Abstract
Therapeutic strategies are being clinically tested either to eradicate the latent HIV reservoir or to achieve virologic control in the absence of antiretroviral therapy (ART). Attaining this goal will require a consensus on how best to measure the levels of persistently-infected cells with the potential to cause viral rebound upon ART cessation to assess the results of cure-directed strategies in vivo. Current measurements assess different aspects of the HIV provirus and its functionality, and produce divergent results. Here, we provide the collective insight and position from the BEAT-HIV Martin Delaney Collaboratory on which viral measurements to prioritize in HIV cure-directed clinical trials.
Keywords: HIV latency, HIV reservoirs, Viral measurements, HIV Cure, HIV persistence, Replication-competent HIV
Antiretroviral therapy (ART) has dramatically reduced morbidity and mortality for people living with HIV (PWH) by effectively suppressing viral replication to undetectable levels in plasma.1–4 However, ART requires lifelong administration and does not eradicate HIV, which continues to cause immune activation, chronic inflammation, and ongoing damage to multiple organs systems.5–8 Therefore, effective therapeutic strategies are needed to cure HIV infection.
The main obstacle to cure HIV is that the virus establishes stable reservoirs of persistently-infected cells, in blood and different anatomical sites.9 These reservoirs persist despite long-term ART and can be broadly divided into two groups. The “latent reservoir” in which cells carry an integrated copy of the viral genome that does not express viral transcripts or proteins. These latent proviruses are not affected by ART or cleared by the immune system; however, they can revert to a viral productive state upon stimulation.10–12 In addition to the latent reservoir, the active reservoir is a minor population of transcriptionally-active cells, producing HIV RNA/proteins and possibly virions, that can be detected in PWH despite long-term ART. Targeting and eliminating both types of reservoirs is the focus of the quest for an HIV cure.13
Currently, several clinical trials are being conducted to evaluate the impact of potential therapeutic strategies to cure HIV. The success of these trials hinges on the ability to measure the size of HIV reservoirs before and after such interventions as a guide to ART interruption. However, there is no consensus on how to use the variety of assays currently available to evaluate the size of HIV reservoirs with the potential to cause viral rebound in the absence of ART. In this Perspective, we provide our collective view on which viral measurements to prioritize in HIV cure-directed clinical trials (Figure 1).
Figure 1.
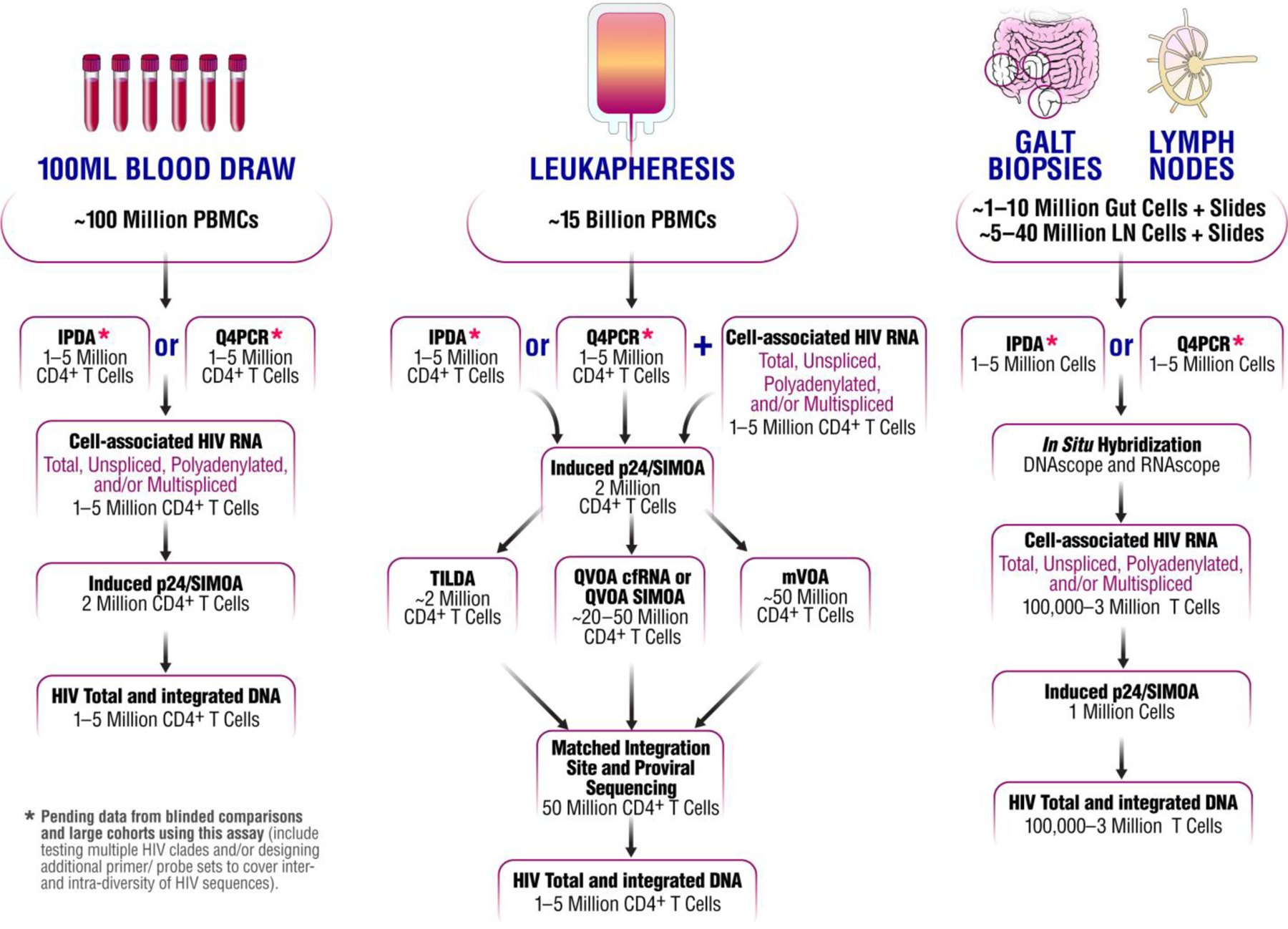
The collective view of the BEAT-HIV Collaboratory on the priority for measuring HIV reservoir size, in blood and tissues, during HIV cure-related clinical trials.
Challenges to measuring the HIV reservoir
There are four major challenges facing clinical trial design when measuring changes in HIV reservoir size on ART: the vast majority of proviruses persisting in ART-treated PWH harbor mutations and/or deletions rendering them defective and unable to replicate; not all intact genomes (genomes with no defective mutations/deletions) are inducible to produce virions; the frequency of latently-infected cells is very low; and the vast majority of HIV reservoirs are in tissues that cannot be accurately sampled with current specimen collection approaches.14–20
HIV cure-directed studies in ART-suppressed PWH currently measure levels of total proviral DNA, intact provirus, transcriptionally-competent provirus (viral RNA), translationally-competent provirus (protein), and replication-competent virus. These clinical studies primarily measure changes in the aforementioned viral measures using four specimen types: peripheral blood mononuclear cells (PBMCs) obtained either by phlebotomy or leukapheresis, and biopsies from gut-associated lymphoid tissue (GALT) or lymph nodes (LN). It’s probable that no measurement of a single compartment can fully inform on the totality of reservoir dynamics under ART. Most clinical trial designs mainly collect peripheral blood for the ease of longitudinal sampling, the willingness of participants to donate blood frequently, its low cost, high throughput, and ease of collection at clinical sites around the world. As mentioned, GALT and LN biopsies are also collected in some clinical trials. While, HIV might persist in other tissues, the collection of these other tissues is challenging.
