Abstract
Aloe vera is used for its large variety of biological activities such as wound healing, anti-fungal, anti-inflammatory, hypoglycemic, immunomodulatory, gastroprotective, and anti-cancer. Although the beneficial effects of Aloe vera on wound healing have been proven, little is known about its effects at the cellular level. In this study, we evaluated the angiogenic and migrative effects of Aloe vera gel on fibroblasts and endothelial cells. Fibroblasts and endothelial cells were cultured in monolayer conditions with low glucose DMEM with 10% serum and 1% penicillin-streptomycin. Fresh and mature leaves of Aloe vera were used for gel preparation. Cell proliferation and morphology were studied by an inverted microscope. The migration of fibroblasts was assessed by scratch assay. MTT assay was performed for cell viability assessment, and real-time RT-PCR was used for evaluation of PECAM-1, integrin α1 and β1 transcription. After two days, the protein level of PECAM-1 was detected by flow cytometry. Our results showed that Aloe vera has a higher proliferative effect on fibroblasts in comparison with endothelial cells. Aloe vera also induced the migration of fibroblasts. The viability of both types of cells was similar to control ones. Integrin α1, β1 and PECAM-1 gene expression increased significantly (P <0.005) in Aloe vera treated fibroblasts and endothelial cells in comparison with the control groups. However, the expression of these genes was significantly higher in fibroblasts in comparison with endothelial cells. Protein levels of PECAM-1 showed no change in both cell types upon Aloe vera treatment. Aloe vera gel induced angiogenic and cell adhesion properties in fibroblasts more than endothelial cells. Further investigations are needed to show the main role of fibroblasts rather than endothelial cells in wound healing by Aloe vera administration.
Key Words: Aloe vera gel, fibroblast, endothelial cells, integrin, PECAM-1
Wound healing is a dynamic process that is directed by multiple cell types in four phases, hemostasis, inflammation, proliferation, and remodeling(1). Resident cells of the tissue as well as recruited cells participate in the healing process, and these local epithelial cells, fibroblasts, dendritic cells, and endothelial cells secret angiogenic factors (2). Fibroblasts also produce extra cellular matrix (ECM) for promoting angiogenesis (3). Each healing cell type and angiogenic factor have unique function time points for the healing process; for example, stimulation and migration of endothelial cells occur in the proliferation phase (2). Angiogenesis occurs in the proliferation phase to compensate for vascular injury and accelerate the healing of wounds (4). It has also been found that cluster of differentiation 31 (CD31)also known as Platelet endothelial cell adhesion molecule 1 (PECAM-1) is involved in endothelial cell migration and angiogenesis (5).
Integrins play an important role in wound healing, and help to express the genes associated with this process (6). Cell surface integrins are also essential for cell migration (7), proliferation, and angiogenesis (6). Integrins α1β1 are expressed in microvascular endothelial cells (8), and they provide the essential support for angiogenesis and migration of these cells (9). Antibody against integrin α1 inhibited fibroblast migration in collagen substratum (10). Integrins α1β1 and α2β1 are known as a major receptor in regulating ECM remodeling (11). Matrix remodeling is vital for wound healing. It has been found that integrins play a crucial role in re-epithelialization due to increasing proliferation in keratinocytes. Integrins control cell migration via the ECM as well (12).
Medicinal plants are used for accelerating wound healing, and Aloe vera (Aloe barbadensis Miller from Liliaceae family) is one of the most useful plants (13). Traditionally, it is used for a large variety of biological activities such as wound healing, anti-fungal activity, anti-inflammatory activity, hypoglycemic, immunomodulatory, gast-roprotective, anti-cancer (14), and antibacterial effects (15, 16). It is believed that these activities of Aloe vera are due to the synergistic action of many bioactive compounds present in the gel (17). The gel is the inner layer of Aloe vera leaves, which contains amino acids, anthraquinones, carbohy-drates, chromones, dietary fibers, enzymes, hormones, minerals, sterols, proteins, and organic compounds (18).
Anthraquinones are derivatives of anthracene from the quinone group, and are mainly recognized as laxative compounds. Recently, studies have shown that anthraquinones have antioxidant, antiviral, cytotoxic, and anti-inflammatory effects on squamous cell carcinoma cells and a bacteriostatic effect on Streptococcus. Aloin and aloe-emodin are the two main anthraquinones of Aloe vera (14). Aloin accelerates wound healing by increasing the proliferation of endothelial cells and fibroblasts and also inducing the expression of epidermal growth factor (19).
Carbohydrates include monosaccharides and polysaccharides. Acemannan is one of the most important polysaccharide compounds found in leaf gels of Aloe vera. These compounds have immunomodulatory, anti-cancer, antioxidant, wound healing, and neuroprotective effects (20). Another polysaccharide of Aloe vera is a water-soluble compound called glucomannan, which can increase fibroblasts proliferation, and accelerate wound healing (21). Today, glucomannan is used as a supplement to lose weight, regulate cholesterol levels, and treat constipation, diabetes, and atherosclerosis. New research showed that glucomannan of Aloe vera can also be used as an anti-tumor compound (22). Enzymes include catalase, amylase, oxidase, cellulase, lipase, and carboxypeptidase. Calcium, chlorine, chromium, copper, iron, magnesium, manganese, phosphorus, potassium, sodium, and zinc are the main mineral compounds of Aloe vera gel (22). Aloe vera has 4 sterols, lupeol, sitosterol, cholestrol, and campesterol. These sterols have anti-inflammatory properties. Lupeol also has antiseptic and analgesic effects (23).
