Significance
The most useful information about the anatomy of human memory comes from postmortem neurohistological analysis of patients who have been well-studied during life. Because of practical difficulties, this has rarely been accomplished. Here, we describe neuropsychological and neuropathological findings for a memory-impaired patient whom we studied for 24 y. He had a moderately severe memory impairment in new learning and retrograde amnesia for about 5 y. His neuropathology was unique among memory-impaired patients in involving both medial temporal lobe and diencephalic structures that have been associated with memory function.
Keywords: hippocampus, diencephalon, amnesia
Abstract
We report neuropsychological and neuropathological findings for a patient (A.B.), who developed memory impairment after a cardiac arrest at age 39. A.B. was a clinical psychologist who, although unable to return to work, was an active participant in our neuropsychological studies for 24 y. He exhibited a moderately severe and circumscribed impairment in the formation of long-term, declarative memory (anterograde amnesia), together with temporally graded retrograde amnesia covering ∼5 y prior to the cardiac arrest. More remote memory for both facts and autobiographical events was intact. His neuropathology was extensive and involved the medial temporal lobe, the diencephalon, cerebral cortex, basal ganglia, and cerebellum. In the hippocampal formation, there was substantial cell loss in the CA1 and CA3 fields, the hilus of the dentate gyrus (with sparing of granule cells), and the entorhinal cortex. There was also cell loss in the CA2 field, but some remnants remained. The amygdala demonstrated substantial neuronal loss, particularly in its deep nuclei. In the thalamus, there was damage and atrophy of the anterior nuclear complex, the mediodorsal nucleus, and the pulvinar. There was also loss of cells in the medial and lateral mammillary nuclei in the hypothalamus. We suggest that the neuropathology resulted from two separate factors: the initial cardiac arrest (and respiratory distress) and the recurrent seizures that followed, which led to additional damage characteristic of temporal lobe epilepsy.
Since the earliest descriptions of human memory impairment (1, 2), it has been appreciated that clinical cases can provide useful information about normal memory and its anatomical substrates (3–5). Well-studied individual cases in particular (6–8), together with related work in nonhuman primates (9, 10), have illuminated the organization of brain structures essential for memory. For example, this work identified the components of a medial temporal lobe memory system comprised of the hippocampal formation (the dentate gyrus, hippocampus, subiculum, presubiculum, parasubiculum and entorhinal cortex) and adjacent structures, the perirhinal and parahippocampal cortices. Other clinicopathologic studies identified important structures in the diencephalon: the mediodorsal and anterior nuclei of the thalamus and the mammillary nuclei of the posterior hypothalamus (11–13).
The most detailed information about the functional neuroanatomy of human memory comes from postmortem neurohistological analysis of patients who have been well-studied during life. This is difficult and rarely accomplished as it depends on cooperation from many directions, especially on being able to obtain the brain shortly after death. Among single cases with pathology limited largely to the medial temporal lobe, one can identify eight particularly instructive cases: R.B. and G.D. (CA1 lesions) (7, 14); a case of hippocampal lesions, no initials given (15); W.H., L.M., N.C. (hippocampal lesions) (14, 16); E.P. (medial temporal lobe lesion) (17); and H.M. (an initial description of his medial temporal lobe lesion) (18). Among single cases involving the diencephalon, one can identify six particularly instructive cases: E.A. and H.J. (19); B.C. and J.W. (20); and M.G. and P.N. (16).
Here, we report neuropsychological and neurohistological data for patient A.B. who, at the age of 39, developed moderately severe memory impairment following a cardiac arrest and respiratory distress. His postarrest hospitalization was marked initially by seizures that continued for up to 2 d. After his recovery, he survived for 29 y, during which time he contributed to 75 published studies. Two factors make this case of interest. A.B. was a cooperative and intelligent participant with a Ph.D. in clinical psychology, whose memory impairment occurred as a circumscribed deficit against a background of good intellectual and perceptual function. In addition, his condition was likely the result of two different factors: circulatory and respiratory insufficiency due to the heart attack itself and the effects of the seizures that followed. Although the placement of a pacemaker precluded magnetic resonance imaging (MRI) during life, his brain was subjected to comprehensive microscopic analysis after death to define regions of pathology that could be associated with his memory impairment. This report provides a summary of his neuropsychological findings and neuropathology, which involved both the medial temporal lobe and the diencephalon.
Case History
A.B. was a right-handed, Caucasian male born in 1937 as the youngest of three siblings in an intact Catholic family. He obtained a Ph.D. in 1965 in clinical psychology and worked at a university in the Midwest. He married and had seven children. A.B. had no history of stroke or brain injury but did have a myocardial infarction in 1974 from which he recovered. He also had a history of cigarette smoking (prior to 1974) and moderate alcohol use. The family moved to San Diego in 1975 where he worked as a clinical psychologist. He had always been active and hard-working.
In September 1976, while jogging on the beach, A.B. suffered a cardiac arrest. He was given immediate cardiopulmonary resuscitation by his wife (a nurse) and by a physician and was admitted to hospital within 30 min. Soon after admission, he began having frequent, generalized seizures, which, according to his medical chart, occurred “nearly every other minute.” These slowed and then ceased after the second hospital day. He was comatose for 10 d. By 3 wk after admission, he was stable but had cognitive and motor deficits. He was transferred to a rehabilitation facility in November 1976 with a diagnosis of organic brain damage secondary to cerebral anoxia. He was subsequently prescribed antiseizure medication (300 mg of Dilantin) and continued at this dose for the rest of his life. He was also maintained on a regimen of antihypertensive medication.
A.B.’s physical condition improved during the next few months, but cognitive deficits persisted, along with a rigid, shuffling gait. During this early period, he also suffered from depression and agitation. Neuropsychological testing (conducted in 1978 before we met him) documented impaired motoric speed and impaired fine motor coordination bilaterally. His most severe impairment was described as “short-term and intermediate range memory function.” His remote memory appeared “essentially intact,” and he retained “a wealth of knowledge and information that had been acquired prior to his cardiac arrest.” In 1984, A.B. had bypass surgery, and a pacemaker was implanted, which precluded MRI analysis of his brain. A computed tomography (CT) scan in 2001 detected lesions in the region of the optic radiations, as well as atrophy of the cerebral cortex.
After the onset of memory impairment, A.B. continued to live at home with his wife and their children. His wife described him as pleasant but detached in the presence of other people. He was unable to return to work and struggled with the fact that he was disabled. On typical days, he took walks around his neighborhood, sometimes stopping for coffee at a local shop. He was able to carry out basic activities of daily living and spent most of his time in his office in the lower level of the family home. He appeared to enjoy neuropsychological test sessions and was attentive and cooperative. A.B. participated in our studies from 1981 to 2005, and his memory impairment was stable during this period. On the morning of December 4, 2005, at the age of 68, A.B. suffered a cardiac arrest near his home and could not be resuscitated.
Neuropsychology
Intellectual and Perceptual Functions.
In 1978, A.B. obtained a full-scale IQ score of 119 on the Wechsler Adult Intelligence Scale (WAIS) and 119 again in 1982 on the WAIS-Revised (WAIS-R). His Verbal IQ remained stable during the years that we tested him (121, 119, and 122 in 1982, 1991, and 1997, respectively). However, his Performance IQ scores were lower, likely reflecting his motor slowness (114, 87, and 89 in 1982, 1991, and 1997). He scored 134 (healthy controls, 139.7) on the Dementia Rating Scale (DRS) (maximum score = 144) (21), with 6 points lost on the memory subportion (22). A.B. also performed well on tests sensitive to frontal lobe dysfunction (22). He scored 35 (maximum score = 37) on the Initiation-Perseveration subportion of the DRS. On the Wisconsin Card Sorting Test (23), he sorted five categories and averaged only 9% perseverative errors (healthy controls sorted 4.0 categories and averaged 20.2% perseverative errors) (22). A.B. was also intact on difficult tests of visual discrimination (24). On three tasks that required discriminating among pictures of six visually degraded three-dimensional (3D) objects, he scored 74.9% correct (controls, 73.8%). On a task that required discriminating among six photographs of male faces that appeared in different orientations, A.B. scored 63.3% correct (controls, 58.0% correct).
Semantic Knowledge.
