Abstract
Oleuropein is one of the main phenolic secoiridoid of the olive leaf extract, which is known for its antioxidant and anti-inflammatory effects. The main objective of the present study was to investigate the effectiveness of oleuropein in the ulcerative colitis treatment. An experimental study was designed on rats, which were divided into three groups, group 1 (normal control), group 2 (induced for ulcerative colitis and untreated), and group 3 (induced for ulcerative colitis and treated with oleuropein). Colonic tissue samples were collected from all studied groups and the oxidative stress and antioxidant activity were assessed by evaluating malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), myeloperoxidase (MPO), and nitric oxide (NO) levels. The expression levels of pro-inflammatory cytokines such as IL-1β, TNF-α, IL-10, COX-2, iNOS, TGF-β1, MCP-1, and NF-κB, the pro-apoptotic gene Bax, and the anti-apoptotic gene Bcl2 were assessed in colon tissues to evaluate the effectiveness of oleuropein treatment. Oleuropein was effective on reducing the mortality rate and disease activity index. Oleuropein caused a significant reduction in colon MDA, MPO, and NO levels and a significant elevation in SOD, CAT, and GPX levels and induced the down regulation of analyzed proinflammatory cytokines. Also, downregulation of Bax and upregulation of Bcl2 were observed as a result of oleuropein treatment in comparison with untreated acetic acid induced ulcerative colitis group. Oleuropein showed intestinal anti-inflammatory, antioxidant, and anti-apoptotic effects in ulcerative colitis experimental model.
Key Words: Ulcerative colitis, oleuropein, anti-inflammatory, antioxidant, Bax, Bcl2
Inflammatory bowel disease (IBD) is a group of long- lasting disorders categorized into two major types: ulcerative colitis (UC) and Crohn’s disease, characterized by a recurrent idiopathic inflammation and linked with immune system dysregulation (1, 2). The etiology of IBD especially UC is associated with both genetic and environmental factors with not fully understood pathogenesis (3, 4). Ulcerative colitis (UC) affects the large intestine (colon) and rectum; the most common disease symptoms include abdominal pain, bloody diarrhea, weight loss, fever, and anemia. The main complications of this disease include megacolon, colon cancer, and extra-intestinal complications such as osteoarticular and skin involvement (2, 5). Oxidative stress is one of the main participating factors involved in the disease complication and development. Oxidative stress results from either reactive oxygen species (ROS) overproduction or a decreased antioxidant activity (6, 7). The antioxidant system comprises enzymes such as malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), myeloperoxidase (MPO), catalase (CAT), and nitric oxide (NO) (8). UC is associated with the release of pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, and nuclear factor (NF)-κB (9). The available treatments for UC include aminosalicylates, corticosteroids, immunosupp-ressants, and antibiotics (10). Recently, botanical drugs have shown a distinguished safe effect through modulating the inflammatory response (11). Olea europaea L. is one of the most common available botanical drugs; olive leaf extracts have several effects including antihypertensive, antiatherogenic, anti-inflammatory, hypoglycemic, and hypocholesterolemic activities (12). The olive leaf extracts contain several bioactive compounds, especially phenolic compounds such as phenolic acids, phenolic alcohols, flavonoids and secoiridoids (oleuropein) (13). Oleuropein has an antioxidant effect; it reduces the levels of ROS in pressure ulcers (14). Oleuropein also has anti-inflammatory effect, due to its ability to inhibit pro-inflammatory cytokines synthesis (15, 16). The primary objectives of the present study are to evaluate the effect of oleuropein olive leaf extract in UC treatment by measuring antioxidative stress and antioxidant activity including (MDA, MPO, NO, SOD, CAT, and GPX) in colonic tissue of the rats. Secondly we also aimed to perform a molecular analysis of pro-inflammatory cytokines such as (IL-1β, TNF-α, Il-10, COX-2, iNOS, TGF-β1, MCP-1, and NF-κB) in addition to the pro-apoptotic gene Bax and the anti-apoptotic gene Bcl2 in colonic tissues.
