Abstract
Pulmonary hypertension (PH) is an independent risk factor for adverse clinical outcome, particularly in left heart disease (LHD) patients. Recent advances have clarified the mean pulmonary artery pressure (mPAP) range that is above normal and associated with clinical events, including mortality. This progress has for the first time resulted in a new clinical definition of PH that is evidenced-based, inclusive of mPAP >20 mmHg, and emphasizes early diagnosis. Additionally, pulmonary vascular resistance (PVR)=2.2–3.0 WU, considered previously to be normal, appears to associate with elevated clinical risk. A revised approach to classifying PH patients as pre-capillary, isolated post-capillary, or combined pre-/post-capillary PH now guides point-of-care diagnosis, risk stratification, and treatment. Exercise hemodynamic or confrontational fluid challenge studies may also aid decision-making for patients with PH-LHD or otherwise unexplained dyspnea. This collective progress in pulmonary vascular and heart failure medicine reinforces the critical importance of accurate hemodynamic assessment (Central Illustration).
Keywords: Pulmonary hypertension, heart failure, hemodynamics
CONDENSED ABSTRACT:
The definition for diagnosing pulmonary hypertension (PH) has been revised to include mean pulmonary artery pressure (mPAP) >20 mmHg. This is consistent with a wider paradigm shift emphasizing earlier diagnosis to improve outcome. Adding pulmonary vascular resistance >2.2 WU to mPAP >20 mmHg may further optimize risk assessment, particularly in patients with PH and left heart disease (LHD). Additionally, the utility of invasive exercise and fluid challenge testing to profile PH-LHD patients has come into clearer focus. Overall, lowering the cardiopulmonary hemodynamic thresholds used clinically reinforces the importance of accurate right heart catheterization data collection and interpretation.
Introduction
Pulmonary hypertension (PH) affects a major segment of the population, including >50% of patients with left heart disease (LHD). Advances characterizing the cardiopulmonary hemodynamic clinical risk spectrum have led to a new PH definition, which for the first time is evidence-based and uses mPAP >20 mmHg determined by RHC to emphasize early diagnosis (1). Cardiologists will also encounter patients with pulmonary arterial hypertension (PAH), other discrete PH subtypes, or unexplained dyspnea in routine practice. These disorders must be distinguished from PH-LHD to ensure proper diagnosis and treatment, which is best accomplished by careful RHC assessment, including exercise or confrontational fluid testing in some patients. However, a knowledge gap around RHC data interpretation is reported among practicing cardiologists (2). To address this opportunity, the contemporary approach to PH diagnosis is summarized here, emphasizing cutting-edge knowledge on RHC parameters that are used to classify PH patients appropriately.
New Data on the Clinical Risk Related to Cardiopulmonary Hemodynamics Pulmonary artery pressure
Background.
Elevated mPAP is a reproducible risk factor for right heart failure and adverse clinical outcome. The origins of this observation are from patients with end-stage PAH, which is a distinct although uncommon subgroup of PH. However, greater emphasis on early PH diagnosis has emerged recently. This strategic shift is based on clinical trial data supporting up-front aggressive pharmacotherapy to mitigate clinical deterioration in PAH, but also recognition that perinatal developmental events promote early adult-onset PH, the observation that subtly elevated mPAP is an independent risk factor for mortality in heart failure populations, and longitudinal data showing that a subgroup of mild PH patients ultimately develop severely abnormal mPAP (3–6).
Changes to Clinical Practice.
With greater emphasis on early PH, it became important to clarify the mPAP spectrum of clinical risk. In 2009, a comprehensive retrospective meta-analysis involving 1,187 healthy volunteers showed that the median mPAP upper normal limit is ~20 mmHg, which is independent of sex or ethnicity but did rise slightly with increasing age (7).These data converged with results from a subsequent national referral cohort study of 21,727 patients enriched with a history of heart failure and systemic hypertension showing a continuous relationship between adjusted all-cause mortality and mPAP that begins at 19–20 mmHg (8). Mildly elevated mPAP (19–24 mmHg) was common, affecting 23% of the overall study population, and among that subgroup the mortality risk was increased by 23% compared with patients with normal mPAP. These findings were validated in a second large referral cohort (5), and later in a meta-analysis of 15 studies showing a risk ratio for mortality that was 1.52 among patients with mPAP 19–24 mmHg versus those with lower pressures (9).
In well-phenotyped populations at risk for PH, mPAP ~20–25 mmHg is also important for prognosticating exercise intolerance, hospitalization and mortality. This includes patients with systemic sclerosis, sickle cell disease, post-myocardial infarction, and mixed cardiac and lung disease cohorts (10). Although it is tempting to ascribe outcome differences in mild PH patients to study limitations or comorbidities, recent reports from echocardiography registries suggest otherwise. From a national Australian cohort (11) and Vanderbilt University (12), subtle elevation in estimated PAP was associated with right atrial and RV enlargement, and decreased RV systolic function measured quantitatively. However, echocardiography generates PAP estimates that correlate only modestly with RHC findings in individual patients, and PVR, pulmonary artery wedge pressure (PAWP) or right atrial pressure (all prognostic in PH) cannot be determined precisely non-invasively. Thus, optimal hemodynamic phenotyping requires RHC. Functional studies also show that perturbations to normal pulmonary vascular mechanics in the setting of mildly elevated mPAP may disrupt the RV-pulmonary arterial coupling relationship (13), predisposing patients to future right heart failure. Further, pre-heart transplant mPAP <25 mmHg is associated with increased clinical risk in subpopulations (14). These collective data suggest that at mildly elevated mPAP, important pulmonary vascular pathology may exist and bear on outcome in cardiovascular disease.
Pulmonary vascular resistance
Background.
The pulmonary vasculature is a low-resistance circuit, responsive to environment cues via arterial recruitment and flow-mediated dilation that reflects intrinsic pulmonary vascular distensibility. The PVR informs the ratio of change in pulmonary artery pressure to mean pulmonary blood flow, and is calculated in clinical practice as: ([mPAPPAWP]/CO in Wood Units [WU]).Kovacs and colleagues first reported differences in the upper limit of normal PVR across age groups: 0.76±0.3 vs. 0.86±0.4 vs. 1.1±0.2 vs. 1.1±0.5 WU for <24, 24–50, 51–69, ≥70 yrs, respectively (15). There is a (very) mild curvilinearity in the relationship between CO and mPAP. This may account for the observation that exercise (in which CO may be 5-fold above resting levels) and other physiological high blood volume states do not induce a pathological degree of RV afterload. Nonetheless, it is notable any PVR decrease during exercise in young, healthy individuals is minimal (15, 16).
Changes to Clinical Practice.
All PH clinical phenotypes involve a vasculopathy (remodeling or functional) that affects distal pulmonary arterioles, which, over time, is expected to increase PVR. Increasingly, hypertrophic and sclerotic pulmonary venule remodeling is also associated with increased PVR among PH-LHD patients. Indeed, PVR ≥3.0 WU is a traditional (i.e., historical) criterion for diagnosing PAH and prognosticating PH-LHD and chronic obstructive pulmonary disease among others (1). Clarifying the spectrum of risk related to PVR is especially relevant in PH-LHD since pulmonary vascular disease is already an independent risk factor for hard clinical events in this population (17).