Measuring levels of replication-competent virus or intact HIV genomes
Quantitative viral outgrowth assay (QVOA) and its derivates
Until recently, the QVOA and its derivatives (see Table 1) were the standard measurements of the replication-competent HIV reservoir.21,22 However, we think that QVOA is not well suited for cure-directed studies due to its high cost, labor requirements, need for a high number of cells (limiting its use to peripheral blood cells), and biosafety containment requirements. Moreover, QVOA underestimates the size of the replication-competent viral reservoir, as not all intact HIV proviruses can be induced to release virus following a single round of T cell activation.20,23
Table 1:
Current viral measurements and strategies to estimate HIV reservoir size.
| Assay | What it measures? | Advantages | Drawbacks | Involves stimulation and/or expansion? |
|---|---|---|---|---|
| Assays measuring levels of replication-competent virus or intact HIV genomes | ||||
| Quantitative Viral Outgrowth assay (QVOA).3,20,21,23,111 | Frequencies of cells harboring replication-competent HIV that can be induced ex vivo to produce infectious virus. | Has been regarded as the definitive assay to measure the size of the replication-competent HIV reservoir; clade independent. | Underestimates the size of the replication-competent HIV reservoir; requires a large number of cells; time-consuming and labor-intensive; cost-intensive. | ex vivo stimulation followed by expansion. |
| Modified QVOA using cell lines supporting HIV replication.112 | Frequencies of cells harboring replication-competent HIV that can be induced ex vivo to produce infectious virus. | Less labor-intensive and more consistent compared to the traditional QVOA; clade independent. | Underestimates the size of replication-competent HIV reservoir; requires a large number of cells; cost-intensive. | ex vivo stimulation followed by expansion. |
| The qualitative and quantitative viral outgrowth assay (Q2VOA).24 | Frequency of cells encoding HIV proviral DNA that can be induced ex vivo, as well as genetic and potentially phenotypic characterization of latent viruses. | Provides additional insights on the qualitative nature of HIV proviral DNA. | Underestimates the size of the replication-competent virus as it misses replication-competent non-induced proviruses, similar to traditional QVOA. Labor, time, and cost-intensive; the sequencing part of the assay could be clade dependent. | ex vivo stimulation followed by expansion. |
| Intact proviral DNA assay (IPDA): HIV DNA by digital droplet PCR targeting multiple regions of proviral DNA to exclude deleted and hypermutated proviruses.27 | Frequency of cells encoding intact and defective proviral HIV DNA. | Eliminates 97% of defective proviruses and is predicted to overestimate the size of the latent reservoir by only ~ 1.5 fold; simple, medium cost, and fast compared to traditional QVOA. | Sequence polymorphisms could preclude amplification in some patients; alternative primers/probes needed; clade-dependent; does not measure the inducibility of the proviruses. | No stimulation or expansion. |
| HIV DNA by limiting dilution four-probe qPCR assay followed by sequence verification of reactions positive for two or more probes (Q4PCR).28 | Frequency of cells encoding intact and defective proviral HIV DNA confirmed by sequencing. | Estimates levels of replication-competent virus; results are verified by sequencing; sensitive. | Lower throughput and more time-consuming, and labor-intensive compared to IPDA; cost-intensive; clade dependent; does not measure the inducibility of the proviruses; does not provide absolute quantification. | No stimulation or expansion. |
| Near full-length individual proviral sequencing (FLIP-Seq).20,25 | Levels and genetic characteristics of genetically-intact proviruses. | Measures the levels of genetically-intact proviruses in a definitive manner. | Time-consuming and labor-intensive; cost-intensive; requires custom design of primers. | No stimulation or expansion. |
| Proviral ultra-deep sequencing.113 | Could measure levels of genetically-intact proviruses. | Cost-effective; simple compared to single-genome sequencing. | Potential template resampling and PCR errors/bias; cost-intensive; clade dependent. | No stimulation or expansion. |
| High throughput integration site sequence analysis.114,115 | Distributions of integration HIV proviruses, with linked information on clonal abundance | Efficiently reports locations of integration sites, and associations of insertional mutagenesis and clonal expansion | Requires specialized expertise; cost-intensive; requires workable numbers of proviruses to analyze in samples | No stimulation or expansion. |
| Matched integration site and proviral sequencing (MIP-Seq). 39 | Individual proviral sequences and corresponding chromosomal integration site. | Investigate chromosomal positioning and integration site features of intact HIV-1 proviruses. | Technically complex and cost-intensive. | No stimulation or expansion. |
| Murine viral outgrowth assay (mVOA).33 | Presence/absence of replication-competent virus within the number of cells/tissues tested. | Can detect low levels of latent infection; clade independent. | Multiple animals are required per sample if a quantitative value is needed; needs a large number of cells; cost-intensive. | In vivo expansion. |
| Assays measuring inducible translationally- or transcriptionally-competent virus | ||||
| Modified QVOA using ultra-sensitive readouts (including QVOA p24 SIMOA and QVOA cfRNA).57–59 | Frequencies of cells harboring transcription-competent and translation-competent HIV that can be induced ex vivo. | Simple, fast, and requires fewer cells compared to QVOA; detects transcriptional-competent and translation-competent virus. | Can detect cells infected with a replication-incompetent virus that is able to produce viral RNA or protein; Semi-labor intensive unless automated; cost-intensive. | ex vivo stimulation followed by expansion (unless done in the presence of ART). |
| Cell-associated p24 protein quantification. 116 | Productive HIV p24 expression in the presence or absence of ex-vivo stimulation. | Sensitive; simple; fast; relatively cost-effective; requires relatively few cells; application across sample types; measurement of productively expressing “active” and “inducible” reservoirs; closer to replication competence when compared to measuring HIV transcripts; biologically relevant target for immune response and immune-based interventional approaches. | May overestimate reservoir size as detects translation-competent and replication-competent provirus rather than the replication-competent virus. | Can be done without stimulation but usually done with short ex vivo stimulation but no expansion. |
| Induction-based viral RNA reactivation assays (including TILDA).57,66,117 | The number of cells harboring transcriptionally reactivatable HIV tat/rev msRNA. | Simpler, faster, and requires fewer cells compared to QVOA; can be useful in measuring the response to LRAs ex vivo. | Measure inducible levels of HIV transcripts (transcriptional competence) without distinction of defective or replication-competent (replication competence); cost-intensive; semi-labor intensive unless automated. | short ex-vivo stimulation but no expansion. |
| Single-cell analysis of inducible virus (including FISH/flow). 56 | Measures the frequency of cells undergoing HIV transcription and/or translation upon stimulation at the single-cell level. | Single-cell insights of HIV transcription and/or translation and phenotypic characterization of individual cells; clade independent; relatively cost-effective. | Do not measure replication competence. | Can be done without stimulation but usually done with short ex-vivo stimulation but no expansion. |
| Assays measuring constitutive levels of HIV DNA and cell-associated HIV RNA | ||||
| Cell-associated HIV RNA by real-time or droplet digital PCR.53,61–64,75,93,118 | Levels of HIV transcripts (a surrogate of transcription-competent cellular HIV) without distinction of defective, translation-competent, or replication-competent. | Fast, relatively cost-effective, and sensitive; cell-sparing; application across sample types; Different HIV transcripts can be measured to indicate the degree of HIV transcriptional activity; may provide a surrogate measure for reservoir size as levels of HIV RNA during ART predict time to viral rebound upon treatment cessation. | Overestimates the size of the latent HIV reservoir. Doesn’t measure translation- or replication-competence; clade dependent. | No stimulation or expansion. |
| HIV DNA by real-time PCR or droplet digital PCR.34,53,72–76,79,81,93 | Levels of selected regions of total, integrated, or circular HIV DNA measure without distinction of defective or replication-competent provirus. | Fast, cost-effective, and sensitive; cell-sparing, application across sample types; most available assays span across different clades. | Vastly overestimates the size of the HIV reservoir, as only a small fraction of HIV genomes is able to be reactivated upon ex vivo stimulation to produce replication-competent virus. | No stimulation or expansion. |
| In situ hybridization-based assays, e.g., DNAscope and RNAscope.53,70,71 | Cellular location and levels of HIV sequences associated with integration (DNA) or transcription (RNA) without distinction of defective or replication-competent. | Visualize and phenotypically characterize infected cells in tissues; allow for a better understanding of the anatomical distribution of infected cells in vivo; cost-effective; near sensitivity to qRT-PCR. | Do not measure replication competence; clade dependent. | No stimulation or expansion. |
| Assays measure the in vivo burden of HIV reservoirs | ||||
| Ultra-sensitive residual viremia.67–69 | The release of HIV particles in vivo during ART, without distinction of tissue origin. | Could represent virus release from stable reservoirs, including these in tissues; relatively cost-effective. | Requires large volumes of plasma or body fluid; limited dynamic range; the relationship between the HIV reservoir and low-level viremia during ART viremia is unclear; clade dependent. | No stimulation or expansion. |
|
Common strategy to test for
viral burden on ART yet not an “Assay,” included
here for comparison only: Analytical Treatment Interruptions (ATI).98,110 |
Systemic virus replication in vivo after ART-cessation, without distinction of tissue origin. | Determines the duration of HIV remission upon the cessation of ART. It is the most clinically relevant measure of the impact of interventions on the total body burden of HIV infection. | Potential clinical risks to individuals stopping therapy and to their partners; clinically demanding; substantial reservoir reductions are needed to produce significant delays in viral rebound, so unlikely any current interventions could result in a significant delay in viral rebound. Effort and monitoring cost-intensive | In vivo expansion. |
Other assays have been developed based on QVOA, such as Q2VOA. Q2VOA has the same limitations of QVOA in under-estimating reservoir size; however, it provides added sequence information in addition to an estimate of reservoir size.24 Independently from QVOA or other similar outgrowth-based assays, single-genome, near full-length proviral sequencing does allow for a detailed analysis of the proviral landscape such as an ability to identify the frequency of intact and defective proviral species, phylogenetic sequence analysis and detection of clonal intact proviral sequences.19,20,25,26 However, near full-length proviral sequencing is laborious and time-intensive, rendering it unfeasible for large HIV cure-directed clinical trials. In addition, methods relying on long-distance PCR may not be highly quantitative due to the possible inefficiency of such reactions.
