Abstract
IMPORTANCE
Cutaneous manifestations of chronic graft-vs-host disease (GvHD) are highly variable and may recapitulate well-characterized autoimmune diseases, including systemic sclerosis and Sjögren syndrome. However, vitiligo and alopecia areata (AA) have not been well characterized in the chronic GvHD setting.
OBJECTIVE
To determine laboratory markers, transplant-related factors, and other systemic manifestations associated with vitiligo and/or AA in patients with chronic GvHD.
DESIGN, SETTING, AND PARTICIPANTS
A cross-sectional, retrospective study conducted by the National Institutes of Health (NIH) of 282 adult and pediatric patients with chronic GvHD seen under the NIH natural history protocol between 2004 and 2013.
MAIN OUTCOMES AND MEASURES
Demographic, clinical, and laboratory data, including measures of 11 antibodies, were included in the analysis. Patients with vitiligo and/or AA were identified from dermatologist documentation and photographic evidence. Univariate and multivariable logistic regression analyses were used to determine risk factors for vitiligo and AA development.
RESULTS
Fifteen (5.3%) of 282 patients demonstrated vitiligo (14 of 282; 4.9%) and/or AA (2 of 282; 0.7%) (1 patient had both vitiligo and AA). Univariate analysis identified female donor to male recipient sex mismatch (P = .003), positive test results for anticardiolipin (ACA) IgG (P = .03) or antiparietal antibody (P = .049), elevated CD19 level (P = .045), and normal or elevated IgG level (P = .02) as risk factors for vitiligo or AA. Female donor to male recipient sex mismatch (P = .003) and positive findings for ACA-IgG (P = .01) retained significance in the multivariable analysis.
CONCLUSIONS AND RELEVANCE
Female donor and female donor to male recipient sex mismatch, in particular, are significantly associated with the development of vitiligo and/or AA. Further studies are needed to explore transplant-related risk factors that may lead to better understanding of the pathomechanisms of chronic GvHD.
Chronic graft-vs-host disease (GvHD) is one of the most frequent and devastating complications arising after allogeneic hematopoietic stem cell transplantation(HSCT) and is the major cause of mortality and late non–relapse-associated morbidity in long-term survivors.1 Occurring in up to 80% of allogeneic HSCT recipients,2 chronic GvHD is a multiorgan disease that is associated with immune dysfunction and often significantly impacts quality of life.3 The skin is the most commonly affected organ—presentations range from nonsclerotic epidermal involvement (such as lichen planus–like eruptions or poikiloderma) to morphea-like or deep sclerotic disease resembling fasciitis.4
The underlying biology of chronic GvHD has not been fully elucidated; however, many of its cutaneous and histologic features recapitulate those of well-characterized autoimmune diseases such as systemic sclerosis and Sjögren syndrome. Other autoimmune manifestations, including autoimmune cytopenias, myasthenia gravis, and autoimmune thyroid diseases, are also increasingly recognized after allogeneic HSCT.5 Several case reports and small series have reported vitiligo6–19 or alopecia areata (AA)18–20 following HSCT, most occurring in the setting of GvHD, further supporting the role of GvHD in the development of cutaneous autoimmune disease.21 However, the frequency of skin autoimmune manifestations and associated risk factors have not been well described.
In this retrospective cross-sectional analysis, we examine the prevalence of autoantibodies and other risk factors for the development of vitiligo and/or AA in a cohort of 282 patients with chronic GvHD who were comprehensively evaluated as part of a National Institutes of Health (NIH) chronic GvHD natural history study.
Methods
The study was approved by the institutional review board of the National Cancer Institute, and all participants provided written informed consent.
Patient Population and Chronic GvHD Assessment
A total of 282 adult and pediatric patients with a diagnosis of chronic GvHD, as defined by the NIH Consensus Group Criteria,22 and referred to the NIH Clinical Center between 2004 and 2013 were included in this cross-sectional analysis (Figure 1). All participants were enrolled in an NIH chronic GvHD natural history protocol (clinicaltrials.gov Identifier: NCT00331968) and were comprehensively evaluated by a multidisciplinary team during a week-long visit in which demographic, clinical, photographic, imaging, and laboratory data were obtained.23 Comprehensive skin assessment included full body skin examination, body surface area scoring, and skin biopsy.
Figure 1.
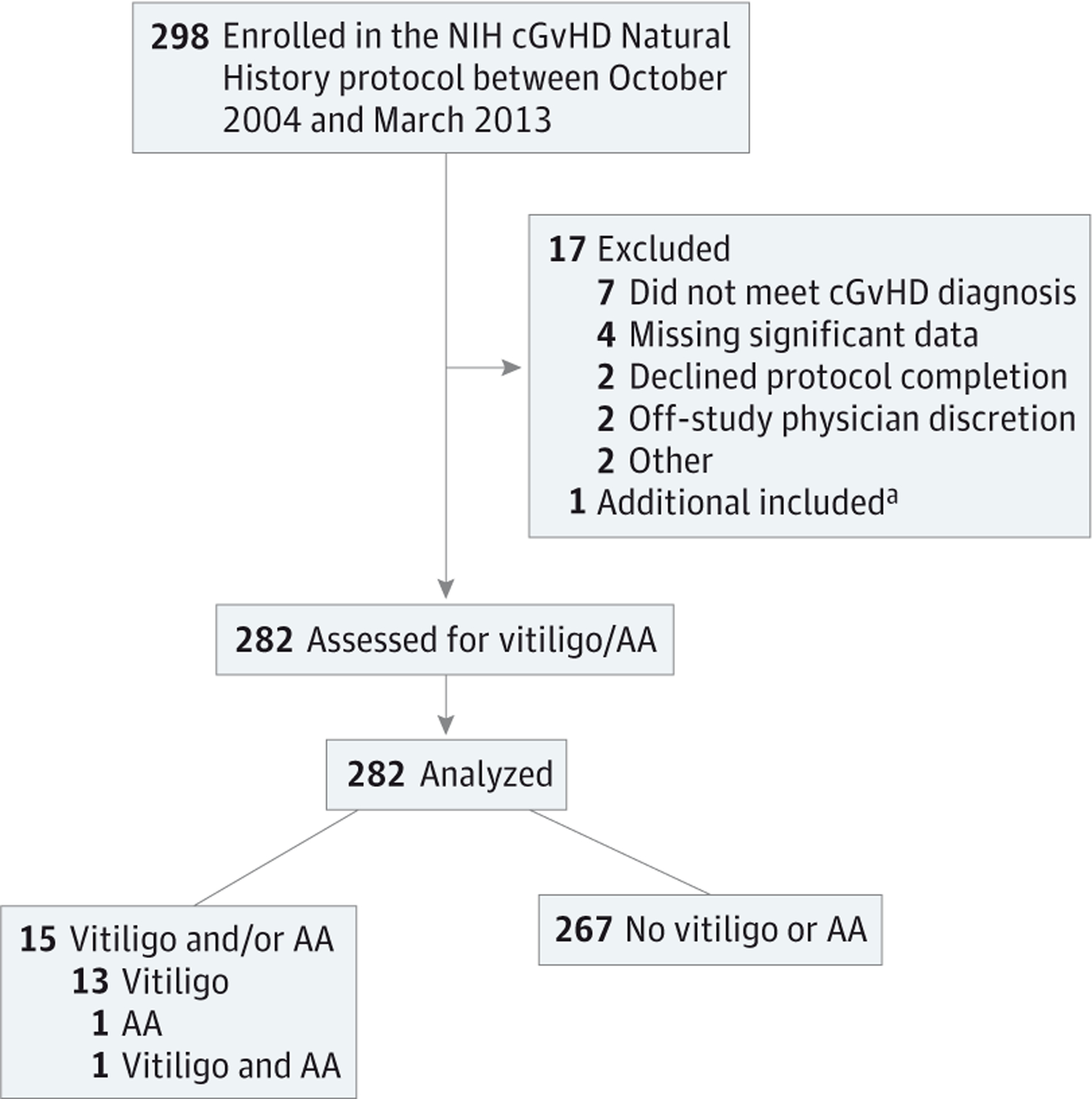
Flow Diagram for Patient Enrollment and Progress Through the Study
AA indicates alopecia areata; cGvHD, chronic graft-vs-host disease; NIH, National Institutes of Health.
aOne additional patient (with vitiligo) was identified for inclusion in this study just prior to statistical analysis.
