Abstract
Excessive alcohol (ethanol) consumption negatively impacts social, emotional, as well as cognitive function and well-being. Thus, identifying behavioral and/or biological predictors of excessive ethanol consumption is important for developing prevention and treatment strategies against alcohol use disorders (AUDs). Sex differences in alcohol consumption patterns are observed in humans, primates, and rodents. Selectively bred high alcohol-drinking rat lines, such as the “HAD-1” lines are recognized animal models of alcoholism. The present work examined sex differences in alcohol consumption, object recognition, and exploratory behavior in male and female HAD-1 rats. Naïve male and female HAD-1 rats were tested in an object recognition test (ORT) prior to a chronic 24 h intermittent ethanol access procedure for five weeks. Object recognition parameters measured included exploratory behavior, object investigation, and time spent near objects. During the initial training trial, rearing, active object investigation and amount of time spent in the object-containing section was significantly greater in female HAD-1 rats compared to their male counterparts. During the subsequent testing trial, time spent in the object-containing section was greater in female, compared to male, rats; but active object investigation and rearing did not statistically differ between females and males. In addition, female HAD-1 rats consumed significantly more ethanol than their male counterparts, replicating previous findings. Moreover, across all animals there was a significant positive correlation between exploratory behavior in ORT and ethanol consumption level. These results indicate there are significant sex differences in cognitive performance and alcohol consumption in HAD-1 rats, which suggests neurobiological differences as well.
Keywords: Object recognition test, Selectively bred high alcohol-drinking rats, Alcohol use disorders, Exploratory behaviour, Novel object recognition, Attention
1. Introduction
Alcohol use disorder (AUD) is a widely prevalent and debilitating health condition with significant negative medical consequences and vast societal socioeconomic costs. In the US, the estimated cost associated with excessive alcohol consumption in 2010 was $249 billion [1]. The identification of behavioral and biological predictors of AUD is an important goal in the field, as it would facilitate determination of at-risk individuals and novel intervention strategies. Although a variety of genetic and environmental factors associated with AUD and dependence have been identified [2,3], how these factors differ by sex remains unclear [4,5].
Clinically, males consume more alcohol than females, however current studies show that the gap between male and female alcohol consumption rates has narrowed in recent decades [6,7]. In rodents, females tend to drink more than their male counterparts in varying ethanol access procedures [8]. Our laboratory recently reported this sex difference in ethanol consumption by selectively bred High Alcohol-Drinking (HAD-1) rats, with females drinking more than males [9]. While little is known about sex differences in the HAD-1 line several studies indicate novelty-associated behaviors may be reliable predictors for ethanol consumption in humans and rodents [10–13]. This suggests that differences in baseline cognitive function and/or emotional reactivity may be associated with AUD and ethanol consumption in this rat line. The present study sought to determine whether baseline cognitive performance differed between male and female HAD-1 rats and identify behaviors that may be associated with ethanol consumption. Here, male and female HAD-1 rats were tested on an object recognition test (ORT) that measures attention, exploratory activity, and working memory [14] prior to a three-bottle choice protocol of ethanol access. Exploratory behavior, object investigation, and time near object were the dependent measures for ORT, with ethanol consumption measured subsequently.
