Summary
We have previously shown that HOMER2 (Homer scaffolding protein 2), a protein coding gene, was highly expressed in low grade (LG) endometrioid adenocarcinoma (EAC) of the uterus. The role of HOMER2 in endometrial cancer (EC) is widely unknown; therefore, the aim of this study was to determine the expression and the predictive value of HOMER2 protein expression in series of patients with EC. HOMER2 protein expression was detected on paraffin-embedded tissues from 336 cases using immunohistochemistry (IHC). Tumours were categorised in two groups; group 1 (EAC, FIGO grade 1 and 2; n = 191) and group 2 (all other subtypes including grade 3 EAC; n = 145). Statistical analysis was performed to evaluate associations between HOMER2 protein expression and pathological parameters (histological type, grade, stage, lymphovascular invasion, myometrial depth of invasion) and patient outcome [progression-free survival (PFS) and cancer-specific survival (CSS)]. HOMER2 was significantly overexpressed in group 1 compared to group 2 cancers (67% versus 30%; p < 0.001) and with low tumour grade (p < 0.001). In group 1, HOMER2 overexpression was an independent prognostic factor for improved CSS (adjusted-hazard ratio 0.28; 95% confidence interval 0.08–0.96; p = 0.042). HOMER2 expression was not associated with survival in group 2 (p > 0.05). This is the first study of HOMER2 protein expression in EC. We speculate that HOMER2 may be involved in tumourigenesis of endometrioid uterine tumours and suggest that HOMER2 should be studied further for potential clinical and therapeutic applications.
Keywords: HOMER2, endometrial cancer, endometrioid adenocarcinoma, immunoexpression, low grade, prognostic factor
INTRODUCTION
Endometrial adenocarcinoma (EC) is the fourth most common malignancy in women in the US, with more than 61,000 new cases expected in 2017.1 Endometrial adenocarcinomas are broadly categorised into two groups, based on cellular morphologies, genetic fingerprints and patient outcomes. Group 1 consists of endometrioid adenocarcinoma (EAC) and mucinous adenocarcinoma histological subtypes. They are more likely to occur in young woman, they are highly associated with obesity, and they commonly harbour mutations in the PTEN, PI3K/AKT, PIK3CA and KRAS genes.2,3 Patients with EAC have low grade, early stage tumours, and they have very good prognosis. On the other hand, group 2 endometrial adenocarcinoma consists of serous and clear cell type histological subtypes. They occur in older women, more commonly in African-American women. They present at late stage disease and have poor prognosis. Tumours often carry p53 gene mutation.3,4 However, this categorisation has begun to shift with deeper molecular characterisation of EC subtypes. Retrospective analyses as well as molecular analysis demonstrated that patients diagnosed with the International Federation of Obstetrics and Gynecology (FIGO) grade 3 (G3) EACs tend to have similar outcomes to patients with serous and clear cell subtypes, rather than endometrioid cancers.5–7 Consequently, in this study EAC-G3 were grouped in the same categories as serous and clear cell subtypes.
The standard treatment for early stage tumours is hysterectomy and bilateral salpingo-oophorectomy with or without brachytherapy. Chemotherapy and pelvic radiation therapy is used in group 2 cancer and advanced stage group 1 in an adjuvant setting.8–10 Hormonal therapy is another option for patients with low grade endometrioid adenocancarcinomas, however a high rate of relapse is seen with this treatment. Hence, treatment options are limited in patients who are high risk for surgery, or those who wish to preserve fertility.11–13 Recently, there have been advances in molecular characterisation of ECs in an effort to find targeted therapies, and many clinical trials are now underway. In a previous study our group also showed that a novel protein coding gene, HOMER2 (Homer scaffolding protein 2) mRNA, was highly expressed in low grade endometrioid endometrial carcinoma.14
HOMER2 is an adaptor protein. There are three HOMER family proteins in mammals, each characterised by a conserved amino-terminal domain and a Homer-specific carboxy-terminal domain.15 The function of HOMER proteins is best characterised in neurons, where they are thought to act as adaptor proteins for many post-synaptic density proteins. HOMER proteins link many critical synaptic proteins, including those involved in glutamate signalling, implicating them in many different brain functions and diseases.16–18 The role of HOMER2 in EC has not been previously studied; therefore, the aim of the current study was to evaluate HOMER2 protein expression and its prognostic value in a series of patients with EC.
