Significance
Forests are experiencing growing risks of drought-induced mortality in a warming world. Yet, ecosystem dynamics following drought mortality remain unknown, representing a major limitation to our understanding of the ecological consequences of climate change. We provide an emerging picture of postdrought ecological trajectories based on field indicators of forest dynamics. Replacement patterns following mortality indicate limited short-term persistence of predrought dominant tree species, highlighting the potential for major ecosystem reorganization in the coming decades. The great variability of the observed dynamics within and among species reinforces the primary influence of drought characteristics and ecosystem legacies, modulated by land use, management, and past disturbances, on ongoing drought-related species turnover and their potential implications for future forest biodiversity and ecosystem services.
Keywords: drought-induced mortality, forest dynamics, forest resilience, global tree mortality, climate change
Abstract
Forest vulnerability to drought is expected to increase under anthropogenic climate change, and drought-induced mortality and community dynamics following drought have major ecological and societal impacts. Here, we show that tree mortality concomitant with drought has led to short-term (mean 5 y, range 1 to 23 y after mortality) vegetation-type conversion in multiple biomes across the world (131 sites). Self-replacement of the dominant tree species was only prevalent in 21% of the examined cases and forests and woodlands shifted to nonwoody vegetation in 10% of them. The ultimate temporal persistence of such changes remains unknown but, given the key role of biological legacies in long-term ecological succession, this emerging picture of postdrought ecological trajectories highlights the potential for major ecosystem reorganization in the coming decades. Community changes were less pronounced under wetter postmortality conditions. Replacement was also influenced by management intensity, and postdrought shrub dominance was higher when pathogens acted as codrivers of tree mortality. Early change in community composition indicates that forests dominated by mesic species generally shifted toward more xeric communities, with replacing tree and shrub species exhibiting drier bioclimatic optima and distribution ranges. However, shifts toward more mesic communities also occurred and multiple pathways of forest replacement were observed for some species. Drought characteristics, species-specific environmental preferences, plant traits, and ecosystem legacies govern postdrought species turnover and subsequent ecological trajectories, with potential far-reaching implications for forest biodiversity and ecosystem services.
Climate-induced forest mortality is an emerging global phenomenon (1) with major consequences for the functioning of these key ecosystems (2). Reported increases in tree mortality point toward accelerating global forest vulnerability under hotter temperatures and longer, more intense droughts associated with increased climatic variability (2–5). Abrupt drought-induced forest mortality can trigger substantial changes in the composition and structure of ecosystems, altering carbon storage and cycling (6), plant productivity (7), ecosystem–atmosphere exchanges (8), hydrological cycles (9), and ecosystem resilience to subsequent disturbances (10, 11). Global trends in the potential ecological consequences of increased tree mortality, however, remain largely unknown.
Research on recent drought-induced vegetation change (since the 1970s) has mostly focused on plant mortality processes and not on community recovery, which still represents a research frontier in our understanding of forest mortality (2, 12, 13). Performance and sensitivity of the postdrought community to contemporary and future climatic conditions are predicted to have a lasting effect on ecosystem dynamics (14), which in turn are central to projecting climate-induced vegetation change and Earth system feedbacks (15). Thus, characterizing ecological communities following forest mortality is crucial to understanding subsequent consequences for ecosystems.
Postdisturbance recruitment is a key ecological process, as it sets the stage for long-term ecosystem recovery and dynamics. Initial recruitment stages are, however, highly sensitive to environmental constraints, typically exhibiting high mortality rates (16) that could be exacerbated under climate change (17). In addition, the climatic requirements and tolerance of juveniles often differ from those of adults (18). Alternatively, the assessment of intermediate stages, such as saplings or subcanopy trees representing dominant and well-established plants occupying the space and using resources released by dead trees, can provide better short-term indicators of forest dynamics and ecosystem resistance (19) following mortality events. Forest ecosystems comprise long-lived species and, in the absence of further disturbance, we can expect that the influence of the persisting species [i.e., disturbance legacies (10)] and their demographic responses will remain for decades (13, 16).
Here, we assess short-term replacement patterns following tree mortality associated with drought in 131 forest and woodland sites encompassing a wide range of vegetation types across multiple biomes, from dry tropical to temperate and boreal systems but excluding species-rich tropical forests (Fig. 1 and Dataset S1). In all sites studied, tree mortality was linked to drought evidence regardless of among-site differences in drought intensity, duration, seasonality, and pre- and postdrought conditions relative to the reported mortality events (i.e., drought characteristics; SI Appendix, Fig. S1 and Datasets S1 and S2). Postmortality surveys (mean 5 y, range 1 to 23 y after mortality; SI Appendix, Fig. S2) were performed to assess dominant woody species composition, allowing characterization of the degree of self-replacement (i.e., replacement of a dominant tree species with itself), replacement by other woody species including trees and shrubs, or lack of replacement by woody vegetation. We use a joint compositional and structural community resemblance index to quantify forest and woodland resistance or potential vegetation-type conversion based on the relative cover and identity of neighboring and understory trees, shrubs, and well-established saplings occupying the space released by dead trees. We then examine the influence of drought characteristics and site climate, dominant species, management, and concurrent biotic disturbances on the observed replacement patterns and thus ecosystem resistance. Finally, we use the bioclimatic niches of predrought dominant and postdrought replacing species in relation to precipitation regime and aridity to determine shifts toward more xeric or more mesic communities and to ascertain which factors (bioclimatic optimum, driest edge, range, successional index) are associated with the observed shifts. Despite the limited temporal perspective of the available information, our approach provides an emerging picture of current postdrought ecological trajectories across a broad range of global forest biomes.
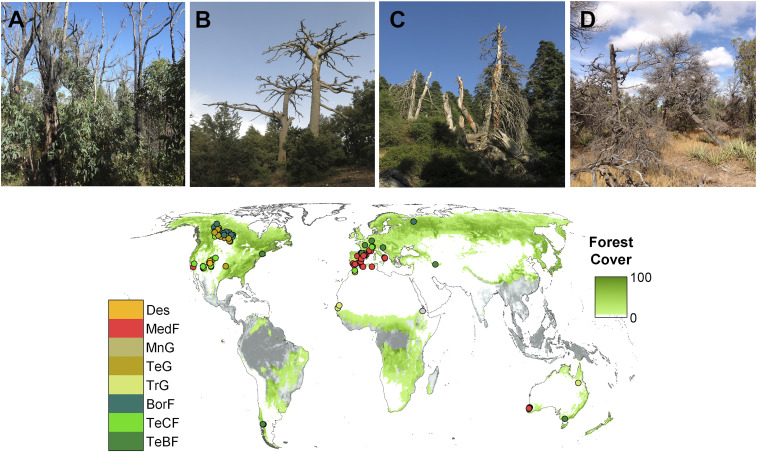
Fig. 1.
