INTRODUCTION
Minimally invasive surgical equipment and techniques have revolutionized spinal surgery. Although initially popularized in surgery for degenerative spinal disease and trauma, these techniques have also become widely applied in spinal tumor and deformity surgery. Among patients with malignant spinal tumors, minimizing the interruption or delay in systemic and radiation therapy plays a critical role in survival improvement. Utilization of minimally invasive techniques that facilitate postoperative recovery and decrease the risk of complications and systemic surgical stress may result in improved survival for patients with cancer and certainly plays a role in expediting their return to home and continuation of oncologic treatment.
The principal indications for surgery among patients with spinal tumors include tumor control, decompression of the spinal cord, and restoration of mechanical stability of the spinal column. Surgery for primary tumors may be undertaken with a curative intent, whereas metastatic tumor surgery is performed for symptom palliation. The key components of minimal access surgery include percutaneous spinal instrumentation, tubular and expandable retractors that facilitate transmuscular approaches, and neuronavigation. Endoscopic and robot-assisted surgery shows promise in further expanding the minimally invasive surgery (MIS) tumor surgery capabilities (Figs. 1–3).
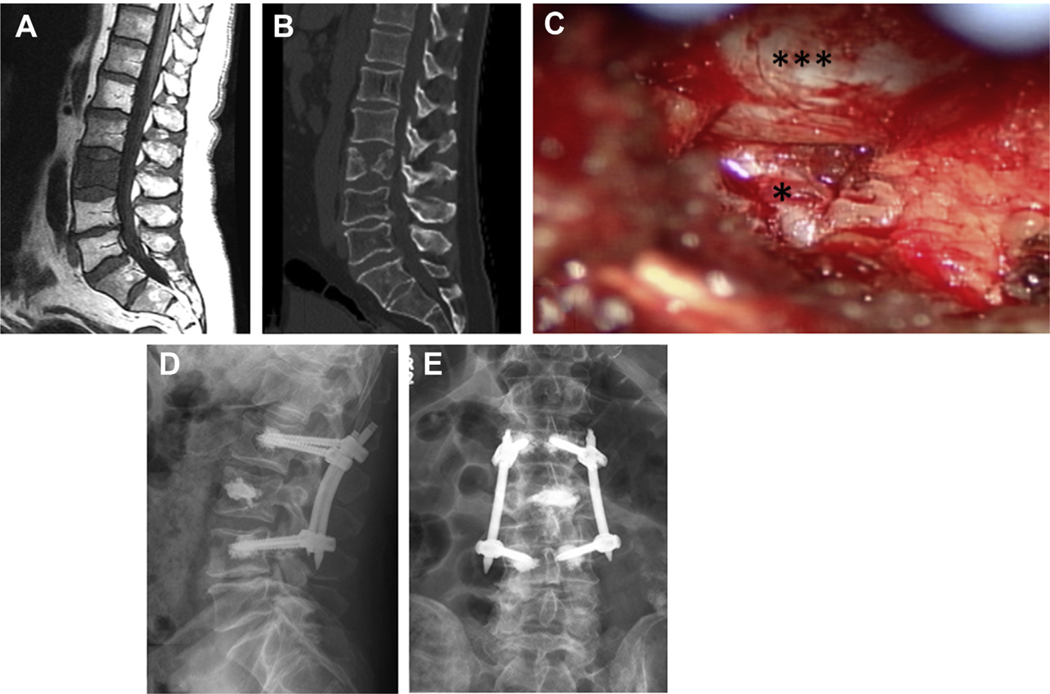
Fig. 1.
A 61-year-old man with widely metastatic urothelial carcinoma treated with Pembrolizumab and concomitant radiation. He underwent SBRT to L3 vertebral body metastases with no significant compression fracture and no epidural disease (A). Several weeks after radiation, he experienced severe lower back pain and left-sided mechanical radiculopathy. CT scan showed progression of his L3 fracture with a fragment compressing the exiting nerve root (B). He underwent L2-L4 percutaneous stabilization with cement augmentation, L3 kyphoplasty, and left L3-4 minimally invasive facetectomy. Intraoperative image demonstrating the decompressed thecal sac (triple asterisk) and exiting nerve root (asterisk) (C). Postoperatively, his pain significantly improved, and he was discharged home on postoperative day 2. Postoperative anteroposterior (AP) (D) and lateral (E) views demonstrate the stabilizing construct.