MATERIALS AND METHODS
Patient population
This study was conducted at the University of Southern California with approval from the Institutional Review Board (IRB). Patient data were de-identified prior to analysis. EC cases were selected from the pathology archives at Los Angeles Country Hospital and University of Southern California Keck School of Medicine. The study included a total of 336 cases of EC.
Table 1 illustrates the demographic description of the patient population. Cases were divided into two groups based on histology; group 1 (n = 191 cases) comprised EAC G1/G2, and group 2 (n = 145 cases) comprised all others including uterine serous carcinoma (USC), clear cell carcinoma (CCC), carcinosarcoma (CS) as well as EAC G3. As previously described, we categorised EAC G3 in the group 2 tumours.7 Median patient age was 63.5 years. A total of 220 cases were early stage (stage I and II) and 116 were advanced stage (stage III and IV). The median follow-up period was 54.2 months.
Table 1.
Demographic data of the study group (n = 336)
| Characteristic | n (%) |
|---|---|
| Age, years | 63.5 (±12.8) |
| ≥60 | 130 (38.7%) |
| <60 | 206 (61.3%) |
| Histology type | |
| Grade 1–2 endometrioid | 191 (56.8%) |
| Grade 3 endometrioid | 50 (14.9%) |
| Serous | 57 (17.0%) |
| Clear cell | 20 (6.0%) |
| Carcinosarcoma | 18 (5.4%) |
| Tumour type | |
| Endometrioid adenocarcinoma G1/G2 | 191 (56.8%) |
| All other subtypes | 145 (43.2%) |
| Tumour size, cm | |
| ≤2.0 | 60 (17.9%) |
| 2.1–4.0 | 111 (33.0%) |
| 4.1–6.0 | 82 (24.4%) |
| >6.0 | 83 (24.7%) |
| LVI | |
| Negative | 220 (65.7%) |
| Positive | 115 (34.3%) |
| Depth of invasion | |
| None | 43 (12.8%) |
| 1–50% | 154 (45.8%) |
| >50% | 139 (41.4%) |
| Lymph node metastasis | |
| Negative | 142 (42.4%) |
| Positive | 80 (23.9%) |
| Not sampled | 113 (33.7%) |
| Stage | |
| I | 187 (55.7%) |
| II | 33 (9.8%) |
| III | 79 (23.5%) |
| IV | 37 (11.0%) |
LVI, lymphovascular invasion.
Tissue microarray and immunohistochemistry
Paraffin-embedded tissues from 336 patient samples were included in this study. A tissue microarray was constructed as described previously by Kononen et al.19 Briefly, after carefully choosing a morphologically representative region from the haematoxylin and eosin (H&E) section, a 1 mm core was punched from the paraffin-embedded block (donor block), and transferred to the receiver paraffin-embedded block (receiver block). To overcome tumour heterogeneity, core biopsies were performed from three different areas of each tumour. One section was stained with H&E to confirm the presence of the tumour by light microscopy.
