Abstract
Epilepsy is a chronic clinical syndrome of brain function which is caused by abnormal discharge of neurons. MicroRNAs (miRNAs) are small non-coding RNAs which act post-transcriptionally to regulate negatively protein levels. They affect neuroinflammatory signaling, glial and neuronal structure and function, neurogenesis, cell death, and other processes linked to epileptogenesis. The aim of this study was to explore the possible role of miR-125a and miR-181a as regulators of inflammation in epilepsy through investigating their involvement in the pathogenesis of epilepsy, and their correlation with the levels of inflammatory cytokines. Thirthy pediatric patients with epilepsy and 20 healthy controls matched for age and sex were involved in the study. MiR-181a and miR-125a expression were evaluated in plasma of all subjects using qRT-PCR. In addition, plasma levels of inflammatory cytokines (IFN-γ and TNF-) were determined using ELISA. Our findings indicated significantly lower expression levels of miR-125a (P=0.001) and miR-181a (P=0.001) in epileptic patients in comparison with controls. In addition, the production of IFN-γ and TNF- was non-significantly higher in patients with epilepsy in comparison with the control group. Furthermore, there were no correlations between miR-125a and miR-181a with the inflammatory cytokines (IFN-γ and TNF-) in epileptic patients. MiR-125a and miR-181a could be involved in the pathogenesis of epilepsy and could serve as diagnostic biomarkers for pediatric patients with epilepsy.
Key Words: Epilepsy, MiR-181a, MiR-125a, inflammation, TNF-α, IFN-γ
Epilepsy is a chronic neurological disorder characterized by frequent aberrant electrical activity in brain, which is estimated to affect about 65 million individuals' worldwide (1). This complex disorder is associated with comprehensive changes in brain function at the molecular, cellular, and circular levels. It can occur due to genetic defects or it can be acquired through epileptogenic insults, such as "traumatic brain injury, brain infections, stroke, or status epilepticus" (2). In recent years, some clinical and experimental studies have found that encephalitis is an essential manifestation of pathological brain tissue of high risk in pharmaco-resistant epilepsy from various etiologies (3).
MicroRNAs (miRNAs) are small non-coding RNAs that control gene expression post-transcriptionally, and either encourage the degradation of their target mRNAs or promote translational suppression (4). They mainly regulate the innate immune response through modifying astrocyte-mediated inflammation, and are involved in regulating the function of T lymphocytes in the immune response. Unsurprisingly, dysregulation of miRNAs was observed in various diseases expanded from cancer and immune disorders to brain diseases (5). MiR181 and miR-125 families are highly preserved miRNAs in humans; miR181 family has four members miR181a, miR181b, miR181c, and miR181d (6). Abnormal expression of miR-181 family is associated with many nervous system disorders. Also, it plays a major role in immune cells development and function, including differentiation and activities of B and T lymphocytes (7). Previous studies demonstrated that miR-181a can prevent negative regulatory factors in the pathway of T-cell receptor signals (8), and through targeting IFN-γ, it influences on the naïve CD4+ T cells differentiation, prevents cell proliferation, and promotes programmed cell death, and thus, affects the function of T cells (9). On the other hand, miRNA-125 family consists of miR-125a and miR-125b (10). MiR-125a has shown to inhibit innate macrophage responses through suppressing macrophage differentiation (11). A large number of studies showed high levels of specific inflammatory mediators, and noticed upregulation of their correlated receptors in the chronic epileptic brain, which indicates that some of the proinflammatory pathways are mostly activated in foci of seizure (12).
Nudelman et al. first indicated relations between seizures and altered miRNAs expression (13). It has been demonstrated that modulation and inflammation of the neuron morphology may be two of the greatest significant controlling roles of miRNAs in epilepsy formation (14). In addition, miRNAs are known as chief regulators for the production of protein during and after seizures; so, miRNAs might regulate neuronal excitability and also remodeling responses (15). Therefore, miRNAs may be related to epilepsy pathogenesis and therapies (16). In nearly 30% of all epileptic individuals, seizures cannot be controlled with the currently available medications (17). Thus, studies in epilepsy that revealed altered expression and function of miRNAs, which are supposed to regulate many downstream targets and cellular processes simultaneously, have aroused great interest in miRNAs defects as pathological mechanisms and potential therapeutic targets in epilepsy (5). Consequently, the aim of this study was to determine the association of circulating miR-125a and miR-181a expression with the pathogenesis of epilepsy and with the production of IFN-γ and TNF-α in pediatric patients with epilepsy.