Materials and methods
The study groups
Male laboratory albino rats (n = 30) of similar weight (6-8 weeks old) were purchased from the animal house of the Faculty of Veterinary Medicine, Kafr Al-shiekh University, Egypt. All rats were kept in specific pathogen free cages maintained on standard clean rodent food and tap water ad libitium. All procedures of the present study were conducted in accordance with the Institutional Animal Ethics Committee, Kafr Al-shiekh University guidelines. Study period for all groups was 1 week. Rats were divided into three groups which include 10 rats each. Group 1 (normal control); group 2 (positive control) received 2 ml intrarectal acetic acid 4% to induce colitis according to Kojima et al. (17). Briefly, rats were anaesthetized and a medical polyurethane canal was inserted into their anus up to 6 cm, and 2 ml of 4% acetic acid (Sigma Chemical Co. Egypt) was introduced into the colon. Group 3 (treated with oleuropein after 1 h acetic acid administration) received HPLC oleuropein 70% (Phytochem science Inc., China) at a dose of 350 mg/kg diluted in 1 ml normal saline. Oleuropein was given orally for 7 successive days. All groups were sacrificed after 1 week.
Biochemical analyses
The homogenized colon supernatants were used to determine the levels of the lipid peroxidation biomarkers MDA, SOD, CAT, GPX, MPO, and NO according to the manufactures' instructions (Bio-Diagnostics Co, Egypt). All biochemical parameters were determined spect-rophotometrically on their specific wavelength (18).
RNA extraction and molecular analyses
Total RNA was extracted from the colonic tissue samples using RNA extraction kit (Qiagen, Valencia, CA, USA, cat. No, 74704) according to the manufacturers' instructions. RNA purity and quantity were determined by Q5000 137 nanodrop (Quawell, USA). Reverse transcription was conducted to synthesize the complementary DNA (cDNA) using Reverse Transcription kit (Qiagen, Valencia, CA, USA, Cat. No, 218161) according to the manufacturer’s instructions. The total reaction volume was 20 µL containing 100 ng of the total RNA. qPCR was conducted for pro inflammatory cytokines such as (IL-1β, TNF-α, IL-10, Cox2, iNOS, TGF-β1, MCP-1, and NF-κB), and apoptotic genes (Bax, Bcl2). β- actin was the reference housekeeping gene. Specific primers were designed (Table 1) and used via Maxima SYBR Green/ROX qPCR Master Mix (Thermo Scientific, USA) in a StepOnePlus real time thermal cycler (Applied Biosystems, Life technology, USA). The PCR was performed according to the following conditions (95 ˚C for 15 s, 60 ˚C for 30 s, 72 ˚C for 30 s) for 40 cycles after initial denaturation (95 ˚C, 10 min). The critical threshold (Ct) values of target genes were normalized according to the formula x =2−ΔΔCt, where x = fold change relative to control.
Table 1.
Specific primer sequences used for real-time PCR
| Primer name | Forward (5′- 3′) | Reverse (5′- 3′) |
|---|---|---|
| TNF-α | ATGAGCACAGAAAGCATGA | AGTAGACAGAAGAGCGTGGT |
| IL-1β | TCCAGGATGAGGACATGAGCAC | GAACGTCACACACCAGCAGGTTA |
| COX-2 | CCC CAT TAG CAG CCA GTT | CAT TCC CCA CGG TTT TGA |
| iNOS | GTT CTC AGC CCA ACA ATA CAA GA | GTG GAC GGG TCG ATG TCA C |
| IL-10 | ATAACTGCACCCACTTCCCA | GGGCATCACTTCTACCAGGT |
| MCP-1 | GCTCAGCCAGATGCAGTTAA | TCTTGAGCTTGGTGACAAAAACT |
| TGF-β1 | CCTGCAAGACCATCGACATG | TGTTGTACAAAGCGAGCACC |
| NF-κB | GTGGTGCCTCACTGCTAACT | GGATGCACTTCAGCTTCTGT |
| β-actin | AAGTCCCTCACCCTCCCAAAAG | AGCAATGCTGTCACCTTCCC |
| Bax | CCTGTGCACCAAGGTGCCGGAACT | CCACCCTGGTCTTGGATCCAGCCC |
| Bcl-2 | TGTGGCCTTCTTTGAGTTCGGTG | GGTGCCGGTTCAGGTACTCAGTCA |
Disease activity index (DAI) and mortality rate
The main clinical signs of UC were determined by evaluating the disease activity index (DAI) including stool consistency, rectal hemorrhage, and body weight loss (summarized in Table 2). DAI in all groups was observed daily after induction of colitis by acetic acid. The normal control group (G1) and the treated group with oleuropein (G3) showed no mortality during the whole experiment period. While, positive control group (G2) showed 20% mortality rate (two rats died of the overall 10 rats).