The Boston Naming Test (25) asks for the names of 60 drawings of common and uncommon objects (e.g., broom, trellis). A.B. correctly identified 57 items (controls, 55.7) (26). In another study, A.B. read 65 ambiguous and 25 nonambiguous sentences (27). He detected 55 of the ambiguous sentences and correctly explained the ambiguity for 54 (controls detected a mean of 54 and explained a mean of 49). A.B. was also given nine tests based on 48 line drawings of 24 animals and 24 objects from the Semantic Test Battery (28). With one exception, he matched or exceeded control performance on each test (29). For example, he pointed to the correct drawings given their names or descriptions. He also named items correctly in response to drawings or definitions, and he provided definitions in response to names or drawings. He was, however, slower than controls when asked to provide examples from each of eight categories of living or nonliving things (1-min time limit per category). A.B. generated 96 items (controls, 128.9 ± 5.3).
Immediate Memory.
A.B. performed comparably to controls on several tests of verbal and nonverbal immediate memory. He was given the digit span test on seven occasions, averaging 6.4 digits (controls, 6.8 ± 0.4) (30). In the spatial span task from the Wechsler Memory Scale (WMS-III), A.B. tried to touch an array of nine blocks in the same sequence as had just been demonstrated by the experimenter. A.B.’s span was five blocks; controls, 5.9 blocks (range, four to seven). In other tasks (30), A.B. was also able to remember the size of an angle across 0.5- to 1-s delays (A.B., 15.0% error; controls, 11.7 ± 2.9%) and to judge whether just-seen geometric designs were now displayed as correct mirror-image reversals (A.B., 12.8% error; controls, 18.4 ± 3.6%). Finally, A.B. remembered four complex designs after a 0- to 2-s delay (A.B., 69.8% correct; controls, 67.2 ± 2.4%) (31), and he remembered single faces after delays of 2 or 7 s (A.B., 99.0%; controls, 98.2 ± 2.1%) (32).
Declarative Memory (New Learning).
A.B.’s principal impairment was in forming long-term declarative memory. His impairment was evident on tests of both recall and recognition and across a broad range of material. Table 1 and Fig. 1 show his performance on four standard memory tests. For comparison, scores are included as well for patient L.M., whose circumscribed hippocampal lesions were documented by postmortem neurohistology (14). In addition to these four tests, A.B. demonstrated markedly impaired performance on 18 different recognition memory tests (20 or 24 words tested after a 5- to 10-min delay by yes–no or forced choice recognition) (33). Across all 18 tests, he scored 61.3% correct (controls = 82.6 ± 2.2% correct, chance = 50%). In another task (34), he studied an array of 16 toy objects on a table and, 5 min later, was tested for object name recall, eight-choice object name recognition (chance = 12.5%), and for recall of object location (i.e., he attempted to reconstruct the layout of the objects). A.B. recalled only five object names (controls, 9.2 objects) and recognized 50.0% of the object names (controls = 98.4%). Memory for the original spatial location of the items was also impaired, measured as the mean displacement of the 16 items from their original locations (A.B., 20.9 cm; controls, 9.5 ± 4.4 cm).
Table 1.
Performance on tests of anterograde memory
| Words | Faces | Prose recall | Paired-associate learning | |||
| Trial 1 | Trial 2 | Trial 3 | ||||
| A.B. | 31 | 33 | 0 | 1 | 1 | 2 |
| L.M. | 29 | 37 | 0 | 1 | 1 | 1 |
| CON | 41.1 | 38.1 | 6.4 | 6.0 | 7.6 | 8.9 |
The scores for controls (CON, n = 8) are from Squire and Shimamura (68). For comparison to A.B., scores are included for another patient (L.M.) whose hippocampal damage was confirmed by postmortem histology (14). The Words and Faces scores are based on a two-choice recognition test given after a 24-h delay [Warrington (69); chance = 25]. The prose recall scores are based on the number of story segments recalled after a 12-min delay. The paired-associate scores are the number of word pairs recalled out of 10 on three successive learning trials.
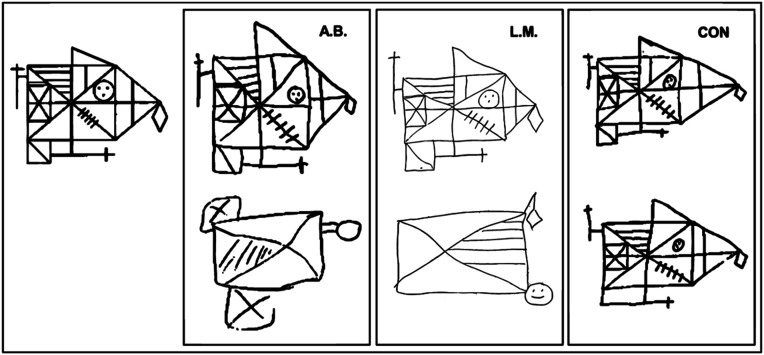
Fig. 1.
Participants were instructed to copy the figure (67) at the Top Left and 5 to 10 min later to reproduce it from memory. Copies (Top) and reproductions (Bottom) are shown for A.B., for another memory-impaired patient (L.M.) (14), and for a representative control (CON). Although the patients could copy the figure accurately (copy scores: A.B. = 32; L.M. = 33; CON = 31; maximum = 36), they were impaired at reproducing it from memory (reproduction scores: A.B. = 4; L.M. = 6; CON = 26).
A.B. also was impaired on memory tests outside the visual modality. Like other memory-impaired patients, he failed to acquire trace eyeblink conditioning (35). In another task, he was impaired on a yes–no recognition test for 10 nonsense sounds (e.g., tones, short novel melodies, or gurgling sounds) that had been presented 5 min earlier (36); he scored 56.0% correct (controls, 68.5 ± 2.3%). In still another task, he was impaired on a yes–no recognition test for 12 odors that had been sampled 1 h earlier (37). He scored 68.8% correct (controls, 75.4 ± 1.7% correct).
Retrograde Memory.
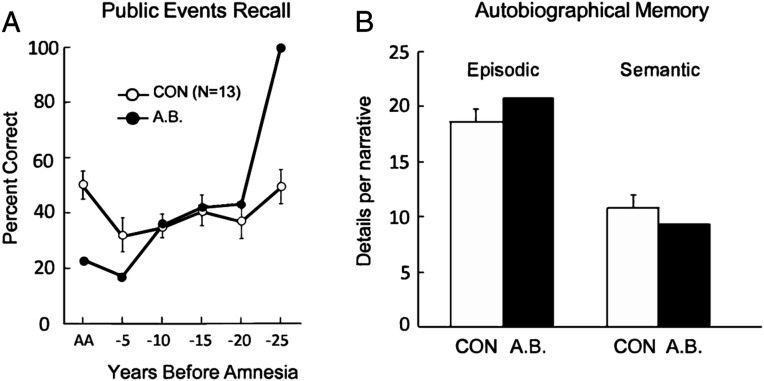
A.B.’s memory impairment began in 1976 when he was 39 y old. Tests of retrograde memory therefore focused on the period 1950 to 1975 (adolescence and adulthood). Tests involving knowledge of famous faces, new vocabulary words, and public events (26, 38–40) demonstrated good memory for premorbid material. Tests with improved year-by-year temporal resolution (40) revealed retrograde amnesia within the 5 y immediately preceding the onset of his memory impairment (Fig. 2A). There were only 11 test items for the most remote time period so his excellent score for those items is perhaps not surprising given his advanced education.
Fig. 2.
Retrograde memory. (A) Recall performance on a test of news events that occurred from 1952 to 2005 (data from ref. 40). The scores for A.B. show his performance for the period of anterograde amnesia (AA, 1977 to 2005) and for 5-y intervals preceding the onset of amnesia. The scores for controls show performance for the corresponding time periods. (B) Number of details contained in remote autobiographical recollections by A.B. and controls (CON, n = 10) (42). A.B. was given cue words (e.g., river, bird, nail) and tried to recollect an event from early life related to each word. Narratives were scored separately for details that described a specific event (episodic) and for details that were part of the narrative but were not unique to the event (semantic). Brackets show SEM.
In other studies (40–42), A.B. produced as many well-formed autobiographical memories from the past as controls in response to cue words, descriptions of possible scenarios, and in response to standardized questions from the Autobiographical Memory Interview (43). In one study (42), A.B. was able to construct recollections from early life in response to 21 of 24 cue words (controls = 23.0), and his recollections contained as many episodic and semantic details as did control recollections (Fig. 2B).
Nondeclarative Memory.