Materials and methods
Study subjects
This study follows the guidelines of the ethics committee of National Research Centre, Giza, Egypt, and written informed consents were collected from the parent/guardian of all children involved in our study before their enrollment. The study involved 50 subjects; their age ranged from 5 to 15 years. They included 2 groups: group 1 involved 30 patients with epilepsy; group 2 involved 20 healthy subjects with the same age range, as a control group.
Patients were recruited from Pediatrics Neurology clinic, Al-Zahraa University Hospital, Cairo, Egypt during January 2019 to June 2019. Inclusion criteria: presence of idiopathic epilepsy by clinical examination and electroencephalogram (EEG). Patients with other chronic diseases or psychiatric disorders, history of autoimmune diseases, history of infection 2 weeks before sample collection were excluded from the study. Patients in this study were subjected to full medical history with special emphasis on character of seizures (type, age of onset, and duration of the seizures), and full neurological examination. Intelligence quotient (IQ) of children was assessed by Stanford Binet test. Clinical characteristics and treatments of epileptic patients are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of subjects
| Characteristic | Epileptic patients | Normal healthy controls | |
|---|---|---|---|
| No of cases | 30 | 20 | |
| Gender, no. male/female | 20/10 | 11/9 | |
| Age range (years) | 5-15 | 5-15 | |
| Disease duration (years), mean±S.E. | 6.8±0.95 | - | |
| IQ (% of patients) | Low average | 5 | |
| Average | 45 | ||
| High average | 25 | ||
| Excellent | 15 | ||
| Very excellent | 10 | ||
| Medications (tegretol, depakine, tiratam) | 23/30 | 0/20 | |
IQ: Intelligence quotient
RNA extraction and quantitative real - time PCR
MiRNAs were isolated and extracted from plasma of all subjects of the study using miRNeasy Mini kit (Qiagen, Germany), and by following the manufacturer’s instructions. For miRNA-specific reverse transcription, using TaqMan® MicroRNA Reverse Transcription Kit and specific primers (Applied Biosystems, USA) and by following the manufacturer’s instructions, miRNAs were reverse-transcribed to cDNA. Reverse transcription was done under the following conditions: 30 min at 16 °C, 30 min at 42 °C, followed by 5 min at 85 °C, and the cDNA was stored at −80 °C until use.
A real-time quantitative PCR (qRT-PCR) was done using TaqMan® MicroRNA Assay kit (miR-125a, assay ID: 002198; miR-181a, assay ID: 000480; Applied Biosystems, USA) and TaqMan® Universal Master Mix (Applied Biosystems, USA) to measure the expression levels (in triplicate) of mature miR-181a and miR-125a using 7500 fast real-time PCR system by following the manufacturer’s instructions. The endogenous control used was RNU48 to normalize the expression levels of target miRNAs. We calculated the relative quantification (Rq) of miRNA expression using the 2−ΔΔCT threshold cycle method. ΔCt was obtained by subtracting the Ct values for RUN48 from the Ct values for the gene of interest. qRT-PCR was done under the following conditions: 2 min at 50 °C, 10 min at 95 °C, followed by 50 cycles at 95 °C for 15 s and at 60 °C for 1 min (18).
Enzyme-linked immunosorbent assay (ELISA)
Plasma IFN-γ and TNF- levels of all study subjects were determined in duplicate using Human IFN-γ and TNF- ELISA kit (Elabscience, Elabscience Biotechnology Co., Ltd) by following the manufacturer’s protocol.
Statistical analysis
By using SPSS version 19.0 software (SPSS Inc., Chicago, Illinois, USA), data were statistically analyzed. Non-parametric Mann-Whitney U Test was used for comparing the expression levels among groups, and Spearmans rank correlation to test the association of clinical data and inflammatory cytokines of patients with miRNA expression levels. Data were presented as median. A p value <0.05 was considered statistically significant. Receiver operating characteristic (ROC) curve was made for each miRNA to assess the efficacy of miRNAs as biomarkers for epileptic patients against controls. Area under curve (AUC) values, sensitivity, specificity, and 95 % confidence interval to each miRNA was calculated.