Participants with vitiligo and/or AA were compared with participants in the cohort with documented chronic GvHD of the skin or other organ system who did not manifest vitiligo or AA. Diagnosis of skin chronic GvHD was determined by NIH Consensus Criteria.22 Diagnostic skin manifestations included poikiloderma, lichen planus–like eruption, morphea-like superficial sclerotic features, lichen sclerosus (LS)-like lesions, and deep sclerotic features. According to NIH Consensus Criteria, pigmentary changes and alopecia are not a diagnostic feature of chronic GvHD—that is, they may occur in the setting of chronic GvHD, but other histologic or laboratory criteria in the skin or other organs are required to render a diagnosis of chronic GvHD.22
The onset of chronic GvHD was classified as quiescent, de novo, or progressive according to history of prior acute GvHD. Quiescent onset was defined as the occurrence of chronic GvHD after complete resolution of acute GvHD; de novo onset indicated the appearance of chronic GvHD with no prior acute GvHD; and progressive onset indicated the onset of chronic GvHD without resolution of existing acute GvHD. The NIH Global Severity Score reflects the number of organs or sites affected with chronic GvHD, the disease severity at each affected organ, and the clinical effects of this involvement on the patient’s functional status.22 The NIH scores were categorized as mild (1–2 organs with a score of 1), moderate (≥3 organs with a score 1, any organ with a score of 2, or a lung with a score of 1), and severe (any organ with a score of 3 or a lung with a score ≥2).
Statistical Analysis
Univariate analysis was initially performed to screen for associations between factors of interest and development of vitiligo and/or AA. Relevant patient demographics, transplant history, chronic GvHD characteristics, and laboratory data were analyzed. Eleven autoantibodies were examined: antinuclear antibody, rheumatoid factor, anticyclic citrullinated peptides, antiparietal, anticentromere, antiribonucleoprotein, anti-smith, anticardiolipin (ACA) IgM, ACA-IgG, antiex-tractable nuclear antigens, and anti-thyroid peroxidase auto-antibodies. Comparisons were made between patients with vitiligo and/or AA and patients without vitiligo and/or AA using the Wilcoxon rank sum test for continuous variables. The Cochran-Armitage test was used for ordered categorical variables,24 and the Mehta modification of the Fisher exact test was used for unordered categorical variables.25 Dichotomous variables were compared between the 2 groups using the Fisher exact test. Following an initial screening by univariate methods, multivariable logistic regression analysis was used to identify factors associated with the development of vitiligo and/or AA.
In view of the number of univariate tests performed, a 2-tailed P value such that P < .005 was considered statistically significant. All P values reported are 2 tailed and presented without any formal adjustment for multiple comparisons.
Results
Patient Demographic and Clinical Characteristics and Laboratory Parameters
Fifteen (5.3%) of 282 participants with vitiligo (14 of 282; 4.9%) and/or AA (2 of 282; 0.7%) were identified among 282 patients with chronic GvHD (Table 1). One patient had both vitiligo and AA. The median age of affected participants at enrollment was 38 years (age range, 9–69 years), and there was a male preponderance (10 of 15; 66.7%). Three patients (20.0%) were younger than 18 years. The most common indications for transplantation were chronic myelogenous leukemia (CML) (5 of 15; 33.3%) and acute leukemia or myelodysplastic syndrome (5 of 15; 33.3%). Most patients (13 of 15; 86.7%) had an HLA-identical donor and received peripheral blood stem cell transplantation (9 of 15; 60.0%). Eleven patients (73.3%) manifested concomitant skin chronic GvHD at the time of evaluation, most often sclerotic-type chronic GvHD (9 of 15; 60.0%). In 5 patients with documented onset of skin depigmentation, pigmentary changes developed a median of 41 months (range, 24–84 months) after transplant. Depigmentation was classic periorbital, perioral, acrofacial involvement in 6 patients (Figure 2A), generalized in 6 patients, and torso predominant in 2 patients. Trichrome vitiligo was present in 3 patients (Figure 2B), and poliosis occurred in 5 patients. Skin biopsies from depigmented skin were performed in 3 patients, revealing an absence of melanocytes in the basal layer confirmed by Melan-A immunohistochemical analysis. Hair loss was localized to the scalp in both patients with AA (Figure 2C).
Table 1.
Characteristics of Patients With cGvHD and Vitiligo and/or AA
| Vitiligo and/or AA | cGvHD Involvement | |||||||
|---|---|---|---|---|---|---|---|---|
| Patient No./Sexa,b,c | Disease | Transplant Conditioning | Graft Type | Time to Occurrence, mo | Distribution | Vitiligo Type | Skin | Other Organs |
| 1/M | CML | Bu/Cyc | BM | 24 | Perioral, torso, R buttock, pretibial legs | Torso | Epid | Eyes, lungs, liver |
| 2/M | APL | … | PB | … | Periorbital/oral, head/neck, back, fingers | Classicd | Bothe | Eyes, oral, GI |
| 3/F | FNHL | Flu/Cyc | PB | … | Periorbital, perioral, L cheek, back | Classicd | Epid | Eyes |
| 4/M | RMSA | Flu/Cyc/Mel | PB | … | B/L cheeks, head/neck, upper chest, UE, LE | Generalized | Bothe | Eyes, oral, lungs, joint |
| 5/M | CML | Bu/Cyc/VP-16 | BM | … | Perioral | Classicd | Bothe | Eyes, oral, GI, joint |
| 6/M | CML | Bu/Flu/ATG | BM | 36 | Perioral, cheeks, acral, UE, genitalia | Classicd | Bothe | Eyes, oral, GI, liver, genital |
| 7/F | CML | Bu/Flu/ATG | PB | … | Trichrome face, chest, upper back, B/L thighs | Classicd | None | Eyes, oral, lungs, GI, joint |
| 8/Ma,b | WAS | … | Cord | … | Diffuse trichrome vitiligo | Generalized | Bothe | Lungs, joint |
| 9/F | AML | Flu/TBI | PB | 36 | Face, chest, lower back, B/LUE, LE | Generalized | Scl | Eyes, oral, lungs, GI, joint |
| 10/Fb,c | AML | Flu/Cyc/TBI | Cord | … | Vitiligo: face, postauricular, neck, trunk, UE, LE AA: scalp vertex | Generalized | Epid | Lungs, vulvovaginal |
| 11/M | CLL | Flu/Cyc | PB | … | Face, scalp, neck, anterior trunk | Generalized | Bothe | Eyes, oral, liver, joint |
| 12/M | B-cell ALL | Cyc/TBI | PB | … | Periorbital, perioral, scalp, chin | Classicd | Epid | Oral, lungs |
| 13/F | CTCL | Flu/Mel | PB | … | Neck, trunk, proximal UE | Torso | Bothe | Eyes, lungs, GI, liver, genital |
| 14/M | CML | Bu/Cyc | PB | 84 | Trichrome face, trunk, B/LUE | Generalized | Bothe | Eyes, oral, lungs, liver |
| 15/Mc | ALL | Cyc/VP-16/TBI | BM | 24 | Scalp | NA | None | Lungs, GI |
Abbreviations: AA, alopecia areata; ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; APL, acute promyelocytic leukemia; ATG, antithymocyte globulin; B/L, bilateral; BM, bone marrow; Bu, busulfan; cGvHD, chronic graft-vs-host disease; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; CTCL, cutaneous T-cell lymphoma; Cyc, cyclophosphamide; epid, epidermal; Flu, fludarabine; FNHL, follicular non-Hodgkin lymphoma; GI, gastrointestinal tract; L, left; LE, lower extremities; Mel; melphalan; NA, not applicable; PB, peripheral blood; R, right; RMSA, rhabdomyosarcoma; Scl, sclerotic; TBI, total body irradiation; UE, upper extremities; VP-16, etoposide; WAS, Wiskott-Aldrich syndrome; …, not known.