Location of the 131 field sites (Dataset S1) for which this research assessed tree species replacement patterns after mortality concomitant with drought. The analysis considers forest and woodland sites across Earth’s forested biomes, excluding species-rich tropical biomes (gray areas in the map). Global forest cover is based on Global Forest Watch (http://globalforestwatch.org). Biome classification (61): BorF, boreal forests/taiga; Des, deserts and xeric shrublands; MedF, Mediterranean forests, woodlands, and scrub; MnG, montane grasslands; TeBF, temperate broadleaf and mixed forests; TeCF, temperate conifer forests; TeG, temperate grasslands, savannas, and shrublands; TrG, tropical and subtropical grasslands, savannas, and shrublands. Photos exemplifying the four replacement processes considered are as follows. (A) Self-replacement; E. marginata, Northern Jarrah Forest, Australia (G.M., 2014). (B) Replacement by another tree species; Cedrus atlantica, Middle Atlas, Morocco (E.B., 2017). (C) Replacement by shrub species; Abies pinsapo, Sierra de las Nieves, Spain (E.B., 2017). (D) No replacement by woody vegetation; P. edulis, New Mexico, USA (F.L., 2012).
Forest and Woodland Replacement Patterns
Following tree mortality concomitant with drought, persistence of dominant forest and woodland species is limited across our sites. Although predrought dominant tree species are present in the community after mortality episodes in 63% of the sites, self-replacement of the dominant species is only prevalent in 33% of these cases (21% out of the total; Fig. 2A). Replacement by other woody species (trees and shrubs) is the dominant process in 69% of the sites. Of those, shrubs are the main replacing species in 42% of the cases (29% of total cases) following mortality, pointing to important postdrought alterations of ecosystem structure and function (11). Further, in 10% of the sites (Fig. 2A), no replacement by woody vegetation prevailed, indicating at least a transient loss of forest and woodland cover promoted by drought-related mortality (up to ∼20 y following drought; Dataset S1). Such replacement patterns are consistent regardless of recovery time and forest successional state (SI Appendix, Fig. S3 and Dataset S1). Ecologists increasingly recognize that long-term outcomes of ecological succession can be disproportionately shaped by early patterns of species occupancy and ecosystem legacies (20–22), particularly under directional climate change (10). Drought represents, therefore, a likely driving factor of species turnover and structural change with strong influences on ecosystem resistance and thus short-term resilience (2, 19). Whether such changes correspond to transient communities within successional trajectories or to stable state shifts will only be unraveled through long-term monitoring, and is a key future research direction.
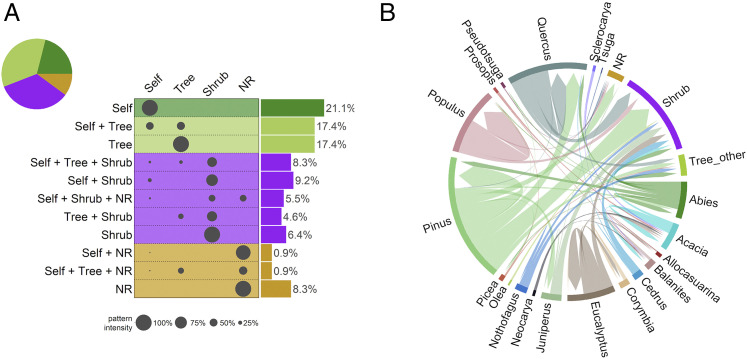
Fig. 2.
Postdrought replacement patterns by vegetation replacement type (NR, no replacement by woody vegetation; Self, self-replacement; Shrub, replacement by shrublands; Tree, replacement by other tree species) and by tree genus. In A, each bar depicts the possible combinations of replacement by the different types (e.g., Self + Tree corresponds to sites in which self-replacement and replacement by other tree species are observed), whereas the proportion of each replacement type across all sites is depicted by the size of the gray dots. The overall proportion of sites showing a given replacement type is shown (Right). Colors depict major replacing categories in which trees (green), shrubs (violet), or lack of replacement by woody vegetation (brown) dominate. In B, outer-level colored bars show the dominant (predrought) genus and the most important replacing woody genera (NR, lack of replacement by woody vegetation; Shrub, replacement by shrub species; Tree_other, replacement by other scarcely represented tree genera: Acer, Arbutus, Austrocedrus, Betula, Carya, Dasyphyllum, Fagus, Ilex, Lomatia, Sorbus, Ulmus, Weinmannia). Inner links are directional, joinning predrought dominant genera (flat ends) and postdrought replacing genera (arrow ends). The inner links depict replacement proportions, so link width is proportional to the number of cases showing any given replacement pattern.
Short-term forest and woodland replacement patterns are not consistently related to the bioclimatic characteristics (drier versus wetter) of the affected sites or species (SI Appendix, Fig. S4). This is consistent with the equivalent relationships between mortality and drought intensity reported among biomes and between major plant taxonomic levels (4) (angiosperms versus gymnosperms). However, replacement patterns are significantly modulated by drought characteristics (Fig. 3 and SI Appendix, Fig. S5). Specifically, wetter conditions after drought-related mortality are associated with smaller compositional and structural differences between pre- and postdrought community. Conversely, the less favorable postmortality drought conditions are relative to the drought event, the greater the community change irrespective of the intensity of the event. This suggests stronger and more sustained shifts under directional precipitation changes (e.g., longer-term, increasing drought trends). Premortality drought conditions are not significantly related to the replacement patterns reported here. Regardless, the influence of postmortality drought conditions on forest and woodland replacement patterns highlights the potential of drought-induced ecosystem reorganizations under ongoing trends of increased aridity (1, 5).
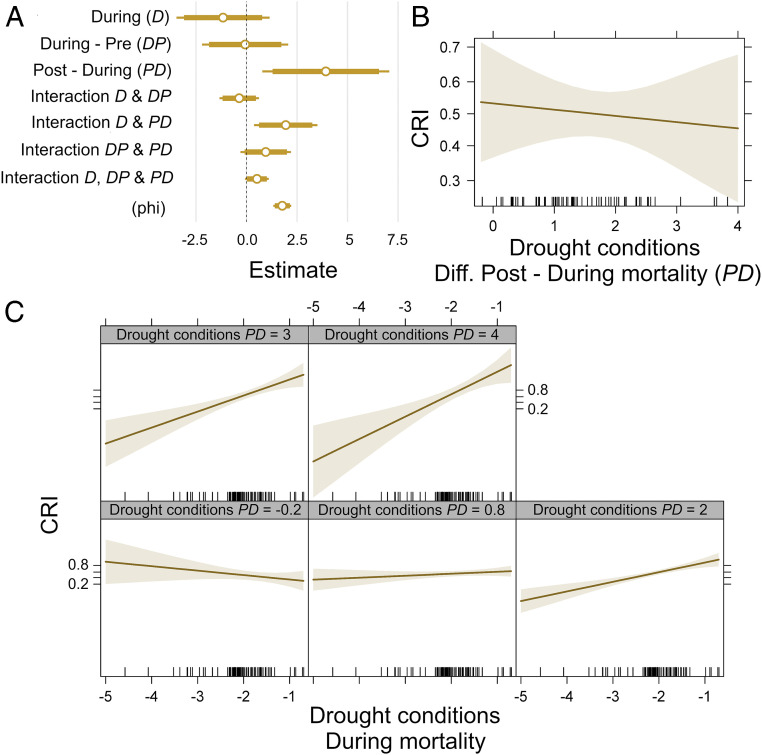
Fig. 3.