Table 2.
Disease activity index (DAI) score
| Score | Weight loss % | Stool consistency | Occult/gross bleeding |
|---|---|---|---|
| 0 | 0 | Normal | Normal |
| 1 | 1-5% | - | - |
| 2 | 5-10% | Loose stools | Occult blood |
| 3 | 10-15% | - | - |
| 4 | >20% | Diarrhea | Gross bleeding+ mucous |
Statistical analysis
The results were expressed as mean ± standard error of mean (SEM). Data were statistically analyzed by One-way ANOVA followed by Dunnett’s multiple comparisons test using GraphPad Prism version 8.0.0 for Windows, GraphPad Software, San Diego, California USA. Statistical significance was set at P ≤0.05.
Results
Evaluation of treatment with oleuropein on disease activity index (DAI)
The effect of oleuropein on DAI of experimentally induced colitis is summarized in Table 3. The obtained data exhibited a significant increase in DAI in acetic acid-induced colitis group (G2) in comparison with the normal control group (G1) (P≤0.05). The treated group with oleuropein (G3) showed significant reduction in DAI score (P≤0.05).
Table 3.
Effect of oleuropein on disease activity index (DAI) of experimental colitis
| G1 | G2 | G3 | |
|---|---|---|---|
| Initial weight(g) | 123.4 ± 4.2 | 124.3 ± 3.8 | 124.7 ± 3.9 |
| Induction weight (g) | 164.5 ±10.61 | 163.1 ±10.2 | 165.0 ± 9.8 |
| Final weight (g) | 165.9 ± 9.83 | 144.8 ± 8.90 | 154.8 ± 7.6 |
| Body weight Score | 0 ± 0 | 3.6 ± 0.12 | 2.1 ± 0.10 |
| Stool score | 0 ± 0 | 2.5 ± 0.13 | 2.3 ± 0.14 |
| Blood score | 0 ± 0 | 2.4 ± 0.17 | 1.0 ± 0.08 |
| Disease activity index | 0 ± 0 | 2.94± 0.16 a | 1.46 ± 0.11 b |
G1: normal control group; G2: acetic acid-induced colitis group; G3: oleuropein treated group. Data were statistically analyzed as mean ± SEM. Rows carrying different superscript letters [a (highest) – b (lowest)] are significantly different at P ≤ 0.05.
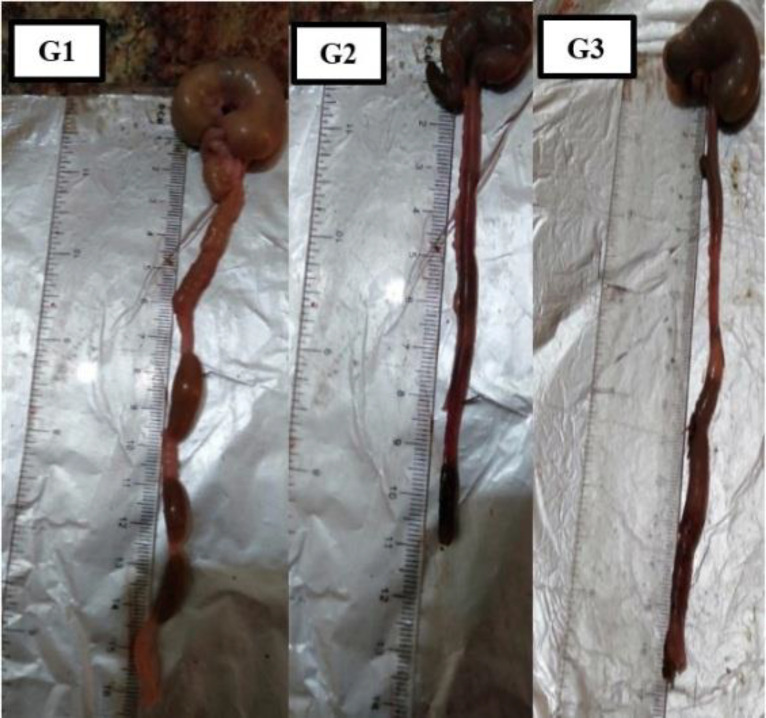
Colon weight and length
Table 4 shows the effect of oleuropein on colon weight and length. The obtained results showed a significant (P ≤0.05) increase in weight and a decrease in length in acetic acid group (G2) as compared to the normal control group (G1). This increased weight was significantly reduced following the administration of oleuropein (G3). No significant difference was noticed between G1 and G3 (Figure 1).
