Significance
Bacterial promoters are often predicted by similarity to the Escherichia coli −10 and −35 consensus elements. Although these elements are highly conserved in diverse bacterial phyla, only 30 to 43% of promoters we analyzed from Rhodobacter sphaeroides and two other α-proteobacteria contained −7T, a base in the −10 element present in 90 to 99% of promoters from non–α-proteobacteria. Expression from 15 of 16 R. sphaeroides promoters tested in vitro that lacked −7T was very weak, but we identified an essential transcription factor, CarD, that activated all 15 of these promoters. We suggest that promoters lacking a critical base in a consensus element can shape the transcriptome by coordinating expression of large numbers of genes by a single transcription factor.
Keywords: CarD, RNA polymerase, −10 element, Rhodobacter sphaeroides, promoter
Abstract
Using an in vitro transcription system with purified RNA polymerase (RNAP) to investigate rRNA synthesis in the photoheterotrophic α-proteobacterium Rhodobacter sphaeroides, we identified a surprising feature of promoters recognized by the major holoenzyme. Transcription from R. sphaeroides rRNA promoters was unexpectedly weak, correlating with absence of −7T, the very highly conserved thymine found at the last position in −10 elements of promoters in most bacterial species. Thymine substitutions for adenine at position −7 in the three rRNA promoters strongly increased intrinsic promoter activity, indicating that R. sphaeroides RNAP can utilize −7T when present. rRNA promoters were activated by purified R. sphaeroides CarD, a transcription factor found in many bacterial species but not in β- and γ-proteobacteria. Overall, CarD increased the activity of 15 of 16 native R. sphaeroides promoters tested in vitro that lacked −7T, whereas it had no effect on three of the four native promoters that contained −7T. Genome-wide bioinformatic analysis of promoters from R. sphaeroides and two other α-proteobacterial species indicated that 30 to 43% contained −7T, whereas 90 to 99% of promoters from non–α-proteobacteria contained −7T. Thus, promoters lacking −7T appear to be widespread in α-proteobacteria and may have evolved away from consensus to enable their coordinated regulation by transcription factors like CarD. We observed a strong reduction in R. sphaeroides CarD levels when cells enter stationary phase, suggesting that reduced activation by CarD may contribute to inhibition of rRNA transcription when cells enter stationary phase, the stage of growth when bacterial ribosome synthesis declines.
The α-proteobacteria are a Gram-negative, metabolically diverse, biotechnologically important class of bacteria. Although regulation of transcription has been well characterized in the γ-proteobacterium Escherichia coli, much less is known about transcription and its regulation in α-proteobacteria or in most other bacterial classes and phyla. Rhodobacter sphaeroides is a purple nonsulfur α-proteobacterium capable of aerobic and anaerobic respiration, photosynthesis, and CO2 and N2, as well as H2 and polyhydroxybutyrate, production (1, 2). Aerobic growth is similar to that of other Gram-negative bacteria. However, when oxygen levels decline, R. sphaeroides switches to photosynthetic growth, completely remodeling its intracellular membrane and creating pigments necessary to capture light energy (3). In addition to its use as a photosynthetic α-proteobacterial model system, R. sphaeroides has also been studied extensively for its potential in bio-based production of fuels and chemicals (1, 4, 5). A better understanding of its basic transcription properties would improve the usefulness of R. sphaeroides for bioproduction as well as our understanding of α-proteobacterial gene regulation in general.
In E. coli, the synthesis of the translation machinery is tightly regulated at the level of rRNA transcription initiation in order to ensure a sufficient number of ribosomes to support the cellular growth rate. Study of rRNA transcription from the promoters for the seven E. coli rRNA operons has led to many general insights about transcription and its regulation, including the discovery that the nucleoid protein Fis is an important transcription factor (6), that the α-subunit of RNAP is a DNA-binding protein that contributes to specific promoter recognition (7), and that DksA is a transcription factor that functions in conjunction with the second messenger ppGpp to regulate large numbers of bacterial promoters (8, 9). Therefore, we focused on studying the properties of R. sphaeroides rRNA promoters to obtain insights about the mechanism of transcription initiation and its control in α-proteobacteria.
In contrast to E. coli, R. sphaeroides 2.4.1 has two chromosomes and only three rRNA operons, rrnA on chromosome 1 and rrnB and rrnC on chromosome 2. The transcription start sites for the rrnA, rrnB, and rrnC promoters were mapped previously by primer extension (10, 11). In several bacterial species, including Mycobacterium tuberculosis, Thermus thermophilus, and Myxococcus xanthus, rRNA promoters are activated in vitro by CarD, a small protein that binds to the lobe of the RNAP β-subunit and interacts with promoter DNA just upstream of the −10 hexamer (12–16). In the α-proteobacterium Caulobacter crescentus, the CarD homolog localizes to rRNA promoters, and depletion decreases rRNA transcription (17). (The CarD homologs in M. xanthus and C. crescentus are sometimes referred to as CdnL.)
CarD family members are found in the Actinomycetes, Firmicutes, Deinococcus/Thermus, Spirochaetes, δ-proteobacteria, and most classes of α-proteobacteria, but are not found in β- and γ-proteobacteria (13, 14, 17). Although carD is essential in some species, and its depletion or deletion affects expression of many genes in M. tuberculosis and C. crescentus (12, 17–19), a direct, widespread role for CarD in transcription from non-rRNA promoters has not been demonstrated previously with purified components in vitro.
We report here that R. sphaeroides rRNA promoters are activated by CarDRsp in vitro, at least in part because these promoters lack a critical thymine at the last position on the nontemplate strand of the −10 hexamer (−7T) that is present in almost all E. coli promoters recognized by the major holoenzyme. The T at −7 in E. coli promoters fits tightly in a pocket in σ70, an interaction that is critical for transcription initiation (20). Not only do R. sphaeroides rRNA promoters utilize CarDRsp to compensate, at least in part, for the absence of this −7T interaction with σ, but we also show here that many other R. sphaeroides promoters are activated by CarDRsp. In fact, bioinformatic analyses indicate that the majority of promoters in R. sphaeroides, as well as in two other α-proteobacterial species analyzed, lack a T at the last position in the −10 element, suggesting that many promoters in α-proteobacteria may also utilize CarD to increase transcription initiation.
Results
R. sphaeroides rRNA Promoters Initiate Poorly In Vitro with R. sphaeroides RNAP.
To purify R. sphaeroides RNAP for analysis of regulation of R. sphaeroides rRNA promoters in vitro, we inserted sequences coding for a C-terminal 10× histidine tag into the rpoC gene in the R. sphaeroides chromosome by homologous recombination, then purified R. sphaeroides RNAP (RNAPRsp) by nickel-affinity chromatography (SI Appendix, Fig. S1A and Expanded Materials and Methods). The resulting purified RNAPRsp preparation contained proteins of the sizes expected for the β, β′, α, and ω subunits, as well as the major σ subunit, σ93. The identity of σ93 was confirmed by Western blotting with a polyclonal anti-E. coli σ70 antibody that cross-reacted with R. sphaeroides σ93 (SI Appendix, Fig. S1B). Unless otherwise indicated, we use “RNAPRsp” to refer to the holoenzyme containing σ93.
In vitro transcription with the three R. sphaeroides rRNA promoters was carried out with supercoiled plasmids containing rRNA promoter fragments (nontemplate strand sequences shown from −57 to +1 with respect to the transcription start sites in Fig. 1A) inserted into plasmid pRLG770 (6). Transcript lengths of ∼200 nt were predicted based on termination at the E. coli rrnB T1 terminator sequence downstream of the promoter fragment insertion site in the plasmid. Few transcripts of this size were detected from the R. sphaeroides rrnA, rrnB, and rrnC promoters with RNAPRsp (Fig. 1B, lanes 1 to 6) or RNAPEco (Fig. 1C, lanes 1 to 6) under conditions where transcription was detected from the RNA I promoter, part of the replication control system encoded by the plasmid templates (Fig. 1 B and C). In contrast, an abundant transcript of the expected size was detected in reactions containing the E. coli rrnB P1 promoter and either R. sphaeroides or E. coli RNAP (Fig. 1 B and C, lanes 7 and 8). RNAPRsp also produced transcripts from two mutant E. coli or phage promoters that were tested, lacUV5 and T7A1lacO34 (SI Appendix, Fig. S2), consistent with the conclusion that the lack of transcription from the R. sphaeroides rRNA promoters was not a result of inactivity of the purified RNAPRsp holoenzyme.