Effects of drought conditions before (pre), during, and after (post) tree mortality on the reported replacement patterns. The panels show the results of a beta regression model where replacement pattern (included in the model as the community resemblance index) is the dependent variable and drought conditions are the explanatory variables. The CRI is a joint compositional and structural index that quantifies the vegetation-type change in initial tree forest composition. CRI = 0 reflects no change in composition or structure (complete self-replacement by neighboring canopy trees) and CRI = 1 corresponds to the maximum possible change (no woody replacement). (A) The model’s coefficient estimates. (B) Influence of the difference between postmortality and during-mortality drought conditions on CRI; larger values correspond thus to more favorable conditions after mortality. (C) Influence of the interaction between during-mortality drought and the difference between postmortality and during-mortality drought conditions on CRI. Model pseudo-R2 = 0.274.
Replacement patterns are often influenced by major species-specific traits such as resprouting capacity or light requirements (Fig. 2B and SI Appendix, Fig. S6). Resprouting tree species (e.g., Populus spp., Eucalyptus spp., Quercus spp.) tend to show higher levels of self-replacement than obligate seeding species (e.g., Pinus spp., Abies spp.), although mortality rates concomitant with drought are similar among them (Fig. 2B and SI Appendix, Fig. S6). Additionally, community-level resprouting capacity increases as a result of species-specific replacement patterns (SI Appendix, Fig. S6). Other functional attributes, such as light requirements, will also mediate forest recovery patterns. Shade-tolerant plants present in the understory increase in abundance following canopy mortality (SI Appendix, Fig. S6). Therefore, it is unlikely that either resprouting or seeding becomes the prevalent regenerative strategy of the dominant postmortality species under the stochastic spatiotemporal patterns of extreme droughts (23). Moreover, the interaction of drought with other disturbances, such as fire, may also lead to different outcomes in the prevalence of regenerative traits (24, 25).
Persistence versus turnover of dominant tree species is modulated by the influence of past and concurrent codrivers such as management type, management intensity, and biotic disturbances (26, 27) (Fig. 4). Although highly variable, replacement by shrubs following drought tends to be highest in communities dominated by naturalized species originated by historical planting (Fig. 4 and SI Appendix, Fig. S7), likely reflecting the absence of other tree species in these areas and a stronger disequilibrium between predrought dominant species and the climatic regime in these sites (28). In such cases, replacement by shrubs may initiate successional trajectories toward communities better adapted to the conditions of the affected sites. Prescribed burning, thinning, and, to a lesser degree, grazing, deplete plant density and biomass and can enhance ecosystem water availability (29). These mechanisms, together with the unintended selection by these practices of species resistant to disturbance, could explain higher self-replacement patterns associated with these management types. Nevertheless, high management intensity, irrespective of management type, is associated with higher replacement by shrubs compared with low or moderate management intensities (Fig. 4 and SI Appendix, Figs. S7 and S8). Whether and how postdrought communities differ from recovery patterns associated with management practices inducing tree mortality (e.g., timber harvest) is uncertain but may be a function of biome, climate, and management type and goals, among others. For instance, in forests dominated by shade-tolerant species such as Picea abies or Pinus edulis, advanced regeneration in the understory would lead to self-replacement following any disturbance affecting overstory trees (e.g., selective thinning, drought). However, even in these systems, climate trends (e.g., warming) may preclude self-replacement where increased moisture stress results in recruitment failure and high juvenile mortality (30). As another example, microenvironmental conditions generated by tree mortality associated with drought can be clearly different from those generated after harvesting. In Nothofagus dombeyi forests, dead trees remain standing for many years following drought, generating less direct radiation but high levels of diffuse radiation, favoring replacement by tree species with intermediate shade tolerance, rather than shade-tolerant or shade-intolerant species (31).
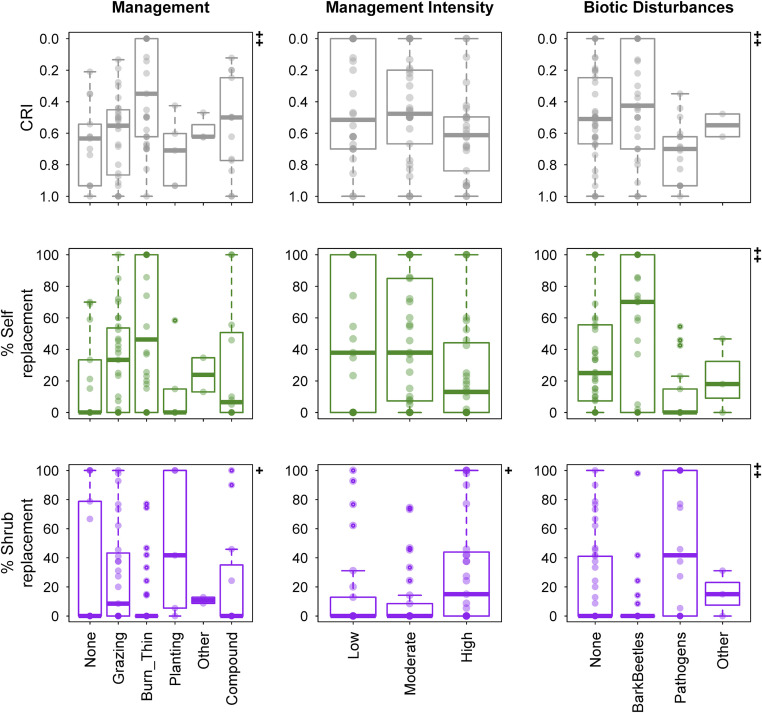
Fig. 4.
Postdrought replacement patterns in relation to management, management intensity, and biotic disturbances. The CRI is a joint compositional and structural index that quantifies the vegetation-type change in initial tree forest composition (Fig. 3). The symbols (Top Right) in each plot show significant differences among the different classes in each panel: +P < 0.05, ++P < 0.01.
Biotic disturbances contemporaneous with the drought event show the strongest effect on replacement patterns (Fig. 4). When fungi and other pathogens act as codrivers of tree mortality, the highest levels of shrub replacement and the lowest levels of self-replacement are observed. In contrast, bark beetles do not seem to systematically relate to low levels of self-replacement. Thus, together with climate, replacement patterns may reflect distinct ecosystem legacies depending on the type of biotic agent involved. For example, insects may have tree size requirements which, unlike pathogens and fungi, may leave smaller conspecific trees intact in the understory (32). Additionally, fungal pathogens could be primary factors of tree mortality and vegetation changes by themselves but empirical and experimental evidence supports in many cases higher mortality rates when strong physiological stress (e.g., drought) limits the vigor of trees and predisposes them to parasite attacks (27). Overall, these results highlight that interacting disturbances can disrupt ecosystem resistance and amplify processes that can lead to state changes in forest ecosystems (26).