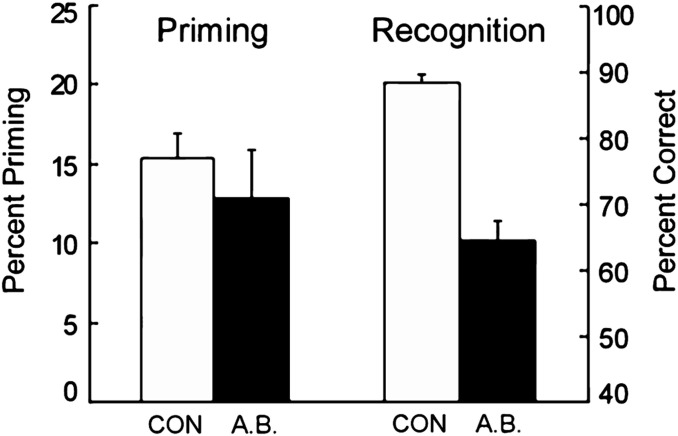
A.B. exhibited intact nondeclarative memory on a number of different tasks, including perceptual priming of words, orthographically regular nonwords, nonpronounceable letter strings (44–46), pictures of objects (47), line drawings (48), and conceptual priming (49). He also succeeded at perceptual learning (50), adaptation-level effects (51), learning to perceive random-dot stereograms (51), and delay eyeblink conditioning (35). For example, in the case of word priming (45), in 12 separate tests, A.B. studied 24 words and, after 5 min, tried to identify 48 words presented briefly for 25 to 28 ms (Fig. 3). A.B. exhibited the same advantage as controls at identifying recently presented words in comparison to newly presented words (A.B., 48% vs. 35%; controls, 48% vs. 32%). By contrast, on six parallel tests of declarative memory, A.B. was poor at recognizing words he had studied 5 min earlier (A.B., 64.6% correct; controls, 88.3%; P < 0.01; chance = 50%) (Fig. 3).
Fig. 3.
Performance of A.B. and controls (CON, n = 7) on 12 tests of perceptual identification priming and six parallel tests of recognition memory (45). Priming was scored as percent correct identification of 24 study words, presented briefly at test, minus percent correct identification of briefly presented 24 new words. Two-alternative, forced-choice recognition was scored as mean percent correct on six tests that presented 24 pairs of old and new words. Brackets indicate SEM.
A.B. also was intact at probabilistic classification learning, a task intended to assess habit learning (52). A.B. learned to predict which of two outcomes would occur on each trial, given which combination of four different cues was presented. A.B. gradually learned the task across 50 training trials. He scored 50.0% correct in the first block of 10 trials (controls, 59.6%) and gradually reached a score of 70.8% correct in the last block of 10 trials (controls, 70.3%). By contrast, A.B. was impaired on a multiple-choice task of declarative memory that asked about the task (36.5% correct; controls, 76.9% correct; chance = 25%).
Neuropathology.
Neuropathology was observed among many brain regions. To describe the salient features of A.B.’s neuropathology in the context of his memory impairment, we begin by describing changes in the hippocampal formation and other regions of the medial temporal lobe. We then proceed to findings in the thalamus, mammillary nuclei, and cerebral cortex. These are followed by observations of the basal ganglia and the cerebellum. Sections from A.B.’s brain were compared with similarly prepared sections from an age-matched control brain, IML-13, as well as other control brains in our collection.
Hippocampal Formation.
Dentate gyrus.
The granule cell layer of the dentate gyrus is present at all levels but appears thin and disorganized. The cells of the polymorphic layer that lie adjacent to the granule cell layer are almost totally absent bilaterally throughout much of the rostrocaudal extent of the dentate gyrus; a few cells remain in the most caudal 20% of the dentate gyrus (Figs. 4–6).
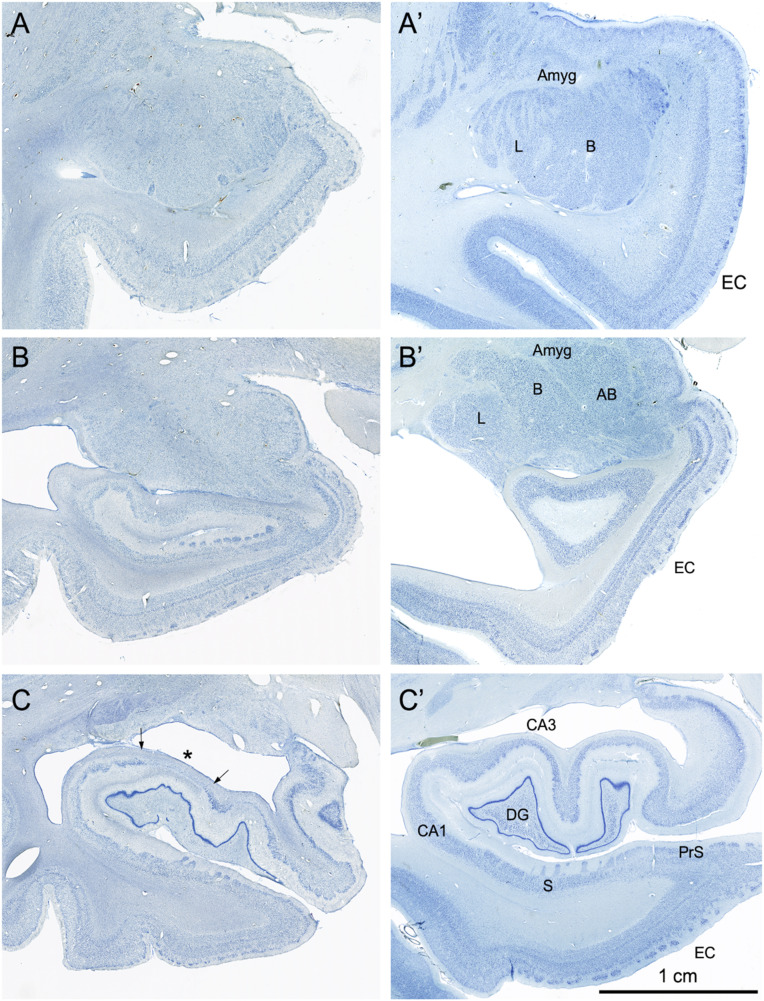
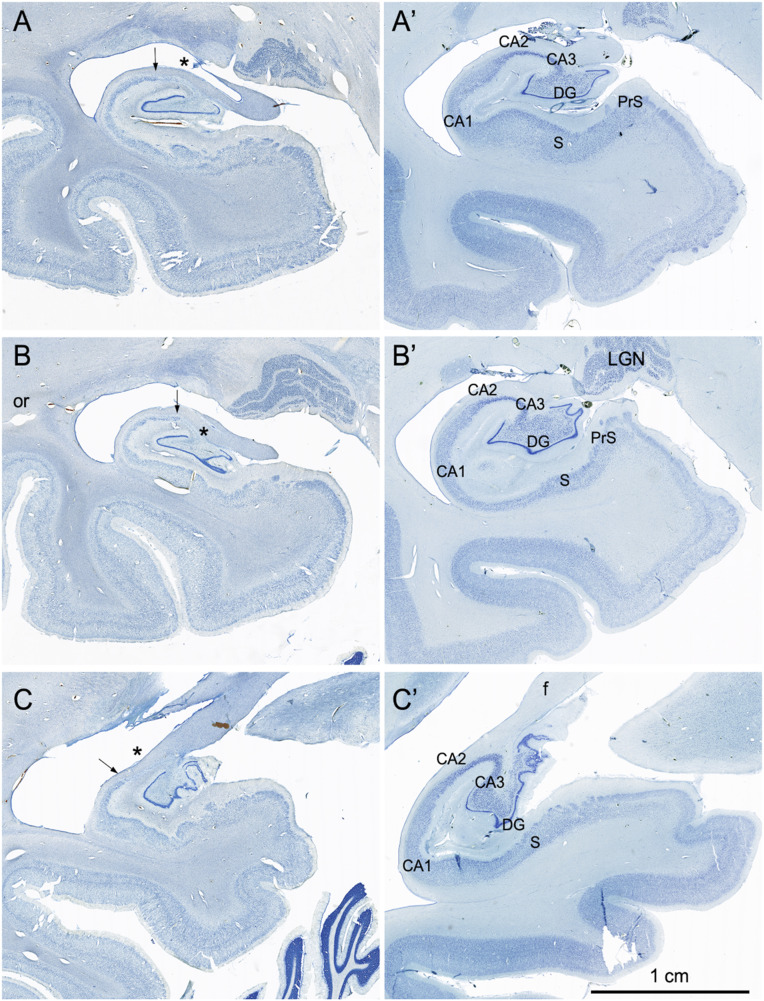
Fig. 4.