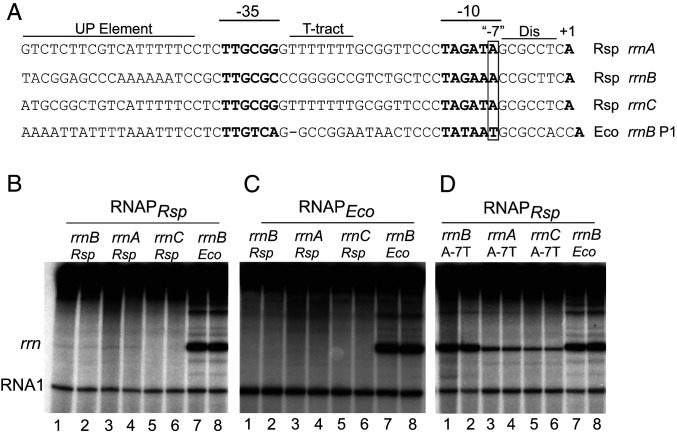
Fig. 1.
Activity of R. sphaeroides rRNA promoters with either R. sphaeroides RNAP or E. coli RNAP. (A) Sequences of the three R. sphaeroides rRNA promoters, rrnA, rrnB, and rrnC, from −57 to the transcription start site, +1, and the E. coli rrnB P1 promoter. The discriminator (Dis) region, T-tract sequence, and UP element are indicated, in addition to the −10 and −35 RNAP recognition hexamers and the transcription start site, which are in bold. The last bp in the −10 element is referred to as the “−7” position (boxed), although it is 8 or 9 bp rather than 7 bp upstream from the transcription start site due to the larger-than-consensus number of bp between the −10 element and the TSS in these promoters. (B) In vitro transcription of the indicated rRNA promoters from plasmid templates with R. sphaeroides RNAP in buffer containing 170 mM NaCl (SI Appendix, Expanded Materials and Methods). Duplicate lanes are shown for each promoter. The RNA I promoter and transcript are part of the plasmid replication control system (SI Appendix, Expanded Materials and Methods). (C) In vitro transcription of the indicated promoters as in B, but with E. coli RNAP. (D) In vitro transcription with R. sphaeroides RNAP as in B, but with the A-7T promoter variants of the three R. sphaeroides rRNA promoters or with the wild type E. coli rrnB P1 promoter. A higher concentration of R. sphaeroides RNAP (50 nM) than E. coli RNAP (10 nM) was used to ensure that the absence of transcription from the R. sphaeroides rRNA promoters was not a result of limiting RNAPRsp. Robust, approximately equivalent transcription was observed from the E. coli rrnB promoter at 50 nM RNAPRsp and 10 nM RNAPEco (B–D).
The inactivity of the R. sphaeroides rRNA promoters was unexpected given that rRNA promoters are very active in moderate- to fast-growing bacterial cells (11, 21). In addition, the R. sphaeroides rRNA promoters contain several features characteristic of E. coli rRNA promoters (11), including a TTG sequence (nontemplate strand) at the upstream end of a putative −35 element, a TA sequence at the upstream end of a likely −10 element, an A+T-rich UP element-like sequence upstream of the −35 element (22), and a G+C-rich sequence immediately downstream from the −10 element, corresponding to the discriminator sequence in E. coli rRNA promoters (23) (Fig. 1A). The high A+T content of the UP element-like sequence and the 7-nt T-tract in the spacer region of rrnA and rrnC were especially striking given the ∼70% G+C content of the R. sphaeroides genome (1).
In contrast to E. coli rRNA promoters (and most other Eσ70-dependent E. coli promoters), each of the R. sphaeroides rRNA promoters contains an A rather than the highly conserved T at the last position in the −10 element (designated here as position −7; Fig. 1A). In fact, the predicted rRNA promoters from other sequenced R. sphaeroides strains also lack a T at −7 (SI Appendix, Fig. S3). The significance of the conserved T at −7 for Eσ70-dependent promoter activity in E. coli was demonstrated long ago by genetic analyses and by structural studies showing that only the thymine, and not other bases at position −7, is compatible with binding in a pocket in σ70 (20, 24–26). The σ-residues that contribute to the −7 pocket are conserved in the major RNAPRsp, consistent with the ability of RNAPRsp to recognize E. coli promoters (SI Appendix, Fig. S4A). Also consistent with the absence of −7T as a determinant of the low activity of the R. sphaeroides rRNA promoters in vitro, we found that a −7T substitution increased transcription from each of the rRNA promoters with RNAPRsp (Fig. 1D), although the mutated rrnA(A–7T) and rrnC(A–7T) promoters were much weaker than rrnB(A–7T) or E. coli rrnB P1. Taken together, these results suggested that R. sphaeroides rRNA promoters might require a transcription activator for function.
R. sphaeroides CarD Activates rRNA Promoters.
Previous studies have reported that the CarD protein from M. tuberculosis, Mycobacterium smegmatis, T. thermophilus, and M. xanthus stimulates rRNA transcription in vitro (12, 13, 27, 28). Among the proteobacteria, CarD is found in the α and δ, but not in the β and γ classes (i.e., not in E. coli) (13, 14). The R. sphaeroides CarD homolog (rsp_2425) is similar in size (169 amino acids), is 25 to 60% identical to CarD proteins from the other bacteria (SI Appendix, Fig. S5), and is essential (5), consistent with a role in one or more critical functions including rRNA transcription.
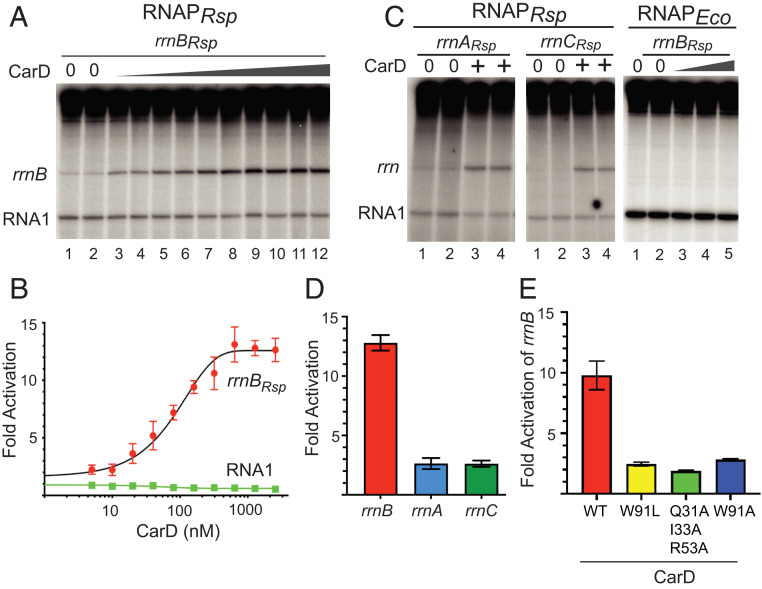
To test this idea, we purified R. sphaeroides CarD after overexpression in E. coli and measured its effects on R. sphaeroides rRNA promoters in vitro (Fig. 2A). CarDRsp activated transcription from the R. sphaeroides rrnB promoter at least 10-fold in vitro, with a half-maximal stimulatory effect (EC50) at 85 nM (Fig. 2B). When added to RNAPRsp, CarDRsp also activated the R. sphaeroides rrnA and rrnC promoters, although transcription from rrnA and rrnC was weaker than from rrnB, and the fold activation by CarDRsp was smaller (Fig. 2 C and D). These results are consistent with a previous report that the R. sphaeroides rrnB promoter was stronger than the rrnA promoter in vivo based on measurements of promoter–xylE fusions (11).
Fig. 2.
Activities of the R. sphaeroides rRNA promoters in vitro with or without purified R. sphaeroides CarD. (A) In vitro transcription of the R. sphaeroides rrnB promoter with 20 nM R. sphaeroides RNAP with or without CarDRsp (wedge indicates CarDRsp range of 5 to 2,560 nM) in buffer with 170 mM NaCl. (B) Fold activation of the R. sphaeroides rrnB promoter by CarDRsp (with/without the indicated concentration of CarDRsp) from experiments like that in A. The RNA I transcript is from a plasmid-encoded promoter. Error bars indicate SD from n = 3 separate assays. (C) In vitro transcription of the R. sphaeroides rrnA or rrnC promoters with R. sphaeroides RNAP as in A with or without 1,280 nM CarD or of the R. sphaeroides rrnB promoter with 10 nM E. coli RNAP with or without 20, 40, or 80 nM CarD. (D) Fold activation of the R. sphaeroides rrnA, rrnB, and rrnC promoters by 1,280 nM CarD with SD from n = 3 assays for each promoter. (E) Fold activation of the R. sphaeroides rrnB promoter by RNAPRsp and 1,280 nM wild-type CarD or W91A, W91L, or Q31A/I33A/R53A triple-mutant CarD (SD from n = 3 assays). Wild-type CarD used in B and E was from different protein preparations, so differences in specific activities could account for the slight differences in fold activation.