Acceleration of wound healing has occurred by oral and local administration of Aloe vera gel (24). It had shown that the oral administration of Aloe vera gel increased angiogenesis and accelerated the acute radiation-delayed wound healing process (25). In wound healing, Aloe vera promotes epidermal keratinocytes proliferation and migration (26). In corneal inflammation, transpla-ntation of human corneal endothelial cells plays an important role in the regeneration of the cornea (27). Although these cells have limited capacity to proliferate (28), Aloe vera decreases inflammation by the release of IL-1β from human corneal cell line (27). Aloe-emodin, one of Aloe vera constituents was shown to increase white blood cells count after 6 weeks in fish (29). In vivo studies showed that wound healing was promoted using the mesenchymal stem cell (MSCs) (30). When adipose-derived stem cells were loaded on Aloe vera gel, burn wounds healed with decreased scar formation (31). We previously found that Aloe vera /collagen increased PECAM-1 and integrins α1β1 in adipose stem cells (32). A vast majority of studies illustrated the therapeutic activity of Aloe vera on the regeneration of tissues (16). Since little is known about the effect of Aloe vera on the different cells of a tissue, this study aimed to evaluate the proliferative, angiogenic, and migrative effect of Aloe vera gel on fibroblasts and endothelial cells, and to compare the gene expression level of PECAM-1, integrin α1β1in these cell lines.
Materials and methods
Aloe vera gel preparation
Aloe vera gel was extracted from a fully mature Aloe vera plant as a previously published method (33). In brief, the mature leaves were removed after surface washing, and their shell was removed under clean conditions. White pulp Aloe vera gel was homogenized by a mixer and centrifuged at 12,000 rpm for 30 min at 4 °C to divide the fibers. The supernatant was transferred into a new falcon, and sterilized with chloroform (10% gel volume) and stored in a refrigerator.
Cell culture
Human fibroblast cell line IBRC C10003 and endothelial IBRC C10638 were purchased from Pasteur Institute Cell Bank, Iran. Fibroblasts and endothelial cells were cultured at 37 °C, 5% CO2 in low glucose DMEM (Gibco, UK) with 10% placental serum. After 24 h, the culture media was replaced, and then the culture medium was changed every 2–3 days. During the cell culture, the morphology of cells was determined microsco-pically. The cells were trypsinized (0.25% trypsin/0.2% EDTA; Sigma, USA) and counted. Every 70-100 thousand cells were transferred to a flask, and 5 ml medium was added to them (32).
Hematoxylin and eosin staining
The cells (4000 cells/cm2) were cultured on the coverslip glass, in 6 well plates. In the first step, the supernatant has been removed from the cells, and the cells have been washed by PBS and fixed with 4% paraformaldehyde after emptying the PBS, and washed once with PBS for one min.
To perform the hematoxylin and eosin (H&E) staining, cells on coverslips have been transferred to hematoxylin solution for 10 min, and after washing were immersed in eosin solution for 15 s. At the end, coverslips were inversely mounted on slides, and studied under the microscope. The cells were stained three times (34).
Cell viability evaluation
The cells of all groups were cultured in 96-well plates (3000 cells per well), and a working medium containing Aloe vera (10%) was added to the treated cells group. After 24 h, culture media was removed and 200 µl serum-free DMEM medium and 20 µl 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) solution (5 mg/ml, Sigma USA) were added to the cells. After 4 h, 100 µL dimethyl sulfoxide (DMSO, Sigma, Germany) was added to the chambers to dissolve the formazan crystals. After 30 min in a dark room, the optical density was recorded by ELISA reader at 540 nm (32). This experiment was repeated three times.
Scratch test
Fibroblasts were cultured by the mentioned method until 90% confluency was reached. At this point, the fibroblasts were split into 2 groups and Aloe vera gel was added to the experiment group. A part of the pellet was scratched using a sampler head. Cells were incubated for 24 h, and then the number of migrated fibroblasts was determined using a microscope. This experiment was conducted three times (35).
RNA extraction, cDNA generation, and quantitative reverse transcriptase-polymerase chain reaction (real-time RT-PCR)
The RNX-Plus solution (phenol + guanidine isothiocyanate) for total RNA extraction was the product of SinaClon (Cat.no: PS4131). In brief, 24 h after treatment, the culture medium was removed from groups, and total RNA was extracted from cells using the RNX-Plus Solution Kit, according to the manfacturer’s protocol. After purification and quantification, RNA concentration was determined by measuring the optical density at 260 and 280 nm using Nanodrop (Nanodrop- ND-1000, Thermo Fisher Scientific, USA). PrimeScript reagent kit (Cat.no: RR037Q, Takara, Japan) was used for cDNA synthesis. Real Q Plus 2x and the first strand of cDNA was generated from 500 ng of extracted total RNA using the Takara Prime Script reagent kit according to the protocol provided by the manufacturer. Master Mix Green High ROX (Cat.no: A 325402, Ampliqon, Denmark) was used for real time PCR analysis. SYBER green and ROX were used as the reporter and reference dies, respectively. The relative amount of mRNA for each target was normalized to the gene expression. Gene-specific primer sets used in this study are shown in Table 1. Experiments were repeated three times.
Table 1.