Table 4.
Colon weight and length in all study groups
| Colon weight (g) | Colon length (cm) | |
|---|---|---|
| Mean± SEM | Mean± SEM | |
| G1 | 1.06 ± 0.06 b | 13.95 ± 0.72 a |
| G2 | 1.82 ± 0.09 a | 8.73 ± 0.67 b |
| G3 | 1.19 ± 0.07 b | 14.10 ± 0.73 a |
G1: normal control group; G2: acetic acid-induced colitis group; G3: oleuropein treated group. Mean values with different superscript letters [a (highest) – b (lowest)] in the same column are significantly different at (P ≤ 0.05). Data are presented as (Mean ± SEM); SEM = standard error of mean.
Fig. 1.
Colon length in cm for all groups. G1: normal control group; G2: acetic acid induced group; G3: treated with oleuropein; x1
Oxidative stress and antioxidant activity
Administration of acetic acid (G2) resulted in a significant elevation (P≤0.05) in colon MDA, MPO, and NO levels and a significant decline (P ≤ 0.05) in colon SOD, CAT, and GPX levels in comparison with the normal control group (G1) (Table 5). In contrast, administration of oleuropein (G3) resulted in a significant reduction (P≤0.05) in colon MDA, MPO, and NO levels and a significant elevation (P≤0.05) in SOD, CAT, and GPX levels. The treatment did not return the disrupted oxidant/antioxidant parameter level to the normal level as noticed in G1.
Table 5.
The Levels of MDA, MPO, SOD, CAT, GPX, and NO in colon tissues
|
Malondial
dehyde nmol/g tissue |
Myeloperoxidase U/mg tissue |
Superoxide Dismutase
U/g tissue |
Catalase
U/g tissue |
Glutathione Peroxidase
U/g tissue |
Nitric Oxide mol/g tissue | |
|---|---|---|---|---|---|---|
| Mean± SEM | Mean± SEM | Mean± SEM | Mean± SEM | Mean± SEM | Mean± SEM | |
| G1 | 0.17± 0.02 c | 0.07± 0.005 c | 6.27± 0.36 a | 0.14± 0.01 a | 0.14± 0.01 a | 26.14± 1.2 c |
| G2 | 0.94±0.06 a | 0.59±0.020 a | 2.29±0.13 c | 0.07±0.003 c | 0.07±0.003 c | 59.33±2.81 a |
| G3 | 0.49±0.04 b | 0.24±0.010 b | 3.81±0.19 b | 0.1±0.005 b | 0.1±0.005 b | 40.18±1.6 b |
G1: normal control group; G2: acetic acid-induced colitis group; G3: oleuropein treated group. Mean values with different superscript letters [a (highest) – c (lowest)] in the same column are significantly different at (P≤0.05). Data are presented as (Mean ± SEM); SEM = standard error of mean.
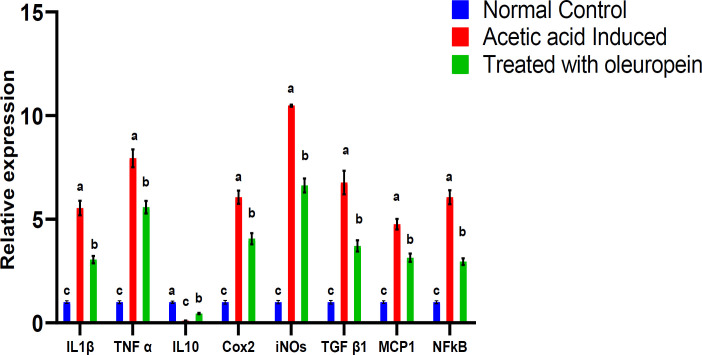
Oleuropein effect on the relative expression of pro-inflammatory cytokines
The qPCR data revealed a significant (P≤0.05) upregulation in the expression levels of IL-1β, TNF-α, IL-10, COX-2, iNOS, TGF-β1, MCP-1, and NF-κB genes in colon tissues of acetic acid-induced UC rats (G2) in comparison with the normal control (G1) group (Figure 2). This expression was significantly downregulated following the administration of oleuropein (G3). The expression levels in the treated group G3 remained significantly higher than that in the normal control group (G1).
Fig. 2.