Data assembled recently from 40,082 patients (many with prevalent LHD) referred for RHC in the Veterans Affair system were used to model the relationship between PVR and all-cause mortality (18). A rise in the mortality hazard among patients with elevated mPAP emerged beginning at PVR ~2.2 WU, and was consistent with the upper normal limit for the age range of the study population. The hazard ratio for adjusted all-cause mortality and heart failure hospitalization were 1.47 (95% CI: 1.42–1.53, P<0.0001) and 1.17 (95% CI: 1.11–1.24, P<0.0001), respectively, in patients with PVR ≥2.2 WU vs. <PVR 2.2 WU. Directionally similar findings were observed when analyzing the association between PVR ≥2.2 WU and outcome in a sex-balanced validation cohort from Vanderbilt University (18).
Using PVR >2.2 WU captured ~55% more at-risk patients across the two study populations compared with ≥3.0 WU, suggesting that missed opportunity may exist to optimize early PH diagnosis in many PH-LHD patients. However, it is notable that major differences in the magnitude of this association between PVR ≥2.2 WU were observed when stratifying patients at the time of RHC by PAWP ≤15 vs. >15 mmHg (Figure 1). These findings demonstrate that elevated PVR in the absence of pulmonary vascular congestion is especially worrisome and that even a subtle PVR rise in PH-LHD has important implications on prognosis. The importance of PVR <3.0 WU has also been reported recently in at-risk subpopulations outside left heart failure, including systemic sclerosis (19) and PAH (20). Notwithstanding these recent data, PVR ≥3.0 WU is the current standard for defining patients with elevated mPAP and a pre-capillary component (see below).
Figure 1. The continuum of clinical risk between pulmonary vascular resistance and mortality in patients with pulmonary hypertension.
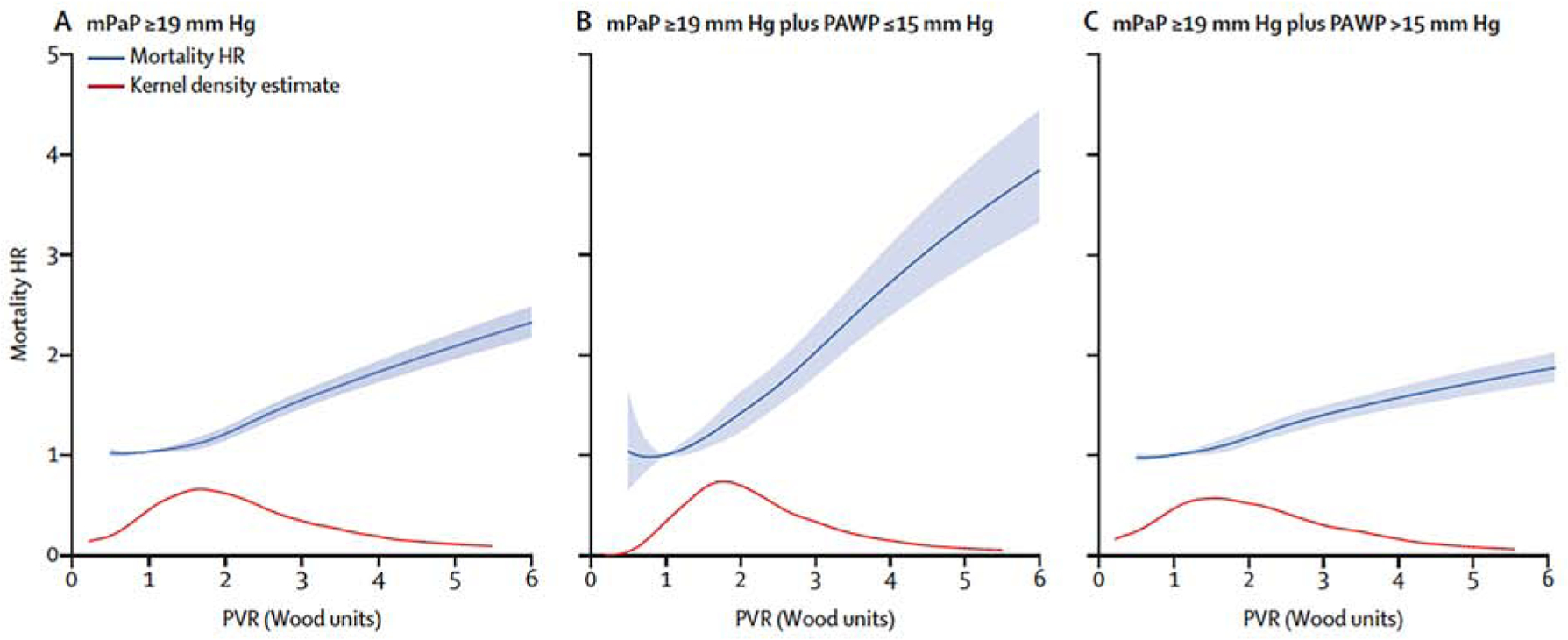
(A) The PVR continuum of all-cause mortality risk in patients with elevated mPAP (defined in the dataset shown here as ≥19 mmHg) begins at ~2.2 WU and increases through 6 WU. However, the slope of this relationship hinges substantially on the absence or presence of pulmonary venous hypertension at the time of right heart catheterization, depicted in (B) and (C) after stratifying the population by low and high pulmonary artery wedge pressure (PAWP), respectively. WU, Wood unit. The grey line inset is the kernel density estimate, representing the relative density of patients across PVR values. Reproduced with permission from Ref. 18.
Pulmonary Arterial Compliance and Elastance
PVR is a measure of the steady-state load imposed on the RV. However, pulsatile blood flow is associated with reflective waves that contribute to RV afterload. Hemodynamic parameters that incorporate these pulsatile components include pulmonary arterial compliance (estimated as stroke volume/pulmonary artery pulse pressure) and pulmonary arterial elastance (estimated as systolic pulmonary artery pressure/stroke volume).Clinicians should be aware that these parameters have stronger associations with both RV dysfunction and heart failure prognosis than for PVR (4, 21). Because these measures are substantially influenced by passive elevations in left heart filling pressures (22), they are less useful in defining pre-capillary components of PH. Further, pulmonary arterial compliance and PVR are inversely related, with compliance falling precipitously with just mild increases in PVR. Accordingly, future studies should assess if compliance is a superior marker of mild (and presumably early) pulmonary vascular disease.
Patient Classification
The three modern cardiopulmonary hemodynamic phenotypes are: pre-capillary, isolated post-capillary (IpcPH), and combined pre-/post-capillary PH (CpcPH) (Central Illustration) (1). Pre-capillary and post-capillary PH is delineated by the absence or presence, respectively, of a resting PAWP >15 mmHg. In IpcPH, elevated mPAP is merely reflective of (and proportional to) passive elevation in LV filling pressure. In CpcPH, the mPAP is typically higher than expected (“out of proportion”) based on the LV filling pressures alone, and PVR is elevated (≥3.0 WU). Previously, the diastolic pressure gradient (DPG) was used to differentiate these two entities (with levels <7 and ≥7 mmHg diagnostic for IpcPH and CpcPH, respectively). However, subsequent controversy over the prognostic utility of DPG (23, 24) and concerns related to measurement fidelity have steered focus back to PVR solely (24).