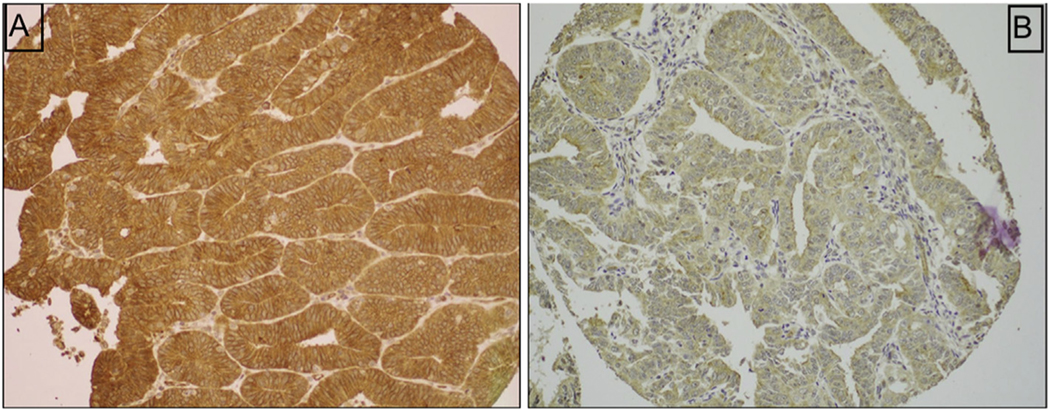
HOMER2 protein expression was detected by immunohistochemistry (IHC) using a polyclonal antibody. Immunohistochemistry was performed as described in an earlier study.14 Briefly, 4 μm paraffin embedded tissue microarray (TMA) sections were deparaffinised, dehydrated with alcohol and treated with 3% H2O2 to block endogenous peroxidase. Non-specific binding sites were blocked using a serum-free protein block (DakoCytomation, Denmark). Antigen retrieval was performed by heating in the microwave for 20 min, before incubating the sections with a polyclonal recombinant protein HOMER2 antibody (1:50, 60 min; Novus Biologicals, USA). The diaminobenzidine complex was used as chromogen, and staining was performed with an automated Bond III instrument (Leica, Germany). Normal pancreas was used as a positive control as recommended by the manufacturer and the negative control was adding rabbit serum IgG and omitting the primary antibody was simultaneously stained. HOMER2 exhibited a cytoplasmic staining pattern. Whole sections from 10 cases included in the TMA were evaluated for staining pattern. The staining was homogenous throughout the tumour. IHC was scored based on the intensity and percentage, with staining intensity categorised as negative (0), weak (1+), moderate (2+), strong (3+), and percentage as negative 0%, 1–10%, 11–25%, 26–50% and >50%. The immunostains were evaluated by one pathologist at 2 months interval. Discrepancies between the first and second read were about 5%. The majority of the discrepancies were between intensity 1+ and 2+. Because the vast majority of the cases had >50% positive staining, the percentage was not taken into consideration for the statistical study.
Statistical consideration
For statistical purposes, tumours were classified as negative (0/1+) or positive (2+/3+) expression for HOMER2 (Fig. 1). Statistical analysis was performed to evaluate the association of HOMER2 protein expression and pathological parameters [histological type, grade, stage, lymphovascular invasion (LVI), myometrial depth of invasion], and to patients’ survival outcomes. One-way ANOVA and chi-square tests were used for univariate analysis as appropriate. The Kaplan–Meier method was used for plotting the survival curves, and Cox proportional hazard regression models with conditional backward analysis were utilised to identify the independent prognostic factors on multivariate analysis. In this model, covariates with p < 0.10 on univariate analysis were initially entered in the model, and least significant covariates were removed from the model until the final model retained only the significant covariate with p value of less than 0.05. The relatively liberal p value cut-off for covariate selection was due to small sample size in this study population. Magnitude of statistical significance was expressed with hazard ratio (HR) and 95% confidence interval (CI). All hypotheses were two-tailed, and data analysis was performed using IBM SPSS Statistics 24.0 software (IBM, USA).
Fig. 1.
(A) Strong (2–3+) immunohistochemical staining with HOMER2. (B) Negative (0–1+) immunohistochemical staining with HOMER2.