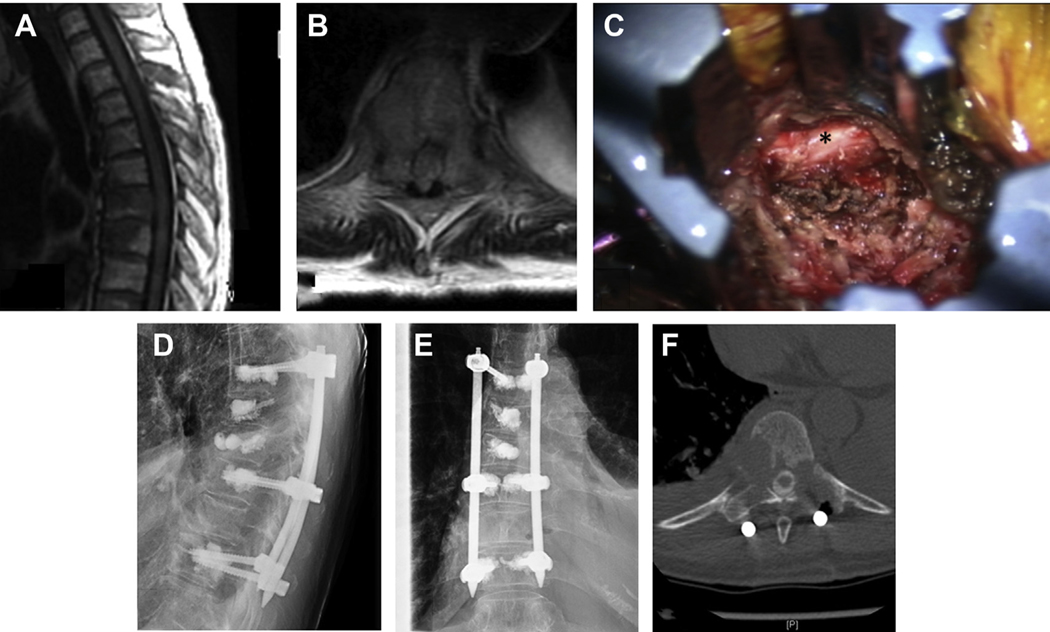
Fig. 3.
A 67-year-old man with metastatic renal cell carcinoma to lungs, thoracic lymph nodes, adrenal glands, and T6 vertebra presenting with subacute movement-related back pain radiating to the bilateral chest wall, found to have a T6 metastasis (A) with epidural spinal cord compression 1C cord compression (B). Kyphoplasty was not performed because of the posterior vertebral body involvement extending into the canal. Patient underwent T5-T7 percutaneous stabilization with cement augmentation followed by adjuvant SBRT. Postoperative AP (C) and lateral (D) radiographs demonstrate the stabilization construct. At 1-year follow-up, the patient remains pain free with durable local tumor control.
MINIMALLY INVASIVE SPINAL TUMOR SURGERY TECHNIQUES
Spinal Stabilization
Vertebral cement augmentation provides reliable pain relief for patients with simple compression fractures without extensive cortical destruction or extension into the posterior elements. Kyphoplasty and vertebroplasty can be performed in the outpatient setting, representing the most enduring and the least-invasive spinal stabilization technique. The cement is injected into the vertebral body through a percutaneously placed needle and can be injected directly into the cancellous bone in vertebroplasty or into a cavity created with an inflatable balloon in kyphoplasty. In cases of epidural tumor extension, injection of cement into the vertebral body can displace the epidural tumor further into the canal and should be done with caution and avoided in cases of high-grade epidural disease.1
Percutaneous pedicle screw instrumentation (PPSI) permits minimally invasive stabilization of pathologic fractures that are not amenable to stand-alone percutaneous cement augmentation. The technique requires minimal approach-associated soft tissue disruption compared with open posterior instrumentation placement, avoiding muscle necrosis, devascularization, and denervation, and minimizing blood loss. Various techniques for radiographic or navigation guidance for PPSI placement have been described.
The use of cement augmentation can be used to provide additional spinal stability for patients requiring PPSI. Patients with cancer frequently suffer from impaired bone density, placing the patients at high risk for screw pull-out. Augmentation of osseous screw purchase with cement has been demonstrated to be an effective technique in over-coming this challenge. Fenestrated screws permit cement injection through the screw directly into the surrounding bone in the vertebral body.2 Although traditionally, open pedicle screw instrumentation is placed at least 2 levels above and below the tumor in order to diminish the risk of screw pull-out, the use of fenestrated screws permits the use of short constructs with screws placed 1 level above and below the tumor.2,3 In cases of significant lytic destruction of the vertebral body, cement augmentation of the tumor level can provide additional stability of the anterior column.4
Tumor Excision and Decompression
Among patients with primary tumors, surgical excision might be undertaken with curative intent. However, patients with metastatic tumors undergo surgery for the purpose of symptom palliation. In both patient populations, the goals of excisional surgery include meaningful local control and decompression of the spinal cord and nerve roots.