Vegetation Dynamics and Bioclimate
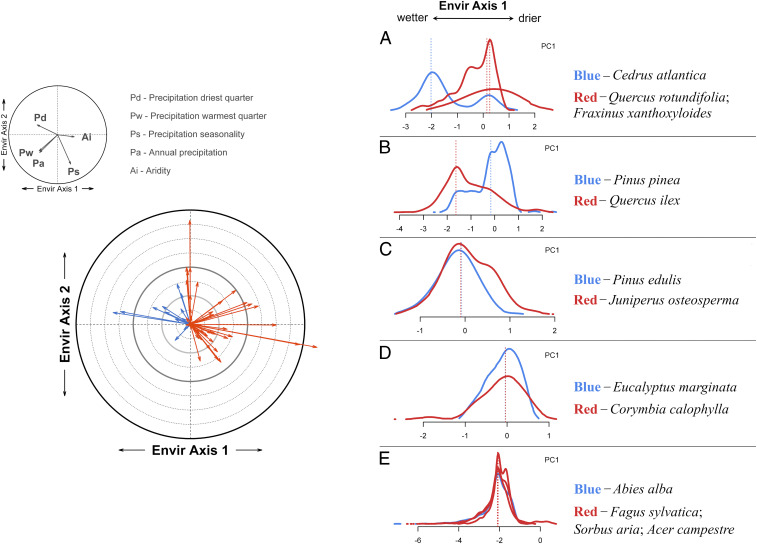
Across multiple forested biomes, drought-related mortality mostly promoted shifts toward more xeric communities, although shifts toward more mesic communities also occurred. This is illustrated by the bioclimatic characteristics of the predrought dominant and postdrought replacing species along the environmental variability axis encompassing aridity (Fig. 5 and SI Appendix, Figs. S9–S12). Changes indicative of shifts toward more xeric postdrought communities (55% of the assessed cases) reflect increased bioclimatic optima in terms of aridity levels and reduced optima in relation to precipitation amount and seasonality of the replacing woody species (Fig. 5A). This is consistent with the potential for abrupt vegetation transitions toward drought-tolerant species formerly reported by Martínez-Vilalta and Lloret (13). It is also consistent with increases in population-level drought tolerance that results from shifts in tree species composition due to incremental increases in climatic water deficit [e.g., United States (33, 34)]. Extreme drought events may accelerate, in some cases, the effect of long-term climate trends on the species replacement process, which may have initiated prior to the onset of drought (35). Yet, pulses of forest mortality concomitant with drought show highly variable population responses. In 24% of the study sites, replacement involved species with less arid bioclimatic optima (Fig. 5B). Finally, in 21% of the cases, no changes in bioclimatic characteristics occurred after forest mortality, corresponding both to self-replacement and to replacement by species with equivalent bioclimatic characteristics following drought (Fig. 5E).
Fig. 5.
Community bioclimatic shift as a result of forest mortality associated with drought. (Left) Relative change within the environmental space defined by precipitation regime and aridity. Environmental axes 1 and 2 encompass 82.4% of the variability of individual variables (PCA-derived axes). Each arrow represents the bioclimatic shift for a given forest site computed as the difference between the bioclimatic centroids of the dominant (predrought) and the replacing woody species weighted by the relative abundance of each species at the site. Orange and blue arrows illustrate shifts toward more xeric and more mesic communities, respectively. (Right) From A to E are examples of bioclimatic niches of the predrought dominant (blue) and postdrought replacing (red) species. Solid lines show the abundance and distribution range of each species along environmental axis 1, whereas the dotted vertical lines correspond to the species’ bioclimatic optima (center of mass of the distribution).
Although the occurrence of mortality events coincident with drought is observed irrespective of site-level and species-specific bioclimatic characteristics (SI Appendix, Fig. S4), our analyses at the community level reveal that sites with a higher dominance of mesic species tend to shift consistently to more xeric communities (Fig. 6A). Such shifts are imparted by replacing species exhibiting distribution limits over drier bioclimatic conditions than the replaced species, likely due to a higher tolerance to water deficit (Figs. 5C and 6B). This higher tolerance to arid conditions may be behind some of the iconic cases of drought-related forest transformation such as P. edulis replacement by Juniperus osteosperma, Juniperus monosperma, and Purshia tridentata in North America, Pinus sylvestris replacement by Quercus pubescens or Quercus ilex in Europe, and Eucalyptus marginata replacement by Corymbia calophylla in Australia (Fig. 5 C and D). In contrast, shifts toward more mesic communities are imparted by replacing species exhibiting distributions over a wider range of climatic conditions in relation to precipitation regimes and aridity (Figs. 5B and 6C and SI Appendix, Fig. S13). This has occurred, for instance, in Mediterranean landscapes, where Pinus pinea has been replaced by Q. ilex, and Quercus cerrioides by Buxus sempervirens (SI Appendix, Fig. S13).
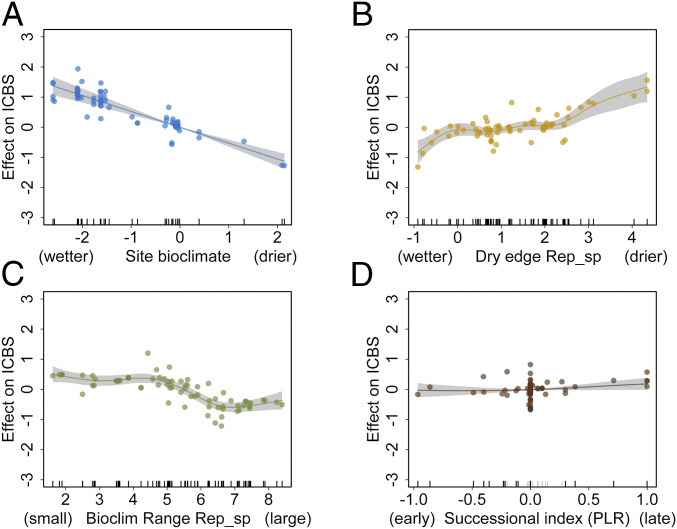
Fig. 6.
Contribution of (A) the bioclimatic characteristics of the study sites, (B) the dry bioclimatic edge of the replacing species (Rep_sp), (C) the range of the bioclimatic distribution of the replacing woody species, and (D) the successional index of the replacing species versus the dominant (predrought) species on the bioclimatic shift index. ICBS is the difference between the bioclimatic optima of the replacing woody species and the bioclimatic optima of the dominant (predrought) species along environmental axis 1 (Fig. 4). Positive ICBS values indicate shifts toward more xeric communities, whereas negative values indicate shifts toward more mesic communities. A shows a linear fit and B–D depict the component smooth functions of a generalized additive model fitted using the four variables depicted here; model R2 = 0.612, explained deviance = 67.2%; all variables are significant at P < 0.05.
Replacement by early- versus late-successional species also appears to influence the observed bioclimatic shifts (Fig. 6D). Shifts to more xeric communities can be associated with replacing species that are late-successional. This is consistent, for instance, with ongoing replacement patterns in some European temperate forests where P. sylvestris, a pioneer conifer that has been favored by silvicultural practices, is replaced by late-successional species such as Q. pubescens. However, successional trajectories can also result in shifts toward more mesic communities when mortality of the forest canopy favors shade-tolerant, more mesic species growing in the understory, for example the replacement of Pinus flexilis by Abies lasiocarpa or Picea engelmannii in western North America. Therefore, community-level bioclimatic changes resulting from successional trajectories are driven, among other factors, by the interplay of climate variability (e.g., drought), the available species pool and their bioclimatic characteristics, and management- or disturbance-caused modifications to stand structure and competition processes (13, 36).
Future Perspectives
We found limited short-term persistence of dominant tree species after drought-related mortality across major forested and woodland biomes. Our work thus represents an important step in documenting the ecological consequences of forest mortality associated with climate variability at the multibiome scale (1, 2, 13, 37). Empirical understanding of long-term trajectories of species persistence versus vegetation shifts following mortality, however, remains very limited. This is particularly true for species-rich tropical forests, not included in this analysis, where the lack of data and the high biological and functional diversity pose additional challenges to understanding community dynamics following drought. Remote sensing approaches to develop composite and multiindicator drought monitoring at broad spatial scales (38), together with the design and implementation of field-based monitoring systems under standardized protocols (12, 13), are needed to better understand the potential long-term consequences of mortality events related to drought.