Gene-specific primer sets used for real-time RT-PCR
| ITGA1-F | 5’-CGGTACAATCATACAGGCCA-3’ |
| ITGA1-R | 5’-TTGCTCCTCCTTCTCTGTTC-3’ |
| ITGB1-F | 5’-AATGCCTACTTCTGCACGAT-3’ |
| ITGB1-R | 5’-GCTTCTCTGCTGTTCCTTTG-3’ |
| PECAM1-F | 5’-CTGGGAGGTCGTCCATGT-3’ |
| PECAM1-R | 5’-CACAGGACTCTCGCAATCC-3’ |
Flow cytometric evaluation
Fibroblasts and endothelial cells were cultured with and without 10% Aloe vera for 24 h, and trypsinized cells were transferred into flow cytometry tubes. The cells (105) were washed with 1% BSA PBS. The staining of cells was performed with 3 μl of phycoerythrin (PE) labeled antibody (Biolegend, London, UK). The cells were kept in the dark at room temperature for 30 min, and then washed with 1% BSA PBS. The cellular deposition was resuspended in 0.5 ml 1% BSA PBS. Finally, the expression of CD31 was analyzed by winmdi software, and overlaid on unstained cell histograms (36).
Statistical analysis
Results are presented as means SEM. Statistical differences between different groups were tested by One-way analysis of variance (ANOVA) using Graph Pad Prism software. A p <0.05 was determined as significant (32).
Results
Normal morphological appearance of fibroblasts and endothelial cells in the presence of Aloe Vera
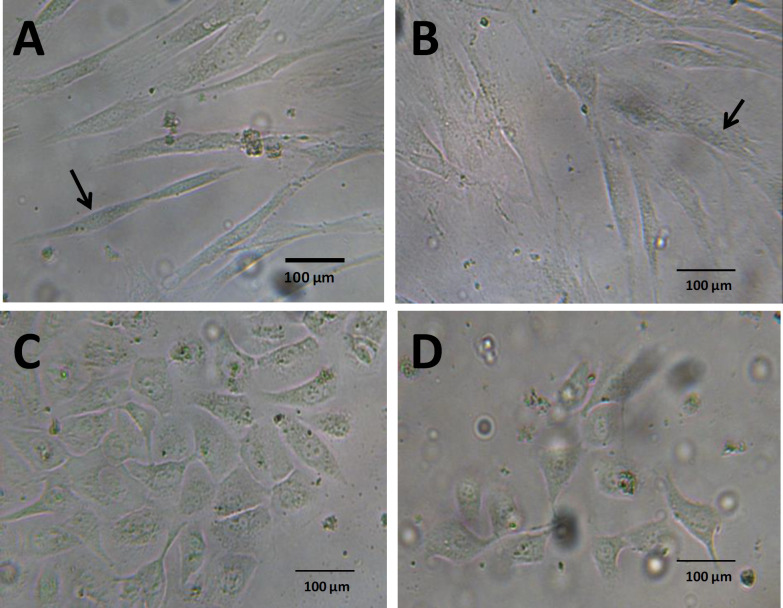
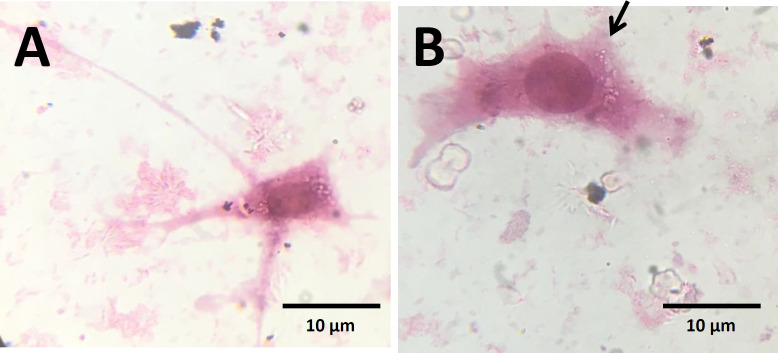
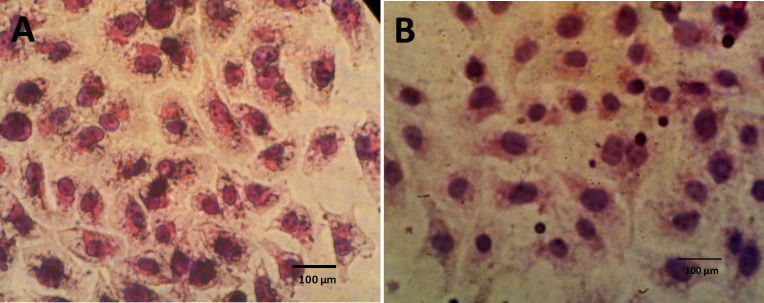
The morphology of fibroblasts and endothelial cells with and without Aloe vera was observed using the inverted microscope, and no morphological change was detected in Aloe vera treated endothelial cells in comparison with the control group. However, treated fibroblasts appeared more widespread in comparison with the spindle shape of fibroblasts in the control group (Figure 1 A and B). High magnification of fibroblasts by H&E staining showed fan shape morphology of Aloe vera treated fibroblasts (Figure 2B). The migration of cells was induced by Aloe vera treatment (Figure 2B). There was no obvious change in Aloe vera treated endothelial cells at high magnification of H&E staining (Figure 3A and B).
Fig. 1.
Morphology of fibroblasts and endothelial cells under inverted microscope. A) Fibroblasts of control group; B) Fibroblasts in the presence of Aloe vera; C) Endothelial cells of control group; D) endothelial cells in the presence of Aloe vera
Fig. 2.
Morphology of fibroblasts by H&E staining. A) Fibroblasts of control group; B) Fibroblasts in the presence of Aloe vera. Migrating fan shape fibroblasts were observed in the presence of Aloe vera. Arrow indicates the migrating side of the cell
Fig. 3.