The expression levels of cytokines in colon tissues and the effect of oleuropein. Values are expressed as mean ± SEM. For each parameter, mean values within columns carrying different superscript letters [a (highest) – c (lowest)] were significantly different at P ≤ 0.05
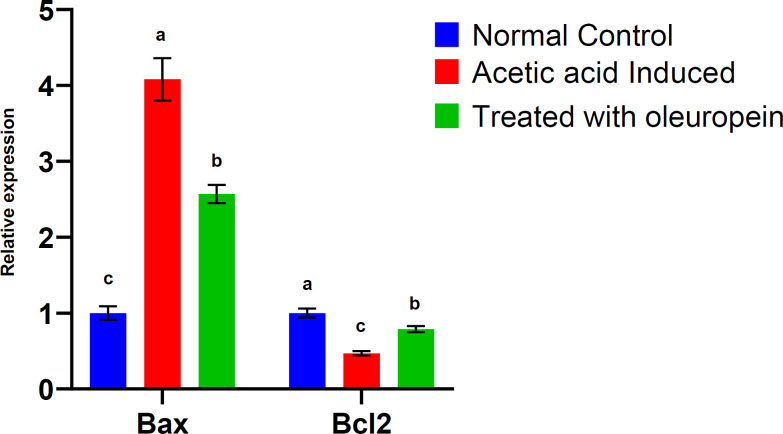
Oleuropein effect on the relative expression of apoptotic genes in colon tissues
The qPCR data revealed a significant (P≤0.05) upregulation of Bax and downregulation of Bcl2 genes in colon tissues of acetic acid-induced UC rats (G2) as compared to the normal control (G1) (Figure 3). This expression was significantly (P≤0.05) upregulated for Bcl2 and downregulated for Bax genes following administration of oleuropein (G3) compared to G2. The expression level of Bax in the treated group G3 remained significantly (P≤0.05) higher than that in the normal control group (G1).
Fig. 3.
The expression levels of apoptotic genes in colon tissues and the effect of oleuropein. Values were expressed as mean ± SEM. For each parameter, mean values within columns carrying different superscript letters [a (highest) – c (lowest)] were significantly different at P ≤ 0.05.
significantly (P≤0.05) higher than that in the normal control group (G1).
Discussion
Until now, the main treatment of UC aimed to reduce the symptoms, and improve the quality of life by decreasing the morbidity rate. DAI is astandard for scoring according to the symptoms. DAI is known as a main indicator for judging the disease degree, and estimating the treatment effectiveness (19). Previous studies have demonstrated that treating the animal with acetic acid to develop colitis closely resembles that of UC in humans (20, 21). In the present study, acetic acid induced colitis (G2) caused body weight loss (as indicated by increased DAI scores), expansion of ulcerative areas, and increased colon weight in comparison with the normal control group (G1). We also demonstrated the oleuropein effectiveness in treating UC, and its effect on the antioxidative stress and antioxidant activity through measuring MDA, MPO, NO, SOD, CAT, and GPX levels. Also, molecular analyses for pro-inflammatory cytokines such as IL-1β, TNF-α, IL-10, COX-2, iNOS, TGF-β1, MCP-1, and NF-κB were performed. In addition, the pro-apoptotic gene Bax and the anti-apoptotic gene Bcl2 expressions were assessed in rats’ colon tissues. Our findings showed that there was not any mortality in the normal control group (G1) and the group treated with oleuropein (G3) during the whole experiment period. While the positive control group (G2) showed 20% mortality rate. Our results revealed the positive effect of oleuropein in reducing the mortality rate as a result of UC. Among UC symptoms, weight loss was detected in 10-15% of G3 animals. Loose stool was also detected in G3, but other symptoms (occult/gross bleeding) were not detected in this group with significant reduction in DAI score (P≤0.05), in agreement with Abu-Gharibeh et al. who reported the reduction in DAI score in mice due to the antioxidant effect of olive oil (22). Moreover, our results revealed that the colon weight was lower in the treated group than in the positive control group, while the colon length decreased in the positive control group and increased in the treated group. Our results were in line with previous studies which reported that the average colon length significantly decreased in the positive control mice (23, 24). Activated phagocytic leukocytes and neutrophils are capable of overproducing ROS in inflamed mucosa; excessive levels of which contribute to the pathogenesis of inflammatory ulcerative disease (8). Furthermore, both the activation of iNOS, COX-2, and the subsequent elevation of end products, such as NO, damage the large intestinal mucous membranes by producing free radicals, and suppressing the antioxidative system (25). Our findings showed significant elevation in colon MDA, MPO, and NO levels and a significant decline in colon SOD, CAT, and GPX in group 2 in comparison with the