2. Materials and methods
2.1. Subjects
Eight male and eight female high-alcohol-drinking rats (HAD-1 generation = 68; four weeks of age) from the Alcohol Research Resource Center at the Indiana University School of Medicine were used in this study. Animals were housed under a reverse light/dark cycle (lights out at 10:00 AM) and were group- and single-housed in plastic cages (22 × 44 × 20 cm) with wood chip bedding. Animals were handled daily (approx. 10 min/day) for four weeks prior to the start of the experiment in a behavioral testing room separate from the vivarium. Animals were group-housed by sex in wire-topped plastic cages until one week prior to the start of the experiment when they were single-housed and remained single-housed thereafter. Rats received food and water ad libitum throughout the experiment, except during ORT. The experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas at Austin. In addition, all procedures were conducted in accordance with principles outlined in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
2.2. Novel object recognition test
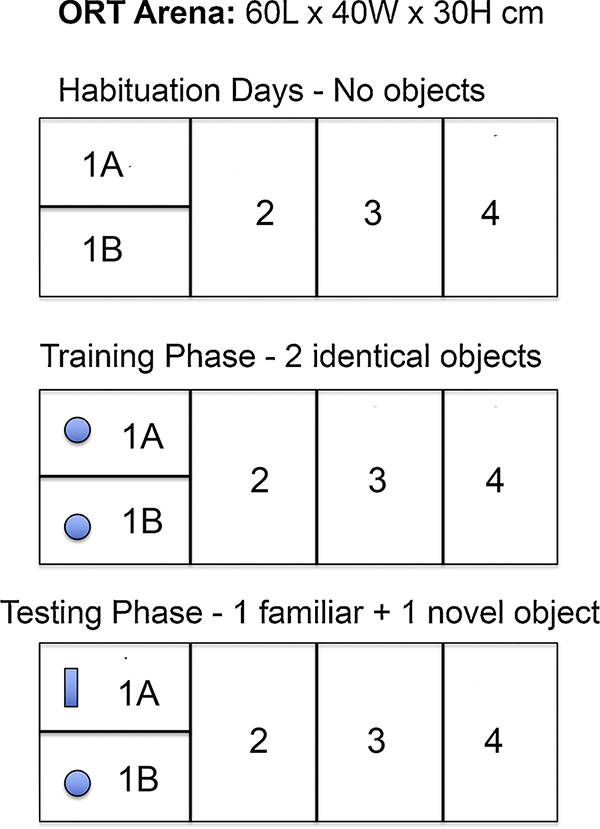
Prior to ORT testing, animals were habituated to the ORT arena (60 l × 40 W × 30H cm) for 20 min per day for two days. The floor of the arena was equally divided into four sections (e.g., each 15 cm in length) labeled 1–4 (see Fig. 1). During the 2 Habituation days, no objects were present in the ORT arena. On the third day, the ORT was performed in two phases, a Training Phase and a Testing Phase. For the Training Phase, two identical objects were placed in section 1 (1A vs 1B) of the arena. Each rat was then placed in the center of the arena and allowed to explore for 10 min. After 10 min the rat was removed from the arena and placed back in the home cage. After 60 min, the Test Phase was conducted. For the Test Phase, one of the two identical objects was replaced with a different (e.g. novel) object. The novel object was of similar size to the original object but differed in texture and shape. Other than the introduction of a novel object, the Test Phase procedure was identical to the Training Phase, in that animals were placed in the center of the arena and were allowed to explore the space for 10 min. During the Training and Testing Phases, rats were videotaped from above the arena for later determination of the amount of time each rat engaged in investigating the objects, the amount of time occupying each of the numbered sections of the arena and the number of rearing behaviors occurring during the sessions. Two-way ANOVA was used to assess the effect of sex on the recorded behaviors. Student’s t-tests were used to assess sex differences in behaviors within the object containing quadrant (1A vs 1B).
Fig. 1. ORT arena layout.
Animals were habituated to the ORT arena for 20 min per day for two days with no objects were present. On the third day, the ORT was performed in two phases, a Training Phase and a Testing Phase. For the Training Phase, two identical objects were placed in section 1 (1A vs 1B) of the arena. Each rat was then placed in the center of the arena and allowed to explore for 10 min. After 10 min the rat was removed from the arena and placed back in the home cage. After 60 min, the Test Phase was conducted. For the Test Phase, one of the two identical objects was replaced with a different (e.g. novel) object.
2.3. Ethanol availability sessions
Following the ORT test, rats were given access to 24 -h chronic intermittent ethanol sessions 3 days/week for 5 weeks using a three-bottle choice paradigm. The sessions began at the beginning of the dark cycle. The rats were weighed at the beginning of each session and received 3 bottles containing water, 15 % and 30 % ethanol. The bottles were removed 24 h later and weighed to measure ethanol and water consumption. Repeated-measures ANOVA was used to assess the effect of sex on ethanol consumption and total fluid intake. Linear regression models were used to assess potential correlations between object recognition behaviors and ethanol consumption levels.