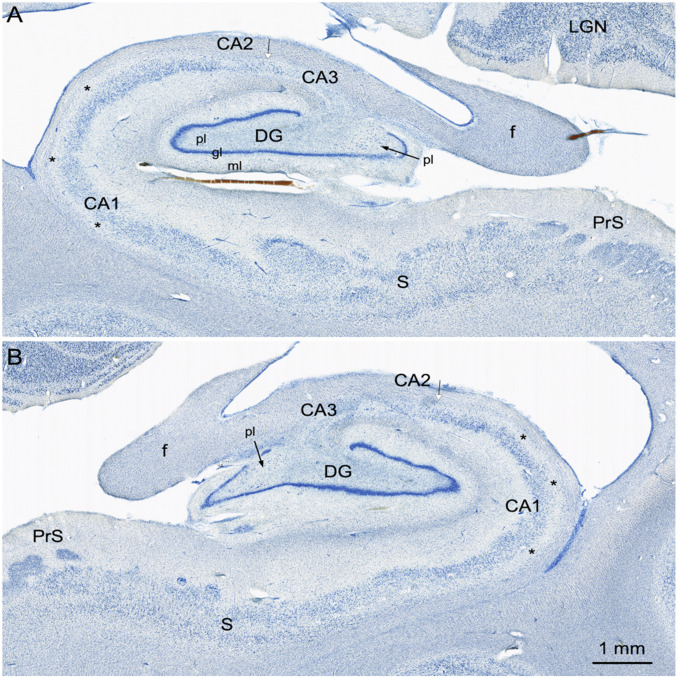
Coronal, Nissl-stained sections of the rostral, anterior, medial temporal lobe arranged from rostral (A) to caudal (C). All images are from the right hemisphere. (Left) Images from the brain of A.B. (Right) Images from the control comparison brain (IML-13) at approximately the same level. (A and A′) These sections illustrate a level through the rostral amygdala (Amyg) and demonstrate cell loss throughout. The entorhinal cortex (EC), located ventromedially, demonstrates patchy cell loss in A.B., particularly in layer III. (B and B′) This section is at a level through the caudal amygdala and at the rostral-most portion of the hippocampus. In the control section (B′), the lateral (L), basal (B), and accessory basal (AB) nuclei are easily differentiable. In A.B., there is generalized neuronal loss in the amygdala (B) which is replaced by a background of gliosis. The layers of the entorhinal cortex are apparent in A.B. but are overall less distinct due to patchy cell loss. (C and C’) This section is at the uncal level of the hippocampal formation and illustrates the rostral dentate gyrus (DG), hippocampus (CA3 and CA1), and components of the subicular complex (subiculum [S] and presubiculum [PrS]). Neurons of the polymorphic layer of the dentate gyrus are completely missing at this level as is the CA3 field of the hippocampus (asterisk between the two arrows in C). There is also neuronal loss in the deep portion of the CA1 pyramidal cell layer, which is better seen in Fig. 6).
Fig. 6.
Higher magnification images of the right (A) and left (B) hippocampal formation in A.B. These sections are taken at a level through the rostral lateral geniculate nucleus (LGN). Here, it is clear that the granule cell layer (gl) of the dentate gyrus (DG) is irregular. Much of the polymorphic layer (pl) of the dentate gyrus is depopulated of neurons, but there are some scattered cells in the medial portion of the polymorphic layer (arrows). There appear to be remaining neurons in the CA2 field of the hippocampus (arrow with white arrowhead). It is also easy to appreciate that the deep portion of the CA1 pyramidal cell layer (asterisks) is devoid of neurons. The subiculum (S) and presubiculum (PrS) have an essentially normal appearance with occasional patches of cell loss. Additional abbreviation: ml, molecular layer of the dentate gyrus.
Hippocampus.
The CA3 field of the hippocampus is entirely absent throughout its full rostrocaudal extent bilaterally (Figs. 4–6). In the caudal half of the hippocampus, a few pyramidal cells are present close to the CA2/CA1 border (Fig. 6 A and B). These are likely remnants of the CA2 field (Fig. 6). Given the disruption of the CA3 region, it is difficult to define with certainty the border between CA3 and CA2. What is clear is that there are large cells at the border of CA1 in the region where CA2 normally appears. In the CA1 field of the hippocampus, there are patches of complete cell loss throughout its rostrocaudal extent bilaterally. These patches are not confined to any particular transverse portion of the field. There is also a more widely distributed loss of neurons in the deep half of the CA1 pyramidal cell layer (Fig. 6 A and B). Many of the remaining CA1 neurons appeared to be shrunken compared to those in the control brain.
Subiculum.
The subiculum is largely intact although there is patchy cell loss at all levels. The presubiculum and parasubiculum had an overall normal appearance.
Entorhinal cortex.
The entorhinal cortex is thin at all levels and appears particularly abnormal at rostral levels (Fig. 4). Loss of cells is most obvious rostrally in layer III, extending also into layers V and VI. The islands of cells that constitute layer II are apparent at all levels of the entorhinal cortex. As one progresses through the caudal half, all layers are present although they appear thinner than in the control (IML-13) and in other control cases.
Perirhinal and parahippocampal cortices.
The perirhinal cortex adjacent to the rostral entorhinal cortex also has some cell loss throughout. The parahippocampal cortex appears essentially normal but has occasional patchy cell loss.
Amygdala.
There is profound cell loss in the amygdala throughout all rostrocaudal levels bilaterally (Fig. 4 A and B). The cell loss is not confined to any particular nucleus but is particularly obvious in the deep nuclei (lateral, basal, and accessory basal) where loss of the larger cells is easily observable. There are islands of intact cells in the region of the deep nuclei, but it is difficult to determine in which nuclei the islands are located. Superficial regions, such as the periamygdaloid cortex, appear to be more intact although the central nucleus is difficult to identify.
Thalamus.
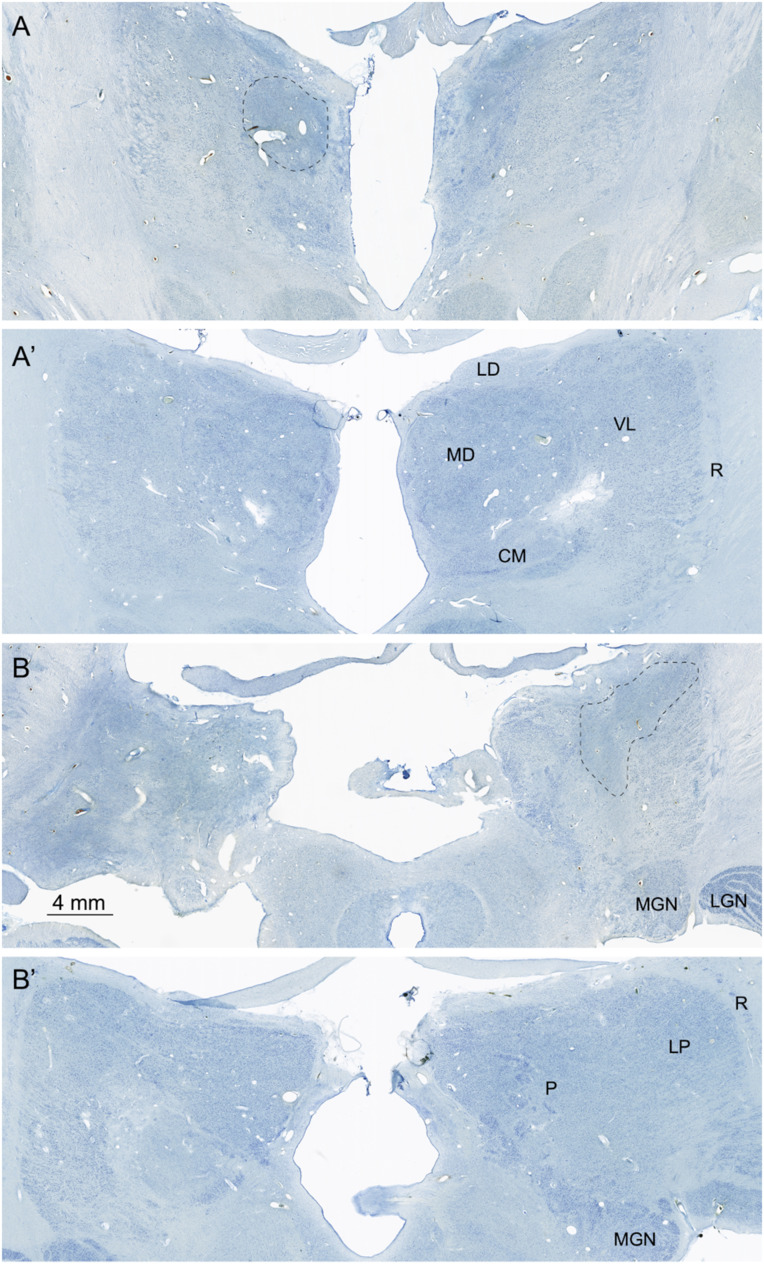
The thalamus has patchy cell loss throughout its rostrocaudal extent and bilaterally (Fig. 7). Cell loss extends rostrally from the anterior nuclear complex to the pulvinar caudally. At rostral levels, cell loss is more evident medially than laterally, but, at more caudal levels, cell loss extends throughout the full width of the thalamus (Fig. 7B). At rostral levels, the anterior nuclear complex and the mediodorsal nucleus appear to have profound cell loss. The substance of the thalamus is so disrupted as to make it difficult to determine which nuclei remaining cells belong to. Because of their unique architecture and location, it is clear that the lateral and medial geniculate nuclei are present and have a generally normal appearance.