Results
Dysregulation of plasma miR-125a and miR-181a expression pattern in patients with epilepsy
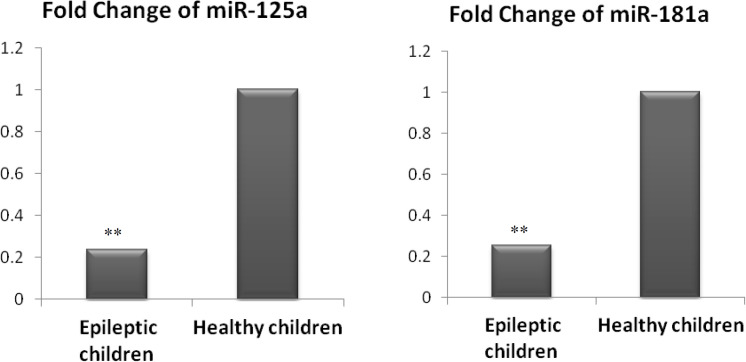
Our findings indicated highly significant down-regulation of miR-125a (4.2-fold) in epileptic patients in comparison with controls (Figure 1).
Fig. 1.
Fold change of miR-125a and miR-181a in epileptic children relative to healthy controls. Bars show the median of fold change. (**: significant at P < 0.01 versus controls, by non-parametric Mann-Whitney U test).
In addition, the results of this study demonstrated that miR-181a showed significantly lower (3.9 fold) expression levels in epileptic patients in comparison with healthy controls (Figure 1).
Association of plasma miR-125a and miR-181a expression with demographic and clinical features
Association analysis showed that miR-125a expression levels have a significant positive association with the age and weight of epileptic patients but have no association with other demographic or clinical data (gender, seizure duration, and IQ). Furthermore, no association was found between miR-181a expression levels and any demographic or clinical data of studied patients (Table 2).
Table 2.
Association of plasma miR-125a and miR-181a expression levels with clinical data of epileptic patients
| MiR-125a | MiR-181a | |||
|---|---|---|---|---|
| R (Spearman correlation) | P value | R (Spearman correlation) | P value | |
| Age | 0.667 | 0.050* | 0.233 | 0.546 |
| Gender | 0.000 | 1 | -0.087 | 0.825 |
| Weight | 0.723 | 0.028* | 0.100 | 0.797 |
| Seizure duration | 0.050 | 0.899 | 0.211 | 0.586 |
| IQ | -0.343 | 0.367 | -0.052 | 0.894 |
*: Correlation is significant at 0.05 level (2-tailed).
Correlation of miR-125a and miR-181a with inflammatory cytokines in patients with epilepsy
The findings of the study clarified that the production of IFN-γ and TNF- was non-significantly higher in patients with epilepsy in comparison with the control group (Figure 2).
Fig. 2.
Plasma levels of IFN-γ and TNF- in epileptic and healthy children. Bars show the results as the median. (Non-significant versus controls, P = 0.435 for IFN-γ, and p = 0.172 for TNF- , by non-parametric Mann-Whitney U test).
In addition, our data indicated that there were no correlations between miR-125a and miR-181a with the inflammatory cytokines (IFN-γ and TNF -) in epileptic patients (Table 3).
Table 3.
Correlations of plasma miR-125a and miR-181a expression levels with plasma inflammatory cytokines of epileptic patients
| MiR-125a expression | MiR-181a expression | |||
|---|---|---|---|---|
| R | P value | R | P value | |
| IFN-γ | -0.317 | 0.406 | 0.050 | 0.898 |
| TNF- | 0.250 | 0.516 | 0.183 | 0.637 |
P value was calculated using Spearman correlation analysis.
ROC curve of miR-125a and miR-181a
ROC curve analysis showed that miR-125a has an AUC value of 0.815 (95 % CI 0.593-1.036), a sensitivity of 70%, and a specificity of 83% (P = 0.045) (Figure 3). In addition, the ROC curve of miR-181a has an AUC value of 0.704 (95 % CI 0.429–0.978), a sensitivity of 66.7%, and a specificity of 67% (P = 0.195) (Figure 4).
Fig. 3.