Emerging PCR-based techniques to measure intact HIV DNA levels
The above limitations prompted the development of more sensitive and straightforward assays to distinguish between intact and defective proviruses. New assays, including the intact proviral DNA assay (IPDA),27 and the Quadruplex qPCR (Q4PCR) assay,28 now offer promising strategies that require a relatively small number of cells to estimate the size of genetically-intact HIV populations in a labor and cost-effective manner, when compared to QVOA or near full-length HIV provirus genome sequencing. Based on the specific requirements of these two assays, we think that IPDA is best suited for high-throughput analysis of large interventional or observational clinical studies; for instance, a recent longitudinal cohort analysis evaluated the decay rate of intact and defective proviruses in a large cohort of patients using IPDA.29 However, these studies have also revealed that <2% of estimated intact proviruses are expected to be induced ex vivo. This observation raises the question whether we would also need to add an inducibility index to IPDA measures as defined by a concurrent viral outgrowth assays (discussed below) to define the relative frequency of cells producing replication-competent virus.30 On the other hand, the Q4PCR assay interrogates four regions of the genome after a near-full-length PCR, providing more detailed information on proviral integrity and direct confirmation of viral sequences. These added steps in Q4PCR may provide valuable information in smaller trials or trial designs where viral sequencing is important, but near full-length sequencing is prohibitively costly or time-consuming. For example, HIV cure-directed studies that use a lentiviral vector as a delivery mechanism such as the CD4 CAR (NCT03617198) may best be served by the Q4PCR assay to distinguish between circulating HIV and lentiviral vector sequences.
While these two newer assays are promising, future studies are needed to: validate them in large independent cohorts; perform blinded comparisons between laboratories; assess relationship with induction potential; and establish if levels of intact proviral HIV DNA in the blood and/or tissues correlate with time to or magnitude of viral rebound during an analytical treatment interruption (ATI). As with any sequence-based assay, sequence polymorphisms preclude amplification in a fraction of PWH and may require alternative primer/probe sets. It will be critical to validate the ability of the primer/probe sets used in these assays to overcome the intra- and inter-individual diversity in HIV sequences. This also includes validating these assays among different HIV clades as most studies to date have been performed in regions with dominant clade B. Importantly, these assays can be performed on frozen cells; therefore, we suggest storing of samples from clinical trials until adequate validation of these assays is completed. With regards to the future use of these assays using GALT biopsies, we currently recommend the analysis of ileum tissue due to higher lymphocyte-rich regions, and the pre-enrichment of CD45+ leukocytes as it has been shown that this step increases the sensitivity of detecting persistent HIV measures.31,32
The murine viral outgrowth assay (mVOA)
mVOA, in which large numbers of cells from PWH can be adoptively transferred to NSG [immunodeficient non-obese diabetic (NOD)] mice and monitored for viremia, represents another tool to assess levels of replication-competent HIV proviruses.33,34 This assay may be more sensitive than traditional ex vivo outgrowth assays in detecting replication-competent HIV from PWH with very low to undetectable levels of HIV DNA and RNA in peripheral blood mononuclear cell (PBMC), due to the in vivo setting.33,34 This assay, by testing a large number of cells in an in vivo environment that may support amplification over time, maybe useful for confirming the absence of replication-competent cells when (1) no other assays detect infectious virus or intact provirus, and (2) no ATI is included in the trial design. However, it is important to note that this assay is laborious, costly, and time-consuming.
HIV integration sites analysis
It is also possible to measure the distribution of HIV DNA integration sites in PWH samples, which can provide information on reservoir structure and maintenance. Quantitative understanding of HIV integration sites can identify HIV-infected cells which are preferentially proliferated in vivo. Understanding why certain HIV-infected cells preferentially proliferate in vivo can provide important information for prioritizing the elimination strategies targeting the proliferating HIV latent reservoir, a current major challenge in the HIV cure field. Second, integration site data may also be linked to studies of provirus inducibility.35,36 For example, HIV is known to favor integration in active transcription units, and associated features,37,38 and this may influence subsequent proviral gene activity.39,40 Finding ways to target proviruses that are not readily induced may be key to reaching ART-free viral control (functional cure). An integrative understanding of HIV integration site and proviral genome integrity (by using MIP-seq39 or MDA-SGS41) or HIV integration site and HIV-driven aberrant cancer gene expression (by using HIV SortSeq42) may provide new therapeutic insights into targeting intact and inducible HIV reservoirs. This level of analysis requires a large number of cells (i.e., leukapheresis) and a high investment (labor and cost) per study participant analyzed.
The BEAT-HIV Collaboratory position on assays measuring the amount of putative intact HIV proviral genomes is to give priority to either the IPDA or Q4PCR approach using both blood and tissue samples (pending validation; Figure 1) when assessing the success of a potential curative strategy in targeting the levels of intact HIV DNA. When cell numbers allow and undetectable levels of HIV are noted in trial designs that do not include a treatment interruption phase to assess viral rebound after ART interruption, the inclusion of mVOA (for understanding inducible reservoirs) and MIP-Seq (for more in-depth qualitative insights on viral sequences) on a limited subgroup of clinical trial participants should be considered.
Measuring levels of translationally-competent virus
Studying cellular reservoirs capable of producing HIV antigen, e.g., gag p24 protein, is biologically and scientifically relevant as protein is more likely, compared to RNA transcripts, to trigger innate and adaptive immune responses, lead to persistent immune activation, and play a role in HIV pathogenesis. HIV antigen is also the target of several immune-based curative strategies.43,44 Furthermore, since viral protein production requires intact viral reading frames, viral protein detection would be expected to identify inducible proviruses relatively frequently, albeit not always as defective proviruses can also express viral proteins.20,45,46 Thus, evaluating the translational competency of HIV reservoirs (latent or active) complements measures of genetically intact, replication-competent virus.