To determine whether the 7-nt T-tract found in the spacer region of R. sphaeroides rrnA (and rrnC, which is identical to rrnA), but not in rrnB, could account for the promoter activity difference, we constructed a derivative of rrnA containing a replacement of the T-tract with the corresponding spacer sequence of rrnB. Replacement of the T-tract resulted in a threefold increase in basal rrnA promoter activity but did not alter the extent of activation by CarD (two- to threefold; Fig. 2 and SI Appendix, Fig. S6). Thus, the T-tract accounts, in part, for the reduced activities of rrnA and rrnC but does not account for the difference in activation by CarD (2- to 3-fold for rrnA vs. 10- to 12-fold for rrnB).
CarDRsp did not increase transcription from the plasmid-encoded RNA I promoter when added to RNAPRsp (Fig. 2 A–C) or from R. sphaeroides rrnB using E. coli RNAP (Fig. 2C). The failure of CarDRsp to stimulate E. coli RNAP is consistent with previous reports that effects of CarD are species-specific because the interacting residues in the CarD RID domain and in the β-subunit of RNAP are not highly conserved (28, 29).
We note that a low level of R. sphaeroides rrnB transcript was detected in a previous study with R. sphaeroides RNAP (30). Based on the results reported here, we suggest that the observed activity may have resulted from the presence of small amounts of CarD in the RNAP used in the previous study. In fact, using an antibody raised against CarDRsp (see below), we detected a very low level of CarD in our purified R. sphaeroides RNAP (∼0.01 mol CarDRsp per mol RNAP). In our in vitro transcription experiments, the concentration of CarD introduced from RNAPRsp was well below the EC50 determined for CarD (85 nM) but perhaps sufficient to account for the previously reported transcription from rrnB (SI Appendix, Fig. S1C, lanes 7 and 8).
To address whether the mechanism of activation by CarDRsp is similar to that by CarDMtb, we created substitutions in CarDRsp at positions analogous to those previously shown to affect the DNA interaction or RNAP interaction domain (RID) activities of CarDMtb (13, 14). CarDMtb interacts with DNA at the upstream edge of the −10 hexamer using residue W85 to help wedge open the −10 hexamer. Alignment of the amino acid sequences from Rhodobacter, Mycobacterium, Thermus, Myxococcus, and Caulobacter CarD homologs indicated that CarDRsp-W91 corresponds to CarDMtb-W85 (14, 31) (SI Appendix, Fig. S5). Substitutions for CarDRsp-W91 (CarDRsp-W91L or -W91A) greatly reduced activation of R. sphaeroides rrnB transcription, decreasing the 10-fold effect observed with wild-type CarDRsp to only ∼2.5-fold (Fig. 2E and SI Appendix, Fig. S5), a partial defect similar to that observed for CarDMtb W85A and CarDTth W86A (14). Conserved basic residues in CarD also interact with the DNA near W86 in the T. thermophilus CarD-RNAP-promoter DNA complex structure (13), suggesting that the partial activity of the W91A or W91L CarDRsp proteins can be attributed to DNA interactions that are weakened but not eliminated, rather than to general folding defects. A CarDRsp variant with three substitutions, Q31A, I33A, R53A, corresponding to T. thermophilus CarD RID domain residues R25, I27, and R47, activated rrnBRsp transcription only ∼twofold (Fig. 2E and SI Appendix, Fig. S5). We tentatively conclude that CarDRsp uses a mechanism similar to that of previously characterized CarD proteins.
CarD Directly Activates Other R. sphaeroides Promoters.
Depletion of CarD from M. smegmatis and M. tuberculosis in vivo (12, 18), or deletion of the C. crescentus homolog (19), suggested that CarD affects gene expression broadly, although the approaches did not distinguish direct from indirect effects. CarD colocalized with M. smegmatis RNAP in ChIP-seq experiments at all promoters recognized by the primary σ-factor in vivo (14, 32). Two M. tuberculosis promoters, the AP3 rRNA promoter and the VapB promoter, were also shown to be activated by CarD in vitro (16).
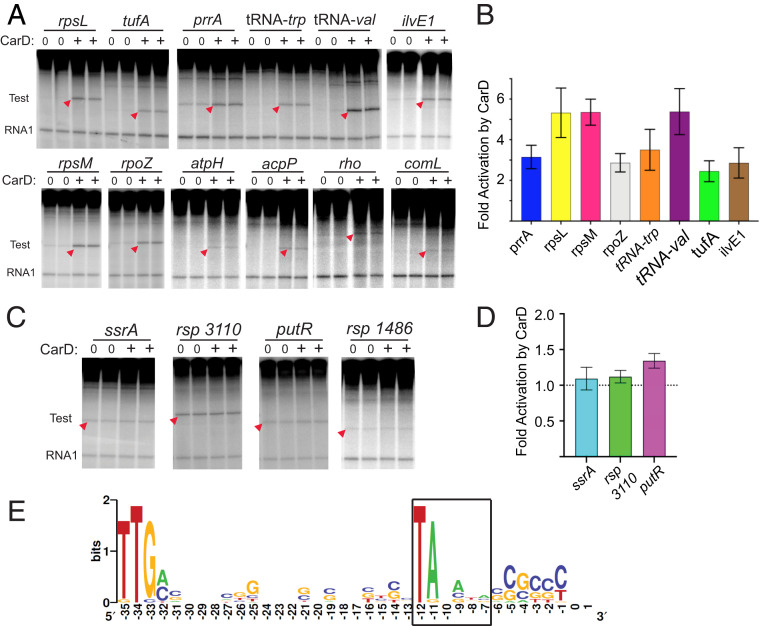
To evaluate whether CarD has a more general role in R. sphaeroides gene expression, we tested its direct effects on transcription in vitro from a set of previously uncharacterized R. sphaeroides promoters. Sixteen additional R. sphaeroides promoters were chosen based on several criteria: genome-wide ChIP signals for σ93 from an R. sphaeroides ChIP-seq dataset (3), proximity to mapped R. sphaeroides transcription start sites (TSSs) (33), and sequence similarity to elements of an E. coli Eσ70 promoter consensus (25). We note that 13 of the 16 non-rRNA promoters lacked −7T (SI Appendix, Fig. S7), even though they were not chosen based on that criterion. PCR-derived fragments containing sequences from ∼150 bp upstream to 30 to 50 bp downstream of the proposed TSSs were cloned into pRLG770, the same plasmid described above for measuring transcription from rRNA promoters, and promoter activities were determined with and without 1,280 nM purified CarDRsp.
The sizes of the transcripts produced in these assays relative to standards of known length confirmed the predicted locations of the promoters (Fig. 3 and SI Appendix, Fig. S7). As with the rRNA promoters, CarDRsp increased the activities of 12 of the 16 non-rRNA promoters. The 12 non-rRNA promoters that were activated by CarDRsp included two for ribosomal protein operons (rpsM and rpsL), two for tRNAs (tRNAtrp and tRNAval), rpoZ (ω-subunit of RNAP), tufA (translation factor EF-Tu), prrA (transcription factor PrrA), rho (transcription termination factor Rho), ilvE1 (a putative aminotransferase), acpP (acyl carrier protein), comL (branched-chain amino acid BamD subunit), and atpH (ATP synthase F1 δ-subunit; Fig. 3A). For the promoters whose basal activities were high enough to quantify, activation by CarDRsp ranged from two- to almost sixfold (Fig. 3B).
Fig. 3.
Activation of R. sphaeroides non-rRNA promoters in vitro with CarDRsp. (A) Transcription in vitro with RNAPRsp (20 nM) and CarDRsp (1,280 nM) in buffer with 100 mM NaCl (SI Appendix, Expanded Materials and Methods). Duplicate lanes with or without CarD are shown for each promoter. Transcripts derived from test promoters are indicated with red arrowheads. Gel images also show position of RNA 1 transcript from the plasmid promoter. SI Appendix, Fig. S7, shows promoter sequences. (B) Fold activation by CarD (+CarD/no CarD) for promoters shown in A whose activities were high enough to quantify accurately. SDs are shown from n = 3 to 6 assays except for ilvE1 (range from two assays). (C) Transcription in vitro of four R. sphaeroides promoters whose activities were unaffected by CarD under the same conditions as in B. SI Appendix, Fig. S7, shows promoter sequences. (D) Fold activation of promoters in C with or without CarD. The SDs shown are from n = 5 assays for ssrA, and the ranges are shown from n = 2 assays for rsp_3110 and putR. (E) WebLogo (34) representation of consensus sequence for 15 promoters whose transcription was activated by CarD in vitro (3 rRNA promoters and the 12 promoters shown in A). The −10 hexamer (−12 to −7) is boxed.
Four of the 16 non-rRNA promoters, including ssrA (tmRNA), putR (a putative transcription factor), rsp_3110 (a putative GST), and rsp_1486 (tetR family homolog), were unaffected by CarDRsp (Fig. 3C). CarDRsp increased transcription less than 1.4-fold from three promoters whose basal activities were high enough to quantify the fold effect (Fig. 3D). In addition, three of the four E. coli promoters tested with RNAPRsp were unaffected by CarDRsp (lacUV5, T7A1lacO34, RNA I; SI Appendix, Fig. S2). Thus, positive regulation by CarDRsp is promoter-specific and not limited to rRNA promoters, and not all promoters are dependent on CarDRsp for activity.