RESULTS
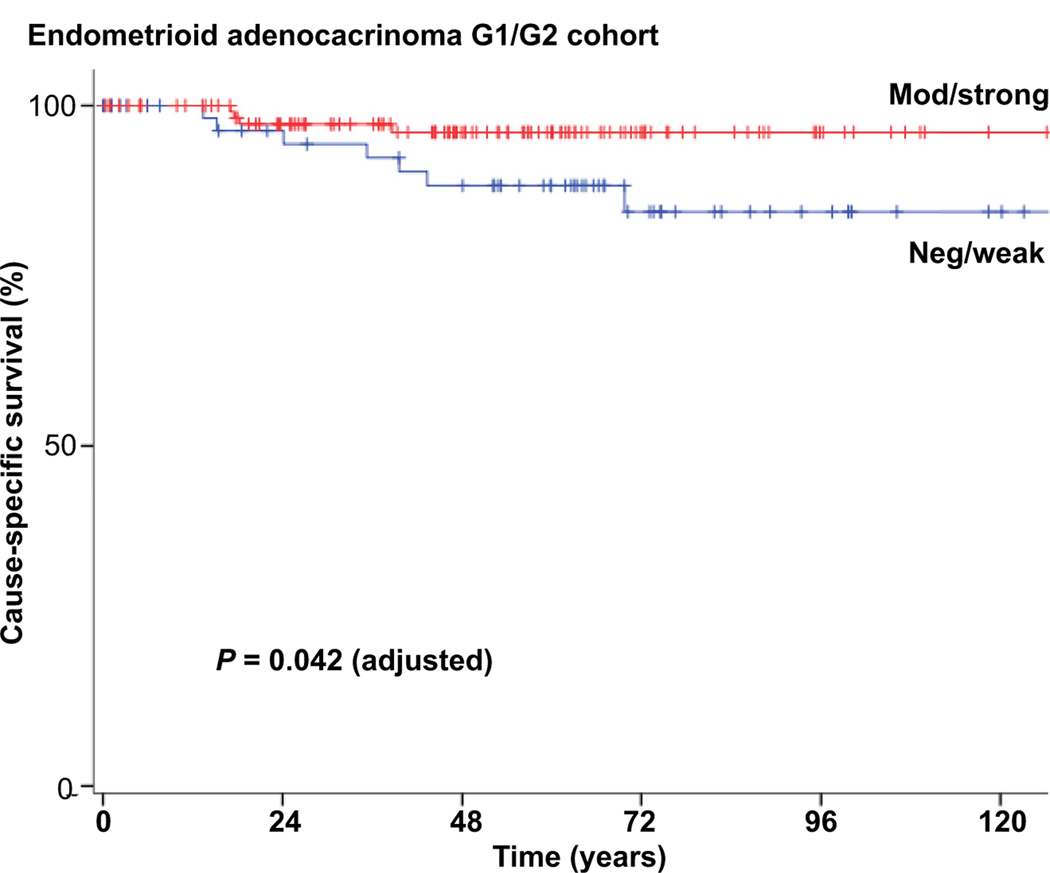
HOMER2 expression was distributed as follows; 0 or negative (n = 51), weak or 1+ (n = 94), moderate or 2+ (n = 69), strong or 3+ (n = 122). HOMER2 expression was associated with patient age (p = 0.003), and histological subtype (p < 0.001), meaning tumours of patients younger than 60 years of age, and with endometrioid histological subtype, had higher HOMER2 immunoexpression than their counterparts. HOMER2 did not have any association with other variates such as tumour size (p = 0.61), tumour stage (p = 0.61), LVI (p = 0.55), depth of myometrial invasion (p = 0.91) and lymph node status (p = 0.10) (Table 2; Supplementary Table 1, Appendix A). On univariate analysis, women in group 1 who had positive HOMER2 expression had a higher 5-year cancer-specific survival (CSS) rate compared to those with negative HOMER2 expression, although it did not reach statistical significance (96.0% versus 88.2%, p = 0.054, Fig. 2). On multivariate analysis, HOMER2 remained an independent prognostic factor for longer CSS in patients with EAC (adjusted HR 0.28; 95% CI 0.08–0.96; p = 0.042; Table 3). However, HOMER2 failed to show any predictive value for progression-free survival (PFS). Tumour stage was an independent predictive factor for CSS and PFS. Conversely to group 1, HOMER2 was not associated with CSS and PFS in group 2 (p = 0.70 and p = 0.50, respectively). Supplementary Table 2 (Appendix A) shows the CSS and PFS for all cohort of 336 patients in univariate and multivariate analysis.
