Abstract
Nonsurgical treatment options for uterine fibroids are uterine artery embolization (UAE), high intensity focused ultrasound ablation (HIFUA), and percutaneous microwave ablation (PMWA). Magnetic resonance imaging (MRI) is the reference standard imaging method before and after these procedures. Contrast-enhanced ultrasound (CEUS) has been studied as an alternative to MRI for evaluating the fibroids’ characteristics and responses to nonsurgical treatments. English literatures investigating the application of CEUS as an adjunct to monitor UAE, HIFUA or PMWA in uterine fibroid treatments in human were searched in PubMed, Ovid Medline, and Scopus databases from January 2000 through June 7th, 2020. Two independent reviewers analyzed 128 publications, out of which 17 were included. Based on this systematic review, CEUS provides detailed data about fibroid volume and vascularization prior, during, and post UAE, and it helps determine the endpoint of the procedure. HIFUA with Intraprocedural CEUS has faster volume shrinkage over a shorter time period with less needed energy and provides early detection of residual tissue after HIFUA. CEUS and contrast-enhanced MRI (CE-MRI) have sufficient agreement to be used interchangeably in the clinic to evaluate the therapeutic effect of PMWA and HIFUA of fibroids.
Keywords: Uterine fibroid, contrast-enhanced ultrasound, uterine artery embolization, percutaneous microwave ablation, High-intensity focused ultrasound ablation
Introduction:
Fibroids are the most common benign tumors of the uterus in women over the age of 40 years. They are monoclonal tumors arising from a single smooth muscle cell of the myometrium (Holdsworth-Carson et al. 2014). Uterine fibroids may exhibit symptoms like excessive bleeding and anemia, pelvic pain, bowel and bladder dysfunction, pregnancy losses and infertility depending on their size, number and location (Brolmann & Huirne. 2008). Hysterectomy is the most common surgical procedure in fibroids with abnormal uterine bleeding or bulk-related symptoms (Borah et al. 2016), but there are alternative nonsurgical treatment options such as uterine artery embolization (UAE), high intensity focused ultrasound ablation (HIFUA), and percutaneous microwave ablation (PMWA) (Sohn et al. 2018). UAE is a technique performed to induce volume reduction in uterine fibroids by obstructing bilateral uterine arteries via micro catheter under fluoroscopy (Keung et al. 2018), while HIFUA is a therapeutic modality that induces thermal necrosis of biological tissues by focusing high-energy ultrasound waves at one specific target (Kim 2017). PMWA is also a thermal ablation technique in which the electromagnetic energy is delivered to the target tissue by a needle like antenna, then microwave radiation induces coagulative necrosis in the tissue surrounding the antenna (Carrafiello et al. 2008)
Currently, MRI without and with contrast is the reference standard for evaluating uterine fibroids prior to any of these treatments (Williams et al. 2011), and interventional radiologist are turning to post-procedural MRI as a follow-up post treatment to determine treatment efficacy (Chrisman et al. 2009). However, MRI is quite expensive, time consuming, and not always available in all medical centers. Moreover, insurance coverage for post-procedure MRIs is becoming a rarity, which is a concern for many patients. Thus, a more available, time-saving, and cost-effective imaging modality is highly needed for assisting those non-surgical treatments.
CEUS, which is a relatively new technique in this field, has been studied as an alternative to MRI because of its lower cost, real-time analysis, and fewer side effects. CEUS uses ultrasound contrast agents (UCAs) which are gas-filled microbubbles with diameters less than 8 μm, and a lipid, protein, or a polymer shell (Lyshchik 2019). UCAs strongly scatter the incident pulse due to marked difference in acoustic impedance at the blood gas interface (Chong et al. 2018). They also undergo volumetric oscillation due to high compressibility of the gas inside the microbubbles, and enhance the backscattered ultrasound signals much more than the surrounding tissues. Contrast specific imaging modes such as harmonic imaging can distinguish the backscattered echoes of the UCAs from received echoes of the tissue (Stride et al. 2003). Since microbubble are the same size as red blood cells, they do not extravasate into the interstitial space and are limited to the intravascular space, which makes them perfect for imaging the micro and macro vasculature. The signal intensity is proportional to the amount of microbubbles in the analyzed tissue and is related to the perfusion in the region of interest (Dietrich et al. 2012). Currently, there are three UCAs approved by the FDA. Definity (Lantheus Medical Imaging, N Billerica, MA) and Optison (GE Healthcare, Princeton, NJ) are approved for echocardiography only, but can be used off-label elsewhere. Lumason (Bracco SpA, Milan, Italy) - known as Sonovue in the rest of the world-has been approved for cardiac and non-cardiac use in the United States in 2016 (Chong et al. 2018).
The vascular enhancement pattern of uterine fibroid with CEUS has been reported in a few studies (Zhang et al. 2010; Stoelinga et al. 2018). There are two characteristic phases -early and late-in the enhancement process of fibroids. The early phase begins after UCA injection, when the enhancement is first observed in the fibroids until it reaches its maximum level (Zhang et al. 2010). During the late phase, the enhancement returns to the baseline level (i.e., wash out).-The fibroids enhance earlier than surrounding myometrium and display faster washout than the myometrium in around 95% of the cases, leaving a black-hole impression (hypo-enhancement) facilitating the determination of the location, size, and number of the tumors, since the contour of the tumors become clearly visible (Zhang et al. 2010;Stoelinga et al. 2018). CEUS provides additional details in terms of fibroids’ pseudo-capsule, central necrosis, and intra-lesion vascularity pattern (quantified based on time–intensity curves), compared to grey-scale ultrasound, sonoelastography, and color/power ultrasound (Stoelinga et al. 2018) (figure 1).
Figure 1.
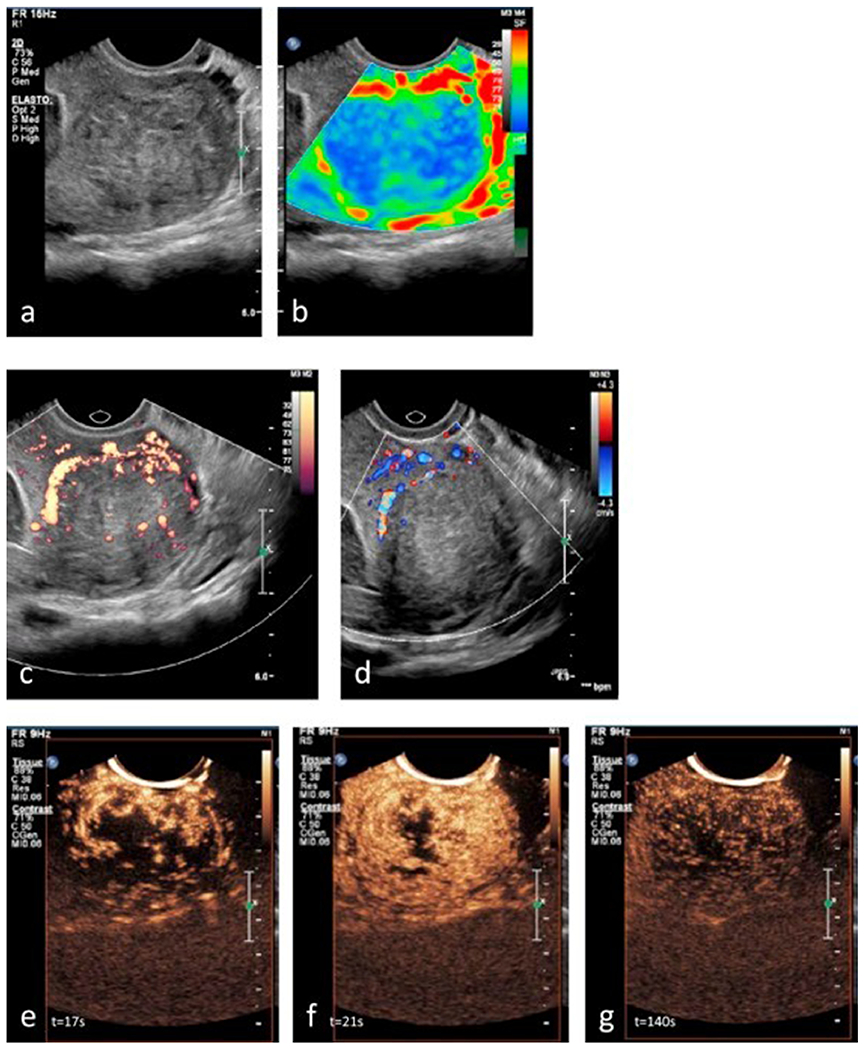
Adopted from (Stoelinga et al, 2018) with permission. All sonographic examinations were performed using a transvaginal probe from the same patient (a) Grey-scale ultrasound image revealing a well-delineated subserosal fibroid (4.2 cm) (b) Sonoelastography image revealing the fibroid’s center in blue (stiff tissue) with a pseudo-capsule in red (soft tissue). Small green areas indicating softer tissue are present in the fibroid’s center (c) Power Doppler image revealing the peripheral vascular network (pseudo-capsule) and a few larger vessels in the fibroid’s center (d) Color Doppler imaging revealing the proximal part of the pseudo-capsule (e)CEUS image obtained 17 s after contrast injection (t = 17 s), revealing peripheral enhancement with vessels from the exterior to interior of the fibroid during wash-in of contrast (f) CEUS image at t = 21 s revealing heterogeneous enhancement of the entire fibroid with hypo-echogenic, avascular areas in the fibroid’s center (g) CEUS image at t = 140 s revealing gradual wash-out of contrast from the fibroid.