Roc curve of miR-125a for epileptic patients versus normal controls. AUC = 0.815 (95 % CI 0.593-1.036); sensitivity = 70%; specificity = 83%. (Statistically significant versus normal controls, P = 0.045, under the non-parametric assumption).
Fig. 4.

Roc curve of miR-181a for epileptic patients versus normal controls. AUC = 0.704 (95 % CI 0.429–0.978); sensitivity = 66.7%; specificity = 67% (Non-significant versus normal controls, P = 0.195, under the non-parametric assumption)
Discussion
Epilepsy is considered as a chronic clinical syndrome of brain function, which is caused by abnormal discharge of neurons. Regarding the clinical manifestations of epilepsy, about 30-40% of patients are often associated with attention dispersion, memory impairment,and other cognitive dysfunctions (19). Unfortunately, one third of the patients are usually resistant to currently existing antiepileptic drugs (AEDs) which act mainly on the brain to reduce the severity and frequency of seizures (17). The potential role of miRNAs in the therapies and pathogenesis of epilepsy has been provided by many recent studies. Most of those studies usually used animal models of epilepsy (20). By using these models, more recent work has determined that the loss of miRNA biogenesis components by the mature brain will result in progressive tissue dysmorphogenesis, neurodege-neration, and seizures (21).
The majority of expressed miRNAs were upregulated in status epilepticus (SE) mice, while in tolerant mice only 18% of the expressed miRNAs were upregulated and 82% were down regulated (22).
The results of our study showed highly significant lower expression levels of miR-125a and miR-181a in epileptic patients in comparison with controls. In addition, the production of IFN-γ and TNF- was non-significantly higher in patients with epilepsy in comparison with the control group. Furthermore, there were no correlations between miR-125a and miR-181a with the inflammatory cytokines (IFN-γ and TNF-) in epileptic patients. These findings agree with what was previously found by Dan et al. (2015) who verified that miR-181a was up-regulated and acted as negative regulators in lipopolysaccharide mediated inflammation via targeting TNF-α mRNA (23). Relatively, Jing et al. (2017) studied the effects of high mobility group box-1 protein (HMGB1) on the maturation and activation of splenic dendritic cells from mice, and proposed that miR-181a-5p may bind the 3′UTR of TNF-α mRNA to negatively regulate its expression (24). Furthermore, Sang et al. (2015) reported that, miR-181a affects the function of T lymphocytes by down-regulating IFN-γ in a dose-dependent manner (9). On the other side, Saki et al. (2015) stated that, miR-125 may reduce the effector function and activation of T-cells, which is shown by decreased levels of intracellular IFN-γ and IL-13 (25).
In a previous study, Liu et al., (2015) found that miR-181a expression was significantly up-regulated in memory impairment group of the pentylenetetrazol (PTZ)-induced epileptic rats, but it remains unknown whether miR-181a can influence the cognitive function of PTZ-induced epileptic rats. This is in contrast with our results which demonstrated that the level of miR-181a expression was significantly lower in epileptic patients. In patients with epilepsy, the miR-181a expression level was 3.9-fold lower than the control group (26). In another study, Ashhab et al. (2013) observed a significant upregulation in the chronic stage, downregulation in the acute stage, and no change in the latent stage of miR-181a in comparison with the control group in a rat model (27). This phenomenon was similarly found in children. Inflammation related to miR-181a can cause this condition (28). It is essential to investigate the role of miR-181a in different stages of epilepsy in the future.
It was observed that miR-125a, was significantly deregulated in rat peripheral blood and in hippocampal tissues at 24 h following SE, which suggests a role for this miRNA in epilepsy diagnosis. At 24 h after epileptic seizures, Liu et al. (2010) identified 147 miRNAs in blood and 91 miRNAs in brain which showed abnormal relative quantification > 1.5 in three different statuses of brain damage including intercerebral ischemic injury, hemorrhagic injury, and kainate seizures in rats. Among them, seven down-regulated miRNAs were found in the blood (one of them is miR-125a-5p.33) and other miRNAs were demonstrated to have defective expression in blood and brain tissue of all three conditions (29). This is in agreement with our findings that indicated a significant lower expression level of miR-125a in epilepsy patients. Moreover, association analysis showed that miR-125a expression levels have a significant positive association with the age and weight of epilepsy patients.