Central illustration. Contemporary model of pulmonary hypertension assessment in left heart disease.
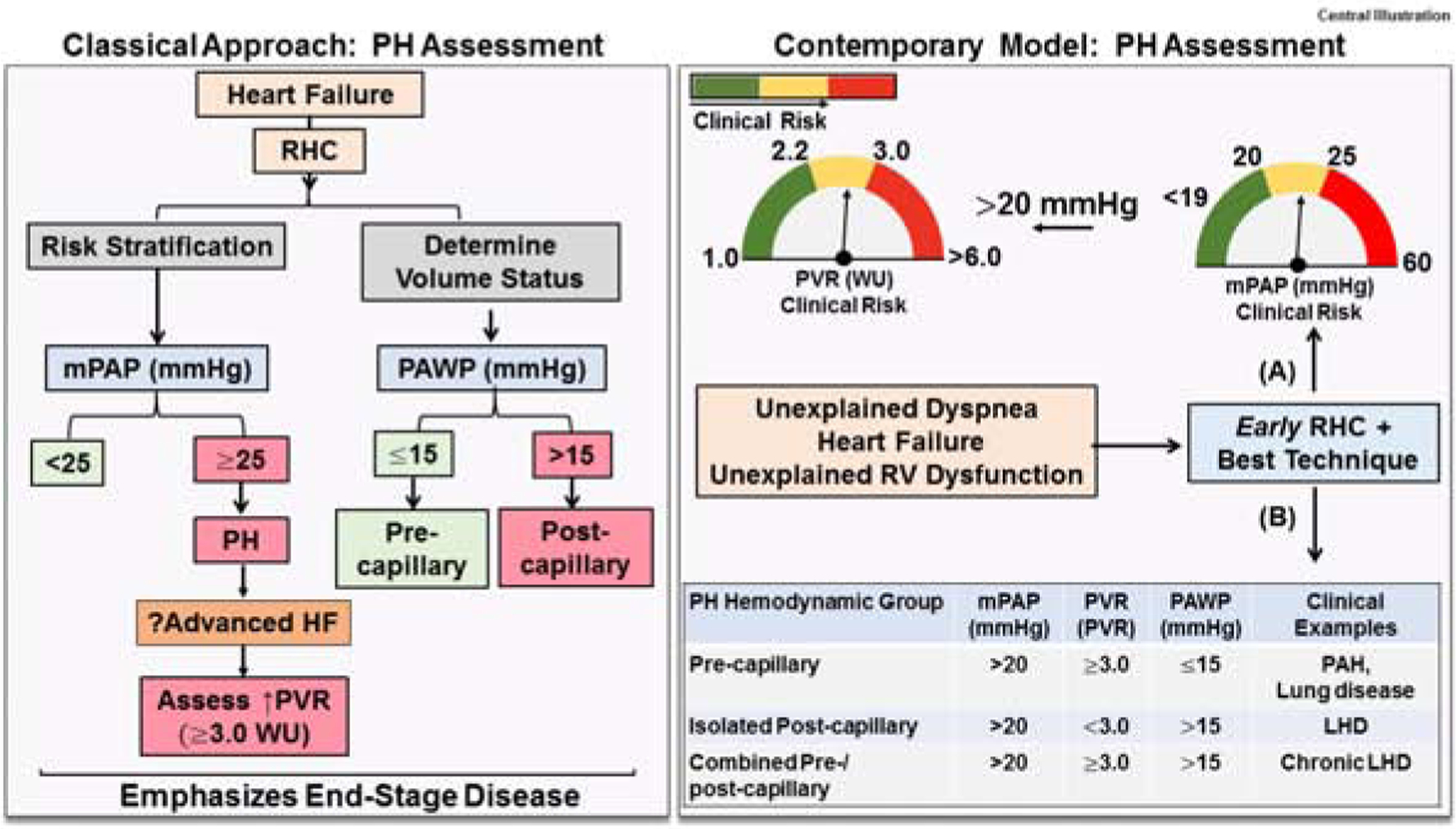
(Left) The classical approach to pulmonary hypertension (PH) assessment in left heart disease (LHD) emphasized data from right heart catheterization (RHC) to delineate patients with mean pulmonary artery pressure (mPAP) ≥25 mmHg and a post-capillary component (PAWP >15 mmHg). An elevated PVR ≥3.0 WU associated with advanced heart failure (HF) clinically. Overall, data collection emphasized end-stage disease. (Right) The contemporary approach to PH-LHD focuses on early RHC, which is based on (A) data demonstrating that the hemodynamic continuum of clinical risk relative to mortality and heart failure hospitalization includes mPAP >20 mmHg and PVR ≥2.2 WU. (B) Data from RHC is also used to classify patients into one of three established PH hemodynamic subtypes. RV, right ventricle. Table: Adapted from Ref. 1.
A PVR ≥3.0 WU is used to identify IpCPH and CpcPH patients presently; however, the association between mortality and PVR varies by PAWP level (Figure 1B, C). Therefore, differing PVR levels may be needed to improve the specificity of hemodynamic criteria that define these PH subgroups. Organized data profiling the factors that influence PAWP (and in turn bear on PH patient classification) are needed, including loading conditions and the potential role of ventricular interdependence. Hemodynamics including the PAWP should always be considered in the context of the overall clinical scenario. Ultimately, integrating the hemodynamic phenotype with individual patient-level information is necessary to determine the best PH clinical group (Figure 2). Given that PAH-specific therapies are formally contraindicated for use in post-capillary PH, the CpcPH classification is more relevant for risk stratification, implementation of conventional risk reduction measures (e.g., dietary adherence, physical activity, and maximized standardized heart failure therapies), and to prompt early referral to expert PH centers of excellence with experience in left heart failure. This enables evaluation for advanced heart failure therapies and clinical trial enrollment.
Figure 2. Integrating the cardiopulmonary hemodynamic and clinical profile of patients with pulmonary hypertension (PH).