Our systematic review aimed to evaluate the application and safety of CEUS as an adjunct to monitoring UAE, HIFUA, and PMWA for fibroid treatment. All included studies had been approved by an ethics committee or institutional review board.
Material and Methods:
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA) (Moher et al. 2009) (Figure 2). The current study is a systematic review of previously published studies institutional review board approval and patient consent were not necessary.
Figure 2.
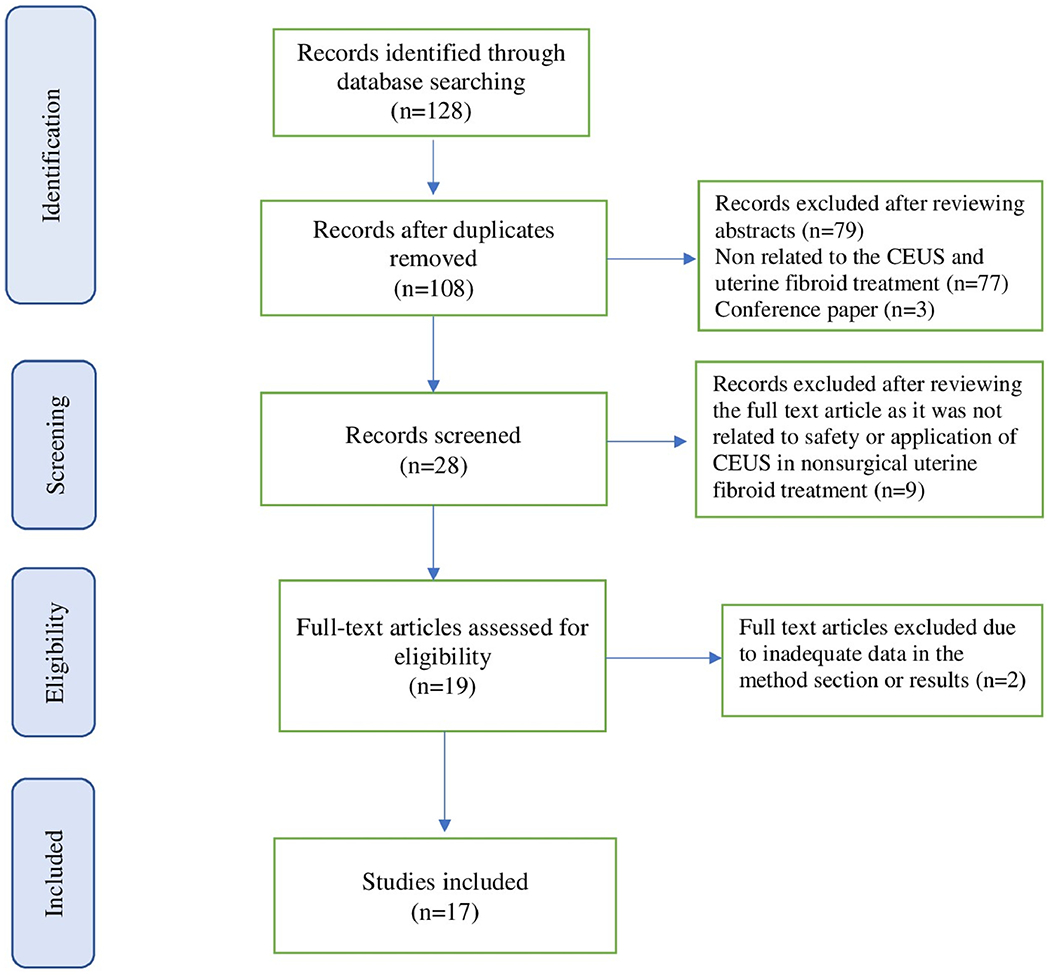
The PRISMA diagram of literature retrieved.
Search strategy and study selection
English literature investigations of the safety and application of CEUS as an adjunct to monitor UAE, HIFUA or PMWA in uterine fibroid treatments in human were searched for in PubMed, Ovid Medline, and Scopus databases from January 2000 through June 7th, 2020. The search strategy was (Contrast enhanced ultrasound OR contrast-enhanced ultrasound OR contrast enhanced ultrasonography OR contrast-enhanced ultrasonography OR contrast-enhanced sonography OR contrast enhanced sonography OR CEUS) AND (uterine fibroid OR uterine myoma OR uterine myoma OR uterine fibroid OR uterine leiomyoma) AND (Uterine artery embolization OR UAE OR Percutaneous microwave ablation OR HIFU OR High intensity focused ultrasound). The detailed search strategy is shown in Supplementary Table 1.
The initial search and assessment of the eligibility of the studies were independently reviewed by two authors (M.T., P.M.) and disagreements were resolved by a third reader (F. F.). Studies were eligible for inclusion if they had addressed the feasibility, efficiency and/or safety of the peri-procedural CEUS in nonsurgical uterine fibroid treatment. Only human studies with full text in English were assessed for inclusion. Studies without detailed method and result section were excluded. In addition, the reference lists of all identified articles were examined to identify studies not captured by electronic searches.
Results
One hundred twenty-eight potential studies were identified and retrieved for evaluation. After removing the duplicates and exclusion of studies based on the title and abstract, 28 remained for full text review. After two authors independently reviewed the articles, nine did not directly discuss the safety or application of CEUS in nonsurgical treatments of uterine fibroids and further two were excluded, due to inadequate data in method section or results. When consensus was reached by the authors, 17 publications met the inclusion criteria. Among the selected 17 papers, 6 studies focused on the usage of CEUS in UAE, 9 studies were discussing the applications of CEUS in HIFUA, and 2 studies focused on the usage of CEUS in PMWA. (Tables 1–3)
Table 1.