The observed variability in short-term replacement patterns and associated bioclimatic shifts reported here indicates that the ecological outcomes of extreme climatic events, such as drought, depend on the relative strength of multiple ecological processes. On one hand, ongoing trends of increasing aridity (5) can promote mortality of major forest species (1), hinder their recovery, and favor replacement by drought-resistant tree and shrub species (i.e., species with broader bioclimatic niches or drier ranges). Mortality linked to climate variability could thus lead to biogeographical shifts or permanent range contractions of tree species with climate niches less suitable to current and future conditions (2, 39) or to geographic shifts in vegetation at the biome level (40). Species functional traits other than those directly related to drought resistance (e.g., resprouting capacity, plant light requirements, dispersal and colonizing ability) may have, however, paramount importance in modulating community dynamics and the compositional and functional resilience capacity of the system (4, 10). On the other hand, drought-related forest mortality may enhance transitions within typical successional pathways by removing early-successional dominant species and favor replacing communities better suited to the climatic regime of the affected area. By contrast, drought could also disrupt successional pathways by removing late-successional species, potentially promoting shifts to divergent trajectories and alternative vegetation states that could be maintained by emerging climates or subsequent disturbances. Further, extreme drought may also act as an environmental filter for species that are particularly sensitive to water deficit (41) irrespective of successional status. Therefore, drought may not necessarily lead to altered trajectories in comparison with succession following other disturbance types, but drought-associated tree mortality could accelerate the spatial extent and temporal rate of vegetation turnover, with broad ecological impacts (6–11, 14, 15). Future research is needed to unravel how postdrought vegetation patterns differ from those following tree mortality from other types of disturbance, such as wildfire, pest infestations, or windthrow, particularly under a changing climate.
Disturbance-related changes in the relative abundance of native and newly introduced or invasive species could lead to new species combinations (i.e., novel ecosystems), representing future, unknown ecological pathways for specific forest ecosystems (42). Similarly, interactions among disturbance events (e.g., drought, fire, insect and pathogen outbreaks) may trigger compositional and structural changes affecting ecological trajectories (26, 43). A key constraint on postdrought dynamics and vegetation-type transitions may thus be the availability of climatically suitable species at a given site (13) as suggested by the great variability of observed dynamics among and within species, including multiple pathways of forest replacement for some species. Overall, short-term replacement patterns reinforce the primary influence of drought characteristics and ecosystem legacies, modulated by land-use changes, management history, and past disturbances, on ongoing species turnover associated with drought and their potential implications for future forest biodiversity and ecosystem services.
Materials and Methods
Mortality Dataset.
This work is based on information from 131 forest and woodland sites (Fig. 1) in which drought is considered to have played a relevant role in tree mortality (SI Appendix, Fig. S1 and Datasets S1 and S2). Therefore, the sites exhibited mortality events exceeding the presumed baseline or background tree mortality rates in the absence of drought (standing mortality: mean 37%; median 31%; range 5 to 97%). Nevertheless, other agents (e.g., insects, diseases) could have acted as codrivers of the observed forest mortality. Basic pre- and postdrought community data (Dataset S3) came from the contributing authors, who performed field-based studies in which mortality of dominant tree species concomitant with drought and subsequent replacing species was assessed (Dataset S1). Additional data come from authors’ observations within or near the field sites, from the CIPHA database (Climate Impacts on the Productivity and Health of Aspen; Natural Resources Canada) (44), and from previously unreported field data collected within the project BIOCLIM (CGL2015-67419-R; principal investigator F.L.). Each contributing author provided data on major site characteristics (location, past management and disturbance legacies) and tree mortality and replacement patterns of the dominant and replacing woody species (Datasets S1 and S3). The average lag between the last year of reported tree mortality and assessment of replacement patterns is 5 y (range 1 to 23 y after mortality; mean 11 y; range 1 to 37 y from the initial year of tree mortality; SI Appendix, Fig. S2).
A comprehensive search through the Thomson Reuters Web of Science was performed on 2 May 2017 with the following keywords (topic search): (vegetation OR forest OR woodland OR shrubland OR biome) AND (shift$ OR change$ OR transition$ OR replacement$ OR substitution$ OR succession) AND drought$ AND (mortality OR die-off OR dieoff OR decline OR die-back OR dieback) AND (regeneration OR recruitment). The search yielded 351 studies, from which we selected field-based studies reporting drought-related mortality after the 1950s, excluding species-rich tropical forests (e.g., ref. 1; see next paragraph). The selection included research from the contributing authors of this study and several additional potential works (n = 168 sites). However, we received no response in some cases (32 studies) and data were not suitable for this survey in others (5 studies). Eventually, data from 131 forest and woodland sites were compiled for this assessment.
Within our framework, dominant (predrought) species refers to all tree species with a canopy cover >25% of total canopy cover of the site that were affected by mortality. Following this definition, we excluded species-rich tropical forests affected by mortality coincident with drought where many species are present. The high levels of biological diversity and functional composition in these forests would have required a different approach from the one presented here. Replacing woody species refers to those species already growing in the gaps produced by the death of the dominant tree species and, therefore, occupying the space released by the mortality event associated with drought. Replacing species include understory trees, shrubs, and well-established saplings (with measurable diameter at breast height) or, alternatively, neighboring trees whose crown occupied the space released by the dead trees. Importantly, young seedlings which we assume to have uncertain fate (16) were not considered here. The replacement of the dominant species with the same species (i.e., by itself) is referred to as self-replacement. Drought within the reporting sites was inferred, nonexclusively, from tree-ring analysis (n = 43), instrumental long-term climate records (n = 61), or a combination of local weather surveys and symptomatic (observational) tree dieback and mortality (n = 106). The standardized precipitation evapotranspiration index [SPEI (45)] at a 1° spatial resolution was obtained from the Global Drought Monitor (http://spei.csic.es/map; accessed March 2019) and used to characterize drought (i.e., pre-, during, and postmortality drought conditions) at each site in relation to the period of observed tree mortality. We selected the SPEI temporal scale that matched the period of tree mortality concomitant with drought as described by the individual study authors (SI Appendix, Fig. S1). Premortality drought conditions correspond to mean SPEI values of 1, 5, and 10 y prior to tree mortality, whereas during-mortality drought conditions correspond to the minimum SPEI value (i.e., the most extreme water deficit experienced in each site) during the period in which tree mortality was reported. Postmortality drought conditions correspond to mean SPEI values after mortality and until postmortality vegetation surveys were conducted (up to 10 y after mortality). Negative and positive SPEI values correspond to dry and wet conditions, respectively. The differences between premortality and during-mortality drought conditions and between during-mortality and postmortality drought conditions were used, together with during-mortality drought conditions, to quantify drought in each site (Dataset S1).
Bioclimatic Niche Characterization.