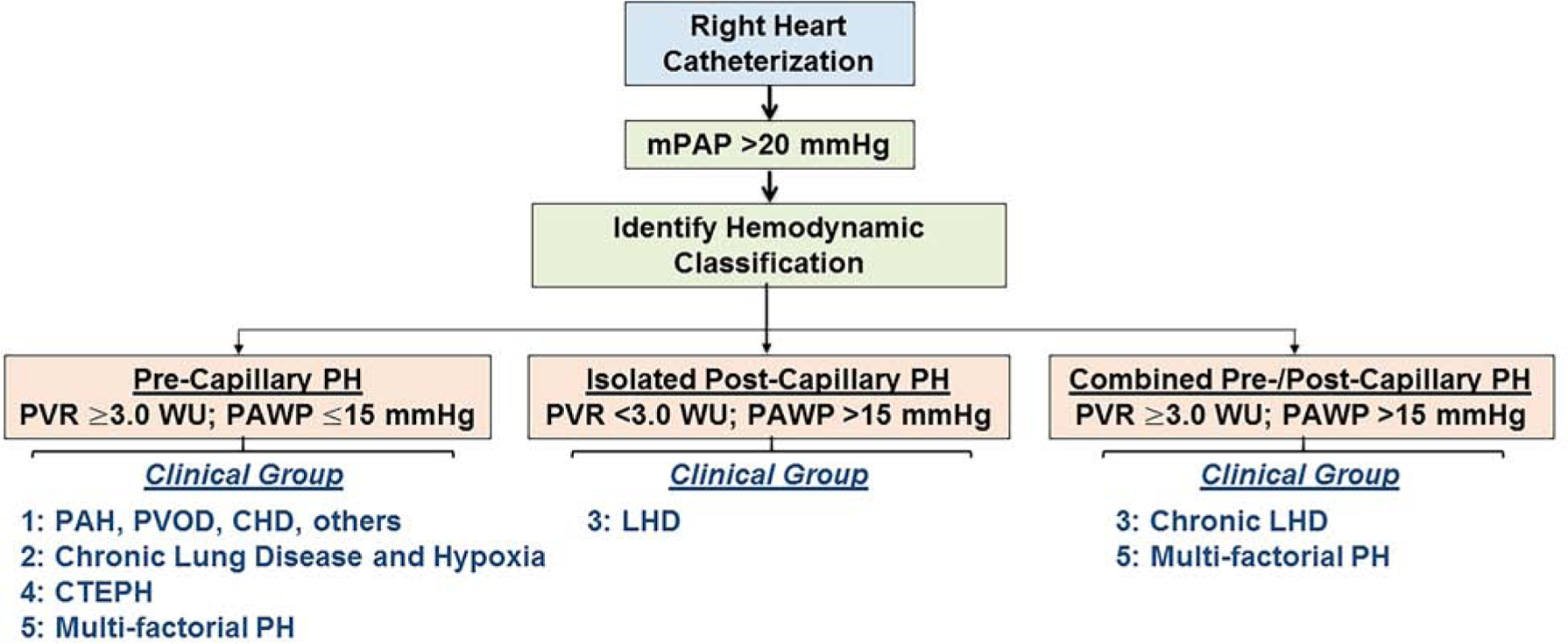
Diagnosing PH is achieved by right heart catheterization, and requires a mean pulmonary artery pressure (mPAP) >20 mmHg. Patients are then classified by hemodynamic category, which together with the clinical profile and other supporting data (e.g., chest imaging, serology, genetic testing) is used to determine the PH clinical group. Certain clinical groups are incompatible with hemodynamic classifications; for example, pulmonary arterial hypertension (PAH) cannot be diagnosed in patients with isolated post-capillary or combined pre-/post-capillary PH hemodynamics. Treatment is based on clinical group, as reviewed in Refs. 1 and 35.Overall, pulmonary vasodilator therapies are not indicated in PH due to left heart disease (LHD), regardless of PVR. PVR, pulmonary vascular resistance; PAWP, pulmonary artery wedge pressure; PVOD, pulmonary veno-occlusive disease; CTEPH, chronic thromboembolic PH.
Diagnosing Pulmonary Hypertension in Heart Failure
A right heart catheterization (RHC) should always be performed prior to initiation of therapy if a diagnosis of pre-capillary PH is suggested by non-invasive imaging. The newly defined risk spectrum related to mPAP and PVR may also support earlier hemodynamic evaluations (Figure 3) (24). Although RHCs are commonly performed after substantial diuresis, this practice may complicate PH classification, particularly in those with preserved LV ejection fraction. We recommend that in patients suspected of incident heart failure, RHC should be considered prior to volume status optimization if safe. In addition to inspecting the waveform to determine current location of the catheter, operators should examine the pressure tracing quality looking carefully for signs of over- or under-dampening (Figure 4). Other best practices for hemodynamic evaluation are summarized in Table 1.
Figure 3. Indications for right heart catheterization (RHC).
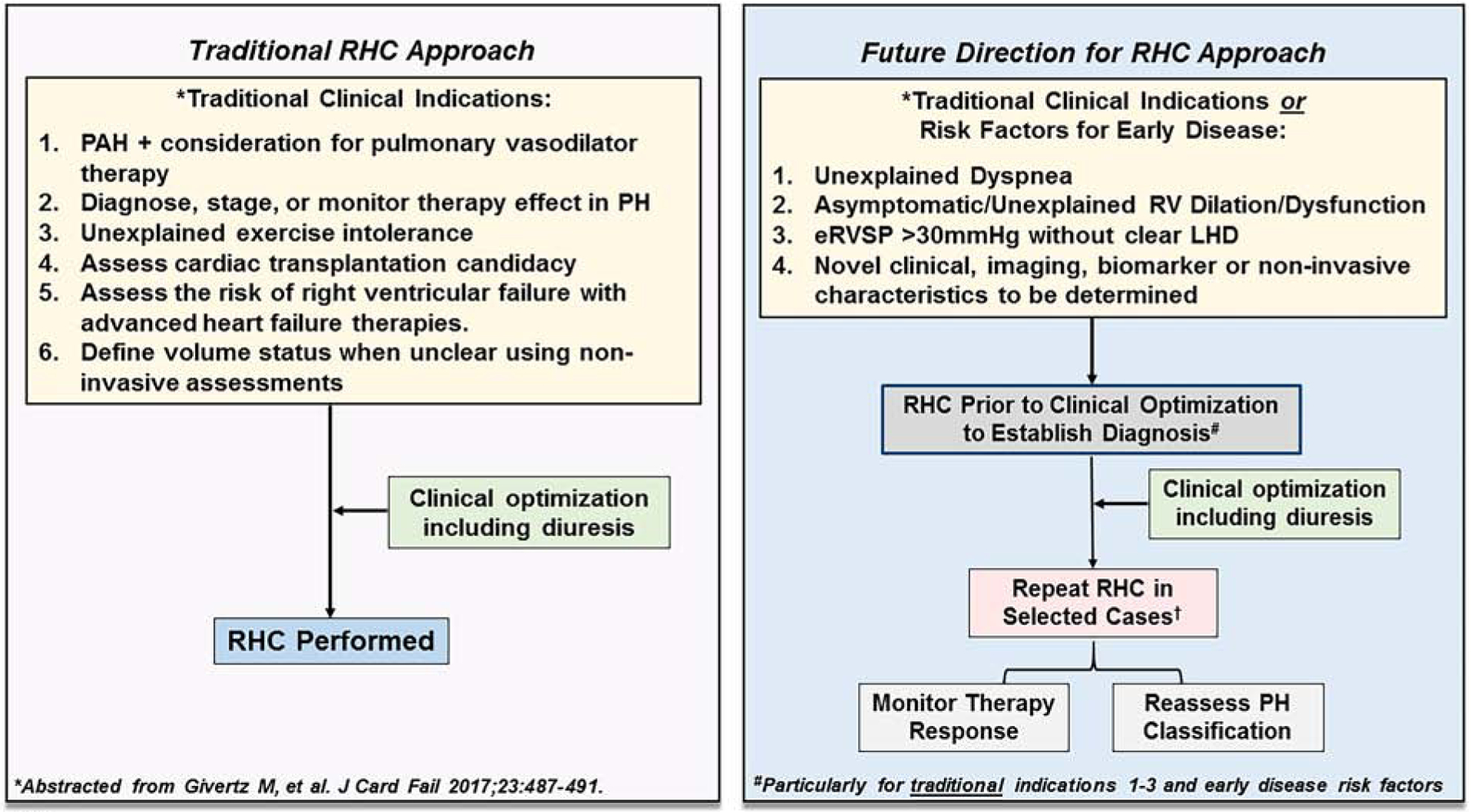
(Left) In the traditional approach to RHC, hemodynamic assessment is performed in patients with a clinical indication following clinical optimization including diuresis. PAH, pulmonary arterial hypertension. (Right) A future direction for RHC approach considers patients with a traditional indication for RHC as well as patients with clinical parameters suggestive of early or unexplained pulmonary hypertension (PH). In this scenario, RHC prior to diuresis may be considered to clarify the PH hemodynamic classification of patients (particularly post-capillary PH vs. combined pre- and post-capillary PH). In select patients, repeat RHC may be indicated to monitor therapy response or reassess PH hemodynamic classification. Unexplained dyspnea is dyspnea without a clear etiology based on results from standard non-invasive testing (e.g., echocardiography, cardiopulmonary exercise testing, others); eRVSP, estimated right ventricular systolic pressure by echocardiography; LHD, left heart disease.