Application of CEUS in UAE for uterine fibroid treatment
| Author Year Country | Objective of the study | Study design | Mean age of patients (years) | Number of patients | Number of fibroids | Uterine fibroids’ mean diameter or volume by ultrasou nd (pre-UAE) | Equipment used for CEUS - Probe used for CEUS | Ultrasound contrast agent dose, brand, and the protocol of use | Adverse reaction to UCA | Main results of the study |
|---|---|---|---|---|---|---|---|---|---|---|
| Marret et al. 2004 France | Evaluate the feasibility of using perioperative CEUS to assess the efficacy of UAE | Case report | 50 | 1 | 3 | 35 mm (range: 25-64) | Esatune* ultrasound scanner - Convex probe | 1 ml of SonoVue† was injected before UAE, after left UAE and after bilateral UAE | None | CEUS can be used to monitor UAE perioperative and immediately post-operative to prevent ischemic complications |
| Dorenberg et al. 2007 Norway | Evaluate the feasibility of using CEUS during UAE to define the correct end-point of the procedure | Prospective | 38.7 | 10 | NR | NR | Sequoia 512 scanner**-4C1 curved-array probe | 2.4 ml SonoVue†† (IV bolus) followed by a flush of 5 ml saline solution was injected before and immediately after UAE | NR | CEUS during UAE may adjust the end-point of procedure and decrease the risk of clinical failure, the need for reinterventions, and the radiation dose |
| Sconfienza et al. 2008 Italy | Analyze the potential value of pre-and posttreatment CEUS to assess UAE outcomes | Prospective | NR | 12 | 21 | 52 mm (range: 35-90 mm) | Philips iU22 scanner***-Transabdominal probe | 4.8 ml SonoVue†† (IV bolus) followed by a flush of 5 ml saline solution Immediately before and immediately after UAE | NR | CEUS is effective for assessing the completeness of vascular occlusion following UAE and it is comparable with clinical and MRI results |
| Dorenberg et al. 2012 Norway | Determine feasibility of the use of CEUS during UAE | Retrospective | 41 | 30 | NR | 120 cm3 (range: 66-238 cm3) | Sequoia 512 scanner**-Transabdominal probe | 2.4 ml SonoVue†† (IV bolus) followed by a flush of 10 mL saline solution was injected at the angiographie endpoint of UAE | NR | CEUS was technically successful during the UAE in all patients and adjusted the end-point of procedure in 5 cases |
| Marret et al. 2014 France | Investigate the fibroids vascularization distribution patterns using CEUS before and after UAE, with the evaluation of radiologic predictive factors for successful embolization | Prospective | 44 ± 6 | 40a | NR | 311 cm3 (range: 167-613 cm3) | Technos MPX scanner*-Transabdominal and transvaginal probes | 2.4 ml SonoVue†† (IV bolus) followed by a flush of 5 ml saline solution was injected one day before, one day after, and 6-12 months later | NR | Partial or total enhancement on CEUS after UAE can be evidence of probable clinical failure in the intermediate term, however no quantitative vascular indicator predicting the clinical results was found |
| Pesapane et al. 2020 Italy | Using CEUS perioperatively to assess the target uterine fibroid’s vascularity and determine the embolization endpoint | Case report and literature review | 39 | 1 | Multiple fibroids | 9.5cm by MRI | ACUSON 3000§§ | 2.4 ml SonoVue†† (IV bolus) followed by a flush of 10 mL saline solution was injected Pre UAE, during arterial phase, and immediately at the angiographie endpoint of UAE | NR | CEUS is feasible and practical to assess the vascularity of uterine fibroids during UAE Caution should be taken to avoid misinterpretation of complete devscularization in cases of multiple fibroids (some fibroids may obscure other vascularized fibroids on CEUS) |
CEUS: contrast enhanced ultrasound; UAE: uterine artery embolization; UCA: ultrasound contrast agent; IV: intravenous; MRI: magnetic resonance imaging; NR: not reported
(Bracco International BV, Amsterdam, The Netherlands),
(SonoVue, Bracco, Milano, Italy)
(Esaote, Genova, Italy) and dedicated software (CnTI, Esaote)
(Siemens, Erlangen, Germany) and dedicated software (Cadence)
(Koninklijke Philips Electronics, Eindhoven, The Netherlands)
(Philips, Bothell, WA, USA)
(Siemens, Erlangen, Germany)
Table 3.
Application of CEUS in PMWA for uterine fibroid treatment
| Author Year Country | Objective of the study | Study design | Number of patients-fibroids | Uterine fibroids’ mean diameter by ultrasound | Mean age of patients (years) | UCA dose, brand, and the protocol of use | Adverse reaction to UCA | Equipment used for CEUS | Results of the study |
|---|---|---|---|---|---|---|---|---|---|
| Wang et al. 2012 China | Evaluate image changes and the relationship between conventional ultrasonography and CEUS in the perioperative period of PMWA for efficacy assessment | NR | 29-31 | NR | 40±5.25 | 2.4 ml of SonoVue* (IV bolus) was injected, followed by a flush of 5.0 mL saline | NR | Siemens sequoia 512 ultrasound system (the probe frequency was 2.5–4.5 MHz) | Non-enhanced CEUS areas immediately after PMW, correlated with hyperechoic area on grayscale ultrasound, representing the ablated area CEUS displays sharp and distinct boundaries of ablated area 12-24 hours after ablation and can be used for efficacy assessment |
| Lei et al. 2014 China | CEUS and CEMRI comparison after PMWA for uterine fibroid treatment | Retrospective | 18-20 | 5.56 ± 1.26 cm | 39.83 ± 5.83 | 2.4 ml of SonoVue* (IV bolus) was injected, followed by a flush of 5.0 ml saline | NR | Siemens Sequoia 512 ultrasound system (the probe frequency was 2.5–4.5 MHz) | CEUS and CEMRI agreed sufficiently to be used interchangeably in evaluating the ablated volumes of uterine fibroids treated with PMWA |
CEUS: contrast enhanced ultrasound; PMWA: percutaneous microwave ablation
(Bracco, Milan, Italy)
(Acuson, Mountain View, CA, USA)
CEUS in UAE treatment of fibroids
In 2004, Marret et al reported a completely perfused uterus with enlarged areas, due to the fibroids observed in the pre-UAE CEUS exam and persistent perfusion in the myometrium but not in the fibroids on the post-UAE CEUS exam (CEUS was performed before UAE, after left UAE, and after bilateral UAE) (Marret et al. 2004). Thus, the authors concluded that CEUS could be used for monitoring UAE, at the end of embolization, and 24 hours later. They added that CEUS could check for myometrial perfusion to avoid over-embolization and confirm that redistribution of the embolic material has not occurred. An example from our practice of this pre- and post UAE CEUS enhancement pattern is shown in Figure 3.
Figure 3.
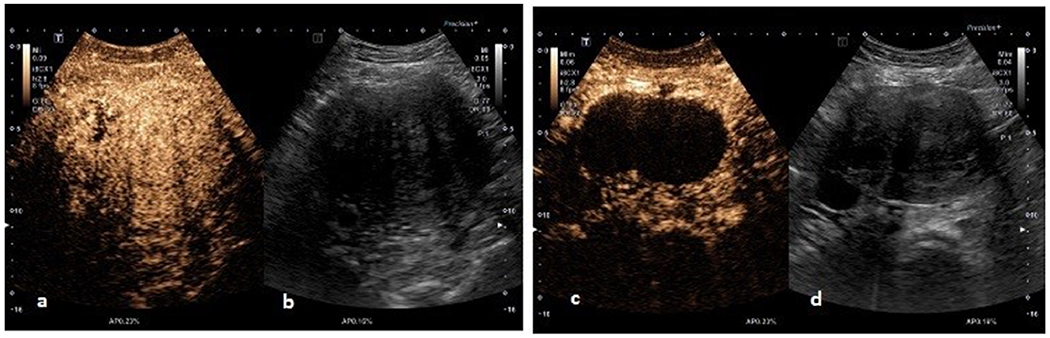
(a, b). CEUS image and grey-scale ultrasound image of an intramural fibroid before UAE revealing vascularity inside the fibroid, (c, d) CEUS image and grey-scale ultrasound image of an intramural fibroid three months after UAE revealing loss of vascularity inside the fibroid.