Digital p24 Single Molecule Assay Technology (SIMOA) immunoassay
Current protein-based measurements take advantage of the natural amplification provided by the high number of HIV gag molecules generated per cell or per virion, together with emerging ultra-sensitive methods for protein detection. The enhanced digital p24 SIMOA immunoassay provides rapid and direct quantification of viral antigen from single HIV+ cell in the absence or presence of CD4+ T cell stimulation (i.e., steady-state or inducible reservoirs, respectively).43,47–50 Advantages of this assay include measurement of virus with sufficient genetic integrity to drive transcription, translation, and downstream processing, however, this approach will likely overestimate the size of the replication-competent reservoir as the increased sensitivity of this assay may allow detection of virion production by cells harboring replication-defective proviruses (e.g., a provirus with packaging signal deletions).20,46 In all outgrowth assays, a failure to document exponential increases in viral RNA or protein over time in culture may contribute to an over-estimation of reservoirs if only measured by ultra-sensitive p24 SIMOA.51–53
Induced cell-associated p24 quantification may also inform on the relationships between HIV persistence and immunological responses as translational-competent cells may contribute to chronic inflammation and immune activation during ART. Lastly, the digital p24 SIMOA assay requires a small number of cells and is applicable to a variety of specimens from various anatomical compartments (blood or tissue). Therefore, our collective recommendation is to include the induced p24 expression as measured by the SIMOA immunoassay (Induced p24/SIMOA) as one of the primary readouts for quantifying levels of steady-state/inducible translationally-competent virus in blood and/or tissues during HIV cure-directed clinical trials (Figure 1).
Single-cell FISH-flow cytometry and other assays measure translationally-competent virus
Apart from the induced p24/SIMOA assay, assessments of induced p24 expression can also be performed using live cells by single-cell FISH-flow cytometry allowing for the added assessment of the frequency of infected cells.54–56 Other assays of the translationally-competent virus, such as ultrasensitive QVOA p24 SIMOA (measuring p24) or QVOA cfRNA (measuring cell-free HIV RNA; a surrogate of HIV virions produced upon ex vivo stimulation of resting CD4+ T cells) have been developed using a limiting dilution step of input cells.57–59 Compared to the QVOA, flow cytometry-based assays are simpler, have higher throughput and sensitivity, and require few cells. However, in contrast to the QVOA, they measure the frequency of cells that are inducible ex vivo to produce viral virions and/or HIV protein [whether harboring defective or replication-competent (intact) virus]. Further studies are needed to evaluate whether the measurements from any of these assays measuring the levels of translationally-competent provirus can reflect the dynamics of an ATI viral rebound in vivo.
The BEAT-HIV Collaboratory position on assays measuring the amount of translationally-competent virus is to give priority to direct p24 immunoassay quantification (either without stimulation or following short ex vivo stimulation to reactivate virus without expansion) followed by measuring changes in inducible infected cell frequency by QVOA cfRNA or QVOA p24 SIMOA (measuring frequency of cells producing virions or viral proteins followed ex vivo stimulation and expansion) (Figure 1). It is important to note that it remains undetermined how different stimuli or repeated stimulations may affect the reactivation potential of different infected cells to express p24, therefore, stimuli used and limitations of each assay should be carefully considered.60
Measuring levels of transcriptionally-competent virus
Viral RNA assays have been used broadly to measure both productive infection and latency reversal; however, given that >90% of proviral DNA is defective, these measures overestimate the size of the reservoir capable of producing infectious virions and are inadequate for determining the effectiveness of interventions targeting translationally- or replication-competent reservoirs.19,20,27 In contrast, measuring cell-associated HIV RNA transcripts that persist during ART (total, elongated, unspliced, polyadenylated, and multi-spliced) or the ratio of HIV RNA to HIV DNA might indicate the degree of HIV residual transcriptional activity.59,61–63 These measures could provide an early indication of change following therapeutic interventions that aim to either stimulate or block viral transcription. Such assays provide 1–2 log10 greater sensitivity over assays of intact or replication-competent virus by providing a greater dynamic range to measure the impact of an effective intervention. Moreover, transcriptionally-competent HIV infected cells may be a significant contributor to chronic inflammation and immune activation during ART, which could impact the efficacy of curative strategies. Importantly, levels of cell-associated HIV RNA during ART (both unspliced and multi-spliced) can serve as predictors of viral rebound after therapy interruption,64,65 supporting the biological and clinical relevance of these HIV RNA measures in measuring the size of HIV reservoirs.
TILDA and HIV-RNAscope
It should be noted that qPCR and droplet digital PCR assays measuring levels of HIV transcripts without a serial dilution step of input cells only evaluate the levels of HIV RNA in bulk cells, but do not determine the frequency of cells that are transcriptionally-competent. Characterizing the frequency of transcriptionally-competent cells before and after interventions can be an important parameter, especially in the context of clinical interventions expected to eliminate cells that are HIV transcriptionally active. If HIV RNA+ cell frequency is needed, we suggest the inclusion qPCR and droplet digital PCR assays measuring levels of HIV transcripts with a serial dilution step of input cells, such as the Tat/Rev Induced Limiting Dilution Assay (TILDA) 66 or similar assays 59 (Figure 1). In addition, measuring levels of residual viremia may be useful when evaluating strategies aimed to reactivate the latent reservoir (shock and kill strategies). However, in the absence of a reactivation strategy, the dynamic range of assays measuring residual plasma HIV RNA after ART limits the use of this assay in cure-directed study design67–69. Finally, for tissue-based specimens, direct in situ hybridization-based assays, such as HIV-DNAscope and HIV-RNAscope, have high priority, as isolation of substantial numbers of CD4+ T cells and macrophages from biopsies may be difficult. These assays provide an important spatial context of HIV reservoirs as well as an understanding of the microenvironments/cellular immune neighborhoods in which HIV RNA+ and DNA+ reservoirs reside within tissue (i.e., providing a superior understanding of the cellular distribution, the phenotype and transcriptional state of persistently infected cells in vivo).53,70,71 However, the ability of these assays to quantify total tissue reservoirs is unknown and unlikely considering the limited tissue sampling available from participants in a clinical trial.
The BEAT-HIV Collaboratory position on assays measuring the amount of transcriptionally-competent virus is to give priority to the use of RNA (and DNA)-scope (when using tissue specimens) assays to be followed by levels of HIV transcripts and the frequency of HIV transcriptionally competent cells, particularly if the strategy is expected to stimulate or block HIV transcription.
Measuring total and integrated levels of HIV DNA
Reservoir size has been characterized by PCR quantification of the total, integrated, and episomal HIV DNA. Interpretation of these measures is complicated by the differential decay rates of HIV DNA and the fact that the vast majority of proviral DNA is profoundly defective.19,20,27 Despite this limitation, total HIV DNA has been measured in many settings and provided important insights on HIV persistence.53,72–76 In addition, measuring total HIV DNA levels could be the only assay possible when a limited cell number is available as other assays might not be sensitive enough for quantitative measurement. In addition to levels of total HIV DNA, specific assays have been developed to measure integrated HIV DNA and episomal HIV DNA (2-LTR circles). Since post-integration latency is thought to represent the major form of viral persistence, levels of integrated HIV DNA provide a more accurate measure of HIV reservoir size than total HIV DNA and in steady-state conditions are correlated with IPDA measures.77 Integrated HIV DNA assays require either a pre-PCR separation of high molecular weight DNA78 or a pre-amplification step with primers for genomic Alu elements and HIV sequences.72,79–81 In summary, based on the goal of cure-directed studies to detect a change in replication-competent provirus and availability of assays such as IPDA or Q4PCR that are able to estimate a change in putative intact HIV provirus numbers irrespective of total HIV DNA with predominant defective proviruses, it is our collective view to place a lower priority for measuring levels of integrated HIV DNA in clinical trials unless available cell number is limited (Figure 1).