Table 2.
Correlation of HOMER2 expression and clinical and pathological data
| Characteristic | Negative/weak | Moderate/strong | p value |
|---|---|---|---|
| Number | 145 (43.2%) | 191 (56.8%) | |
| Age, years | 65.9 (±12.2) | 61.7 (±12.9) | 0.003 |
| <60 | 47 (36.2%) | 83 (63.8%) | |
| Histology type | |||
| ≥60 | 98 (47.6%) | 108 (52.4%) | |
| Histology type | <0.001 | ||
| Grade 1–2 endometrioid | 63 (33.0%) | 128 (67.0%) | |
| Grade 3 endometrioid | 16 (32.0%) | 34 (68.0%) | 0.90a |
| Serous | 37 (64.9%) | 20 (35.1%) | <0.001a |
| Clear cell | 15 (75.0%) | 5 (25.0%) | 0.001a |
| Carcinosarcoma | 14 (77.8%) | 4 (22.2%) | 0.001a |
| Tumour type | <0.001 | ||
| Endometrioid adenocarcinoma G1/G2 | 63 (33.0%) | 128 (67.0%) | |
| All other subtypes | 82 (56.6%) | 63 (43.4%) | |
| Tumour size, cm | 0.78 | ||
| ≤2.0 | 23 (38.3%) | 37 (61.7%) | |
| 2.1–4.0 | 51 (45.9%) | 60 (54.1%) | |
| 4.1–6.0 | 34 (41.5%) | 48 (58.5%) | |
| >6.0 | 37 (44.6%) | 46 (55.4%) | |
| LVI | 0.35 | ||
| Negative | 91 (41.4%) | 129 (58.6%) | |
| Positive | 54 (47.0%) | 61 (53.0%) | |
| Depth of invasion | 0.72 | ||
| None | 21 (48.8%) | 22 (51.2%) | |
| 1–50% | 65 (42.2%) | 89 (57.8%) | |
| >50% | 59 (42.4%) | 80 (57.6%) | |
| Lymph node metastasis | 0.06 | ||
| Negative | 66 (46.5%) | 76 (53.5%) | |
| Positive | 40 (50%) | 40 (50%) | |
| Not sampled | 39 (34.5%) | 74 (65.5%) | |
| Stage | 0.57 | ||
| I | 79 (42.2%) | 108 (57.8%) | |
| II | 18 (54.5%) | 15 (45.5%) | |
| III | 32 (40.5%) | 47 (59.5%) | |
| IV | 16 (43.2%) | 21 (56.8%) |
LVI, lymphovascular invasion.
Compared to grade 1–2 endometrioid.
Fig. 2.
Survival curve for group 1 cohort showing better cause-specific survival in patients with a strong HOMER2 expression.
Table 3.
Multivariate analysis for endometrial cancer-specific survival in endometrioid adenocarcinoma
| Characteristic | n | 3-yr (%) | 5-yr (%) | Univariate |
Multivariate (backward) |
||
|---|---|---|---|---|---|---|---|
| HR (95%CI) | p value | HR (95%CI) | p value | ||||
| FIGO grade | |||||||
| 1 | 120 | 96.90% | 95.70% | 1 | |||
| 2 | 68 | 93.20% | 88.50% | 3.22 (0.94–11.0) | 0.062 | ||
| Tumour size, cm | |||||||
| ≤4.0 | 107 | 98.80% | 98.80% | 1 | |||
| >4.0 | 81 | 91.20% | 85.70% | 6.59 (1.42–30.7) | 0.016 | ||
| LVI | |||||||
| Negative | 157 | 96.10% | 95.20% | 1 | |||
| Positive | 30 | 92.60% | 82.30% | 4.71 (1.42 –15.6) | 0.011 | ||
| Stage | |||||||
| I – II | 151 | 97.60% | 95.70% | 1 | 1 | ||
| III – V | 37 | 87.10% | 82.30% | 6.30 (1.91 –20.9) | 0.003 | 6.59 (2.00–21.7) | 0.002 |
| HOMER2 | |||||||
| Negative/weak | 63 | 92.20% | 88.20% | 1 | 1 | ||
| Moderate/strong | 125 | 97.20% | 96.00% | 0.30 (0.09–1.02) | 0.054 | 0.28 (0.08–0.96) | 0.042 |
CI, confidence interval; HR, hazard ratio; LVI, lymphovascular invasion.