Typically, the angiographic endpoint of the UAE procedure is occlusion of the fibroids’ vessels, while a sluggish forward flow is maintained in the main uterine arteries (Pelage et al. 2005). Dorenberg and colleagues evaluated the feasibility of using CEUS during UAE to correctly define the endpoint of embolization (complete devascularization of all fibroids) (Dorenberg et al. 2007). CEUS was performed reaching the angiographic endpoint, while the angiographic catheters in both uterine arteries were still in place, and the procedure was continued until CEUS showed complete lack of enhancement of the fibroids, or continuing the procedure was no longer technically possible. When compared with post-procedural MRI studies, the CEUS findings matched with the post-procedural CE-MRI in terms of the degree of devascularization after UAE, supporting CEUS as a feasible technique during UAE (Dorenberg et al. 2007). Between the years 2006 to 2009, this group of researchers also performed 30 UAE studies with CEUS and found that CEUS changed the endpoint of embolization in 17% of patients, and 97% of patients showed complete infarction of all fibroids on follow-up MRI after 3 months (Dorenberg et al. 2012).
Similarly, Sconfienza et al carried out a study during which CEUS prior to, immediately after, 1 and 6 months after UAE, and dynamic MR prior to and 3 months after UAE were performed. In each examination, the size and perfusion of the fibroids, the devascularized area, and areas of persistent perfusion after treatment were evaluated. In all cases, CEUS findings were similar to those of the CE-MRI examination, indicating that CEUS could provide good visualization of fibroid size and vascularity prior to and after UAE. Moreover, they also found performing CEUS while the catheter was still in the uterine artery provided the opportunity to directly evaluate the interruption of the fibroid’s blood supply instead of indirect angiographic evaluation of devascularization, which also decreased the radiation exposure time and amount of embolic material needed during UAE (Sconfienza et al, 2008).
Subsequently, CEUS was applied to understand the characteristics of fibroid microvascularization before and after UAE, how fibroids can be embolized without embolization of the myometrium, the radiologic predictive factors for successful embolization and to evaluate the concordance with CE-MRI (Marret et al, 2014). Compared to conventional pre-embolization Doppler ultrasound that only showed rim vascularity in 50% of uterine fibroids, pre-embolization CEUS and CE-MRI provided detailed data for fibroids’ vascularization location and quantification with total enhancement rates of 82.5% and 80%, respectively. There was also a perfect concordance between the pre-embolization perfusion observed with CE-MRI and CEUS. However, in the post-embolization period, Marret et al. found that enhancement in CEUS on post-operative day 1 after UAE could be evidence of probable clinical failure in the intermediate term, while CEUS results at 6 months after UAE did not correlate with clinical failure nor agreed with the MRI results. With CEUS, myometrium was totally perfused in 70% and 100% of patients in one day and six months post-embolization, respectively. No quantitative vascular indicators predicting the clinical results were found (Marret 2014).
Recently, the discordance between CEUS and MRI was presented in a case where a 39-year-old woman with an enlarged uterus underwent UAE with the use of intraoperative CEUS to evaluate the uterine fibroids’ vascularity and determine the endpoint of embolization peri-operatively. While CEUS findings immediately after UAE were interpreted as ‘complete devascularization’ of the fibroids, the 6 months follow-up MRI findings showed a remaining smaller fibroid with enhancement at the inferolateral of the posterior aspect of the uterus (Pesapane et al. 2020). The authors explained that the misinterpretation of CEUS findings as complete devascularization in cases with multiple fibroids in a same ultrasound plan may be related to ‘satisfaction of search’ of the operators following recognizing the bulk of devascularized fibroids and caution should be made to alleviate this risk. No adverse reaction has been reported in the studies reviewed above.
CEUS in HIFUA treatment of fibroids
Typically, with CE-MRI after HIFUA, the unenhanced part of the treated area represents the non-perfused volume (NPV) and the fractional ablation (NPV/uterine fibroid volume before HIFUA) is the most important factor for the long-term clinical outcomes of HIFUA; completely ablated lesions do not enhance, which reflects the disappearance of the blood supply and subsequent necrosis, while any residual tumor is observed as a focal enhancing area within the ablated areas, and diffused enhancement indicates failed treatment (Funaki et al. 2007; Stewart et al. 2007). In 2007 Zhou and colleagues conducted the first study comparing CEUS with MRI within 1 week after HIFUA in patients with uterine fibroids. This study demonstrated the same results with CEUS and CE-MRI in terms of typical coagulative necrosis and vascular damage, which were further confirmed by pathology. The authors concluded that CEUS is useful to evaluate the early therapeutic effect of HIFUA and has 100% negative predictive value for evaluating the residual unablated tumor, suggesting that immediate MRI follow-up after HIFUA is not necessary. CEUS may also help in targeting the residual tumor foci during the additional ablation procedure in fibroids requiring repeat HIFUA (Zhou et al. 2007).
UCAs has been demonstrated to collapse when exposed to higher acoustic pressures (typically > 500 kPa), causing local mechanical injury to the ablated tissue and also enhancing the heating effect of high intensity focused ultrasound (HIFU) (Kajiyama et al. 2010). In 2012, in order to compare the therapeutic response to HIFUA in fibroid treatment, Peng et al. conducted a retrospective study of 162 HIFUAs performed 10 minutes after SonoVue injection and HIFUA in 129 patients without SonoVue injection. They found the median sonication time for ablating 1 cm3 of fibroid volume was significantly shorter in the group with SonoVue (16 seconds) than without it (26 seconds; p = 0.005), and the median fractional ablation immediately after HIFU treatment was 86.0% in the group using SonoVue, and 83.0% in the group without SonoVue (p = 0.025). When fibroids were categorized based on their size, the sonication time to achieve the greyscale changes was significantly different between two groups in fibroids between 4-8 cm in diameter (p < 0.001), but the rate of massive grey scale changes, fractional ablation, and non-perfused volume were not significantly different between the two groups when fibroids were >4 cm in diameter. Therefore, Peng and colleagues concluded that microbubbles are safe to use and enhance the effect of HIFUA in uterine fibroid treatment (Peng et al. 2012).
More prospective studies were conducted to investigate the effect of SonoVue in HIFUA treatment. In 2014, Jiang et al. conducted a randomized trial with 40 patients in the SonoVue group and 40 patients in the control group (Jiang et al. 2014). SonoVue was injected 5 minutes before starting HIFUA in the active group and immediately after the ablation in both groups. A post-HIFUA CE-MRI was performed 1 day after treatment to evaluate the fractional ablation. Results showed the rate of greyscale changes was significantly greater and had an early occurrence in the group receiving SonoVue before HIFUA than in the control group (p < 0.003), and significantly less acoustic energy was needed for ablating 1 mm3 of fibroid volume in the SonoVue group than in the control group (p = 0.029). Thus, Jiang et al. concluded that SonoVue may assist in achieving greater fractional ablation, long term symptomatic relief, and less additional post-ablation fibroid treatments (Jiang et al. 2014).
In 2015, Orsi et al. conducted the first blinded randomized study comparing ultrasound guided HIFUA and a control group without intraprocedural CEUS (Orsi et al. 2015). This study found that intraprocedural CEUS has faster volume shrinkage over a shorter time period with less needed energy, confirming the results of Jiang and colleagues’ study. Also, they found intraprocedural CEUS could provide early detection of residual tissue after ablation, which reduced the need for additional treatments. The same results were achieved when Isern and colleagues retrospectively analyzed data of 390 uterine fibroids, among which 155 were treated with SonoVue and 235 were treated without SonoVue during HIFUA (Isern et al. 2015). Their results also showed the total ablation time to achieve the same NPV and the average acoustic energy used for ablating 1 mm3 was significantly less in the SonoVue group (p=0.001), and the NPV by post-HIFU MRI was significantly higher in the SonoVue group (p = 0.031), while fibroids’ vascularity and size made no significant difference to the results (Isern et al. 2015)
In 2018, Chen et al also conducted a controlled randomized trial on 120 patients with a single uterine fibroid treated with HIFUA. Subjects were randomly divided into 4 groups with SonoVue used in Groups A and B and saline used in Groups C and D as controls. Results showed that NPV ratio was significantly higher in the SonoVue group than that in the saline group (p = 0.006), and starting HIFUA earlier after SonoVue injection (6 minutes in groups A &C vs. 10 minutes in groups B&D) was safe and could significantly shorten the treatment time (p = 0.013) and sonication time (p = 0.04) (Chen et al. 2018).