Fig. 7.
Nissl-stained images of the thalamus. (A and A′) This section is at a mid-rostrocaudal level with A showing A.B. and A′ showing a similar level from the control brain. There is substantial disruption of the structure of the thalamus in A.B. At this level, the mediodorsal (MD) nucleus is nearly entirely depopulated of neurons, and areas of gliosis (outlined region) are extensive. The ventral lateral (VL) nucleus located lateral to the mediodorsal nucleus is somewhat better preserved. The reticular nucleus (R) that surrounds the main portion of the thalamus cannot be distinguished in the section from A.B. (B and B′) These sections are taken through a caudal level of the thalamus that includes the pulvinar (P) and lateral posterior (LP) nuclei. The medial geniculate nucleus (MGN) can also be seen at this level. Virtually all of the pulvinar in A.B. is devoid of neurons, and there are large gliotic patches (outlined area). The medial (MGN) and lateral (LGN) geniculate nuclei of the thalamus are present in the section from A.B. and have a normal appearance. Additional abbreviations: CM, centre median nucleus of the thalamus; LD, lateral dorsal nucleus of the thalamus.
Mammillary Nuclei.
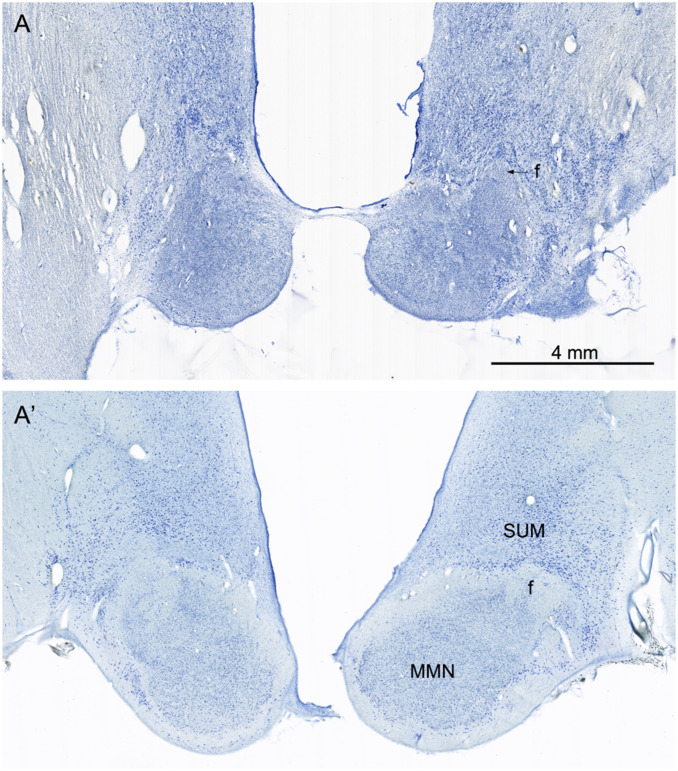
The medial mammillary nucleus is profoundly atrophic with little evidence of healthy neurons (Fig. 8). The lateral mammillary nucleus is also atrophic. The fibrous capsule of the normal medial mammillary nucleus (Fig. 8A), which is comprised of incoming fornix fibers and outgoing mammillothalamic fibers, is virtually absent in A.B. and replaced by gliosis. The profound changes of the mammillary nuclei are likely due to retrograde degeneration consequent to damage of the anterior thalamic complex. The subiculum, which is the source of the fornix fibers to the medial mammillary nucleus, is not heavily damaged in A.B.
Fig. 8.
Nissl-stained sections from A.B. (A) and the control (A′) at a level through the main portion of the medial mammillary nucleus (MMN). In the control brain (A′), there are numerous neurons in the MMN. These are surrounded by a capsule of fibers that include a contribution from the incoming fornix (f) and collecting fibers that will contribute to the mammillothalamic tract. There are also larger and more darkly stained neurons located dorsal to the MMN that constitute the supramammillary region (SUM). In the section from A.B., the MMN is shrunken, and neurons are not detectable. The fibrous capsule is not obvious, and what appears to be the fornix is mainly gliotic. The neurons of the SUM are apparent but appear to be denser due to shrinkage of the lateral hypothalamic tissue. The arrow points to the shrunken fimbria in A.
Cerebral Cortex.
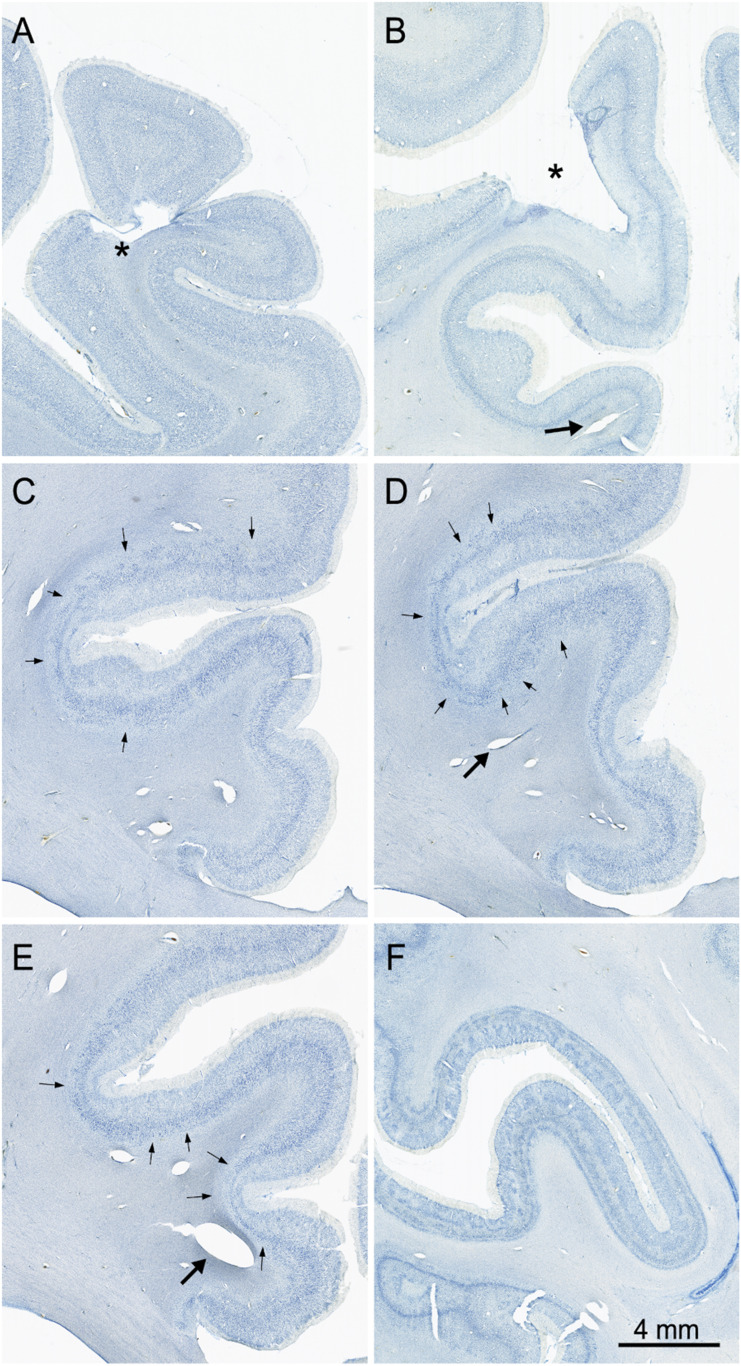
The cerebral cortex shows regions of various forms of focal neuropathology that are widely distributed. Damage ranges from clear infarcts that remove cortical tissue (Fig. 9 A and B) to regions of patchy cell loss, particularly in the cingulate cortex (Fig. 9 C–E). Patchy cell loss is common in the frontal cortex, particularly ventromedially and in the orbitofrontal region. Patches of cell loss are also common in the temporal cortex as well as in the insular cortex. The occipital lobe demonstrates damage to the optic radiations (Fig. 5B) and an unusual patchy appearance that affected area V1 and adjacent visual areas (Fig. 9F). These changes did not appear to have affected A.B.’s visual acuity (53). The cerebral cortex also demonstrates regions of expanded blood vessels. Many of these are within the gray matter and extend vertically through all cortical layers (Fig. 9B). Expanded blood vessels are also observed within the substance of the subcortical white matter (Fig. 9 D and E).