Sources of cells
HIV latency has been extensively characterized in resting CD4+ T cells, which typically do not produce virions or viral antigens ex vivo before activation by a latency-reversing agent.12 HIV can also be detected in cells expressing classical activation markers such as HLA-DR.12,53,82 However, the extent to which a steady-state of activated infected CD4+ T cells contribute to HIV persistence during ART remains unknown as these cells are expected to have a short half-life by expressing viral antigens exposing these cells to viral cytopathic effects and/or immune clearance. HIV has also been detected in different memory cell subsets.83 It is important to note that recent studies show that the markers used to define memory cell populations change with activation state and that intact proviruses are found in most if not all T cell subsets.30,56,83 For example, naïve CD4+ T cells can harbor a small but significant portion of HIV replication-competent virus in vivo.84 Given the absence of a single marker that can identify latently infected CD4+ T cells, and the wide distribution of the reservoir between T cell subsets, we suggest performing the aforementioned measures first on total CD4+ T cells. Analysis of total CD4+ T cells would more accurately reflect the steady-state of HIV persistence, with the caveat that virus detected may originate from both latently-infected cells and recently activated CD4+ T cells (latent and active reservoirs). When larger quantities of CD4+ T cell are available, as in the case of leukapheresis or lymph node biopsy, measurements can be performed on different CD4+ T cell subsets,83 including resting, naïve, central memory, effector memory, follicular helper CD4+ T cells, resident memory CD4+ T cells, regulatory T cells, and other relevant cell populations.
Finally, we acknowledge that CD4+ T cells may not constitute the sole viral reservoir in vivo as other cellular compartments such as tissue-resident myeloid cells may harbor persistent HIV after ART.85,86 However, the difficulty in isolating myeloid cells from tissue compartments limits the analysis of these cell types to in situ hybridization approaches (i.e., HIV-DNAscope and HIV-RNAscope) and non-human primates. Therefore, our current inability to account for the potential retention of persistent HIV in myeloid subsets after ART highlights a gap in our ability to monitor this compartment in clinical trial design. Finally, rapid autopsies are useful to obtain viable deep tissues from PWH on ART participating at the end of life studies, but they are likely not useful in interpreting change in reservoirs after an intervention as an autopsy provides a one-time measurement.87
Host surrogates of reservoir size
To gain insight into the host pressures that control HIV persistence, latency and reactivation, a comprehensive examination of host factors and cell subsets contributing to viral outgrowth ex vivo and rebound viruses in vivo after an ATI is needed. Regarding host factors, rebound HIV isolates after an ATI, but not those activated to by outgrowth ex vivo in a QVOA, are uniformly resistant to the antiviral activities of type 1 interferons (IFN-I). This observation indicates that tissue-specific innate responses limit the viruses that successfully reactivate from latency.88 Phenotypic analyses of QVOA and rebound isolates, including a comparison of their replicative fitness, cell tropism, glycosylation changes, and neutralization sensitivity can provide further insight into the host factors that maintain the latent HIV reservoir and may allow tracking the provenance of the rebounding virus.89 In addition to the phenotypic analyses of QVOA and rebound, several studies have shown associations between host immune-related factors and HIV persistence during ART. Cell surface expression of negative immune checkpoints (PD-1, TIGIT, and LAG-3) correlates with levels of HIV DNA and RNA during ART.90–92 The expression of select anti-HIV host restriction factors are also associated with levels of cell-associated HIV RNA during ART.93 Levels of anti-HIV antibodies can reflect both the degree of HIV persistence and low-level viral replication.94–96 While we have focused this perspective on virological measurements of persistent HIV reservoir, it is our view that concurrent measures of host factors associated with changes in viral reservoirs or rebound could be a critical component in understating the impact of potential interventions on the totality of the body’s HIV reservoirs during a cure-directed trial.
Analytical Treatment Interruptions (ATI)
The likelihood of rebound is thought to be a function of both the size of the inducible replication-competent reservoir and the strength of the immune responses. Clinical data have as yet failed to reveal a threshold of the number of the virus measured while ART-suppressed by any laboratory viral assay below which rebound will not occur.34,97 The inability of viral measures alone to predict a lack of rebound may reflect the absence of taking into account host immune measures. With regards to viral measures alone, modeling studies suggest that a very large reduction in the replication-competent virus levels would be needed to significantly delay viral rebound in vivo.98 However, the limitation of modeling studies focused on changes of viral reservoir measures during ART is most evident with interventions designed to protect cells from infection, such as zinc finger nuclease induced cleavage of CCR5,99 or modulation of immune functions, such as chimeric antigen receptor (CAR) modified T-cells. These interventions would require an ATI to assess outcomes.
In addition, recent data using QVOA have revealed another potentially broader limitation of evaluating HIV within any one tissue compartment (i.e., peripheral blood). Specifically, QVOA HIV sequences induced from isolated CD4+ T cells from PBMC and expanded ex vivo differ, in some cases, from predominant HIV sequences detected in blood after ATI.100–102 The explanation for this discordance remains under investigation but may reflect: inadequate sampling depth of the peripheral reservoir to detect rare cells that may cause rebound during ATI; distinct latent virus populations in the peripheral blood versus the tissues from which virus rebounds;103 differences between ex vivo stimulation in the presence of a uniform activation (e. g. mitogens or anti-CD3/28/cytokines) versus host factors driving in vivo reactivation and viral amplification; and the absence of immune selection pressure in static ex vivo cultures when compared to the dynamic innate, humoral, and cell-mediated pressure influencing viral rebound in vivo.
Importantly, the onset of rebound during an ATI may be subject to sampling bias of circulating virus alone without accounting for changes in viral persistence within mucosal tissue compartments that house the majority of the potential reservoir as demonstrated by a broad sampling of tissue sites for HIV viral RNA and DNA.17 These studies revealed that 98% of the viral DNA and RNA is detected in the gut and lymph node. The inability of the field to accurately quantify changes in these tissue-resident reservoirs represents a major obstacle to the clinical development of approaches to shrink the reservoir. With regards to host factors, innate (plasmacytoid dendritic cell) and adaptive variables have been noted to predict viral rebound.104–106 Furthermore, the glycosylation signatures of plasma antibodies and other glycoproteins on ART associate with time-to-viral-rebound and viral setpoints upon ART cessation.107 Modeling efforts to best integrate the contribution of viral and host surrogate measures to predict viral reservoir levels and viral rebound remain to be developed.
Because of its high information value, we endorse the continued use of closely monitored ATIs with defined safety-based criteria for re-starting ART in cure-directed studies.101,108,109 Criteria on how to address ATIs in study design were addressed in a recent consensus conference.110 It is the position of the BEAT-HIV Collaboratory that only HIV cure-directed strategies with pre-existing evidence of a significant reduction in levels of HIV persistence in vivo or antiviral mechanism of action in animal models, should proceed to include an ATI in trial design in order to establish a definitive measure on the impact on HIV control and/or reservoir size.
Conclusions
We provide the BEAT-HIV Collaboratory view for prioritization of currently available viral measures in the design of HIV cure-directed clinical trials as follows (Figure 1):
For a 100 ml blood draw prioritize the IPDA or Q4PCR (pending validation of either assay), followed by cell-associated RNA, then an inducible p24 assay, to be followed by measuring levels of integrated HIV DNA.
For leukapheresis products first prioritize Q4PCR or IPDA, then cell-associated RNA, followed with inducible p24 assay, and then confirmatory assays using either QVOA p24 SIMOA or QVOA cfRNA. We also support the TILDA when evaluating strategies aimed to stimulate or block HIV transcription. We further support the usage of the mVOA assay as a confirmatory test if there is a lack of replication-competent virus or HIV DNA/RNA and an absence of an ATI in the trial design. If resources are available and the strategy is expected to result in a subset of controllers, we support the inclusion of full-genome sequencing, including integration site and orientation analysis in order to investigate the qualitative potential for HIV reactivation by residual reservoirs that persists after viral control.
For tissues, prioritize the IPDA or Q4PCR, then DNA/RNAscope in situ hybridization, followed by both cell-associated RNA and inducible p24 assay with added measures for levels of HIV DNA, where feasible. Importantly, HIV reservoir reduction determined by any of the aforementioned assays does not indicate potential durable viral control or eradication without the inclusion of an ATI in trial design as no viral measurement has yet predicted a lack of viral rebound after discontinuation of ART.