Promoter Features that Correlate with Increased Transcription by CarDRsp.
An image depicting the degree of sequence conservation at each promoter position in the 15 R. sphaeroides promoters activated by CarDRsp (the 12 above plus the 3 rRNA promoters) is shown in Fig. 3E, and promoter sequences are shown in SI Appendix, Fig. S7. None of the 15 promoters activated by CarDRsp contained a T at −7. Each of the other three bases at this position (A, G, and C) was represented in the activated promoter population, suggesting that the absence of −7T rather than the presence of another specific base was the relevant determinant for activation by CarDRsp. In contrast, three of the four R. sphaeroides promoters unaffected by CarDRsp contained a T at −7 (Fig. 3C and SI Appendix, Fig. S7).
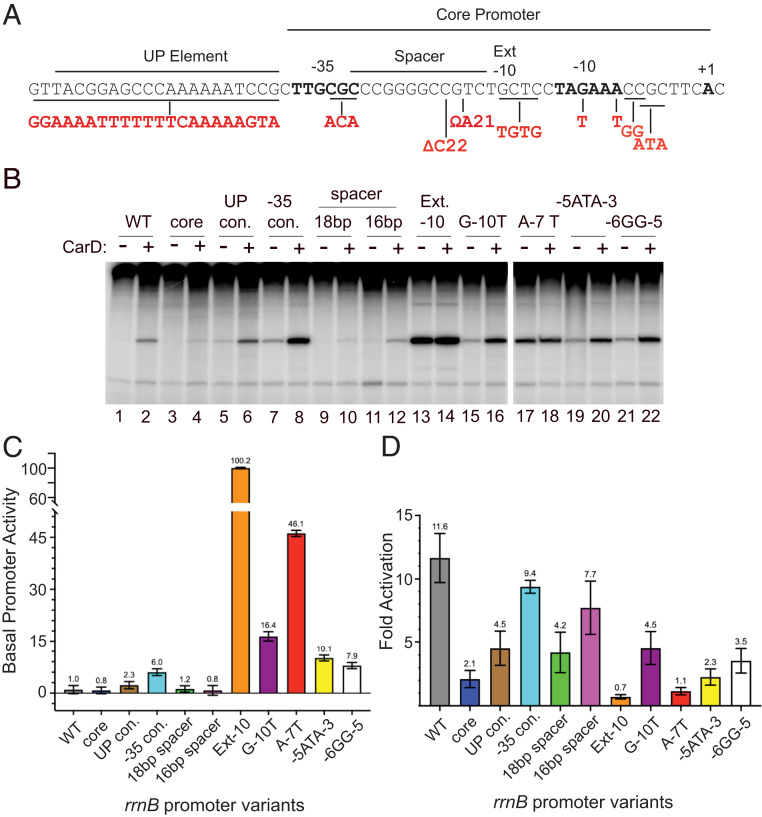
To address whether sequence features in addition to the absence of −7T might also contribute to activation by CarDRsp, we constructed R. sphaeroides rrnB promoter variants with increased or decreased similarity to the E. coli consensus for the UP element, −35 hexamer, extended −10 element, −10 hexamer, discriminator region, and −10/−35 spacer length (35) (Fig. 4A). Transcription activities of the variant promoters were compared to the wild-type promoter in the absence and presence of 1,280 nM CarDRsp in vitro (Fig. 4B).
Fig. 4.
Effects of individual R. sphaeroides rrnB promoter mutations on activation by CarD. (A) Promoter recognition elements (UP element, −35 hexamer, spacer, extended −10, −10 hexamer, and core promoter) are indicated above the R. sphaeroides rrnB promoter sequence. Mutations to create promoter variants are indicated in red (SI Appendix, Tables S2 and S3). Each mutation creates the E. coli σ70 consensus element sequence at the indicated position except the spacer insertion or deletion for which the wild-type R. sphaeroides promoter has the E. coli 17-bp consensus spacing. Triple-mutation −5 ATA −3 alters the base composition in the discriminator region. (B) In vitro transcription of each promoter mutant with RNAPRsp (20 nM) with or without CarD (1,280 nM) in buffer with 170 mM NaCl. (C) Basal activities of mutant rrnB promoters relative to transcription from the wild-type promoter, with SDs from n = 3 to 6 assays for each promoter. (D). Fold activation of each promoter by CarD (+CarD/no CarD). SDs are from n = 3 to 6 assays for each promoter.
We did not detect changes in basal promoter activity resulting from replacement of the rrnB UP element region with either a non-UP element-like sequence or with the E. coli UP element consensus because transcription was too weak for quantitation (Fig. 4 B and C). However, in the presence of CarDRsp, it was apparent that either the native UP element sequence or the consensus UP element increased transcription (Fig. 4 B and D), consistent with conservation of the DNA binding residues in the R. sphaeroides and E. coli α-subunit C-terminal domains (22) (SI Appendix, Fig. S4B).
Substitutions toward consensus for recognition by RNAP in the −35 hexamer, −10 hexamer, and discriminator region increased basal R. sphaeroides rrnB promoter activity (Fig. 4 B and C, compare lane 1 with lanes 7, 13, 16, 17, 19, and 21). Not surprisingly, since the wild-type R. sphaeroides rrnB promoter already has consensus −10/−35 spacing (17 bp), changing the spacing to either 16 bp or 18 bp did not increase basal promoter activity (Fig. 4 B and C, compare lane 2 with lane 10 or 12). Each of these R. sphaeroides rrnB promoter variants was activated by CarDRsp (Fig. 4 B and D), suggesting that they altered transcription without fully bypassing the step(s) affected by CarDRsp. In contrast, the improved extended −10 element and the A–7T mutations increased basal promoter activity to a much greater extent than any of the other substitutions (Fig. 4 B and C, compare lane 1 with lanes 13 and 17), and these promoter variants were no longer activated at all by CarDRsp (Fig. 4 B and C). We conclude that the absence of −7T or an extended −10 element can create a barrier to transcription initiation, and that CarDRsp or mutations to consensus or both can alleviate this barrier.
Context Dependence of Promoter Sequences that Bypass the Effect of CarD on rrnB.
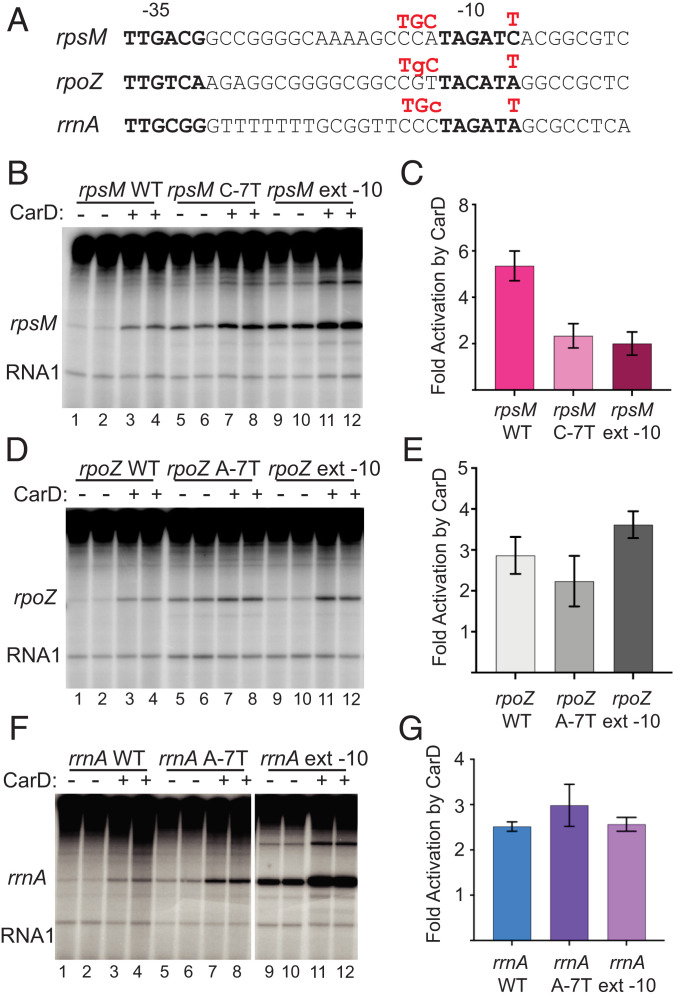
We next addressed whether the −7T or extended −10 substitutions would bypass the requirement for CarD in three other contexts, the rpsM, rpoZ, and rrnA promoters (Fig. 5A). Both substitutions increased the basal activity of the rpsM promoter substantially, and although the substitutions reduced the extent of activation by CarDRsp from ∼fivefold to ∼twofold (Fig. 5 B and C), they did not completely eliminate the effect of CarDRsp. As with the rpsM and rrnB promoters, both substitutions increased the basal activities of the rrnA and rpoZ promoters. However, unlike rpsM and rrnB, the rpoZ and rrnA mutant promoters containing the extended −10 element or −7T substitutions were still activated by CarDRsp almost to the same extent as with the wild-type promoters (Fig. 4 B–D).