In another study, Liu et al. (2019) detected miR-125a-5p down-regulation in the hippocampus of PTZ-induced epilepsy rats. They observed that overexpression of miR-125a-5p reduced seizures and diminished inflammation in the hippocampus of PTZ-induced rats by suppressing its target gene, calmodulin-dependent protein kinase IV (CAMK4) (30).
The comparative analysis of miRNAs in the hippocampus from epilepsy model showed that miR-125a-5p increased. This study also presented the results of miRNAs profiling in peripheral blood samples of the same epileptic model. MiR-125a was confirmed to be increased. The deregulated miRNAs were predictable to control the mitogen-activated protein kinase (MAPK)-signaling pathway, tight junction pathway, TGF-β-signaling pathway, long-term potentiation pathway, axon guidance and glycan structure-biosynthesis (31), which is in contrast with our results that demonstrated low expression of miR-125a. These differences in results may be due to different disease stages or different sample size.
MiRNAs have an important role in inflammatory pathways that have been discovered to be involved in epilepsy (32). Pharmacological modulation of proinflammatory signaling that inhibited seizures and changes in the risk of developing seizures in transgenic mice, proved the concept that brain inflammation might have a role in the etiopathogenesis of seizures (33).
TNF-α and IFN-γ have been involved in seizure generation and were shown to be up-regulated during a seizure (34, 35). This is in agreement with our findings which clarified that the production of IFN-γ and TNF- was non-significantly higher in patients with epilepsy in comparison with the control group. Wang et al. (2015) noticed that serum levels of IFN-γ were correlated with seizure severity (36). According to our data, there were no correlations between miR-125a and miR-181a with the inflammatory cytokines IFN-γ in epileptic patients. On the other hand, other miRNAs may have correlations with increased IFN-γ levels in epilepsy as miR-146a (32) and miR-155 (37).
TNF-α is an inflammatory marker, and its level is also increased after seizures (38, 39). As our results indicated that there were no correlations between miR-125a and miR-181a with TNF- in epileptic patients, other miRNAs such as miR 155 might have a regulatory role on TNF-α in nervous tissue (40).
It has been proven that miR-181a-5p suppresses IFN-γ in human CD4+ T cells. I Infection of the activated human CD4+ T lymphocytes with a lentivirus encoding pre-miR-181a, significantly decreased the protein level of that cytokine in both CD4+ T cells and culture media (41, 9).
In the present study, miR-125a showed a sensitivity of 70% and a specificity of 83% (P < 0.05) while miR-181a showed a sensitivity of 66.7% and a specificity of 67% (P < 0.05).
Any discrepancy between our results and others may be due to assessing their levels at different stages of epilepsy, or to different sample sizes. Thus, miR-125a and miR-181a may be considered as promisingly sensitive, easily detectable, and specific biomarkers that may improve the diagnosis and treatment outcome of epilepsy.
In conclusion, miRNAs and brain inflammation might endorse epileptogenesis and epileptic seizures, and could be considered as diagnostic biomarkers for epilepsy. MiR-125a and miR-181a might serve as regulators of inflammation in epilepsy pediatric patients. Thus, further studies are needed to clarify whether prohibition of inflammatory signals activation and follow-up of miRNAs are useful as potential targets for pharmacological intervention, especially for patients with epilepsy who are resistant to antiepileptic drugs.
Acknowledgment
We thank the National Research Centre (NRC) (in-house office for research projects) for the research grant that supported this work. This work was supported by NRC (Grant No.11010172).
Conflict of interest
There is no conflict of interest for any of the authors.