3. Results
3.1. ORT training phase
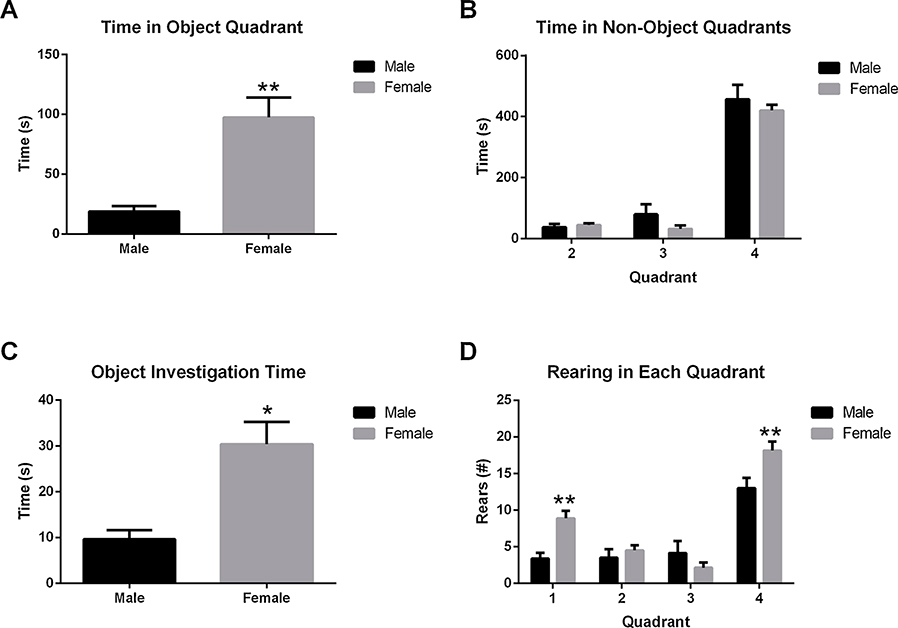
During the initial Training Phase, female HAD-1 rats spent significantly more time in the object-containing section of the arena (i.e., Quadrant 1 vs the combination of Quadrants 2, 3, and 4: Fig. 2A; t14 = 4.514, p < 0.001) and more time investigating the two identical objects (i.e., Quadrant 1A and Quadrant 1B: Fig. 2C; t14 = 3.914, p < 0.01) compared to male HAD-1 rats. Despite this, no significant sex differences were observed in total time spent in the other quadrants (Fig. 2B). Moreover, there was a significant Sex*Quadrant interaction (Fig. 2D; F3,56 = 4.926, p < 0.01) and a main effect of sex (F1,56 = 8.923, p < 0.01) on the total number of rears. Post-hoc analyses revealed that female HAD-1 rats made more rears than male HAD-1 rats in the object-containing areas (t56 = 3.414, p < 0.001) and the opposite end of the arena (i.e., Quadrant 4: t56 = 3.181, p < 0.001).
Fig. 2. Sex differences in exploratory activity during the sample trial of ORT.
A) Female rats spent more time investigating the objects than male rats (* = p < 0.01). B) There were no sex differences in the time spent in the other three quadrants. C) Female rats reared more in the object quadrant than male rats (** = p < 0.001). D) There was a significant Sex*Quadrant interaction (* = p < 0.01) and a main effect of sex (* = p < 0.01) on rearing behaviors during the sample trial. Post-hoc analysis revealed that female rats made more rears in than male rats in first (** = p < 0.001) and the fourth (** = p < 0.001) quadrants.