Fig. 5.
Context dependence of effects of −7T or extended −10 mutants. (A) Positions in three CarD-activated R. sphaeroides promoters where mutations were made are indicated in red above each promoter sequence, either a T at −7 or a TGC at −15, −14, −13. (B) In vitro transcription with rpsM, rpsM C-7T, and rpsM extended −10 with or without 1,280 nM CarD with 20 nM R. sphaeroides RNAP in buffer with 100 mM NaCl. Duplicate samples are shown for each. (C) Fold activation (+CarD/no CarD) with SD from three assays like that shown in B. (D) Same as B except the rpoZ, rpoZ A-7T, and rpoZ extended −10 promoter plasmids were used as templates. (E) Fold activation (+CarD/no CarD) with SD from three assays like that shown in D. (F) Same as B and D except the rrnA, rrnA A-7T, and rrnA extended −10 promoter plasmids were used as templates and the buffer contained 170 mM NaCl. All lanes are from the same gel, with intervening lanes removed for clarity. (G) Fold activation (+CarD/no CarD) with range from two assays like that shown in F.
Thus, there are promoters lacking −7T (i.e., rsp_3110, above) that are not activated by CarDRsp, and there are mutant promoters containing −7T that are still activated, at least to some extent, by CarDRsp. These “context effects” indicate that CarDRsp can compensate for rate-determining steps in promoter complex formation that derive from other promoter sequence features in addition to or instead of −7T. Such context effects have long been recognized in studies of effects of promoter substitutions on transcription (24, 36). The mechanistic explanations for the context effects reported here are described more extensively in the Discussion.
The Majority of R. sphaeroides Promoters Lack the T at the Last Position in the −10 Element.
To identify likely −10 elements used by R. sphaeroides RNAP genome-wide, we analyzed available R. sphaeroides transcription start site (TSS) data obtained from cells in exponential growth where most transcription is likely by Eσ93, the major holoenzyme (33). This analysis was followed by a bioinformatic search for a TA sequence corresponding to the highly conserved TA at positions −12 and −11 in E. coli −10 hexamers, located at an appropriate distance upstream of each TSS (Dataset S1). The A at position −11 is almost universally conserved in E. coli promoters utilizing the major σ-factor (25) and is essential for promoter activity with RNAPEco (24, 37). Residues involved in recognition of A-11 are conserved in the R. sphaeroides primary sigma factor (σ93; SI Appendix, Fig. S4A). We confirmed that −11A is essential for activity of the R. sphaeroides rrnB promoter with RNAPRsp by showing that substitutions for −11A eliminated transcription (SI Appendix, Fig. S5C).
In Fig. 6, we analyze the promoters in R. sphaeroides that contain −7T genome-wide, based on the presence of the TA motif appropriately positioned upstream of the experimentally defined TSSs, as described above. In support of the interpretation that this promoter collection consists primarily of σ93-dependent promoters, the genome of R. sphaeroides does not contain a gene coding for a σS homolog (11), and other holoenzymes containing alternative σ-factors (at least in E. coli) do not contain a TA motif at the upstream end of their −10 elements. The Bioinformatic Analysis section in SI Appendix, Expanded Materials and Methods, contains further details about the promoters included in the genome-wide promoter analysis.
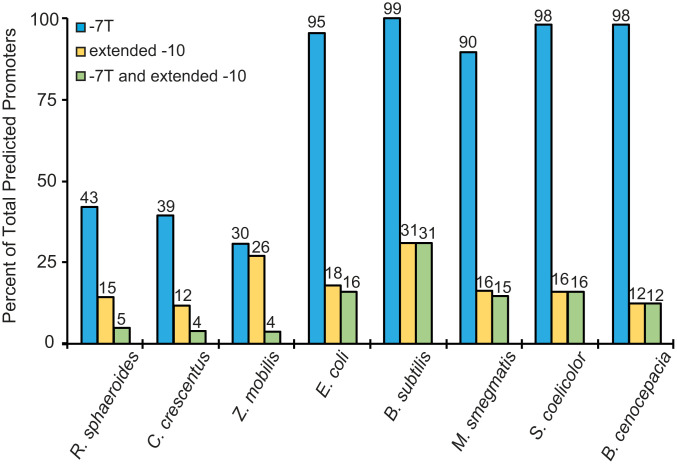
Fig. 6.
Percentage of promoters in indicated bacterial species containing a thymine at the last position of the −10 hexamer (−7) or an extended −10 consensus (TG) at −14, −15, or both. Percentages of promoters with identifiable −10 elements are shown on the y axis. Percentages of those promoters with −7T or extended −10 elements or both are indicated at the top of each bar and are relative to all promoters with identified −10 elements for that species. Promoters were identified based on published TSS data (cited in the main text) and sequence similarity to consensus E. coli −10 element as described in SI Appendix, Expanded Materials and Methods.
In contrast to E. coli promoters, where 95% contain a T at −7, only 43% of R. sphaeroides promoters contained −7T (Fig. 6). As with the R. sphaeroides promoters lacking −7T that we analyzed in vitro, each of the other three bases at position −7 (A, G, and C) was represented in the genome-wide collection of R. sphaeroides promoters lacking −7T, indicating the importance of the absence of −7T rather than the presence of another specific base (Dataset S1 and Bioinformatic Analysis section in SI Appendix, Expanded Materials and Methods).
We also used available TSS data to analyze the predicted −10 elements from a selection of other bacterial species, including the α-proteobacteria C. crescentus (38) and Zymomonas mobilis (39), the γ-protobacterium E. coli (40), the firmicute Bacillus subtilis (41), the actinobacteria Streptomyces coelicolor and M. smegmatis (42, 43), and the β-proteobacterium Burkholderia cenocepacia (44). This analysis showed that ∼39% of the predicted promoters in C. crescentus and 30% in Z. mobilis contain −7T, whereas 95%, 99%, 90%, 98%, and 98% of the predicted promoters from E. coli, B. subtilis, M. smegmatis, S. coelicolor, and B. cenocepacia, respectively, contain −7T (Fig. 6). Thus, although the number of species that we analyzed is limited, our results strongly suggest that the −7T is much less conserved in α-proteobacterial promoters than in promoters from other bacterial phyla or classes.
Because the presence of an extended −10 element eliminated activation of the R. sphaeroides rrnB promoter by CarDRsp (Fig. 4), we also analyzed the percentage of promoters with this element in the genomes of R. sphaeroides and the other bacterial species analyzed above (Fig. 6). Unlike −7T, the extended −10 motif was found in about the same percentage of promoters from both α-proteobacteria and non–α-proteobacteria. The number of promoters with an extended −10 element was much smaller than the percentage with a −7T in most of the species analyzed (Fig. 6).
Although an extended −10 element compensated for the absence of −7T in an rrnB mutant promoter variant, and this promoter was no longer activated by CarDRsp (Fig. 4), an extended −10 element did not correlate well with activation by CarDRsp in the 19 native promoters tested in vitro, in stark contrast to −7T (Fig. 3 and SI Appendix, Fig. S7). Together with our results that the majority of putative promoters in α-proteobacteria lack an extended −10 element (Fig. 6), that the presence of an extended −10 element in six other native R. sphaeroides promoters tested in vitro did not correlate with activation by CarDRsp (SI Appendix, Fig. S7), and that 85% of R. sphaeroides promoters lack an extended −10 element, we conclude that the absence of an extended −10 element is not predictive of activation by CarDRsp.
Levels of CarDRsp Change In Vivo.