It is also important to note that “one size does not fit all” as the interpretation of viral measures (latent or active reservoirs) on ART and how they are to be prioritized and analyzed during HIV cure-related clinical trials may differ relative to the mechanism anticipated for viral clearance; emerging methods for added measures of the HIV reservoirs using single-cell technologies; and resources available and sample availability. In conclusion, study plans need to carefully weigh the ultimate mechanistic insight and impact of performing any selected assay relative to the value of banking samples for future interrogation.
BOX 1: BEAT-HIV Collaboratory.
The BEyond Anti-retroviral Therapy Martin Delaney Collaboratory to Cure HIV-1 Infection by Combination Immunotherapy (BEAT-HIV Collaboratory) is a consortium of HIV researchers from leading academic research institutions working with government, nonprofit organizations, and industry partners. BEAT-HIV Collaboratory objectives are to advance basic cure-directed research and to conduct HIV cure-directed clinical trials using immune-based therapies. The BEAT-HIV Collaboratory is supported by NIAID, NIMH, NINDS, and NIDA, and The Philadelphia Foundation. For added information and full membership go to beat-hiv.org.
BOX 2: Key terms.
HIV reservoir:
collective term for cells infected with replication-competent HIV in blood and various anatomical sites that persist despite long-term antiretroviral therapy and cause viral rebound upon therapy interruption.
HIV latent reservoir:
the major component of the HIV reservoir and defined as quiescent cells carrying an integrated copy of the viral genome that does not express viral transcripts or proteins.
HIV active reservoir:
A minor population of transcriptionally-active cells, producing HIV RNA/proteins and possibly virions, that can be detected in people living with HIV despite long-term therapy.
HIV proviral/provirus:
viral DNA integrated into the infected cell genome.
Near full-length HIV proviral sequencing:
Sequencing of almost the entire integrated viral genome within the infected cell genome.
Intact HIV proviruses or genomes:
HIV proviral sequence that does not contain massive deletions or mutations that can render them defective, and therefore these intact proviruses have a high potential for replication competency.
Defective HIV provirus:
HIV provirus that is inserted into the genome of an infected cell yet harbors mutations and/or deletions rendering them defective and unable to replicate
Inducible HIV provirus:
HIV provirus that able to be induced to produce viral RNA, viral protein, and/or virions.
Transcriptionally-competent HIV provirus:
HIV provirus that can produce viral RNA transcripts.
Translationally-competent HIV provirus:
HIV provirus that can produce viral proteins (such as p24).
Replication-competent HIV provirus:
HIV provirus that can produce and release virions able to replicate, resulting in productive infection and viral amplification.
Clonal HIV proviral expansion:
Expansion of a specific HIV provirus due to the proliferation of an HIV-infected cell carrying this provirus.
Outgrowth assays:
Methods to induce expression/replication of HIV ex vivo using cells obtained from an individual living with HIV (typically ART-suppressed individual).
Analytical Therapy Interruption (ATI):
cessation of antiretroviral therapy followed by monitoring viral rebound.
ACKNOWLEDGMENTS
This work was supported by the NIH-funded BEAT-HIV Martin Delaney Collaboratory to cure HIV-1 infection (1UM1Al126620). LJM is also supported by NIH R01 AI065279, U01 AI065279, R01 DA048728, R01 DA049666, Kean Family Professorship, and the Philadelphia Foundation (Roberts I. Jacobs Fund). M-AM is supported by NIH grants (DK123733, AG062383, NS117458, AI143385, AI129636, and NS106970), The Foundation for AIDS Research (amfAR) impact grant # 109840–65-RGRL, and W.W. Smith Charitable Trust grant # A1901, Wistar Cancer Center Support Grant P30 CA010815–49S2, and the Penn Center for AIDS Research (AI 045008). MJB is supported by The Miguel Servet program funded by the Spanish Health Institute Carlos III (CP17/00179). M. L. Is supported by NIH grants AI117841, AI120008, AI124776, AI130005, AI122377, and AI135940. XGY is supported by NIH grants AI116228, AI078799, HL134539, AI125109, and DA047034. RS supported by AI126603, AI126620 and AI12661, AI094189, 43222 Howard Hughes Medical Institute, and the Bill and Melinda Gates Foundation (OPP1115715). VP supported by AI143567, AI124843. Y-C Ho supported by Yale Top Scholar, Rudolf J. Anderson Fellowship, AI141009, DA047037, AI118402, W.W. Smith AIDS Research Grant, Gilead AIDS Research Grant, Gilead Research Scholar Grant, AI150464, AI094189, AI14868. J.D.E is supported by NIH and the Bill and Melinda Gates Foundation grants 75N93019C00070, AI133706, AI110164, AI141258, AI143411, AI149672, CA206466, DK119945, INV-002704, and OD011092–60, and OPPO1108533.
Footnotes
Competing interests:
BH, DH are employees of Merck & Co.; RFS is an inventor on a patent application on the IPDA filed by JHU and licensed to AccelevirDx (RFS holds no equity on AccelevirDx).
References and Notes:
- 1.Chargin A, et al. Identification and characterization of HIV-1 latent viral reservoirs in peripheral blood. Journal of clinical microbiology 53, 60–66 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chun TW, et al. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proceedings of the National Academy of Sciences of the United States of America 95, 8869–8873 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong JK, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278, 1291–1295 (1997). [DOI] [PubMed] [Google Scholar]
- 4.Wandeler G, Johnson LF & Egger M Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: comparisons with general population. Current opinion in HIV and AIDS 11, 492–500 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biggar RJ, Chaturvedi AK, Goedert JJ, Engels EA & Study HACM AIDS-related cancer and severity of immunosuppression in persons with AIDS. Journal of the National Cancer Institute 99, 962–972 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Carbone A, Volpi CC, Gualeni AV & Gloghini A Epstein-Barr virus associated lymphomas in people with HIV. Current opinion in HIV and AIDS 12, 39–46 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Zucchetto A, et al. Non-AIDS-Defining Cancer Mortality: Emerging Patterns in the Late HAART Era. Journal of acquired immune deficiency syndromes 73, 190–196 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Rodger AJ, et al. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. Aids 27, 973–979 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Eisele E & Siliciano RF Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity 37, 377–388 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crooks AM, et al. Precise Quantitation of the Latent HIV-1 Reservoir: Implications for Eradication Strategies. The Journal of infectious diseases 212, 1361–1365 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finzi D, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nature medicine 5, 512–517 (1999). [DOI] [PubMed] [Google Scholar]
- 12.Chun TW, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387, 183–188 (1997). [DOI] [PubMed] [Google Scholar]
- 13.Richman DD, et al. The challenge of finding a cure for HIV infection. Science 323, 1304–1307 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Banga R, et al. PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nature medicine 22, 754–761 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Chun TW, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. The Journal of infectious diseases 197, 714–720 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Yukl SA, et al. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. The Journal of infectious diseases 202, 1553–1561 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estes JD, et al. Defining total-body AIDS-virus burden with implications for curative strategies. Nat Med 23, 1271–1276 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pantaleo G, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature 362, 355–358 (1993). [DOI] [PubMed] [Google Scholar]
- 19.Bruner KM, et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nature medicine 22, 1043–1049 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho YC, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155, 540–551 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300 (1997). [DOI] [PubMed] [Google Scholar]