Morphology of endothelial cells by H&E staining. A: Endothelial cells of control group, B: Endothelial cells in the presence of Aloe vera
Aloe vera induced migration of fibroblasts
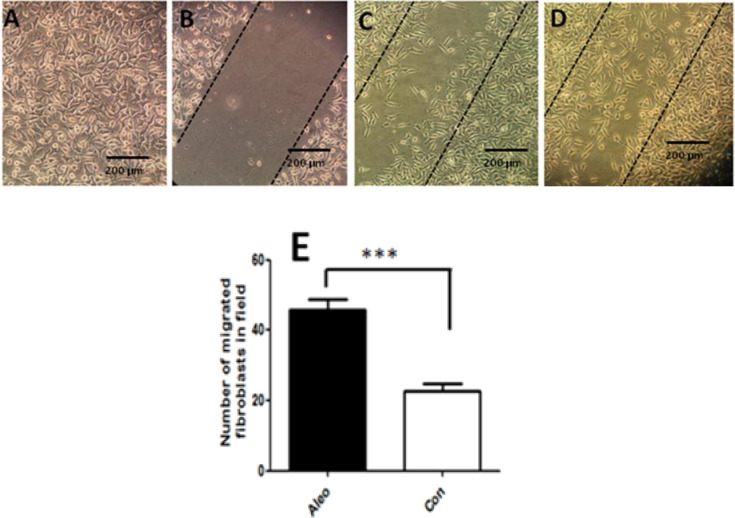
Our finding showed that fibroblasts proliferated and migrated toward the scratch area during 24 h in the treated group (Figure 4). Statistical analysis revealed that the number of migrated fibroblasts in Aloe vera treated groups was significantly (P<0.0005) higher than controls (Figure 4 E)
Fig. 4.
Scratch assay micrographs. A) Confluent fibroblasts; B) Immediately after scratch; C) Control fibroblasts 24 h after scratch; D) Aleo vera treated fibroblasts 24 h after scratch; E) Quantification of migrated fibroblasts to scratch area using image j software (*** P < 0.001).
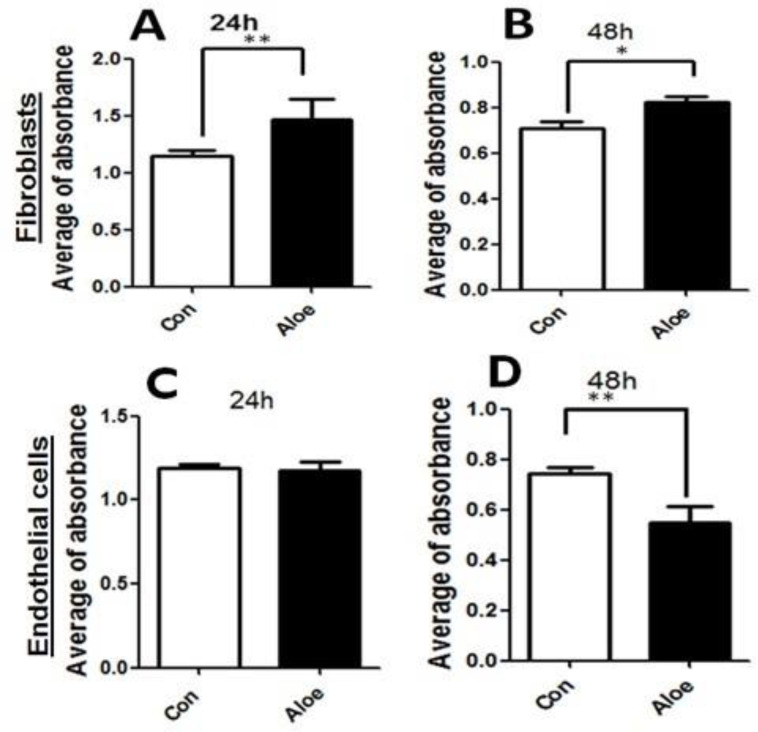
Aloe vera preserved the viability of fibroblasts and endothelial cells
The toxicity and proliferation rate of Aloe vera was evaluated by MTT assay for both cell types. MTT assay revealed that Aloe vera has no toxicity on both cell types after 24 and 48 h. Fibroblasts significantly proliferated in the first 24 h but the proliferation rate decreased slightly at 48 h. However, it was still higher in the Aloe vera treated group in comparison with the control group. Endothelial cells did not proliferate in the first 24 h, and also the optical density of endothelial Aloe vera treated cells significantly (P <0.005) decreased after 48 h (Figure 5).
Fig. 5.
The effects of Aloe vera gel on fibroblasts and endothelial cells after 24 and 48 h. Cell viability was determined by MTT assay. Cell viability rate in Aloe vera groups fibroblasts (A and B) and endothelial cells (C and D) have been compared to controls. Error bars represent the SEM. (* P < 0.05) and (** P < 0.001).