normal control (G1). While animals treated with oleuropein showed significant reduction in colon MDA, MPO, and NO levels and a significant elevation in SOD, CAT, and GPX levels. El-Akabawy and El-Sherif revealed that MDA, a commonly used indicator of lipid peroxidation and oxidative stress, was elevated in acetic acid induced rats, while the enzymatic activity of antioxidants such as SOD, CAT, and GSH were reduced (26). Our results are also in agreement with the findings of Ebrahimi and Hajizadeh who reported that treatment with olive leaf methanolic extract restored the activity of antioxidant enzymes SOD, GPX, GRX, and CAT and also decreased MDA (27). This suggests that oleuropein has a great antioxidant and radical scavenging activity. The intense local immune response involved in UC includes recruitment of lymphocytes and macrophages, followed by release of pro- inflammatory cytokines and inflammatory mediators (28). Many studies have shown that acetic acid colitis mimics acute human intestinal inflammation by stimulating vasopermeability, prolonging neutrophil infiltration, and inducing the production of inflammatory mediators (29-31). Our gene expression analyses revealed a significant (P≤0.05) up-regulation of IL-1β, TNF-α, IL-10, COX-2, iNOS, TGF-β1, MCP-1, and NF-κB in colon tissues of acetic acid-induced UC rats (G2) as compared to the normal control (G1), while the expression was significantly downregulated in oleuropein treated group (G3) which demonstrated that oleuropein can modulate pro-inflammatory cytokines and mediators in an animal model of colitis. In agreement with Ahmed who reported that olive leaf extract is effective in reducing IL-1β and TNF-α levels (32), Larussa et al. reported that the expression of COX-2 significantly decreased in subjects treated with olive leaf extract (4). Also, Vajdian et al.reported IL-10 downregulation as a result of olive leaf extract treatment (33). Serra et al. reported that the phenolic extract inhibited iNOS induction (34) and Al-rasheed et al. reported the downregulation of NF-κB and TGF-β as a result of treatment with different natural antioxidants (35). Since we found that the expression of Cox2 and iNos significantly reduced in oleuropein treated group (G3), this suggests that the oleuropein may induce anti-inflammatory effects in colonic tissue. Our results support the concept that inhibitors of both inducible enzymes are effective anti-inflammatory agents, which represents an important mechanism to improve the UC development. Also, our study revealed a significant (P≤0.05) upregulation of Bax and downregulation of Bcl2 in acetic acid treated group (G2) in comparison with the normal control group (G1), thereby decreasing the Bcl2/Bax ratio. However, oleuropein treatment elevated the Bcl2/Bax ratio in G3 in comparison with G2, suggesting an antiapoptotic property for oleuropein. Similarly, Almeer and Abdel Moneim reported that olive leaf extract treatment inhibited apoptosis by up-regulating Bcl-2 and downregulating Bax (36). Al-Quraishy et al. also stated that the antiapoptotic activity of olive leaf extract is due to its antioxidant and anti-inflammatory properties (37).
Our study concludes the beneficial effects of oleuropein, as it reduces the mortality rate and DAI in comparison with the positive control group. We demonstrated the ability of oleuropein to counteract oxidative stress and inflammation. Oleuropein caused a significant reduction in colon MDA, MPO, and NO levels and a significant elevation in SOD, CAT, and GPX levels and caused down regulation of proinflammatory cytokines (IL-1β, TNF-α, IL-10, COX-2, iNOS, TGF-β1, MCP-1, and NF-κB). Also, the pro-apoptotic gene Bax was downregulated and the anti-apoptotic gene Bcl2 was upregulated as a result of oleuropein treatment in comparison with untreated group (G2). Further clinical and experimental trials are required to clarify the exact molecular mechanisms of oleuropein action in UC treatment.
Conflict of interest
Authors declare no conflict of interest.