All patients whose donor sex was known received transplants from female donors; donor sex for patient 8 was not reported.
Thirteen of the 15 patients had an HLA-identical donor; a mismatch occurred only with patients 8 and 10.
Thirteen of the 15 patients had only vitiligo; patient 10 had both vitiligo and AA; patient 15 had only AA.
Classic indicates predominantly perioral, periorbital, and acrofacial involvement; torso, predominantly truncal involvement; generalized, diffuse involvement.
Both indicates both sclerotic and epidermal cGvHD involvement.
Figure 2.
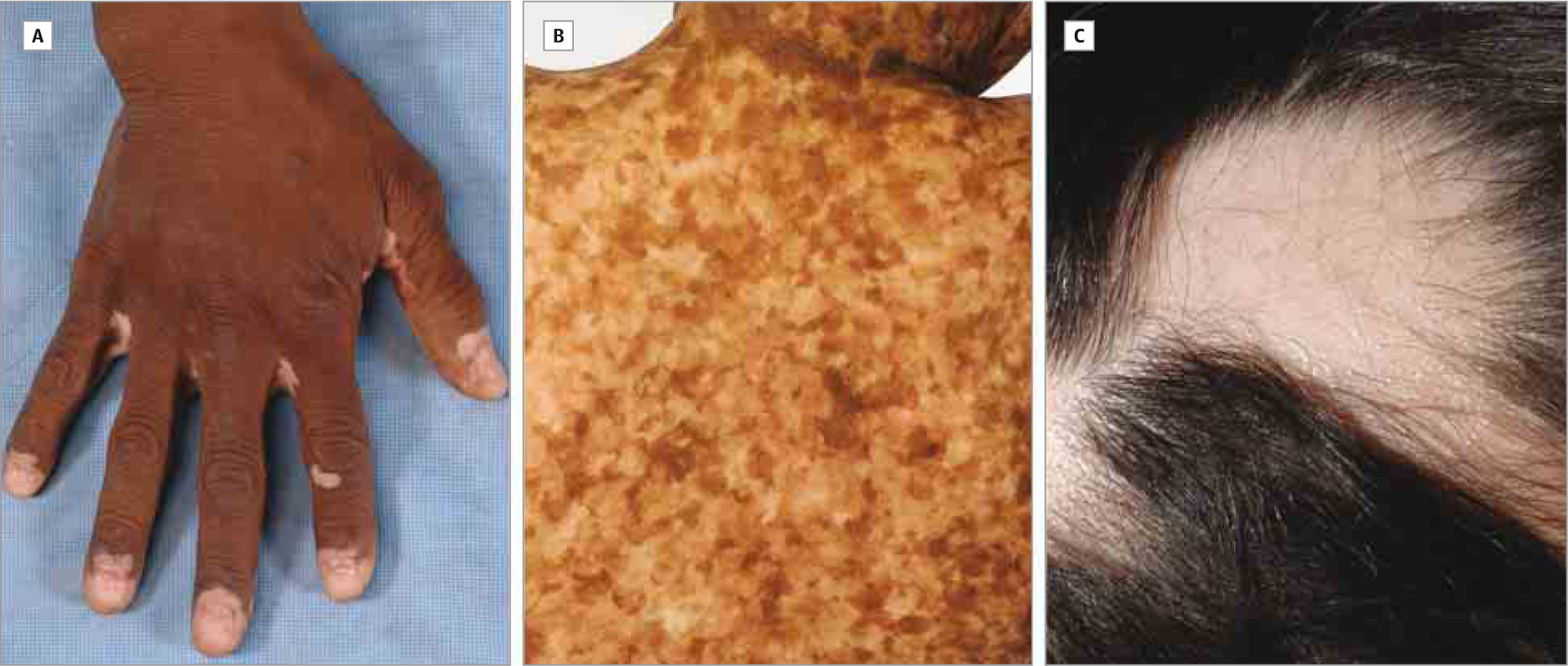
Clinical Spectrum of Vitiligo and Alopecia Areata in the Setting of Chronic Graft-vs-Host Disease
A, Depigmentation involving the distal ends of the fingers in this clinical image of classic vitiligo. B, An intermediate zone of hypopigmentation is present between depigmented and normal skin in trichrome vitiligo. C, Patchy oval areas of hair loss on the scalp characterize classic-appearing alopecia areata.
Patient demographics, chronic GvHD characteristics, and laboratory parameters of patients with and without vitiligo and/or AA are listed in Table 2. In univariate analysis, female donor to male recipient (FtoM) sex mismatch (P = .003), positive ACA-IgG findings (P = .03), positive antiparietal antibody findings (P = .049), elevated CD19 level (P = .045), and normal or elevated IgG level (P = .02) were associated with vitiligo and/or AA. All 14 of 15 patients with vitiligo and/or AA with known donor sex had a female donor (P < .001). One patient with vitiligo was missing donor sex information. Of 3 donors with available parity data, 2 were previously pregnant.
Table 2.