3.2. Testing phase
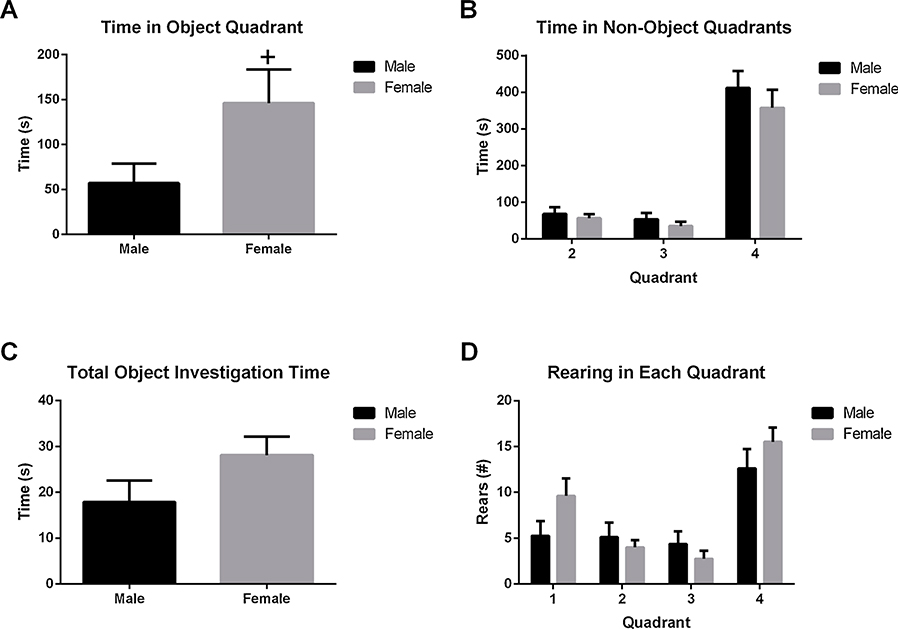
During the subsequent Testing Phase, the sex difference in amount of time spent in the object-containing quadrant reached marginal significance (i.e., Quadrant 1 vs the combination of Quadrants 2, 3, and 4: Fig. 3A; t14 = 2.063, p = 0.058), with female HAD-1 rats occupying the object-containing quadrant longer than male HAD-1 rats. No further sex differences were observed in the time spent in other quadrants (Fig. 3B), time spent investigating the objects (Fig. 3C) or total number of rears made during the test session (Fig. 3D). Both male and female HAD-1 rats tended to investigate the novel object (female: 17.75 ± 3.05 s; male: 10.88 ± 3.32 s) more than the familiar object (female: 10.38 ± 2.86 s; male: 7.00 ± 1.89 s). This suggests no significant sex difference in the ability to discriminate a novel vs familiar object.
Fig. 3. Sex differences in exploratory activity during the test trial of ORT.
A) Female rats spent more time exploring the object quadrant than male rats (+ = p @ 0.058). There were no further sex differences in B) the time spent in the other three quadrants, C) time exploring the objects, or D) rearing behaviors during the test trial.
3.3. Ethanol consumption
Ethanol consumption was calculated by combining g/kg intake levels from the 15 % and 30 % EtOH-containing bottles. Male HAD rats consumed more alcohol from the 30 % bottle (82.69 ml on average) than from the 15 % bottle (62.70 ml on average), however this difference did not reach significance (t(5) < 1. Although female HAD rats consumed less alcohol from the 30 % bottle (71.38 ml on average) than from the 15 % bottle (98.77 ml on average) this difference did not reach significance (t(5) < 1.