- 22.Siliciano JD, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 9, 727–728 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Hosmane NN, et al. Proliferation of latently infected CD4(+) T cells carrying replication-competent HIV-1: Potential role in latent reservoir dynamics. The Journal of experimental medicine 214, 959–972 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenzi JC, et al. Paired quantitative and qualitative assessment of the replication-competent HIV-1 reservoir and comparison with integrated proviral DNA. Proceedings of the National Academy of Sciences of the United States of America 113, E7908–E7916 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee GQ, et al. Clonal expansion of genome-intact HIV-1 in functionally polarized Th1 CD4+ T cells. The Journal of clinical investigation 127, 2689–2696 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinzone MR, et al. Longitudinal HIV sequencing reveals reservoir expression leading to decay which is obscured by clonal expansion. Nat Commun 10, 728 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruner KM, et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 566, 120–125 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaebler C, et al. Combination of quadruplex qPCR and next-generation sequencing for qualitative and quantitative analysis of the HIV-1 latent reservoir. J Exp Med 216, 2253–2264 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peluso MJ, et al. Differential decay of intact and defective proviral DNA in HIV-1-infected individuals on suppressive antiretroviral therapy. JCI Insight 5(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon KJ, et al. Different human resting memory CD4(+) T cell subsets show similar low inducibility of latent HIV-1 proviruses. Sci Transl Med 12(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moron-Lopez S, et al. Switching From a Protease Inhibitor-based Regimen to a Dolutegravir-based Regimen: A Randomized Clinical Trial to Determine the Effect on Peripheral Blood and Ileum Biopsies From Antiretroviral Therapy-suppressed Human Immunodeficiency Virus-infected Individuals. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 69, 1320–1328 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moron-Lopez S, et al. Sensitive quantification of the HIV-1 reservoir in gut-associated lymphoid tissue. PloS one 12, e0175899 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metcalf Pate KA, et al. A Murine Viral Outgrowth Assay to Detect Residual HIV Type 1 in Patients With Undetectable Viral Loads. The Journal of infectious diseases 212, 1387–1396 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henrich TJ, et al. HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study. PLoS medicine 14, e1002417 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewinski MK, et al. Genome-wide analysis of chromosomal features repressing human immunodeficiency virus transcription. J Virol 79, 6610–6619 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherrill-Mix S, et al. HIV latency and integration site placement in five cell-based models. Retrovirology 10, 90 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroder AR, et al. HIV-1 integration in the human genome favors active genes and local hotspots. Cell 110, 521–529 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Wang GP, Ciuffi A, Leipzig J, Berry CC & Bushman FD HIV integration site selection: analysis by massively parallel pyrosequencing reveals association with epigenetic modifications. Genome Res 17, 1186–1194 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Einkauf KB, et al. Intact HIV-1 proviruses accumulate at distinct chromosomal positions during prolonged antiretroviral therapy. J Clin Invest 129, 988–998 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohn LB, et al. HIV-1 integration landscape during latent and active infection. Cell 160, 420–432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patro SC, et al. Combined HIV-1 sequence and integration site analysis informs viral dynamics and allows reconstruction of replicating viral ancestors. Proc Natl Acad Sci U S A 116, 25891–25899 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cesana D, et al. HIV-1-mediated insertional activation of STAT5B and BACH2 trigger viral reservoir in T regulatory cells. Nat Commun 8, 498 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu G, et al. HDAC inhibition induces HIV-1 protein and enables immune-based clearance following latency reversal. JCI Insight 2(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pitman MC, Lau JSY, McMahon JH & Lewin SR Barriers and strategies to achieve a cure for HIV. The lancet. HIV 5, e317–e328 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pollack RA, et al. Defective HIV-1 Proviruses Are Expressed and Can Be Recognized by Cytotoxic T Lymphocytes, which Shape the Proviral Landscape. Cell host & microbe 21, 494–506 e494 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imamichi H, et al. Defective HIV-1 proviruses produce viral proteins. Proceedings of the National Academy of Sciences of the United States of America 117, 3704–3710 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Passaes CPB, et al. Ultrasensitive HIV-1 p24 Assay Detects Single Infected Cells and Differences in Reservoir Induction by Latency Reversal Agents. Journal of virology 91(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai Y, et al. BCL6 Inhibitor-Mediated Downregulation of Phosphorylated SAMHD1 and T Cell Activation Are Associated with Decreased HIV Infection and Reactivation. J Virol 93(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fidler S, et al. Antiretroviral therapy alone versus antiretroviral therapy with a kick and kill approach, on measures of the HIV reservoir in participants with recent HIV infection (the RIVER trial): a phase 2, randomised trial. Lancet 395, 888–898 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Ruiz A, et al. Antigen Production After Latency Reversal and Expression of Inhibitory Receptors in CD8+ T Cells Limit the Killing of HIV-1 Reactivated Cells. Frontiers in immunology 9, 3162 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bertagnolli LN, et al. The role of CD32 during HIV-1 infection. Nature 561, E17–E19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Descours B, et al. CD32a is a marker of a CD4 T-cell HIV reservoir harbouring replication-competent proviruses. Nature 543, 564–567 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Abdel-Mohsen M, et al. CD32 is expressed on cells with transcriptionally active HIV but does not enrich for HIV DNA in resting T cells. Sci Transl Med 10(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baxter AE, et al. Multiparametric characterization of rare HIV-infected cells using an RNA-flow FISH technique. Nature protocols 12, 2029–2049 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grau-Exposito J, et al. A Novel Single-Cell FISH-Flow Assay Identifies Effector Memory CD4(+) T cells as a Major Niche for HIV-1 Transcription in HIV-Infected Patients. mBio 8(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pardons M, et al. Single-cell characterization and quantification of translation-competent viral reservoirs in treated and untreated HIV infection. PLoS Pathog 15, e1007619 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cillo AR, et al. Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proceedings of the National Academy of Sciences of the United States of America 111, 7078–7083 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plantin J, Massanella M & Chomont N Inducible HIV RNA transcription assays to measure HIV persistence: pros and cons of a compromise. Retrovirology 15, 9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Massanella M, et al. Improved assays to measure and characterize the inducible HIV reservoir. EBioMedicine 36, 113–121 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen HC, Martinez JP, Zorita E, Meyerhans A & Filion GJ Position effects influence HIV latency reversal. Nature structural & molecular biology 24, 47–54 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Pasternak AO, et al. Highly sensitive methods based on seminested real-time reverse transcription-PCR for quantitation of human immunodeficiency virus type 1 unspliced and multiply spliced RNA and proviral DNA. J Clin Microbiol 46, 2206–2211 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pasternak AO, Lukashov VV & Berkhout B Cell-associated HIV RNA: a dynamic biomarker of viral persistence. Retrovirology 10, 41 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yukl SA, et al. HIV latency in isolated patient CD4(+) T cells may be due to blocks in HIV transcriptional elongation, completion, and splicing. Sci Transl Med 10(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li JZ, et al. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. Aids 30, 343–353 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pasternak AO, et al. Cell-associated HIV-1 RNA predicts viral rebound and disease progression after discontinuation of temporary early ART. JCI Insight 5(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Procopio FA, et al. A Novel Assay to Measure the Magnitude of the Inducible Viral Reservoir in HIV-infected Individuals. EBioMedicine 2, 874–883 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dinoso JB, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci U S A 106, 9403–9408 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hong F, et al. Associations between HIV-1 DNA copy number, proviral transcriptional activity, and plasma viremia in individuals off or on suppressive antiretroviral therapy. Virology 521, 51–57 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Palmer S, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol 41, 4531–4536 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deleage C, Chan CN, Busman-Sahay K & Estes JD Next-generation in situ hybridization approaches to define and quantify HIV and SIV reservoirs in tissue microenvironments. Retrovirology 15, 4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deleage C, et al. Defining HIV and SIV Reservoirs in Lymphoid Tissues. Pathog Immun 1, 68–106 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Doherty U, Swiggard WJ, Jeyakumar D, McGain D & Malim MH A sensitive, quantitative assay for human immunodeficiency virus type 1 integration. Journal of virology 76, 10942–10950 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Strain MC & Richman DD New assays for monitoring residual HIV burden in effectively treated individuals. Curr Opin HIV AIDS 8, 106–110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu JJ, et al. A more precise HIV integration assay designed to detect small differences finds lower levels of integrated DNA in HAART treated patients. Virology 379, 78–86 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eriksson S, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS pathogens 9, e1003174 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Williams JP, et al. HIV-1 DNA predicts disease progression and post-treatment virological control. Elife 3, e03821 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Emmanouil Papasavvas LA, Ross Brian N., Fair Matthew, Yuan Zhe, Gyampoh Kwasi, Mackiewicz Agnieszka, Sciorillo Amanda C., Paggliuzza Amelie, Lada Steven, Wu Guoxin, Shih Lin Goh Carolyn Banck-Teets, Holder Daniel J., Zuck Paul D., Damra Mohammad, Lynn Kenneth M., Tebas Pablo, Mounzer Karam, Kostman Jay R., Mohamed Abdel-Mohsen Douglas Richman, Chomont Nicolas, Howell Bonnie J., Montaner Luis J.. Intact HIV reservoir associates with levels of total and integrated proviruses in the blood during suppressive antiretroviral therapy. Clinical Infectious Diseases in press (2020). [DOI] [PMC free article] [PubMed]