References
- 1.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–25. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 2.Larussa T, Imeneo M, Luzza F. Olive Tree Biophenols in Inflammatory Bowel Disease: When Bitter is Better. Int J Mol Sci. 2019:20. doi: 10.3390/ijms20061390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knights D, Lassen KG, Xavier RJ. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut. 2013;62:1505–10. doi: 10.1136/gutjnl-2012-303954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larussa T, Oliverio M, Suraci E, et al. Oleuropein Decreases Cyclooxygenase-2 and Interleukin-17 Expression and Attenuates Inflammatory Damage in Colonic Samples from Ulcerative Colitis Patients. Nutrients. 2017:9. doi: 10.3390/nu9040391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu YR, Rodriguez JR. Clinical presentation of Crohn's, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin Pediatr Surg. 2017;26:349–55. doi: 10.1053/j.sempedsurg.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Rana SV, Sharma S, Prasad KK, et al. Role of oxidative stress & antioxidant defence in ulcerative colitis patients from north India. Indian J Med Res. 2014;139:568–71. [PMC free article] [PubMed] [Google Scholar]
- 7.Balmus IM, Ciobica A, Trifan A, et al. The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: Clinical aspects and animal models. Saudi J Gastroenterol. 2016;22:3–17. doi: 10.4103/1319-3767.173753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian T, Wang Z, Zhang J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid Med Cell Longev. 2017;2017:4535194. doi: 10.1155/2017/4535194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge H, Tang H, Liang Y, et al. Rhein attenuates inflammation through inhibition of NF-kappaB and NALP3 inflammasome in vivo and in vitro. Drug Des Devel Ther. 2017;11:1663–71. doi: 10.2147/DDDT.S133069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernstein CN. Treatment of IBD: where we are and where we are going. Am J Gastroenterol. 2015;110:114–26. doi: 10.1038/ajg.2014.357. [DOI] [PubMed] [Google Scholar]
- 11.Algieri F, Rodriguez-Nogales A, Rodriguez-Cabezas ME, et al. Botanical Drugs as an Emerging Strategy in Inflammatory Bowel Disease: A Review. Mediators Inflamm. 2015;2015:179616. doi: 10.1155/2015/179616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vezza T, Algieri F, Rodriguez-Nogales A, et al. Immunomodulatory properties of Olea europaea leaf extract in intestinal inflammation. Mol Nutr Food Res. 2017:61. doi: 10.1002/mnfr.201601066. [DOI] [PubMed] [Google Scholar]
- 13.Talhaoui N, Vezza T, Gómez-Caravaca AM, et al. Phenolic compounds and in vitro immunomodulatory properties of three Andalusian olive leaf extracts. J Funct Foods. 2016;22:270–7. [Google Scholar]
- 14.Ahamad J, Toufeeq I, Khan MA, et al. Oleuropein: A natural antioxidant molecule in the treatment of metabolic syndrome. Phytother Res. 2019;33:3112–28. doi: 10.1002/ptr.6511. [DOI] [PubMed] [Google Scholar]
- 15.Impellizzeri D, Esposito E, Mazzon E, et al. The effects of oleuropein aglycone, an olive oil compound, in a mouse model of carrageenan-induced pleurisy. Clin Nutr. 2011;30:533–40. doi: 10.1016/j.clnu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Nediani C, Ruzzolini J, Romani A, et al. Oleuropein, a Bioactive Compound from Olea europaea L as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants (Basel) 2019:8. doi: 10.3390/antiox8120578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kojima R, Hamamoto S, Moriwaki M, et al. [The new experimental ulcerative colitis model in rats induced by subserosal injection of acetic acid] Nihon Yakurigaku Zasshi. 2001;118:123–30. doi: 10.1254/fpj.118.123. [DOI] [PubMed] [Google Scholar]
- 18.Altintas R, Polat A, Vardi N, et al. The protective effects of apocynin on kidney damage caused by renal ischemia/ reperfusion. J Endourol. 2013;27:617–24. doi: 10.1089/end.2012.0556. [DOI] [PubMed] [Google Scholar]