We obtained global presence data for the 122 species constituting the dominant (predrought) tree species and the replacing woody species from the Global Biodiversity Information Facility (GBIF; https://www.gbif.org/). In all cases, data from botanical gardens were excluded and, when available, coordinate uncertainty was used to filter out data with geolocation errors >10 km. Only occurrence data from the 1950-to-2017 period were retained. Subsequently, a 95% density distribution kernel was used as the CI to systematically remove outliers according to the bulk of occurrences for each species (SI Appendix, Fig. S14). This method was used to remove single-occurrence points far from the major species distribution clusters (46) (SI Appendix, Fig. S14). To correct for geographical bias in sampling efforts within the GBIF data and to remove points that were too close to each other, we resampled occurrence data falling within 10 arc-min (∼18.5 km) distance from each other as described in ref. 46. This enabled us to obtain a balanced dataset with a minimum number of occurrence data to characterize the bioclimatic niche of each species.
The environmental space used to define species’ bioclimatic niches incorporated four variables to characterize precipitation regimes [precipitation of the driest and warmest quarters, precipitation seasonality, annual precipitation; WorldClim 2.0; https://worldclim.org (47)] and the aridity index [AI; Global Potential Evapotranspiration database, Consortium of International Agricultural Research Centers for Spatial Information (CGIAR-CSI); https://cgiarcsi.community (48)]. The spatial resolution of the AI was adjusted to the 10 × 10-km resolution of the precipitation data and, for each pixel, the aridity level was computed as 1 − AI (46). The four precipitation variables and the aridity values were standardized, and the climate data from species-rich tropical biomes (Fig. 1) were excluded from the datasets, before principal-component analysis (PCA) was performed to reduce the dimensionality of the five climate variables included in this analysis. We retained the first two PCA axes (PC1 and PC2), which comprise two orthogonal axes of variation of precipitation regimes and aridity explaining 82.4% of the variability within the original climate dataset (PC1, 60.2%, mostly related to aridity levels [AI], and PC2, 22.2%, related to precipitation amount and seasonality). Subsequently, PCA loadings were used to compute the PC1 and PC2 values for each pixel to map the environmental information to geographic space.
Occurrence data and the defined environmental space were then used to characterize the mean features of the bioclimatic niche—or bioclimate—for each species (SI Appendix, Fig. S14). Features incorporated in our analysis were: 1) the bioclimatic optimum—defined as the weighted centroid in environmental joint PC1–PC2 space [center of mass of the distribution kernel of each species; COGravity function, SDMTools package (49)]; 2) the driest bioclimatic edge—defined as the maximum value of the species distribution along PC1; and 3) the bioclimatic range—defined as the width of the climate envelope in PC1. The bioclimatic edge and range were incorporated to better characterize the capacity of species to endure different levels of aridity and precipitation variation. For very widely distributed species (e.g., P. sylvestris), the bioclimatic optima may be less representative of a “typical” climate niche. However, this was partially ameliorated by using the 95% distribution kernel to define the species’ bioclimatic optima and ranges (SI Appendix, Fig. S14). In addition, uncertainty in niche characterization is directly related to the number of occurrence records for each species (SI Appendix, Fig. S15). Also, niche estimations presented here correspond to the realized species niche that, among others, reflects all past processes that have influenced current species distributions (e.g., land use).
Replacement Pattern Analysis.
First, for each site and affected dominant species, we characterized replacement following drought-related mortality by assessing the proportion of mortality-created gaps in which the dominant vegetation occupying the gaps corresponded to the same tree species (self-replacement), other tree species (tree replacement), shrub species (shrub replacement), and lack of replacement by woody vegetation (NR). Species-specific data from all sites were then aggregated to obtain a summary of the replacement patterns by these four replacement typologies. Subsequently, we summarized the replacement patterns at the genus level by computing the overall proportion of sites where each dominant (predrought) genus was present and the identity of replacing woody genera in each case. We used a circular layout [R package circlize (50)] as an efficient method for visualizing the multidimensional replacement patterns across the multiple biomes included in this study.
Second, replacement patterns were quantified by means of a community resemblance index (CRI). The CRI corresponds to a joint measure of community resemblance in terms of composition and structure, in which these two ecosystem properties have the same weight. Compositional and structural differences were characterized by means of Bray–Curtis dissimilarity, an index that is appropriate to measure community resemblance in terms of size structure and species composition (51). Pre- and postdrought compositional differences were computed with the function vegdist from the R package vegan (52) on the basis of community data matrices (i.e., matrices with the relative abundance of predrought dominant and postdrought replacing woody species in each site). Structural differences were computed with the function vegdiststruct from the R package vegclust (53) on the basis of five structural typologies or size classes of replacement: neighboring canopy, (subcanopy) tree replacement, sapling replacement, shrub replacement, and no woody replacement. We assumed that replacement by neighboring canopy trees represents the minimum structural change and no woody replacement represents the maximum change. Tree, sapling, and shrub replacement represent intermediate structural changes between the two extremes. The CRI is the mean of the compositional and structural dissimilarities, taking a value of 0 when there is neither change in composition nor in structure (i.e., replacement by neighboring canopy trees of the same species) and a value of 1 when the change is largest (i.e., no woody replacement).
Third, we used the CRI to assess the relationship between replacement patterns and drought conditions before, during, and after mortality by means of beta regression models. To do this, CRI values (y) were previously transformed as (y·(n − 1) + 0.5)/n, where n is the sample size (54) to obtain values in the open standard unit interval (0,1). We also used the CRI to examine the relationship between replacement patterns and the bioclimatic characteristics of the study sites, as inferred from their geographical location and also in relation to the bioclimatic optima of the dominant (predrought) species. We mapped PCA loadings to extract the PC1 and PC2 values at the x–y coordinates of the sites, and subsequently computed the environmental centroid of each site as the weighted mean of the bioclimatic optima of the dominant (predrought) species based on their abundance in each site. Finally, we used the CRI and the proportion of self-replacement and replacement by shrubs to assess the influence of codrivers such as management type, management intensity, and the occurrence of biotic disturbances (Dataset S1) in the overall replacement patterns. Management intensity was characterized by means of a semiquantitative index (low, moderate, high) based on the author’s expert knowledge of the study sites and on-site evidence (e.g., stumps, trampling). For instance, high thinning intensity would correspond to a forest site with abundant stumps whereas a low thinning intensity would imply a much lower density of stumps. However, given the diversity of the assessed forests, a one-size-fits-all threshold to define intensity (e.g., stump density) is hard to establish and that is why such estimates rely on authors’ expertise in the study areas. A Kruskal–Wallis rank-sum test was applied to perform a nonparametric one-way ANOVA and determine whether there are significant differences in the observed replacement patterns in relation to the management and biotic agent codrivers. Also, we used beta regression models and multiple comparisons (Tukey honest significant differences) to assess pairwise differences on the mean levels of replacement between management and disturbance types. Note that the proportion of self-replacement and replacement by shrubs was rescaled between 0 and 1 before applying beta regression models and that rescaled variables were transformed to obtain values in the open unit interval (0,1) as described above for the CRI. Beta regression models were computed with the R package betareg (55) and pairwise differences with the package multcomp (56). These analyses were performed for 102 (out of the 131) forest sites with detailed quantitative information on replacement patterns (Dataset S1).