The mini-open approach combines PPSI with conventional midline or anterior approaches for tumor excision and spinal cord decompression. This approach generally requires a 1- or 2-level midline approach for tumor exposure and decompression, while sparing the rest of the midline musculature that would have been dissected and retracted for the purpose of posterior instrumentation placement. Anterior mini-open approaches have also been described, largely using the thoracotomy or retroperitoneal approaches while limiting the skin and muscle dissection. The extent of decompression and tumor excision range from laminectomy to en bloc corpectomy and can be tailored to the oncologic goals of the operation. Matched comparison of mini-open approaches with conventional open surgery demonstrated decreased intraoperative blood loss, postoperative pain, and hospitalization duration after the mini-open approach.5,6
Tubular and expandable retractors provide a transmuscular exposure through serial muscle dilation, thus allowing greater muscle sparing compared with the midline mini-open approach. Such retractors have been used to remove intradural and extradural tumors. Fixed tubular retractors come in a range of diameters and lengths, allowing retractor selection specific to the patient’s anatomy and surgical plan. Expandable retractors provide a wider surgical field and have mobile retractor blades that can be adjusted as the surgery progresses. These retractors can be placed through the incisions used for PPSI at a trajectory that allows targeted decompression or through a separate incision. Retractor trajectory is critical in providing adequate exposure and safe conditions for tumor removal. These retractors are placed over serial dilator tubes that are docked on the lamina. In patients with tumors causing lytic destruction of the lamina, serial dilation must be performed with great caution in order to avoid unintended entry in the spinal canal. The use of fixed and expandable transmuscular retractors has been described in a wide range of tumor operations from limited laminectomy to intralesional corpectomy for extradural tumors as well as for the removal of intradural tumors.
Endoscopy represents the next step in decreasing the invasiveness of spinal surgery. Current endoscopic systems provide a subcentimeter channel for visualization and singleport dissection. The popularity of such systems for the treatment of degenerative spinal problems has dramatically grown, with excellent data supporting the safety and efficacy of endoscopic spinal surgery. The use of thoracoscopy for anterior-approach corpectomy has been described in several case series and technical notes.7,8 On the other hand, the use of endoscopic systems for posterior-approach spinal tumor work has been limited, described in only a few case reports.9–12 However, the posterior-approach endoscopic technique holds promise for targeted tumor excision and bone removal for decompression of the spinal cord and nerve roots, and these applications will gain prominence as the technology improves and surgeons continue to gain experience with spinal endoscopy. Currently available endoscopic instruments have very limited capacity to control tumor bleeding, thus significantly limiting the role of endoscopy in tumor work; however, improved coagulation devices are expected to enter the market soon.
Robot-assisted surgery has gained great prominence in a broad range of surgical subspecialties, including thoracic, colorectal, genitourinary, and head and neck surgery. Most robot-assisted operations use transcavitary approaches that permit a working space for several robotic arms and a camera, allowing outstanding visualization of the surgical anatomy as well as controlled and precise movements of surgical instruments in small spaces. Robot-assisted spinal surgery has not been performed for posterior-approach spinal surgery; however, several reports describe its use in the removal of paraspinal thoracic and presacral tumors.13–17
Various ablation techniques have also been used in order to obtain local tumor control. The use of radiofrequency (RF), laser, microwave ablation, and cryoablation for the treatment of benign osseous spinal tumors has been described.18–21 Ablation is often performed in combination with vertebral cement augmentation and radiotherapy. Proximity of tumors to nerve roots and vascular structures limits the utilization of percutaneous ablation; however, various organ displacement techniques and improved neuromonitoring might allow broader application. Multiple case series suggest that RF ablation provides pain relief in the treatment of metastatic spinal tumors; however, convincing data about the role of RF ablation in providing local tumor control are lacking. Laser ablation permits real-time monitoring of the ablation zone, improving the safety of the procedure. Spinal laser interstitial thermoablation combined with stereotactic body radiotherapy (SBRT) has been successfully used to treat spinal metastatic tumors, reported in large patient series.22,23
MINIMALLY INVASIVE SPINAL TUMOR INDICATIONS
Benign Epidural Tumors of the Spinal Column
Surgical treatment of benign osseous spinal tumors is indicated in cases of symptomatic or growing tumors and is generally undertaken with curative intent. Multiple reports describe curative treatment of osteoid osteomas and osteoblastomas using computed tomographic (CT)-guided percutaneous ablation techniques.18 These series report complete pain relief with durable follow-up of up to 5 years without periprocedural complications or evidence of tumor recurrence. Because these lesions are frequently located in the posterior elements, including the pedicle and articular processes, open surgical excision may be associated with iatrogenic instability and the need for spinal stabilization. Percutaneous ablation avoids instability. Although proximity of these tumors to the spinal cord, nerve roots, and the vertebral artery may limit the use of ablation, modern neurologic monitoring techniques generally provide accurate information about the safety of the ablation.21,24