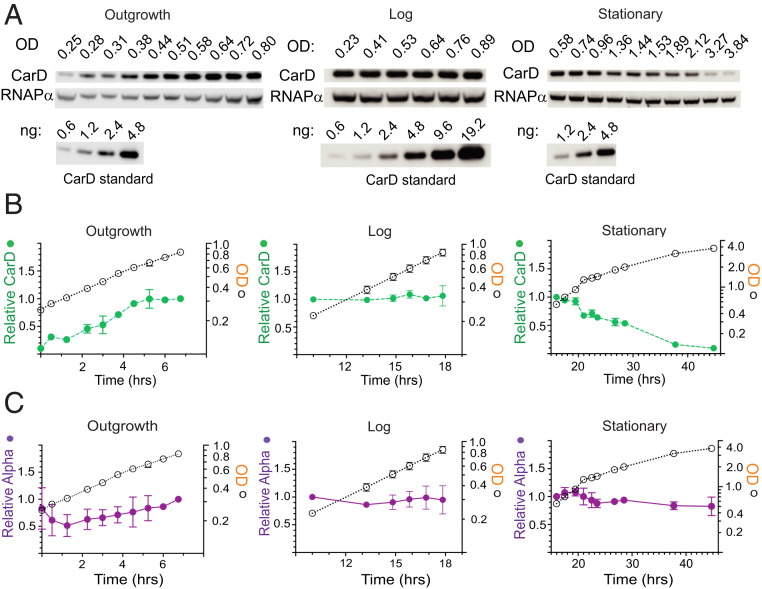
One prediction of our data is that promoters lacking −7T may have evolved in α-proteobacteria to be regulated by changing concentrations of CarD. To test whether CarD levels change in vivo, we used purified CarDRsp to develop a CarD-specific antibody (SI Appendix, Expanded Materials and Methods), and CarDRsp levels were analyzed by Western blot during aerobic growth in minimal medium as cells emerged from stationary phase (outgrowth), grew throughout log phase (log; Fig. 7 A–C), and went into and remained in stationary phase (stationary; Fig. 7 A–C). During outgrowth, the CarDRsp concentration rose gradually to the level present in log phase, whereas the level of the α-subunit of RNAP changed little. The levels of CarD and α remained constant per OD600 (an estimate of total protein concentration) during log phase. However, the CarD concentration dropped more than 10-fold during stationary phase, whereas the RNAP α-subunit concentration decreased only slightly. We analyzed the cellular CarD concentration relative to colony-forming units and found that the decrease in CarD concentration did not reduce cell viability (SI Appendix, Fig. S8). Based on the purified CarDRsp standards run in parallel (Fig. 7A) and a volume of 0.87 μm3 for aerobically grown log-phase cells (45), we estimate that the concentrations of CarD are ∼1.1 µM in log phase and <100 nM in stationary phase (calculation provided in SI Appendix, Expanded Materials and Methods), in the range of the concentrations of CarD that affected transcription in vitro. Taken together, our data indicate that CarD concentrations change in vivo and thus have the potential to regulate transcription in cells. Future studies will investigate the mechanisms responsible for regulating the concentrations (or activities) of CarD under different conditions.
Fig. 7.
CarDRsp levels at different phases of growth. (A) Western blots were performed from R. sphaeroides aerobic cultures grown at 30 °C, diluted from stationary phase into fresh minimal medium at time 0, and sampled at the indicated times. Separate experiments were conducted for each growth phase (outgrowth, log, and stationary; detailed in SI Appendix, Expanded Materials and Methods). For each sample, the OD600 was measured, CarD levels were determined by Western blotting with anti-CarDRsp antibody, and RNAPRsp levels were determined with an anti–α-subunit antibody. Purified CarD standards were analyzed in parallel for quantitation of CarD levels. (B) Relative CarD levels and OD600 values for cultures sampled during outgrowth, log, and stationary phase. (C) Relative RNAPRsp α-subunit levels in the same samples analyzed for CarD levels. Further details are provided in SI Appendix, Expanded Materials and Methods. Values for CarDRsp, RNAPRsp α-subunit, and OD600 were plotted on the same scale by normalizing to the CarD level at an overlapping OD600. Error ranges were determined from two experiments for each.
Discussion
The Absence of −7T Is a Major Determinant of the R. sphaeroides Transcriptome.
We discovered an unexpected feature of R. sphaeroides promoters and the promoters of two other α-proteobacterial species, C. crescentus (38) and Z. mobilis (39). Only 30 to 43% of the promoters from these three α-proteobacterial species contain a thymine base at promoter position −7, the most downstream position in the −10 element, whereas this base is 90 to 99% conserved in promoters in other bacterial phyla (Fig. 6). Most of the 19 R. sphaeroides promoters tested in vitro were very poorly transcribed by R. sphaeroides RNAP and lacked −7T, suggesting an activator might be required to compensate for the absence of the −7T interaction with σ. We suggest that activation of the large number of promoters lacking −7T by transcription factor(s) makes a major contribution to shaping the transcriptome of α-proteobacteria.
Role of −7T in Promoter Function.
Promoters recognized by the major holoenzyme, Eσ70 in E. coli or Eσ93 in R. sphaeroides, have sequences that vary except at six very highly conserved positions, three each in the −35 and −10 hexamers (35), one of which is the thymine at −7. Transcription initiation is characterized by a series of conformational changes in the promoter and in RNAP, driven by binding free energy, in which RNAP first binds to the promoter to form a closed complex, followed by a series of steps in which RNAP melts the DNA strands to form an open complex (35, 46–48). The −7T plays an important role in this multistep process, fitting base-specifically into a conserved pocket formed by the major σ-factor in promoter complexes in very diverse bacterial phyla (e.g., E. coli, T. thermophilus, and M. smegmatis) (20, 26, 49). This interaction with σ occurs subsequent to the formation of an initial partially opened complex containing a 5-nt transcription bubble (47). The −7T interaction with σ facilitates further DNA strand separation, displacement of the N-terminal domain of σ (σ1.1) from the main DNA channel, and stabilization of the open complex to yield a 13-nt bubble (47, 48). The absence of the −7T interaction in the majority of R. sphaeroides promoters would be expected to reduce the rate of formation and stability of the open complex, creating the need for a transcription factor to enhance the rate-limiting kinetic step(s) at these promoters and thereby enhance promoter activity.
CarD Facilitates a Step in the Mechanism of Promoter Opening that Is Affected by the RNAP Interaction with Promoter Position −7T.
We identified a strong correlation between the absence of −7T and activation of native R. sphaeroides promoters by the transcription factor CarD. CarD increased the activity of 15 of the 16 promoters that lacked −7T, whereas it had no effect on 3 of the 4 promoters that contained −7T (Fig. 3 and SI Appendix, Fig. S7). R. sphaeroides CarD shares significant similarity with other CarD homologs from different phyla (e.g., Mycobacteria and Thermus). Transcription activation by CarDRsp required a highly conserved tryptophan residue (W91) in the predicted DNA-binding domain, corresponding to W85 in Mycobacteria and W86 in Thermus, suggesting that CarDRsp has mechanistic features in common with these previously characterized CarD proteins (Fig. 2) (13, 14, 27). Previous structural studies indicated that CarDTth interacts sequence-nonspecifically with promoter DNA at the junction of double-stranded and single-stranded DNA at the upstream end of the −10 hexamer (12–16, 27), adjacent to position −11, where strand separation initiates. Kinetic studies with the Mycobacterial and Thermus CarDs indicated that they affect isomerization step(s) during open complex formation, stimulating formation of a partially melted intermediate and stabilizing open complexes (13, 16, 27, 50).
Thus, CarD and −7T both act on isomerization steps involving extension of a partially open to a fully open transcription bubble and stabilization of the open complex, consistent with the ability of CarDRsp to compensate, at least in part, for the absence of −7T in many promoters.
Context Affects Promoter Activity.
Our in vitro experiments established a strong correlation between promoters lacking −7T and activation by CarDRsp. The high percentage of R. sphaeroides promoters genome-wide lacking −7T is consistent with the relative ease with which we identified specific promoters activated directly by CarDRsp, even though the promoters were not chosen for study because they lacked −7T. However, as described below, the absence of −7T is not a perfect predictor of activation by CarD in all promoter contexts.
As a result of the multistep nature of promoter complex formation and the influence of multiple RNAP interactions with other sequences in the promoter, substitutions at the same position in different promoters have long been known to exhibit different effects on transcription output (context effects; e.g., refs. 24, 36). Promoter interactions with RNAP containing the primary σ-factor involve not only the specific base interactions at the critical conserved positions in the consensus elements, but also phosphate backbone interactions within and outside of the consensus hexamers and base-specific interactions in regions of the promoter that may only be present in a subset of promoters. These interactions nevertheless collectively contribute to the rate of open complex formation and can lead to differences in the rate-determining steps that limit promoter activity at different promoters (48).
Transcription can only be activated by a factor that acts on a rate-determining kinetic step. In some contexts, an interaction involving a particular promoter position may be the major determinant of the step affected by the activator, but in other contexts, another promoter interaction, or more than one interaction, may limit promoter activity, and the activator may have little or no effect on transcriptional output (35, 46). The one promoter in our cohort of 16 native R. sphaeroides promoters examined in vitro that lacked −7T but was not activated by CarDRsp is an example of such a context effect. This promoter, rsp_3110, contains an extended −10 element that we suggest bypasses the requirement for CarDRsp by facilitating strand opening, just as a mutation to −7T in some promoter contexts (e.g., rpoZ and rrnA) does not fully eliminate activation by CarDRsp.
Taken together, our observations suggest that the absence of −7T is a major contributor to the dependence of many R. sphaeroides promoters on an activator for transcription. CarDRsp may not be the transcription factor responsible for regulating every promoter lacking −7T that requires an activator, nor is the presence/absence of −7T likely to be the only determinant of a response to the regulator(s) in all promoter contexts. Thus, prediction of the promoters activated by CarDRsp genome-wide, as well as prediction of the magnitude of the effect of CarDRsp on a specific promoter, will be more complex than simply identifying promoters lacking −7T.