To further study the correlation between CEUS and CE-MRI, Peng et al (2015) conducted a study where 68 patients underwent CE-MRI before and 1 day after HIFUA for uterine fibroid treatment and CEUS pretreatment, intraprocedural, and immediate post HIFUA. Results showed CEUS and CE-MRI correlated well in terms of size and volume of the fibroids, and NPV (r > 0.94), and CEUS was reliable for assessing the treatment response (Peng et al. 2015).
CEUS to predict treatment response before HIFUA
HIFUA exerts its therapeutic effects by thermal ablation of the tissue causing greyscale changes visualized on conventional ultrasound (figure 4) (Zhang et al. 2017). Since blood perfusion in the target tissue is an important factor influencing the energy distribution of the thermal ablation, an accurate, repeatable, and feasible assessment of blood perfusion in uterine fibroid before HIFUA is essential for predicting the therapeutic outcomes (Kim 2017). Achieving a fractional ablation higher than 70% indicates safer and more effective HIFUA for uterine fibroid treatment (Wei et al. 2018). In 2019, Wang and co-workers tried to quantitatively assess the perfusion characteristics of uterine fibroids using CEUS before HIFUA and determined its ability to predict the outcomes of HIFUA in patients with uterine fibroids (Wang et al. 2019). Using an automatic contrast quantification software built into the HIFU equipment, time intensity parameters from recorded video clips of CEUS were measured. Post treatment volume and NPV were also measured from CE-MRI, considering the fractional ablation ≥70% as a favorable therapeutic outcome. Results revealed that patients in the group with a fractional ablation of ≥ 70 % had a longer mean arrival time, peak time, and enhancement time alongside with lower mean enhancement intensity and enhancement rate when compared to those with a fractional ablation of <70% (p < 0.05). Wang and colleagues concluded that the enhancement rate by CEUS had the highest area under the curve in the ROC curve analysis, followed by the time to peak and enhancement intensity, suggesting the enhancement rate (or estimated perfusion) as the best indicator of diagnostic efficacy, and of the perfusion characteristics of uterine fibroids which may be a valuable indicator for predicting the ablation efficacy of HIFUA.
Figure 4.
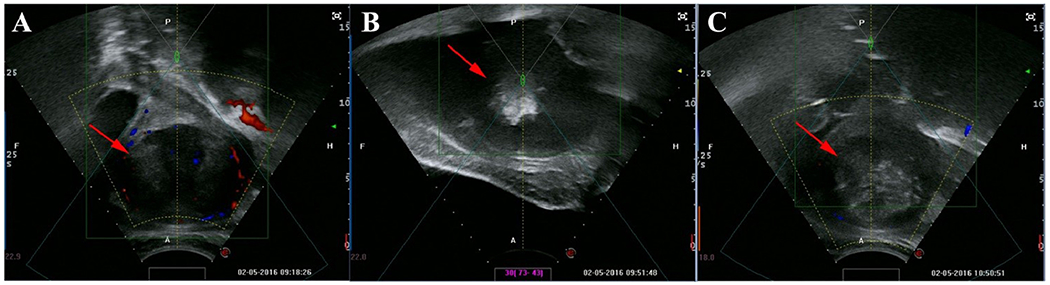
Adopted from (Zhang et al, 2017) with permission. Large greyscale changes during treatment of a 26-year-old patient with a 7.5-cm fibroid. (A) Ultrasound image with Doppler obtained prior to HIFUA showing a hypoechoic fibroid with surrounding small blood vessel (red arrow). (B) Large grey-scale change arising within targeted area during treatment (red arrow). (C) Ultrasound image with Doppler obtained at the end of HIFUA showing a complete large greyscale change inside fibroid (red arrow) with disappearance of surrounding blood supply
Safety Concerns of the use of CEUS during HIFUA
There were also concerns about the safety of using CEUS during HIFUA of uterine fibroids. Cheng et al. published a retrospective study in 2015 showing that the intra-operative HIFU-related adverse effects, such as leg pain, sacrum/buttock pain, groin pain, pain in the treated region, and discomfort on skin were more frequent among patients who were injected with Sonovue (n = 844) than those who were not (n= 819) (p < 0.05), while the post-procedural HIFU-related complications showed no significant difference between these two groups except for higher rate of pain in the lower abdominal wall in the Sonovue group (p < 0.05). Additionally, among the group exposed to SonoVue, two patients experienced acute renal failure with elevated creatinine levels, one at day 2 and one at day 5 post-procedure. One patient had history of hypertension, and the other had a 6-year history of using nonsteroidal anti-inflammatory drugs (NSAIDS). Both of them underwent continuous renal replacement therapy and recovered after two weeks. It should be mentioned that the routine bowel preparation before HIFU could make some patients dehydrated. Therefore, Cheng et al. concluded that using SonoVue to assess HIFU treatment efficacy is safe, but may increase the occurrence of some common yet tolerable HIFU-related side effects. They also recommended renal function monitoring in patients with a history of hypertension or taking NSAIDS (Cheng et al. 2015).
CEUS in PMWA treatment of fibroids
In 2012, Wang et al. conducted a study to evaluate the imaging manifestations and relationships between conventional ultrasound and CEUS in PMWA treatment of fibroids. During PMWA, microbubbles in boiling tissue caused enhancing hyperechoic signals on grayscale ultrasound started from the microwave emission point until covered the entire fibroid. Upon stopping the emission, the hyperechoic microbubbles in the target tissue gradually vanished, but echoes in ablated areas were still significantly higher than the adjacent myometrium. Results showed hyperechoic area in grayscale was significantly larger than non-enhancement area by CEUS, when compared immediately after PMWA (p<0.01). CEUS non-enhanced area immediately after ablation was also slightly smaller than CEUS non-enhanced area 12–24 hours after ablation; albeit not a significant difference. Wang and colleagues thus, concluded that conventional ultrasound can depict the thermal field range immediately after microwave ablation although the boundaries are not as clear as on CEUS images, and CEUS displays ablated area as a clear enhancement void with distinct boundaries 12-24 hours after the ablation. CEUS enhanced area immediately after PMWA may be influenced by remnant microbubbles in the thermal field and result in underestimating the ablation range for inexperienced operators (Wang et al. 2012).
Lei et al. (2014) compared ablation volumes obtained from CEUS and CE-MRI using the intraclass correlation coefficient (ICC) and Bland-Altman analysis. All patients (N = 18) underwent preoperative ultrasound guided uterine biopsy, PMWA, postoperative ultrasound guided uterine biopsy, CEUS 7 days after ablation, and CE-MRI within 3 days after CEUS. ICC, Bland-Altman analysis and Bradley-Blackwood test results showed an excellent agreement between ablated volumes obtained from CEUS and CE-MRI, so that CEUS and CEMRI could be used interchangeably in the clinic to evaluate the therapeutic effect and follow up in PMWA of fibroids (Lei et al. 2014).
Conclusion:
According to the reviewed studies, CEUS provides detailed and clinically useful data about fibroid volume and vascularization prior to UAE, helps with determining the endpoint of embolization during the procedure, and can be used as a convenient follow-up imaging technique post UAE to evaluate the efficacy of the procedure. CEUS can minimize the radiation exposure time for patients whenever used as an alternative for angiography during the procedure.