References
- 1.Moshé SL, Perucca E, Ryvlin P, et al. Epilepsy: new advances. The Lancet. 2015;385:884–98. doi: 10.1016/S0140-6736(14)60456-6. [DOI] [PubMed] [Google Scholar]
- 2.Tatum WO. Mesial temporal lobe epilepsy. J Clin Neurophysiol. 2012;29:356–65. doi: 10.1097/WNP.0b013e31826b3ab7. [DOI] [PubMed] [Google Scholar]
- 3.Vezzani A, French J, Bartfai T, et al. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–79. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 5.Tiwari D, Peariso K, Gross C. MicroRNA-induced silencing in epilepsy: Opportunities and challenges for clinical application. Dev Dyn. 2018;247:94–110. doi: 10.1002/dvdy.24582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen CZ, Li L, Lodish HF, et al. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–6. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Ortiz CJ, Baglietto-Vargas D, Martinez-Coria H, et al. Upregulation of miR-181 decreases c-Fos and SIRT-1 in the hippocampus of 3xTg-AD mice. J Alzheimers Dis. 2014;42:1229–38. doi: 10.3233/JAD-140204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lykken EA, Li QJ. microRNAs at the regulatory frontier: an investigation into how microRNAs impact the development and effector functions of CD4 T cells. Immunol Res. 2011;49:87–96. doi: 10.1007/s12026-010-8196-4. [DOI] [PubMed] [Google Scholar]
- 9.Sang W, Zhang C, Zhang D, et al. MicroRNA-181a, a potential diagnosis marker, alleviates acute graft versus host disease by regulating IFN-gamma production. Am J Hematol. 2015;90:998–1007. doi: 10.1002/ajh.24136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin H, Sun Y, Wang X, et al. Progress on the relationship between miR-125 family and tumorigenesis. Exp Cell Res. 2015;339:252–60. doi: 10.1016/j.yexcr.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Lee HM, Kim TS, Jo EK. MiR-146 and miR-125 in the regulation of innate immunity and inflammation. BMB Rep. 2016;49:311–8. doi: 10.5483/BMBRep.2016.49.6.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holtman L, van Vliet EA, van Schaik R, et al. Effects of SC58236, a selective COX-2 inhibitor, on epileptogenesis and spontaneous seizures in a rat model for temporal lobe epilepsy. Epilepsy Res. 2009;84:56–66. doi: 10.1016/j.eplepsyres.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Nudelman AS, DiRocco DP, Lambert TJ, et al. Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus. 2010;20:492–8. doi: 10.1002/hipo.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukasiuk K, Becker AJ. Molecular biomarkers of epileptogenesis. Neurotherapeutics. 2014;11:319–23. doi: 10.1007/s13311-014-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alsharafi WA, Xiao B, Abuhamed MM, et al. miRNAs: biological and clinical determinants in epilepsy. Front Mol Neurosci. 2015;8:59. doi: 10.3389/fnmol.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henshall DC. Manipulating MicroRNAs in Murine Models: Targeting the Multi-Targeting in Epilepsy. Epilepsy Curr. 2017;17:43–7. doi: 10.5698/1535-7511-17.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wahab A. Difficulties in Treatment and Management of Epilepsy and Challenges in New Drug Development. Pharmaceuticals (Basel) 2010;3:2090–110. doi: 10.3390/ph3072090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amr KS, Bayoumi FS, Eissa E, et al. Circulating microRNAs as potential non-invasive biomarkers in pediatric patients with celiac disease. Eur Ann Allergy Clin Immunol. 2019;51:159–64. doi: 10.23822/EurAnnACI.1764-1489.90. [DOI] [PubMed] [Google Scholar]
- 19.Ouellet DL, Perron MP, Gobeil LA, et al. MicroRNAs in gene regulation: when the smallest governs it all. J Biomed Biotechnol. 2006;2006:69616. doi: 10.1155/JBB/2006/69616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kretschmann A, Danis B, Andonovic L, et al. Different microRNA profiles in chronic epilepsy versus acute seizure mouse models. J Mol Neurosci. 2015;55:466–79. doi: 10.1007/s12031-014-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiorenza A, Lopez-Atalaya JP, Rovira V, et al. Blocking miRNA Biogenesis in Adult Forebrain Neurons Enhances Seizure Susceptibility, Fear Memory, and Food Intake by Increasing Neuronal Responsiveness. Cereb Cortex. 2016;26:1619–33. doi: 10.1093/cercor/bhu332. [DOI] [PubMed] [Google Scholar]