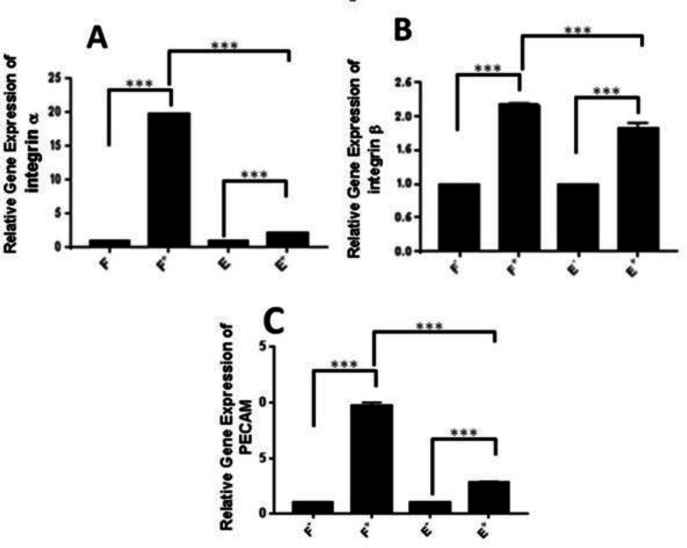
Aloe vera enhanced the gene expression levels of integrin α1, β1 and PECAM-1 ( CD31 ), in fibroblasts and endothelial cells
We performed real-time RT PCR analysis to evaluate the gene expression of integrins α1 and β1 to investigate the migrative effect of Aloe vera gel. According to the results, Aloe vera increased the expression of integrins α1 and β1 in fibroblasts and endothelial cells. The ratio of integrins α1 and β1 gene expression in fibroblasts containing Aloe vera relative to the control group was 19.8 and 2.17, and for endothelial cells was 2.21 and 2.16, respectively (Figure 6 A and B). Therefore, the fold change of integrins α1 and β1 gene expressions was significantly (P <0.0001) higher in fibroblasts in comparison with endothelial cells 8.95 and 1.18, respectively (Figure 6 A and B).
Fig. 6.
Relative gene expressions in fibroblasts and endothelial cells by real time RT-PCR. A) integrin α1; B) integrin β1; C: PECAM-1. F) Fibroblasts; E) Endothelial cells; cells cultured without Aloe vera; +: Aloe vera treated cells. Error bars represent SEM (*** P < 0.0005).
Gene expression evaluation of PECAM-1 in fibroblasts and endothelial cells resulted in significantly higher gene expression in Aloe vera treated fibroblasts (P<0.0001) and endothelial cells (P <0.0001) versus controls. Interestingly, Aloe veratreated fibroblasts expressed more (P <0.0001) PECAM-1 gene in comparison with endothelial cells (Figure 6C).
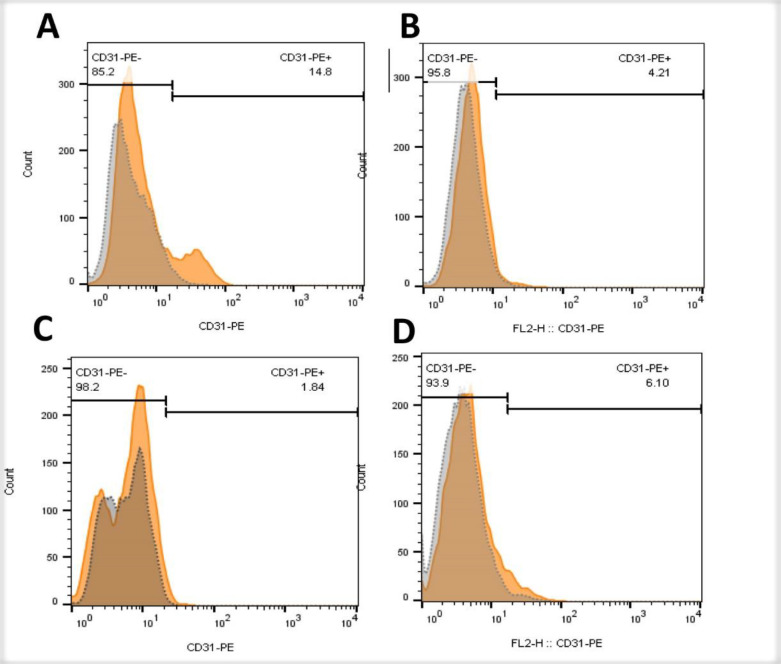
Protein levels of PECAM-1 (CD31) in fibroblasts and endothelial cells by flow cytometry
Flow cytometry of fibroblasts and endothelial cells was performed 48 h after the culture of these cells to examine the expression of CD31 protein. Our data suggested that the expression of CD31 in fibroblasts was 8 times higher than endothelial cells. However, the level of CD31 in Aloe vera-treated fibroblasts and endothelial cells had not increased in comparison with their respective control group (Figure 7)
Fig. 7.
Immunophenotyping of fibroblasts and endothelial cells for PECAM-1 (CD31). A) fibroblasts of control group; B) fibroblasts in the presence of Aloe vera; C) endothelial cells of control group; D) endothelial cells in the presence of Aloe vera. Gray histograms indicate unstained cells and orange histograms show CD31-PE stained cells
Discussion
This study has compared fibroblast and endothelial cell properties after Aloe vera administration. Our findings showed that the morphological changes have occurred in fibroblasts, and Aloe vera-treated cells were fan shaped flat cells which are characteristic of migratory cells. These results are in line with our gene expression data that revealed that the expression of integrins α1β1 increased in fibroblasts, but their increase in fibroblasts was significantly higher than endothelial cells. Our findings illustrated that the viability of endothelial cells decreased after 48 h treatment with Aloe vera gel. It seems that among the two important cell types of tissue, the wound healing effects of Aloe vera is more pronounced in fibroblasts than endothelial cells. However, further in vivo studies will be necessary to determine the importance of each cell.
Medicinal plants are frequently used by patients because of their low cost, compatibility with the human body, and fewer side effects (37). Aloe vera is one of these herbs that has been used for wound healing from ancient times (38). Several mechanisms such as antioxidant activity (39), increase of matrix metalloproteinases-2 (MMP2), collagen bundles formation (40), and anti-inflammatory process (41) have been suggested for tissue repair properties of Aloe vera.