HIFUA of uterine fibroids with intraprocedural CEUS imaging by SonoVue has shown faster volume reduction in a shorter term with less required energy. Data obtained from CEUS and CE-MRI before and after HIFUA showed good correlation, presenting CEUS as a cost-effective alternative for pre and post treatment imaging method of choice, especially when MRI is contraindicated or is not available or covered by insurance. The enhancement rate (as a measure of perfusion) obtained by CEUS before HIFUA may be an indicator for predicting the efficacy of HIFUA, where CEUS after HIFUA of uterine fibroid, might help with predicting clinical therapeutic response. Of note, using SonoVue to assess HIFU treatment efficacy may increase the occurrence of some common yet tolerable HIFU-related side effects in exposed patients or impair renal function in those with pre-existing hypertension or long history of NSAIDS usage. Thus, adequate hydration and appropriate pain control should be practiced during the procedure.
Based on available data, CEUS and CE-MRI have also shown sufficient agreement to be used interchangeably in the clinic to evaluate the therapeutic effect of PMWA of fibroids. Twelve to 24 hours after PMWA, CEUS displays a sharp and distinct boundary of ablated area with no blood supply and can confirm either successful ablation or residual unablated tissue.
Compared to MRI, CEUS is an inexpensive and better accessible imaging technique. However, comparing it with conventional sonography, adding an IV contrast agent is quite a difference from patient and logistic perspective and not all sonographers and physicians are familiar with this technique. There are also some intrinsic limitation same as conventional sonography. For example, the scanning field is small and only one uterine fibroid or several uterine fibroids on the same plane can be observed with a single injection of contrast agent.
With the growing trend toward non-surgical uterine fibroid treatments and necessity of an available cost-effective follow-up imaging method, there is need for future research in this field to recognize the valid advantages and possible pitfalls of CEUS as an adjunct to UAE, HIFUA, and PMWA. Characterizing fibroids based on quantitative CEUS findings and evaluating the corresponding therapeutic results might also be a promising topic for future research.
Supplementary Material
Table 2.
Application of CEUS in HIFUA for uterine fibroid treatment
| Author Year Country | Objective of the study | Study design | Number of patients-fibroids | Uterine fibroids’ mean diameter or volume by ultrasound | Mean age of patients (years) | UCA dose, brand, and the protocol of use | Adverse reaction to UCA | Equipment used for CEUS | Results of the study |
|---|---|---|---|---|---|---|---|---|---|
| Zhou et al. 2007 China | Evaluate the utility of CEUS in assessing the therapeutic response to HIFUA | Prospective | 64 - 64 | 53 ± 12 mm (range: 32-89 mm) | 39.1 ± 5.6 | 2.4 ml of SonoVue* (IV bolus) was injected, followed by a flush of 5.0 mL saline | None | Toshiba Aplio 80** | CEUS showed 100% NPV for finding any residual unablated tumor in the HIFU ablated zone CEUS can help to target the residual tumor during the additional ablation procedure |
| Peng et al. 2012 China | Evaluate the clinical value of the contrast agent SonoVue* in the treatment of uterine fibroids with therapeutic HIFUA | Retrospective | Group of patients treated by HIFUA with SonoVue* | 2.0 ml of SonoVue* (IV bolus) was injected 10 minutes before HIFUA 1.5 ml of SonoVue* (IV bolus) during or/and immediately after HIFUA | There were no significant differences in adverse effects between the two groups | My-Lab 70† ultrasoun d imaging machine | CEUS can be used to assess the extent of ablation during HIFUA Non-perfused volume/fibroid volume was 86.0% in the group with SonoVue* and 83.0% in the group without SonoVue* The sonication time for ablating 1 cm3 of fibroid volume was significantly shorter in the group with SonoVue than without it | ||
| 162-162 | 56 mm (range: 29–120 mm) | 36.9 ± 6.0 | |||||||
| Group of patients treated by HIFUA without SonoVue* | |||||||||
| 129-129 | 46 mm (range:21–128 mm) | 40.0 ± 5.8 | |||||||
| Jiang et al. 2014 China | Evaluate the role of the ultrasound contrast agent SonoVue* in enhancing the effects of HIFUA | Prospective | Group of patients treated by HIFUA with SonoVue* | 2.0 ml of SonoVue* (IV bolus) was injected 5 minutes before HIFUA in SonoVue* group 1.5 ml of SonoVue* (IV bolus) was injected immediately after HIFUA to assess the therapeutic response in both SonoVue* and control groups | There were no significant differences in adverse effects between the two groups | My-Lab 70† ultrasoun d imaging machine | SonoVue* is able to safely decrease the sonication time and acoustic energy required in HIFUA for uterine fibroids treatment | ||
| 40-40 | 47 ± 17 mm (range:21–88 mm) | 39.2 ± 5.4 | |||||||
| Group of patients treated by HIFUA without SonoVue* | |||||||||
| 40-40 | 51 ± 23 mm (range:20–99 mm) | 40.5 ± 5.7 | |||||||
| Orsi et al. 2015 Italy | Evaluate safety and effective ne ss of CEUS on HIFUA of uterine fibroids | Prospective (blind randomized) | Group of patients treated by HIFUA with SonoVue* | 2.4 of SonoVue* (IV bolus) was injected before, during and after HIFUA | There were no significant differences in adverse effects between the two groups | My-Lab 70† ultrasound imaging machine | CEUS was safe and effective in enhancing ultrasound guidance during HIFUA for uterine fibroids treatment CEUS reduced the treatment time and treatment repetitions (for incomplete fibroid ablation) CEUS during HIFUA increased the volume reduction at 1 and 3 months, but not at 6 months, between the two groups at MRI | ||
| 17-20 | 419 cm3 (range: 47.3–1865.0 cm3) | 43.1 | |||||||
| Group of patients treated by HIFUA without SonoVue* | |||||||||
| 16-17 | 189.5 cm3 (range: 34.6–709.5 cm3) | 42 | |||||||
| Peng et al. 2015 China | Investigate CEUS to evaluate treatment response of uterine fibroids to HIFUA | Prospective | 68-68 | 75.2 cm3 (range: 34.2–127.3 cm3) | 39.3 ± 6.6 | 2.0 ml of SonoVue (IV bolus) was injected 8 minutes before HIFUA 1.5 ml of SonoVue (IV bolus) was injected during and/or immediately after HIFUA to assess the therapeutic response | None | My-Lab 70† ultrasound imaging machine | CEUS clearly showed the size of fibroids and the non-perfused areas of the fibroid during or immediately after HIFUA |
| Cheng et al. 2015 China | Evaluate adverse effects of HIFUA for fibroids in a comparison between procedures with and without the use of UCA | Retrospective | Group of patients treated by HIFUA with SonoVue* | 2.0 ml of SonoVue* (IV bolus) was injected 5 minutes before procedure and 1.5 ml of SonoVue* (IV bolus) was injected immediately after HIFUA to assess the therapeutic response in the group of patients treated by HIFUA with SonoVue | Intraprocedural sacrum/butto ck pain, groin pain, leg pain, pain in the treated region, and discomfort on the skin, and post procedural lower abdominal pain are significantly higher in the group of patients treated by HIFUA with SonoVue Two acute renal failures also occurred in the group of patients treated by HIFUA with SonoVue | My-Lab 70† ultrasound imaging machine | SonoVue may increase the incidence rates of some common intraprocedural HIFUA-related adverse effects, however it can be safely used to assess HIFUA treatment efficacy | ||