- 78.Lada SM, et al. Quantitation of Integrated HIV Provirus by Pulsed-Field Gel Electrophoresis and Droplet Digital PCR. Journal of clinical microbiology 56(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liszewski MK, Yu JJ & O’Doherty U Detecting HIV-1 integration by repetitive-sampling Alu-gag PCR. Methods 47, 254–260 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vandergeeten C, et al. Cross-clade ultrasensitive PCR-based assays to measure HIV persistence in large-cohort studies. Journal of virology 88, 12385–12396 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Butler SL, Hansen MS & Bushman FD A quantitative assay for HIV DNA integration in vivo. Nat Med 7, 631–634 (2001). [DOI] [PubMed] [Google Scholar]
- 82.Lee E, et al. Memory CD4 + T-Cells Expressing HLA-DR Contribute to HIV Persistence During Prolonged Antiretroviral Therapy. Frontiers in microbiology 10, 2214 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chomont N, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 15, 893–900 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zerbato JM, McMahon DK, Sobolewski MD, Mellors JW & Sluis-Cremer N Naive CD4+ T Cells Harbor a Large Inducible Reservoir of Latent, Replication-competent Human Immunodeficiency Virus Type 1. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 69, 1919–1925 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abreu CM, et al. Myeloid and CD4 T Cells Comprise the Latent Reservoir in Antiretroviral Therapy-Suppressed SIVmac251-Infected Macaques. mBio 10(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ganor Y, et al. HIV-1 reservoirs in urethral macrophages of patients under suppressive antiretroviral therapy. Nat Microbiol 4, 633–644 (2019). [DOI] [PubMed] [Google Scholar]
- 87.Chaillon A, et al. HIV persists throughout deep tissues with repopulation from multiple anatomical sources. The Journal of clinical investigation 130, 1699–1712 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gondim M, S.S.-M., Saag M, Nussenzweig M, Silicano J, Sharp P, Borrow P, Montaner L, Bar1 K, Hahn B. MARKED VARIATION IN THE SUSCEPTIBILITY OF HIV-1 TO TYPE 1 INTERFERON INHIBITION DURING EARLY, LATE AND REBOUND INFECTION. Journal of Virus Eradication 5 supplement 3(2019). [Google Scholar]
- 89.Andrade VM, et al. A minor population of macrophage-tropic HIV-1 variants is identified in recrudescing viremia following analytic treatment interruption. Proc Natl Acad Sci U S A 117, 9981–9990 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hatano H, et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. The Journal of infectious diseases 208, 50–56 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chew GM, et al. TIGIT Marks Exhausted T Cells, Correlates with Disease Progression, and Serves as a Target for Immune Restoration in HIV and SIV Infection. PLoS Pathog 12, e1005349 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fromentin R, et al. CD4+ T Cells Expressing PD-1, TIGIT and LAG-3 Contribute to HIV Persistence during ART. PLoS pathogens 12, e1005761 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abdel-Mohsen M, et al. Select host restriction factors are associated with HIV persistence during antiretroviral therapy. Aids 29, 411–420 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Keating SM, et al. HIV Antibody Level as a Marker of HIV Persistence and Low-Level Viral Replication. The Journal of infectious diseases 216, 72–81 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burbelo PD, et al. HIV antibody characterization as a method to quantify reservoir size during curative interventions. J Infect Dis 209, 1613–1617 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yukl SA, et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS pathogens 9, e1003347 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luzuriaga K, et al. Viremic relapse after HIV-1 remission in a perinatally infected child. The New England journal of medicine 372, 786–788 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hill AL, Rosenbloom DI, Fu F, Nowak MA & Siliciano RF Predicting the outcomes of treatment to eradicate the latent reservoir for HIV-1. Proceedings of the National Academy of Sciences of the United States of America 111, 13475–13480 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tebas P, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. The New England journal of medicine 370, 901–910 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cohen YZ, et al. Relationship between latent and rebound viruses in a clinical trial of anti-HIV-1 antibody 3BNC117. The Journal of experimental medicine 215, 2311–2324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Salantes DB, et al. HIV-1 latent reservoir size and diversity are stable following brief treatment interruption. J Clin Invest 128, 3102–3115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vibholm LK, et al. Characterization of Intact Proviruses in Blood and Lymph Node from HIV-Infected Individuals Undergoing Analytical Treatment Interruption. Journal of virology 93(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.De Scheerder MA, et al. HIV Rebound Is Predominantly Fueled by Genetically Identical Viral Expansions from Diverse Reservoirs. Cell host & microbe 26, 347–358 e347 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pacanowski J, et al. Early plasmacytoid dendritic cell changes predict plasma HIV load rebound during primary infection. The Journal of infectious diseases 190, 1889–1892 (2004). [DOI] [PubMed] [Google Scholar]
- 105.Papasavvas E, et al. Plasmacytoid dendritic cell and functional HIV Gag p55-specific T cells before treatment interruption can inform set-point plasma HIV viral load after treatment interruption in chronically suppressed HIV-1(+) patients. Immunology 145, 380–390 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tomescu C, et al. A correlate of HIV-1 control consisting of both innate and adaptive immune parameters best predicts viral load by multivariable analysis in HIV-1 infected viremic controllers and chronically-infected non-controllers. PloS one 9, e103209 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Giron LB, et al. Plasma and antibody glycomic biomarkers of time to HIV rebound and viral setpoint. Aids 34, 681–686 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Papasavvas E, et al. Analytical antiretroviral therapy interruption does not irreversibly change preinterruption levels of cellular HIV. Aids 32, 1763–1772 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Lunzen J & Hoffmann C Virological rebound and its consequences during treatment interruption. Curr Opin HIV AIDS 2, 1–5 (2007). [DOI] [PubMed] [Google Scholar]
- 110.Julg B, et al. Recommendations for analytical antiretroviral treatment interruptions in HIV research trials-report of a consensus meeting. The lancet. HIV 6, e259–e268 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chun TW, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A 94, 13193–13197 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Laird GM, et al. Rapid quantification of the latent reservoir for HIV-1 using a viral outgrowth assay. PLoS Pathog 9, e1003398 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee SK, et al. Quantification of the Latent HIV-1 Reservoir Using Ultra Deep Sequencing and Primer ID in a Viral Outgrowth Assay. Journal of acquired immune deficiency syndromes 74, 221–228 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sherman E, et al. INSPIIRED: A Pipeline for Quantitative Analysis of Sites of New DNA Integration in Cellular Genomes. Mol Ther Methods Clin Dev 4, 39–49 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Berry CC, et al. INSPIIRED: Quantification and Visualization Tools for Analyzing Integration Site Distributions. Mol Ther Methods Clin Dev 4, 17–26 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cabrera C, Chang L, Stone M, Busch M & Wilson DH Rapid, Fully Automated Digital Immunoassay for p24 Protein with the Sensitivity of Nucleic Acid Amplification for Detecting Acute HIV Infection. Clin Chem 61, 1372–1380 (2015). [DOI] [PubMed] [Google Scholar]
- 117.Bullen CK, Laird GM, Durand CM, Siliciano JD & Siliciano RF New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med 20, 425–429 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kumar AM, Borodowsky I, Fernandez B, Gonzalez L & Kumar M Human immunodeficiency virus type 1 RNA Levels in different regions of human brain: quantification using real-time reverse transcriptase-polymerase chain reaction. Journal of neurovirology 13, 210–224 (2007). [DOI] [PubMed] [Google Scholar]