- 19.He Z, Zhou Q, Wen K, et al. Huangkui Lianchang Decoction Ameliorates DSS-Induced Ulcerative Colitis in Mice by Inhibiting the NF-kappaB Signaling Pathway. Evid Based Complement Alternat Med. 2019;2019:1040847. doi: 10.1155/2019/1040847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Low D, Nguyen DD, Mizoguchi E. Animal models of ulcerative colitis and their application in drug research. Drug Des Devel Ther. 2013;7:1341–57. doi: 10.2147/DDDT.S40107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Randhawa PK, Singh K, Singh N, et al. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol. 2014;18:279–88. doi: 10.4196/kjpp.2014.18.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abu-Gharbieh E, Bayoumi FA, Ahmed NG. Alleviation of antioxidant defense system by ozonized olive oil in DNBS-induced colitis in rats. Mediators Inflamm. 2014;2014:967205. doi: 10.1155/2014/967205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy AS, Simon O, Shelly J, et al. 6-Shogaol reduced chronic inflammatory response in the knees of rats treated with complete Freund's adjuvant. BMC Pharmacol. 2006;6:12. doi: 10.1186/1471-2210-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassan SM, Hassan AH. The possibility of using shogaol for treatment of ulcerative colitis. Iran J Basic Med Sci. 2018;21:943–9. doi: 10.22038/IJBMS.2018.28616.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sklyarov AY, Panasyuk NB, Fomenko IS. Role of nitric oxide-synthase and cyclooxygenase/lipooxygenase systems in development of experimental ulcerative colitis. J Physiol Pharmacol. 2011;62:65–73. [PubMed] [Google Scholar]
- 26.El-Akabawy G, El-Sherif NM. Zeaxanthin exerts protective effects on acetic acid-induced colitis in rats via modulation of pro-inflammatory cytokines and oxidative stress. Biomed Pharmacother. 2019;111:841–51. doi: 10.1016/j.biopha.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Ebrahimi A, Hajizadeh Moghaddam A. The effect of olive leaf methanolic extract on hippocampal antioxidant biomarkers in an animal model of Parkinson’s disease. Journal of Basic andClinical Pathophysiology. 2017;5:9–14. [Google Scholar]
- 28.Guan Q, Zhang J. Recent Advances: The Imbalance of Cytokines in the Pathogenesis of Inflammatory Bowel Disease. Mediators Inflamm. 2017;2017:4810258. doi: 10.1155/2017/4810258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gopu B, Dileep R, Rani MU, et al. Protective Role of Curcumin and Flunixin Against Acetic Acid-Induced Inflammatory Bowel Disease via Modulating Inflammatory Mediators and Cytokine Profile in Rats. J Environ Pathol Toxicol Oncol. 2015;34:309–20. doi: 10.1615/jenvironpatholtoxicoloncol.2015013049. [DOI] [PubMed] [Google Scholar]
- 30.Araujo DFS, Guerra GCB, Junior RFA, et al. Goat whey ameliorates intestinal inflammation on acetic acid-induced colitis in rats. J Dairy Sci. 2016;99:9383–94. doi: 10.3168/jds.2016-10930. [DOI] [PubMed] [Google Scholar]
- 31.Suluvoy JK, Sakthivel KM, Guruvayoorappan C, et al. Protective effect of Averrhoa bilimbi L fruit extract on ulcerative colitis in wistar rats via regulation of inflammatory mediators and cytokines. Biomed Pharmacother. 2017;91:1113–21. doi: 10.1016/j.biopha.2017.05.057. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed KM. The effect of olive leaf extract in decreasing the expression of two pro-inflammatory cytokines in patients receiving chemotherapy for cancer A randomized clinical trial. Saudi Dent J. 2013;25:141–7. doi: 10.1016/j.sdentj.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vajdian R, Mosaffa N, SEYYED TJ, et al. Effect of Olive Leaf Extract on Cytokines Secreted by Macrophage. Novelty in Biomedicine. 2016;4:116–20. [Google Scholar]
- 34.Serra G, Incani A, Serreli G, et al. Olive oil polyphenols reduce oxysterols-induced redox imbalance and pro-inflammatory response in intestinal cells. Redox Biol. 2018;17:348–54. doi: 10.1016/j.redox.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Rasheed NM, Fadda LM, Al-Rasheed NM, et al. Down-regulation of NFKB, Bax, TGF-β, Smad-2mRNA expression in the livers of carbon tetrachloride treated rats using different natural antioxidants. Braz arch biol technol. 2016;59:e16150553. [Google Scholar]
- 36.Almeer RS, Abdel Moneim AE. Evaluation of the Protective Effect of Olive Leaf Extract on Cisplatin-Induced Testicular Damage in Rats. Oxid Med Cell Longev. 2018;2018:8487248. doi: 10.1155/2018/8487248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Quraishy S, Othman MS, Dkhil MA, et al. Olive (Olea europaea) leaf methanolic extract prevents HCl/ethanol-induced gastritis in rats by attenuating inflammation and augmenting antioxidant enzyme activities. Biomed Pharmacother. 2017;91:338–49. doi: 10.1016/j.biopha.2017.04.069. [DOI] [PubMed] [Google Scholar]