The Absence of a Conserved RNAP Recognition Feature in a Large Set of Promoters Creates a Regulon Controlled by a Transcription Factor.
The unexpectedly low percentage of R. sphaeroides promoters containing −7T (43%) stands in stark contrast to the very high percentage (90 to 99%) containing −7T in other bacterial phyla (Fig. 6). Together, the strong correlation between the absence of −7T and activation by CarDRsp in our in vitro experiments (Figs. 2 and 3 and SI Appendix, Fig. S7), along with the observations that CarD is essential in R. sphaeroides (5) and that CarD concentrations vary with growth phase (Fig. 7), suggest that CarD plays a role in activating many promoters lacking −7T. Most bacterial phyla (e.g., β- and γ-Proteobacteria, Firmicutes, Actinobacteria, Thermus-Deinococcus) have promoters containing −7T. How such a large subset of promoters lacking −7T evolved in α-proteobacteria whereas diverse bacterial phyla contain promoters with −7T remains a question for future exploration. We suggest that regulons in other bacterial phyla that derive from utilization of a transcription factor that compensates for the absence of a crucial base in the core promoter, not necessarily −7T, could be a common mechanism contributing to regulation.
The Role of CarD and −7T in Other α-Proteobacteria and Other Bacterial Phyla.
Our bioinformatic analysis of published genome-scale TSS data indicated that the absence of −7T is a feature of a majority of promoters in R. sphaeroides and the two other α-proteobacterial species for which we were able to predict −10 element sequences genome-wide. Consensus sequences for promoters in the α-proteobacteria C. crescentus and Sinorhizobium meliloti have been proposed (38, 51–53). These sequence predictions included a −10 element consensus element consisting only of positions matching bases near the upstream end of the E. coli −10 element. Furthermore, the rrnA promoter from the δ-proteobacterium M. xanthus lacks −7T and is activated by CarD (14). Together, these studies are consistent with our proposal that CarD activates transcription in many other species. However, the previous studies did not correlate the absence of −7T with activation by CarD. Deletion of C. crescentus CarD (CdnL) was reported to affect transcription from many promoters in vivo, but it was proposed that the number of promoters regulated directly by CarD was small and that most effects of CarD on transcription were likely to be indirect (19). In contrast, our data suggest that α-proteobacterial CarD is likely to affect transcription initiation from many promoters directly.
In Mycobacterial systems, where CarD has been most thoroughly investigated in vitro, most promoters including the rRNA promoter AP3 contain −7T, and thus a role for CarD in compensating for the −7T interaction with σ was not proposed. Other interactions must be rate-limiting in Mycobacterial promoters as a result of DNA sequence differences in the promoter and/or amino acid sequence variation in RNAP among Mycobacteria, Thermus, and proteobacteria (13, 14, 27, 54). The effects of CarD on the M. tuberculosis rRNA promoter are also amplified by a second transcription factor, RbpA, not found in R. sphaeroides (16, 50).
Implications for Regulation of Synthesis of the R. sphaeroides Translation Apparatus.
The R. sphaeroides promoters activated by CarD include a variety of housekeeping genes, including several involved in synthesis and assembly of the translation apparatus (rRNAs, ribosomal proteins, tRNAs, transcription factors; Fig. 3 and SI Appendix, Fig. S7). Consistent with its critical role in translation, carD is an essential gene in R. sphaeroides (5). The decrease in CarD levels in stationary phase may contribute to the decrease in expression of rRNA and other translation-related gene products at this stage in growth.
Additional transcription factors are also likely to contribute to rRNA transcription regulation in R. sphaeroides. In E. coli, the stringent response factors DksA and ppGpp, like CarD in R. sphaeroides, interact directly with RNAP and affect specific promoters because of their specific kinetic properties, in contrast to classical transcription factors whose promoter specificity results from DNA binding sites adjacent to individual promoters (8, 35, 46, 50). R. sphaeroides DksA was previously shown to regulate transcription by E. coli RNAP in vitro (55), suggesting that R. sphaeroides uses ppGpp/DksA as well as CarD to regulate rRNA transcription. In E. coli, the Fis protein activates rRNA promoters by binding to sites upstream of their −35 elements, contributing to their regulation (6). R. sphaeroides rRNA promoters could also respond to transcription factor(s) yet to be identified that bind upstream of the core promoter, since deletions upstream of the −35 element reduce rRNA promoter activity in vivo (11). The integration of effects of CarD and other regulators of R. sphaeroides rRNA transcription will be subjects of a separate study.
Materials and Methods
Further details for each section are provided in SI Appendix, Expanded Materials and Methods.
Bacterial Strains.
E. coli and R. sphaeroides strains are listed in SI Appendix, Table S1.
Bacterial Growth.
R. sphaeroides was grown aerobically in a succinate-based minimal medium (56).
Purification of R. sphaeroides RNAP.
An R. sphaeroides 2.4.1 derivative for purification of RNAP was constructed by creating a His10 tag fused to the C terminus of the β′ subunit and introducing it into the R. sphaeroides chromosome using the nonreplicative plasmid pk18mobsacB (57) and a two-step recombination method. RNAP holoenzyme containing σ93 was purified by Ni-affinity chromatography from aerobically grown cells.
Construction and Purification of R. sphaeroides Sumo-Tagged CarD.
A codon-optimized carD gene was inserted into pETSUMO for purification of an N-terminally tagged His10-SUMO–tagged CarD. Wild type and variant CarD proteins were overexpressed and purified from E. coli BL21 (DE3) pLysS cells (14).
In Vitro Transcription.
Test promoter fragments were PCR-amplified from R. sphaeroides 2.4.1 chromosomal DNA and inserted into pRLG770 (6), and promoter variants were constructed using Multi Site Lightning Quick Change Mutagenesis (Agilent) using primers listed in SI Appendix, Table S2. Transcription was performed with either R. sphaeroides RNAP holoenzyme containing the major σ-factor σ93 or E. coli Eσ70 as indicated.
Western Blot Analysis of CarD.
CarD concentrations in aerobically grown R. sphaeroides cells were determined throughout a growth curve using a polyclonal antibody raised against CarD without a SUMO tag. Polyclonal antibody raised against the α-subunit of R. sphaeroides RNAP was used for comparison.
Bioinformatic Analysis of Promoter Elements.
We used available transcription start site (TSS) information in the literature to determine the likely −10 elements of promoters from the bacterial species indicated in Fig. 6, first identifying the bases most likely corresponding to −11A and −12T and then the DNA sequences most likely corresponding to −7T and the extended −10 element, as described in detail in SI Appendix, Expanded Materials and Methods. The TSSs for R. sphaeroides were determined as previously described (33).
Supplementary Material
Acknowledgments
We thank A. Mehle for access to the Li-Cor Fc instrument used for imaging Western blots and D. Grainger for providing genome-wide B. subtilis transcription start site information prior to publication. We also thank F. Alberge for measurements of R. sphaeroides cell volume. Work in the R.L.G. laboratory is supported by NIH R01 GM37048. K.K.H. was also supported by fellowships from the Jack Kent Cooke Foundation, an NIH Molecular Biosciences Training Grant (T32GM007215), and the Department of Bacteriology. Work in the T.J.D. and R.L. labs is supported by US Department of Energy, Office of Biological and Environmental Research, under Award DE-SC0018409.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2010087117/-/DCSupplemental.
Data Availability.
All study data are included in the article and supporting information.