Fig. 9.
Images of Nissl-stained sections showing neuropathology in the cerebral cortex. (A) Infarct (*) in the left dorsomedial cortex. (B) Infarct (*) in the left posterior parietal cortex. (C–E) Sections arranged from rostral (C) through caudal (E) through the right cingulate cortex. Arrows point to patches of cell loss that occurred in all layers and was particularly consistent (and bilateral) in the cingulate cortex. Large arrows in panels B, C, and D point to expanded blood vessels in the white matter. (F) Radially oriented and laminar patches of cell loss in V1 cortex along both banks of the calcarine sulcus. The radial appearance of much of this pathology resembles ocular dominance columns. This may be due to damage to the optic radiations, which is observable in the right hemisphere (Fig. 5B).
Fig. 5.
Coronal Nissl-stained sections of the medial temporal lobe of the right hemisphere arranged from rostral (A) to caudal (C). (Left) Images from the brain of A.B. (Right) Images from the control comparison brain (IML-13) at approximately the same level. The major loss of neurons in the sections from A.B. was in the CA3 region of the hippocampus (asterisk and up to arrow) and in the polymorphic layer of the dentate gyrus. (A and A′) Images of the hippocampal formation just caudal to the uncus. There are scattered cells at the arrow in a region containing the CA2 portion of the hippocampus. There is also cell loss in the deep portion of the pyramidal cell layer in the CA1 region of the hippocampus (better seen in Fig. 6). Some patchy cell loss is also observed in the subiculum (S). The presubiculum has a normal appearance. The parahippocampal region, like many other cortical areas, appears to be thinner than usual with patches of cell loss. (B and B′) Images of the hippocampal formation at the level of the caudal lateral geniculate nucleus (LGN). The pathology is similar to what was described in A. An expanded space in the region of the optic radiations (or) may correspond to the damage identified in an early CT scan of A.B. It may also be the cause of the patchy appearance of the cortex in V1 and adjacent visual areas (Fig. 9F) (C and C′) Images of the caudal pole of the hippocampal formation as the fimbria/fornix (f) ascends. The CA3 field of the hippocampus is completely devoid of pyramidal neurons (asterisk), and the polymorphic layer of the dentate gyrus is also totally depopulated of neurons.
Basal Ganglia.
Both the caudate nucleus and the putamen demonstrate extensive loss of neurons that is not restricted to one region. By comparison with the control brain, it is clear that there is a paucity of the larger striatal neurons, but the background of smaller cells also appears depopulated. The small bundles of fibers that penetrate the caudate and putamen and that appear as cell-free zones in the control brain are highly gliotic in A.B.’s brain. The globus pallidus is also shrunken and depopulated of neurons.
Cerebellum.
There is substantial loss of Purkinje cells in the cerebellum that appears throughout much of the structure.
Discussion
A.B. was a clinical psychologist who suffered a cardiac arrest at the age of 39. He was resuscitated but then had recurrent epileptic seizures for up to 2 d. His resulting disability prevented his return to work, but he was an earnest and cooperative participant in our research program for 24 y until his death in 2005. During this time, we comprehensively evaluated his memory and other cognitive functions. A.B. had a moderately severe impairment in the ability to form long-term declarative memory, which was evident in tests of both recall and recognition and in multiple modalities. His memory impairment occurred against a background of intact intellectual and perceptual functions and intact immediate memory. He was also intact at tests of skill learning, habit learning, priming, and simple classical conditioning (nondeclarative memory). In addition to his impairment in new learning (anterograde amnesia), he had temporally graded retrograde amnesia covering approximately the 5 y prior to the onset of his memory impairment. More remote memory for both facts and autobiographical events was intact. Lastly, he exhibited a persisting, shuffling gait and was slow in his movements.
A.B.’s pacemaker precluded magnetic resonance imaging for evaluation of brain damage. Thus, the locus and extent of his damage could be appreciated only after his death when a detailed neuropathological evaluation was carried out. Within the hippocampal formation, damage was particularly evident in the CA1 and CA3 fields and in the hilus (the polymorphic layer) of the dentate gyrus. The cells of the CA3 field were almost entirely absent bilaterally and only small patches of polymorphic cells remained in the dentate gyrus. The narrow CA2 field also had neuronal loss, but there were patches of cells at the border of CA1 that are likely remnants of CA2. CA2 is the so-called “resistant sector” that has been known for decades to demonstrate some residual neurons in temporal lobe epilepsy (54). The CA1 field was still apparent but had substantial loss of neurons in the deep half of the pyramidal layer throughout its transverse extent. The entorhinal cortex also demonstrated thinning, likely due to neuronal cell loss. The cerebral cortex had various forms of pathology that ranged from focal infarcts to areas of enlarged blood vessels, to areas of patchy cell loss that were scattered from the frontal to the occipital lobes. The amygdala, particularly the lateral, basal, and accessory basal nuclei had significant cell loss. Cell loss was also evident in the basal ganglia. In the diencephalon, both the thalamus (notably the anterior nuclear complex, mediodorsal nucleus, and pulvinar) and the hypothalamus (mammillary nuclei) were depopulated of neurons and shrunken. The cerebellum had a noticeable reduction of Purkinje cells. Given this widespread pathology of structures in the medial temporal lobe and the diencephalon, as well as fairly substantial cortical pathology, it is notable that A.B.’s memory impairment was as selective as it was.
In the medial temporal lobe, damage to the hippocampus and entorhinal cortex, as in A.B., has been associated with moderately severe memory impairment in both humans (7, 14, 15) and monkeys (55, 56). In the case of the medial temporal lobe, the deficit can be more profound (10), but only when the damage substantially involves the parahippocampal gyrus as in patients H.M. (57) and E.P. (17). A.B.’s pathology also includes regions of the diencephalon where damage has been associated with memory impairment: the anterior thalamic nucleus (58), the mediodorsal thalamic nucleus (13), and the mammillary nuclei (19).
It is instructive to compare the severity of A.B.’s memory impairment to the impairment exhibited by other patients where neuropsychological and anatomical information was available. In an earlier study, nine patients with damage limited to the hippocampal formation, and two with larger medial temporal lobe lesions, were given five tests of anterograde memory (59). A.B.’s impaired new learning ability as measured by the same five tests was comparable to the impairment in the nine patients with hippocampal damage (for A.B., z-score = −4.06; for the nine patients, mean z-score = −4.12 ± 0.22). [Impairment can be more severe, as it was for the two patients with larger lesions, G.P. and E.P (17), who obtained z-scores of −5.42 and −7.89, respectively.] In any case, A.B.’s considerable diencephalic damage did not noticeably exacerbate what would have been expected from his hippocampal lesions alone. A possible explanation is that these hippocampal and diencephalic structures operate as a single functional unit such that, when sufficient damage occurs to one of these regions, additional damage to the other region does not increase the deficit.
The vulnerability of neurons in the CA1 field of the hippocampus to anoxia–ischemia has been well documented (7, 55, 60). Cerebellar Purkinje cells are also vulnerable. In contrast, in temporal lobe epilepsy, neurons in the CA3 field and the hilus of the dentate gyrus are particularly vulnerable, along with neurons in CA1, with relative sparing of dentate granule cells and the CA2 field (61, 62). It is also the case in temporal lobe epilepsy that hippocampal damage is often accompanied by additional damage: for example to the entorhinal cortex, medial thalamus, and amygdala (63–65).
In our experience, A.B. is unique among memory-impaired patients in having damage both to the medial temporal lobe memory system as well as to diencephalic structures that have been strongly implicated in memory function. We suggest that these two regions were damaged in A.B. as a result of two factors: the cardiac arrest and the seizures that followed. This sequence of events could account for the loss of cells from the CA1 field and the loss of cerebellar Purkinje cells (from respiratory distress), as well as damage to the CA3 field, the hilus, the thalamus, and additional structures (from recurrent seizures). A.B.’s neuropathology also went well beyond these regions and involved widespread areas of the cerebral cortex, basal ganglia, and cerebellum. Considering the extent of brain damage, it is notable that he functioned so well for so long. This may reflect his intelligence and his ability to devise alternative strategies to negotiate aspects of everyday life. Nevertheless, he was not able to overcome his memory deficit, which attests to the specific and essential role of medial temporal lobe and diencephalic structures for the formation of enduring declarative memory.