| 844-NR | 57.2 cm3 | NR | |||||||
| Group of patients treated by HIFUA without SonoVue* | |||||||||
| 819-NR | 57.4 cm3 | NR | |||||||
| Isern et al. 2015 Spain | Assessment of the therapeutic effect of SonoVue on HIFUA fo different subtypes of uterine fibroids | Retrospective | Group of patients treated by HIFUA with SonoVue* | 2.0 ml of SonoVue* (IV bolus) was injected 2 minutes before procedure | No serious adverse reaction to UCA was reported and no significant difference between two groups in terms of reported adverse events | My-Lab 70† ultrasound imaging machine | Therapeutic SonoVue significantly decreases the time and energy needed for HIFUA of the same fibroid volume in all types of fibroid. | ||
| 124-155 | 87cm3 (range: 2-982) | 41±6 (range: 25-53) | |||||||
| Group of patients treated by HIFUA without SonoVue* | |||||||||
| 196-235 | 127 cm3 (range: 2-736) | 40±6 (range: 26-54) | |||||||
| Chen et al. 2018 China | Investigate UCA effects on HIFUA for uterine treatment, with dosage comparison | Prospective (randomized controlled trial) | Group of patients treated by HIFUA with SonoVue* (6 minutes before HIFUA) | 1.5 ml of SonoVue* (IV bolus) was injected, followed by a flush of 5 ml saline solution 6 minutes before HIFUA | Radiation pain, sacrococcygeal/buttock pain, groin pain discomfort on the on the skin, and lower abdominal pain were transient There were no significant difference in the rate of adverse effects among the four groups (p > 0.05 | My-Lab 70† ultrasound imaging machine | SonoVue enhanced the effect of HIFUA for uterine fibroids treatment, shortened HIFU treatment time and sonication time, with an increase in the rate of grey-scale changes and non-perfused volume ratio HIFUA was safe and more effective as it was started earlier after SonoVue injection | ||
| 30-30 | 79.6 ± 81.8 cm3 | 40.3 ± 6.7 | |||||||
| Group of patients treated by HIFUA with SonoVue* (10 minutes before HIFUA) | 1.5 ml of SonoVue* was injected (IV bolus), followed by a flush of 5 ml saline solution 10 minutes before HIFUA | ||||||||
| 30-30 | 58.7 ± 41.8 cm3 | 40.1 ± 4.7 | |||||||
| Group of patients treated by HIFUA with saline 6 minutes before HIFUA (without SonoVue*) | 1.5 ml of saline (IV bolus) was injected 6 minutes before HIFUA | ||||||||
| 30-30 | 66.3 ± 49.9 cm3 | 40.3 ± 5.9 | |||||||
| Group of patients treated by HIFUA with saline 10 minutes before HIFUA (without SonoVue*) | 1.5 ml of saline (IV bolus) was injected 10 minutes before HIFUA | ||||||||
| 30-30 | 81.5 ± 98.3 cm3 | 42.1 ± 4.8 | |||||||
| Wang et al. 2019 China | Assess quantitative perfusion parameters from CEUS for the clinical outcome of HIFUA in uterine fibroid treatment | Retrospective | 263-263 | 81.2 ± 38.7 cm3 | 38.2 ± 5.6 | 2.0 ml SonoVue* (IV bolus) was injected, followed by a flush of 5 ml saline solution immediately before HIFUA | None | NR | CEUS derived quantitative parameters such as higher time parameters and lower intensity parameters are linked to a better treatment outcome |
CEUS: contrast enhanced ultrasound; CEMRI: contras enhanced magnetic resonance imaging; HIFUA: high intensity focused ultrasound ablation; HIFU: high-intensity focused ultrasound; NPV: negative predictive value; UCA: Ultrasound contrast agent; IV: intravenous
(SonoVue, Bracco, Milan, Italy)
(Toshiba, Tokyo, Japan)
(Esaote; Genoa, Italy
Acknowledgement:
We would like to thank Dr. Kibo Nam for her great support during the writing of this manuscript.
Funding: Supported in part by R03 EB028464.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of interest statement: The authors report no conflict of interest.
References:
- Borah BJ, Laughlin-Tommaso SK, Myers ER, Yao X, Stewart EA. Association Between Patient Characteristics and Treatment Procedure Among Patients With Uterine Leiomyomas. Obstet Gynecol. 2016; 127(1):67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brolmann H, Huirne J. Current treatment options and emerging strategies for fibroid management. The Internet Journal of Gynecology and Obstetrics. 2008; 10(1), 2. [Google Scholar]
- Carrafiello G, Laganá D, Mangini M, Fontana F, Dionigi G, Boni L, Rovera F, Cuffari S, Fugazzola C. Microwave tumors ablation: principles, clinical applications and review of preliminary experiences. Int J Surg. 2008; 6 Suppl 1:S65–9. doi: 10.1016/j.ijsu.2008.12.028. Epub 2008 Dec 14. Review. [DOI] [PubMed] [Google Scholar]
- Chen Y, Jiang J, Zeng Y, Tian X, Zhang M, Wu H, Zhou H. Effects of a microbubble ultrasound contrast agent on high-intensity focused ultrasound for uterine fibroids: a randomised controlled trial. Int J Hyperthermia. 2018; 34(8):1311–1315. doi: 10.1080/02656736.2017.1411620. [DOI] [PubMed] [Google Scholar]
- Cheng CQ, Zhang RT, Xiong Y, Chen L, Wang J, Huang GH, Li KQ, Zhang L, Bai J. Contrast-enhanced ultrasound for evaluation of high-intensity focused ultrasound treatment of benign uterine diseases: retrospective analysis of contrast safety. Medicine (Baltimore). 2015; 94(16):e729. doi: 10.1097/MD.0000000000000729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong WK, Papadopoulou V, Dayton PA. Imaging with ultrasound contrast agents: current status and future. Abdom Radiol. 2018; 43(4):762–772. doi: 10.1007/s00261-018-1516-1 [DOI] [PubMed] [Google Scholar]
- Chrisman HB, Rajeswaran S, Dhand S, Nikolaidis P, Corpuz B, Vogelzang RL, Omary RA. Effect of post-procedural pelvic MR imaging on medical decision-making in women who have undergone uterine artery embolization. J Vase Interv Radiol. 2009; 20(7):977–980. doi: 10.1016/j.jvir.2009.03.041 [DOI] [PubMed] [Google Scholar]
- Dietrich CF, Averkiou MA, Correas JM, Lassau N, Leen E, Piscaglia F. An EFSUMB introduction into Dynamic Contrast-Enhanced Ultrasound (DCE-US) for quantification of tumour perfusion. Ultraschall Med. 2012; 33(4):344–51. [DOI] [PubMed] [Google Scholar]
- Dorenberg EJ, Jakobsen JA, Brabrand K, Hafsahi G, Smith HJ. The feasibility of contrast-enhanced ultrasound during uterine artery embolization: a pilot study. Cardiovasc Intervent Radiol. 2007; 30(5):882–7. [DOI] [PubMed] [Google Scholar]
- Dorenberg EJ, Hol PK, Jakobsen JA, Ring E. (2012). Improved infarction rates in fibroids after the introduction of contrast-enhanced ultrasound during uterine artery embolization. Acta Radiol. 2012; 1; 53(1):34–8. doi: 10.1258/ar.2011.110331. [DOI] [PubMed] [Google Scholar]
- Funaki K, Fukunishi H, Funaki T, Sawada K, Kaji Y, Maruo T. Magnetic resonance-guided focused ultrasound surgery for uterine fibroids: relationship between the therapeutic effects and signal intensity of preexisting T2-weighted magnetic resonance images. Am J Obstet Gynecol. 2007; 196(2):184.e1–6. [DOI] [PubMed] [Google Scholar]
- Hloldsworth-Carson SJ, Zaitseva M, Vollenhoven BJ, Rogers PA. Clonality of smooth muscle and fibroblast cell populations isolated from human fibroid and myometrial tissues. Mol Hum Reprod. 2014; 20(3):250–259. doi: 10.1093/molehr/gat083. [DOI] [PubMed] [Google Scholar]