Figure 4. Pulmonary Artery Pressure Waveforms.
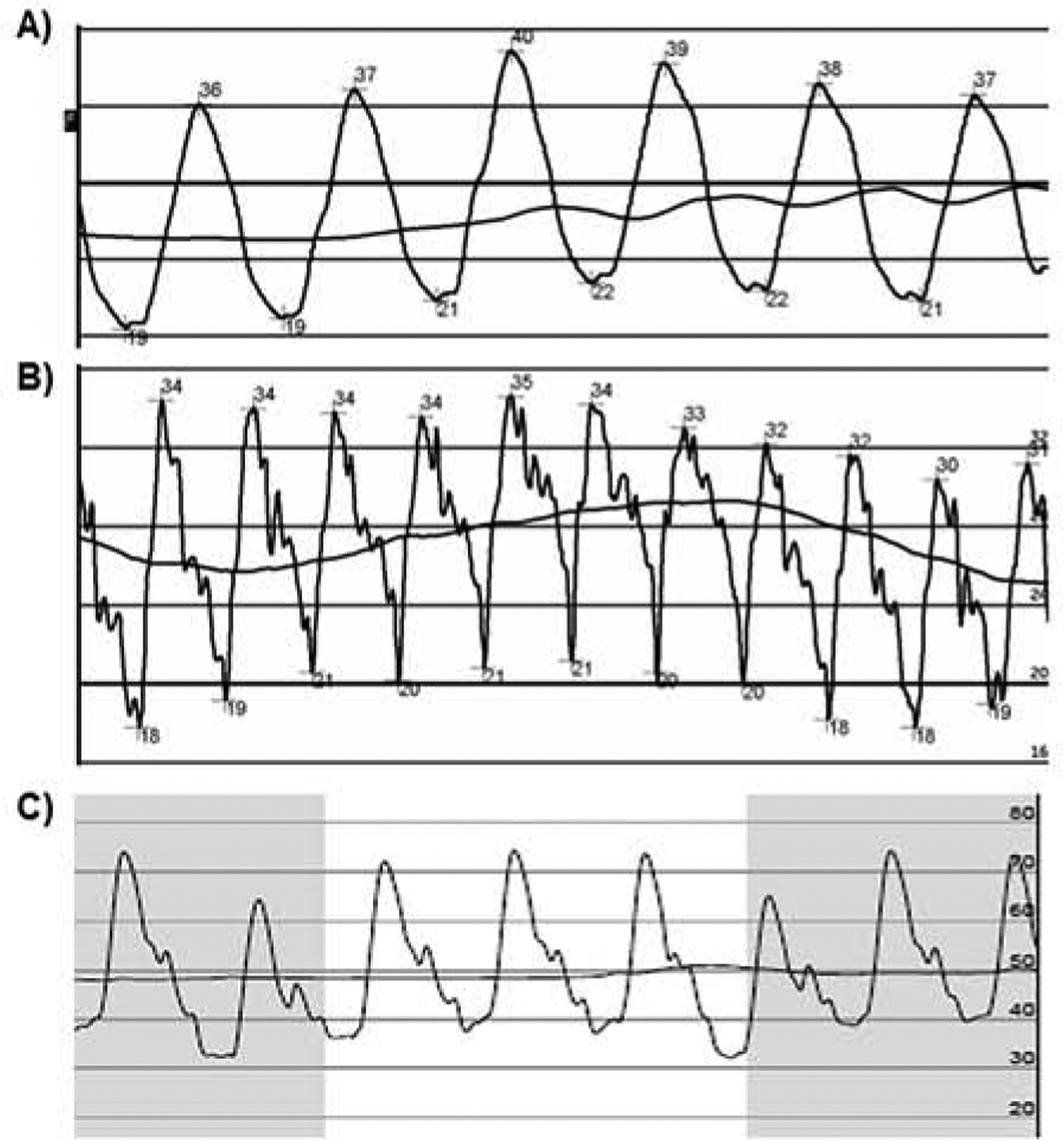
(A)Over-dampening. Note that characteristic dichrotic notch is not seen. Over-dampening occurs when air is introduced into the catheter or tubing and may result in reduction of the overall amplitude of the pressure tracing (mean pressure is usually not affected). This can be addressed by flushing the catheter or tubing. (B) Under-dampening. Also called catheter ringing and occurs when the frequency of the transmitted waveform (heart rate) approximates the natural resonance frequency of the transducer system. This falsely increases the amplitude of the waveform. Similar to over-dampening, mean pressure is usually not affected. Ringing artifact may be reduced by introducing a small amount of a denser fluid, such as blood or contrast, into the catheter, with careful attention not to overdamp the signal (C) Example of an optimal pulmonary artery pressure waveform with preserved dichroic notch but minimal systolic and diastolic ringing artifact.
Table 1.
Best practice technique for right heart catheterization.
| Best Practice Technique | |
|---|---|
| General considerations | |
| Preparation |
|
| Patient positioning | Supine position with legs flat; avoid recording while patient is talking, coughing, or under duress |
| Sedation | Minimize systemic sedation when possible to avoid altered breathing patterns |
| Leveling | Pressure transducer should be zeroed to atmospheric pressure at level of left atrium (halfway between the anterior sternum and table surface) |
| Tracing quality | Watch for signs of over- or under-dampening (see Figure 3). If under-dampening is present, catheter ringing can be reduced by introducing a small amount of blood or contrast into the fluid filled catheter |
| Respiratory Cycle Considerations |
|
| PAWP Measurement | |
| Confirmation of complete PA occlusion | Measure PAWP SaO2 |
| “Normal” PAWP but risk factors for PH-LHD | Consider provocative maneuvers (i.e. saline bolus) |
| Presence of large V waves | Suggestive of LHD and/or mitral regurgitation and should be reported |
| Cardiac Output Measurement | |
| In absence of intracardiac shunting, thermodilution is preferred over indirect Fick for estimation of cardiac output and associated hemodynamic calculation. |
ECG, electrocardiogram; PAWP, pulmonary artery wedge pressure; SaO2, mixed venous oxyhemoglobin saturation; LHD, left heart disease; PH, pulmonary hypertension.