Clinical Characteristics and Laboratory Data of Patients With cGvHD With and Without Vitiligo and/or Alopecia Areataa
| Characteristic | Total (n = 282) | No Vitiligo or AA (n = 267) | Vitiligo and/or AA (n = 15) | P Value |
|---|---|---|---|---|
| Age, median (range), y | 46 (4–70) | 46 (4–70) | 38 (9–69) | .30 |
| ≤18 y | 20/282 (7.09) | 17/267 (6.37) | 3/15 (20.00) | … |
| Sex | ||||
| Male | 160/282 (56.73) | 150/267 (56.18) | 10/15 (66.67) | .59 |
| Female | 122/282 (43.26) | 117/267 (43.82) | 5/15 (33.33) | |
| Primary disease | ||||
| ALL/AML/MDS | 128/282 (45.39) | 123/267 (46.07) | 5/15 (33.33) | .22 |
| CML | 39/282 (13.82) | 34/267 (12.73) | 5/15 (33.33) | |
| CLL | 17/282 (6.03) | 16/267 (5.99) | 1/15 (6.67) | |
| Malignant lymphoma | 64/282 (22.69) | 62/267 (23.22) | 2/15 (13.33) | |
| Otherb | 34/282 (12.06) | 32/267 (11.99) | 215 (13.33) | |
| Conditioning regimen | ||||
| TBI−/MAC− | 100/279 (35.84) | 94/265 (35.47) | 6/14 (42.86) | .93 |
| TBI+/MAC− | 24/279 (8.60) | 23/265 (8.68) | 1/14 (7.14) | |
| TBI−/MAC+ | 74/279 (26.52) | 70/265 (26.42) | 4/14 (28.57) | |
| TBI+/MAC+ | 81/279 (29.03) | 78/265 (29.43) | 3/14 (21.43) | |
| MAC | 155/279 (55.56) | 148/265 (55.85) | 7/14 (50.00) | .78 |
| RIC | 124/279 (44.44) | 117/265 (44.15) | 7/14 (50.00) | … |
| TBI | 105/279 (37.63) | 101/265 (38.11) | 4/14 (28.57) | .58 |
| DLI post transplant | ||||
| Yes | 25/262 (9.54) | 24/247 (9.72) | 1/15 (6.67) | >.99 |
| No | 247/262 (94.27) | 223/247 (9.28) | 14/15 (93.33) | |
| Stem cell source | .05 | |||
| BM | 53/282 (18.79) | 49/267 (18.35) | 4/15 (26.67) | .49 |
| PB | 219/282 (77.66) | 210/267 (78.65) | 9/15 (60.00) | .11 |
| Cord | 10/282 (3.55) | 8/267 (3.00) | 2/15 (13.33) | .09 |
| Donor relationship | ||||
| Related | 174/281 (61.92) | 163/266 (61.28) | 11/15 (73.33) | .42 |
| Unrelated | 107/281 (38.08) | 103/266 (38.72) | 4/15 (26.67) | |
| HLA matched | ||||
| Yes | 230/275 (83.64) | 217/260 (83.46) | 13/15 (86.67) | >.99 |
| No | 45/275 (16.36) | 43/260 (16.54) | 2/15 (13.33) | |
| Donor sex | ||||
| Male | 135/255 (52.94) | 135/241 (56.02) | 0/14 | <.001 |
| Female | 120/255 (47.06) | 106/241 (43.98) | 14/14 (100) | |
| Donor to patient sex match or mismatch | <.001 | |||
| M to M | 76/256 (30.08) | 76/242 (31.82) | 0/14 | … |
| F to F | 57/256 (21.88) | 52/242 (21.07) | 5/14 (35.71) | … |
| M to F | 55/256 (21.88) | 55/242 (22.73) | 0/14 | .05 |
| F to M | 68/256 (26.17) | 59/242 (24.38) | 9/14 (64.29) | .003 |
| cGvHD duration, y | ||||
| ≤1 | 79/282 (28.01) | 76/267 (28.46) | 3/15 (20.00) | .57 |
| ≥1 | 203/282 (71.99) | 191/267 (71.54) | 12/15 (80.00) | |
| cGvHD onset | ||||
| Quiescent | 77/280 (27.50) | 75/265 (28.30) | 2/15 (13.33) | .31 |
| De novo | 92/280 (32.86) | 88/265 (33.21) | 4/15 (26.67) | |
| Progressive | 111/280 (39.64) | 102/265 (38.49) | 9/15 (60.00) | |
| Intensity of immunosuppressionc | ||||
| None or mild | 70/281 (24.91) | 66/266 (24.81) | 4/15 (26.67) | >.99 |
| Moderate | 100/281 (35.59) | 95/266 (35.71) | 5/15 (33.33) | |
| High | 111/281 (39.5) | 105/266 (39.47) | 6/15 (40.00) | |
| Presence of skin cGvHDd | ||||
| Yes | 215/281 (76.51) | 202 (75.70) | 13/15 (86.67) | .53 |
| No | 66/281 (23.49) | 64/266 (24.06) | 2/15 (13.33) | |
| Median BSA, % | ||||
| Erythema | 0.81 | 0.86 | 0.54 | .96 |
| Sclerosis | 0 | 2.16 | 0.36 | .81 |
| NIH Global Severity score | ||||
| Moderate | 81/278 (29.14) | 74/264 (71.97) | 7/14 (50.00) | .13 |
| Severe | 197/278 (70.86) | 190/264 (71.97) | 7/14 (50.00) | |
| Laboratory parameters | ||||
| Presence of autoantibodies | 133/279 (47.67) | 124/264 (46.97) | 9/15 (60.00) | .43 |
| ANA | 90/278 (32.37) | 85/264 (32.20) | 5/14 (35.71) | .78 |
| RF | 35/275 (12.73) | 31/260 (11.92) | 4/15 (26.67) | .11 |
| Anti-CCP | 20/277 (7.22) | 18/262 (6.87) | 2/15 (13.33) | .30 |
| Antiparietal | 7/277 (2.53) | 5/262 (1.91) | 2/15 (13.33) | .05 |
| Anticentromere | 9/277 (3.25) | 8/262 (3.05) | 1/15 (6.67) | .40 |
| Anti-RNP | 14/279 (5.02) | 12/264 (4.55) | 2/15 (13.33) | .17 |
| Anti-smith | 3/279 (1.08) | 2/264 (0.76) | 1/15 (6.67) | .15 |
| ACAIgM | 22/277 (7.94) | 21/262 (8.02) | 1/15 (6.67) | >.99 |
| ACAIgG | 23/277 (8.30) | 19/262 (7.25) | 4/15 (26.67) | .03 |
| Anti-ENA | 23/279 (8.24) | 21/264 (7.95) | 2/15 (13.33) | .36 |
| Anti-TPO | 3/59 (5.08) | 2/51 (3.92) | 1/8 (12.50) | .36 |
| Low CD3 (<1000/UL) | 156/261 (59.77) | 149/247 (60.32) | 7/14 (50.00) | .58 |
| Low CD4 (<250/UL) | 80/263 (30.42) | 77/249 (30.92) | 3/14 (21.43) | .56 |
| Low CD19 (<400/UL) | 199/282 (70.57) | 192/267 (71.91) | 7/15 (46.67) | .05 |
| High IgG (>1200 mg/dL) | 46/278 (16.55) | 41/263 (15.59) | 5/15 (33.33) | .02e |
Abbreviations: AA, alopecia areata; ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; ANA, antinuclear antibody; BM, bone marrow; BSA, body surface area; CCP, cyclic citrullinated peptides; cGvHD, chronic graft-vs-host disease; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; DLI, donor lymphocyte infusion; ENA, extractable nuclear antigens; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; NIH, National Institutes of Health; PB, peripheral blood; RIC, reduced-intensity conditioning; RF, rheumatoid factor; RNP, ribonucleoprotein; TBI, total body irradiation; TPO, thyroid peroxidase; …, analysis not performed.
Unless otherwise indicated, data are reported as number of participants/total number in the category (percentage).
The “other” category includes patients with multiple myeloma, sarcoma, immunodeficiency, aplastic anemia and/or paroxysmal nocturnal hemoglobinuria, and nonmalignant diseases.
Mild immunosuppression defined as single-agent prednisone at less than 0.5 mg/kg/d; moderate, prednisone at 0.5 mg/kg/d or higher and/or any other single agent or modality; high, 2 or more agents or modalities with or without prednisone at 0.5 mg/kg/d.
Indicates both sclerotic and/or nonsclerotic dermatologic manifestations of cGvHD.
P value relates to the comparison across low vs normal vs high IgG.