An in vivo study demonstrated that oral administration of Aloe vera enhanced TGF-β1 and VEGF protein in diabetic rats (42). Also, it has shown that Aloe vera induced the expression of bFGF and TGFβ1 in fibroblasts (17). Prakasso showed that the ratio of CD4+ to CD8+ cells increased in the wound area following Aloe vera administration, and suggested that the ability of Aloe vera in wound healing may be due to an increase in lymphocytes (43). Integrins are key mediators for binding to the ECM. Heterodimers of α1β1 integrins play an important role in cell migration and angiogenesis (44). Beta integrins are highly expressed in fibroblasts (45). Integrins of the β1 family are involved in wound re-epithelialization (46), myofibroblast differentiation, and in granulation tissue synthesis and remodeling (47). These mechanisms are related to fibroblast function. Cell migration is an integrin mediated multi-stage process that is effective in wound healing (6). Fibroblasts migrate to the wound site 48-72h after injury, and play an important role in all stages of wound healing. In line with our results, Negahdari et al. found that Aloe vera can increase fibroblast cell migration and proliferation (48). An in vitro study confirmed the above data and showed that Aloe vera enhances fibroblast and keratinocyte cell migration and proliferation (49). In this study, we evaluated the gene expression of α1 and β1 integrins as factors involved in cell migration in fibroblast and endothelial cells. The expression of both integrins in fibroblast cells was greater than endothelial cells which confirm that the migration of fibroblasts is higher than endothelial cells upon Aloe vera gel treatment. Our morphologic and gene expression findings indicated that Aloe vera could accelerate wound healing by increasing rather fibroblast cells migration than endothelial cells. Angiogenesis plays a vital role in wound healing process by forming new blood vessels from prior vessels then invading to wound clot and organizing the microvascular network through the granulation tissue. This dynamic process is highly regulated by signals from both serum and the surrounding ECM environment (50). Boudreau and Beland indicated that Aloe vera gel treatment could shorten wound healing time by increasing angiogenesis (51). Choi et al. found that beta sitosterol of Aloe vera enhanced angiogenesis by increasing VEGF gene expression in chick embryo chorioallantoic membrane assay (52). Aloesin an anthraquinone of Aloe vera leaves induced angiogenesis via activation of SMAD and MAPK signaling proteins in endothelial cells (53). An in vivo study confirmed the above data and demonstrated that oral administration of Aloe vera enhanced Tgf-β1 and Vegf protein in diabetic rats (42). Aloe vera gel could up-regulate Tgfβ1 gene expression with a dose-dependent and time-dependent manner in mouse embryonic fibroblast cells as well (17).
In 1997, DeLisser et al. introduced PECAM-1 as an angiogenic factor (54). The absence of PECAM-1 in endothelial cells results in the increase of cell motility and poor migration in wound healing (3). Our previous study demonstrated that Aloe vera/collagen blended can be useful in the tissue engineering process by enhancing PECAM-1 gene expression in adipose-derived stem cells (32). PECAM-1 was upregulated by Aloe vera gel in fibroblasts. However, flow cytometric data were controversial probably due to undetectable protein expression level during 48 h treatment with Aloe vera. It seems that longer treatment time should be investigated to evaluate the post-translational regulation of PECAM-1
In general, the effect of Aloe vera on proliferation, angiogenesis, and cell migration in fibroblasts seems to be more than endothelial cells. Therefore, the wound healing effect of Aloe vera was probably due to the effect of this plant on fibroblast cells. We concluded that Aloe vera could accelerate wound healing by increasing α1β1 integrins and PECEM-1 expression. However, more investigations are needed to show the main role of fibroblasts in wound healing in comparison with endothelial cells by Aloe vera administration.
Acknowledgment
We would like to thank the Zanjan University of Medical Sciences for funding this research and their great support in study development. The eEthics Committee of Zanjan University of Medical Sciences approved the study protocol with code number 243.
Conflict of interest
No conflict of interest in terms of scientific collaboration and financial benefits is declared by the authors of the study.
References
- 1.Serra MB, Barroso WA, da Silva NN, et al. From Inflammation to Current and Alternative Therapies Involved in Wound Healing. Int J Inflam. 2017;2017:3406215. doi: 10.1155/2017/3406215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lingen MW. Role of leukocytes and endothelial cells in the development of angiogenesis in inflammation and wound healing. Arch Pathol Lab Med. 2001;125:67–71. doi: 10.5858/2001-125-0067-ROLAEC. [DOI] [PubMed] [Google Scholar]
- 3.DeLisser HM. Modulators of endothelial cell filopodia: PECAM-1 joins the club. Cell Adh Migr. 2011;5:37–41. doi: 10.4161/cam.5.1.13575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broughton G, 2nd , Janis JE, Attinger CE. The basic science of wound healing. Plast Reconstr Surg. 2006;117:12S–34S. doi: 10.1097/01.prs.0000225430.42531.c2. [DOI] [PubMed] [Google Scholar]