Although typical and atypical vertebral hemangiomas generally do not require treatment, they may occasionally cause pain, epidural venous congestion, and fractures. Most symptomatic hemangiomas can be treated with percutaneous cement injection.25 Occasionally, the cement injection is performed in combination with alcohol ablation or transarterial embolization.26
Transtubular or endoscopic tumors excision provides another minimally invasive alternative to percutaneous ablation. Tubular retractors have been used to excise anterior cervical benign tumors using the anterior approach to C2 and the subaxial cervical spine, along with the posterior approach to thoracic and lumbar posterior element tumors.27 Thoracoscopy has also been used for the removal of anteriorly located thoracic osteoid osteomas.28
Intradural Tumors
Similarly to benign osseous tumors, benign intradural intramedullary and extramedullary tumors are excised when they grow or cause symptoms. Surgery is undertaken with the goal of safe gross total excision and usually results in cure. Surgery for spinal astrocytomas may be limited to biopsy in cases of higher-grade tumors. The most commonly described minimal access approach to the intradural compartment involves a para-median incision with sequential muscle dilation and the placement of a fixed tubular or expandable retractor. A hemilaminectomy with undercutting of the spinous process is performed. The intradural dissection is performed according to conventional microsurgical technique. Several techniques for dural closure through a tubular dilator have been described. Numerous case series and technical reports describe excellent outcomes after minimal access excision of intradural schwannomas, meningiomas, ependymomas, hemangioblastomas, and cavernous malformations.29–34
Metastatic Tumors
Instability
The diagnosis of spinal mechanical instability among patients with spinal metastatic tumors is facilitated using the Spinal Instability Neoplastic Score.35 Generally, patients with pathologic fractures causing pain that is exacerbated by movement benefit from spinal stabilization. A prospective randomized trial demonstrated that balloon-assisted kyphoplasty provides superior pain relief and functional restoration compared with patients treated using noninterventional methods.36 Cement augmentation works well among patients with simple pathologic compression fractures limited to the vertebral body.
Patients with thoracolumbar fractures, significant epidural tumor extension, fracture fragment retropulsion, fracture extension into the posterior elements, or severe lytic cortical destruction generally require instrumented stabilization because adequate cement fill may not be feasible. PPSI provides an excellent alternative to stand-alone cement augmentation, through stabilizing the anterior and posterior spinal elements. The small incisions and minimal muscle disruption required for PPSI lead to very low risk of wound complications and rapid postoperative recovery. The efficacy and safety of PPSI in stabilization of tumor-associated fractures have been reported by multiple investigators. Short-segment PPSI stabilization of painful pathologic fractures provides excellent pain relief when used as stand-alone or with fracture and screw cement augmentation.3,37,38 Cement augmentation of the screw-bone interface using fenestrated screws has been shown to provide durable construct stability, with very low complication risk.2 In addition to the use of cement for screw augmentation, cement stabilization of the fractured level may be performed using kyphoplasty or vertebroplasty technique in combination with PPSI in order to provide additional support to the anterior column.3,4
Various MIS pedicle screw placement strategies have been reported in the cancer literature. Most commonly, separate small skin incisions are made for each screw. However, in cases of multilevel constructs or decompressive surgery, some investigators advocate for a midline skin incision and transfacial transmuscular placement of the screws.5,6 If a common midline incision is selected, a subcutaneous drain is usually required.
Metastatic epidural spinal cord compression and radiculopathy
Patients with symptomatic spinal cord compression benefit from surgical decompression. Furthermore, patients with fractures causing mechanical radiculopathy require nerve root decompression in order to attain pain relief.39 Decompression strategies range from limited laminectomy or facetectomy to corpectomy, and all have been performed using various MIS techniques.
The midline mini-open approach has been used in order to perform tumor excision for circumferential spinal cord decompression in combination with posterior spinal instrumentation. Chou and Lu40 used the mini-open approach to perform a transpedicular corpectomy with expandable cage and transfascial pedicle screw stabilization. They subsequently demonstrated that patients who under-went mini-open surgery had lower blood loss and shorter hospital stay compared with patients who underwent open corpectomy.6 Similar comparison by Saadeh and colleagues5 confirmed the reduction in blood loss, hospitalization duration, and postoperative pain.
Tubular retractors have been used in order to provide a posterior approach for the purpose of transpedicular vertebrectomy. Deutsch and colleagues41 described transpedicular thoracic vertebrectomy using a 22-mm tubular retractor. Massicotte42 has reported a series of patients who underwent thoracic and lumbar PPSI placement and corpectomies via an 18-mm tubular retractor in the outpatient setting.