Most patients with vitiligo and/or AA had been diagnosed as having chronic GvHD for more than 1 year (12 of 15; 80.0%) and had progressive-onset disease (9 of 15; 60.0%). As summarized in Table 2, autoantibodies were detected in 9 patients (60.0%; P = .43 vs those without vitiligo and/or AA). The autoantibodies detected most frequently were antinuclear antibodies (5 of 14, 35.71%; P = .78), rheumatoid factor (4 of 15, 26.67%; P = .11), and ACA-IgG (4 of 15, 26.67%; P = .03). There were no significant differences in sex, indication for transplantation, conditioning regimen, chronic GvHD onset (quiescent, de novo, or progressive), chronic GvHD duration, or intensity of immunosuppression when patients with vitiligo and/or AA were compared with those without. Stem cell source (bone marrow, peripheral blood, or cord), donor and/or patient relationship (related or unrelated), HLA mismatch, receipt of donor lymphocyte infusion(s), type of skin chronic GvHD (sclerotic or nonsclerotic) and NIH Global Severity Score were also not associated with vitiligo and/or AA.
Multivariable Predictive Model of Vitiligoand/or AA Development
Number of CD19 cells, prevalence of antiparietal and ACA-IgG antibodies, and FtoM sex mismatch were included in a multivariable predictive model using logistic regression with backward selection. Donor sex (P < .001) was excluded as a potential parameter for inclusion in this model as a result of its infinite hazard ratio. The final model after backward selection included FtoM sex mismatch (P = .003) and ACA-IgG (P = .01). This model, when applied to the same patients on which the model was derived, correctly predicted 78.6% of patients with vitiligo and/or AA and 70.6% of those without vitiligo and/or AA.
Discussion
Allogeneic HSCT is a potentially curative treatment for refractory nonmalignant and malignant hematologic diseases; how-ever, its use is limited by the morbidity and mortality of acute and chronic GvHD. The pathogenesis of chronic GvHD involves dysregulation of both alloimmune and autoimmune mechanisms, resulting in autoantibody production, profibrotic pathways, and defective thymic function.26,27
Patients with vitiligo are at risk of AA and vice versa, and may also share common immunologic pathomechanisms. The prevalence of vitiligo and AA in the general population is 0.5% to 1.0% and 0.1%, respectively. Coexistence of vitiligo in patients with AA has been reported to be 3% to 8%.28 Adaptive immunity, particularly infiltrative cytotoxic CD8+ and CD4+ T cells, very likely plays a key role in the destruction of melanocytes in vitiligo and hair loss in AA.29–31
Vitiligo and/or AA following allogeneic HSCT has been described in 15 case reports and/or series in the literature, 81% of which reported concurrent or prior skin GvHD (Table 3). In our cohort, CML and acute leukemia (acute lymphoblastic leukemia and/or acute myelogenous leukemia and/or myelodysplastic syndrome) were the most common disease indications for transplant. In previous reports, 38% of patients with chronic GvHD and vitiligo and/or AA underwent HSCT for CML, a frequency comparable with our findings. Notably, CML accounts for only about 300 of 7892 (3.8%) allogeneic HSCTs per year in the United States, a relative minority among indicated diseases.32
Table 3.
Reported Cases of Non–Donor Transferred Vitiligo and/or AA Occurring After Allogeneic HSCT
| Vitiligo and/or AA | ||||||||
|---|---|---|---|---|---|---|---|---|
| Source, Year | Patient Sex/Age, y | Donor Sex | Disease | Transplant Conditioning | Condition | Time to Occurrencea | Distribution | Type of Skin GvHD |
| Nagler et al,13 1996 | M/14 | NR | SAA | TLI/Cyc | Vitiligo | 3 mo | Total leukoderma, leukotrichia | cGvHD-Lich |
| Aubin et al,7 2000 | M/44 | F | CML | Cyc/Thiotepa/TBI | Vitiligo | 5 mo | Generalized | cGvHD-Lich |
| Au et al,6 2001 | M/35 | NR | CML | NR | Vitiligo | 3 y | Generalized, leukotrichia | aGvHD grade 4 |
| F/36 | NR | ALL | NR | Vitiligo | 3–5 y | Generalized | aGvHD grade 3 | |
| Jacobsohn et al,11 2002 | M/14 | NR | CML | Bu/Ara-C/TBI | Vitiligo | 5 mo | Total leukoderma, leukotrichia | cGvHD-Lich |
| Sanli et al,19 2004 | M/26 | NR | AML | Bu/Cyc | AA | 6 mo | Left parietal, vertex, lateral right eyebrow | cGvHD-Lich |
| M/19b | NR | CML | Bu/Cyc | Vitiligo and AA | 8 mo | Vitiligo: face, neck, trunk, UE/LE; AA: temporal/occipital | NR | |
| M/31 | NR | CML | Bu/Cyc | AA | 3 mo | Eyebrows, forearms | NR | |
| Cho et al,9 2005 | M/15 | NR | ALL | Cyc/TBI | Vitiligo | 3 mo | Generalized | aGvHD |
| M/30 | F | CML | Cyc/TBI | Vitiligo | 2 y | Generalized | aGvHD | |
| M/28 | F | ALL | Cyc/TBI | Vitiligo | 26 mo | Generalized | cGvHD-Scl | |
| M/43 | F | MDS | Bu/TBI/Cyc | Vitiligo | 3 mo | Face, UE | cGvHD | |
| Williams et al,17 2008 | M/55 | NR | MDS | NR | Vitiligo | 3 y | Total leukoderma, leukotrichia | cGvHD-Epid |
| M/66 | NR | MDS | RIC | Vitiligo | 3 y | Generalized | cGvHD | |
| Cathcart and Marrell,8 2007 | M/15 | M | FA/AML | Flu/TBI/Cyc/AT G | Vitiligo | 4 y | Hands, arms, neck, trunk, genitalia | aGvHD |
| Sanli etal,18 2008 | M/34 | M | CML | Bu/Cyc | Vitiligo | 4 y | Generalized, leukotrichia | cGvHD-Lich |
| M/19b | M | CML | Bu/Cyc | Vitiligo and AA | 8 mo | AA: temporal, occipital; Vitiligo: face, neck, trunk, UE/LE | NR | |
| M/44 | F | CML | Bu/Cyc | Vitiligo | 7 mo | Face, hair, eyelids, trunk, UE/LE | cGvHD-Fol | |
| M/44 | F | CML | Bu/Cyc | Vitiligo | 4 yc | Face, dorsum of hands | cGvHD-Scl | |
| F/41 | M | CML | Bu/Flu | Vitiligo | 6 mo | Face | NR | |
| Heath et al,10 2009 | F/2 | NR | SCID | Bu/Cyc/ATG | Vitiligo | 4 mo | Chest, arms, legs, face, scalp | aGvHD |
| M/5 | NR | X-SCID | Bu/Cyc | Vitiligo | NR | Diffuse depigmentation, scattered leukotrichia | aGvHD | |
| Lee etal,12 2009 | M/23 | F | SAA | Cyc | Vitiligo | 2 y | Face, trunk, UE/LE | NR |
| Tan et al,16 2011 | F/48 | M | MDS | Cyc/Bu | Vitiligo | 5 yd | Face, trunk, UE/LE | cGvHD-Lich, Scl |
| Kamińska et al,20 2012 | M/38 | NR | aApAn | Flu/Alemtuzumab/Mel | AA | 6 mo | Diffuse alopecia | cGvHD |
| Oh and Lee,15 2013 | F/36 | NR | ALCL | NR | Vitiligo | 3 wk | UE/LE, trunks | NR |
| Nambudiri et al,14 2013 | M/15 | F | X-SCID | None | Vitiligo | 1 mo | Total leukoderma, leukotrichia | cGvHD-Scl |
Abbreviations: AA, alopecia areata; aApAn, acquired aplastic anemia; aGvHD, acute graft-vs-host disease; ALCL, anaplastic large cell lymphoma; ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; Ara-C, cytarabine; ATG, antithymocyte globulin; Bu, busulfan; cGvHD, chronic graft-vs-host disease; CML, chronic myelogenous leukemia; Cyc, cyclophosphamide; Epid, epidermal; FA, Fanconi anemia; Flu, fludarabine; HSCT, hematopoietic stem cell transplantation; LE, lower extremities; Lich, lichenoid; MDS, myelodysplastic syndrome; Mel, melphalan; NR, not reported; RIC, reduced-intensity conditioning; SAA, severe aplastic anemia; SCID, severe combined immunodeficiency; Scl, sclerotic; TBI, total body irradiation; TLI, total lymph node irradiation; UE, upper extremities; X-SCID, x-linked SCID.