- 5.Cao G, Fehrenbach ML, Williams JT, et al. Angiogenesis in platelet endothelial cell adhesion molecule-1-null mice. Am J Pathol. 2009;175:903–15. doi: 10.2353/ajpath.2009.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koivisto L, Heino J, Hakkinen L, et al. Integrins in Wound Healing. Adv Wound Care (New Rochelle) 2014;3:762–83. doi: 10.1089/wound.2013.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2010;188:11–9. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bazan-Socha S, Zuk J, Plutecka H, et al. Collagen receptors alpha(1)beta(1) and alpha(2)beta(1) integrins are involved in transmigration of peripheral blood eosinophils, but not mononuclear cells through human microvascular endothelial cells monolayer. J Physiol Pharmacol. 2012;63:373–9. [PubMed] [Google Scholar]
- 9.Ghatak S, Niland S, Schulz JN, et al. Role of Integrins alpha1beta1 and alpha2beta1 in Wound and Tumor Angiogenesis in Mice. Am J Pathol. 2016;186:3011–27. doi: 10.1016/j.ajpath.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Hu S, Cui D, Yang X, et al. The crucial role of collagen-binding integrins in maintaining the mechanical properties of human scleral fibroblasts-seeded collagen matrix. Mol Vis. 2011;17:1334–42. [PMC free article] [PubMed] [Google Scholar]
- 11.Yue J, Zhang K, Chen J. Role of integrins in regulating proteases to mediate extracellular matrix remodeling. Cancer Microenviron. 2012;5:275–83. doi: 10.1007/s12307-012-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in Wound Healing: A Comprehensive Review. Adv Wound Care (New Rochelle) 2014;3:445–64. doi: 10.1089/wound.2013.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shedoeva A, Leavesley D, Upton Z, et al. Wound Healing and the Use of Medicinal Plants. Evid Based Complement Alternat Med. 2019;2019:2684108. doi: 10.1155/2019/2684108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hes M, Dziedzic K, Gorecka D, et al. Aloe vera (L ) Webb: Natural Sources of Antioxidants - A Review. Plant Foods Hum Nutr. 2019;74:255–65. doi: 10.1007/s11130-019-00747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehsani M, Amin Marashi M, Zabihi E, et al. A Comparison between Antibacterial Activity of Propolis and Aloe vera on Enterococcus faecalis (an In Vitro Study) Int J Mol Cell Med. 2013;2:110–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez M, Gonzalez-Burgos E, Iglesias I, et al. Pharmacological Update Properties of Aloe Vera and its Major Active Constituents. Molecules. 2020:25. doi: 10.3390/molecules25061324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hormozi M, Assaei R, Boroujeni MB. The effect of aloe vera on the expression of wound healing factors (TGFbeta1 and bFGF) in mouse embryonic fibroblast cell: In vitro study. Biomed Pharmacother. 2017;88:610–6. doi: 10.1016/j.biopha.2017.01.095. [DOI] [PubMed] [Google Scholar]
- 18.Maan AA, Nazir A, Khan MKI, et al. The therapeutic properties and applications of Aloe vera: A review. J Herb Med. 2018;12:1–10. [Google Scholar]
- 19.Li L-J, Gao S-Q, Peng L-H, et al. Evaluation of efficacy of aloin in treating acute trauma in vitro and in vivo. Biomed Pharmacother. 2017;88:1211–9. [Google Scholar]
- 20.Liu C, Cui Y, Pi F, et al. Extraction, Purification, Structural Characteristics, Biological Activities and Pharmacological Applications of Acemannan, a Polysaccharide from Aloe vera: A Review. Molecules. 2019:24. doi: 10.3390/molecules24081554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thakare PO. Aloe Vera: A Plant with Many Uses. International Journal of Progressive Research in Science and Engineering. 2020;1:82–4. [Google Scholar]
- 22.Li JY, Sun F, Zhou HF, et al. A Systematic Review Exploring the Anticancer Activity and Mechanisms of Glucomannan. Front Pharmacol. 2019;10:930. doi: 10.3389/fphar.2019.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh N, Iqbal Z, Ansari TA, et al. The portent plant with a purpose: Aloe vera. J Pharmacogn Phytochem. 2019;8:4124–30. [Google Scholar]
- 24.Jamil M, Mansoor M, Latif N, et al. Review Effect of Aloe vera on Wound Healing. Biological Sciences-PJSIR. 2020;63:48–61. [Google Scholar]
- 25.Atiba A, Nishimura M, Kakinuma S, et al. Aloe vera oral administration accelerates acute radiation-delayed wound healing by stimulating transforming growth factor-beta and fibroblast growth factor production. Am J Surg. 2011;201:809–18. doi: 10.1016/j.amjsurg.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Moriyama M, Moriyama H, Uda J, et al. Beneficial Effects of the Genus Aloe on Wound Healing, Cell Proliferation, and Differentiation of Epidermal Keratinocytes. PLoS One. 2016;11:e0164799. doi: 10.1371/journal.pone.0164799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mimura T, Yamagami S, Amano S. Corneal endothelial regeneration and tissue engineering. Prog Retin Eye Res. 2013;35:1–17. doi: 10.1016/j.preteyeres.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Wen Y, Luo W, et al. Human Amniotic Epithelial Cells Promote the Proliferation of Human Corneal Endothelial Cells by Regulating Telomerase Activity via the Wnt/beta-catenin Pathway. Curr Eye Res . 2020:1–9. doi: 10.1080/02713683.2020.1792508. [DOI] [PubMed] [Google Scholar]