Expandable retractors provide a wider and more flexible working corridor compared with fixed tubular retractors and have been used for posterior, posterolateral, and lateral approaches to spinal tumors. Taghva and colleagues43 used dual expandable retractors to perform a bilateral T4 and T5 decompression, with a transpedicular approach on 1 side and a costotransversectomy approach on the contralateral side. A series of extracavitary corpectomies through an expandable retractor with short segment PPSI was also reported by Smith and colleagues.44 The use of expandable retractors for facetectomy or transpedicular corpectomy with cage reconstruction in the lumbar spine has also been reported by several investigators.45–47 Expandable retractors have also been used in transthoracic, transplural, and retroperitoneal approaches to the thoracic and lumbar spine in order to perform anterior corpectomy and cage reconstruction.48,49 Thoracoscopy has also been used in order to perform anterior thoracic corpectomy.7
Finally, with popularization of SBRT, separation surgery, involving circumferential epidural decompression without corpectomy, has become a commonly performed operation for patients with metastatic epidural spinal cord compression. Because SBRT provides reliable tumor control without the need for extensive tumor excision, the goal of separation surgery is to create favorable conditions for SBRT by providing a separation between the tumor and the spinal cord.50,51 The use of fixed tubular and expandable retractors in separation surgery has been described, followed by SBRT.52,53 Tatsui and colleagues54 have used LITT in order to perform focused ablation of epidural tumor in order to provide the separation necessary for successful SBRT. LITT is described in detail in a dedicated article in the current issue and provides a promising alternative to surgical tumor excision.
Algorithm for Minimally Invasive Surgery Strategy Selection in Patients with Metastatic Tumors
Clearly, surgeons use myriad MIS options in the treatment of spinal metastatic tumors. The choice of technique is dictated by the surgical indication, tumor location and morphology, hemorrhagic potential, prior surgery, and the degree of spinal cord compression. Not every tumor is amenable to safe and effective minimally invasive treatment. For example, a patient with renal cell carcinoma metastasis causing multilevel spinal cord compression might be better treated with open surgery after tumor embolization. On the other hand, focal tumors with low vascularity represent ideal targets for MIS decompression. The surgeon’s experience with the MIS technique plays a critical role in the approach selection.
The authors have developed a treatment algorithm that considers the extent of epidural tumor extension, symptoms, tumor histology, and fracture morphology in order to facilitate and clarify MIS strategy selection.47 They treat patients with solid tumors causing symptomatic spinal cord compression with PPSI and decompression through midline mini-open or transmuscular approach using an expandable retractor. Patients with lumbar tumors causing mechanical radiculopathy are treated with PPSI and facetectomy through a tubular or expandable retractor. Patients with mechanical instability caused by radiosensitive tumors with high-grade epidural extension are treated with PPSI with screw cement augmentation. In the absence of high-grade epidural extension, mechanically unstable fractures with extension into the posterior elements are treated with PPSI with screw and fracture cement augmentation. Finally, simple compression fractures can be treated with kyphoplasty.
SUMMARY
MIS presents clear advantages for patients with spinal tumors. For patients with benign osseous tumors, the minimal disruption of muscle and osseous structural elements may avoid iatrogenic instability and the need for fusion and diminish the risk of chronic pain. Patients with metastatic tumors usually require postoperative radiation and systemic therapy. The minimal soft tissue disruption with the use of MIS techniques facilitates healing and allows the patients to proceed to postoperative therapy with minimal delay. Finally, MIS diminishes the systemic surgical stress and facilitates postoperative recovery, thereby providing surgical options to patients at risk for prioprative complications after open surgery and expediting their return to home. The use of MIS techniques will continue to expand in spine tumor surgery, and new techniques, such as endoscopy and robot-assisted surgery, will only improve outcomes and patient access to surgery.
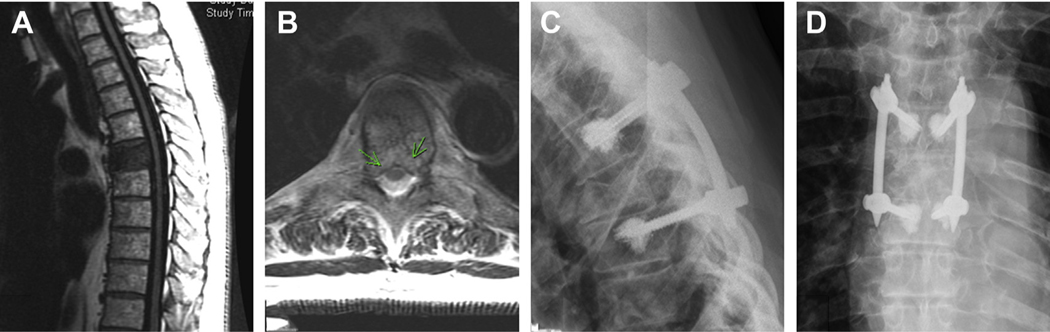
Fig. 2.
A 66-year-old woman with no previous history of cancer, presented with severe movement-related back pain and imaging suggestive of metastatic lung cancer involving spine, liver, lung, lymph nodes, and left pleura. Her MRI scan was significant for multiple pathologic fractures (A) and multilevel epidural disease, notably high-grade compression with bilateral pedicle and joint involvement at the level of T10 (B). Given her mechanical instability and high-grade cord compression, she underwent T6-T10 percutaneous stabilization, T7 and T8 kyphoplasty, and a left-sided T10 minimally invasive decompression. The left caudal screw incision was extended, and decompression was performed via an expandable retractor (C; asterisk, thecal sac). Postoperative AP (D) and lateral (E) radiographs demonstrate the stabilizing construct, and postoperative CT myelogram (F) demonstrates the left-sided pediculectomy, partial facetectomy, and reconstitution of the thecal sac at the level of T10.