Indicates time from allogeneic HSCT to onset of vitiligo or AA.
The case by Sanli et al 2008 may represent the same patient as the 19-year-old patient in Sanli et al 2004.
The donor (sister) of this patient had generalized vitiligo for 10 y. The authors postulate that the development of vitiligo 4 years after transplant was not likely from passive donor transfer.
This patient received 2 allogeneic HSCTs; the time shown refers to time after the second allogeneic HSCT.
Allogeneic HSCT-associated vitiligo has been reported as localized disease (eg, face, trunk, limbs), generalized involvement, and total leukoderma. Our cohort manifested a variety of vitiligo presentations, most frequently generalized and classic periorbital, perioral, and acrofacial distributions (Table 1). Trichrome vitiligo was observed in 3 patients. Total leukoderma, reported in 4 prior cases, was not seen in our cohort.
In contrast to prior cases, a large percentage (60.0%) of our cohort had sclerotic chronic GvHD, likely owing to the refractory nature of this skin manifestation and the referral pattern to the NIH. Sclerotic chronic GvHD manifests as a spectrum of clinical presentations, including localized fibrotic plaques resembling morphea with concomitant pigmentary changes as well as fibrosis limited to the papillary dermis resulting in white, shiny, guttate papules and plaques resembling LS.4 It may be clinically challenging, then, to differentiate cutaneous chronic GvHD pigmentary abnormalities from true depigmentation suggestive of vitiligo. Interestingly, LS and vitiligo have been associated in the nonallogeneic HSCT setting (vitiligoid LS).33 Lichenoid inflammation associated with LS triggering an autoimmune reaction against melanocytes has been proposed as a pathogenic mechanism.34
To our knowledge, this is the first study to describe an association between donor-recipient sex mismatch and the development of concomitant autoimmunity in patients with chronic GvHD. It is notable that in our cohort, all 14 patients with vitiligo and/or AA who had information on donor sex received their graft from a female donor. Nine of the 14 recipients were male, resulting in FtoM sex mismatch in 64.0% cases. Of 13 participants with chronic GvHD and vitiligo and/or AA in the literature with donor sex information, 8 (62%) had female donors and all were FtoM sex mismatch (Table 3).
Use of parous female donor and donor-recipient sex mismatch are important transplant risk factors for both acute and chronic GvHD.35–38 Use of FtoM results in a higher probability of acute and chronic GvHD as well as poorer outcomes.35–37,39–41 Interestingly, Kamoi et al42 found a significant association between severe dry eye in patients with chronic GvHD and FtoM sex mismatch marrow transplant.
The risk of autoimmunity in FtoM transplants may reflect the activity of skin-homing donor T cells specific for recipient minor histocompatibility antigens encoded by Y-chromosome genes,39 a mechanism previously implicated in both GvHD and graft-vs-tumor responses. In addition, prior studies indicate that presence of donor B-cell antibodies against the H-Y minor histocompatibility antigens may also contribute to the pathogenesis of chronic GvHD.42,43 Tan et al16 proposed that T-cell recognition of foreign melanocyte antigens may elicit a persistent immune response against host melanocytes. Likewise, a hallmark of active AA is the presence of CD8+ T cells around the bulb region of anagen hair follicles.44 Nonetheless, it is possible that the high percentage of FtoM sex mismatch reflects an epiphenomenon related to the high proportion of patients in our cohort with late-stage or treatment-refractory chronic GvHD.
Several additional theories have been proposed for depigmentation after allogeneic HSCT, including donor-transferred vitiligo,6,45–48 chemoradiation therapy and/or donor lymphocyte infusion (DLI)–induced vitiligo,6,11,13 and GvHD-associated vitiligo.6,8,9,13,16,18 Adoptive transfer alloimmune destruction of melanocytes from donors with vitiligo has been described in several reports.6,45–48 No information regarding history of vitiligo from graft donors was available in our patients. A role of DLI has been hypothesized,6 but we did not find a significant association between receipt of DLI(s) and vitiligo and/or AA. Interestingly, T cell–mediated vitiligo has been described in a patient who developed melanocyte destruction after antigen-specific immunotherapy form melanoma.49 By the same token, the pretransplant conditioning regimen may render melanocytes susceptible to immune-mediated destruction through melanocyte-specific T cells or antimelanocyte antibodies triggered by chronic GvHD and/or thymic damage.8,9,13 Sanli et al18 suggested tumor necrosis factor (TNF) as a possible mediator of vitiligo. Elevations of TNF and interleukin 6 have been found in vitiligo skin50 and observed in the induction of chronic GvHD.51 Four patients in our cohort did not manifest concomitant chronic GvHD skin involvement, supporting the theory that vitiligo and/or AA can be triggered or persist without localized chronic GvHD skin involvement. Genetic risk factors of both the host and donor, specifically HLA predispositions to vitiligo, may also play a role in disease pathogenesis. In 3 patients with vitiligo in whom HLA recipient data was available, each had 1 or more HLA alleles (DRB1*04, −07, −07:01) that have been associated with vitiligo in specific Asian and European populations.52,53 Interestingly, these 3 individuals also demonstrated HLA alleles (DRB1*07:01, DQB1*02:02, DQB1*06:02) that have been associated with alopecia areata.54
Outside the setting of HSCT, vitiligo and AA are frequently associated with autoantibody formation. Autoantibodies, particularly low-titer antinuclear antibody, are commonly detected in chronic GvHD55; however, their clinical significance is unclear because they lack the specificity seen in other well-characterized autoimmune conditions and are not associated with disease severity. In our cohort, the only antibody significantly associated with vitiligo and/or AA development was ACA-IgG. Antiphospholipid antibodies recognize phospholipid protein complexes and include lupus anticoagulant, anticardiolipin, and anti-β2-glycoprotein I antibodies. Anticardiolipins have been detected in patients with systemic lupus erythematosus, systemic sclerosis, scleroderma, and Sjögren syndrome.56–58 Our study suggests that ACA-IgG in patients with chronic GvHD may be a risk factor for cutaneous autoimmunity.
Vitiligo and AA are associated with other autoimmune disorders, including thyroid disease, diabetes mellitus, and pernicious anemia.59 In our chronic GvHD cohort, patients 8 and 9 had a diagnosis of Hashimoto thyroiditis prior to transplantation, and patient 8 had associated anti-thyroid peroxidase antibodies. Patients 2, 4, and 10 were found to have elevated levels of thyroid stimulating hormone with no documented evidence of thyroid disorders prior to transplantation. Thus, it is reasonable to screen for thyroid function abnormalities and other autoimmune diseases in patients with chronic GvHD vitiligo and/or AA.