Cardiac Output.
Accurate assessment of CO is critical for calculation of hemodynamic parameters used in disease classification and risk stratification. The direct Fick method is the gold standard for determining CO, but requires specialized equipment to directly measure oxygen consumption. Therefore, thermodilution (TD) and the indirect Fick methods are used more commonly. The indirect Fick method relies on estimated values for total body oxygen consumption, which, unfortunately, is often inaccurate in patients with heart failure, PH, and obesity. Although TD is perceived to be less accurate in the setting of tricuspid regurgitation and low CO, studies have shown good correlation with TD and direct Fick under these circumstances (25).A recent analysis of >15,000 patients undergoing RHC confirmed a poor correlation between TD and indirect Fick methods (r=0.65) with one third of the cohort differing >20% between methods (26). A cardiac index <2.2 L/min/m2 measured by the TD method may be superior in predicting 90-day mortality compared with the indirect Fick method. These data support TD as the preferred method of measuring CO and calculation of associated hemodynamic parameter with the exception of patients with intracardiac shunt, for whom direct measurement of oxygen consumption, comprehensive oxyhemoglobin saturation sampling, or cardiac MRI may be useful. Though its use in CO calculation is limited by inaccuracies in oxygen consumption estimation, mixed venous oxygen saturation may have added prognostic value, particularly in PAH (27).
Interpreting the PAWP tracing.
In most situations, the PAWP should be recorded at end-expiration (but without breath hold maneuvers which can prompt Valsalva physiology). At that point, intrathoracic pressure closely approximates zero and, thus, exerts minimal influence on intracardiac pressures. In severe lung disease or morbid obesity, large respiratory pressure variation may be present and intrathoracic pressure at end-expiration may be greater than zero. Reporting an average pressure over the respiratory cycle is preferred in these cases, and we typically report both end-expiratory and averaged values when significant respiratory variation is observed. As long as both mPAP and PAWP are measured at the same point in the respiratory cycle, PVR accuracy is not affected.
The mitral valve is open at end-diastole; thus, left atrial pressure (and PAWP) should be equal to LV end-diastolic pressure (LVEDP) in the absence of mitral stenosis. Because the c-wave (mitral valve closure) may be difficult to identify on the PAWP tracing, the peak and trough of the a-wave is averaged and correlates with the pre-c-wave value (Figure 5A). This value is the best estimate of LVEDP. With atrial fibrillation, the a-wave is absent and end diastolic PAWP is measured 130–160 msec after QRS complex onset and before the v-wave (24). Mean PAWP, or PAWP averaged over the cardiac cycle, encompasses the pressure waveform during both systole and diastole, and best represents the ambient pressure of the pulmonary venous circulation. Although in many instances mean PAWP approximates end diastolic PAWP, the presence of large v waves or atrial fibrillation often lead to a mean PAWP that is greater than the end-diastolic PAWP (Figure 5B).With the shift away from the use of DPG (where end-diastolic PAWP or LVEDP is the proper comparator to diastolic PAP) and renewed focus on PVR as the pre-capillary marker, we believe mean PAWP should be used to calculate PVR in clinical practice. Finally, large amplitude V waves are highly suggestive of LHD (including mitral regurgitation) even when resting PAWP is normal, and should therefore be reported (24).
Figure 5. Pulmonary Artery Wedge Pressure (PAWP) Waveforms.
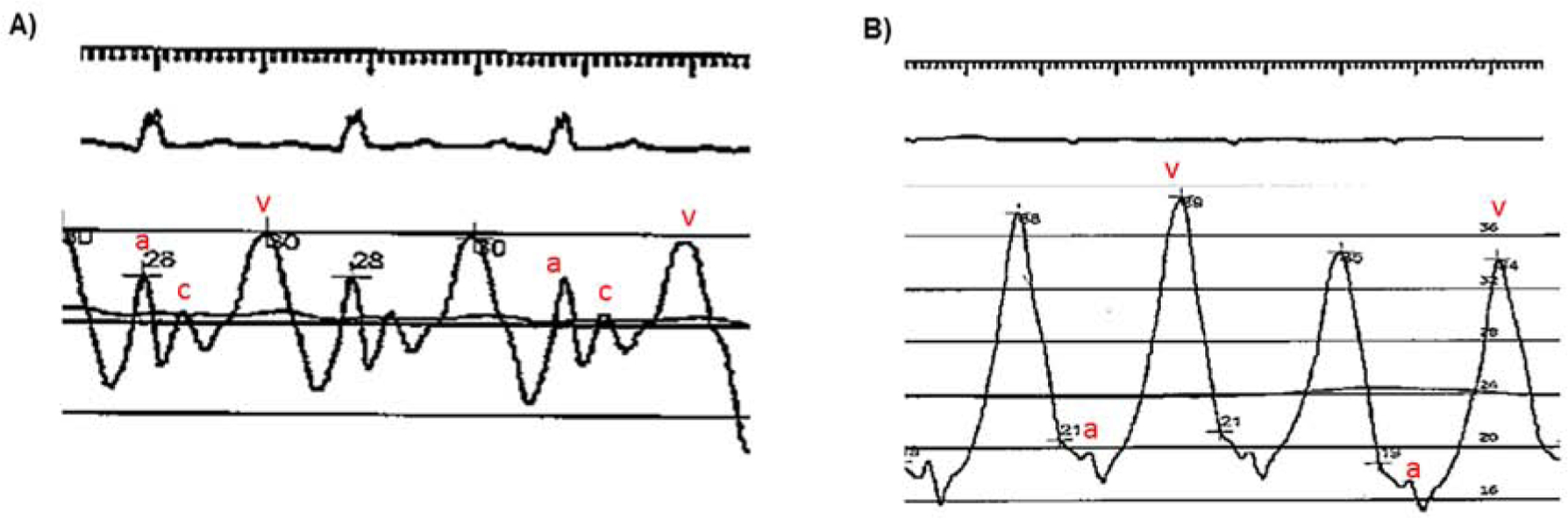
(A)Typical PAWP tracing. A-wave, c-wave, and v-wave as noted in red. End-diastole occurs just prior to the c-wave, and thus, pressure at this point correlates best with left ventricular end-diastolic pressure. When the c-wave is not well seen, averaging the peak and trough of the a-wave is recommended. In this example, the v-wave is normal amplitude. Mean PAWP (black horizontal line), which an average pressure over the entire cardiac cycle, is essentially equal to end-diastolic PAWP. (B) A-wave, and v-wave as noted in red (c-wave not appreciated).Very large amplitude V waves are present. Here, mean PAWP (black horizontal line) is significantly greater than end-diastolic PAWP.