References
- 1.Mackenzie C., et al. , Postgenomic adventures with Rhodobacter sphaeroides. Annu. Rev. Microbiol. 61, 283–307 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Imam S., Noguera D. R., Donohue T. J., Global insights into energetic and metabolic networks in Rhodobacter sphaeroides. BMC Syst. Biol. 7, 89 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imam S., Noguera D. R., Donohue T. J., Global analysis of photosynthesis transcriptional regulatory networks. PLoS Genet. 10, e1004837 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeilstra-Ryalls J. H., Gomelsky M., Yeliseev A. A., Eraso J. M., Kaplan S., Transcriptional regulation of photosynthesis operons in Rhodobacter sphaeroides 2.4.1. Methods Enzymol. 297, 151–166 (1998). [DOI] [PubMed] [Google Scholar]
- 5.Burger B. T., Imam S., Scarborough M. J., Noguera D. R., Donohue T. J., Combining genome-scale experimental and computational methods to identify essential genes in Rhodobacter sphaeroides. mSystems 2, e00015–e00017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross W., Thompson J. F., Newlands J. T., Gourse R. L., E.coli Fis protein activates ribosomal RNA transcription in vitro and in vivo. EMBO J. 9, 3733–3742 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross W., et al. , A third recognition element in bacterial promoters: DNA binding by the alpha subunit of RNA polymerase. Science 262, 1407–1413 (1993). [DOI] [PubMed] [Google Scholar]
- 8.Gourse R. L., et al. , Transcriptional responses to ppGpp and DksA. Annu. Rev. Microbiol. 72, 163–184 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez-Vazquez P., Dewey C. N., Kitten N., Ross W., Gourse R. L., Genome-wide effects on Escherichia coli transcription from ppGpp binding to its two sites on RNA polymerase. Proc. Natl. Acad. Sci. U.S.A. 116, 8310–8319 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dryden S. C., Kaplan S., Localization and structural analysis of the ribosomal RNA operons of Rhodobacter sphaeroides. Nucleic Acids Res. 18, 7267–7277 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dryden S. C., Kaplan S., Identification of cis-acting regulatory regions upstream of the rRNA operons of Rhodobacter sphaeroides. J. Bacteriol. 175, 6392–6402 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stallings C. L., et al. , CarD is an essential regulator of rRNA transcription required for Mycobacterium tuberculosis persistence. Cell 138, 146–159 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae B., et al. , CarD uses a minor groove wedge mechanism to stabilize the RNA polymerase open promoter complex. eLife 4, 08505 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srivastava D. B., et al. , Structure and function of CarD, an essential mycobacterial transcription factor. Proc. Natl. Acad. Sci. U.S.A. 110, 12619–12624 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallego-García A., et al. , Structural insights into RNA polymerase recognition and essential function of Myxococcus xanthus CdnL. PLoS One 9, e108946 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hubin E. A., et al. , Structure and function of the mycobacterial transcription initiation complex with the essential regulator RbpA. eLife 6, e22520 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallego-García A., et al. , Caulobacter crescentus CdnL is a non-essential RNA polymerase-binding protein whose depletion impairs normal growth and rRNA transcription. Sci. Rep. 7, 43240 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu D. X., Garner A. L., Galburt E. A., Stallings C. L., CarD contributes to diverse gene expression outcomes throughout the genome of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 116, 13573–13581 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woldemeskel S. A., et al. , The conserved transcriptional regulator CdnL is required for metabolic homeostasis and morphogenesis in Caulobacter. PLoS Genet. 16, e1008591 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feklistov A., Darst S. A., Structural basis for promoter-10 element recognition by the bacterial RNA polymerase σ subunit. Cell 147, 1257–1269 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryals J., Little R., Bremer H., Control of rRNA and tRNA syntheses in Escherichia coli by guanosine tetraphosphate. J. Bacteriol. 151, 1261–1268 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estrem S. T., Gaal T., Ross W., Gourse R. L., Identification of an UP element consensus sequence for bacterial promoters. Proc. Natl. Acad. Sci. U.S.A. 95, 9761–9766 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haugen S. P., et al. , rRNA promoter regulation by nonoptimal binding of sigma region 1.2: An additional recognition element for RNA polymerase. Cell 125, 1069–1082 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Moyle H., Waldburger C., Susskind M. M., Hierarchies of base pair preferences in the P22 ant promoter. J. Bacteriol. 173, 1944–1950 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shultzaberger R. K., Chen Z., Lewis K. A., Schneider T. D., Anatomy of Escherichia coli sigma70 promoters. Nucleic Acids Res. 35, 771–788 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y., et al. , Structural basis of transcription initiation. Science 338, 1076–1080 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis E., Chen J., Leon K., Darst S. A., Campbell E. A., Mycobacterial RNA polymerase forms unstable open promoter complexes that are stabilized by CarD. Nucleic Acids Res. 43, 433–445 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.García-Moreno D., et al. , CdnL, a member of the large CarD-like family of bacterial proteins, is vital for Myxococcus xanthus and differs functionally from the global transcriptional regulator CarD. Nucleic Acids Res. 38, 4586–4598 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westblade L. F., et al. , Structural basis for the bacterial transcription-repair coupling factor/RNA polymerase interaction. Nucleic Acids Res. 38, 8357–8369 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karls R. K., Jin D. J., Donohue T. J., Transcription properties of RNA polymerase holoenzymes isolated from the purple nonsulfur bacterium Rhodobacter sphaeroides. J. Bacteriol. 175, 7629–7638 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss L. A., et al. , Interaction of CarD with RNA polymerase mediates Mycobacterium tuberculosis viability, rifampin resistance, and pathogenesis. J. Bacteriol. 194, 5621–5631 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landick R., Krek A., Glickman M. S., Socci N. D., Stallings C. L., Genome-wide mapping of the distribution of CarD, RNAP σA, and RNAP β on the Mycobacterium smegmatis chromosome using chromatin immunoprecipitation sequencing. Genom. Data 2, 110–113 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers K. S., et al. , Genome-wide identification of transcription start sites in two alphaproteobacteria, rhodobacter sphaeroides 2.4.1 and novosphingobium aromaticivorans DSM 12444. Microbiol. Resour. Announc. 9, e00880-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crooks G. E., Hon G., Chandonia J. M., Brenner S. E., WebLogo: A sequence logo generator. Genome Res. 14, 1188–1190 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haugen S. P., Ross W., Gourse R. L., Advances in bacterial promoter recognition and its control by factors that do not bind DNA. Nat. Rev. Microbiol. 6, 507–519 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graña D., Gardella T., Susskind M. M., The effects of mutations in the ant promoter of phage P22 depend on context. Genetics 120, 319–327 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heyduk E., Kuznedelov K., Severinov K., Heyduk T., A consensus adenine at position -11 of the nontemplate strand of bacterial promoter is important for nucleation of promoter melting. J. Biol. Chem. 281, 12362–12369 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Zhou B., et al. , The global regulatory architecture of transcription during the Caulobacter cell cycle. PLoS Genet. 11, e1004831 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vera J. M., et al. , Genome-scale transcription–translation mapping reveals features of Zymomonas mobilis transcription units and promoters. mSystems 5, e00250-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomason M. K., et al. , Global transcriptional start site mapping using differential RNA sequencing reveals novel antisense RNAs in Escherichia coli. J. Bacteriol. 197, 18–28 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warman E., Forrest D., Wade J.T., Grainger D.C., Widespread divergent transcription from prokaryotic promoters. bioRxiv:10.1101/2020.01.31.928960 (2 Feb 2020).
- 42.Jeong Y., et al. , The dynamic transcriptional and translational landscape of the model antibiotic producer Streptomyces coelicolor A3(2). Nat. Commun. 7, 11605 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X., et al. , Transcriptome landscape of Mycobacterium smegmatis. Front. Microbiol. 8, 2505 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sass A. M., et al. , Genome-wide transcription start site profiling in biofilm-grown Burkholderia cenocepacia J2315. BMC Genomics 16, 775 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lemmer K. C., et al. , The NtrYX two-component system regulates the gram-negative cell envelope. mBio 11, e00957-20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galburt E. A., The calculation of transcript flux ratios reveals single regulatory mechanisms capable of activation and repression. Proc. Natl. Acad. Sci. U.S.A. 115, E11604–E11613 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen J., et al. , Stepwise promoter melting by bacterial RNA polymerase. Mol. Cell 78, 275–288.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruff E. F., Record M. T. Jr, Artsimovitch I., Initial events in bacterial transcription initiation. Biomolecules 5, 1035–1062 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyaci H., Chen J., Jansen R., Darst S. A., Campbell E. A., Structures of an RNA polymerase promoter melting intermediate elucidate DNA unwinding. Nature 565, 382–385 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rammohan J., Ruiz Manzano A., Garner A. L., Stallings C. L., Galburt E. A., CarD stabilizes mycobacterial open complexes via a two-tiered kinetic mechanism. Nucleic Acids Res. 43, 3272–3285 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malakooti J., Wang S. P., Ely B., A consensus promoter sequence for Caulobacter crescentus genes involved in biosynthetic and housekeeping functions. J. Bacteriol. 177, 4372–4376 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacLellan S. R., MacLean A. M., Finan T. M., Promoter prediction in the rhizobia. Microbiology (Reading) 152, 1751–1763 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Schlüter J. P., et al. , Global mapping of transcription start sites and promoter motifs in the symbiotic α-proteobacterium Sinorhizobium meliloti 1021. BMC Genomics 14, 156 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kouba T., et al. , The core and holoenzyme forms of RNA polymerase from Mycobacterium smegmatis. J. Bacteriol. 201, e00583-18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lennon C. W., et al. , A Rhodobacter sphaeroides protein mechanistically similar to Escherichia coli DksA regulates photosynthetic growth. mBio 5, e01105–e01114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sistrom W. R., A requirement for sodium in the growth of Rhodopseudomonas spheroides. J. Gen. Microbiol. 22, 778–785 (1960). [DOI] [PubMed] [Google Scholar]
- 57.Schäfer A., et al. , Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: Selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145, 69–73 (1994). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and supporting information.