There are several limitations of this study. First, the cross-sectional design restricts our evaluation to chronic GvHD manifestations and, specifically, cutaneous features at a single point in time. Similarly, laboratory data, including all blood analyses, may not be reflective of earlier points in each patient’s disease course. Furthermore, the cross-sectional design does not establish causality of the relationship between donor-recipient sex combinations and development of vitiligo. In addition, date of vitiligo onset was determined from review of patient records and was only available in a minority of patients in our subset. The NIH chronic GvHD natural history protocol referral pattern is also highly enriched for longstanding, treatment-refractory chronic GvHD and therefore is not reflective of all patients with chronic GvHD, particularly those with less severe disease. Nonetheless, the cross-sectional study design provides a powerful method to detect rare skin manifestations in a large patient cohort.
Conclusions
Vitiligo and/or AA are uncommon phenomena seen in allogeneic HSCT recipients and are likely secondary to the long-term immune dysregulation of chronic GvHD. In our cohort, ACA and female donor sex, in particular FtoM sex mismatch, were associated with risk of vitiligo and/or AA. Although vitiligo and AA are not life threatening, the psychological consequences in patients with chronic GvHD can further impair quality of life. Future studies are needed to clarify whether the risk factors identified in this study could lead to better understanding of other autoimmune manifestations in the setting of chronic GvHD.
Funding/Support:
This study was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), the National Cancer Institute, and the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc, The Doris Duke Charitable Foundation, The Alexandria Real Estate Equities Inc, Mr and Mrs Joel S. Marcus, and the Howard Hughes Medical Institute, as well as other private donors.
Role of the Funder/Sponsor: The NIH played a central role in the design and conduct of the study.
All other funding institutions had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None reported.
Contributor Information
Seth M. Steinberg, Biostatistics and Data Management Section, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Kristin Baird, Pediatric Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Sandra A. Mitchell, Outcomes Research Branch, Applied Research Program, Division of Cancer Control and Population Science, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Edward W. Cowen, Dermatology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9(4):215–233. [DOI] [PubMed] [Google Scholar]
- 2.Baird K, Pavletic SZ. Chronic graft versus host disease. Curr Opin Hematol. 2006;13(6):426–435. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell SA, Reeve BB. Health-related quality of life in chronic graft vs host disease In: Vogelsang GB, Pavletic SZ, eds. Chronic Graft vs Host Disease: Principles and Practice of Interdisciplinary Management. New York, NY: Cambridge University Press; 2009:335–348. [Google Scholar]
- 4.Hymes SR, Alousi AM, Cowen EW. Graft-vs-host disease: part I, pathogenesis and clinical manifestations of graft-vs-host disease. J Am Acad Dermatol. 2012;66(4):515.e1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holbro A, Abinun M, Daikeler T. Management of autoimmune diseases after haematopoietic stem cell transplantation. Br J Haematol. 2012;157(3): 281–290. [DOI] [PubMed] [Google Scholar]
- 6.Au WY, Yeung CK, Chan HH, Lie AK. Generalized vitiligo after lymphocyte infusion for relapsed leukaemia. Br J Dermatol. 2001;145(6):1015–1017. [DOI] [PubMed] [Google Scholar]
- 7.Aubin F, Cahn JY, Ferrand C, Angonin R, Humbert P, Tiberghien P. Extensive vitiligo after ganciclovir treatment of GvHD in a patient who had received donor T cells expressing herpes simplex virus thymidine kinase. Lancet. 2000;355(9204):626–627. [DOI] [PubMed] [Google Scholar]
- 8.Cathcart S, Morrell D. Vitiligo as a post-bone marrow transplantation complication. J Pediatr Hematol Oncol. 2007;29(7):485–487. [DOI] [PubMed] [Google Scholar]
- 9.Cho SB, Roh MR, Chung KY, Lee KH, Park YK. Generalized vitiligo after allogeneic bone marrow transplantation. Acta Derm Venereol. 2005;85(1): 82–83. [DOI] [PubMed] [Google Scholar]
- 10.Heath CR, Burk CJ, Lawley LP, Mancini AJ, Connelly EA. Severe combined immunodeficiency (SCID)-associated dyschromia with subsequent repigmentation: a report of two patients. Pediatr Dermatol. 2009;26(2):162–168. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsohn DA, Ruble K, Moresi JM, Vogelsang GB. Rapid-onset leukoderma associated with graft-versus-host disease. Bone Marrow Transplant. 2002;30(10):705–706. [DOI] [PubMed] [Google Scholar]
- 12.Lee HT, Chen WS, Chou CT, Chen MH, Tsai CY. Chronic graft-vs-host disease mimicking rapid progressive rheumatoid arthritis with atlantoaxial subluxation. BMJ Case Rep; 2009;2009(pii): bcr06.2009.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagler A, Goldenhersh MA, Levi-Schaffer F, Bystryn JC, Klaus SN. Total leucoderma: a rare manifestation of cutaneous chronic graft-versus-host disease. Br J Dermatol. 1996;134(4):780–783. [PubMed] [Google Scholar]
- 14.Nambudiri VE, Tsiaras WG, Schmidt BA, Huang JT. Total leukoderma and leukotrichia in a child after hematopoietic SCT: report of a case and review of the literature. Bone Marrow Transplant. 2014;49(3): 460–462. [DOI] [PubMed] [Google Scholar]
- 15.Oh CC, Lee HY. Treatment of eczematoid and vitiliginous graft vs host disease with extracorporeal photopheresis and alemtuzumab. J Am Acad Dermatol. 2013;68(4)(suppl 1):AB142. [Google Scholar]
- 16.Tan AW, Koh LP, Goh BK. Leucoderma in chronic graft-versus-host disease: excellent repigmentation with noncultured cellular grafting. Br J Dermatol. 2011;165(2):435–437. [DOI] [PubMed] [Google Scholar]
- 17.Williams JS, Mufti GJ, du Vivier AW, Salisbury JR, Creamer D. Leucoderma and leucotrichia in association with chronic cutaneous graft-versus-host disease. Br J Dermatol. 2008;158(1):172–174. [DOI] [PubMed] [Google Scholar]
- 18.Sanli H, Akay BN, Arat M, et al. Vitiligo after hematopoietic cell transplantation: six cases and review of the literature. Dermatology. 2008;216(4): 349–354. [DOI] [PubMed] [Google Scholar]
- 19.Sanli H, Kusak F, Arat M, Ekmekci P, Ilhan O. Simultaneous onset of chronic graft versus host disease and alopecia areata following allogeneic haematopoietic cell transplantation. Acta Derm Venereol. 2004;84(1):86–87. [DOI] [PubMed] [Google Scholar]
- 20.Kamińska EC, Larson RA, Petronic-Rosic V. Amelanocytic anhidrotic alopecia areata-like phenotype after allogeneic hematopoietic cell transplant. Arch Dermatol. 2012;148(8):931–934. [DOI] [PubMed] [Google Scholar]
- 21.Sanz J, Arriaga F, Montesinos P, et al. Autoimmune hemolytic anemia following allogeneic hematopoietic stem cell transplantation in adult patients. Bone Marrow Transplant. 2007;39(9):555–561. [DOI] [PubMed] [Google Scholar]
- 22.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease, I: diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. [DOI] [PubMed] [Google Scholar]
- 23.Martires KJ, Baird K, Steinberg SM, et al. Sclerotic-type chronic GVHD of the skin: clinical risk factors, laboratory markers, and burden of disease. Blood. 2011;118(15):4250–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agresti A Categorical Data Analysis. New York, NY: John Wiley and Sons, Inc; 1990:79–129. [Google Scholar]
- 25.Mehta CR, Patel NR. A network algorithm for performing Fisher’s exact test in r × c contingency tables. J Am Stat Assoc. 1983;78(382):427–434. [Google Scholar]
- 26.Schroeder MA, DiPersio JF. Mouse models of graft-versus-host disease: advances and limitations. Dis Model Mech. 2011;4(3):318–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaglowski SM, Devine SM. Graft-versus-host disease: why have we not made more progress? Curr Opin Hematol. 2014;21(2):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller SA, Winkelmann RK. Alopecia areata: an evaluation of 736 patients. Arch Dermatol. 1963; 88:290–297. [DOI] [PubMed] [Google Scholar]
- 29.Harris JE. Vitiligo and alopecia areata: apples and oranges? Exp Dermatol. 2013;22(12):785–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Boorn JG, Konijnenberg D, Dellemijn TA, et al. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol. 2009;129(9):2220–2232. [DOI] [PubMed] [Google Scholar]
- 31.Gilhar A, Paus R, Kalish RS. Lymphocytes, neuropeptides, and genes involved in alopecia areata. J Clin Invest. 2007;117(8):2019–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR summary slides. http://www.cibmtr.org/pages/index.aspx. Accessed July 11, 2014.