DISCUSSION
Recently the Homer family of proteins has been implicated in various cancers. Based on RNA-sequencing data from The Cancer Genome Atlas project, the HOMER2 gene was found to be specifically overexpressed in EAC, but not in serous or mixed ECs.14 Along the same lines, two studies by Mori et al. and Shah et al. showed hypermethylation of HOMER2 in colon cancer in comparison to normal mucosa.20 However, information on the role of HOMER2 in cancer and in particular in endometrial cancer is widely lacking.
This study is the first to evaluate HOMER2 expression in large series of patients with EC, as a follow-up to our previous report showing HOMER2 gene expression to be elevated (p = 0.009, fold change 0.15) in EAC in comparison to USC in a small patient cohort. Herein, we aimed at validating protein expression as well as determining the prognostic value of HOMER2 in a large cohort of patients with EAC with comprehensive clinical and pathological annotation and adequate follow-up. By studying HOMER2 protein expression using immunohistochemistry on 336 patients, we confirmed that HOMER2 protein was expressed in endometrioid histological type, and low grade (G1/G2) tumours. As for outcome analysis, HOMER2 protein was found to be an independent prognostic factor for CSS in these patients. Low grade EAC patients with tumours expressing HOMER2 have a longer CSS rate. However, HOMER2 failed to show any value in predicting PFS. In addition, but unsurprisingly, HOMER2 failed to show any role in serous, clear cell and G3 EAC. Further studies will be necessary to determine the mechanistic role of HOMER2 in EC, but knockdown studies in the HEC1B cell line failed to show an effect on cell proliferation (data not shown), suggesting interrogation of other phenotypes may be required. A study conducted by Ajima et al. found HOMER2 to be involved in suppressing anchorage independent growth in tumour cells, and loss of this gene may lead to growth deregulation in tumours.21 Factors upstream of HOMER2 are also poorly understood. One study reported hypermethylation at this locus in colon cancer and in adenoma but not in normal colonic epithelium, and one can speculate that HOMER2 gene silencing through hypermethylation might have a role in endometrial cancer but this has yet to be explored.20
Modulating HOMER2 expression in vivo could be a potential therapeutic agent for selected patients including young women, and extremely obese patients who are not good candidates for hysterectomy. The common understanding is that low grade, early stage EAC is an indolent disease where hysterectomy alone would be the treatment of choice. However, EAC can occur in young women, a group of patients with high desire for fertility sparing surgery. In this setting, progestin therapy is the treatment of choice, but with limited results. Another potential indication of HOMER2 as a therapeutic agent is for recurrent disease where salvage therapy is not very effective.
Our study provides compelling evidence to support further exploration of the role of HOMER2 in EC. Mechanistic studies should be performed to determine the action of HOMER2 in cell culture and animal models. In addition, it will be useful to determine whether HOMER2 is expressed early in cancer development, in endometrial hyperplasia, and if it has a predictive factor in the progression of endometrial hyperplasia to EC.
Supplementary Material
Footnotes
Conflicts of interest and sources of funding: The authors state that there are no conflicts of interest to disclose.