KEY POINTS.
Innovation in surgical technique and contemporary spinal instrumentation paired with intraoperative navigation/imaging concepts allows for safer and less-invasive surgical approaches.
The combination of stereotactic body radiotherapy, contemporary surgical adjuncts, and less-invasive techniques serves to minimize blood loss, soft tissue injury, and length of hospital stay without compromising surgical efficacy, potentially enabling patients to begin adjuvant treatment sooner.
Footnotes
DISCLOSURE
Dr I. Laufer is Consultant for SpineWave, Depuy/Synthes, Medtronic, Globus, and Brainlab. Dr Bilsky Royalties from Globus and Depuy, Brainlab and Varian speaker’s bureau. Dr J.E. O’Toole Globus - Consulting/royalties, RTI Surgical - Royalties, Theracell: stock ownership.
REFERENCES
- 1.Lis E, Laufer I, Barzilai O, et al. Change in the cross-sectional area of the thecal sac following balloon kyphoplasty for pathological vertebral compression fractures prior to spine stereotactic radiosurgery. J Neurosurg Spine 2018;30(1):111–8. [DOI] [PubMed] [Google Scholar]
- 2.Barzilai O, McLaughlin L, Lis E, et al. Utility of cement augmentation via percutaneous fenestrated pedicle screws for stabilization of cancer related spinal instability. Oper Neurosurg (Hagerstown) 2018;16(5):593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moussazadeh N, Rubin DG, McLaughlin L, et al. Short-segment percutaneous pedicle screw fixation with cement augmentation for tumor-induced spinal instability. Spine J 2015;15(7):1609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang CW, Fu TS, Lin DY, et al. Percutaneous balloon kyphoplasty and short instrumentation compared with traditional long instrumentation for thoracolumbar metastatic spinal cord compression. World Neurosurg 2019;130:e640–7. [DOI] [PubMed] [Google Scholar]
- 5.Saadeh YS, Elswick CM, Fateh JA, et al. Analysis of outcomes between traditional open versus mini-open approach in surgical treatment of spinal metastasis. World Neurosurg 2019;130:e467–74. [DOI] [PubMed] [Google Scholar]
- 6.Lau D, Chou D. Posterior thoracic corpectomy with cage reconstruction for metastatic spinal tumors: comparing the mini-open approach to the open approach. J Neurosurg Spine 2015;23(2):217–27. [DOI] [PubMed] [Google Scholar]
- 7.Ray WZ, Schmidt MH. Thoracoscopic vertebrectomy for thoracolumbar junction fractures and tumors: surgical technique and evaluation of the learning curve. Clin Spine Surg 2016;29(7): E344–50. [DOI] [PubMed] [Google Scholar]
- 8.Ragel BT, Amini A, Schmidt MH. Thoracoscopic vertebral body replacement with an expandable cage after ventral spinal canal decompression. Neurosurgery 2007;61(5 Suppl 2):317–22 [discussion 322–3]. [DOI] [PubMed] [Google Scholar]
- 9.Archavlis E, Schwandt E, Kosterhon M, et al. A modified microsurgical endoscopic-assisted transpedicular corpectomy of the thoracic spine based on virtual 3-dimensional planning. World Neurosurg 2016;91:424–33. [DOI] [PubMed] [Google Scholar]
- 10.Dhandapani S, Karthigeyan M. “Microendoscopic” versus “pure endoscopic” surgery for spinal intradural mass lesions: a comparative study and review. Spine J 2018;18(9):1592–602. [DOI] [PubMed] [Google Scholar]
- 11.Telfeian AE, Choi DB, Aghion DM. Transforaminal endoscopic surgery under local analgesia for ventral epidural thoracic spinal tumor: case report. Clin Neurol Neurosurg 2015;134:1–3. [DOI] [PubMed] [Google Scholar]
- 12.Xie T, Xiu P, Song Y, et al. Percutaneous endoscopic excision and ablation of osteoid osteoma of the lumbar spine and sacrum: a technical note and outcomes. World Neurosurg 2019;133:121–6. [DOI] [PubMed] [Google Scholar]
- 13.Yin J, Wu H, Tu J, et al. Robot-assisted sacral tumor resection: a preliminary study. BMC Musculoskelet Disord 2018;19(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez-Cruet MJ, Welsh RJ, Hussain NS, et al. Use of the da Vinci minimally invasive robotic system for resection of a complicated paraspinal schwannoma with thoracic extension: case report. Neurosurgery 2012;71(1 Suppl Operative):209–14. [DOI] [PubMed] [Google Scholar]
- 15.Chinder PS, Hindiskere S, Doddarangappa S, et al. Robotic surgery assisted staged en-bloc sacrectomy for sacral chordoma: a case report. JBJS Case Connect 2019;9(2):e0240. [DOI] [PubMed] [Google Scholar]
- 16.Bederman SS, Lopez G, Ji T, et al. Robotic guidance for en bloc sacrectomy: a case report. Spine 2014; 39(23):E1398–401. [DOI] [PubMed] [Google Scholar]