- 33.Attili VR, Attili SK. Vitiligoid lichen sclerosus: a reappraisal. Indian J Dermatol Venereol Leprol. 2008;74(2):118–121. [DOI] [PubMed] [Google Scholar]
- 34.Carlson JA, Grabowski R, Mu XC, Del Rosario A, Malfetano J, Slominski A. Possible mechanisms of hypopigmentation in lichen sclerosus. Am J Dermatopathol. 2002;24(2):97–107. [DOI] [PubMed] [Google Scholar]
- 35.Loren AW, Bunin GR, Boudreau C, et al. Impact of donor and recipient sex and parity on outcomes of HLA-identical sibling allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12(7):758–769. [DOI] [PubMed] [Google Scholar]
- 36.Kollman C, Howe CW, Anasetti C, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98(7): 2043–2051. [DOI] [PubMed] [Google Scholar]
- 37.Gahrton G Risk assessment in haematopoietic stem cell transplantation: impact of donor-recipient sex combination in allogeneic transplantation. Best Pract Res Clin Haematol. 2007;20(2):219–229. [DOI] [PubMed] [Google Scholar]
- 38.Flowers ME, Inamoto Y, Carpenter PA, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011; 117(11):3214–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Randolph SS, Gooley TA, Warren EH, Appelbaum FR, Riddell SR. Female donors contribute to a selective graft-versus-leukemia effect in male recipients of HLA-matched, related hematopoietic stem cell transplants. Blood. 2004; 103(1):347–352. [DOI] [PubMed] [Google Scholar]
- 40.Nannya Y, Kataoka K, Hangaishi A, Imai Y, Takahashi T, Kurokawa M. The negative impact of female donor/male recipient combination in allogeneic hematopoietic stem cell transplantation depends on disease risk. Transpl Int. 2011;24(5): 469–476. [DOI] [PubMed] [Google Scholar]
- 41.Remberger M, Kumlien G, Aschan J, et al. Risk factors for moderate-to-severe chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2002;8(12):674–682. [DOI] [PubMed] [Google Scholar]
- 42.Kamoi M, Ogawa Y, Uchino M, et al. Donor-recipient gender difference affects severity of dry eye after hematopoietic stem cell transplantation. Eye (Lond). 2011;25(7):860–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105(7):2973–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hordinsky MK. Overview of alopecia areata. J Investig Dermatol Symp Proc. 2013;16(1):S13–S15. [DOI] [PubMed] [Google Scholar]
- 45.Neumeister P, Strunk D, Apfelbeck U, Sill H, Linkesch W. Adoptive transfer of vitiligo after allogeneic bone marrow transplantation for non-Hodgkin’s lymphoma. Lancet. 2000;355 (9212):1334–1335. [DOI] [PubMed] [Google Scholar]
- 46.Alajlan A, Alfadley A, Pedersen KT. Transfer of vitiligo after allogeneic bone marrow transplantation. J Am Acad Dermatol. 2002;46(4): 606–610. [DOI] [PubMed] [Google Scholar]
- 47.Mellouli F, Ksouri H, Dhouib N, et al. Possible transfer of vitiligo by allogeneic bone marrow transplantation: a case report. Pediatr Transplant. 2009;13(8):1058–1061. [DOI] [PubMed] [Google Scholar]
- 48.Campbell-Fontaine A, Coad JE, Kovach R, Ericson SG. Adoptive transfer of vitiligo after allogeneic peripheral blood stem cell transplant. Bone Marrow Transplant. 2005;36(8):745–746. [DOI] [PubMed] [Google Scholar]
- 49.Yee C, Thompson JA, Roche P, et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of T cell-mediated vitiligo. J Exp Med. 2000;192(11): 1637–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moretti S, Spallanzani A, Amato L, et al. New insights into the pathogenesis of vitiligo: imbalance of epidermal cytokines at sites of lesions. Pigment Cell Res. 2002;15(2):87–92. [DOI] [PubMed] [Google Scholar]
- 51.Cavet J, Dickinson AM, Norden J, Taylor PR, Jackson GH, Middleton PG. Interferon-gamma and interleukin-6 gene polymorphisms associate with graft-versus-host disease in HLA-matched sibling bone marrow transplantation. Blood. 2001;98(5): 1594–1600. [DOI] [PubMed] [Google Scholar]
- 52.Singh A, Sharma P, Kar HK, et al. ; Indian Genome Variation Consortium. HLA alleles and amino-acid signatures of the peptide-binding pockets of HLA molecules in vitiligo. J Invest Dermatol. 2012;132(1):124–134. [DOI] [PubMed] [Google Scholar]
- 53.Taştan HB, Akar A, Orkunoğlu FE, Arca E, Inal A. Association of HLA class I antigens and HLA class II alleles with vitiligo in a Turkish population. Pigment Cell Res. 2004;17(2):181–184. [DOI] [PubMed] [Google Scholar]
- 54.Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128(1):240–243. [DOI] [PubMed] [Google Scholar]
- 55.Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34(3):389–396. [DOI] [PubMed] [Google Scholar]
- 56.Hunt RD, Robinson M, Patel R, Franks AG Jr. Antiphospholipid-antibody-associated panniculitis. Dermatol Online J. 2012;18(12):18. [PubMed] [Google Scholar]
- 57.Love PE, Santoro SA. Antiphospholipid antibodies: anticardiolipin and the lupus anticoagulant in systemic lupus erythematosus (SLE) and in non-SLE disorders: prevalence and clinical significance. Ann Intern Med. 1990;112(9): 682–698. [DOI] [PubMed] [Google Scholar]
- 58.Sato S, Fujimoto M, Hasegawa M, Takehara K. Antiphospholipid antibody in localised scleroderma. Ann Rheum Dis. 2003;62(8):771–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alikhan A, Felsten LM, Daly M, Petronic-Rosic V. Vitiligo: a comprehensive overview, part I: introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65(3):473–491. [DOI] [PubMed] [Google Scholar]