Whenever the PAWP tracing morphology is atypical or pre-capillary PH is suspected clinically despite a measured PAWP >15 mmHg, the PAWP blood sample oxyhemoglobin saturation (SaO2) content should be analyzed (24). A truly wedged catheter will yield an SaO2 reflective of the post-capillary pulmonary bed, typically >90–95%. Lower values should prompt repeat attempts to wedge including alternate vascular areas or consideration to direct LV measurement.
Diagnostic Insights from Provocative Maneuvers During Right Heart Catheterization.
In the early stages of cardiopulmonary disease or as a consequence of diuretic use and/or pre-procedure fasting, resting cardiopulmonary hemodynamics may be normal despite underlying pulmonary vascular (arterial or venule) or cardiac pathology. In these situations, hemodynamic derangements may only become apparent with provocation. Saline loading or dynamic exercise are point-of-care tools used increasingly to unmask occult LHD, differentiate PAH from heart failure with preserved ejection fraction (HFpEF), or diagnose exercise PH.
Exercise RHC.
Hemodynamic assessment methodology during exercise remains controversial, including patient position, exercise type and duration, and normative (28). However, averaging PAWP over several respiratory cycles during exercise is recommended widely due to limited accuracy of end-expiratory measurements under these conditions. Exercise RHC permits assessment of hemodynamic changes during real-world exertion and an expansive range of physiologic data. When combined with a metabolic cart, ventilatory inefficiency, anaerobic threshold, and many other parameters are collected that inform musculoskeletal deconditioning, anemia, or alternative contributions to multifactorial dyspnea.
The precise combination of exercise RHC variables used to diagnose and prognosticate PH-LHD is evolving. Eisman and colleagues reported that a PAWP/CO slope >2 mmHg/L/min is consistent with occult LHD and appears more reflective of pathology than a single exercise PAWP cutoff (29). Similarly, the hazard for cardiovascular events or death is 2-fold greater in PH-LHD patients (HFpEF) with PAP/CO slope >3 compared with <3 mmHg/l/min on invasive cardiopulmonary exercise testing (30). Exercise RHC testing may be helpful for identifying patients with early stage pulmonary vascular disease, as increased CO provokes abnormal pulmonary impedance and altered pulmonary vasoregulation in some at-risk patients, which are not evident at rest (31). For example, an initial impairment in pulmonary arterial compliance (with normal PVR) may shift the mPAP/CO slope rate upward (32), signaling underlying pulmonary vascular disease. The direct Fick method to assess CO during exercise is preferred, as TD may underestimate CO at higher outputs (33).
Confrontational saline challenge.
In contrast to exercise RHC, saline loading is less complex technically, requires less specialized equipment, and is more widely available. There is also greater consensus on what qualifies as an abnormal test result: PAWP >18 mmHg after ~500cc of normal saline over 5 min (34). In a study of thoroughly screened healthy subjects, none, irrespective of sex or age, increased PAWP >18 mmHg with this degree of fluid loading (35). In a separate study, D’alto and colleagues arrived at similar cut-off of 18 mmHg for diagnosis of occult PH-LHD (34). Further, the influence of saline loading on PAWP appears to be more age-independent than exercise. For these reasons, it has been recommended that patients with PH and intermediate-to-high pretest probability of occult LHD (especially left atrial enlargement) with PAWP 13–15 mmHg undergo a fluid challenge rather exercise testing (24).Outcome data on use of PAH specific therapy in individuals with normal resting PAWP but an abnormal response to exercise or saline challenges are lacking. Thus, these patients should be referred to a PH center of excellence should consideration to PAH-specific therapy be deemed appropriate. Similarly, exercise RHC testing rather than fluid challenge should be considered at expert centers in the evaluation of unexplained dyspnea or exercise-induced PH.
Vasoreactivity testing.
Vasoactive testing is recommended in individuals with idiopathic, heritable or drug-induced PAH using inhaled nitric oxide or similar pulmonary vasodilator (reviewed in refs. 36, 37).By contrast, vasoreactivity testing is typically not indicated in PH-LHD, unless it is being performed in the context of heart transplantation evaluation (24). In that setting, systemic vasodilators such as nitroprusside is preferred, whereas selective pulmonary vasodilators should be avoided given the theoretic risk of pulmonary edema.
Conclusions
The hemodynamic definition of PH has been revised to mPAP >20 mmHg, which is based on clinically relevant data and emphasizes early diagnosis in at-risk populations. Patients with elevated mPAP are classified further into pre-capillary-PH, Ipc-PH, or CpC-PH subtypes based on PVR and PAWP levels. This step is critical to diagnose, prognosticate, and treat patients accurately. Emergent data on the cardiopulmonary hemodynamic risk continuum may refine the hemodynamic subtypes, thereby assisting clinicians with diagnosing PH-LHD. This includes a lower PVR threshold of 2.2 WU or above that is associated with increased clinical events. With these collective advances, the importance of standardized and meticulous RHC assessment comes into sharp focus as a fundamental necessity for optimizing patient classification with the overarching goal of expanding risk profiles to better detect early disease.
Main Messages.
Recent data on the cardiopulmonary hemodynamic clinical risk spectrum have changed the definition of pulmonary hypertension.
Lower pulmonary artery pressure and pulmonary vascular resistance criteria focus attention on early diagnosis of pulmonary hypertension.
Exercise and fluid challenge studies could facilitate classification of unexplained dyspnea in patients with pulmonary hypertension and heart failure.
Funding:
B.A.M.: R01HL139613-01, R01HL1535-02, U54HL119145, R21HL145420; Cardiovascular Medical Research Education Foundation, and McKenzie Family Charitable Trust, Boston Biomedical Innovations Center.
Abbreviations
- PH
pulmonary hypertension
- PAH
pulmonary arterial hypertension
- LHD
left heart disease
- RHC
right heart catheterization
- mPAP
mean pulmonary artery pressure
- PAWP
pulmonary artery wedge pressure
- PVR
pulmonary vascular resistance
- CO
cardiac output
- LV
left ventricle
- RV
right ventricle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Tweet: Resuscitating back to life the lost art of hemodynamic assessment…welcome to the new world of pulmonary hypertension diagnosis.
Disclosures: B.A.M., consultant for Actelion, and co-inventor on the following patents or patent application that are related to pulmonary hypertension (U.S. Patent #9,605,047; PCT/US2015/029672; Provisional ID: #62475955; Provisional ID: #24624; Provisional ID: #24622).Dr. Tedford reports no direct conflicts relevant to this manuscript. Other general conflicts include consulting relationships with Medtronic, Aria CV Inc., Arena Pharmaceuticals and United Therapeutics. Dr. Tedford is on a steering committee for Medtronic and a research advisory board for Abiomed. He also does hemodynamic core lab work for Actelion and Merck. Dr. Vaidya reports consulting for Bayer, United Therapeutics, Liquidia, and Actelion, and no promotional speakers bureau for Bayer, Actelion, United Therapeutics. Dr. Bhatt discloses the following relationships - Advisory Board: Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Level Ex, Medscape Cardiology, PhaseBio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, Tobe Soft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees),Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, Takeda.