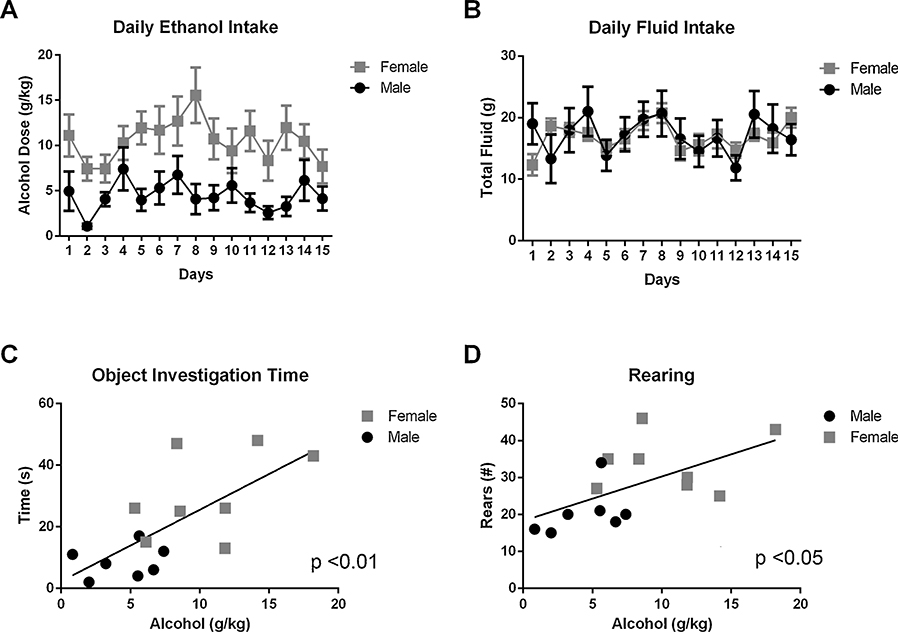
Female HAD-1 rats consumed significantly higher amounts of alcohol (g/kg) than the male rats throughout the 5-week (3 days/wk) ethanol drinking experiment (Fig. 4A; F1,195 = 70.08, p < 0.0001). There were no sex differences in total fluid consumption during the ethanol drinking experiment (Fig. 4B). Linear regression modeling showed a significant correlation between ethanol consumption and the object investigation time (Fig. 4C; r = .70, F1,13 = 13.08, p < 0.01), as well as rearing behavior (Fig. 4D; r = .57, F1,13 = 6.74, p < 0.05) during the training phase.
Fig. 4. ORT exploratory activity associated with future alcohol consumption.
A) Female rats drank significantly more alcohol than male rats (p < 0.0001). B) There were no sex differences in total fluid intake. C) There was a significant correlation between object investigation time during the sample trial and total alcohol consumption (p < 0.01). D) There was a significant correlation between total rearing during the sample trial and total alcohol consumption (p < 0.05).
4. Discussion
The identification of behavioral predictors of high alcohol consumption is important for treatment and prevention strategies. The present study showed that there are sex differences in exploratory and investigative behaviors at baseline in the HAD-1 line of rats. Further, these behavioral parameters correlated with subsequent ethanol consumption. Here, female rats showed greater exploratory activity and object investigation during the initial training phase of ORT that significantly correlated with subsequent ethanol consumption. Together these results suggest that exploratory rearing and object investigation may be a predictor for the propensity to consume excessive ethanol HAD-1 rats.
Several studies have shown that female rats typically consume higher levels of ethanol than their male counterparts [15,16]. The findings in the present work are consistent with these reports and our previous work comparing HAD-1 males and females [9], but did differ from another HAD-1 study that did not detect sex differences in ethanol consumption [17]. One potential explanation for these contrasting findings may be due to procedural differences between the studies. For example, the Dhaher et al. study [17] utilized a two-bottle choice paradigm with continuous 22-hr per day access to 15 % ethanol for 30 days, whereas the present study used a three-bottle choice design (water, 15 % and 30 % ethanol) and provided access to ethanol 3 days per week for 5 weeks. Another difference is that the finding of greater ethanol consumption by male HAD-1 rats was primarily restricted to adolescent, and not adult, rats. Interestingly, another study in adolescent and adult HAD rats showed the same sex difference in adolescent, but not adult, rats [18]. In the current study, HAD-1 females attended to novel and familiar objects for longer periods and displayed more exploratory rearing compared to HAD-1 males. Therefore, it is possible that the presence of an extra bottle and the disruption associated with the removal and reintroduction of the bottles may differentially favor increased consumption in the female rats due to their heightened attention and exploratory activity levels. These hypotheses will need to be directly tested in future studies, to address the sex-dependent differences observed herein.