- 17.Finley D, Sherman JH, Avila E, et al. Thorascopic resection of an apical paraspinal schwannoma using the da Vinci surgical system. J Neurol Surg A Cent Eur Neurosurg 2014;75(1):58–63. [DOI] [PubMed] [Google Scholar]
- 18.Faddoul J, Faddoul Y, Kobaiter-Maarrawi S, et al. Radiofrequency ablation of spinal osteoid osteoma: a prospective study. J Neurosurg Spine 2017; 26(3):313–8. [DOI] [PubMed] [Google Scholar]
- 19.Gazis AN, Beuing O, Franke J, et al. Bipolar radiofrequency ablation of spinal tumors: predictability, safety and outcome. Spine J 2014;14(4):604–8. [DOI] [PubMed] [Google Scholar]
- 20.Tomasian A, Wallace A, Northrup B, et al. Spine cryoablation: pain palliation and local tumor control for vertebral metastases. AJNR Am J Neuroradiol 2016;37(1):189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomasian A, Wallace AN, Jennings JW. Benign spine lesions: advances in techniques for minimally invasive percutaneous treatment. AJNR Am J Neuroradiol 2017;38(5):852–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghia AJ, Rebueno NC, Li J, et al. The use of image guided laser interstitial thermotherapy to supplement spine stereotactic radiosurgery to manage metastatic epidural spinal cord compression: proof of concept and dosimetric analysis. Pract Radiat Oncol 2016;6(2):e35–8. [DOI] [PubMed] [Google Scholar]
- 23.Tatsui CE, Lee SH, Amini B, et al. Spinal laser interstitial thermal therapy: a novel alternative to surgery for metastatic epidural spinal cord compression. Neurosurgery 2016;79(Suppl 1):S73–82. [DOI] [PubMed] [Google Scholar]
- 24.Noel MA, Segura MJ, Sierre S, et al. Neurophysiological monitoring in radiofrequency ablation of spinal osteoid osteoma with a progressive time and temperature protocol in children. Spine Deform 2017;5(5):351–9. [DOI] [PubMed] [Google Scholar]
- 25.Hadjipavlou A, Tosounidis T, Gaitanis I, et al. Balloon kyphoplasty as a single or as an adjunct procedure for the management of symptomatic vertebral haemangiomas. J Bone Joint Surg Br 2007;89(4): 495–502. [DOI] [PubMed] [Google Scholar]
- 26.Premat K, Clarencon F, Cormier E, et al. Long-term outcome of percutaneous alcohol embolization combined with percutaneous vertebroplasty in aggressive vertebral hemangiomas with epidural extension. Eur Radiol 2017;27(7):2860–7. [DOI] [PubMed] [Google Scholar]
- 27.Amendola L, Cappuccio M, Boriani L, et al. Endoscopic excision of C2 osteoid osteoma: a technical case report. Eur Spine J 2013;22(Suppl 3):S357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campos WK, Gasbarrini A, Boriani S. Case report: curetting osteoid osteoma of the spine using combined video-assisted thoracoscopic surgery and navigation. Clin Orthop Relat Res 2013;471(2): 680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kruger MT, Steiert C, Glasker S, et al. Minimally invasive resection of spinal hemangioblastoma: feasibility and clinical results in a series of 18 patients. J Neurosurg Spine 2019;1–10. [DOI] [PubMed] [Google Scholar]
- 30.Afathi M, Peltier E, Adetchessi T, et al. Minimally invasive transmuscular approach for the treatment of benign intradural extramedullary spinal cord tumours: technical note and results. Neurochirurgie 2015;61(5):333–8. [DOI] [PubMed] [Google Scholar]
- 31.Formo M, Halvorsen CM, Dahlberg D, et al. Minimally invasive microsurgical resection of primary, intradural spinal tumors is feasible and safe: a consecutive series of 83 patients. Neurosurgery 2018;82(3):365–71. [DOI] [PubMed] [Google Scholar]
- 32.Ogden AT, Fessler RG. Minimally invasive resection of intramedullary ependymoma: case report. Neurosurgery 2009;65(6):E1203–4 [discussion: E1204]. [DOI] [PubMed] [Google Scholar]
- 33.Winkler EA, Lu A, Rutledge WC, et al. A mini-open transspinous approach for resection of intramedullary spinal cavernous malformations. J Clin Neurosci 2018;58:210–2. [DOI] [PubMed] [Google Scholar]
- 34.Tredway TL. Minimally invasive approaches for the treatment of intramedullary spinal tumors. Neurosurg Clin N Am 2014;25(2):327–36. [DOI] [PubMed] [Google Scholar]
- 35.Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine 2010;35(22):E1221–9. [DOI] [PubMed] [Google Scholar]
- 36.Berenson J, Pflugmacher R, Jarzem P, et al. Balloon kyphoplasty versus non-surgical fracture management for treatment of painful vertebral body compression fractures in patients with cancer: a multicentre, randomised controlled trial. Lancet Oncol 2011;12(3):225–35. [DOI] [PubMed] [Google Scholar]