References
- 1.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deaño RC, Glassner-Kolmin C, Rubenfire M, et al. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral study. JAMA Intern Med 2013;173:887–93. [DOI] [PubMed] [Google Scholar]
- 3.Galie N, Barbera JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015;373:834–44. [DOI] [PubMed] [Google Scholar]
- 4.Vanderpool RR, Saul M, Nouraie M, Gladwin MT, Simon MA. Association between hemodynamic markers of pulmonary hypertension and outcomes in heart failure with preserved ejection fraction. JAMA Cardiol 2018;3:298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assad TR, Maron BA, Robbins IM, et al. Prognostic effect of longitudinal hemodynamic assessment of borderline pulmonary hypertension. JAMA Cardiol 2017;2:1361–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maron BA, Abman SH. Translational advances in the field of pulmonary hypertension. focusing on developmental origins and disease inception for the prevention of pulmonary hypertension. Am J Respir Crit Care Med 2017;195:292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: A systematic review. Eur Respir J 2009;34:888–894. [DOI] [PubMed] [Google Scholar]
- 8.Maron BA, Hess E, Maddox TM, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: Insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. Circulation 2016;133:1240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolte D, Lakshmanan S, Jankowich MD, Brittain EL, Maron BA, Choudhary G. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc 2018;7:e009729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovacs G, Douschan P, Maron BA, Condliffe R, Olschewski H. Mildy increased pulmonary aterial pressure: a new disease entity or just a marker of poor prognosis. Eur J Heart Fail 2019;21:1057–1061. [DOI] [PubMed] [Google Scholar]
- 11.Strange G, Stewart S, Celermajer DS, et al. Threshold of pulmonary hypertension associated with increased mortality. J Am Coll Cardiol 2019;73:2660–72. [DOI] [PubMed] [Google Scholar]
- 12.Huston JH, Maron BA, French J, et al. Association of mild echocardiographic pulmonary hypertension with mortality and right ventricular function. JAMA Cardiol 2019;4:1112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vriz O, Pirisi M, Bossone E, et al. Right ventricular-pulmonary arterial uncoupling in mild-to-moderate systemic hypertension. J Hypertens 2020;38:274–281. [DOI] [PubMed] [Google Scholar]
- 14.Crawford TC, Leary PJ, Fraser CD, et al. Impact of the new pulmonary hypertension definition on heart transplant outcomes: expanding the hemodynamic risk profile. Chest 2020;157:151–161. [DOI] [PubMed] [Google Scholar]
- 15.Kovacs G, Olschewski A, Berghold A, Olschewski H. Pulmonary vascular resistance during exercise in normal subjects: a systemic review. Eur Respir J 2012;39:319–328. [DOI] [PubMed] [Google Scholar]
- 16.Wolsk E, Bakkestrom R, Thomsen JH, et al. The influence of age on hemodynamic parameters during rest and exercise in healthy individuals. JACC Heart Fail 2017;5:337–346. [DOI] [PubMed] [Google Scholar]
- 17.Miller WL, Grill DE, Borlaug BA. Outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: Pulmonary hypertension and heart failure. JACC: Heart Fail 2013;1:290–299. [DOI] [PubMed] [Google Scholar]
- 18.Maron BA, Brittain EL, Hess E, et al. The association between pulmonary vascular resistance and clinical outcomes in patients with pulmonary hypertension: A retrospective cohort study. Lancet Respir Med 2020;8:873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xanthouli P, Jordan S, Milde N, et al. Haemodynamic phenotypes and survival in patients with systemic sclerosis: the impact of the new definition of pulmonary arterial hypertension. Ann Rheum Dis 2020;79:370–378. [DOI] [PubMed] [Google Scholar]
- 20.Ratwatte S, Anderson J, Strange G, et al. Pulmonary arterial hypertension with below threshold pulmonary vascular resistance. Eur Respir J 2020:1901654. [DOI] [PubMed] [Google Scholar]
- 21.Tampakakis E, Shah SJ, Borlaug BA et al. Pulmonary Effective Arterial Elastance as a Measure of Right Ventricular Afterload and Its Prognostic Value in Pulmonary Hypertension Due to Left Heart Disease. Circ Heart Fail 2018;11(4):e004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tedford RJ, Hassoun PM, Mathai SC, et al. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading / clinical perspective. Circulation 2012;125:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tampakakis E, Leary PJ, Selby VN, et al. The diastolic pulmonary gradient does not predict survival in patients with pulmonary hypertension due to left heart disease. JACC: Heart Failure 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vachiery JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J 2019;53:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoeper MM, Maier R, Tongers J, et al. Determination of cardiac output by the Fick Method, thermodilution, and acetylene rebreathing in pulmonary hypertension. Am J Respir Crit Care Med 1999;160:535–541. [DOI] [PubMed] [Google Scholar]
- 26.Opotowsky AR, Ojeda J, Rogers F, et al. A simple echocardiographic prediction rule for hemodynamics in pulmonary hypertension. Circ Cardiovasc Imaging 2012;5:765–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khirfan G, Almoushef A, Naal T, et al. Mixed venous oxygen saturation is a better prognosticator than cardiac index in pulmonary arterial hypertension. Chest 2020;S20012–3692(20)31850-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovacs G, Herve P, Barbera JA, et al. An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J 2017;50:1700578. [DOI] [PubMed] [Google Scholar]
- 29.Eisman AS, Shah RV, Dhakal BP, et al. Pulmonary capillary wedge pressure patterns during exercise predict exercise capacity and incident heart failure. Circ Heart Fail 2018;11:e004750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho JE, Zern EK, Lau ES, et al. Exercise pulmonary hypertension predicts clinical outcomes in patients with dyspnea on effort. J Am Coll Cardiol 2020;75:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau EM, Humbert M, Celermajer DS. Early detection of pulmonary arterial hypertension. Nat Rev Cardiol 2015;12:143–55. [DOI] [PubMed] [Google Scholar]
- 32.Gorter TM, Obokata M, Reddy YNV, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J 2018;39:2825–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu S, Brusca SB, Rhodes PS, et al. Use of thermodilution cardiac output overestimates diagnoses of exercise-induced pulmonary hypertension. Pulm Circ 2017;7(1):253–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Alto M, Romeo E, Argiento P, et al. Clinical relevance of fluid challenge in patients evaluated for pulmonary hypertension. Chest 2017;151:119–126. [DOI] [PubMed] [Google Scholar]
- 35.Fujimoto N, Borlaug BA, Lewis GD, et al. Hemodynamic responses to rapid saline loading: the impact of age, sex, and heart failure. Circulation. 2013;127(1):55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maron BA, Galie N. Diagnosis, treatment, and clinical management of pulmonary arterial hypertension in the contemporary era: A review. JAMA Cardiol 2016;1:1056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maron BA, Bhatt DL, Nykiel M, Kinlay S, Waxman AB. Protocol for vasoreactivity testing with epoprostenol in pulmonary hypertension. Crit Pathw Cardiol 2012;11:40–2. [DOI] [PubMed] [Google Scholar]