Materials and Methods
Acquisition of Postmortem Brain.
Plans to obtain the brain upon A.B.’s death were approved by the Institutional Review Board at the University of California, San Diego, and consent for brain donation was obtained from A.B. and his family. Approximately 12 h after death on 4 December 2005, the brain was removed and suspended by vessels of the circle of Willis in a cold solution of 10% phosphate-buffered formalin. On the following day, the brain was transferred to fresh 4% paraformaldehyde in 0.1 M phosphate buffer and then placed in fresh solution after 3 d, again after 1 mo, and then two additional times before further processing. The brain was stored at 4 °C.
Preparation of Brain for Sectioning and Histological Processing.
Histological processing of A.B.’s brain followed an established protocol (17). After removal of the surface vasculature and leptomeninges, the brain was photographed and then embedded in a cylindrical cast of 10% gelatin that was hardened in a solution of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.2) for 7 d. The embedded brain was cut coronally into three thick tissue blocks parallel to each other. The anterior cut was made just in front of the temporal pole. The posterior cut was made at the level of the splenium of the corpus callosum. Given this blocking scheme, the middle tissue block included the temporal lobes and all subcortical structures (with the exception of the pulvinar). The brain stem and cerebellum were disconnected at the level of the midbrain.
The three blocks of tissue were cryoprotected in increasingly concentrated phosphate-buffered solutions of sucrose (10 to 30% by volume). After the blocks were completely infiltrated, they were frozen rapidly in a chilled bath of isopentane (−40 °C) following the method of Rosene et al. (66). Whole-brain coronal sections were cut at a thickness of 70 µm using a custom-engineered, large-format, sliding microtome (modified from a Leica SM 2500; Leica Microsystems Inc., Buffalo Grove, IL). Tissue sections were stored in sequential order at 4 °C in 0.1 M phosphate buffer and 0.01% sodium azide. One in every 24 sections was mounted on 5 × 7-inch glass slides, dried at room temperature for 48 h, and stained for Nissl substance using a 0.2% solution of thionin in acetate buffer. Briefly, batches of 50 sections were defatted in 100% alcohol/chloroform and then rehydrated in a progressive series of decreasing ethanol solutions. A 10-s immersion in the thionin solution was followed by differentiation in 70% ethanol and dehydration in 100% ethanol. The tissue was cleared in xylenes, and the slides were coverslipped using DPX (Sigma-Aldrich) as mountant.
For comparison with the sections from A.B., and documentation of neuropathology, a second brain was prepared from a donor with no known psychiatric or neurological disorders (IML-13). IML-13 was from a male donor who died at 68 y of age following cardiac infarction. The brain weight was 1,380 g. This case was processed slightly differently from A.B. in that the brain was cut into 1-cm slabs, each of which was cryoprotected and frozen. The slabs were sectioned on a freezing, sliding microtome at 70 µm (similar to A.B.), and a one in eight series of sections were mounted and stained with thionin.
For photographic documentation, sections were scanned using a Huron Instruments high resolution TissueScope scanner. Images were edited in Photoshop (v 21.1.1), and illustrations were composed.
Acknowledgments
This work was supported by the Medical Research Service of the Department of Veterans Affairs (Program 5IK6CX001644) and National Institute of Mental Health Grant 24600. We thank Helen Scharfman for her generous advice and Joyce Zouzounis and Nicola Broadbent for their assistance. We also thank forensic pathologists Trinidad Argente del Castillo and Joaquín Garijo González (Instituto de Medicina Legal de Albacete) for control material used for comparison.
Footnotes
The authors declare no competing interest.
Data Availability.
Study data are available upon request.
References
- 1.Winslow F., On Obscure Diseases of the Brain and Disorders of the Mind (John W. Davies, London, ed. 2, 1861). [Google Scholar]
- 2.Ribot T., Les Maladies de la Memoire (Appleton-Century-Crofts, New York, 1881) [English translation: Diseases of Memory]. [Google Scholar]
- 3.Whitty C. W. M., Zangwill O. L., Amnesia (Butterworths, London, 1966), p. 217. [Google Scholar]
- 4.Milner B., Disorders of learning and memory after temporal lobe lesions in man. Clin. Neurosurg. 19, 421–466 (1972). [DOI] [PubMed] [Google Scholar]
- 5.Squire L. R., The neuropsychology of human memory. Annu. Rev. Neurosci. 5, 241–273 (1982). [DOI] [PubMed] [Google Scholar]
- 6.Scoville W. B., Milner B., Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20, 11–21 (1957). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zola-Morgan S., Squire L. R., Amaral D. G., Human amnesia and the medial temporal region: Enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J. Neurosci. 6, 2950–2967 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tulving E., Schacter D. L., McLachland D., Moscovitch M., Priming of semantic autobiographical knowledge: A case study of retrograde amnesia. Brain Cogn. 8, 3–20 (1988). [DOI] [PubMed] [Google Scholar]
- 9.Mishkin M., A memory system in the monkey. Phil. Trans. Roy. Soc. London. Series B 1089, 83–95 (1982). [DOI] [PubMed] [Google Scholar]
- 10.Squire L. R., Zola-Morgan S., The medial temporal lobe memory system. Science 253, 1380–1386 (1991). [DOI] [PubMed] [Google Scholar]
- 11.von Cramon D. Y., Hebel N., Schuri U., A contribution to the anatomical basis of thalamic amnesia. Brain 108, 993–1008 (1985). [DOI] [PubMed] [Google Scholar]
- 12.Squire L. R., Amaral D. G., Zola-Morgan S., Kritchevsky M., Press G. A., Description of brain injury in the amnesic patient N.A. based on magnetic resonance imaging. Exp. Neurol. 105, 23–25 (1989). [DOI] [PubMed] [Google Scholar]
- 13.Victor M., Adams R. D., Collins G. H., The Wernicke-Korsakoff Syndrome and Related Neurological Disorders due to Alcoholism and Malnutrition (F.A. Davis, Philadelphia, ed. 2, 1989). [Google Scholar]
- 14.Rempel-Clower N. L., Zola S. M., Squire L. R., Amaral D. G., Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J. Neurosci. 16, 5233–5255 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Victor M., Agamanolis D., Amnesia due to lesions confined to the hippocampus: A clinical-pathological study. J. Cogn. Neurosci. 2, 246–257 (1990). [DOI] [PubMed] [Google Scholar]
- 16.Gold J. J., Squire L. R., The anatomy of amnesia: Neurohistological analysis of three new cases. Learn. Mem. 13, 699–710 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Insausti R., Annese J., Amaral D. G., Squire L. R., Human amnesia and the medial temporal lobe illuminated by neuropsychological and neurohistological findings for patient E.P. Proc. Natl. Acad. Sci. U.S.A. 110, 1953–1962 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Annese J., et al. , Postmortem examination of patient H.M.’s brain based on histological sectioning and digital 3D reconstruction. Nat. Commun. 5, 3122 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mair W. G. P., Warrington E. K., Weiskrantz L., Memory disorder in Korsakoff psychosis. A neuropathological and neuropsychological investigation of two cases. Brain 102, 749–783 (1979). [DOI] [PubMed] [Google Scholar]
- 20.Mayes A. R., Meudell P. R., Mann D., Pickering A., Location of lesions in Korsakoff’s syndrome: Neuropsychological and neuropathological data on two patients. Cortex 3, 367–388 (1988). [DOI] [PubMed] [Google Scholar]
- 21.Mattis S., “Dementia rating scale” in Geriatric Psychiatry, Karasu B., Bellack R., Eds. (Grune and Stratton, New York, 1976), pp. 77–121. [Google Scholar]
- 22.Janowsky J. S., Shimamura A. P., Kritchevsky M., Squire L. R., Cognitive impairment following frontal lobe damage and its relevance to human amnesia. Behav. Neurosci. 103, 548–560 (1989). [DOI] [PubMed] [Google Scholar]
- 23.Milner B., Effects of different brain lesions on card sorting. Arch. Neurol. 9, 90–100 (1963). [Google Scholar]
- 24.Stark C. E. L., Squire L. R., Intact visual perceptual discrimination in humans in the absence of perirhinal cortex. Learn. Mem. 7, 273–278 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan E., Goodglass H., Weintraub S., The Boston Naming Test (Lea Febiger, Philadelphia, 1983). [Google Scholar]