Fourth, a generalized additive model [GAM; R package mgcv (57)] was used to determine which factors, when replacement by other woody vegetation occurred, were associated with shifts toward more xeric or more mesic woody replacing communities. We used the site-level community bioclimatic shift index (ICBS) as the response variable, which was calculated as the difference between the bioclimatic optima of the replacing woody species and the bioclimatic optima of the predrought forest along PC1, the axis mainly driven by differences in aridity. As a result, positive ICBS values indicate a shift toward more xeric species whereas negative values indicate a shift toward more mesic species. When multiple replacing-dominant species were present, we used a weighted mean procedure based on species abundance in each site to obtain a site-level ICBS. Four explanatory variables were used in the GAM: the bioclimatic optimum of the predrought forest, the driest bioclimatic edge of the replacing woody species, the bioclimatic range of the replacing woody species, and the successional index of the replacing woody species versus the predrought forest, characterized as the difference between plant light requirements (PLRs) of the replacing community and PLRs of the predrought community. Species-specific PLRs and the other plant traits (e.g., resprouting capacity) used in our study were obtained from the publicly available, open-access TRY database [https://www.try-db.org (58)]. PLR was converted to a quantitative index, where 1 indicates full light, 2 indicates half-shadowy, and 3 indicates shadowy (following notation in TRY). Therefore, the successional index or PLR difference between post- and predrought community yields negative values when replacing species are early-successional and positive values when replacing species are late-successional. For those species where PLRs were missing, we used the R package mice (59) (multivariate imputation chained equations) to create multiple imputations (n = 100) for multivariate missing data. For the multivariate imputation, we used a suite of 13 plant traits, including specific leaf area, plant vegetative height, plant height growth, seed dry mass, seedling growth rate, plant resprouting capacity, plant light requirements, wood density, leaf dry mass, leaf dry matter content, leaf lifespan, seedbank type, and seed longevity. Subsequently, we used the mean of the imputed values at the species level to assign the imputed PLR values. The mean, mode, and median of the imputed values yielded equivalent results. The same imputation procedure was used to obtain missing data for plant resprouting capacity of all species.
Data treatment, analyses, and visualization were all performed in R (60).
Supplementary Material
Acknowledgments
We thank two anonymous reviewers who helped to improve the manuscript and also acknowledge M. De Cáceres for his assistance with community indices. E.B. thanks the support of Project CGL2017-87176-P, and E.B. and F.L. thank the support of Project CGL2015-67419-R and research group 2017 SGR 1001. W.R.L.A. acknowledges funding from the University of Utah Global Change and Sustainability Center (NSF Grants 1714972 and 1802880) and the United States Department of Agriculture National Institute of Food and Agriculture, Agricultural and Food Research Initiative Competitive Program, Ecosystem Services and Agro-Ecosystem Management (Grant 2018-67019-27850). A.V.-C. was funded by the European Union’s Horizon 2020 research and innovation programme under Marie Skłodowska-Curie Grant Agreement 656300, and the 50th Anniversary Fellowship program of the University of Stirling. G.S.-B. is supported by a Juan de la Cierva-formación Grant (FJCI 2016-30121) from the Spanish Ministry of Science and Innovation.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2002314117/-/DCSupplemental.
Data Availability.
All data related to this paper are available in the main text, SI Appendix, and Datasets 1–3.
References
- 1.Allen C. D., Breshears D. D., McDowell N. G., On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6, 1–55 (2015). [Google Scholar]
- 2.Anderegg W. R. L., Kane J. M., Anderegg L. D. L., Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Chang. 3, 30–36 (2013). [Google Scholar]
- 3.Williams A. P., et al. , Temperature as a potent driver of regional forest drought stress and tree mortality. Nat. Clim. Chang. 3, 292–297 (2013). [Google Scholar]
- 4.Greenwood S., et al. , Tree mortality across biomes is promoted by drought intensity, lower wood density and higher specific leaf area. Ecol. Lett. 20, 539–553 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Intergovernmental Panel on Climate Change (IPCC) , “Summary for policymakers” in Global Warming of 1.5°C. An IPCC Special Report on the Impacts of Global Warming of 1.5°C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty, Masson-Delmotte V., Ed. et al. (World Meteorological Organization, Geneva, Switzerland, 2018), pp. 1–32. [Google Scholar]
- 6.Frank D., et al. , Effects of climate extremes on the terrestrial carbon cycle: Concepts, processes and potential future impacts. Glob. Change Biol. 21, 2861–2880 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang L., et al. , Drought dominates the interannual variability in global terrestrial net primary production by controlling semi-arid ecosystems. Sci. Rep. 6, 1–7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vicca S., et al. , Remotely-sensed detection of effects of extreme droughts on gross primary production. Sci. Rep. 6, 28269 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilcox B. P., Transformative ecosystem change and ecohydology: Ushering in a new era for watershed management. Ecohydrology 3, 126–130 (2010). [Google Scholar]
- 10.Johnstone J. F., et al. , Changing disturbance regimes, ecological memory, and forest resilience. Front. Ecol. Environ. 14, 369–378 (2016). [Google Scholar]
- 11.Seidl R., et al. , Forest disturbances under climate change. Nat. Clim. Chang. 7, 395–402 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann H., et al. , Research frontiers for improving our understanding of drought-induced tree and forest mortality. New Phytol. 218, 15–28 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Martínez-Vilalta J., Lloret F., Drought-induced vegetation shifts in terrestrial ecosystems: The key role of regeneration dynamics. Global Planet. Change 144, 94–108 (2016). [Google Scholar]
- 14.Anderson-Teixeira K. J., et al. , Altered dynamics of forest recovery under a changing climate. Glob. Change Biol. 19, 2001–2021 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Hartmann H., Adams H. D., Anderegg W. R. L., Jansen S., Zeppel M. J. B., Research frontiers in drought-induced tree mortality: Crossing scales and disciplines. New Phytol. 205, 965–969 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Canham C. D., Murphy L., The demography of tree species response to climate: Sapling and canopy tree survival. Ecosphere 8, e01701 (2017). [Google Scholar]
- 17.Dobrowski S. Z., et al. , Forest structure and species traits mediate projected recruitment declines in western US tree species. Glob. Ecol. Biogeogr. 24, 917–927 (2015). [Google Scholar]
- 18.Bell D. M., Bradford J. B., Lauenroth W. K., Early indicators of change: Divergent climate envelopes between tree life stages imply range shifts in the western United States. Glob. Ecol. Biogeogr. 23, 168–180 (2014). [Google Scholar]
- 19.Hodgson D., McDonald J. L., Hosken D. J., What do you mean, ‘resilient’? Trends Ecol. Evol. 30, 503–506 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Pickett S. T. A., White P., The Ecology of Natural Disturbance and Patch Dynamics (Academic Press, 2013). [Google Scholar]