- 29.Devi G, Harikrishnan R, Paray BA, et al. Effects of aloe-emodin on innate immunity, antioxidant and immune cytokines mechanisms in the head kidney leucocytes of Labeo rohita against Aphanomyces invadans. Fish Shellfish Immunol. 2019;87:669–78. doi: 10.1016/j.fsi.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 30.de Mayo T, Conget P, Becerra-Bayona S, et al. The role of bone marrow mesenchymal stromal cell derivatives in skin wound healing in diabetic mice. PLoS One. 2017;12:e0177533. doi: 10.1371/journal.pone.0177533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oryan A, Alemzadeh E, Mohammadi AA, et al. Healing potential of injectable Aloe vera hydrogel loaded by adipose-derived stem cell in skin tissue-engineering in a rat burn wound model. Cell Tissue Res. 2019;377:215–27. doi: 10.1007/s00441-019-03015-9. [DOI] [PubMed] [Google Scholar]
- 32.Sigaroodi F, Shafaei H, Karimipour M, et al. Aloe Vera/Collagen Mixture Induces Integrin alpha1beta1 and PECAM-1 Genes Expression in Human Adipose-Derived Stem Cells. Adv Pharm Bull. 2019;9:662–7. doi: 10.15171/apb.2019.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jithendra P, Rajam AM, Kalaivani T, et al. Preparation and characterization of aloe vera blended collagen-chitosan composite scaffold for tissue engineering applications. ACS Appl Mater Interfaces. 2013;5:7291–8. doi: 10.1021/am401637c. [DOI] [PubMed] [Google Scholar]
- 34.Shafaei H, Esfandiari E, Baghernezhad H. Evaluation of morphology and immunophenotype of mesenchymal stem cells after switching of bovine serum of media to human serum. Razi Journal of Medical Sciences. 2017;23:105–13. [Google Scholar]
- 35.Burr SD, Stewart JA Jr. A Cost Effective and Adaptable Scratch Migration Assay. J Vis Exp . 2020 doi: 10.3791/61527. [DOI] [PubMed] [Google Scholar]
- 36.Dizaji Asl K, Shafaei H, Soleimani Rad J, et al. Comparison of Characteristics of Human Amniotic Membrane and Human Adipose Tissue Derived Mesenchymal Stem Cells. World J Plast Surg. 2017;6:33–9. [PMC free article] [PubMed] [Google Scholar]
- 37.Pal SK, Shukla Y. Herbal medicine: current status and the future. Asian Pac J Cancer Prev. 2003;4:281–8. [PubMed] [Google Scholar]
- 38.Sánchez-Machado DI, López-Cervantes J, Sendón R, et al. Aloe vera: Ancient knowledge with new frontiers. Trends Food Sci Technol. 2017;61:94–102. [Google Scholar]
- 39.Radha MH, Laxmipriya NP. Evaluation of biological properties and clinical effectiveness of Aloe vera: A systematic review. J Tradit Complement Med. 2015;5:21–6. doi: 10.1016/j.jtcme.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aro AA, Nishan U, Perez MO, et al. Structural and biochemical alterations during the healing process of tendons treated with Aloe vera. Life Sci. 2012;91:885–93. doi: 10.1016/j.lfs.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Egesie U, Chima K, Galam N. Anti-inflammatory and analgesic effects of aqueous extract of Aloe Vera (Aloe barbadensis) in rats. Afr J Biomed Res. 2011;14:209–12. [Google Scholar]
- 42.Atiba A, Ueno H, Uzuka Y. The effect of aloe vera oral administration on cutaneous wound healing in type 2 diabetic rats. J Vet Med Sci. 2011;73:583–9. doi: 10.1292/jvms.10-0438. [DOI] [PubMed] [Google Scholar]
- 43.Prakoso YA, Kurniasih The Effects of Aloe vera Cream on the Expression of CD4(+) and CD8(+) Lymphocytes in Skin Wound Healing. J Trop Med. 2018;2018:6218303. doi: 10.1155/2018/6218303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paul NR, Jacquemet G, Caswell PT. Endocytic Trafficking of Integrins in Cell Migration. Curr Biol. 2015;25:R1092–105. doi: 10.1016/j.cub.2015.09.049. [DOI] [PubMed] [Google Scholar]
- 45.Geiger B, Yamada KM. Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Longmate WM, Dipersio CM. Integrin Regulation of Epidermal Functions in Wounds. Adv Wound Care (New Rochelle) 2014;3:229–46. doi: 10.1089/wound.2013.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu S, Xu SW, Blumbach K, et al. Expression of integrin beta1 by fibroblasts is required for tissue repair in vivo. J Cell Sci. 2010;123:3674–82. doi: 10.1242/jcs.070672. [DOI] [PubMed] [Google Scholar]
- 48.Negahdari S, Galehdari H, Kesmati M, et al. Wound Healing Activity of Extracts and Formulations of Aloe vera, Henna, Adiantum capillus-veneris, and Myrrh on Mouse Dermal Fibroblast Cells. Int J Prev Med. 2017;8:18. doi: 10.4103/ijpvm.IJPVM_338_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teplicki E, Ma Q, Castillo DE, et al. The Effects of Aloe vera on Wound Healing in Cell Proliferation, Migration, and Viability. Wounds. 2018;30:263–8. [PubMed] [Google Scholar]
- 50.DiPietro LA. Angiogenesis and wound repair: when enough is enough. J Leukoc Biol. 2016;100:979–84. doi: 10.1189/jlb.4MR0316-102R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boudreau MD, Beland FA. An evaluation of the biological and toxicological properties of Aloe barbadensis (miller), Aloe vera. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2006;24:103–54. doi: 10.1080/10590500600614303. [DOI] [PubMed] [Google Scholar]
- 52.Choi S, Kim KW, Choi JS, et al. Angiogenic activity of beta-sitosterol in the ischaemia/reperfusion-damaged brain of Mongolian gerbil. Planta Med. 2002;68:330–5. doi: 10.1055/s-2002-26750. [DOI] [PubMed] [Google Scholar]
- 53.Wahedi HM, Jeong M, Chae JK, et al. Aloesin from Aloe vera accelerates skin wound healing by modulating MAPK/Rho and Smad signaling pathways in vitro and in vivo. Phytomedicine. 2017;28:19–26. doi: 10.1016/j.phymed.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 54.DeLisser HM, Christofidou-Solomidou M, Strieter RM, et al. Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am J Pathol. 1997;151:671–7. [PMC free article] [PubMed] [Google Scholar]