APPENDIX A. SUPPLEMENTARY DATA
Supplementary data related to this article can be found at https://doi.org/10.1016/j.pathol.2018.03.004.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Hong B, Le Gallo M, Bell DW. The mutational landscape of endometrial cancer. Curr Opin Genet Dev 2015; 30: 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol 2014; 15: e268–78. [DOI] [PubMed] [Google Scholar]
- 4.Le Gallo M, Bell DW. The emerging genomic landscape of endometrial cancer. Clin Chem 2014; 60: 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayeni TA, Bakkum-Gamez JN, Mariani A, et al. Comparative outcomes assessment of uterine grade 3 endometrioid, serous, and clear cell carcinomas. Gynecol Oncol 2013; 129: 478–85. [DOI] [PubMed] [Google Scholar]
- 6.Reynaers EA, Ezendam NP, Pijnenborg JM. Comparable outcome between endometrioid and non-endometrioid tumors in patients with earlystage high-grade endometrial cancer. J Surg Oncol 2015; 111: 790–4. [DOI] [PubMed] [Google Scholar]
- 7.Mhawech-Fauceglia P, Wang D, Kesterson J, et al. Gene expression profiles in stage I uterine serous carcinoma in comparison to grade 3 and grade 1 stage I endometrioid adenocarcinoma. PLoS One 2011; 6, e18066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkinson-Ryan I, Binder PS, Pourabolghasem S, et al. Concomitant chemotherapy and radiation for the treatment of advanced-stage endometrial cancer. Gynecol Oncol 2014; 134: 24–8. [DOI] [PubMed] [Google Scholar]
- 9.Shah PH, Kudrimoti M, Feddock J, Randall M. Adjuvant treatment for stage IIIC endometrial cancer: options and controversies. Gynecol Oncol 2011; 122: 675–83. [DOI] [PubMed] [Google Scholar]
- 10.Ly D, Soisson PA, Dodson MK, Sause WT, Gaffney DK. Adjuvant radiation therapy is associated with improved pelvic control and overall survival in FIGO IB endometrial carcinoma with high grade histology. Gynecol Oncol 2015; 138: 526–31. [DOI] [PubMed] [Google Scholar]
- 11.Park JY, Kim DY, Kim TJ, et al. Hormonal therapy for women with stage IA endometrial cancer of all grades. Obstet Gynecol 2013; 122: 7–14. [DOI] [PubMed] [Google Scholar]
- 12.Park JY, Kim DY, Kim JH, et al. Long-term oncologic outcomes after fertility-sparing management using oral progestin for young women with endometrial cancer (KGOG 2002). Eur J Cancer 2013; 49: 868–74. [DOI] [PubMed] [Google Scholar]
- 13.Kalogera E, Dowdy SC, Bakkum-Gamez JN. Preserving fertility in young patients with endometrial cancer: current perspectives. Int J Womens Health 2014; 6: 691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrenson K, Pakzamir E, Liu B, et al. Molecular analysis of mixed endometrioid and serous adenocarcinoma of the endometrium. PLoS One 2015; 10, e0130909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiraishi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biol 2007; 8: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goulding SP, Szumlinski KK, Contet C, MacCoss MJ, Wu CC. A mass spectrometry-based proteomic analysis of Homer2-interacting proteins in the mouse brain. J Proteomics 2017; 166: 127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szumlinski KK, Lominac KD, Oleson EB, et al. Homer2 is necessary for EtOH-induced neuroplasticity. J Neurosci 2005; 25: 7054–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiraishi Y, Mizutani A, Yuasa S, Mikoshiba K, Furuichi T. Glutamate-induced declustering of post-synaptic adaptor protein Cupidin (Homer 2/vesl-2) in cultured cerebellar granule cells. J Neurochem 2003; 87: 364–76. [DOI] [PubMed] [Google Scholar]
- 19.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 1998; 4: 844–7. [DOI] [PubMed] [Google Scholar]
- 20.Mori Y1, Olaru AV, Cheng Y, et al. Novel candidate colorectal cancer biomarkers identified by methylation microarray-based scanning. Endocr Relat Cancer 2011; 18: 465–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ajima R1, Kajiya K, Inoue T, et al. HOMER2 binds MYO18B and enhances its activity to suppress anchorage independent growth. Biochem Biophys Res Commun 2007; 356: 851–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.