- 22.Jimenez-Mateos EM, Engel T, Merino-Serrais P, et al. Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat Med. 2012;18:1087–94. doi: 10.1038/nm.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dan C, Jinjun B, Zi-Chun H, et al. Modulation of TNF-alpha mRNA stability by human antigen R and miR181s in sepsis-induced immunoparalysis. EMBO Mol Med. 2015;7:140–57. doi: 10.15252/emmm.201404797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu J, Wang FL, Wang HB, et al. TNF-alpha mRNA is negatively regulated by microRNA-181a-5p in maturation of dendritic cells induced by high mobility group box-1 protein. Sci Rep. 2017;7:12239. doi: 10.1038/s41598-017-12492-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saki N, Abroun S, Soleimani M, et al. Involvement of MicroRNA in T-Cell Differentiation and Malignancy. Int J Hematol Oncol Stem Cell Res. 2015;9:33–49. [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Wu Y, Huang Q, et al. Grouping Pentylenetetrazol-Induced Epileptic Rats According to Memory Impairment and MicroRNA Expression Profiles in the Hippocampus. PLoS One. 2015;10:e0126123. doi: 10.1371/journal.pone.0126123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashhab MU, Omran A, Gan N. MicroRNAs (9, 138, 181A, 221, and 222) and mesial temporal lobe epilepsy in developing brains. Transl Neurosci. 2013;4:357–62. [Google Scholar]
- 28.Ma Y. The Challenge of microRNA as a Biomarker of Epilepsy. Curr Neuropharmacol. 2018;16:37–42. doi: 10.2174/1570159X15666170703102410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu DZ, Tian Y, Ander BP, et al. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab. 2010;30:92–101. doi: 10.1038/jcbfm.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Q, Wang L, Yan G, et al. MiR-125a-5p Alleviates Dysfunction and Inflammation of Pentylenetetrazol- induced Epilepsy Through Targeting Calmodulin-dependent Protein Kinase IV (CAMK4) Curr Neurovasc Res. 2019;16:365–72. doi: 10.2174/1567202616666190906125444. [DOI] [PubMed] [Google Scholar]
- 31.Hu K, Zhang C, Long L, et al. Expression profile of microRNAs in rat hippocampus following lithium-pilocarpine-induced status epilepticus. Neurosci Lett. 2011;488:252–7. doi: 10.1016/j.neulet.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Yu JT, Tan L, et al. Genome-wide circulating microRNA expression profiling indicates biomarkers for epilepsy. Sci Rep. 2015;5:9522. doi: 10.1038/srep09522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang X, Zhang H, Yang J, et al. Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis. 2010;40:193–9. doi: 10.1016/j.nbd.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marchi N, Granata T, Janigro D. Inflammatory pathways of seizure disorders. Trends Neurosci. 2014;37:55–65. doi: 10.1016/j.tins.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dey A, Kang X, Qiu J, et al. Anti-Inflammatory Small Molecules To Treat Seizures and Epilepsy: From Bench to Bedside. Trends Pharmacol Sci. 2016;37:463–84. doi: 10.1016/j.tips.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Wang D, Guo D. Interictal cytokine levels were correlated to seizure severity of epileptic patients: a retrospective study on 1218 epileptic patients. J Transl Med. 2015;13:378. doi: 10.1186/s12967-015-0742-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kan AA, van Erp S, Derijck AA, et al. Genome-wide microRNA profiling of human temporal lobe epilepsy identifies modulators of the immune response. Cell Mol Life Sci. 2012;69:3127–45. doi: 10.1007/s00018-012-0992-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renno T, Krakowski M, Piccirillo C, et al. TNF-alpha expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. Regulation by Th1 cytokines. J Immunol. 1995;154:944–53. [PubMed] [Google Scholar]
- 39.Probert L, Akassoglou K, Kassiotis G, et al. TNF-alpha transgenic and knockout models of CNS inflammation and degeneration. J Neuroimmunol. 1997;72:137–41. doi: 10.1016/s0165-5728(96)00184-1. [DOI] [PubMed] [Google Scholar]
- 40.Ashhab MU, Omran A, Kong H, et al. Expressions of tumor necrosis factor alpha and microRNA-155 in immature rat model of status epilepticus and children with mesial temporal lobe epilepsy. J Mol Neurosci. 2013;51:950–8. doi: 10.1007/s12031-013-0013-9. [DOI] [PubMed] [Google Scholar]
- 41.Fayyad-Kazan H, Hamade E, Rouas R, et al. Downregulation of microRNA-24 and -181 parallels the upregulation of IFN-gamma secreted by activated human CD4 lymphocytes. Hum Immunol. 2014;75:677–85. doi: 10.1016/j.humimm.2014.01.007. [DOI] [PubMed] [Google Scholar]