Staphylococcus aureus enterotoxins, exfoliative toxins, toxic shock syndrome toxin 1, and methicillin resistance. J. Clin. Microbiol. 2000;38:1032–1035. doi: 10.1128/jcm.38.3.1032-1035.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales P., Fernandez G.E., Nunez M. Caseinolysis in cheese by Enterobacteriaceae strains of dairy origin. Lett. Appl. Microbiol. 2003;37:410–414. doi: 10.1046/j.1472-765x.2003.01422.x. [DOI] [PubMed] [Google Scholar]
- Ombarak R.A., Hinenoya A., Awasthi S.P., Iguchi A., Shima A., Elbagory A.M., Yamasaki S. Prevalence and pathogenic potential of Escherichia coli isolates from raw milk and raw milk cheese in Egypt. Int. J. Food Microbiol. 2016;221:69–76. doi: 10.1016/j.ijfoodmicro.2016.01.009. [DOI] [PubMed] [Google Scholar]
- Osman D.M., Shahin Y.H., Abd El- Galil H.A., Mohran M.A. Chemical and microbiological characteristics of Ras cheese collected from Assiut markets. Assiut J. Agri. Sci. 2011;42:47–54. [Google Scholar]
- Robinson R.K., Tamime A.Y. England, UK: Ellis Horwood Limited; 1991. Feta and related cheeses. [Google Scholar]
- Sambrook J., Fritscgh E.F., Mentiates T. 2nd. Cold Spring Harbor, NY: Cold Spring Harbor Laboratotry Press; 1989. Molecular coloning. A laboratory manual. [Google Scholar]
- Sarowska J., Futoma-Koloch B., Jama-Kmiecik A., Frej-Madrzak M., Ksiazczyk M., Bugla-Ploskonska G., and Choroszy-Krol I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: recent reports. Gut Pathog. 2019;11:1–16. doi: 10.1186/s13099-019-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed M., Abdel-Hameid A., Shaban W. Microbiological evaluation of some Egyptian white soft cheeses. Benha Vet. Med. J. 2011;1:1–6. [Google Scholar]
- Silva N.D., Taniwaki M.H., Junqueira V.C.A., Silveira N.F.D., Okazaki M.M., Gomes R.A.R. Microbiological examination methods of food and water a laboratory manual p. 2nd. London, UK: CRC Press, Taylor & Francis Group; 2019. [Google Scholar]
- Sokurenko E.V., Chesnokova V., Dykhuizen D.E., Ofek I., Wu X., Krogfelt K.A., Struve C., Schembri M.A., Hasty D. Pathogenic adaptation of Escherichia coli by natural variation of the FimH adhesion. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8922–8926. doi: 10.1073/pnas.95.15.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenutz R., Weintraub A., Widmalm G. The structures of Escherichia coli O-polysaccharide antigens. Fed. Eu. Microbiol. Soc. 2006;30:382–403. doi: 10.1111/j.1574-6976.2006.00016.x. [DOI] [PubMed] [Google Scholar]
- Straub J.A., Hertel C., Hammes W.P. A 23S rDNA-targeted polymerase chain reaction–based system for detection of staphylococcus aureus in meat starter cultures and dairy products. J. Food Prot. 1999;62:1150–1156. doi: 10.4315/0362-028x-62.10.1150. [DOI] [PubMed] [Google Scholar]
- Tamime A. Briend cheeses. Hoboken, NJ: Blackwell Publishing Ltd; 2006. [Google Scholar]
- Thong K.L., Lai M.Y., Teh C.S.J., Chua K.H. Simultaneous detection of methicillin-resistant Staphylococcus aureusAcinetobacter baumanniiEscherichia coli Klebsiella pneumoniae and Pseudomonas aeruginosa by multiplex PCR. Trop. Biomed. 2011;28:21–31. [PubMed] [Google Scholar]
- Trmčić A., Chauhan K., Kent D.J., Ralyea R.D., Martin N.H., Boor K.J., Wiedmann M. Coliform detection in cheese is associated with specific cheese characteristics, but no association was found with pathogen detection. J. Dairy Sci. 2016;99:6105–6120. doi: 10.3168/jds.2016-11112. [DOI] [PubMed] [Google Scholar]
- Yaguchi K., Ogitani T., Osawa R., Kawano M., Kokumai N., Kaneshige T., Noro T., Masubuchi K., Shimizu Y. Virulence factors of avian pathogenic Escherichia coli strains isolated from chickens with colisepticemia in Japan. Avian Dis. 2007;51:656–662. doi: 10.1637/0005-2086(2007)51[656:VFOAPE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Zaki N., Shokry Y.M. Chemical and microbiological changes in Mish cheese and Mish during ripening. Egypt. J. Dairy Sci. 1988;16:119–129. [Google Scholar]