- 26.Reed J. M., Squire L. R., Retrograde amnesia for facts and events: Findings from four new cases. J. Neurosci. 18, 3943–3954 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmolck H., Stefanacci L., Squire L. R., Detection and explanation of sentence ambiguity are unaffected by hippocampal lesions but are impaired by larger temporal lobe lesions. Hippocampus 10, 759–770 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Hodges J., Salmon D., Butters N., Semantic memory impairment in Alzheimer’s disease: Failure of access or degraded knowledge? Neuropsychologia 30, 301–314 (1992). [DOI] [PubMed] [Google Scholar]
- 29.Schmolck H., Kensinger E. A., Corkin S., Squire L. R., Semantic knowledge in patient H.M. and other patients with bilateral medial and lateral temporal lobe lesions. Hippocampus 12, 520–533 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Cave C., Squire L. R., Intact verbal and non-verbal short-term memory following damage to the human hippocampus. Hippocampus 2, 151–163 (1992). [DOI] [PubMed] [Google Scholar]
- 31.Buffalo E. A., Reber P. J., Squire L. R., The human perirhinal cortex and recognition memory. Hippocampus 8, 330–339 (1998). [DOI] [PubMed] [Google Scholar]
- 32.Shrager Y., Levy D. A., Hopkins R. O., Squire L. R., Working memory and the organization of brain systems. J. Neurosci. 28, 4818–4822 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stark C. E. L., Squire L. R., Recognition memory and familiarity judgments in severe amnesia: No evidence for a contribution of repetition priming. Behav. Neurosci. 114, 459–467 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Cave C. B., Squire L. R., Equivalent impairment of spatial and nonspatial memory following damage to the human hippocampus. Hippocampus 1, 329–340 (1991). [DOI] [PubMed] [Google Scholar]
- 35.Clark R. E., Squire L. R., Classical conditioning and brain systems: A key role for awareness. Science 280, 77–81 (1998). [DOI] [PubMed] [Google Scholar]
- 36.Squire L. R., Schmolck H., Stark S. M., Impaired auditory recognition memory in amnesic patients with medial temporal lobe lesions. Learn. Mem. 8, 252–256 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levy D. A., Hopkins R. O., Squire L. R., Impaired odor recognition memory in patients with hippocampal lesions. Learn. Mem. 11, 794–796 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Squire L. R., Haist F., Shimamura A. P., The neurology of memory: Quantitative assessment of retrograde amnesia in two groups of amnesic patients. J. Neurosci. 9, 828–839 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manns J. R., Hopkins R. O., Squire L. R., Semantic memory and the human hippocampus. Neuron 38, 127–133 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Bayley P. J., Hopkins R. O., Squire L. R., The fate of old memories after medial temporal lobe damage. J. Neurosci. 26, 13311–13317 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacKinnon D., Squire L. R., Autobiographical memory in amnesia. Psychobiology (Austin Tex.) 17, 247–256 (1989). [Google Scholar]
- 42.Bayley P. J., Hopkins R. O., Squire L. R., Successful recollection of remote autobiographical memories by amnesic patients with medial temporal lobe lesions. Neuron 38, 135–144 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Kopelman M. D., Wilson B. A., Baddeley A. D., The autobiographical memory interview: A new assessment of autobiographical and personal semantic memory in amnesic patients. J. Clin. Exp. Neuropsychol. 5, 724–744 (1989). [DOI] [PubMed] [Google Scholar]
- 44.Hamann S. B., Squire L. R., Intact priming for novel perceptual representations in amnesia. J. Cogn. Neurosci. 9, 699–713 (1997). [DOI] [PubMed] [Google Scholar]
- 45.Hamann S. B., Squire L. R., Intact perceptual memory in the absence of conscious memory. Behav. Neurosci. 111, 850–854 (1997). [DOI] [PubMed] [Google Scholar]
- 46.Haist F., Musen G., Squire L. R., Intact priming of words and nonwords in amnesia. Psychobiology (Austin Tex.) 19, 275–285 (1991). [Google Scholar]
- 47.Cave C., Squire L. R., Intact and long-lasting repetition priming in amnesia. J. Exp. Psychol. Learn. Mem. Cogn. 18, 509–520 (1992). [DOI] [PubMed] [Google Scholar]
- 48.Musen G., Squire L. R., Nonverbal priming in amnesia. Mem. Cognit. 20, 442–448 (1992). [DOI] [PubMed] [Google Scholar]
- 49.Levy D. A., Stark C. E., Squire L. R., Intact conceptual priming in the absence of declarative memory. Psychol. Sci. 15, 680–686 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manns J. R., Squire L. R., Perceptual learning, awareness, and the hippocampus. Hippocampus 11, 776–782 (2001). [DOI] [PubMed] [Google Scholar]
- 51.Benzing W. C., Squire L. R., Preserved learning and memory in amnesia: Intact adaptation-level effects and learning of stereoscopic depth. Behav. Neurosci. 103, 538–547 (1989). [DOI] [PubMed] [Google Scholar]
- 52.Knowlton B. J., Mangels J. A., Squire L. R., A neostriatal habit learning system in humans. Science 273, 1399–1402 (1996). [DOI] [PubMed] [Google Scholar]
- 53.Hamann S. B., Squire L. R., Schacter D. L., Perceptual thresholds and priming in amnesia. Neuropsychology 9, 3–15 (1995). [Google Scholar]
- 54.McLardy T., Ammonshorn pathology and epileptic dyscontrol. Nature 221, 877–878 (1969). [DOI] [PubMed] [Google Scholar]
- 55.Zola-Morgan S., Squire L. R., Rempel N. L., Clower R. P., Amaral D. G., Enduring memory impairment in monkeys after ischemic damage to the hippocampus. J. Neurosci. 12, 1582–2596 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zola-Morgan S., Squire L. R., Ramus S. J., Severity of memory impairment in monkeys as a function of locus and extent of damage within the medial temporal lobe memory system. Hippocampus 4, 483–495 (1994). [DOI] [PubMed] [Google Scholar]
- 57.Corkin S., Amaral D. G., Gonzalez R. G., Johnson K. A., Hyman B. T., H.M.’s medial temporal lobe lesion: Findings from magnetic resonance imaging. J. Neurosci. 17, 3964–3980 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harding A., Halliday G., Caine D., Kril J., Degeneraton of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain 123, 141–154 (2000). [DOI] [PubMed] [Google Scholar]
- 59.Smith C. N., Frascino J. C., Hopkins R. O., Squire L. R., The nature of anterograde and retrograde memory impairment after damage to the medial temporal lobe. Neuropsychologia 51, 2709–2714 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmidt-Kastner R., Genomic approach to selective vulnerability of the hippocampus in brain ischemia-hypoxia. Neuroscience 309, 259–279 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Scharfman H. E., Pedley T. A., “Temporal lobe epilepsy” in Neurobiology of Disease, Gilman A., Ed. (Academic Press, New York, 2006), pp. 349–369. [Google Scholar]
- 62.Blumcke I., Coras R., Miyata H., Ozkara C., Defining clinico-neuropathological subtypes of mesial temporal lobe epilepsy with hippocampal sclerosis. Brain Pathol. 22, 402–411 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwarcz R., Scharfman H. E., Bertram E. H., “Temporal lobe epilepsy: Renewed emphasis on extrahippocampal areas” in Neuropsychopharmacology: The Fifth Generation of Progress, Davis K. L., Charney D., Coyle J. T., Nemeroff C., Eds. (American College of Neuropsychopharmacology, 2002), pp. 1843–1855. [Google Scholar]
- 64.Sinjab B., Martinian L., Sisodiya S. M., Thom M., Regional thalamic neuropathology in patients with hippocampal sclerosis and epilepsy: A postmortem study. Epilepsia 54, 2125–2133 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bertram E. H., Scott C., The pathological substrate of limbic epilepsy: Neuronal loss in the medial dorsal thalamic nucleus as the consistent change. Epilepsia 41, S3–S8 (2000). [DOI] [PubMed] [Google Scholar]
- 66.Rosene D. L., Roy N. J., Davis B. J., A cryoprotection method that facilitates cutting frozen sections of whole monkey brains for histological and histochemical processing without freezing artifact. J. Histochem. Cytochem. 34, 1301–1315 (1986). [DOI] [PubMed] [Google Scholar]
- 67.Osterrieth P. A., Le test de copie d’une figure complexe; contribution à l’étude de la perception et de la mémoire [Test of copying a complex figure; contribution to the study of perception and memory]. Arch. Psychol. (Geneve) 30, 206–356 (1944). [Google Scholar]
- 68.Squire L. R., Shimamura A. P., Characterizing amnesic patients for neurobehavioral study. Behav. Neurosci. 100, 866–877 (1986). [DOI] [PubMed] [Google Scholar]
- 69.Warrington E. K., Recognition Memory Test: Manual (NFER-Nelson, Berkshire, UK, 1984). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Study data are available upon request.