While ORT is most commonly used to test memory, in particular working memory [19], exploratory and investigative behaviors during the training phase can reflect novelty-seeking [20]. Novelty-seeking, in general, has been a good indicator for an initial propensity to take drugs [21], as well as the subsequent tendency for compulsive use and relapse [22]. In the brain, high levels of novelty-induced activity are associated with increased levels of galanin, enkephalin, and orexin in the hypothalamus, a brain region involved in regulating alcohol intake [11,23]. In addition, metabotropic glutamate receptor 5 (mGLUR5) receptors on D-1 expressing neurons have been identified as a common molecular substrate between novelty-seeking behaviors and behaviors associated with alcohol abuse [24]. On the other hand, low levels of neurotensin in the posterior paraventricular nucleus of the thalamus (pPVT) is associated with both increased drinking and increased rearing in a novel chamber [12]. Decreased cholinergic activity is associated with a decrease in exploratory activity as the environment, mediated in part by the hippocampus, becomes familiar [25]. Further, the differences in investigation and exploratory behaviors observed here may be due to potential differences in neural pathways regulating attention and arousal in HAD-1 rats [14]. Our findings provide a rationale for future studies directly exploring these neurocircuitry in HAD-1 rats.
In summary, the present work provided novel evidence of increased exploratory and investigative behavior in female HAD-1 rats, compared to their male counterparts. Moreover, the observed sex differences in novel object investigation and rearing behavior are predictive of increased alcohol consumption behaviors in this rodent line. These behavioral outcomes may represent functional differences in neurochemical substrates underlying alcohol and drug-seeking behaviors. These results highlight the need for direct examination of these systems in male and female HAD-1 rats.
Acknowledgements
Research support: Gates Millennium Scholars Foundation (NM), NIAAA013517 (CLD), AA015512, AA013522 (RLB), University of Texas Waggoner Center for Alcohol and Addiction Research.
References
- [1].Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD, 2010 National and state costs of excessive alcohol consumption, Am. J. Prev. Med 49 (2015) 73–79, 10.1016/j.amepre.2015.05.031. [DOI] [PubMed] [Google Scholar]
- [2].Enoch MA, Genetic influences on response to alcohol and response to pharmacotherapies for alcoholism, Pharmacol. Biochem. Behav 123 (2014) 17–24, 10.1016/j.pbb.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Reilly MT, Noronha A, Goldman D, Koob GF, Genetic studies of alcohol dependence in the context of the addiction cycle, Neuropharmacology (2017), 10.1016/j.neuropharm.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Becker JB, Koob GF, Sex differences in animal models: focus on addiction, Pharmacol. Rev 68 (2016) 242–263, 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Becker JB, McClellan ML, Reed BG, Sex differences, gender and addiction, J. Neurosci. Res. 95 (2017) 136–147, 10.1002/jnr.23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Substance Abuse and Mental Health Services Administration, S.A, M.H.S.S. Administration, Results From the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Ser. H-48, HHS Publ. No. 14–4863, Subst. Abus. Ment. Heal. Serv. Adm, Rockville, MD, 2014, pp. 1–143 https://doi.org/NSDUHSeriesH-41,HHS Publication No. (SMA) 11–4658. [Google Scholar]
- [7].White A, Castle I-JP, Chen CM, Shirley M, Roach D, Hingson R, Converging patterns of alcohol use and related outcomes among females and males in the United States, 2002 to 2012, Alcohol. Clin. Exp. Res 39 (2015) 1712–1726, 10.1111/acer.12815. [DOI] [PubMed] [Google Scholar]
- [8].Juárez J, Barrios de Tomasi E, Sex differences in alcohol drinking patterns during forced and voluntary consumption in rats, Alcohol. 19 (1999) 15–22, 10.1016/S0741-8329(99)00010-5. [DOI] [PubMed] [Google Scholar]