- 21.Turner M. G., Baker W. L., Peterson C. J., Peet R. K., Factors influencing succession: Lessons from large, infrequent natural disturbances. Ecosystems 1, 511–523 (1998). [Google Scholar]
- 22.Seidl R., Rammer W., Spies T. A., Disturbance legacies increase the resilience of forest ecosystem structure, composition, and functioning. Ecol. Appl. 24, 2063–2077 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellingham P. J., Sparrow A. D., Resprouting as a life history strategy in woody plant communities. Oikos 89, 409–416 (2000). [Google Scholar]
- 24.Pratt R. B., et al. , Mortality of resprouting chaparral shrubs after a fire and during a record drought: Physiological mechanisms and demographic consequences. Glob. Change Biol. 20, 893–907 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Henzler J., Weise H., Enright N. J., Zander S., Tietjen B., A squeeze in the suitable fire interval: Simulating the persistence of fire-killed plants in a Mediterranean-type ecosystem under drier conditions. Ecol. Modell. 389, 41–49 (2018). [Google Scholar]
- 26.Clark J. S., et al. , The impacts of increasing drought on forest dynamics, structure, and biodiversity in the United States. Glob. Change Biol. 22, 2329–2352 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Jactel H., et al. , Drought effects on damage by forest insects and pathogens: A meta-analysis. Glob. Change Biol. 18, 267–276 (2012). [Google Scholar]
- 28.Svenning J.-C., Sandel B., Disequilibrium vegetation dynamics under future climate change. Am. J. Bot. 100, 1266–1286 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Giuggiola A., et al. , Improvement of water and light availability after thinning at a xeric site: Which matters more? A dual isotope approach. New Phytol. 210, 108–121 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Redmond M. D., Weisberg P. J., Cobb N. S., Clifford M. J., Woodland resilience to regional drought: Dominant controls on tree regeneration following overstorey mortality. J. Ecol. 106, 625–639 (2018). [Google Scholar]
- 31.Suarez M. L., Kitzberger T., Recruitment patterns following a severe drought: Long-term compositional shifts in Patagonian forests. Can. J. For. Res. 38, 3002–3010 (2008). [Google Scholar]
- 32.Sauvard D., “General biology of bark beetles” in Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis, Lieutier F., Day K., Battisti A., Grégoire J.-C., Evans H., Eds. (Springer, 2004), pp. 63–88. [Google Scholar]
- 33.Zhang T., Niinemets Ü., Sheffield J., Lichstein J. W., Shifts in tree functional composition amplify the response of forest biomass to climate. Nature 556, 99–102 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Trugman A. T., Anderegg L. D. L., Shaw J. D., Anderegg W. R. L., Trait velocities reveal that mortality has driven widespread coordinated shifts in forest hydraulic trait composition. Proc. Natl. Acad. Sci. U.S.A. 117, 8532–8538 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeeman B. J., Lunt I. D., Morgan J. W., Can severe drought reverse woody plant encroachment in a temperate Australian woodland? J. Veg. Sci. 25, 928–936 (2014). [Google Scholar]
- 36.Rigling A., et al. , Driving factors of a vegetation shift from Scots pine to pubescent oak in dry Alpine forests. Glob. Change Biol. 19, 229–240 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Cobb R. C., et al. , Ecosystem dynamics and management after forest die-off: A global synthesis with conceptual state-and-transition models. Ecosphere 8, e02034 (2017). [Google Scholar]
- 38.AghaKouchak A., et al. , Remote sensing of drought: Progress, challenges and opportunities. Rev. Geophys. 53, 1–29 (2015). [Google Scholar]
- 39.Lloret F., Kitzberger T., Historical and event-based bioclimatic suitability predicts regional forest vulnerability to compound effects of severe drought and bark beetle infestation. Glob. Change Biol. 24, 1952–1964 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez P., Neilson R. P., Lenihan J. M., Drapek R. J., Global patterns in the vulnerability of ecosystems to vegetation shifts due to climate change. Glob. Ecol. Biogeogr. 19, 755–768 (2010). [Google Scholar]
- 41.Pérez Navarro M. Á., et al. , Climatic suitability derived from species distribution models captures community responses to an extreme drought episode. Ecosystems 22, 77–90 (2019). [Google Scholar]
- 42.Hobbs R. J., et al. , Novel ecosystems: Theoretical and management aspects of the new ecological world order. Glob. Ecol. Biogeogr. 15, 1–7 (2006). [Google Scholar]
- 43.Batllori E., et al. , Compound fire-drought regimes promote ecosystem transitions in Mediterranean ecosystems. J. Ecol. 107, 1187–1198 (2019). [Google Scholar]
- 44.Michaelian M., Hogg E. H., Hall R. J., Arsenault E., Massive mortality of aspen following severe drought along the southern edge of the Canadian boreal forest. Glob. Change Biol. 17, 2084–2094 (2011). [Google Scholar]
- 45.Vicente-Serrano S., Beguería S., López-Moreno J. I., A multiscalar drought index sensitive to global warming: The standardized precipitation evapotranspiration index. J. Clim. 23, 1696–1718 (2010). [Google Scholar]
- 46.Berdugo M., et al. , Aridity preferences alter the relative importance of abiotic and biotic drivers on plant species abundance in global drylands. J. Ecol. 107, 190–202 (2019). [Google Scholar]
- 47.Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G., Jarvis A., Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005). [Google Scholar]
- 48.Zomer R. J., Trabucco A., Bossio D. A., Verchot L. V., Climate change mitigation: A spatial analysis of global land suitability for clean development mechanism afforestation and reforestation. Agric. Ecosyst. Environ. 126, 67–80 (2008). [Google Scholar]
- 49.VanDerWal J., Falconi L., Januckowski S., Shoo L., Storlie C., SDMTools: Species Distribution Modelling Tools: Tools for Processing Data Associated with Species Distribution Modelling Exercises (R Package Version 1.1-221, 2014). https://CRAN.R-project.org/package=SDMTools. Accessed 20 October 2020.
- 50.Gu Z., Gu L., Eils R., Schlesner M., Brors B., circlize implements and enhances circular visualization in R. Bioinformatics 30, 2811–2812 (2014). [DOI] [PubMed] [Google Scholar]
- 51.De Cáceres M., Legendre P., He F., Dissimilarity measurements and the size structure of ecological communities. Methods Ecol. Evol. 4, 1167–1177 (2013). [Google Scholar]
- 52.Oksanen J., et al. , vegan: Community Ecology Package (R Package Version 2.5-5, 2019). https://CRAN.R-project.org/package=vegan. Accessed 20 October 2020.
- 53.de Cáceres M., Font X., Oliva F., The management of vegetation classifications with fuzzy clustering. J. Veg. Sci. 21, 1138–1151 (2010). [Google Scholar]
- 54.Smithson M., Verkuilen J., A better lemon squeezer? Maximum-likelihood regression with beta-distributed dependent variables. Psychol. Methods 11, 54–71 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Cribari-Neto F., Zeileis A., Beta regression in R. J. Stat. Softw. 34, 1–24 (2010). [Google Scholar]
- 56.Hothorn T., Bretz F., Westfall P., Simultaneous inference in general parametric models. Biom. J. 50, 346–363 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Wood S. N., Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. 73, 3–36 (2011). [Google Scholar]
- 58.Kattge J., et al. , TRY—A global database of plant traits. Glob. Change Biol. 17, 2905–2935 (2011). [Google Scholar]
- 59.van Buuren S., Groothuis-Oudshoorn K., mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67 (2011). [Google Scholar]
- 60.R Core Team , R: A Language and Environment for Statistical Computing, (R Foundation for Statistical Computing, Vienna, 2018). [Google Scholar]
- 61.Olson D. M., et al. , Terrestrial ecoregions of the world: A new map of life on Earth. Bioscience 51, 933–938 (2001). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data related to this paper are available in the main text, SI Appendix, and Datasets 1–3.