- Jiang N, Xie B, Zhang X, Fie M, Li K, Bai J, Wang Z, He J, Zhang L. Enhancing ablation effects of a microbubble-enhancing contrast agent (“SonoVue”) in the treatment of uterine fibroids with high-intensity focused ultrasound: a randomized controlled trial. Cardiovasc Intervent Radiol. 2014;37(5):1321–8. doi: 10.1007/s00270-013-0803-z. [DOI] [PubMed] [Google Scholar]
- Kajiyama K, Yoshinaka K, Takagi S, Matsumoto Y. Micro-bubble enhanced HIFU. Physics Procedia, 2010; 3 (1), 305–314. [Google Scholar]
- Keung JJ, Spies JB, Caridi TM. Uterine artery embolization: A review of current concepts. Best Pract Res Clin Obstet Gynaecol. 2018; 46:66–73. doi: 10.1016/j.bpobgyn. [DOI] [PubMed] [Google Scholar]
- Kim YS. Clinical application of high-intensity focused ultrasound ablation for uterine fibroids. Biomed Eng Lett. 2017; 7(2):99–105. doi: 10.1007/s13534-017-0012-9. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei F, Jing Z, Bo W, Dongmei H, Zhencai L, Xue J, Fang W, Hongyu Z, Jintao R. Uterine myomas treated with microwave ablation: the agreement between ablation volumes obtained from contrast-enhanced sonography and enhanced MRI. Int J Hyperthermia. 2014; 30(1):11–8. doi: 10.3109/02656736.2013.853107. [DOI] [PubMed] [Google Scholar]
- Lénárd ZM, McDannold NJ, Fennessy FM, Stewart EA, Jolesz FA, Hynynen K,Tempany CM. Uterine leiomyomas: MR imaging-guided focused ultrasound surgery--imaging predictors of success. Radiology. 2008; 249(1):187–94. doi: 10.1148/radiol.2491071600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyshchik A Specialty Imaging: Fundamentals of CEUS. Elsevier; Manitoba, Canada: 2019. [Google Scholar]
- Marret H, Eboue F, Bleuzen A, Herbreteau D, Patat F, Tranquart F, Ouldamer L. Contribution of contrast-enhanced ultrasound with Sonovue to describe the microvascularization of uterine fibroid tumors before and after uterine artery embolization. Eur J Obstet Gynecol Reprod Biol. 2014; 181:104–10. doi: 10.1016/j.ejogrb.2014.07.030. [DOI] [PubMed] [Google Scholar]
- Marret H, Tranquart F, Sauget S, Alonso AM, Cottier JP, Herbreteau D. Contrast-enhanced sonography during uterine artery embolization for the treatment of leiomyomas. Ultrasound Obstet Gynecol. 2004; 23(1):77–9. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi F, Monfardini L, Bonomo G, Krokidis M, Della Vigna P, Disalvatore D. Ultrasound guided high intensity focused ultrasound (USgHIFU) ablation for uterine fibroids: Do we need the microbubbles? Int J Hyperthermia. 2015; 31(3):233–9. doi: 10.3109/02656736.2015.1004134. [DOI] [PubMed] [Google Scholar]
- Pelage JP, Cazejust J, Pluot E, Dref OL, Laurent A, Spies JB, Chagnon S, Lacombe P. Uterine fibroid vascularization and clinical relevance to uterine fibroid embolization. Radiographics. 2005; 25 Suppl 1:S99–S117. doi: 10.1148/rg.25si055510 [DOI] [PubMed] [Google Scholar]
- Pesapane F, Leenknegt B, Ammar T, Panella S, Garzillo G, Huang DY. Intraoperative microvascular assessment with contrast-enhanced ultrasound (CEUS) during uterine artery embolisation (UAE): a case report and literature review [published online ahead of print, 2020 Mar 5], J Ultrasound. 2020; 10.1007/S40477-020-00441-2. doi: 10.1007/s40477-020-00441-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Hu L, Chen W, Chen J, Yang C, Wang X, Zhang R, Wang Z, Zhang L. Intraprocedure contrast enhanced ultrasound: the value in assessing the effect of ultrasound-guided high intensity focused ultrasound ablation for uterine fibroids. Ultrasonics. 2015; 58:123–8. doi: 10.1016/j.ultras.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Peng S, Xiong Y, Li K, He M, Deng Y, Chen L, Zou M, Chen W, Wang Z, He J, Zhang L. Clinical utility of a microbubble-enhancing contrast (“SonoVue”) in treatment of uterine fibroids with high intensity focused ultrasound: a retrospective study. Eur J Radiol. 2012;81(12):3832–8. doi:s.1016/j.ejrad.2012.04.030. [DOI] [PubMed] [Google Scholar]
- Sconfienza L, Lacelli F, Gandolfo N, Gazzo P, Perrone N, Serafini G . Contrast-enhanced ultrasound (CEUS) assessment of superselective uterine fibroid embolization (SUFE): Preliminary experience. J Ultrasound. 2008; 11(4):158–61. doi: 10.1016/j.jus.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn GS, Cho S, Kim YM, Cho CH, Kim MR, Lee SR, Working Group of Society of Uterine Leiomyoma. Current medical treatment of uterine fibroids. Obstet Gynecol Sci. 2018; 61(2):192–201. doi: 10.5468/ogs.2018.61.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies JB, Myers ER, Worthington-Kirsch R, Mulgund J, Goodwin S, Mauro M. The FIBROID registry: symptom and quality-of-life status 1 year after therapy. Obstet Gynecol. 2005; 106(6): 1309–1318. [DOI] [PubMed] [Google Scholar]
- Stewart EA, Gostout B, Rabinovici J, Kim HS, Regan L, Tempany CM. Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet. Gynecol. 2007; 110: 279–287. [DOI] [PubMed] [Google Scholar]
- Stoelinga B, Dooper AMC, Juffermans UM, Postema AW, Wijkstra H, Brolmann HAM, Huirne JAF. Use of Contrast-Enhanced Ultrasound in the Assessment of Uterine Fibroids: A Feasibility Study. (2018). Ultrasound Med Biol. 2018; 44(8):1901–1909. doi: 10.1016/j.ultrasmedbio. [DOI] [PubMed] [Google Scholar]
- Stride E, Saffari N. Microbubble ultrasound contrast agents: a review. Proc Inst Mech Eng H. 2003; 217(6):429–47. Review. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhang J, Han ZY, Cheng ZG, Zhou HY, Feng L, Hu DM. Imaging manifestation of conventional and contrast-enhanced ultrasonography in percutaneous microwave ablation for the treatment of uterine fibroids. Eur J Radiol. 2012; 81(11):2947–52. doi: 10.1016/j.ejrad.2011.12.037. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Zhang PH, Zhang R, An PL. Predictive Value of Quantitative Uterine Fibroid Perfusion Parameters From Contrast-Enhanced Ultrasound for the Therapeutic Effect of High-Intensity Focused Ultrasound Ablation. J Ultrasound Med. 2019; 38(6):1511–1517. doi: 10.1002/jum.14838. [DOI] [PubMed] [Google Scholar]
- Wei C, Fang X, Wang CB, Chen Y, Xu X, Dong JN. The predictive value of quantitative DCE metrics for immediate therapeutic response of high-intensity focused ultrasound ablation (HIFU) of symptomatic uterine fibroids. Abdom Radiol (NY). 2018; 43(8):2169–2175. doi: 10.1007/s00261-017-1426-7. [DOI] [PubMed] [Google Scholar]
- Williams PL, Coote JM, Watkinson AF. Pre-uterine artery embolization MRI: beyond fibroids. Cardiovasc Interv Radiol. 2011; 34(6):1143–50. doi: 10.1007/s00270-011-0124-z. [DOI] [PubMed] [Google Scholar]
- Zhang C, Jacobson H, Ngobese ZE, Setzen R. Efficacy and safety of ultrasound-guided high intensity focused ultrasound ablation of symptomatic uterine fibroids in Black women: a preliminary study. BJOG. 2017; 124 Suppl 3:12–17. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Zheng RQ, Yang YB, Huang DM, Song Q, Mao YJ, Li YH, Zheng ZJ. The use of contrast-enhanced ultrasound in uterine leiomyomas. Chin Med J (Engl). 2010; 123(21):3095–9. [PubMed] [Google Scholar]
- Zhou XD, Ren XL, Zhang J, He GB, Zheng MJ, Tian X, Li L, Zhu T, Zhang M, Wang L, Luo W. Therapeutic response assessment of high intensity focused ultrasound therapy for uterine fibroid: utility of contrast-enhanced ultrasonography. Eur J Radiol. 2007; 62(2):289–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