- [9].Mittal N, Thakore N, Bell RL, Maddox WT, Schallert T, Duvauchelle CL, Sex-specific ultrasonic vocalization patterns and alcohol consumption in high alcohol-drinking (HAD-1) rats, Physiol. Behav 203 (2019) 81–90, 10.1016/j.physbeh.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Manzo L, Gómez MJ, Callejas-Aguilera JE, Donaire R, Sabariego M, Fernández-Teruel A, Cañete A, Blázquez G, Papini MR, Torres C, Relationship between ethanol preference and sensation/novelty seeking, Physiol. Behav 133 (2014) 53–60, 10.1016/j.physbeh.2014.05.003. [DOI] [PubMed] [Google Scholar]
- [11].Barson JR, Fagan SE, Chang GQ, Leibowitz SF, Neurochemical heterogeneity of rats predicted by different measures to be high ethanol consumers, Alcohol. Clin. Exp. Res 37 (2013) 141–151, 10.1111/j.1530-0277.2012.01858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pandey S, Badve PS, Curtis GR, Leibowitz SF, Barson JR, Neurotensin in the posterior thalamic paraventricular nucleus: Inhibitor of pharmacologically relevant ethanol drinking, Addict. Biol (2017), 10.1111/adb.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Foulds J, Newton-Howes G, Guy NH, Boden JM, Mulder RT, Dimensional personality traits and alcohol treatment outcome: a systematic review and meta-analysis, Addiction 112 (2017) 1345–1357, 10.1111/add.13810. [DOI] [PubMed] [Google Scholar]
- [14].Antunes M, Biala G, The novel object recognition memory: neurobiology, test procedure, and its modifications, Cogn. Process (2012), 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maldonado-Devincci AM, Alipour KK, Michael LA, Kirstein CL, Repeated binge ethanol administration during adolescence enhances voluntary sweetened ethanol intake in young adulthood in male and female rats, Pharmacol. Biochem. Behav 96 (2010) 476–487, 10.1016/j.pbb.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li TK, Lumeng L, Alcohol preference and voluntary alcohol intakes of inbred rat strains and the National Institutes of Health heterogeneous stock of rats, Alcohol. Clin. Exp. Res 8 (1984) 485–486, 10.1111/j.1530-0277.1984.tb05708.x. [DOI] [PubMed] [Google Scholar]
- [17].Dhaher R, McConnell KK, Rodd ZA, McBride WJ, Bell RL, Daily patterns of ethanol drinking in adolescent and adult, male and female, high alcohol drinking (HAD) replicate lines of rats, Pharmacol. Biochem. Behav 102 (2012) 540–548, 10.1016/j.pbb.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bell RL, Rodd ZA, Hsu CC, Lumeng L, Li TK, Murphy JM, McBride WJ, Effects of concurrent access to a single concentration or multiple concentrations of ethanol on ethanol intake by periadolescent high-alcohol-drinking rats, Alcohol (2004), 10.1016/S0741-8329(04)00096-5. [DOI] [PubMed] [Google Scholar]
- [19].Ennaceur A, Delacour J, A new one - trial test for neurobiological studies of memory in rats. 1: Behavioral data, Behav. Brain Res 31 (1988) 47–59, 10.1016/0166-4328(88)90157-X. [DOI] [PubMed] [Google Scholar]
- [20].Dere E, Huston JP, De Souza Silva MA, The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents, Neurosci. Biobehav. Rev 31 (2007) 673–704, 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- [21].Piazza PV, Deminière JM, Le Moal M, Simon H, Factors that predict individual vulnerability to amphetamine self-administration, Science 245 (1989) 1511–1513, 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- [22].Flagel SB, Waselus M, Clinton SM, Watson SJ, Akil H, Antecedents and consequences of drug abuse in rats selectively bred for high and low response to novelty, Neuropharmacology 76 (2014) 425–436, 10.1016/j.neuropharm.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Barson JR, Leibowitz SF, Hypothalamic neuropeptide signaling in alcohol addiction, Prog. Neuropsychopharmacol. Biol. Psychiatry 65 (2016) 321–329, 10.1016/j.pnpbp.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Parkitna JR, Sikora M, Gołda S, Gołembiowska K, Bystrowska B, Engblom D, Bilbao A, Przewlocki R, Novelty-seeking behaviors and the escalation of alcohol drinking after abstinence in mice are controlled by metabotropic glutamate receptor 5 on neurons expressing dopamine D1 receptors, Biol. Psychiatry (2013), 10.1016/j.biopsych.2012.07.019. [DOI] [PubMed] [Google Scholar]
- [25].Inglis FM, Day JC, Fibiger HC, Enhanced acetylcholine release in hippocampus and cortex during the anticipation and consumption of a palatable meal, Neuroscience 62 (1994) 1049–1056, 10.1016/0306-4522(94)90342-5. [DOI] [PubMed] [Google Scholar]