- 37.Schwab JH, Gasbarrini A, Cappuccio M, et al. Minimally invasive posterior stabilization improved ambulation and pain scores in patients with plasmacytomas and/or metastases of the spine. Int J Surg Oncol 2011;2011:239230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamad A, Vachtsevanos L, Cattell A, et al. Minimally invasive spinal surgery for the management of symptomatic spinal metastasis. Br J Neurosurg 2017;31(5):526–30. [DOI] [PubMed] [Google Scholar]
- 39.Moliterno J, Veselis CA, Hershey MA, et al. Improvement in pain after lumbar surgery in cancer patients with mechanical radiculopathy. Spine J 2014;14(10): 2434–9. [DOI] [PubMed] [Google Scholar]
- 40.Chou D, Lu DC. Mini-open transpedicular corpectomies with expandable cage reconstruction. Technical note. J Neurosurg Spine 2011;14(1):71–7. [DOI] [PubMed] [Google Scholar]
- 41.Deutsch H, Boco T, Lobel J. Minimally invasive transpedicular vertebrectomy for metastatic disease to the thoracic spine. J Spinal Disord Tech 2008; 21(2):101–5. [DOI] [PubMed] [Google Scholar]
- 42.Massicotte E Minimal access spine surgery (MASS) for decompression and stabilization performed as an out-patient procedure for metastatic spinal tumours followed by spine stereotactic body radiotherapy (SBRT): first report of technique and preliminary outcomes. Technol Cancer Res Treat 2012;11(1):15–25. [DOI] [PubMed] [Google Scholar]
- 43.Taghva A, Li KW, Liu JC, et al. Minimally invasive circumferential spinal decompression and stabilization for symptomatic metastatic spine tumor: technical case report. Neurosurgery 2010;66(3):E620–2. [DOI] [PubMed] [Google Scholar]
- 44.Smith ZA, Li Z, Chen NF, et al. Minimally invasive lateral extracavitary corpectomy: cadaveric evaluation model and report of 3 clinical cases. J Neurosurg Spine 2012;16(5):463–70. [DOI] [PubMed] [Google Scholar]
- 45.Donnelly DJ, Abd-El-Barr MM, Lu Y. Minimally invasive muscle sparing posterior-only approach for lumbar circumferential decompression and stabilization to treat spine metastasis–technical report. World Neurosurg 2015;84(5):1484–90. [DOI] [PubMed] [Google Scholar]
- 46.Venkatesh R, Tandon V, Patel N, et al. Solitary plasmacytoma of L3 vertebral body treated by minimal access surgery: common problem different solution! J Clin Orthop Trauma 2015; 6(4):259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barzilai O, McLaughlin L, Amato MK, et al. Minimal access surgery for spinal metastases: prospective evaluation of a treatment algorithm using patientreported outcomes. World Neurosurg 2018;120: e889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uribe JS, Dakwar E, Le TV, et al. Minimally invasive surgery treatment for thoracic spine tumor removal: a mini-open, lateral approach. Spine 2010;35(26 Suppl):S347–54. [DOI] [PubMed] [Google Scholar]
- 49.Serak J, Vanni S, Levi AD. The extreme lateral approach for treatment of thoracic and lumbar vertebral body metastases. J Neurosurg Sci 2019;63(4): 473–8. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Boland P, Mitra N, et al. Single-stage posterolateral transpedicular approach for resection of epidural metastatic spine tumors involving the vertebral body with circumferential reconstruction: results in 140 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine 2004;1(3):287–98. [DOI] [PubMed] [Google Scholar]
- 51.Laufer I, Iorgulescu JB, Chapman T, et al. Local disease control for spinal metastases following “separation surgery” and adjuvant hypofractionated or highdose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J Neurosurg Spine 2013;18(3):207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nasser R, Nakhla J, Echt M, et al. Minimally invasive separation surgery with intraoperative stereotactic guidance: a feasibility study. World Neurosurg 2018;109:68–76. [DOI] [PubMed] [Google Scholar]
- 53.Turel MK, Kerolus MG, O’Toole JE. Minimally invasive “separation surgery” plus adjuvant stereotactic radiotherapy in the management of spinal epidural metastases. J Craniovertebr Junction Spine 2017; 8(2):119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tatsui CE, Stafford RJ, Li J, et al. Utilization of laser interstitial thermotherapy guided by real-time thermal MRI as an alternative to separation surgery in the management of spinal metastasis. J Neurosurg Spine 2015;23(4):400–11. [DOI] [PubMed] [Google Scholar]